Abstract
Background
Therapies to prevent recurrence of Clostridioides difficile infection (CDI) in pediatric patients are needed. Bezlotoxumab is a fully human monoclonal antibody approved for prevention of recurrent CDI in adults. We assessed the pharmacokinetics, safety, tolerability, and efficacy of bezlotoxumab in pediatric patients.
Methods
MODIFY III was a multicenter, double-blind, placebo-controlled study of bezlotoxumab in children (1 to <18 years) receiving antibacterial treatment for CDI. Participants were randomized 3:1 to receive a single infusion of bezlotoxumab (10 mg/kg) or placebo and were stratified by age at randomization (cohort 1: 12 to <18 years, cohort 2: 1 to <12 years). The primary objective was to characterize bezlotoxumab pharmacokinetics to support dose selection for pediatric patients; the primary endpoint was the area under the bezlotoxumab serum concentration–time curve (AUC0-inf). Safety, tolerability, and efficacy were monitored for 12 weeks post-infusion.
Results
A total of 148 participants were randomized and 143 were treated: 107 with bezlotoxumab and 36 with placebo (cohort 1 n = 60, cohort 2 n = 83; median age 9.0 years); 52.4% of participants were male and 80.4% were white. Geometric mean ratios (90% CI) for bezlotoxumab AUC0-inf were 1.06 (0.95, 1.18) and 0.82 (0.75, 0.89) h * μg/mL for cohorts 1 and 2, respectively. Bezlotoxumab 10 mg/kg was generally well-tolerated with an adverse event profile similar to placebo, including no treatment discontinuations due to adverse events. CDI recurrence was low and comparable for bezlotoxumab (11.2%) and placebo (14.7%).
Conclusions
The results of this study support the bezlotoxumab dose of 10 mg/kg for pediatric patients.
Trial registration
Keywords: adolescents, bezlotoxumab, children, Clostridioides difficile infection, recurrence
INTRODUCTION
Clostridioides difficile is an anaerobic, spore-forming gram-positive bacillus. Strains that cause disease in humans produce toxins, in particular C. difficile toxins A and B [1, 2]. C. difficile infection (CDI) occurs when toxigenic strains proliferate in the lower gastrointestinal tract after disruption of normal bacterial colonic flora, typically after exposure to antibiotics. Laboratory diagnosis of CDI requires detection of toxigenic C. difficile or its toxins in stool. CDI results in a wide spectrum of clinical disease ranging from mild diarrhea to severe illness with complications such as ileus, toxic megacolon, or death.
Although CDI more frequently affects adults, and mortality in high-risk patients is strongly associated with advanced age, a minority of cases occur in susceptible pediatric patients. In the US, an estimated 903 cases of CDI occurred among children 1–17 years old in 2018, with an estimated annual incidence of 35.74/100 000 persons, compared with 431.25/100 000 in those ≥65 years of age [3]. Many risk factors for CDI in pediatric patients are similar to those in adults, such as the use of broad-spectrum antibiotics and longer duration of hospital stay. However, CDI in pediatric patients is also strongly associated with additional factors, including malignancy, inflammatory bowel disease, and immune suppression [4–7]. CDI risk is highest in pediatric patients with malignancy [7–10].
A key component of the clinical management of CDI is reducing the risk of recurrence, especially in patients who have already experienced one or more recurrent episodes. Recurrent CDI within 8 weeks of a previous successfully treated CDI has been reported in 10.8%–34.4% of pediatric cases [11–15]. Risk factors for recurrent CDI in children are considered broadly similar to those in adults and include malignancy, recent surgery, exposure to multiple antibiotic classes, immune-suppressive medications, continued systemic antibacterial exposure during initial CDI, severe initial CDI, and infection with ribotype 027 [14–20].
Bezlotoxumab is a fully human monoclonal antibody (mAb) that binds to and neutralizes C. difficile toxin B. Bezlotoxumab does not have antimicrobial activity and is administered with antibacterial treatment for CDI. In adults with CDI, a single IV infusion of bezlotoxumab 10 mg/kg significantly reduced CDI recurrence compared to placebo and was generally well tolerated [21]. Bezlotoxumab is currently approved for the reduction (United States) or prevention (European Union) of recurrent CDI in adults who are receiving antibacterial treatment for CDI and are at high risk for CDI recurrence.
Therapies to prevent CDI recurrence in pediatric patients are needed. Our study was designed to assess the pharmacokinetics (PK) and safety of bezlotoxumab in children 1 to <18 years old, to support the selection of a dose that would achieve bezlotoxumab exposures in pediatric patients similar to those obtained in adults who received the recommended dose (10 mg/kg). A pediatric dose that would provide bezlotoxumab exposures within the clinical comparability bounds (0.6, 1.6) established in adults [22] was predefined as supporting the extrapolation of adult efficacy to children ≥1-year-old, based on similarities in disease pathogenesis, the pharmacology of bezlotoxumab, and the response to bezlotoxumab across these age groups.
METHODS
Study Design and Participants
MK-6072 Protocol 001 (NCT03182907) was a multicenter, randomized, double-blind, placebo-controlled study of bezlotoxumab in children receiving antibacterial treatment for CDI. The study was conducted at 49 centers in 15 countries in accordance with local and/or national regulations, Good Clinical Practice guidelines, and the Declaration of Helsinki principles regarding the protection of human participants in biomedical research. Patients or their guardians provided written informed consent and assent (if appropriate) prior to enrollment.
