Abstract
Objective:
Lexical retrieval deficits are characteristic of a variety of different neurological disorders. However, the exact substrates responsible for this are not known. We studied a large cohort of patients undergoing surgery in the dominant temporal lobe for medically intractable epilepsy (n = 95) to localize brain regions that were associated with anomia.
Methods:
We performed a multivariate voxel-based lesion–symptom mapping analysis to correlate surgical lesions within the temporal lobe with changes in naming ability. Additionally, we used a surface-based mixed-effects multilevel analysis to estimate group-level broadband gamma activity during naming across a subset of patients with electrocorticographic recordings and integrated these results with lesion–deficit findings.
Results:
We observed that ventral temporal regions, centered around the middle fusiform gyrus, were significantly associated with a decline in naming. Furthermore, we found that the ventral aspect of temporal lobectomies was linearly correlated to a decline in naming, with a clinically significant decline occurring once the resection extended 6 cm from the anterior tip of the temporal lobe on the ventral surface. On electrocorticography, the majority of these cortical regions were functionally active following visual processing. These loci coincide with the sites of susceptibility artifacts during echoplanar imaging, which may explain why this region has been previously underappreciated as the locus responsible for postoperative naming deficits.
Significance:
Taken together, these data highlight the crucial contribution of the ventral temporal cortex in naming and its important role in the pathophysiology of anomia following temporal lobe resections. As such, surgical strategies should attempt to preserve this region to mitigate postoperative language deficits.
Keywords: dysnomia, intracranial EEG, language, lesion–symptom mapping, temporal lobe epilepsy
1 ∣. INTRODUCTION
Lexical retrieval is the process of extracting a specific phonological form from a stored lexical concept. The lexicon is a theoretical construct conceived as a hub connecting semantic systems to language-related systems, such as phonology and orthography.1,2 Disruptions in lexical retrieval result in the “tip-of-the-tongue” (TOT) phenomenon, or failure to retrieve a familiar word with partial recall of features associated with the target word.3 Although the TOT state occurs occasionally in healthy individuals, it is a hallmark of pervasive anomia following a variety of different brain injuries.4
Anomia is particularly prevalent in patients with left language-dominant temporal lobe epilepsy, and temporal lobe resections for seizure control further increase the risk of these naming deficits.5-7 However, the precise substrates responsible for this cognitive loss are unclear. In clinical literature, a prominent focus has been on preserving the lateral temporal lobe to prevent dysnomia.8,9 As such, a clear understanding of the most critical constituents of naming might influence the design of surgical strategies to minimize language declines.
A variety of different methods have previously been used to isolate brain regions essential to naming, including lesion deficit mapping,9-13 functional imaging,14-16 and noninvasive electroencephalography (EEG).17 More recently, studies using invasive electrocorticography (ECoG) have yielded a more precise delineation of the neurophysiological basis of word production.18 ECoG provides a direct measurement of cortical activity with high spatial and temporal resolution, which makes it an optimal approach to study distributed language networks. However, it does not provide causal evidence that active brain regions are essential to that process. On the other hand, direct cortical stimulation provides causal support for the involvement of specific brain regions in a given process via transient disruption of targeted focal substrates, and it can be carried out both intraoperatively and extraoperatively with implanted intracranial electrodes. However, interpretation of stimulation-induced deficits can be confounded by limitations in patients' ability to adapt to acute perturbations as well as propagation effects to connected regions.
Voxel-based lesion symptom mapping (VLSM) is a causal approach to localize brain function on a voxel-by-voxel level in the context of chronic lesions and demonstrates the relationship between damage at any given voxel and performance on a behavioral task related to a particular cognitive function.19 Traditionally, VLSM analyses use a mass-univariate approach, where the lesion–deficit relationship is determined one voxel at a time. However, this approach fails to incorporate information regarding the spatial relationship between voxels; it assumes that voxels are statistically independent, which subsequently leads to a loss of statistical power and a potential bias in localization of significant regions. Using a multivariate VLSM approach mitigates these errors by modeling contributions of multiple voxels simultaneously.20 Furthermore, VLSM analyses are often confounded because data are typically collected only after the occurrence of the lesion, and many are limited by smaller patient populations. Thus, a large dataset in which both preoperative and postoperative performance measurements as well as imaging are obtained and in which a heterogenous set of controlled lesions are produced are of particular value in evaluating the role of a given region. Furthermore, the intersection of causal evidence derived from VLSM and correlative measures of brain activity, especially those with high spatiotemporal resolution such as ECoG, not only strengthens support for the involvement of particular brain regions in a given function but also helps identify the specific contributions of individual brain regions to a cognitive process.
We applied multivariate VLSM to a large cohort of patients who underwent surgery in the dominant left temporal lobe for treatment of drug-resistant epilepsy to specifically evaluate which component of the temporal lobe is most critical for naming. We quantified the association between each component of the anterior temporal lobe and the change in picture naming performance to isolate brain regions associated with naming deficits. Additionally, we integrated these results with ECoG recordings during picture naming performed in a subset of patients who underwent intracranial electrode implantation to localize epileptic foci to correlate lesion–deficit findings with brain activity.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Study population
Altogether, 189 patients (105 females, 5–73 years old) underwent surgery in the left temporal lobe for drug-resistant focal epilepsy. Given that the neurosurgeon's practice is chiefly focused on adults, we have very few pediatric patients. Furthermore, the language system is still immature in pediatric patients, and, thus, we excluded all subjects younger than 16 years (n = 7). Left-hemispheric language dominance was confirmed in 146 patients by intracarotid sodium amytal injection,21 functional magnetic resonance imaging (fMRI) laterality index,22 or direct cortical stimulation.8 Patients with right (n = 15), bilateral (n = 3), or inconclusive (n = 25) language dominance were excluded. Additionally, subjects who were not fluent English speakers (n = 3), who had an intelligence quotient (IQ) below 67 (n = 4), or who had previous cortical resections (n = 11) were excluded. Of the remaining 122 patients, 95 patients (53 females, 17–73 years old, mean full-scale IQ = 97 ± 14) underwent neuropsychological testing and MRI prior to and following surgery (Table S1). The other 27 subjects did not have all data points and were excluded.
