Keywords: excitability, ion channels, taste, thermal, voltage gated
Abstract
Temperature strongly influences the intensity of taste, but it remains understudied despite its physiological, hedonic, and commercial implications. The relative roles of the peripheral gustatory and somatosensory systems innervating the oral cavity in mediating thermal effects on taste sensation and perception are poorly understood. Type II taste-bud cells, responsible for sensing sweet, bitter umami, and appetitive NaCl, release neurotransmitters to gustatory neurons by the generation of action potentials, but the effects of temperature on action potentials and the underlying voltage-gated conductances are unknown. Here, we used patch-clamp electrophysiology to explore the effects of temperature on acutely isolated type II taste-bud cell electrical excitability and whole cell conductances. Our data reveal that temperature strongly affects action potential generation, properties, and frequency and suggest that thermal sensitivities of underlying voltage-gated Na+ and K+ channel conductances provide a mechanism for how and whether voltage-gated Na+ and K+ channels in the peripheral gustatory system contribute to the influence of temperature on taste sensitivity and perception.
NEW & NOTEWORTHY The temperature of food affects how it tastes. Nevertheless, the mechanisms involved are not well understood, particularly whether the physiology of taste-bud cells in the mouth is involved. Here we show that the electrical activity of type II taste-bud cells that sense sweet, bitter, and umami substances is strongly influenced by temperature. These results suggest a mechanism for the influence of temperature on the intensity of taste perception that resides in taste buds themselves.
INTRODUCTION
Temperature strongly influences taste perception (1). Cooling temperatures suppress sweet, bitter, and umami taste perception in humans, whereas warmer temperatures enhance it (2–4). For example, ice cream tastes very sweet as it melts in the mouth, but frozen ice cream lacks sweetness; beer tastes more bitter as it gets warmer. Psychophysical and neurophysiological studies have demonstrated that oral surface temperature is an important modulator of both gustatory and trigeminal neural activities and perceptual responses (2, 3, 5–12). Notably, afferent gustatory chorda tympani and glossopharyngeal nerve responses to taste stimuli recapitulate key features observed in human psychophysical studies (13–17). It is likely that at least some of the temperature sensitivities of these peripheral taste-sensitive neurons are mediated by molecular mechanisms in taste buds. Taste buds embedded in the surface of the tongue epithelium sense molecules in foods and drinks and transmit taste information to afferent gustatory nerves. Each taste bud contains ∼100 cells (18) that are categorized into three types (type I, II, and III), with type II cells sensing sweet, umami, and bitter molecules and appetitive NaCl. Type II cells share a common intracellular signal transduction cascade for sweet, umami, and bitter molecules. In brief, taste molecules binding to G-protein coupled receptors (taste GPCRs) in the apical microvilli engage heterotrimeric G-proteins to activate phospholipase C-β2 (PLCβ2), which generates inositol trisphosphate (InsP3) to trigger Ca2+ release from the endoplasmic reticulum through type 3 InsP3 receptors (InsP3R3). The rise of intracellular Ca2+ concentration activates transient receptor potential channel M5 (TRPM5) channels in the basolateral membrane to depolarize the membrane potential to trigger Na+ action potentials. Appetitive NaCl directly depolarizes fungiform papillae type II cells by Na+ permeation through epithelial Na+ channel (ENaC) ion channels, triggering action potentials independent of Ca2+ signaling (19). Action potentials activate large-pore voltage-gated CALHM1/3 heteroligomeric channels to release ATP (20–22), which binds to P2X2/3 receptors on afferent gustatory nerves in a “channel synapse” to transmit taste information to the brain (23–25). All taste stimuli possess a temperature that may stimulate thermosensory mechanisms of the oral skin, including taste buds that completely reside within 100 μm of the surface, to induce thermal sensations that accompany taste. The thermal sensitivity of TRPM5 channel activity in type II cells has been suggested to contribute to the thermal sensitivity of taste perception (24, 26, 27). However, it has been noted that the thermal sensitivity of TRPM5 cannot possibly account for all the reported effects of temperature on GPCR-mediated tastants (1). Notably, temperature strongly influences neuronal excitability (28–30), raising the possibility that other ionic conductances in type II taste-bud cells, as excitable neuroepithelial cells, could also contribute to the thermal sensitivity of taste. Nevertheless, the effects of temperature on type II cell excitability and whole cell conductances have not been previously examined. In the current study, we employed patch-clamp electrophysiology of acutely isolated type II taste-bud cells under different temperature regimes to determine the effects of temperature on action potentials and the underlying voltage-gated Na+ and K+ conductances.
MATERIALS AND METHODS
Animals
TRPM5-GFP mice [green fluorescent protein (GFP) expressed under the Trpm5 promotor] were generously provided by Dr. R. F. Margolskee (31). Mice were housed in a pathogen-free, temperature- and humidity-controlled vivarium on a 12-h light-dark cycle. Diet consisted of standard laboratory chow and double-distilled water. All methods of mouse handling were approved by the University of Pennsylvania’s Animal Care and Use Committee and in accordance with the National Institutes of Health’s Guidelines for the Care and Use of Experimental Animals. TRPM5 is coexpressed with taste-signaling molecules including α-gustducin, Gγ13, PLC-β2, and InsP3R3 specifically in type II taste-bud cells. The Trpm5 promoter drives the expression of GFP, enabling unambiguous identification of type II taste-bud cells (31–34). Only transgenic mice expressing GFP, i.e., expressed in type II taste-bud cells, were used in experiments. All experiments were performed with both sexes of mice that were at least 3 mo old.
Taste-Bud Cell Isolation
Mice were sacrificed by CO2 inhalation and cervical dislocation. The circumvallate taste epithelium was enzymatically delaminated, taste buds were collected from peeled epithelium, and dissociated single taste cells were collected as detailed previously (21). Briefly, 0.5 mL of a mixture of enzymes containing Dispase II (2 mg/mL; Roche), collagenase A (1 mg/mL; Roche), trypsin inhibitor (1 mg/mL; Sigma), elastase (0.2 mg/mL, Sigma), and DNase I (10 µg/ml, Roche) diluted in a Ca2+-Tyrode’s solution (in mM: 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 5 Na-pyruvate, and 10 HEPES, pH of 7.4 adjusted with NaOH) was injected under the lingual epithelium. After 30 min incubation in Ca2+-Tyrode’s solution at room temperature, the epithelium was peeled off and incubated for 15 min in Ca2+-free Tyrode’s solution (in mM: 140 NaCl, 5 KCl, 5 EGTA, 10 glucose, 5 Na-pyruvate, and 10 HEPES, pH of 7.4 adjusted with NaOH). Gentle suction with a glass capillary pipette removed circumvallate cells from the taste buds. The isolated cells were placed on poly-l-lysine-coated coverslips and allowed to settle for ∼60 min before electrophysiological recording.
Electrophysiology and Data Analysis
All experiments were performed on isolated single GFP-expressing type II taste-bud cells dissociated from circumvallate papillae using standard patch-clamp procedures in the whole cell mode as described previously (21). Of note, type II cells from circumvallate papillae do not express ENaC channels (19). Bath solution temperatures at 10°C–20°C were controlled by an in-line solution heater/cooler system (SC-20, Warner Instruments), while temperatures greater than room temperature (25°C and 30°C) were controlled by a warmed platform (WP-10, Warner Instruments). Temperatures greater than 30°C were not studied because of adverse thermal effects on the stability of giga-ohm seals. Data were acquired with an Axopatch 200B amplifier at 5 kHz. Currents were filtered by an eight-pole Bessel filter at 1 kHz and sampled at 5 kHz with an 18-bit A/D converter. Electrode capacitance was compensated electronically, and 60% of series resistance was compensated with a lag of 10 μs. Electrodes were made from thick-walled PG10150-4 glass (World Precision Instruments) and had a resistance of 4–7 MΩ in the recording conditions. HEKA Pulse software (HEKA Eletronik, Germany) was used for data acquisition and stimulation protocols. Igor Pro was used for graphing and data analysis (WaveMetrics, Inc.). Leak subtractions were not applied in the current study. Results are presented as means ± SD As indicated in figure legends, “n” in the current study is the number of independent experiments, for example, n = 3 indicates 3 different type II cells from either the same or different animals. An unpaired Student’s t test was used for statistical analyses.
Recording Solutions
In the whole cell current-clamp mode to record action potentials, and in the whole cell voltage-clamp mode to record ionic conductances, physiological solutions were used: the bath solution contained the following (in mM): 150 Na+, 5.4 K+, 1.5 Ca2+, 1 Mg2+, 150 Cl−, 20 glucose, and 10 HEPES, pH 7.4 adjusted by methanesulfonate, ∼320 mosmol/kgH2O, and the pipette solution contained the following (in mM): 140 K+, 6 Na+, 1 Mg2+, 1 Ca2+, 30 Cl−, 11 EGTA, 3 ATP3-, 0.3 Tris·GTP, 0.01 PIP2, and 10 HEPES, pH 7.3 adjusted by methanesulfonic acid, ∼300 mosmol/kgH2O. For inhibition of voltage-gated K+ currents, and/or recording voltage-gated Na+ currents in type II taste-bud cells, Cs+/TEA+ solutions were used: the bath solution contained the following (in mM): 140 Na+, 5.4 K+, 10 TEA+, 1.5 Ca2+, 1 Mg2+, 1 Cs+, 0.010 CBX (to inhibit CALHM1/3 currents), 150 Cl−, 20 glucose, and 10 HEPES pH 7.4 adjusted by methanesulfonate, ∼330 mosmol/kgH2O; the pipette solution contained the following (in mM): 140 Cs+, 2 TEA+, 1 Ca2+, 11 EGTA, 30 Cl−, and 10 HEPES pH 7.3 adjusted by methanesulfonate, ∼310 mosmol/kgH2O.
RESULTS
Whole Cell Membrane Conductances in Type II Taste-Bud Cells
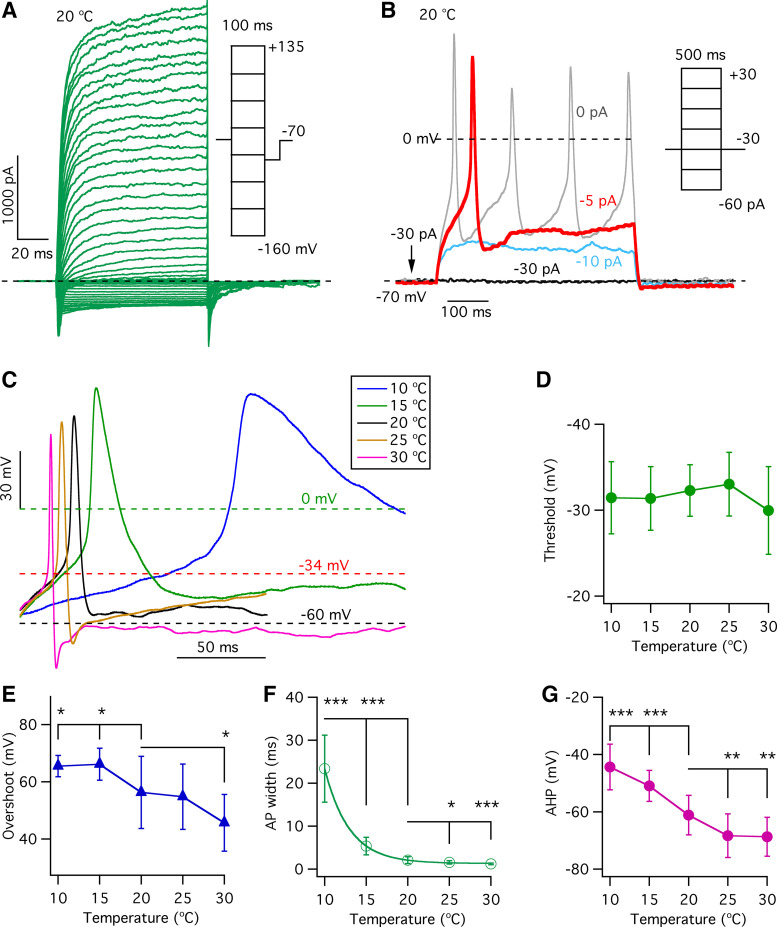
To determine whether and how temperature affects the excitability of type II taste-bud cells, action potentials and membrane conductances were recorded from acutely isolated single GFP-positive type II cells from circumvallate papilla taste buds, using whole cell current- and voltage-clamp modes, respectively (21). A representative family of whole cell currents evoked by 100-ms voltage pulses from −160 mV to +135 mV from a holding potential of −70 mV with physiological bath and pipette solutions (see materials and methods) at 20°C is shown in Fig. 1A. Stable current recordings were obtained over a wide voltage range from −160 mV to +135 mV, which should elicit all possible Na+, K+, or Cl− conductances.