Eligible patients were ≥1 to <18 years of age with suspected or confirmed CDI (Table 1) and receiving or planning to receive a standard-of-care (SOC) antibiotic (oral vancomycin, metronidazole or fidaxomicin, or IV metronidazole with oral vancomycin or fidaxomicin) for 10–21 days. The lower age boundary of 1 year was selected in order to include all potential pediatric patients with clinical CDI, as susceptibility to CDI is established between 12 and 24 months of age [23]. Participants were randomized 3:1 to bezlotoxumab 10 mg/kg or placebo, stratified by age at randomization (Cohort 1: 12 to <18 years, Cohort 2: 1 to <12 years). The diagnosis of CDI had to be confirmed by detection of C. difficile toxin in stool at randomization, and treatment with a SOC antibiotic for CDI had to be ongoing.
Table 1.
Definition of Key Terms
| Term | Definition/Criteria |
|---|---|
| Suspected CDI | A change in normal bowel habits for two or more calendar days with either watery diarrhea or at least six unformed bowel movements within a 48-h period. |
| Severe CDI | The presence of bloody diarrhea, pseudomembranous colitis, diarrhea accompanied by dehydration (as judged by the treating physician), hypoalbuminemia (<2 g/dL), and/or fever (≥38.0°C) with leukocytosis (>15.0 × 109/L) [24]. |
| Initial clinical response | Improvement in the number and character of bowel movements and does not require further CDI therapy within 2 days after completing up to 21 days of antibacterial drug treatment for CDI. |
| CDI recurrence | Diarrhea recurrence within 12 weeks of study treatment, with a positive test for C. difficile toxin in stool, for which the participant received antibacterial treatment. |
| Sustained clinical response | A composite of initial clinical response of the baseline CDI episode and no CDI recurrence within 12 weeks of study treatment. |
| High risk for CDI recurrence | Participants were considered high-risk for CDI recurrence if they were immune-compromised or had any of the following: prior history of CDI, severe baseline CDI episode, C. difficile ribotype 027 isolated during the baseline episode, or treatment with ≥1 systemic antibacterial known to increase CDI risk (eg, clindamycin, fluoroquinolones, cephalosporins, aztreonam, penicillins, macrolides, and carbapenems). |
Participants were enrolled by age cohort with sequential enrollment into two panels, A and B. The purpose of Panel A was to determine the dose for each cohort and required a minimum of 12 participants per cohort to complete all study visits through 12 weeks. Enrollment into Panel B of each cohort began after review of safety and tolerability data from Panel A by an independent Data Monitoring Committee and review of the PK data by an unblinded designee to determine if dose modification was required. At least 24 participants were required in each age cohort at the final age-appropriate dose.
Study Treatments
Bezlotoxumab or placebo (0.9% sodium chloride or 5% dextrose) was administered as a single IV infusion over 60 (±10) min on day 1 of the study. Bezlotoxumab and placebo were dispensed in a blinded fashion through the use of an opaque sleeve over the infusion bag or syringe. The participants (and their parents/guardians) and the investigators involved in treatment administration or participant evaluations remained unaware of the treatment group assignments. Participants were followed for 12 weeks (85 ± 5 days) after the infusion; follow-up visits occurred on day 10 and weeks 4, 8, and 12. Initial clinical response (Table 1) was assessed by the investigator approximately 48 h after the last dose of SOC antibiotic.
Objectives and Endpoints
The primary objective was to characterize bezlotoxumab PK in 2 age cohorts (12 to <18 years and 1 to <12 years) to support dose selection for the pediatric population. The primary PK endpoint was the area under the bezlotoxumab serum concentration–time curve from 0 to infinity (AUC0-inf). The primary safety endpoints were the proportion of participants with adverse events (AEs) through 12 weeks following infusion and the proportion discontinuing study treatment due to AEs.
Secondary endpoints included rates of CDI recurrence and sustained clinical response (overall and in participants at high risk of CDI recurrence), infusion-related reactions, and treatment-emergent positive antibodies to bezlotoxumab. Definitions for CDI recurrence, sustained clinical response, and high risk for CDI recurrence are provided in Table 1.
Analyses
The noncompartmental PK analysis was performed on the per-protocol population, which consisted of 91 participants who received bezlotoxumab and had ≥4 post-dose PK samples. AUC0-inf data were natural-log transformed and compared to historical adult data using an analysis-of-variance model with a factor for age group. A point estimate of the geometric mean ratio (GMR, pediatric/adult) of bezlotoxumab AUC0-inf with 90% confidence interval (CI) was generated from the model for each cohort, and the 90% CIs were compared using prespecified clinical comparability bounds (0.6, 1.6). PK analysis methods are further described in Supplementary Material 1; immunogenicity analyses are described in Supplementary Material 2.