Neuropsychological testing sessions included the Boston Naming Test (BNT), which is a test of confrontational word retrieval used to evaluate aphasia and consists of 60 line drawings of objects that subjects must name within 20 s.23 Given the involvement of all core stages of speech production, picture naming tasks are particularly useful in studying lexical retrieval and its neuroanatomical basis.1,24 In our cohort, postoperative testing was completed at least 3 months following surgery in all patients, with an average of 6.65 ± 3.52 months.
2.2 ∣. MRI acquisition
MRI scans were obtained prior to and following surgery. All scans were obtained using a 3T whole body magnetic resonance scanner (Philips Medical Systems) fitted with a 16-channel SENSE head coil. Images were collected using a magnetization-prepared 180° radiofrequency pulse and a rapid gradient-echo sequence with 1 mm sagittal slices and in-plane resolution of 1 mm isotropic. Pial surface reconstructions were computed with FreeSurfer (v6.0)25 and imported into AFNI (https://afni.nimh.nih.gov).
2.3 ∣. Voxel-based lesion–symptom mapping
Surgical lesions were manually traced on postoperative MRIs in AFNI and verified by an experienced neurosurgeon. Anatomical postoperative scans were aligned to preoperative scans within each subject, and preoperative scans along with registered lesions masks were subsequently warped to a normative space (Montreal Neurological Institute (MNI)) using a nonlinear registration in ANTs (http://stnava.github.io/ANTs/). All coordinates are reported as standard RAS-defined coordinates using the International Consortium for Brain Mapping MNI152 (2009) template.
For VLSM, only voxels in the lesion masks of at least five subjects were analyzed. To reflect relative changes in BNT scores following surgery, preoperative scores were regressed out of postoperative scores using an ordinary least squares linear regression model, and the corrected postperative scores were used as the target. A linear regression model was used given the linear relationship between preoperative and postoperative scores. Additionally, linear regression models are more robust and enable corrected scores to maintain a gaussian distribution. VLSM was implemented using a multivariate support vector regression (SVR) model to correlate the pattern of lesioned voxels to observed deficits using a multivariate lesion symptom mapping toolbox (https://github.com/atdemarco/svrlsmgui.git).20 The SVR model used an ε-insensitive support vector machine algorithm with a radial basis function kernel in MATLAB. Permutation-based cluster level correction with 5000 permutations and a p-value of .05 for voxel-level and subsequent cluster-level correction was used to correct for multiple comparisons.
Given the nature of temporal lobe surgeries, the effect of lesion volume on behavior is complicated by its correlation with location. This confound is not unique to our cohort and is a common challenge with VLSM, such as in stroke patients, where lesions are constrained by vasculature. In these cases, regression of lesion volume can cause a reduction in statistical power and can distort the lesion–deficit relationship.26 Additionally, Schwartz et al.27 found that lesion volume was not a significant predictor of naming errors. As such, VLSM was performed both with and without lesion volume as a covariate.
2.4 ∣. fMRI analysis
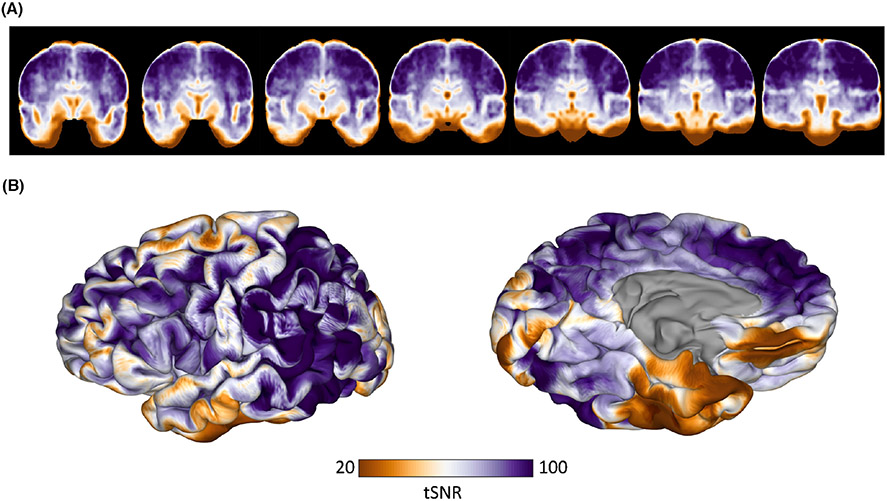
fMRI was acquired in 35 of these patients (19 females, 19–73 years old) using a gradient-recalled echo-planar imaging sequence with 33 axial slices of 3-mm thick-ness and in-plane resolution of 2.75 mm by 2.75 mm (echo time = 30 ms, repetition time = 2015 ms, flip angle = 90°). Stimuli were presented in a block design with 20 s of picture naming interwoven with 14 s of scrambled images. Subjects were instructed to silently name objects if presented with coherent images or to think the word scrambled if presented with incoherent images. Preprocessing was carried out in AFNI and included motion correction via registration of all volumes in the time series to the first volume using a rigid-body transformation, compensation for slice acquisition-dependent time shifts, and registration to the subject's skull-stripped anatomical MRI in AFNI. The temporal signal-to-noise ratio (tSNR) was computed for each voxel as the absolute value of the mean signal divided by its SD following time course normalization. The tSNR volumes were then warped to MNI space using the warp generated for VLSM analyses and averaged across subjects. The average tSNR was assessed in significant regions from VLSM analyses.
2.5 ∣. ECoG acquisition and analysis
Of the 95 patients, 42 (24 females, 18–60 years old) underwent intracranial EEG to localize seizure foci using subdural grid electrodes or depth stereoelectroencephalographic electrodes prior to resection. Subjects performed a picture naming task in which they were instructed to name the object shown if presented with coherent images or to say “scrambled” if presented with incoherent images, which were derived by rearranging the coherent images and comprised one third of the stimuli presented to each patient.23,28 Stimulus presentation software triggered a digital pulse at the start of each trial, and pulses were registered via a digital-to-analog conversion (U3-LV, LabJack). Continuous audio recordings were carried out using an omnidirectional microphone (30–20 000-Hz response, 73-dB SNR, Audio Technica U841A) adjacent to the presentation laptop. Response accuracy and articulation onset were manually labeled in MATLAB.