Figure 1.
Effects of temperature on action potential properties in type II taste-bud cells. A: representative family of whole cell currents from a single type II taste-bud cell recorded at 20°C, evoked by 100-ms voltage pulses from −160 to +135 mV in 5-mV increments from a holding potential of −70 mV. B: representative action potentials in response to 500-ms current injections from the same single type II cell in A. A −30-pA current was injected to hold the membrane potential at −70 mV in current-clamp mode. C: representative single action potentials recorded from a single type II taste-bud cell evoked by 500-ms current injections at indicated temperatures were superimposed to show the effects of temperature on the shape of action potentials. D: action potential thresholds at various temperatures: −31.5 ± 4.2 mV (n = 9, P = 0.54) at 10°C; −31.3 ± 3.7 mV (n = 11, P = 0.40) at 15°C; −32.3 ± 3.0 mV (n = 22) at 20°C; −33.0 ± 3.7 mV (n = 17, P = 0.50) at 25°C; and −30.0 ± 5.1 mV (n = 15, P = 0.09) at 30°C. E: overshooting voltage at different temperatures: 65.5 ± 3.7 mV (n = 9, *P < 0.05) at 10°C; 66.1 ± 5.6 mV (n = 11, *P < 0.05) at 15°C; 56.3 ± 12.6 mV (n = 22) at 20°C; 54.8 ± 11.4 mV (n = 17, P = 0.70) at 25°C; and 45.7 ± 9.9 mV (n = 14, *P < 0.05) at 30°C. F: action potential (AP) width at different temperatures: 23.4 ± 7.8 ms (n = 9, ***P < 0.0005) at 10°C; 5.4 ± 2.1 ms (n = 11, ***P < 0.0005) at 15°C; 2.1 ± 0.8 ms (n = 22) at 20°C; 1.6 ± 0.4 ms (n = 17, *P < 0.05) at 25°C; and 1.2 ± 0.2 ms (n = 15, ***P < 0.0005) at 30°C. G: peak afterhyperpolarization potential (AHP) as a function of temperature: −44.4 ± 8.0 mV (n = 9, ***P < 0.0005) at 10°C; −51.0 ± 5.4 mV (n = 11, ***P < 0.0005) at 15°C; −61.1 ± 6.9 mV (n = 22) at 20°C; −68.3 ± 7.6 mV (n = 17, **P < 0.005) at 25°C; and −68.7 ± 6.8 mV (n = 15, **P < 0.005) at 30°C. P values were obtained from Student’s t test, compared with data at 20°C.
Temperature Alters the Properties of Single Action Potentials in Type II Taste-Bud Cells
Type II cells fire action potentials in response to taste stimuli or electrical stimulation (35). To ask how temperature affects excitability properties of type II cells, action potentials were generated by 500-ms current pulses in the whole cell current-clamp mode in GFP-positive cells from circumvallate papillae taste buds. The properties of a single action potential were determined from analyses of the first action potential in each single cell. Representative action potentials are shown in Fig. 1B, with the action potential colored red chosen for analysis for this single cell. Tastants that activate type II cells were not used in the present study because 1) the complex interplay of temperature and taste concentration on taste perception (1) would require such a range of different tastants and their concentrations as to make the investigations onerous; and 2) our inability to reliability activate tastant GPCRs in whole cell recordings of isolated type II cells (unpublished observations). Representative first action potentials at various temperatures recorded from a single GFP-positive cell are shown in Fig. 1C. The shapes of action potentials are important properties that help to influence their propagation, firing frequency, and activation of molecular mechanisms, including CALHM1/3 channels that mediate communications between cells. Action potential thresholds, detected by measuring the change in dV/dt (21), were not significantly altered by temperature over the range of 10°C to 30°C (Fig. 1D). The action potential overshoot peak voltage, detected by Gaussian fits of the action potential, was a nearly linear inverse function of temperature between 15°C and 30°C, with increasing temperature significantly reducing the overshoot voltage (+65.5 ± 1.3 mV, n = 9, P < 0.05 at 10°C; +66.1 ± 1.8 mV, n = 11, P < 0.05 at 15°C; +56.3 ± 2.7 mV, n = 22 at 20°C; +54.8 ± 2.8 mV, n = 17, P = 0.697 at 25°C; and +45.7 ± 2.8 mV, n = 15, P < 0.05 at 30°C) (Fig. 1E). The action potential half-width, detected by Gaussian fits (21) and used to define the action potential duration, was exponentially reduced by increases in temperature (23.4 ± 2.8 ms, n = 9, P < 0.0005 at 10°C; 5.4 ± 0.7 ms, n = 11, P < 0.0005 at 15°C; 2.1 ± 0.2 ms, n = 22 at 20°C; 1.6 ± 0.1 ms, n = 17, P < 0.05 at 25°C; and 1.2 ± 0.05 ms, n = 15, P < 0.05 at 30°C) (Fig. 1F). Finally, the magnitude of the afterhyperpolarization (AHP) was enhanced as a linear function of temperature over the range of 10°C to 25°C (Fig. 1G). Together, these results demonstrate that temperature significantly affects the properties of single action potentials in type II taste-bud cells, with lower temperatures increasing their duration and amplitude and reducing the AHP.
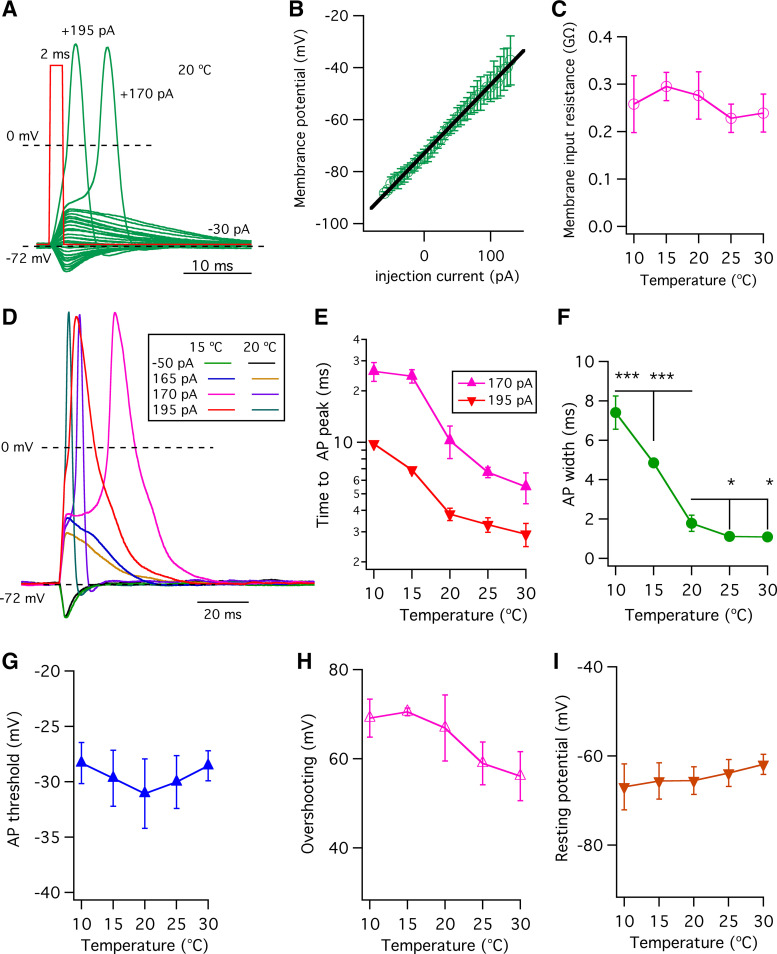
It is possible that a single action potential could activate CALHM ATP-release channels in taste-bud cells (21). Accordingly, we also analyzed single action potentials following application of a brief 2-ms current injection as employed in a previous study (36). A family of membrane potential responses is shown in Fig. 2A. Many current injection pulses were subthreshold that failed to elicit action potentials. After rising above a threshold value in response to stronger current injections, the membrane potential depolarized rapidly, reaching an overshooting peak voltage close to the Na+ equilibrium potential (ENa+), followed by a repolarization phase back to the holding potential. For the current injections that failed to bring the membrane potential to threshold, the membrane potential versus injection current (V-I) relation (Fig. 2B), determined by measurement of the peak of the membrane potential in Fig. 2A, was used to determine the membrane input resistances by linear fits to V-I relations at various temperatures. The V-I relation indicated that temperature does not significantly alter the membrane input resistances during the 2-ms current injections (Fig. 2C). Examples from a single cell are shown in Fig. 2D. A hyperpolarizing 2-ms current injection of −50 pA from a holding current of −30 pA caused a temperature-insensitive transient membrane hyperpolarization (Fig. 2D). Transient depolarizations without firing action potentials were elicited by a +165-pA current injection at both 15°C and 20°C. A first action potential was elicited by a 2-ms depolarizing injection of +170 pA at both 15°C and 20°C, and the last action potential elicited by a +195-pA 2-ms current injection rose to a peak more quickly. Increasing the temperature from 15°C to 20°C shortened the time to reach the overshoot peak (Fig. 2E) and reduced the width (Fig. 2F) of single action potentials, without altering action potential thresholds (Fig. 2G), overshoot peak value (Fig. 2H), or resting membrane potential (Fig. 2I).
Figure 2.
Effects of temperature on single action potentials evoked by 2-ms current injections. A: family of membrane potentials evoked by 2-ms current injections from −80 pA to +195 pA in 5-pA increments from a holding current of −30 pA. Two action potentials are shown with indicated current injections (red trace: 1 representative current injection) at 20°C. B: the membrane potential- injection current (V-I) relation at 20°C, determined by measurement of the peak membrane potential before action potential firing in A. C: membrane input resistances, obtained by linear fits to V-I relations at various temperatures, indicate that temperature does not alter the membrane input resistance. Membrane input resistance (GΩ): 0.26 ± 0.06 (n = 4, P = 0.593) for 10°C; 0.29 ± 0.03 (n = 6, P = 0.434) for 15°C; 0.28 ± 0.05 (n = 9) for 20°C; 0.23 ± 0.03 (n = 4, P = 0.124) for 25°C; and 0.24 ± 0.04 (n = 4, P = 0.266) for 30°C. D: family of membrane potentials in response to a hyperpolarizing current injection of −50 pA from a holding current of −30 pA and in response to depolarizing current injections of various magnitudes: +165 pA that failed to trigger action potentials and action potentials triggered by +170 pA and +195 pA, at 15°C and 20°C as examples. E: time to the overshooting peak of action potentials (ms), determined as the time of stimulus to the peak of the action potential. At +170-pA current injection (which triggers first action potential): 26.0 ± 3.3 (n = 3, P < 0.0005) for 10°C; 24.4 ± 2.2 (n = 4, P < 0.0005) for 15°C; 10.2 ± 2.2 (n = 7) for 20°C; 6.7 ± 0.5 (n = 3, P < 0.05) for 25°C; and 5.5 ± 1.1 (n = 3, P < 0.05) for 30°C. At +195-pA current injection, which triggers the last action potential: 9.8 ± 0.2 (n = 3, P < 0.0005) for 10°C; 6.9 ± 0.3 (n = 4, P < 0.0005) for 15°C; 3.8 ± 0.3 (n = 7) for 20°C; 3.3 ± 0.3 (n = 3, P < 0.05) for 25°C; and 2.9 ± 0.5 (n = 3, P < 0.05) for 30°C. F: half-width of single action potentials (ms) detected by Gaussian fits from threshold to overshooting peak: 7.4 ± 0.8 (n = 3, ***P < 0.0005) for 10°C; 4.8 ± 0.2 (n = 4, ***P < 0.0005) for 15°C; 1.8 ± 0.4 (n = 7) for 20°C; 1.1 ± 0.04 (n = 3, *P < 0.05) for 25°C; and 1.1 ± 0.04 (n = 3, *P < 0.05) for 30°C. G: action potential (AP) thresholds (mV) detected by the change in dV/dt and defined as the voltage reached once dV/dt significantly increased from baseline (see details in Ref. 21): 28.3 ± 1.8 (n = 3, P = 0.201) for 10°C; 29.7 ± 2.5 (n = 4, P = 0.476) for 15°C; 31.1 ± 3.1 (n = 7) for 20°C; 30.0 ± 2.4 (n = 3, P = 0.625) for 25°C; and 28.6 ± 1.4 (n = 3, P = 0.229) for 30°C. H: action potential overshooting peaks (mV) detected by Gaussian fits: 69.1 ± 4.3 (n = 3, P = 0.648) for 10°C; 70.5 ± 0.9 (n = 4, P = 0.362) for 15°C; 66.9 ± 7.4 (n = 7) for 20°C; 59.0 ± 4.8 (n = 3, P = 0.133) for 25°C; and 56.1 ± 5.5 (n = 3, P = 0.055) for 30°C. I: resting potentials (mV) at various temperatures: −67.0 ± 5.2 (n = 3, P = 0.600) for 10°C; −65.6 ± 4.1 (n = 4, P = 0.983) for 15°C; −65.6 ± 3.1 (n = 7) for 20°C; −63.9 ± 3.0 (n = 3, P = 0.446) for 25°C; and −61.9 ± 2.3 (n = 3, P = 0.105) for 30°C. P values were obtained from Student’s t test, compared with data at 20°C.