Safety analyses were performed on the all-patients-as-treated population, which included all randomized participants who received study treatment. The Miettinen–Nurminen asymptotic method [25] was used to calculate treatment-group differences and 95% CIs for AE incidence. Relationship of AE to study drug was determined by the primary investigator at each study site. Serious AEs (SAE) were those that resulted in death, were life-threatening, required or prolonged an inpatient hospitalization, resulted in persistent or significant disability/incapacity, or required medical or surgical intervention.
Efficacy analyses were performed on the modified intent-to-treat (mITT) population, which included all participants who received any amount of study infusion, had a positive test for C. difficile toxin, and were taking protocol-defined antibacterial treatment for CDI on the day of infusion. Two-sided 95% CIs were calculated with the Miettinen–Nurminen method stratified by age cohort using a Cochran–Mantel–Haenszel weight to evaluate the treatment differences for CDI recurrence and sustained clinical response.
RESULTS
Participants
A total of 148 participants were randomized: 111 to bezlotoxumab and 37 to placebo (Table 2), and 143 were treated: 107 with bezlotoxumab and 36 with placebo. Sixty treated participants were in age cohort 1 (12 to <18 years) and 83 were in age cohort 2 (1 to <12 years); five participants were <2 years of age. Demographic and baseline characteristics were generally comparable between the treatment groups in the combined cohorts (Table 3). The median age was 9.0 years; 52.4% of participants were male and 80.4% were white.
Table 2.
Disposition of All Randomized Participants
| Age Cohort 1, 12 to <18 years | Age Cohort 2, 1 to <12 years | |||||||
|---|---|---|---|---|---|---|---|---|
| Bezlotoxumab | Placebo | Bezlotoxumab | Placebo | |||||
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Participants in population | 46 | 16 | 65 | 21 | ||||
| Status for trial | ||||||||
| Completed | 42 | (91.3) | 16 | (100.0) | 61 | (93.8) | 19 | (90.5) |
| Discontinued | 4 | (8.7) | 0 | (0.0) | 4 | (6.2) | 2 | (9.5) |
| Death | 2 | (4.3) | 0 | (0.0) | 1 | (1.5) | 0 | (0.0) |
| Lost to follow-up | 0 | (0.0) | 0 | (0.0) | 1 | (1.5) | 0 | (0.0) |
| Protocol deviation | 2 | (4.3) | 0 | (0.0) | 2 | (3.1) | 0 | (0.0) |
| Withdrawal by parent/guardian | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 2 | (9.5) |
| Status for study treatment | ||||||||
| Completed | 44 | (95.7) | 16 | (100.0) | 63 | (96.9) | 20 | (95.2) |
| Not treated | 2 | (4.3) | 0 | (0.0) | 2 | (3.1) | 1 | (4.8) |
Three deaths occurred prior to the completion of the 12-week study follow-up period and are summarized in this table. Three participants died after completing the 12-week follow-up.
Table 3.
Demographic and Other Baseline Characteristics (All Participants as Treated)
| Characteristic | Bezlotoxumab (N = 107) | Placebo (N = 36) | Total (N = 143) | |||
|---|---|---|---|---|---|---|
| Sex, n (%) | ||||||
| Male | 57 | (53.3) | 18 | (50.0) | 75 | (52.4) |
| Female | 50 | (46.7) | 18 | (50.0) | 68 | (47.6) |
| Age (years) | ||||||
| 1 to <6 | 37 | (34.6) | 13 | (36.1) | 50 | (35.0) |
| 6 to <12 | 26 | (24.3) | 7 | (19.4) | 33 | (23.1) |
| 12 to <18 | 44 | (41.1) | 16 | (44.4) | 60 | (42.0) |
| Median (range) | 10.0 | (1-17) | 8.0 | (1-17) | 9.0 | (1-17) |
| Race, n (%) | ||||||
| American Indian/Alaska Native | 2 | (1.9) | 0 | (0.0) | 2 | (1.4) |
| Asian | 3 | (2.8) | 2 | (5.6) | 5 | (3.5) |
| Black or African American | 6 | (5.6) | 1 | (2.8) | 7 | (4.9) |
| Multiraciala | 9 | (8.4) | 1 | (2.8) | 10 | (7.0) |
| White | 83 | (77.6) | 32 | (88.9) | 115 | (80.4) |
| Hispanic or Latino, n (%) | 28 | (26.2) | 8 | (22.2) | 36 | (25.2) |
| Weight (kg), median (range) | 30.1 | (7.8-108.0) | 24.2 | (8.8-116.9) | 27.0 | (7.8-116.9) |
| Primary treatment for baseline CDI episode, n (%) | ||||||
| Vancomycin—enteral/oral | 44 | (41.1) | 15 | (41.7) | 59 | (41.3) |
| Fidaxomicin—enteral/oral | 14 | (13.1) | 5 | (13.9) | 19 | (13.3) |
| Metronidazole—enteral/oral | 47 | (43.9) | 16 | (44.4) | 63 | (44.1) |
Abbreviation: CDI, Clostridioides difficile infection.
aAll multiracial participants were Black/African American and White.