Data were collected with a sampling rate of 2000 Hz and a bandwidth of .1–700 Hz using NeuroPort NSP (Blackrock Microsystems) or with a sampling rate of 1000 Hz and a bandwidth of .15–300 Hz using Neurofax (Nihon Kohden). Electrode localization was performed via registration of postoperative CT scans with preoperative anatomical MRI scans. Electrodes within abnormal brain tissue or contaminated by line noise were excluded, and each channel was referenced to a common average of all remaining electrodes. Trials containing epileptiform activity or trials in which subjects responded incorrectly or after 2 s were excluded from analysis. The gamma band (65–115 Hz) was extracted from the raw signal using a frequency domain bandpass Hilbert filter.
Analyses were done with trials time-locked to picture onset. The baseline period was defined as −750 to −250 ms relative to stimulus onset. The percent change in broadband gamma activity (BGA) was calculated as the square of the instantaneous power normalized to baseline. Surface-based mixed-effects multilevel analysis (SB-MEMA) was used to estimate power change in BGA at a population level.29 This approach is composed of two levels of analysis using mixed-effects models to assess both within-subject and between-subject variability. Results were propagated onto the cortical surface using the surface recording zone of each electrode, which was computed as a 10-mm region centered around the closest node index for each electrode. Nodes within a given recording zone were weighted as an exponential decay with a full-width half-maximum of 2.3 mm. Patient-specific cortical surfaces were registered to a standard surface for integration across subjects. A familywise error rate correction with an alpha-level of .05 was used to correct for multiple comparisons. SB-MEMA was calculated as a contrast for pictures against scrambled images to control for sensory processing and isolate semantic-specific activity. VLSM results were colocalized to the same standardized cortical surface to compute overlap with SB-MEMA. Additionally, electrodes were indexed to the closest surface node, and electrodes within this overlap were included in the average BGA trace.
3 ∣. RESULTS
3.1 ∣. Voxel-based lesion symptom mapping
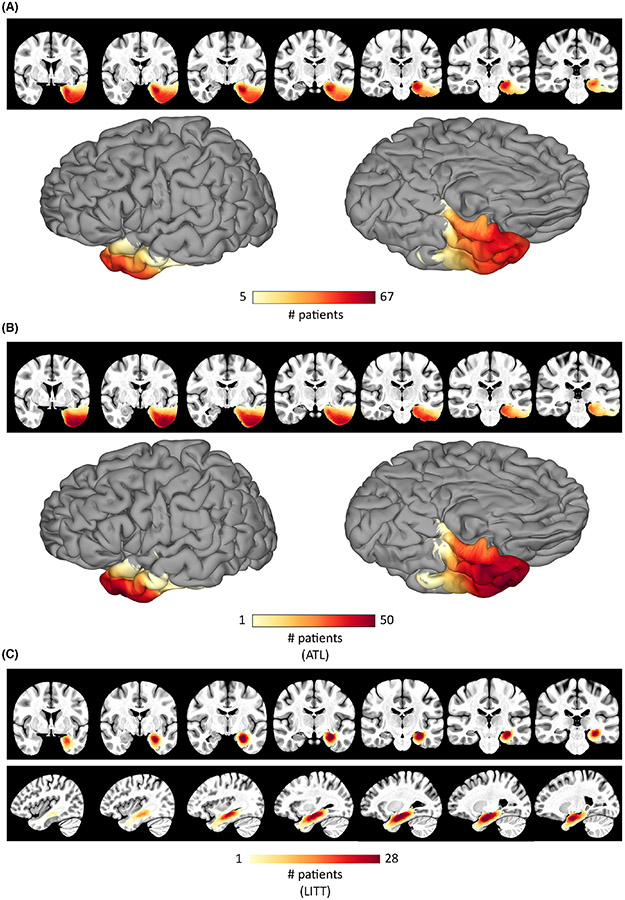
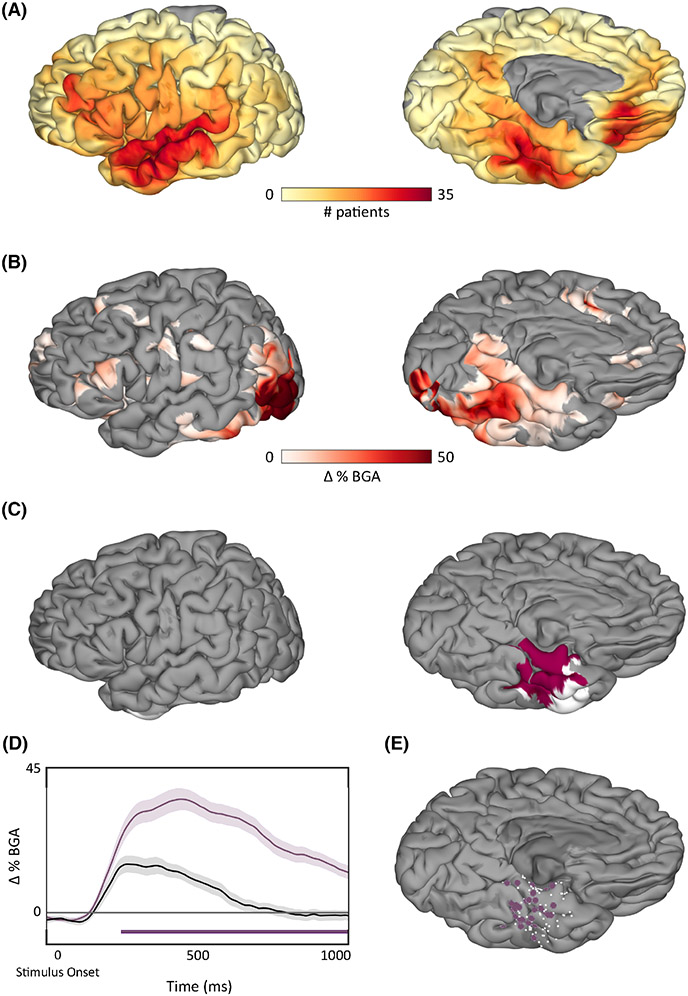
Resection overlap across all patients is shown in Figure 1A. Additionally, resection overlap across all subjects who underwent anterior temporal lobectomies (ATLs) is shown in Figure 1B (n = 49) and across all subjects who underwent laser interstitial thermal ablation (LITT) of the mesial temporal lobe is shown in Figure 1C (n = 31). The remaining patients (n = 15) had more focal resections based on individual clinical evaluations, which were distributed throughout the left temporal lobe.
FIGURE 1.