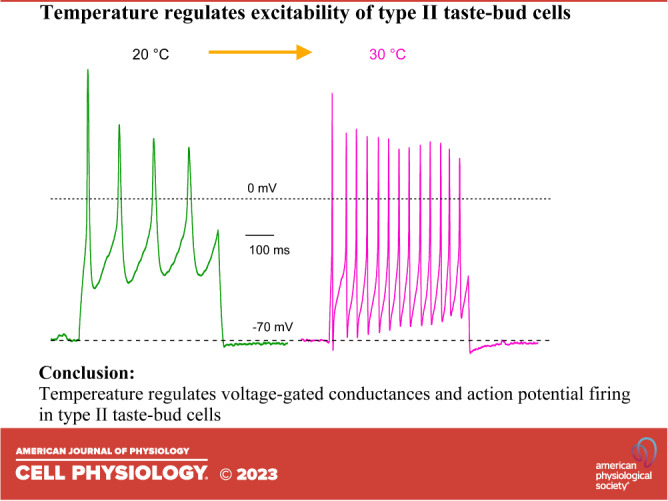
Effects of Temperature on Action Potential Firing Frequency in Type II Taste-Bud Cells
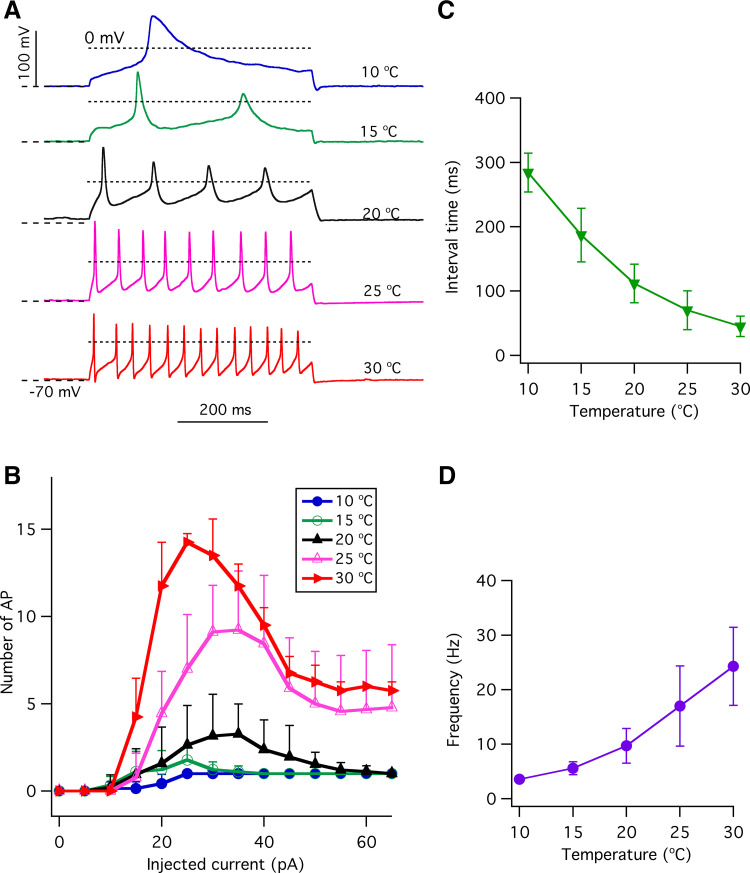
Action potentials are required to activate voltage-gated CALHM1/3 channels to release ATP to mediate neurotransmission in type II taste-bud cells (20, 22, 37, 38). Temperature affects the intensity of taste perception (1), which is encoded by the excitability of upstream transduction mechanisms. Taste stimulation can generate a train of action potentials (23), and the generation of action potentials is sensitive to stimulus duration and intensity (39). With a prolonged stimulus duration (500 ms), only three to approximately four action potentials with a firing frequency of ∼10 Hz at room temperature were observed in previous studies (21, 39, 40). With stronger current injections, the number of overshooting action potentials declined, likely due to Na+ channel inactivation. Here, we found that elevation of the temperature strongly increased the number of overshooting action potentials in response to 500-ms depolarizing current pulses (Fig. 3, A and B), associated with a decreased interval time between action potentials (Fig. 3C) that resulted in enhanced action potential firing frequency (Fig. 3D). Enhanced action potential frequency was due to the observed effects of temperature on the shape of single action potentials (Fig. 1). Together, these results indicate that type II cell excitability is strongly influenced by temperature, suggesting that the thermal perception of taste may be mediated in part by the temperature sensitivities of type II cell ionic conductances in taste buds.
Figure 3.
The effects of temperature on trains of action potentials in type II taste-bud cells. A: representative trains of action potentials in response to 500-ms +30-pA current injections at different temperatures as indicated. Dotted lines indicate −70 mV and 0 mV, respectively. B: number of overshooting action potentials (AP) during a 500-ms depolarizing current injection at various temperatures as indicated (n = 4–18). C: in the cells that generated a maximum number of action potentials, the interval time (ms) between overshooting peaks of first and second action potentials in the train of action potentials were 284.1 ± 30.2 (n = 3, P < 0.0005) at 10°C; 186.9 ± 41.7 (n = 8, P < 0.0005) at 15°C; 111.9 ± 29.8 (n = 22) at 20°C; 70.2 ± 30.2 (n =17, P < 0.0005) at 25°C; 45.3 ± 15.7 (n = 15, P < 0.0005) at 30°C. D: action potential frequency (Hz), the reciprocal of the interval time in C was 3.6 ± 0.4 (n = 3, P < 0.005) for 10°C; 5.6 ± 1.2 (n = 8, P < 0.005) for 15°C; 9.7 ± 3.2 (n = 22) for 20°C; 17.0 ± 7.3 (n =17, P < 0.0005) for 25°C; and 24.3 ± 7.2 (n = 15, P < 0.0005) for 30°C. P values were obtained from Student’s t test, compared with data at 20°C.
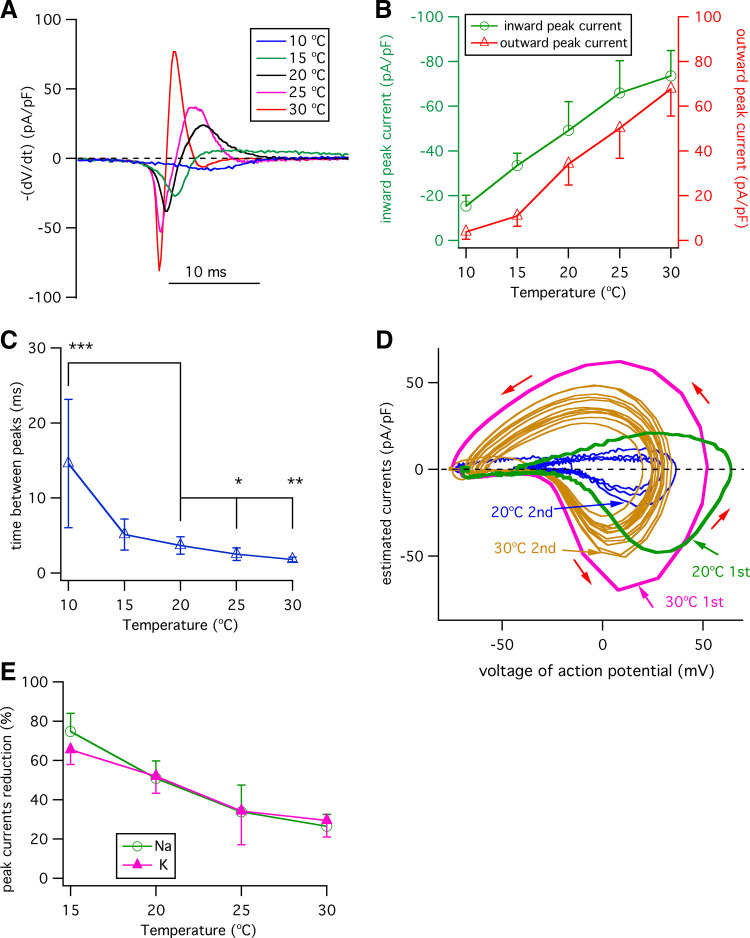
Effects of Temperature on Estimated Na+ and K+ Currents during Action Potentials in Type II Taste-Bud Cells
Voltage-gated Na+ and K+ conductances shape the properties of action potentials, but it is not known how temperature affects these conductances during action potentials in type II taste-bud cells. To investigate this, we took two approaches. First, we estimated the currents using phase plane analyses (41). Here, the voltage-gated transient currents I (pA/pF) were estimated from the relationship I = −dV/dt (pA/pF), where dV/dt is the first derivative of the action potential (21, 41). In this analysis, the ionic current through the membrane (the net current through all channels) is equal and opposite to the capacitive current, providing an estimate of the ionic current as a function of the voltage throughout the action potential (41). Traces of estimated currents shown in Fig. 4A were derived from the single action potentials in Fig. 1C as examples. Changes in the temperature significantly affected both inward currents and outward currents during a single action potential, with increasing temperatures significantly enhancing both peak inward currents and outward currents (Fig. 4B). The magnitudes of these changes cannot be accounted for by the small effects of temperature on the Nernstian potentials for either K+ or Na+. The time between the peak of the inward currents and that of outward currents reflects the relative kinetics of opening and closing of voltage-gated Na+ and opening of K+ channels during an action potential. As demonstrated below in measurements of the kinetics of voltage-gated Na+ and K+ currents at these temperatures in response to various voltages, nearly all the Na+ channels activated and inactivated within 5 ms, whereas the K+ channel activation constant was greater than 10 ms. Accordingly, the peaks of the inward and outward currents in the phase-plane plots reflect good estimates of the peak outward Na+ and inward K+ currents. Elevated temperature shortened the interval between peak inward peak and outward currents (Fig. 4C). This effect is therefore strongly correlated with the observed effect of elevated temperature to shorten the single action potential duration.
Figure 4.