Risk factors for CDI recurrence were present in 94.2% (131/139) of the mITT population and were generally comparable between treatment groups (Table 4). The most common risk factors were immunocompromise (72.7%) and receipt of ≥1 systemic antibacterial known to increase CDI risk (62.6%). Approximately one-third of participants in each treatment group had ≥1 CDI episode prior to the baseline episode. Overall, 18.0% of participants met criteria for severe CDI (bezlotoxumab 19.2%, placebo 14.3%), most commonly bloody diarrhea (10.1%), fever (7.2%), and diarrhea with dehydration (5.0%). Three (2.2%) participants had severe complicated CDI at the baseline episode; all were in the bezlotoxumab group and met criteria for intensive care unit admission.
Table 4.
Risk Factors for CDI Recurrence (Modified Intent-to-Treat Population)
| Risk Factor | Bezlotoxumab (N = 104) | Placebo (N = 35) | Total (N = 139) | |||
|---|---|---|---|---|---|---|
| Immunocompromised | 73 | (70.2) | 28 | (80.0) | 101 | (72.7) |
| ≥1 CDI prior to baseline episode | 33 | (31.7) | 10 | (28.6) | 43 | (30.9) |
| Severe baseline CDI episode | 20 | (19.2) | 5 | (14.3) | 25 | (18.0) |
| C. difficile ribotype 027 | 3 | (2.9) | 1 | (2.9) | 4 | (2.9) |
| Systemic antibacterial treatmenta | 67 | (64.4) | 20 | (57.1) | 87 | (62.6) |
| Number of risk factors for CDI recurrence | ||||||
| 1 | 20 | (19.2) | 9 | (25.7) | 29 | (20.9) |
| 2 | 60 | (57.7) | 20 | (57.1) | 80 | (57.6) |
| 3 | 14 | (13.5) | 5 | (14.3) | 19 | (13.7) |
| 4 | 1 | (1.0) | 0 | (0.0) | 1 | (0.7) |
| 5 | 2 | (1.9) | 0 | (0.0) | 2 | (1.4) |
| No criteria met | 7 | (6.7) | 1 | (2.9) | 8 | (5.8) |
Abbreviation: CDI, Clostridioides difficile infection.
aTreatment with ≥1 systemic antibacterials on or before randomization, along with standard-of-care treatment for CDI baseline episode
A diverse range of C. difficile ribotypes was detected from stool samples collected at baseline in the mITT population (Supplementary Material 3). The most common ribotype was 014, detected in 8.6% of participants (bezlotoxumab 7.7%, placebo 11.4%). The incidence of ribotype 027 was low (2.9% in both groups). The overall mean susceptibility of C. difficile isolates to vancomycin, metronidazole, and fidaxomicin was similar between the treatment groups (Supplementary Material 4).
Study treatment was administered within 10 days of baseline CDI onset in 56.1% of participants in the mITT population. SOC antibiotics for the baseline CDI episode were comparable between treatment groups; the most common were metronidazole (53.2%) and vancomycin (40.3%). Mean duration of SOC antibiotics before study infusion was 7.6 days for bezlotoxumab and 7.8 days for placebo; the mean total duration of SOC antibiotics was 14.7 and 13.4 days, respectively.
Pharmacokinetics
Results of the interim PK analysis (Panel A) supported progression to Panel B at the same dose (10 mg/kg) in both age cohorts. The 90% CI of pediatric/adult GMR for AUC0-inf fell within the prespecified clinical comparability bounds (0.6, 1.6) for each age cohort in Panel A. Corresponding results for maximum serum concentration (Cmax) were comparable for each age cohort and to historical data in adults. In addition, the terminal half-life (t1/2) was generally consistent for each pediatric age cohort and compared with historical data in adults.
Results of the final analysis across the entire per-protocol population (Panels A and B) support the primary hypothesis that AUC0-inf in pediatric participants is similar to historical data in adults (Table 5). Specifically, the 90% CIs of pediatric/adult GMR for bezlotoxumab AUC0-inf were contained within the prespecified clinical comparability bounds of (0.6, 1.6) for both pediatric age cohorts. Corresponding results for Cmax were generally comparable for each age cohort and to historical data in adults.
Table 5.