Lesion overlap. (A) The resection mask coverage across all subjects shown in the volume and on the cortical surface with a threshold applied to show only voxels that were included in the resections of at least five subjects. (B,C) The resection mask coverage across all subjects who underwent (B) a standard anterior temporal lobectomy (ATL) procedure or (C) a selective laser ablation (laser interstitial thermal therapy [LITT]) of the hippocampus and amygdala.
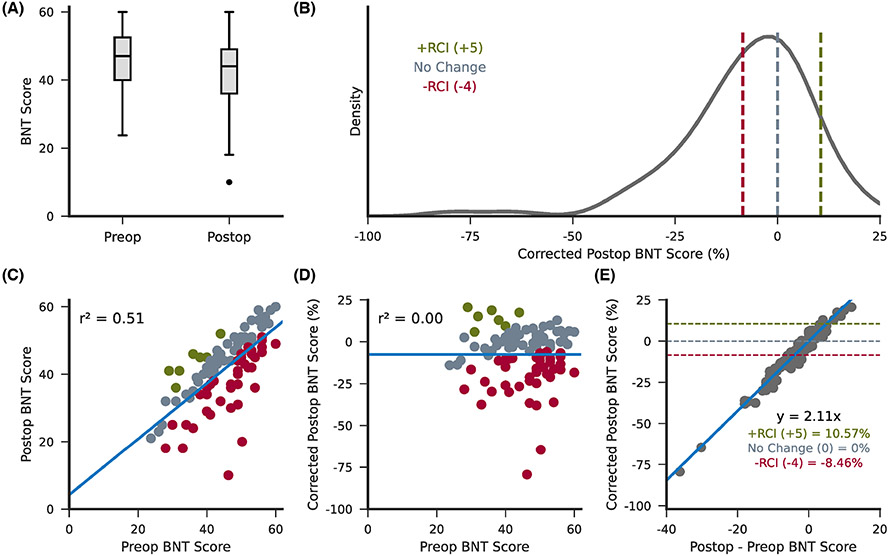
The average raw BNT scores were 45.35 ± 8.85 preoperatively and 41.75 ± 10.30 postoperatively (Figure 2A). Prior to VLSM, preoperative BNT scores were regressed out of postoperative scores using a linear regression. The resulting corrected postoperative BNT scores were represented as a standardized percentage that can be interpreted as a single score representation of BNT performance following surgery relative to preoperative performance (Figure 2B). No change in BNT performance corresponded to 0% (gray), a 5-point increase in BNT performance following surgery (clinically significant improvement) corresponded to 10.57% (green), and a 4-point decrease in BNT performance (clinically significant decline) corresponded to −8.46% (red).30 Figure 2C,D shows the correlation between preoperative and postoperative BNT scores both before and after linear regression. The relationship between corrected postoperative scores and the difference between postoperative and preoperative scores are shown in Figure 2E.
FIGURE 2.
Support vector regression covariates. (A) The distribution of raw preoperative and postoperative Boston Naming Test (BNT) scores across all subjects. (B) The distribution of postoperative BNT scores following regression of preoperative BNT scores displayed as a standardized percentage. Dotted lines indicate corrected scores corresponding to a difference between postoperative and preoperative BNT scores of 0 (no change; gray), +5 (+RCI; green), and −4 (−RCI; red). (C,D) The correlation between preoperative and postoperative BNT scores before (C) and after (D) regression. Scatter points are colored as clinically significant improvement (green), clinically significant decline (red), or clinically insignificant change (gray). (E) The relationship between corrected postoperative BNT scores and the difference between postoperative and preoperative BNT scores. An increase of 5 points postoperatively (significant improvement) corresponded to 10.57%; no change postoperatively corresponded to 0%; and a decrease of 4 points postoperatively (significant decline) corresponded to −8.46%. RCI, reliable change index.
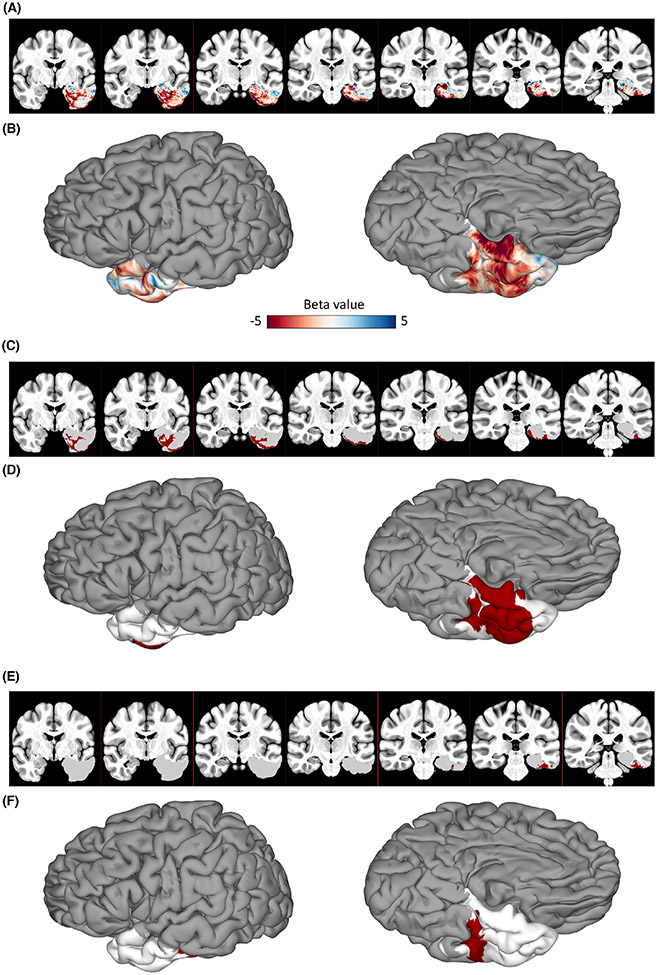
Figure 3A, B shows the unthresholded Beta map where the Beta value of a given voxel represents its contribution to BNT performance, with negative values indicating a decline following resection of the corresponding voxel. The Beta map revealed that resection of more posterior ventral temporal regions was significantly associated with naming deficits. Figure 3C,D shows the most significant cluster contributing to BNT decline (p = .0248), which primarily included the fusiform gyrus, parahippocampal gyrus (PHG), and inferior temporal gyrus (ITG), with a volume of 5656 mm3 and a center of mass in the fusiform gyrus (−33.1, −12.3, −33.4).
FIGURE 3.