Effects of temperature on ionic currents during action potentials. A: traces of estimated currents (pA/pF) [−(dV/dt)] vs. the time of single action potentials at different temperatures as indicated. Currents were superimposed to coincide with the beginning of inwardly current activation (21, 41). B: estimated peak Na+ and K+ currents during a single action potential. Estimated peak inward currents (in pA/pF): −15.4 ± 4.8 (n = 8, P < 0.0005) at 10°C; −33.4 ± 5.5 (n = 8, P < 0.005) at 15°C; −49.2 ± 12.9 (n = 15) for 20°C; −65.9 ± 14.4 (n = 14, P < 0.005) at 25°C; and −73.6 ± 11.3 (n = 7, P < 0.0005) at 30°C. Estimated peak outward currents (in pA/pF): 3.8 ± 3.3 (n = 8, P < 0.0005) at 10°C; 10.9 ± 4.6 (n = 8, P < 0.0005) for 15°C; 34.2 ± 9.4 (n = 15) at 20°C; 50.2 ± 13.5 (n = 14, P < 0.005) at 25°C; and 67.7 ± 12.1 (n = 7, P < 0.0005) at 30°C. C: interval between peak inward current to peak outward current in a single action potential (in ms): 14.6 ± 8.6 (n = 8, P < 0.0005) for 10°C; 5.1 ± 2.1 (n = 8, P < 0.05) for 15°C; 3.7 ± 1.2 (n = 15) for 20°C; 2.50 ± 0.8 (n = 14, P < 0.005) for 25°C; 1.8 ± 0.3 (n = 7, P < 0.0005) for 30°C. D: phase-plane plots of estimated currents [−dV/dt] (pA/pF) vs. voltage of action potentials during trains of action potentials at 20°C and 30°C from Fig, 3A as examples. Estimated currents during action potentials in a train of action potentials are shown. Red arrows indicate direction of the action potential. The estimated currents during the 1st and 2nd action potentials in a train of action potentials are indicated in color. Subsequent action potential estimated currents are shown in different colors (gray for 20°C and yellow for 30°C). E: percent reduction of peak inward and outward currents in the second action potential relative to those in the first action potential, calculated as reduction percent (%) = (1st peak current – 2nd peak current)/(1st peak current) × 100 (%). Peak inward current reduction (%): 74.7 ± 9.3 (n = 8, P < 0.0005) at 15°C; 50.9 ± 8.9 (n = 15) at 20°C; 33.8 ± 13.6 (n = 14, P < 0.0005) at 25°C; 26.5 ± 6.0 (n = 7, P < 0.0005) at 30°C. Peak outward current reduction (%): 65.4 ± 7.5 (n = 8, P < 0.005) at 15°C; 51.9 ± 8.6 (n = 15) at 20°C; 34.2 ± 17.3 (n = 14, P < 0.005) at 25°C; and 29.4 ± 8.4 (n = 7, P < 0.0005) at 30°C. P values were obtained from Student’s t test, compared with data at 20°C.
To understand how elevated temperature modulates voltage-gated Na+ and K+ currents to enhance action potential firing frequency during a train of action potentials (Fig. 3, A and B), the currents during a train of action potentials were estimated during exposure to a range of temperatures. The estimated inward and outward currents during a train of action potentials at 20°C and 30°C are shown as examples in phase-plane plots in Fig. 4D. The magnitudes of the estimated peak Na+ and K+ currents during a train of action potentials were significantly reduced at the cooler temperature (Fig. 4D). Notably, the magnitudes of the peak inward current and peak outward currents in the second action potential relative to those in the first were significantly reduced at both temperatures. Such an effect is most likely due to incomplete recovery from inactivation of voltage-gated Na+ channels induced by the first action potential. Over a range of temperatures from 15°C to 30°C, cooling strongly minimized the peak-current decrements of both the voltage-gated inward and outward currents between the second and first action potential (Fig. 4E). Such an effect is expected to reduce the amplitude of the overshooting voltage and magnitude of AHP in the second action potential compared with the first in a train of action potentials, as observed (Fig. 3A). Subsequent action potentials behaved similarly to the second in a train of action potentials (Fig. 4E). These data suggest that voltage-gated Na+ and K+ currents responsible for action potentials in type II taste-bud cells have temperature sensitivities, consistent with the observed sensitivities of action potentials on temperature.
Effects of Temperature on the Voltage-Gated Outward K+ Currents in Type II Taste-Bud Cells
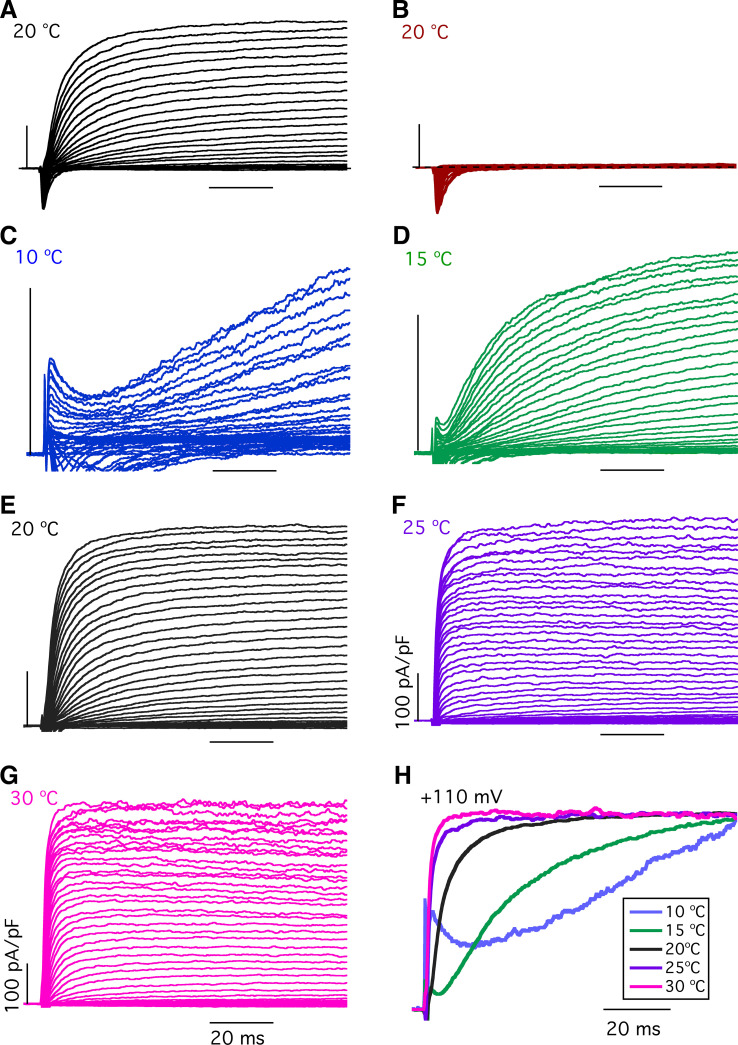
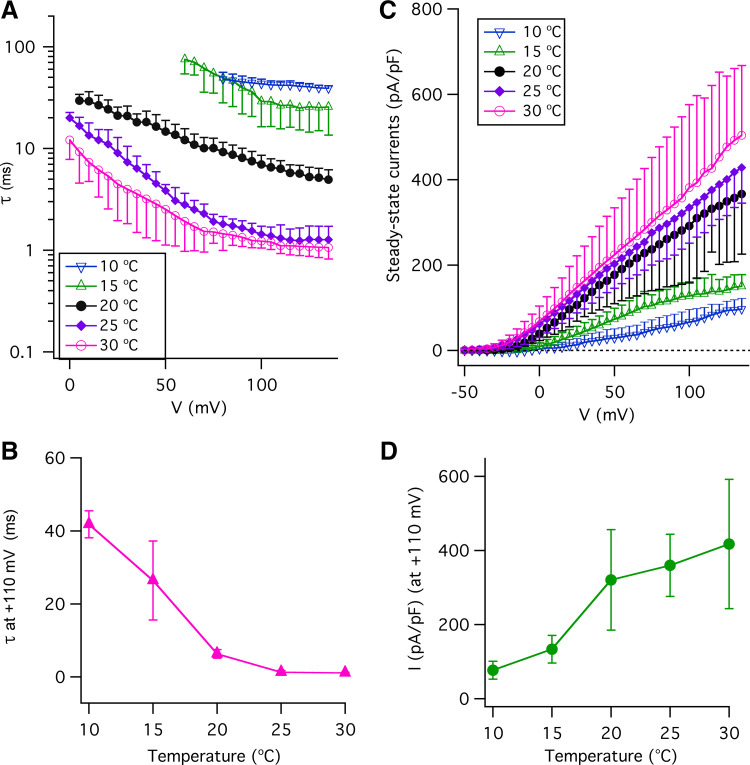
In a second approach to understanding the effects of temperature on the voltage-gated K+ and Na+ conductances underlying action potential properties in type II taste-bud cells, we directly examined the temperature dependence of these currents. Type II cells express voltage-gated K+ channels (42–44) and possess large voltage-gated noninactivating outward K+ currents (20, 21, 39, 45–48). In agreement with previous studies, we observed large voltage-gated outward currents in type II taste-bud cells that are sensitive to TEA+ and Cs+ (Fig. 5, A and B), confirming that they are voltage-gated K+ currents (Fig. 5). We first focused on whether and how temperature affects these voltage-gated K+ currents. Representative families of whole cell outward currents recorded from a single GFP-positive taste-bud cell at several temperatures that encompass a range from 10°C to 30°C are shown in Fig. 5. Noninactivating outward currents evoked by depolarizing voltages developed slowly at 10°C with steady-state activation still incomplete after 100 ms at +135 mV (Fig. 5C). Elevation of the temperature to 15°C significantly accelerated activation kinetics in the same single cell, with further enhancement at higher temperatures (Fig. 5, C–G). The normalized current traces at +110 mV recorded in this single type II cell at various temperatures are shown in Fig. 5H, demonstrating that temperature has significant effects on the activation of outward K+ currents in type II taste-bud cells. A summary of the time courses of current activation at each voltage, described by single exponential functions, over the temperature range of 10°C to 30°C is shown in Fig. 6A. Voltage activation time constants at +110 mV were 41.9 ms at 10°C, 6.3 ms at 20°C, 1.3 ms at 25°C, and 1.1 ms at 30°C (Fig. 6B). A 10°C temperature increase from 20°C to 30°C enhanced activation kinetics by fivefold, while a 10°C temperature reduction from 20°C to 10°C resulted in an approximately fivefold slower activation time constant, giving the temperature coefficient (Q10) of ∼5, indicating that the voltage-activated outward K+ currents in type II taste-bud cells have moderately strong temperature-dependent activation. In addition, temperature strongly affected the steady-state outward K+ currents. Current-density (pA/pF) versus voltage relations (I-V), where the steady-state currents were determined at the end of 100-ms pulses and normalized to the individual whole cell capacitance, suggest that temperatures below room temperature strongly reduce the K+ current magnitudes (Fig. 6C). The current densities (pA/pF) at +110 mV were 417.5 at 30°C, 359.6 at 25°C, 320.2 at 20°C, 133.9 at 15°C, and 76.9 at 10°C (Fig. 6D), suggesting that cool temperatures significantly reduce K+ current densities, consistent with the effects of temperature on the voltage-gated activation kinetics, in which steady-state currents were not achieved at low temperatures by the end of 100-ms voltage pulses (Fig. 5). These effects cannot be accounted for by the small effects of temperature on the K+ equilibrium potential. However, it is likely that single-channel K+ conductances are enhanced by elevated temperatures and contribute to the effects of temperature on K+ current densities observed here. Taken together, these results suggest that temperature strongly affects voltage-gated outward K+ currents in type II taste-bud cells, primarily by affecting the activation kinetics.
Figure 5.