Pharmacokinetic Parameters Following a Single Infusion of 10 mg/kg Bezlotoxumab in Two Age Cohorts of Pediatric Participants Versus Adult Participants in Prior Studies
| Pediatric Participants | Adult Participants | Pediatric/ Adult Participants | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | N | GM | 95% CI | N | GM | 95% CI | GMR | 90% CI | rMSEa |
| Age cohort 1: 12 to <18 years | |||||||||
| AUC0-inf (h * μg/mL) | 36 | 56 100 | (49400, 63700) | 1550 | 52 800 | (51800, 53800) | 1.06 | (0.95, 1.18) | 0.388 |
| Cmax (μg/mL) | 37 | 155 | (145, 166) | 1550 | 184 | (183, 186) | 0.84 | (0.79, 0.89) | 0.217 |
| Tmax (h)b | 37 | 3.00 | (2.67, 3.47) | 1550 | 1.00 | (0.0667, 4.73) | |||
| t1/2 (day)c | 36 | 21.7 | 22.1 | 1550 | 18.7 | 27.7 | |||
| Vd (L)c | 36 | 7.50 | 33.3 | 1550 | 7.34 | 16.3 | |||
| CL (mL/h)c | 36 | 9.99 | 33.7 | 1550 | 13.2 | 40.4 | |||
| Weight-normalized Vd (L/kg)c | 36 | 0.134 | 29.9 | ||||||
| Weight-normalized CL (mL/h/kg)c | 36 | 0.178 | 31.0 | ||||||
| Age cohort 2: 1 to <12 years | |||||||||
| AUC0-inf (h * μg/mL) | 54 | 43 200 | (38900, 47900) | 1550 | 52800 | (51800, 53800) | 0.82 | (0.75, 0.89) | 0.388 |
| Cmax (ug/mL) | 54 | 129 | (122, 137) | 1550 | 184 | (182, 187) | 0.70 | (0.67, 0.74) | 0.220 |
| Tmax (h)b | 54 | 3.00 | (2.67, 285) | 1550 | 1.00 | (0.0667, 4.73) | |||
| t1/2 (day)c | 54 | 18.1 | 33.8 | 1550 | 18.7 | 27.7 | |||
| Vd (L)c | 54 | 2.93 | 54.6 | 1550 | 7.34 | 16.3 | |||
| CL (mL/h)c | 54 | 4.66 | 59.6 | 1550 | 13.2 | 40.4 | |||
| Weight-normalized Vd (L/kg)c | 54 | 0.146 | 28.9 | ||||||
| Weight-normalized CL (mL/h/kg)c | 54 | 0.233 | 34.5 | ||||||
Abbreviations: AUC0-inf, area under the curve from time zero to infinity; BLOQ, below assay limit of quantitation; CI, confidence interval; CL, clearance; Cmax, maximum serum concentration; GM, geometric least-squares mean; t½ = terminal half-life; Tmax = time of peak serum concentration; Vd = volume of distribution.
arMSE: square root of conditional mean squared error (residual error) from the linear fixed-effect model. rMSE * 100% approximates the between-subject percent CV on the raw scale.
bMedian (min, max) reported for Tmax.
cGeometric mean, percent CV reported for apparent terminal t½, Vd, and CL.
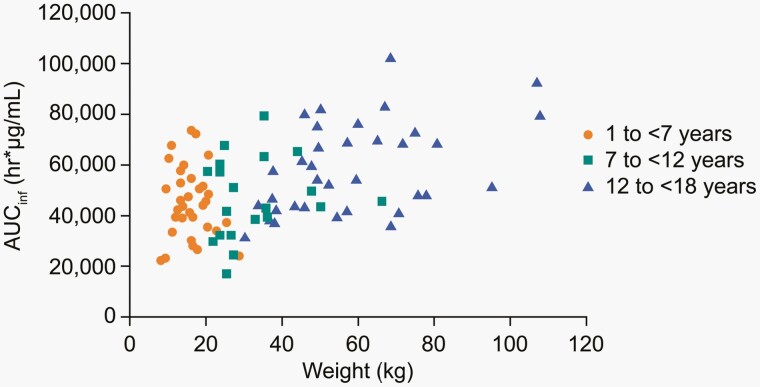
Consistent with expectations based on allometric scaling concepts, mean clearance and volume of distribution (Vd) decreased with increasing body weight, and therefore with increasing age. Clearance and Vd normalized by body weight and t1/2 were generally comparable for each age group. AUC0-inf showed substantial overlap across the entire range of age or body weight (Figure 1). Taken together, these results indicate that weight-based dosing adequately accounts for changes in clearance and Vd with age and body weight.
Figure 1.
Bezlotoxumab AUCinf following a single infusion of 10 mg/kg, by age and weight.
Safety
The most frequently reported AEs were febrile neutropenia (bezlotoxumab 21.5%, placebo 30.6%), pyrexia (17.8%, 30.6%), headache (14.0%, 22.2%), and vomiting (13.1%, 22.2%). The most common AEs had similar incidence rates in the bezlotoxumab and placebo groups (Table 6). No participant discontinued study infusion due to an AE. No clinically meaningful differences in AEs by age cohort were observed between the treatment groups.
Table 6.
Summary of Adverse Events (All Participants as Treated)
| Bezlotoxumab (n = 107) | Placebo (n = 36) | Difference Estimate | |||
|---|---|---|---|---|---|
| Participants With: | n | (%) | n | (%) | % (95% CI) |
| One or more adverse events | 95 | (88.8) | 34 | (94.4) | −5.7 (−14.5, 7.7) |
| Drug-relateda adverse events | 17 | (15.9) | 3 | (8.3) | 7.6 (−7.1, 17.8) |
| Serious adverse events | 57 | (53.3) | 29 | (80.6) | −27.3 (−41.4, −9.3) |
| Serious drug-relateda adverse events | 2 | (1.9) | 0 | (0.0) | 1.9 (−7.9, 6.6) |
| Deathsb | 5 | (4.7) | 1 | (2.8) | 1.9 (−9.8, 8.4) |
| Discontinued due to an adverse event | 0 | (0.0) | 0 | (0.0) | 0.0 (−9.7, 3.5) |
| Most common adverse eventsc | |||||
| Anemia | 8 | (7.5) | 6 | (16.7) | −9.2 (−25.1, 1.8) |
| Febrile neutropenia | 23 | (21.5) | 11 | (30.6) | −9.1 (−27.0, 6.4) |
| Abdominal pain | 15 | (14.0) | 6 | (16.7) | −2.6 (−19.0, 9.4) |
| Diarrhea | 8 | (7.5) | 5 | (13.9) | −6.4 (−21.8, 3.9) |
| Nausea | 8 | (7.5) | 4 | (11.1) | −3.6 (−18.5, 5.9) |
| Vomiting | 14 | (13.1) | 8 | (22.2) | − 9.1 (−26.0, 4.0) |
| Pyrexia | 19 | (17.8) | 11 | (30.6) | −12.8 (−30.5, 2.4) |
| Hypokalemia | 9 | (8.4) | 6 | (16.7) | −8.3 (−24.2, 2.9) |
| Headache | 15 | (14.0) | 8 | (22.2) | −8.2 (−25.2, 5.0) |
aDetermined by the investigator to be related to the study treatment.