Voxel-based lesion symptom mapping results. (A,B) The Beta map computed using a multivariate support vector regression model shown in the volume (A) and on the cortical surface (B). More negative values indicate that resection of the given voxel corresponds to a decline in Boston Naming Test performance. (C,D) The most significant cluster following permutation-based cluster level correction of the Beta map shown in the volume (C) and on the cortical surface (D). (E,F) The largest cluster prior to permutation-based cluster level correction shown in the volume (E) and on the cortical surface (F). Regions included in the cluster are shown in red, and regions not included in the cluster but included in the analysis are shown in white.
To investigate the effect of lesion volume, VLSM was also run with lesion volume regressed out of postoperative BNT scores as well as lesion data. In this model, there were no significant clusters following permutation-based cluster-level correction. Without applying a cluster-wise correction, the largest cluster contributing to BNT decline was similar in location to that of our prior VLSM analysis (Figure 3E,F), with a volume of 1043 mm3 and a center of mass in the fusiform gyrus (−45, −29.4, −26.6).
Less than 3% of voxels within the significant cluster were located in the hippocampus. To further evaluate the role of the hippocampus in naming, we computed the correlation between percentage of the hippocampus removed and corrected postoperative BNT scores (r = −.21, ln[BF10] = −.50; BF = bayes factor). We found that the percentage of the hippocampus removed explained less than 5% of the variance in corrected BNT scores, and there was moderate evidence that it did not affect naming.
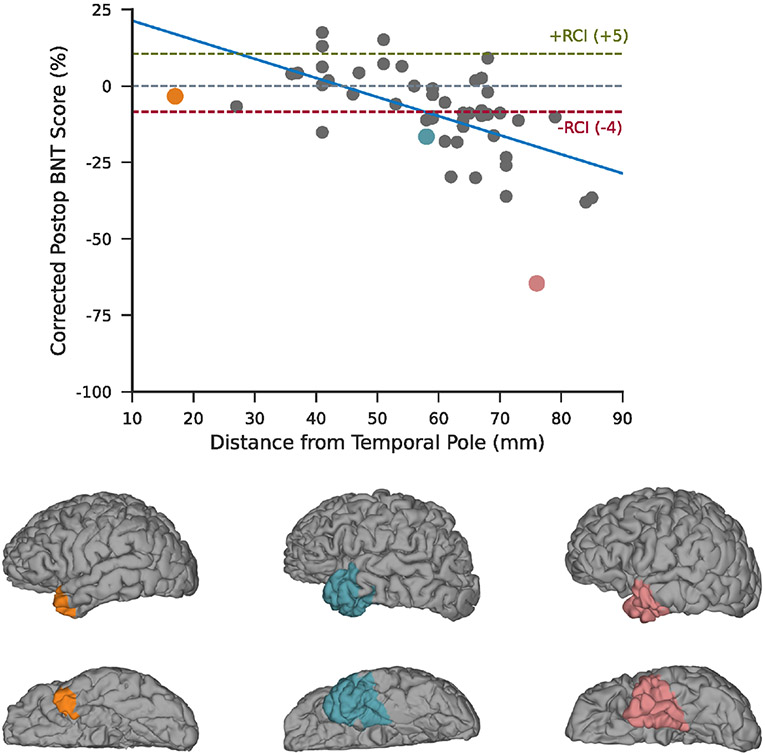
To investigate the nature of the relationship between the extent of ATL resections and naming deficits, we computed the correlation between the distance from the tip of the temporal pole to the most posterior coordinate of resections and corrected postoperative scores across subjects who underwent standard ATL or temporal pole resections (Figure 4). We found strong evidence of an effect of the ventral extent of resections on naming (r = −.58, ln[BF10] = 7.25) with a significant decline (reliable change index [RCI] ≤ −4) occurring once the posterior margin extended beyond 6 cm.
FIGURE 4.
Analysis of posterior extent of anterior temporal lobectomy (ATL) resections. The correlation between corrected Boston Naming Test (BNT) scores and the posterior margin of ATL resections measured as distance from the tip of the temporal pole (r = −.58, ln[BF10] = 7.25). Resections for three exemplar patients are shown on the cortical surface and highlighted in the scatter plot in the corresponding color. RCI, reliable change index.
3.2 ∣. fMRI analysis
Figure 5 shows the average fMRI tSNR. Regions with the lowest tSNR included the orbitofrontal cortex and the ventral temporal cortex. Whole brain tSNR ranged from 2.35 to 131.5, with an average of 79.40 ± 21.73. The mean tSNR of the significant cluster from VLSM was 51.93 ± 21.29.
FIGURE 5.
Functional magnetic resonance imaging (fMRI) temporal signal-to-noise ratio (tSNR). The average tSNR calculated on fMRI scans acquired during a picture naming task is shown in the volume (A) and on the cortical surface (B).
3.3 ∣. ECoG analysis
To integrate lesion-deficit findings with brain activity, ECoG recordings acquired during picture naming in a subset of patients included in our cohort prior to surgery were analyzed. The average reaction time was 1355 ms with a mean accuracy of 93.65% ± .04%. Figure 6A shows the surface recording zones aggregated across all electrodes included in the analysis (n = 5113). SB-MEMA during picture naming showed peak BGA spreading anteriorly along the ventral temporal stream 500–750 ms following picture onset, and regions with greater cortical responses to pictures compared to scrambled images were located primarily within the posterior ventral temporal lobe (Figure 6B). This is concordant with prior work revealing a lexicosemantic-specific increase in activity in this region on both fMRI and ECoG.18 The thresholded SB-MEMA map overlapped with 78.8% of the significant cluster from VLSM (Figure 6C). Overlap was primarily seen in the fusiform gyrus. Figure 6D,E shows the average percent change in BGA relative to picture onset for pictures (purple) and scrambled images (black) of active electrodes within this overlap (ΔBGA > 25%). There was a significant increase in BGA compared to scrambled images beginning around 250 ms following picture onset with a peak increase occurring within 500–750 ms. Significance was computed using an unpaired t-test and a false discovery rate-corrected p-value of .05.
FIGURE 6.