Effects of temperature on voltage-gated outward K+ currents in type II taste-bud cells. A and B: representative families of whole cell currents evoked by 100-ms voltage pulses from −80 mV to +70 mV in 5-mV increments from a holding potential of −70 mV in single type II taste-bud cells in the physiological solutions (A) and in Cs+/TEA+ solutions (B) (described in materials and methods). Voltage-gated outward K+ currents and inward Na+ currents observed in A but only voltage-gated inward Na+ currents observed in B. C–G: families of currents evoked by 100-ms voltage pulses from −160 to +135 mV in 5-mV increments from a holding potential of −70 mV in physiological solutions (described in materials and methods) at different temperatures as indicated. Vertical bars = 100 pA/pF; horizontal bars = 20 ms. H: normalized currents at +110 mV at indicated temperatures. Whole cell capacitances: 4.97 ± 1.38 pF for 10°C (n = 7, P = 0.809); 4.84 ± 1.45 pF for 15°C (n = 9, P = 0.849); 5.11 ± 1.38 pF for 20°C (n = 38); 4.97 ± 1.62 pF for 25°C (n = 7, P = 0.605); and 5.02 ± 1.72 pF for 30°C (n = 13, P =0.802). P values were obtained from Student’s t test, compared with data at 20°C.
Figure 6.
Effects of temperature on features of voltage-gated outward K+ currents. A: voltage-activation time constants were obtained by fitting the outward currents with a single exponential function: 10°C (∇, n = 5); 15°C (Δ, n = 7); 20°C (•, n = 9); 25°C (♦, n = 5); and 30°C (Ο, n = 6). B: voltage-activation time constants (ms) at +110 mV: 10°C (41.8 ± 3.7, n = 5, P < 0.0005); 15°C (26.4 ± 10.8, n = 7, P < 0.0005); 20°C (6.3 ± 1.2, n = 9); 25°C (1.3 ± 0.5, n = 5, P < 0.0005); and 30°C (1.1 ± 0.2, n = 6, P < 0.0005). C: steady-state current-voltage (I-V) relations at different temperatures determined at the end of 100-ms pulses: 10°C (∇, n = 7); 15°C (Δ, n = 7); 20°C (•, n = 27); 25°C (♦, n = 7); and 30°C (Ο, n = 23). D: steady-state currents (pA/pF) at +110 mV: 10°C (76.9 ± 23.6, n = 5, P < 0.0005); 15°C (133.9 ± 37.2, n = 7, P < 0.005); 20°C (320.2 ± 135.6, n = 26); 25°C (359.7 ± 82.8, n = 5, P = 0.502); and 30°C (417.5 ± 174.3, n = 23, P < 0.05). P values were obtained from Student’s t test, compared with data at 20°C.
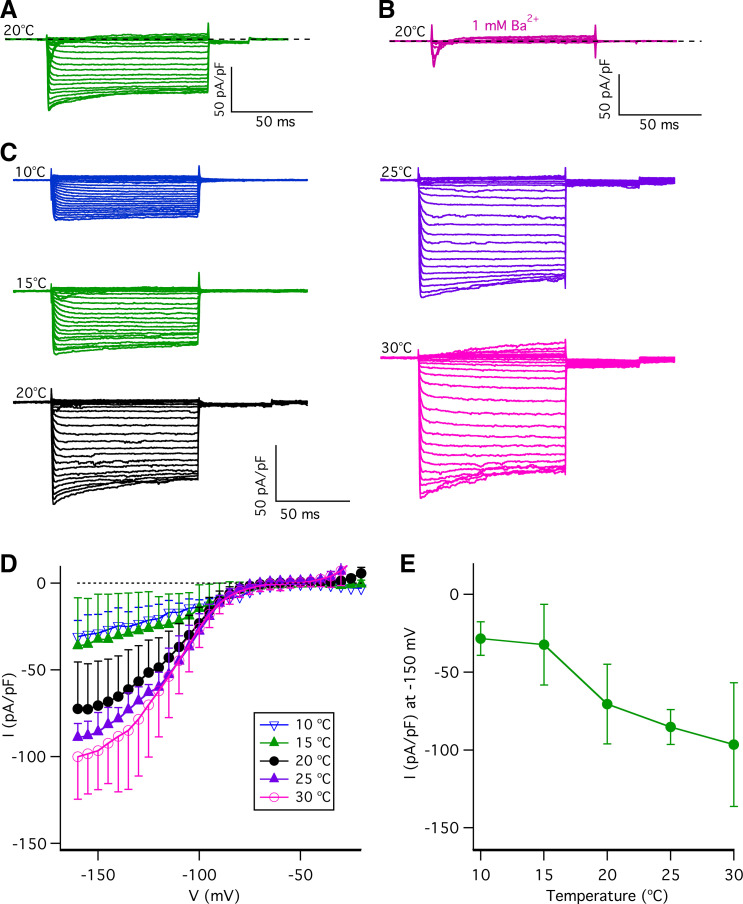
The Temperature Dependence of Inward Rectifying K+ Currents in Type II Taste-Bud Cells
Type II taste-bud cells also express K+ channels that generate large inwardly rectifying currents (21, 45, 49–51). Large steady-state inward currents were evoked by 100-ms voltage pulses from −150 mV to −15 mV from a holding potential of −70 mV (Fig. 7A) from a single type II taste-bud cell. These large steady-state inward currents were sensitive to 1 mM Ba2+ (Fig. 7B) and 1 mM Cs+ (data not shown) but not to 100 µM ZD7288 (a HCN channel blocker; Ref. 52) (data not shown), thus confirming that they are the inwardly rectifying K+ currents. Inwardly rectifying K+ currents play a role in regulating action potential firing in excitable cells by hyperpolarizing the membrane potential. Accordingly, we asked whether temperature alters the inwardly rectifying K+ currents in type II taste-bud cells. Representative whole cell families of currents from a single type II taste-bud cell over a range of temperatures are shown in Fig. 7C. Quantification of the steady-state currents determined at the end of 100-ms pulses (Fig. 7D) revealed that temperature strongly affects the inwardly rectifying K+ currents, with temperatures <20°C markedly suppressing them. The temperature dependence of the currents at –150 mV revealed a Q10 of ∼2.5 (Fig. 7E). These results suggest that the temperature sensitivity of inwardly rectifying K+ channels may also contribute to the effects of temperature on type II-cell electrical excitability.
Figure 7.
The effects of temperature on inwardly rectifying K+ currents. Steady-state inward currents evoked by 100-ms voltage pulses from −150 to −15 mV in 5-mV increments from a holding potential of −70 mV at 20°C before (A) and after (B) application of extracellular 1 mM Ba2+ from a single type II taste-bud cell in physiological pipette and bath solutions (described in materials and methods). Representative of >5 independent experiments. C: representative families of inwardly rectifying K+ currents evoked by 100-ms voltage pulses from −160 to −5 mV in 5-mV increments from a holding potential of −70 mV at various temperatures as indicated in physiological pipette and bath solutions. D: steady-state inward current-voltage (I-V) relations at different temperatures: 10°C (∇, n = 7); 15°C (Δ, n = 7); 20°C (•, n = 27); 25°C (♦, n = 7); and 30°C (Ο, n = 23). Inward currents were determined at the end of 100-ms pulses and normalized to the whole cell capacitance. E: steady-state inward rectifying currents (pA/pF) at −150 mV: 10°C (−28.8 ± 10.8, n = 7, P < 0.0005); 15°C (−32.3 ± 24.9, n = 7, P < 0.005); 20°C (−70.5 ± 25.6, n = 27); 25°C (−85.3 ± 11.2, n = 7, P = 0.149); and 30°C (−95.6 ± 39.7, n = 23, P < 0.05). The current-temperature relation for inward currents at −150 mV indicates an average Q10 of 2.5. P values were obtained from Student’s t test, compared with data at 20°C.
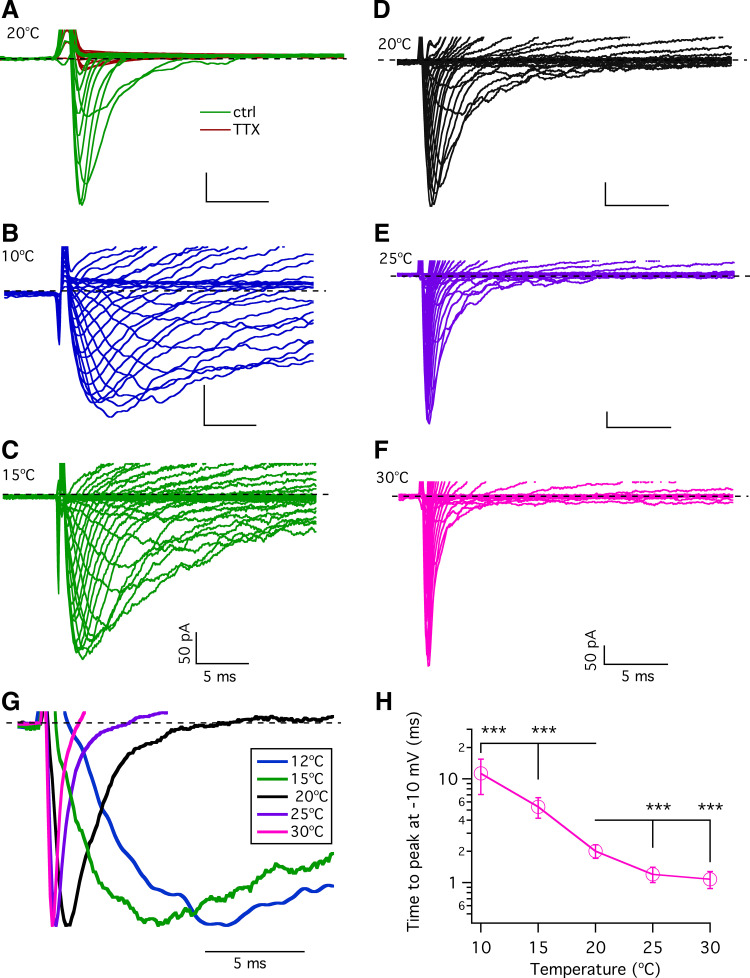
Effects of Temperature on Voltage-Gated Na+ Currents in Type II Taste-Bud Cells
Voltage-gated Na+ channel activity is a fundamental determinant of action potential firing in type II cells in response to tastants and electrical stimuli (53). Taste buds express several types of voltage-gated Na+ channels (54) and possess large voltage-gated Na+ currents (21, 40, 55, 56) mediated by tetrodotoxin (TTX)-sensitive Nav1.2, Nav1.3, and Nav1.7 voltage-gated Na+ channels (40, 56–58). To further understand how temperature influences type II taste-bud cell excitability, we first confirmed that the observed voltage-gated Na+ currents in type II taste-bud cells are sensitive to TTX (Fig. 8A). We next asked whether and how temperature alters the voltage-gated Na+ currents. Representative families of voltage-gated Na+ currents recorded from a single GFP-positive cell at different temperatures are shown in Fig. 8, B–F. Both activation and inactivation were slow at 10°C, markedly accelerated at 20°C, and then further enhanced at 25–30°C. The time to peak inward Na+ currents depends on the kinetics of channel activation and inactivation during a step depolarization, e.g., 100-ms step to −10 mV. We determined the time to reach the maximal peak inward current at each temperature. The normalized currents evoked by depolarization to −10 mV revealed that reducing the temperature from 25°C to 10°C significantly pronged the time to reach the peak inward Na+ current (Fig. 8, G and H). These results suggest that lower temperatures slow the rates of activation and inactivation of the voltage-gated Na+ currents in type II taste-bud cells.
Figure 8.
Effects of temperature on voltage-gated Na+ currents in type II taste-bud cells. A: voltage-gated Na+ currents in type II taste-bud cells were sensitive to tetrodotoxin (TTX). Representative families of whole cell currents from a single type II taste-bud cell (cell capacitance: 4.2 pF) evoked by 100-ms voltage pulses from −120 to + 40 mV in 10-mV increments from a holding potential of −120 mV without (green traces) and with 3 µM TTX (red traces). Bath and pipette solutions contained Cs+ and TEA+ (described in materials and methods). Representative of >5 independent experiments. B–F: representative families of whole cell voltage-gated Na+ currents in a type II taste-bud cell evoked by 100-ms voltage pulses from −80 to +60 mV in 5-mV increments from a holding potential of −70 mV at indicated temperatures. Bath and pipette contained physiological solutions materials and methods). G: normalized currents at −10 mV at different temperatures. H: summary of time (ms) to peak inward current at −10 mV over a range of temperatures: 11.3 ± 4.2 at 10°C (n = 8, ***P < 0.0005); 5.4 ± 1.2 at 15°C (n = 7, ***P < 0.0005); 2.0 ± 0.3 at 20°C (n = 24); 1.2 ± 0.2 at 25°C (n = 6, ***P < 0.0005); and 1.1 ± 0.2 at 30°C (n = 8, ***P < 0.0005). P values were obtained from Student’s t test, compared with data at 20°C.