bThree participants died after completing the 12-week follow-up period.
cEvents occurring in ≥12 participants in the bezlotoxumab group or ≥2 participants in the placebo group.
Relationship of AEs to study drug was determined by the investigator. The incidence of drug-related AEs was numerically higher for bezlotoxumab (15.9%) compared with placebo (8.3%), with a difference of 7.6 (95%CI: −7.1, 17.8). Most drug-related AEs were of mild intensity and resolved. The most frequently reported drug-related AEs in the bezlotoxumab group were increased alanine aminotransferase (ALT) (≤3xULN), increased aspartate aminotransferase (AST) (≤2xULN), and headache, each observed in three participants (2.8%) (Supplementary Material 5). The increased transaminase levels were mild, resolved during the follow-up period, and were not associated with other hepatic test results requiring further evaluation. Two participants (one from each treatment group) had an infusion-related reaction (mild decrease in blood pressure) which resolved within 24 h of infusion onset.
Three participants (all in the bezlotoxumab group) died during the 12-week follow-up period. The causes of death were veno-occlusive disease (after bone marrow transplant), progression of leukemia, and septic shock, respectively. None of the deaths were considered related to study treatment.
The incidence of SAEs was lower in the bezlotoxumab group (53.3%) than in the placebo group (80.6%), with a difference of −27.3 (95% CI: −41.4, −9.3). SAEs with incidence ≥5% in either group were febrile neutropenia (bezlotoxumab 20.6%, placebo 30.6%), pyrexia (3.7%, 8.3%), C. difficile colitis (0.9%, 5.6%), and urinary tract infection (2.9%, 5.6%). Two participants in the bezlotoxumab group each had one SAE that was considered by the investigator to be drug-related: intussusception on day 1 (mechanism unknown) that resolved spontaneously during laparotomy on day 2, and severe nausea on day 4 that resolved within 24 h after hospitalization and treatment with IV glucose.
Efficacy
Among 132 participants from the mITT population who achieved an initial clinical response, the CDI recurrence rate was low and comparable between bezlotoxumab (11.2%) and placebo (14.7%); difference −3.7, 95% CI (−20.0, 8.0). Of the 131 participants at high risk for CDI recurrence, 124 achieved an initial clinical response, and the CDI recurrence rate was comparable for the bezlotoxumab (12.1%) and placebo (15.2%) groups; difference −3.1, 95% CI (−19.9, 9.0). Similarly, the proportion with sustained clinical response was comparable between treatment groups in the mITT population overall (83.7%, 82.9%) and those at high risk for CDI recurrence (82.5%, 82.4%).
Discussion
In this randomized placebo-controlled study, bezlotoxumab exposure following a single infusion of 10 mg/kg in pediatric participants (age 1 to <18 years) was similar to historical data from adults, falling within the clinical comparability bounds of (0.6, 1.6) for both pediatric age cohorts. These results support the extrapolation to the pediatric population of bezlotoxumab efficacy and safety established in adults for the prevention of CDI recurrence. There were no clinically meaningful trends in bezlotoxumab exposure with body weight or age, supporting the conclusion that dosing proportional to body weight is appropriate in the pediatric population, as it is in adults.
The overall incidence of AEs was generally comparable for bezlotoxumab and placebo, and no participants discontinued study treatment because of an AE. SAEs were more common in the placebo group than in the bezlotoxumab group; however, most AEs in both groups were considered not related to study treatment (as assessed by the investigators), and no reason for the difference in SAEs could be identified. The high incidence of AEs and SAEs reported in this study is consistent with a pediatric population with CDI, as CDI typically occurs in pediatric patients with serious underlying medical conditions and associated therapies, in particular malignancy [8–10].
The incidence of drug-related AEs and drug-related SAEs was higher in the bezlotoxumab group than in the placebo group, although the 95% CIs for the difference between groups included zero. The most common drug-related AEs in the bezlotoxumab group were liver enzyme (ALT and AST) increases; these changes were rated mild, resolved during the follow-up period, and were not associated with other hepatic abnormalities. The SAEs that were considered drug-related (intussusception and worsening of nausea) resolved quickly with treatment. None of the deaths that occurred in the bezlotoxumab group during the 12-week follow-up period was considered related to study treatment, and the reported causes of death were consistent with underlying disease rather than CDI. Overall, the safety profile of bezlotoxumab in pediatric participants was consistent with the safety profile previously demonstrated in adults [21].