Surface-based mixed-effects multilevel analysis (SB-MEMA) results. (A) Coverage of surface recording zones for all left hemisphere electrodes across all patients included in the study who underwent intracranial electrode implantation. (B) Surface-based group-level electrocorticographic estimate of group broadband gamma activity (BGA) 500–750 ms following picture onset for pictures of objects > scrambled images. Maps are restricted to regions with significant activity (p < .05, corrected) and BGA change > 2.5%. (C) Regions in pink represent the overlap between active regions as determined by the thresholded SB-MEMA map and the voxel-based lesion symptom mapping (VLSM) significant cluster. Regions in white indicate nodes in the VLSM significant cluster that were not significantly active. (D) Time series average of group estimates of BGA percent change ± 1 SEM following picture onset. Data were smoothed with a Savitsky–Golay filter (third order, 251-ms length). Significant increase from the control condition (scrambled images) is indicated by the horizontal bar (unpaired t-test, p < .05, false discovery rate corrected). (E) Electrodes located within the VLSM cluster and SB-MEMA overlap. Electrodes that had a significant cortical response and were included in the average trace are shown in purple, and electrodes that did not have a significant cortical response are shown in white.
4 ∣. DISCUSSION
We isolated the critical cortical constituents of naming in the dominant temporal lobe using multivariate VLSM and integrated these findings with ECoG within the same patient population to derive quantifiable measures of convergence between lesion-deficit localization and brain activity. We found that damage to the dominant ventral temporal cortex (VTC) was significantly associated with deficits in visual naming, and we also found that the majority of this region was significantly more active when naming pictures compared to scrambled images. Together, these data firmly establish the role of the fusiform gyrus as the critical temporal lobe component responsible for naming deficits.18,31,32
Previous studies using cortical stimulation mapping have highlighted the importance of VTC, also referred to as the basal temporal language area (BTLA), in confrontation naming.18,33 More specifically, it has been shown that disruption of the fusiform gyrus, PHG, and ITG results in speech arrest.33,34 Additionally, Forseth et al.18 observed that stimulation of the fusiform gyrus specifically disrupted object naming for both pictures and auditory descriptions without disruption of sentence repetition or sensorimotor effects, which implicates its role in lexicosemantic processing as opposed to audiovisual integration. Despite clear evidence for acute disruption in language function following electrical stimulation, previous studies have underplayed the role of this region in chronic lesions, reporting that resection of the BTLA results in no significant language impairments or only transient aphasias with the majority of language deficits resolving within 1 month following surgery.33,35,36 It has been hypothesized that these findings might suggest that the BTLA does not play an important role in language37,38 or that its role within language networks is not essential in that function recovers to preresection levels.39,40 However, other studies have found BTLA resections to be associated with more pervasive aphasias.11,14,40 In the recent past, Binder et al.11 and Abdallah et al.12 have highlighted the importance of the BTLA in postoperative naming deficits. Consistent with and elaborating the scope of these studies, we show that resections involving the left dominant BTLA do result in long-term language deficits with many patients reporting noticeable word finding difficulties at the time of postoperative testing, which took place at least 3 months following surgery.
Another potential interpretation of our VLSM results is that our findings are driven by lesion size rather than damage to a particular brain structure. However, this is unlikely for several reasons. First, if our findings were driven by lesion volume, it would be expected that more posterolateral temporal regions would also be significantly associated with naming decline, which is not seen in our VLSM results. Second, VLSM findings were validated by ECoG analyses in the same patient population, which revealed that regions found to be significantly associated with naming deficits with VLSM were also more active when subjects performed a naming task. Given that ECoG data are collected prior to surgery, it is not influenced by resection volume, thus concordant findings between ECoG and VLSM indicate that our results are primarily driven by factors independent of lesion size. Lastly, including lesion volume as a covariate in our VLSM analysis caused a large reduction in statistical power but did not significantly affect the localization of the brain region associated with deficits in naming. As such, our findings support VTC as the primary locus driving postoperative deficits. However, more widespread disruption of the language network as a result of damage to broader, interconnected regions may still contribute to greater language dysfunction.
Despite accumulating evidence implicating VTC in naming, functional neuroimaging studies have reported variable results regarding naming-associated activations in the ventral temporal lobe, and many have localized lexicosemantic function to more lateral temporal regions.41-43 However, limitations of echo-planar imaging may restrict the ability to study the ventral temporal lobe using fMRI. Due to its proximity to both the sinuses and the skull, this area is particularly affected by image distortion and signal loss caused by susceptibility-induced magnetic field gradients, and efforts to minimize these artifacts to improve accuracy often result in a reduced SNR.44,45 We found that ventral temporal regions, especially those identified as significant by our VLSM analysis, were associated with a low SNR on fMRI, which may explain why this region has been underappreciated as the locus responsible for postoperative deficits.
The exact role of the hippocampus in naming is not clearly understood. It has been shown that laser ablation of the hippocampus and amygdala reduces the risk of postoperative naming impairment in comparison to standard ATL procedures.11,46,47 Concordantly, we did not find a significant association between the hippocampus and naming performance, and <3% of voxels included in the significant cluster belonged to the hippocampus. Additionally, we found moderate evidence that the percentage of the hippocampus removed or ablated was not significantly associated with a change in BNT performance. Thus, our results do not support that the hippocampus is an essential component of object naming.
It is well known that dominant ATL resections increase the risk of postoperative naming deficits.7,31 Based on these findings and studies in patients with semantic dementia, it has also been hypothesized that the temporal pole functions as the critical access hub for lexical retrieval.48,49 Our results show that resection of the temporal pole within 3 cm of the anterior tip of the temporal lobe is not significantly associated with postoperative naming deficits. However, we found that the ventral aspect of the posterior margin of ATL resections was linearly correlated with postoperative naming scores, with resections extending more posteriorly resulting in larger naming declines and a clinically significant decline occurring if the resection extended >6 cm from the temporal pole. Although it is possible that language declines may result from smaller temporal pole resections, our results support that the extent of the fusiform gyrus resected clearly worsens the severity of deficits and diminishes recovery. However, the existence of word finding deficits following resections that spare the fusiform gyrus suggests a more complex relationship between the fusiform gyrus and other temporal lobe regions. Trimmel et al.50 found the left VTC to be functionally connected to the left anterior superior temporal gyrus and temporal pole, and it has been suggested that naming decline following dominant ATL resections may also result from partial disconnections between the resected regions and VTC.16,50 Although our data do not support that anterior temporal lobe regions are essential to naming, it is likely these regions support more complex aspects of lexicosemantic processing. As such, language declines may still occur following resection of more anterior portions of the left temporal lobe due to increased disruption of ventral temporal connections, and observed visual naming decline may reflect the proportion of disruption to the anteroposteriory–basal temporal language network.