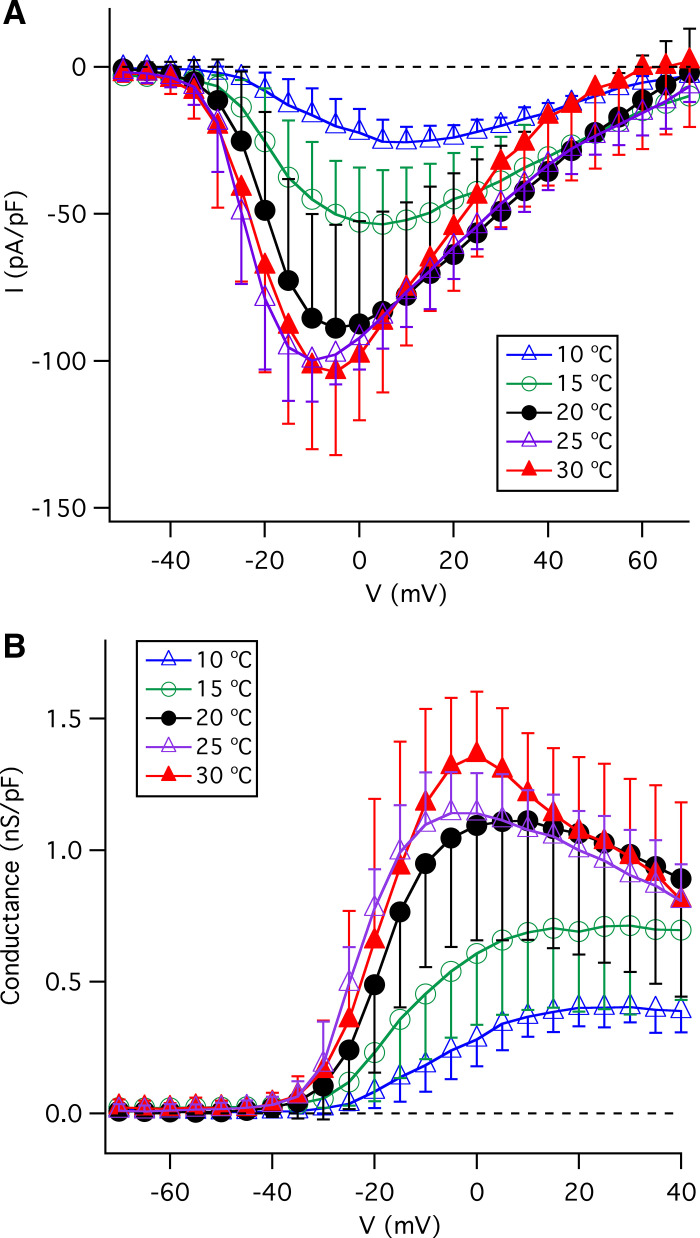
The I-V relations of inward Na+ peak currents at various temperatures are shown in Fig. 9A. Reducing the temperature from 20°C decreased peak inward Na+ currents (pA/pF) at −10 mV from −85.5 ± 35.4 (n = 29) to −45.1 ± 19.4 (n = 8, P < 0.005) at 15°C and −16.4 ± 9.1 (n = 7, P < 0.0005) at 10°C. In contrast, elevated temperatures (25°C and 30°C) did not significantly alter the peak inward currents (Fig. 9A) (−99.9 ± 14.0, n = 7, P = 0.30 at 25°C; and −101.9 ± 28.1; n = 19, P = 0.95 at 30°C). The conductance-voltage (G-V) relations (Fig. 9B) indicate that temperatures greater than room temperature (25°C and 30°C) do not significantly alter the magnitude of the Na+ conductance whereas lower temperatures profoundly decreased it. The influence of temperature in the range from 10°C to 20°C may reflect effects on single-channel conductance. The observed reduction of the maximum Na+ conductance at strongly depolarized voltages at warm temperatures (25°C and 30°C) is likely caused by enhanced Na+ channel inactivation. Together, these results suggest that that the voltage-gated Na+ channels that underly action potentials in type II cells have intrinsic temperature sensitivities that, as for the voltage-gated K+ channels, likely contribute to the temperature sensitivity of type-II cell excitability. In aggregate, these results suggest that the thermal sensitivity of taste perception is likely due, in part, to thermal sensitivities of ion conductances that reside in the taste buds themselves.
Figure 9.
Effects of temperature on voltage-gated Na+ currents in type II taste-bud cells. A: effects of temperature on current-voltage (I-V) relations of voltage-gated Na+ currents, obtained by measurements of peak inward currents normalized to individual whole cell capacitance at indicated temperatures: 10°C (n = 7), 15°C (n = 8), 20°C (n = 29), 25°C (n = 7), and 30°C (n = 19). B: effects of temperature on conductance-voltage relations (G-V) for voltage-gated Na+ currents in type II taste-bud cells.
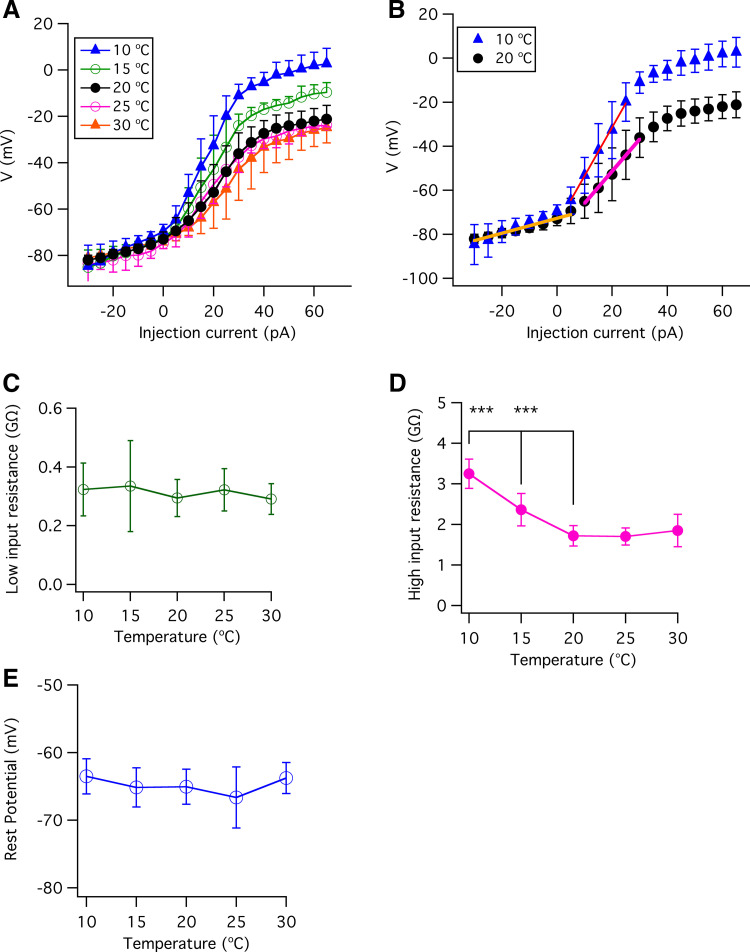
Effects of Temperature on Membrane Input Resistance
Our analyses of the whole cell currents have revealed that temperature affects the whole cell outward and inwardly rectifying K+ conductances as well as voltage-gated Na+ conductances in type II taste-bud cells. Temperature affects the input resistance in neurons (59). The input resistance will influence how much a type II cell will depolarize in response to a steady current, affecting its electrical excitability. Accordingly, we also examined the effects of temperature on type II cell passive membrane properties by determining the input resistance over a temperature range from 10°C to 30°C. Voltage-current (V-I) relations were determined at the end of 500-ms current pulses with 1 µM TTX present to prevent action-potential firing (Fig. 10A). The membrane input resistances were determined as the slopes of linear fits to the V-I data, as shown in Fig. 10B. The membrane input resistance is distinct in two voltage regimes in type II taste-bud cells, with low input resistance observed within a membrane potential range of −80 mV to the resting potential of approximately −65 mV and a higher input resistance observed in a membrane potential range from the resting potential (approximately −65 mV) to −20 mV (Fig. 10B). The membrane properties conferring the low input-resistance were insensitive to temperatures over the full range of temperatures employed (10°C to 30°C; Fig. 10C). In contrast, reducing temperatures below room temperature strongly increased the high input resistance (Fig. 10D). This result indicates that, first, temperature does not significantly alter the membrane conductance near the resting membrane potential of type II taste-bud cells. This is consistent with the observation that the background currents near the resting potential were not significantly different over the range of temperatures we explored (data not shown). Second, the observed effects of temperature on the high input-resistance membrane properties are consistent with our observations that temperatures lower than room temperature, but not elevated temperatures, had significant effects on membrane K+ currents at voltage depolarized from the resting membrane potential of approximately −65 mV (Fig. 6, A–D). In summary, temperature significantly modulates voltage-gated K+ and Na+ currents in type II taste-bud cells and influences the membrane properties in the physiologically relevant range of voltages above the resting membrane potential. In contrast, the temperature does not influence membrane input resistance in the hyperpolarized low input-resistant regime. These results are consistent with the lack of effect of temperature on the resting membrane potential of type II cells (Fig. 10E). Notably, the observed effects of temperature on the steady-state whole cell conductances are consistent with the observed effects of temperature on the Na+ and K+ conductances associated with an action potential.
Figure 10.
Effects of temperature on membrane input resistances in type II taste-bud cells. A: membrane potential vs. injected currents (V-I) at 10°C (n = 8), 15°C (n = 12), 20°C (n = 30), 25°C (n = 9), and 30°C (n = 17), obtained from steady-state voltages at the end of 500-ms current pulses in the presence of TTX to prevent firing action potential firing. B: V-I relations at 10°C and 20°C. Solid lines are linear fits. C: low membrane input resistance, obtained from linear fits to data within −80 mV to −65 mV. The low membrane input resistances (GΩ): 0.323 ± 0.090 for 10°C (n = 8, P = 0.304); 0.335 ± 0.154 for 15°C (n = 12, P = 0.219); 0.295 ± 0.063 for 20°C (n = 30); 0.321 ± 0.725 for 25°C (n = 9, P = 0.268); and 0.291 ± 0.052 for 30°C (n = 17, P = 0.861). D: high membrane input resistances, obtained from linear fits to data within −65 mV to −20 mV. High membrane input resistances (GΩ): 3.25 ± 0.36 for 10°C (n = 8, ***P < 0.0005); 2.36 ± 0.39 for 15°C (n = 12, ***P < 0.0005); 1.72 ± 0.25 for 20°C (n = 30); 1.70 ± 0.21 for 25°C (n = 9, P = 0.844); 1.85 ± 0.40 for 30°C (n = 17, P = 0.160). E: resting membrane potential (mV) over a range of temperatures from 10°C to 30°C: −63.5 ± 2.6 (n = 9, P =0.143) for 10°C; −65.1 ± 2.9 (n = 11, P =0.927) for 15°C; −65.1 ± 2.6 (n = 22) for 20°C; −66.7 ± 4.5 (n = 17, P =0.174) for 25°C; and −63.8 ± 2.3 (n = 15, P =0.130) for 30°C. P values were obtained from Student’s t test, compared with data at 20°C.