This study was designed as a PK and safety bridging study and was not intended nor powered to demonstrate a difference in CDI recurrence; the efficacy endpoints were estimated rather than formally assessed due to sample size limitations. CDI recurrence and sustained clinical response rates were comparable between the treatment groups, both overall and in those at high risk of recurrence. Although most participants had protocol-defined risk factors for recurrence, the CDI recurrence rate was low overall, contributing to a lack of discernible differences between the treatment groups. The possibility that participants who did not achieve an initial clinical response experienced a further post-baseline CDI episode was considered; however, no AEs of CDI were reported among the seven participants who did not achieve an initial clinical response. The high rate of initial clinical response and low incidence of CDI recurrence may reflect the changing epidemiology of pediatric CDI and increased use of vancomycin and fidaxomicin [26, 27]. Very few participants had the hypervirulent and transmissible 027 ribotype at baseline. Additionally, susceptibility of baseline C. difficile isolates to the baseline SOC antibiotic (Supplementary Material 4) was below the breakpoints for resistance in both groups.
Other limitations of this study include the small sample size; however, a larger study was not feasible given the low incidence of CDI in children [3]. The choice of SOC antibiotic was limited to those specified in the protocol, and the selection of SOC antibiotic was at the discretion of the treating clinician rather than standardized. Other therapies currently used for the prevention of recurrent CDI were not allowed; therefore, the combined effect of bezlotoxumab and other approaches (e.g., fecal microbiota transplantation) could not be assessed.
In summary, among pediatric participants (age 1 to <18 years) with CDI, a single intravenous infusion of bezlotoxumab had a PK profile similar to that observed in adults and was generally well tolerated, with a safety profile similar to placebo. The results of this study support a bezlotoxumab pediatric dose of 10 mg/kg, the same dose that has previously demonstrated safety and efficacy for the prevention of CDI recurrence in adults [21].
Supplementary Material
Acknowledgments
The authors thank the members of the MODIFY III Study Group (Supplementary Material 6) as well as the study participants and their families for their contributions to the study. Medical writing and editorial support were provided by Kim M. Strohmaier, MPH, and Carol Zecca, BS, employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Financial support. This work was supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Potential conflicts of interest : T. J. S. member of the Merck Scientific Advisory Committee for this study. C. M. de O. payment from Pfizer/Wyeth and AstraZeneca for lectures. T. M, M. N, A. L., and C. F. G. no conflicts of interest related to this manuscript. M. B. D., G. W., F.-H. S, S. P., D. F., and H. W. current or former employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. S. R. H. current employee of MSD UK.
Contributor Information
Thomas J Sferra, Department of Pediatrics, UH Rainbow Babies & Children’s Hospital and Case Western Reserve University School of Medicine, Cleveland, Ohio, USA.
Tomas Merta, Department of Pediatric Oncology, University Hospital Brno, Brno, Czech Republic.
Michael Neely, Division of Infectious Diseases, Children’s Hospital Los Angeles, Los Angeles, California, USA.
Claudia Murta de Oliveira, Santa Casa de Misericórdia, Belo Horizonte, Brazil.
Alvaro Lassaletta, Pediatric Hematology-Oncology Department, Hospital Niño Jesus, Madrid, Spain.
Mary Beth Dorr, PPDM QP2, Merck & Co., Inc., Rahway, New Jersey, USA.
Gregory Winchell, Biostatistics, Merck & Co., Inc., Rahway, New Jersey, USA.
Feng-Hsiu Su, Clinical Operations, Merck & Co., Inc., Rahway, New Jersey, USA.
Sarah Perko, Clinical Research, MSD UK, London, UK.
Doreen Fernsler, Clinical Research, MSD UK, London, UK.
Hetty Waskin, PPDM QP2, Merck & Co., Inc., Rahway, New Jersey, USA.
Stephen R Holden, Clinical Research, MSD UK, London, UK.