Using both multivariate VLSM and ECoG, we have demonstrated the essential contribution of the fusiform cortex to naming. We found that resection of more posterior inferior temporal regions, centered around the fusiform gyrus, was significantly associated with visual object naming deficits, and the majority of these regions were active during picture naming using ECoG. These findings build on our prior work18 as well as recent studies11,12 elucidating the role of the fusiform gyrus in naming. Altogether, these results support the importance of VTC in the naming network, and further implicate the fusiform gyrus as a critical access hub for lexical retrieval.
Supplementary Material
Key Points.
In a cohort of 95 patients who underwent surgery in the dominant left temporal lobe for epilepsy, we localized the most critical regions within the temporal lobe responsible for postoperative naming deficits
Using both multivariate voxel-based lesion–symptom mapping and electrocorticography, we have shown the essential contribution of the fusiform gyrus to naming and its involvement in the pathophysiology of anomia
Our results indicate that surgical approaches in the dominant temporal lobe should aim to preserve the fusiform gyrus to improve the risk of postoperative language deficits
ACKNOWLEDGMENTS
We thank all the patients who participated in this study, members of the Tandon lab (Oscar Woolnough, Elliot Murphy, and Jessica Johnson), neurologists at the Texas Comprehensive Epilepsy Program who participated in the care of these patients, and all the nurses and technicians in the Epilepsy Monitoring Unit at Memorial Hermann Hospital who helped make this research possible.
FUNDING INFORMATION
This work was supported by the National Institute for Deafness and Other Communication Disorders (DC014589, awarded to N.T.).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors report no conflicts of interest for this work.
PATIENT CONSENT STATEMENT
Study design was approved by the University of Texas Health Science Center's Committee for the Protection of Human Subjects.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92(1):101–44. 10.1016/j.cognition.2002.06.001 [DOI] [PubMed] [Google Scholar]
- 2.Friedmann N, Biran M, Dotan D. Lexical retrieval and its breakdown in aphasia and developmental language impairment. In: Boeckx C, Grohmann KK, editors. The Cambridge Handbook of Biolinguistics. Cambridge Handbooks in Language and Linguistics. Cambridge: Cambridge University Press; 2013. p. 350–74. 10.1017/CBO9780511980435.021 [DOI] [Google Scholar]
- 3.Brown R, McNeill D. The “tip of the tongue” phenomenon. Journal of Verbal Learning and Verbal Behavior. 1966;5(4):325–37. 10.1016/S0022-5371(66)80040-3 [DOI] [Google Scholar]
- 4.Margolin DI, Pate DS, Friedrich FJ, Elia E. Dysnomia in dementia and in stroke patients: different underlying cognitive deficits. J Clin Exp Neuropsychol. 1990;12(4):597–612. 10.1080/01688639008401004 [DOI] [PubMed] [Google Scholar]
- 5.Bell BD, Davies KG. Anterior temporal lobectomy, hippocampal sclerosis, and memory: recent neuropsychological findings. Neuropsychol Rev. 1998;8(1):25–41. 10.1023/A:1025679122911 [DOI] [PubMed] [Google Scholar]
- 6.Schwarz M, Pauli E, Stefan H. Model based prognosis of postoperative object naming in left temporal lobe epilepsy. Seizure - European Journal of Epilepsy. 2005;14(8):562–8. 10.1016/j.seizure.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 7.Hamberger MJ. Object naming in epilepsy and epilepsy surgery. Epilepsy Behav. 2015;46:27–33. 10.1016/j.yebeh.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 8.Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere: an electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71(3):316–26. 10.3171/jns.1989.71.3.0316 [DOI] [PubMed] [Google Scholar]
- 9.Pino D, Mädebach A, Jescheniak JD, Regenbrecht F, Obrig H. BONEs not CATs attract DOGs: semantic context effects for picture naming in the lesioned language network. Neuroimage. 2022;246:118767. 10.1016/j.neuroimage.2021.118767 [DOI] [PubMed] [Google Scholar]
- 10.Warrington EK. The selective impairment of semantic memory. Q J Exp Psychol. 1975;27(4):635–57. 10.1080/14640747508400525 [DOI] [PubMed] [Google Scholar]
- 11.Binder JR. Temporal lobe regions essential for preserved picture naming after temporal epilepsy surgery. Epilepsia. 2020;00:1–10. 10.1111/epi.16643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdallah C, Brissart H, Colnat-Coulbois S, Pierson L, Aron O, Forthoffer N, et al. Stereoelectroencephalographic language mapping of the basal temporal cortex predicts postoperative naming outcome. J Neurosurg. 2021;135(5):1466–76. 10.3171/2020.8.JNS202431 [DOI] [PubMed] [Google Scholar]
- 13.Aabedi AA, Kakaizada S, Young JS, Kaur J, Wiese O, Valdivia C, et al. Convergence of heteromodal lexical retrieval in the lateral prefrontal cortex. Sci Rep. 2021;11(1):6305. 10.1038/s41598-021-85802-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharp DJ, Scott SK, Wise RJS. Retrieving meaning after temporal lobe infarction: the role of the basal language area. Ann Neurol. 2004;56(6):836–46. 10.1002/ana.20294 [DOI] [PubMed] [Google Scholar]
- 15.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–96. 10.1093/cercor/bhp055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonseca ATD, Guedj E, Alario FX, Laguitton V, Mundler O, Chauvel P, et al. Brain regions underlying word finding difficulties in temporal lobe epilepsy. Brain. 2009;132(10):2772–84. 10.1093/brain/awp083 [DOI] [PubMed] [Google Scholar]
- 17.Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron. 2003;38(3):487–97. 10.1016/s0896-6273(03)00197-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forseth KJ, Kadipasaoglu CM, Connor CR, Hickok G, Knight RT, Tandon N. A lexical semantic hub for heteromodal naming in middle fusiform gyrus. Brain. 2018;141:2112–26. 10.1093/brain/awy120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion–symptom mapping. Nat Neurosci. 2003;6(5):448–50. 10.1038/nn1050 [DOI] [PubMed] [Google Scholar]