DISCUSSION
Temperature strongly influences the intensity of taste (1), but it remains understudied despite its physiological, hedonic, and commercial implications. Taste sensing involves the peripheral gustatory system and the somatosensory system innervating the oral cavity (60), but the relative roles of the two sensory systems in mediating thermal effects on this and other taste sensations and perceptions are poorly understood (1, 10). In type II taste-bud cells responsible for sensing sweet, bitter umami, and appetitive NaCl, TRPM5 was suggested to contribute in the gustatory system to the thermal sweet-taste sensitivity (24, 26, 27, 61). However, it was implicated based on studies in mice with TRMP5 genetically deleted (2, 27, 55), where the canonical type II cell signaling pathway was largely eliminated. In principle, because all processes in the taste bud signal transduction should have some thermal sensitivities, it is a challenge to determine which step(s) contribute most importantly. Here we have demonstrated that temperature profoundly affects type II taste-bud cell electrical excitability and voltage-gated membrane conductances. Our data reveal that temperature strongly affects action potential properties and firing, and they suggest that the thermal sensitivities of underlying voltage-gated Na+ and K+ currents in type II taste-bud cells provide a mechanism for how and whether temperature-sensitive ion channels in the peripheral gustatory system contribute to the influence of temperature on taste sensitivity and perception.
A major finding in the present study is that temperature significantly modulates the firing frequency of action potentials in type II taste-bud cells. Taste intensity depends on the frequency and number of action potentials in an action potential train (18, 23) to sustain the activation of voltage-gated CALHM1/3 ATP-release channels. It has been noted that neural firing and the magnitude and behavioral responses to taste stimuli are proportional to stimulus concentration that interacts with temperature to influence gustatory activity (1). Previously, it has been demonstrated that type II cells fire only a few action potentials during a prolonged stimulation (21, 40). However, those studies were performed at room temperature. Under physiological conditions, the temperature of the surface of the tongue surrounding taste buds is likely near or slightly lower than body temperature until the oral mucosa is exposed to cold or warm food and solutions. Here we show that type II cells can fire a sustained train of action potentials in response to a prolonged stimulus when the temperature is warmer than room temperature. Elevated temperature significantly reduced the interval time between action potentials, increasing firing frequency. We used two approaches to understand the ionic mechanisms for the effects of temperature on action potential firing. First, we employed phase-plane analysis (21, 41) to estimate the magnitude and kinetics of ionic currents during a single action potential and a train of action potentials as a function of temperature. This analysis revealed that increased temperatures increased the magnitudes of peak Na+ and K+ currents and reduced the time to reach peak currents during action potentials. These effects resulted in reduced action potential width and increased the AHP. Both effects would be expected to enable a more rapid recovery of Na+ channels from inactivation for their subsequent involvement in the next action potential. Indeed, similar effects were observed during more prolonged stimulation that elicited a train of action potentials. During a train of action potentials, the Na+ and K+ currents during the second action potential were reduced by more than 50% compared to those during first action potential at room temperature. Such an effect is likely mediated primarily by incomplete escape of Na+ channels from voltage-dependent inactivation, with consequent reduced activation of K+ channels due to the reduced overshoot potential. Interestingly, increased temperatures minimized this decrement, which contributes to the ability of elevated temperature to generate higher action-potential firing frequency. In addition, higher temperatures increased the rate of depolarization between action potentials, likely by enhancing Na+ channel escape from inactivation, also strongly contributing to the enhanced firing rate at elevated temperatures.
In the second approach, we directly measured the gating kinetics of the voltage-gated K+ and Na+ currents in type II cells that underlie activity-induced action potentials. We found that temperature modulates voltage-gated outward K+ currents, with a main effect of warm temperatures to enhance voltage-dependent activation, reducing the activation time constant, giving a temperature coefficient (change per 10 degrees, Q10) of ∼5. Temperature affects the rate of conformation transitions by two- to fivefold per 10°C (Q10 = 2∼5) in most ion channels (62, 63). Thus the kinetics of voltage-gated outward K+ currents in type II cells have a similar temperature dependence as other voltage-gated K+ channels (62, 64) but not as strong as some TRP channels, which may have Q10 >10 (65, 66). Increasing the activation rate of voltage-gated outward K+ currents by elevated temperature is predicted to accelerate the hyperpolarization phase of action potentials, increasing the magnitude of the afterhyperpolarization voltage and shortening the width of action potentials. These results are consistent with the observed effects of temperature on the properties and frequencies of the action potentials we recorded.
We also discovered that the conductance of inwardly rectifying K+ currents in type II taste-bud cells was temperature sensitive, with a Q10 of 2.5 over the range of 10°C to 30°C. Previous studies showed that the inwardly rectifying K+ currents in frog skeletal muscle fibers (67), cardiac myocytes (68), guinea pig ventricular cells (69), and mouse olfactory bulb neurons (70) displayed Q10 between 1.5 and 2.5. Thus the temperature sensitivity of inwardly rectifying K+ currents in type II cells is similar to those in other cell types. Inwardly rectifying K+ currents play a role in setting the resting membrane potential (71–73). Nevertheless, we found that temperature did not significantly alter the resting membrane potential of type II cells. Close examination of expanded I-V relations revealed no differences in the current densities over the voltage range from −90 mV to −30 mV that encompasses the resting potential. It is possible that the effects of temperature on both inward and outward K+ currents result in no significant net effect of temperature on the K+ conductance over the range of voltages near the cell resting potential.
We found that temperature profoundly affects the voltage-gated Na+ currents in type II cells. Raising the temperature enhanced their activation and inactivation rates, in agreement with previous observations in taste-bud cells (74). The time to reach peak inward Na+ currents at −10 mV was temperature dependent with a Q10 of ∼2 between 20°C and 30°C and ∼5 between 20°C and 10°C. The Na+ currents and conductance were reduced when the temperature was lowered below room temperature, similar to the effects of temperature on voltage-gated Na+ channels in rat dorsal root ganglion neurons (75). Analysis of the whole cell steady-state recordings suggested that warmer temperatures generate larger and more rapid inward Na+ peak currents, both predicted to shorten the width of action potentials and increase the overshooting voltage. A shortening of the action potential width was indeed observed. In contrast, the value of the overshooting potential was not enhanced at higher temperatures. It is likely that the combined effects of temperature on the rates of both Na+ channel activation and inactivation and of K+ channel activation prevent the overshooting voltage from increasing further as temperature increases. Indeed, phase-plane plots indicate that increasing temperature shortens the time between the peak inward Na+ and outward K+ currents, which is predicted to reduce the magnitude of the peak overshooting voltage.
In summary, we have provided evidence for strong temperature regulation of type II taste-bud cell excitability, and our results provide insights into the underlying mechanisms that involve thermal modulation of voltage-gated K+ and Na+ channel activities that can account for temperature effects on action potential properties and frequencies. These mechanisms enable type II cells to transmit thermal information associated with the ingestion of bitter, sweet, or umami substances and appetitive concentrations of NaCl, influencing the perception of the intensities of these tastants. Rodents have been used extensively to understand the molecular/cellular/biophysical mechanisms involved in taste, as such studies in humans are largely not feasible. Previous studies (5, 27, 56, 76) have suggested that the molecular mechanisms are conserved between rodents and humans. Whereas there is a relative paucity of studies on the thermal responses to taste stimuli in rodents, the data to date suggest conserved responses and mechanisms. In future studies, determination of the thermal sensitivities of other mechanisms in the signal transduction cascade in response to tastants in type II cells, including the responses to GPCR-mediated tastants and their integration with the mechanisms defined here, will help to provide a more complete understanding of the roles and mechanisms of the peripheral taste machinery in the thermal perception of taste.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by a grant from the National Institute on Deafness and Other Communication Disorders Grant DC018278 to J.K.F. and Z.M.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.M., U.P., and J.K.F. conceived and designed research; Z.M. and U.P. performed experiments; Z.M. analyzed data; Z.M. and J.K.F. interpreted results of experiments; Z.M. prepared figures; Z.M. and J.K.F. drafted manuscript; Z.M. and J.K.F. edited and revised manuscript; J.K.F. approved final version of manuscript.
REFERENCES
- 1. Lemon CH. Modulation of taste processing by temperature. Am J Physiol Regul Integr Comp Physiol 313: R305–R321, 2017. doi: 10.1152/ajpregu.00089.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Green BG, Alvarado C, Andrew K, Nachtigal D. The effect of temperature on umami taste. Chem Senses 41: 537–545, 2016. doi: 10.1093/chemse/bjw058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Green BG, Andrew K. Stimulus-dependent effects of temperature on bitter taste in humans. Chem Senses 42: 153–160, 2017. doi: 10.1093/chemse/bjw115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Green BG, Nachtigal D. Temperature affects human sweet taste via at least two mechanisms. Chem Senses 40: 391–399, 2015. doi: 10.1093/chemse/bjv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartoshuk LM, Rennert K, Rodin J, Stevens JC. Effects of temperature on the perceived sweetness of sucrose. Physiol Behav 28: 905–910, 1982. doi: 10.1016/0031-9384(82)90212-8. [DOI] [PubMed] [Google Scholar]
- 6. Calvino AM. Perception of sweetness: the effects of concentration and temperature. Physiol Behav 36: 1021–1028, 1986. doi: 10.1016/0031-9384(86)90474-9. [DOI] [PubMed] [Google Scholar]
- 7. Green BG. The effect of cooling on the vibrotactile sensitivity of the tongue. Percept Psychophys 42: 423–430, 1987. doi: 10.3758/bf03209749. [DOI] [PubMed] [Google Scholar]
- 8. Green BG, Frankmann SP. The effect of cooling on the perception of carbohydrate and intensive sweeteners. Physiol Behav 43: 515–519, 1988. doi: 10.1016/0031-9384(88)90127-8. [DOI] [PubMed] [Google Scholar]
- 9. Green BG, Nachtigal D. Somatosensory factors in taste perception: effects of active tasting and solution temperature. Physiol Behav 107: 488–495, 2012. doi: 10.1016/j.physbeh.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lemon CH. Perceptual and neural responses to sweet taste in humans and rodents. Chemosens Percept 8: 46–52, 2015. doi: 10.1007/s12078-015-9177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson DM, Lemon CH. Modulation of central gustatory coding by temperature. J Neurophysiol 110: 1117–1129, 2013. doi: 10.1152/jn.00974.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilson DM, Lemon CH. Temperature systematically modifies neural activity for sweet taste. J Neurophysiol 112: 1667–1677, 2014. doi: 10.1152/jn.00368.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu B, Breza JM, Contreras RJ. Temperature influences chorda tympani nerve responses to sweet,salty, sour, umami, and bitter stimuli in mice. Chem Senses 41: 727–736, 2016. doi: 10.1093/chemse/bjw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu B, Breza JM, Nikonov AA, Paedae AB, Contreras RJ. Leptin increases temperature-dependent chorda tympani nerve responses to sucrose in mice. Physiol Behav 107: 533–539, 2012. doi: 10.1016/j.physbeh.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 15. Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol 95: 674–685, 2006. doi: 10.1152/jn.00793.2005. [DOI] [PubMed] [Google Scholar]