References
- 1. Voth DE, Ballard JD.. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev 2005; 18:247–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 2005; 366:1079–84. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control. Emerging Infections Program, Healthcare-Associated Infections–Community Interface Report, Clostridioides difficile infection, 2018. https://www.cdc.gov/hai/eip/Annual-CDI-Report-2018.html
- 4. Banaszkiewicz A, Kowalska-Duplaga K, Pytrus T, Pituch H, Radzikowski A.. Clostridium difficile infection in newly diagnosed pediatric patients with inflammatory bowel disease: prevalence and risk factors. Inflamm Bowel Dis 2012; 18:844–8. [DOI] [PubMed] [Google Scholar]
- 5. Hojsak I, Ferenc T, Bojanić K, et al. Incidence of Clostridium difficile infection in children with inflammatory bowel disease compared to oncology and immunocompetent patients. Digestion 2012; 86:6–11. [DOI] [PubMed] [Google Scholar]
- 6. Pant C, Anderson MP, Deshpande A, et al. Health care burden of Clostridium difficile infection in hospitalized children with inflammatory bowel disease. Inflamm Bowel Dis 2013; 19:1080–5. [DOI] [PubMed] [Google Scholar]
- 7. Enoch DA, Butler MJ, Pai S, Aliyu SH, Karas JA.. Clostridium difficile in children: colonisation and disease. J Infect 2011; 63:105–13. [DOI] [PubMed] [Google Scholar]
- 8. Price V, Portwine C, Zelcer S, et al. Clostridium difficile infection in pediatric acute myeloid leukemia: from the Canadian infections in acute myeloid leukemia research group. Pediatr Infect Dis J 2013; 32:610–3. [DOI] [PubMed] [Google Scholar]
- 9. de Blank P, Zaoutis T, Fisher B, Troxel A, Kim J, Aplenc R., Trends in Clostridium difficile infection and risk factors for hospital acquisition of Clostridium difficile among children with cancer. J Pediatr 2013; 163:699–705.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tai E, Richardson LC, Townsend J, Howard E, Mcdonald LC.. Clostridium difficile infection among children with cancer. Pediatr Infect Dis J 2011; 30:610–2. [DOI] [PubMed] [Google Scholar]
- 11. Chandrakumar A, Zohni H, El-Matary W.. Clostridioides difficile infection in children with inflammatory bowel disease. Inflamm Bowel Dis 2020; 26:1700–6. [DOI] [PubMed] [Google Scholar]
- 12. Barbar R, Hayden R, Sun Y, Tang L, Hakim H.. Epidemiologic and clinical characteristics of Clostridioides difficile infections in hospitalized and outpatient pediatric oncology and hematopoietic stem cell transplant patients. Pediatr Infect Dis J 2021; 40:655–62. [DOI] [PubMed] [Google Scholar]
- 13. Lo Vecchio A, Lancella L, Tagliabue C, et al. Clostridium difficile infection in children: epidemiology and risk of recurrence in a low-prevalence country. Eur J Clin Microbiol Infect Dis 2017; 36:177–85. [DOI] [PubMed] [Google Scholar]
- 14. Aldrich AM, Argo T, Koehler TJ, Olivero R.. Analysis of treatment outcomes for recurrent Clostridium difficile infections and fecal microbiota transplantation in a pediatric hospital. Pediatr Infect Dis J 2019; 38:32–6. [DOI] [PubMed] [Google Scholar]
- 15. Nicholson MR, Crews JD, Starke JR, Jiang Z-D, DuPont H, Edwards K.. Recurrent Clostridium difficile infection in children: patient risk factors and markers of intestinal inflammation. Pediatr Infect Dis J 2017; 36:379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tschudin-Sutter S, Tamma PD, Naegeli AN, Speck KA, Milstone AM, Perl TM.. Distinguishing community-associated from hospital-associated Clostridium difficile infections in children: implications for public health surveillance. Clin Infect Dis 2013; 57:1665–72. [DOI] [PubMed] [Google Scholar]
- 17. Kociolek LK, Palac HL, Patel SJ, Shulman ST, Gerding DN.. Risk factors for recurrent Clostridium difficile infection in children: a nested case–control study. J Pediatr 2015; 167:384–9. [DOI] [PubMed] [Google Scholar]
- 18. Kimura T, Snijder R, Sugitani T.. Characterization and risk factors for recurrence of Clostridioides (Clostridium) difficile infection in Japan: a nationwide real-world analysis using a large hospital-based administrative dataset. J Infect Chemother 2019; 25:615–20. [DOI] [PubMed] [Google Scholar]
- 19. Adams DJ, Barone JB, Nylund CM.. Community-associated Clostridioides difficile infection in children: a review of recent literature. J Pediatr Infect Dis Soc 2021; 10:S22–6. [DOI] [PubMed] [Google Scholar]
- 20. Nicholson MR, Thomsen IP, Slaughter JC, Creech CB, Edwards KM.. Novel risk factors for recurrent Clostridium difficile infection in children. J Pediatr Gastroenterol Nutr 2015; 60:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017; 376:305–17. [DOI] [PubMed] [Google Scholar]
- 22. Yee KL, Kleijn HJ, Kerbusch T, Matthews RP, Dorr MB, Garey KW, Wrishko RE. Population pharmacokinetics and pharmacodynamics of bezlotoxumab in adults with primary and recurrent Clostridium difficile infection. Antimicrob Agents Chemother 2019; 63:e01971–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jangi S, Lamont JT.. Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. J Pediatr Gastroenterol Nutrition 2010; 51:2–7. [DOI] [PubMed] [Google Scholar]
- 24. van Dorp SM, Smajlović E, Knetsch CW, Notermans DW, de Greeff SC, Kuijper EJ.. Clinical and microbiological characteristics of Clostridium difficile infection among hospitalized children in the Netherlands. Clin Infect Dis 2017; 64:192–8. [DOI] [PubMed] [Google Scholar]
- 25. Miettinen O, Nurminen M.. Comparative analysis of two rates. Stat Med 1985; 4:213–26. [DOI] [PubMed] [Google Scholar]
- 26. Edwards PT, Thurm CW, Hall M, Nicholson MR.. Clostridioides difficile infection in hospitalized pediatric patients: comparisons of epidemiology, testing, and treatment from 2013 to 2019. J Pediatr 2023; 252:111–116.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haeusler GM, Lehrnbecher T, Agyeman PKA, et al. Clostridioides difficile infection in paediatric patients with cancer and haematopoietic stem cell transplant recipients. Eur J Cancer 2022; 171:1–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.