- 20.DeMarco AT, Turkeltaub PE. A multivariate lesion symptom mapping toolbox and examination of lesion-volume biases and correction methods in lesion-symptom mapping. Hum Brain Mapp. 2018;39:4169–82. 10.1002/hbm.24289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance: experimental and clinical observations. J Neurosurg JNS. 2007;106(6):1117–33. 10.3171/jns.2007.106.6.1117 [DOI] [PubMed] [Google Scholar]
- 22.Ellmore TM, Beauchamp MS, Breier JI, Slater JD, Kalamangalam GP, O'Neill TJ, et al. Temporal lobe white matter asymmetry and language laterality in epilepsy patients. Neuroimage. 2010;49(3):2033–44. 10.1016/j.neuroimage.2009.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan E, Harold G, Sandra W, Harold G. Boston Naming Test. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 24.Herbert R, Hickin J, Howard D, Osborne F, Best W. Do picture-naming tests provide a valid assessment of lexical retrieval in conversation in aphasia? Null. 2008;22(2):184–203. 10.1080/02687030701262613 [DOI] [Google Scholar]
- 25.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- 26.Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, et al. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132(Pt 12):3411–27. 10.1093/brain/awp284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz MF, Kimberg DY, Walker GM, Brecher A, Faseyitan OK, Dell GS, et al. Neuroanatomical dissociation for taxonomic and thematic knowledge in the human brain. Proc Natl Acad Sci U S A. 2011;108(20):8520–4. 10.1073/pnas.1014935108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snodgrass J, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn. 1980;6(2):174–215. 10.1037//0278-7393.6.2.174 [DOI] [PubMed] [Google Scholar]
- 29.Kadipasaoglu CM, Baboyan VG, Conner CR, Chen G, Saad ZS, Tandon N. Surface-based mixed effects multilevel analysis of grouped human electrocorticography. Neuroimage. 2014;101:215–24. 10.1016/j.neuroimage.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 30.Sachs BC, Lucas JA, Smith GE, Ivnik RJ, Petersen RC, Graff-Radford NR, et al. Reliable change on the Boston naming test. J Int Neuropsychol Soc. 2012;18(2):375–8. 10.1017/S1355617711001810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15(11):527–36. 10.1016/j.tics.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woolnough O, Donos C, Rollo PS, Forseth KJ, Lakretz Y, Crone NE, et al. Spatiotemporal dynamics of orthographic and lexical processing in the ventral visual pathway. Nat Hum Behav. 2021;5(3):389–98. 10.1038/s41562-020-00982-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luders H, Lesser RP, Hahn J, Dinner DS, Morris HH, Wyllie E, et al. Basal temporal language area. Brain. 1991;114(2):743–54. 10.1093/brain/114.2.743 [DOI] [PubMed] [Google Scholar]
- 34.Burnstine TH, Lesser RP, Hart J, Uematsu S, Zinreich SJ, Krauss GL, et al. Characterization of the basal temporal language area in patients with left temporal lobe epilepsy. Neurology. 1990;40(6):966–70. 10.1212/WNL.40.6.966 [DOI] [PubMed] [Google Scholar]
- 35.Penfield W, Roberts L. Speech and brain mechanisms. Princeton, NJ: Princeton University Press; 1959. [Google Scholar]
- 36.Loring DW, Meador KJ, Lee GP. Effects of temporal lobectomy on generative fluency and other language functions. Arch Clin Neuropsychol. 1994;9(3):229–38. 10.1093/arclin/9.3.229 [DOI] [PubMed] [Google Scholar]
- 37.Lambon Ralph MA, Cipolotti L, Manes F, Patterson K. Taking both sides: do unilateral anterior temporal lobe lesions disrupt semantic memory? Brain. 2010;133(11):3243–55. 10.1093/brain/awq264 [DOI] [PubMed] [Google Scholar]
- 38.Wilson SM, Lam D, Babiak MC, Perry DW, Shih T, Hess CP, et al. Transient aphasias after left hemisphere resective surgery. J Neurosurg JNS. 2015;123(3):581–93. 10.3171/2015.4.JNS141962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krauss GL, Fisher R, Plate C, Hart J, Uematsu S, Gordon B, et al. Cognitive effects of resecting basal temporal language areas. Epilepsia. 1996;37(5):476–83. 10.1111/j.1528-1157.1996.tb00594.x [DOI] [PubMed] [Google Scholar]
- 40.Langfitt JT, Rausch R. Word-finding deficits persist after left Anterotemporal lobectomy. Arch Neurol. 1996;53(1):72–6. 10.1001/archneur.1996.00550010090021 [DOI] [PubMed] [Google Scholar]
- 41.Thompson-Schill SL. Neuroimaging studies of semantic memory: inferring “how” from “where”. Neuropsychologia. 2003;41(3):280–92. 10.1016/S0028-3932(02)00161-6 [DOI] [PubMed] [Google Scholar]
- 42.Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58(1):25–45. 10.1146/annurev.psych.57.102904.190143 [DOI] [PubMed] [Google Scholar]
- 43.Ralph MAL, Jefferies E, Patterson K, Rogers TT. The neural and computational bases of semantic cognition. Nat Rev Neurosci. 2017;18(1):42–55. 10.1038/nrn.2016.150 [DOI] [PubMed] [Google Scholar]
- 44.Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused Echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6(3):156–67. 10.1006/nimg.1997.0289 [DOI] [PubMed] [Google Scholar]
- 45.Olman CA, Davachi L, Inati S. Distortion and signal loss in medial temporal lobe. PLoS One. 2009;4(12):e8160. 10.1371/journal.pone.0008160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drane DL, Loring DW, Voets NL, Price M, Ojemann JG, Willie JT, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 2015;56(1):101–13. 10.1111/epi.12860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donos C, Breier J, Friedman E, Rollo P, Johnson J, Moss L, et al. Laser ablation for mesial temporal lobe epilepsy: surgical and cognitive outcomes with and without mesial temporal sclerosis. Epilepsia. 2018;59:1421–32. 10.1111/epi.14443 [DOI] [PubMed] [Google Scholar]
- 48.Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8(12):976–87. 10.1038/nrn2277 [DOI] [PubMed] [Google Scholar]
- 49.Lambon Ralph MA. Neurocognitive insights on conceptual knowledge and its breakdown. Philos Trans R Soc Lond B Biol Sci. 2013;369(1634):20120392. 10.1098/rstb.2012.0392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trimmel K, van Graan AL, Caciagli L, Haag A, Koepp MJ, Thompson PJ, et al. Left temporal lobe language network connectivity in temporal lobe epilepsy. Brain. 2018;141(8):2406–18. 10.1093/brain/awy164 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.