- 16. Nakamura M, Kurihara K. Differential temperature dependence of taste nerve responses to various taste stimuli in dogs and rats. Am J Physiol Regul Integr Comp Physiol 261: R1402–R1408, 1991. doi: 10.1152/ajpregu.1991.261.6.R1402. [DOI] [PubMed] [Google Scholar]
- 17. Nagaki J, Yamashita S, Sato M. Neural response of cat to taste stimuli of varying temperatures. Jpn J Physiol 14: 67–89, 1964. doi: 10.2170/jjphysiol.14.67. [DOI] [PubMed] [Google Scholar]
- 18. Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol 190: 285–296, 2010. [Erratum in J Cell Biol 191: 429, 2010]. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nomura K, Nakanishi M, Ishidate F, Iwata K, Taruno A. All-electrical Ca2+-independent signal transduction mediates attractive sodium taste in taste buds. Neuron 106: 816–829 e816, 2020. doi: 10.1016/j.neuron.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 20. Ma Z, Taruno A, Ohmoto M, Jyotaki M, Lim JC, Miyazaki H, Niisato N, Marunaka Y, Lee RJ, Hoff H, Payne R, Demuro A, Parker I, Mitchell CH, Henao-Mejia J, Tanis JE, Matsumoto I, Tordoff MG, Foskett JK. CALHM3 is essential for rapid ion channel-mediated purinergic neurotransmission of GPCR-mediated tastes. Neuron 98: 547–561.e510, 2018. doi: 10.1016/j.neuron.2018.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma Z, Saung WT, Foskett JK. Action potentials and ion conductances in wild-type and CALHM1-knockout type II taste cells. J Neurophysiol 117: 1865–1876, 2017. doi: 10.1152/jn.00835.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495: 223–226, 2013. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murata Y, Yasuo T, Yoshida R, Obata K, Yanagawa Y, Margolskee RF, Ninomiya Y. Action potential-enhanced ATP release from taste cells through hemichannels. J Neurophysiol 104: 896–901, 2010. doi: 10.1152/jn.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang YA, Roper SD. Intracellular Ca2+ and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol 588: 2343–2350, 2010. doi: 10.1113/jphysiol.2010.191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma Z, Tanis JE, Taruno A, Foskett JK. Calcium homeostasis modulator (CALHM) ion channels. Pflugers Arch 468: 395–403, 2016. doi: 10.1007/s00424-015-1757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Z, Zhao Z, Margolskee R, Liman E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci 27: 5777–5786, 2007. doi: 10.1523/JNEUROSCI.4973-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438: 1022–1025, 2005. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 28. Van Hook MJ. Temperature effects on synaptic transmission and neuronal function in the visual thalamus. PLoS One 15: e0232451, 2020. doi: 10.1371/journal.pone.0232451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Austerschmidt LJ, Khan A, Plant DO, Richards EMB, Knott S, Baker MD. The effects of temperatureon the biophysical properties of optic nerve F-fibres. Sci Rep 10: 12755, 2020. doi: 10.1038/s41598-020-69728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kiernan MC, Cikurel K, Bostock H. Effects of temperature on the excitability properties of human motor axons. Brain 124: 816–825, 2001. doi: 10.1093/brain/124.4.816. [DOI] [PubMed] [Google Scholar]
- 31. Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol 4: 7, 2006. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sukumaran SK, Lewandowski BC, Qin Y, Kotha R, Bachmanov AA, Margolskee RF. Whole transcriptome profiling of taste bud cells. Sci Rep 7: 7595, 2017. doi: 10.1038/s41598-017-07746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaske S, Krasteva G, Konig P, Kummer W, Hofmann T, Gudermann T, Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci 8: 49, 2007. doi: 10.1186/1471-2202-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5: 1169–1176, 2002. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 35. Furue H, Yoshii K. In situ tight-seal recordings of taste substance-elicited action currents and voltage-gated Ba currents from single taste bud cells in the peeled epithelium of mouse tongue. Brain Res 776: 133–139, 1997. doi: 10.1016/s0006-8993(97)00974-8. [DOI] [PubMed] [Google Scholar]
- 36. Takeuchi K, Yoshii K, Ohtubo Y. Age-related electrophysiological changes in mouse taste receptor cells. Exp Physiol 106: 519–531, 2021. doi: 10.1113/EP089104. [DOI] [PubMed] [Google Scholar]
- 37. Kinnamon SC, Finger TE. A taste for ATP: neurotransmission in taste buds. Front Cell Neurosci 7: 264, 2013. doi: 10.3389/fncel.2013.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310: 1495–1499, 2005. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 39. Chen Y, Sun XD, Herness S. Characteristics of action potentials and their underlying outward currents in rat taste receptor cells. J Neurophysiol 75: 820–831, 1996. doi: 10.1152/jn.1996.75.2.820. [DOI] [PubMed] [Google Scholar]
- 40. Ohtubo Y. Slow recovery from the inactivation of voltage-gated sodium channel Nav1.3 in mouse taste receptor cells. Pflugers Arch 473: 953–968, 2021. doi: 10.1007/s00424-021-02563-w. [DOI] [PubMed] [Google Scholar]
- 41. Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 8: 451–465, 2007. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- 42. Wang H, Iguchi N, Rong Q, Zhou M, Ogunkorode M, Inoue M, Pribitkin EA, Bachmanov AA, Margolskee RF, Pfeifer K, Huang L. Expression of the voltage-gated potassium channel KCNQ1 in mammalian taste bud cells and the effect of its null-mutation on taste preferences. J Comp Neurol 512: 384–398, 2009. doi: 10.1002/cne.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ohmoto M, Matsumoto I, Misaka T, Abe K. Taste receptor cells express voltage-dependent potassium channels in a cell age-specific manner. Chem Senses 31: 739–746, 2006. doi: 10.1093/chemse/bjl016. [DOI] [PubMed] [Google Scholar]
- 44. Liu L, Hansen DR, Kim I, Gilbertson TA. Expression and characterization of delayed rectifying K+ channels in anterior rat taste buds. Am J Physiol Cell Physiol 289: C868–C880, 2005. doi: 10.1152/ajpcell.00115.2005. [DOI] [PubMed] [Google Scholar]
- 45. Nakao Y, Koshimura M, Yamasaki T, Ohtubo Y. Cell-type-independent expression of inwardly rectifying potassium currents in mouse fungiform taste bud cells. Physiol Res 69: 501–510, 2020. doi: 10.33549/physiolres.934331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Medler KF, Margolskee RF, Kinnamon SC. Electrophysiological characterization of voltage-gated currents in defined taste cell types of mice. J Neurosci 23: 2608–2617, 2003. doi: 10.1523/JNEUROSCI.23-07-02608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci 9: 1, 2008. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McPheeters M, Barber AJ, Kinnamon SC, Kinnamon JC. Electrophysiological and morphological properties of light and dark cells isolated from mudpuppy taste buds. J Comp Neurol 346: 601–612, 1994. doi: 10.1002/cne.903460411. [DOI] [PubMed] [Google Scholar]
- 49. Nishijima K, Atoji Y. Taste buds and nerve fibers in the rat larynx: an ultrastructural andimmunohistochemical study. Arch Histol Cytol 67: 195–209, 2004. doi: 10.1679/aohc.67.195. [DOI] [PubMed] [Google Scholar]
- 50. Zhao FL, Herness S. Resynthesis of phosphatidylinositol 4,5-bisphosphate mediates adaptation ofthe caffeine response in rat taste receptor cells. J Physiol 587: 363–377, 2009. doi: 10.1113/jphysiol.2008.165167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ye W, Chang RB, Bushman JD, Tu YH, Mulhall EM, Wilson CE, Cooper AJ, Chick WS, Hill-Eubanks DC, Nelson MT, Kinnamon SC, Liman ER. The K+ channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction. Proc Natl Acad Sci U S A 113: E229–238, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Knaus A, Zong XG, Beetz N, Jahns R, Lohse MJ, Biel M, Hein L. Direct inhibition of cardiac hyperpolarization-activated cyclic nucleotide-gated pacemaker channels by clonidine. Circulation 115: 872–880, 2007. doi: 10.1161/CIRCULATIONAHA.106.667675. [DOI] [PubMed] [Google Scholar]
- 53. Vandenbeuch A, Kinnamon SC. Why do taste cells generate action potentials? J Biol 8: 42, 2009. doi: 10.1186/jbiol138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gao N, Lu M, Echeverri F, Laita B, Kalabat D, Williams ME, Hevezi P, Zlotnik A, Moyer BD. Voltage-gated sodium channels in taste bud cells. BMC Neurosci 10: 20, 2009. doi: 10.1186/1471-2202-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kimura K, Ohtubo Y, Tateno K, Takeuchi K, Kumazawa T, Yoshii K. Cell-type-dependent action potentials and voltage-gated currents in mouse fungiform taste buds. Eur J Neurosci 39: 24–34, 2014. doi: 10.1111/ejn.12388. [DOI] [PubMed] [Google Scholar]
- 56. Bigiani A, Sbarbati A, Osculati F, Pietra P. Electrophysiological characterization of a putative supporting cell isolated from the frog taste disk. J Neurosci 18: 5136–5150, 1998. doi: 10.1523/JNEUROSCI.18-14-05136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suwabe T, Kitada Y. Voltage-gated inward currents of morphologically identified cells of the frog taste disc. Chem Senses 29: 61–73, 2004. doi: 10.1093/chemse/bjh006. [DOI] [PubMed] [Google Scholar]
- 58. Noguchi T, Ikeda Y, Miyajima M, Yoshii K. Voltage-gated channels involved in taste responses and characterizing taste bud cells in mouse soft palates. Brain Res 982: 241–259, 2003. doi: 10.1016/s0006-8993(03)03013-0. [DOI] [PubMed] [Google Scholar]
- 59. Griffin JD, Boulant JA. Temperature effects on membrane potential and input resistance in rat hypothalamic neurones. J Physiol 488: 407–418, 1995. doi: 10.1113/jphysiol.1995.sp020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang J, Jin H, Zhang W, Ding C, O'Keeffe S, Ye M, Zuker CS. Sour sensing from the tongue to the brain. Cell 179: 392–402 e315, 2019. doi: 10.1016/j.cell.2019.08.031. [DOI] [PubMed] [Google Scholar]
- 61. Skinner M, Eldeghaidy S, Ford R, Giesbrecht T, Thomas A, Francis S, Hort J. Variation in thermally induced taste response across thermal tasters. Physiol Behav 188: 67–78, 2018. doi: 10.1016/j.physbeh.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rodriguez BM, Sigg D, Bezanilla F. Voltage gating of Shaker K+ channels. The effect of temperatureon ionic and gating currents. J Gen Physiol 112: 223–242, 1998. doi: 10.1085/jgp.112.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pusch M, Ludewig U, Jentsch TJ. Temperature dependence of fast and slow gating relaxations of CIC-0 chloride channels. J Gen Physiol 109: 105–116, 1997. doi: 10.1085/jgp.109.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yang F, Zheng J. High temperature sensitivity is intrinsic to voltage-gated potassium channels. eLife 3: e03255, 2014. doi: 10.7554/eLife.03255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lamas JA, Rueda-Ruzafa L, Herrera-Perez S. Ion channels and thermosensitivity: TRP, TREK, or both? Int J Mol Sci 20: 2371, 2019. doi: 10.3390/ijms20102371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Clapham DE. TRP channels as cellular sensors. Nature 426: 517–524, 2003. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 67. Caffier G, Shvinka NE. Effect of temperature on the inward rectifier and gramicidin A-induced channels in the membrane of frog skeletal muscle fibres. Gen Physiol Biophys 5: 47–51, 1986. [PubMed] [Google Scholar]
- 68. McLarnon JG, Hamman BN, Tibbits GF. Temperature dependence of unitary properties of an ATP-dependent potassium channel in cardiac myocytes. Biophys J 65: 2013–2020, 1993. doi: 10.1016/S0006-3495(93)81243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mitsuiye T, Shinagawa Y, Noma A. Temperature dependence of the inward rectifier K+ channel gating in guinea-pig ventricular cells. Jpn J Physiol 47: 73–79, 1997. doi: 10.2170/jjphysiol.47.73. [DOI] [PubMed] [Google Scholar]
- 70. Borin M, Fogli Iseppe A, Pignatelli A, Belluzzi O. Inward rectifier potassium (Kir) current in dopaminergic periglomerular neurons of the mouse olfactory bulb. Front Cell Neurosci 8: 223, 2014. doi: 10.3389/fncel.2014.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90: 291–366, 2010. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 72. Lu Z. Mechanism of rectification in inward-rectifier K+ channels. Annu Rev Physiol 66: 103–129, 2004. doi: 10.1146/annurev.physiol.66.032102.150822. [DOI] [PubMed] [Google Scholar]
- 73. Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol 59: 171–191, 1997. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 74. Ruff RL. Effects of temperature on slow and fast inactivation of rat skeletal muscle Na+ channels. Am J Physiol Cell Physiol 277: C937–C947, 1999. doi: 10.1152/ajpcell.1999.277.5.C937. [DOI] [PubMed] [Google Scholar]
- 75. Sarria I, Ling J, Gu JG. Thermal sensitivity of voltage-gated Na+ channels and A-type K+ channels contributes to somatosensory neuron excitability at cooling temperatures. J Neurochem 122: 1145–1154, 2012. doi: 10.1111/j.1471-4159.2012.07839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cruz A, Green BG. Thermal stimulation of taste. Nature 403: 889–892, 2000. doi: 10.1038/35002581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.