Abstract
Introduction:
Sanfilippo syndrome is a rare disease and fatal genetic disorder with no FDA-approved treatment in the United States (US), and no comprehensive assessment of economic disease burden is available.
Objectives:
To develop a model to estimate the economic burden associated with Sanfilippo syndrome in the US using direct costs, indirect costs and valued intangibles (disability-adjusted life years, or DALYs) from 2023 onward.
Design and Setting:
A multistage comorbidity model was generated based on Sanfilippo syndrome symptoms, and disability weights from the 2010 Global Burden of Disease Study. Attributable increase in caregiver mental health burden were estimated using data from the CDC National Comorbidity Survey and retrospective studies on caregiver burden. Direct costs were approximated from the 2019 EveryLife Foundation survey, and indirect costs were estimated from Federal income data. Monetary valuations were adjusted to USD 2023 and given a 3% discount rate from 2023 onward.
Main Outcome Measures:
Incidence of Sanfilippo syndrome was calculated for each year, and year-over-year DALYs due to patient years lived with disability (YLDs) and years life lost (YLLs) were calculated by comparing to the health-adjusted life expectancy (HALE) in the US. Direct and indirect costs were calculated for each simulated patient from onset of symptoms to death.
Results:
From 2023–2043, overall economic burden in the US attributable to Sanfilippo syndrome was estimated to be $2.04 billion USD present value (2023) with current standard of care. The burden to individual families exceeded $8 million present value from time of birth per child born with Sanfilippo syndrome.
Conclusion:
Sanfilippo syndrome is a rare lysosomal storage disease, however the severe burden associated with the disease for individual families demonstrates a considerable cumulative impact. Our model represents the first disease burden value estimate associated with Sanfilippo syndrome, and underscores the substantial morbidity and mortality burden of Sanfilippo syndrome.
Keywords: MPS III, Sanfilippo syndrome, disease burden, economic burden
Introduction
Sanfilippo syndrome (MPS III) is a rare mucopolysaccharidosis within the lysosomal storage disease category, which is characterized by an inability to break down heparan sulfate, a glycosaminoglycan, which leads to accumulation, inflammation, oxidative stress and ultimately the debilitating manifestations of the disease.[1] MPS III is categorized into four subtypes (A, B, C and D) based on which enzyme is affected in the heparan sulfate breakdown pathway,[2] and hence each would require different biological products to replace the defective enzyme. Each subtype has been described with different levels of severity and life-expectancy,[3] yet clinically they remain virtually indistinguishable without molecular testing.[4] Like many rare diseases, there is currently no cure for the MPS III subtypes, however multiple clinical trials have been initiated to attempt enzyme replacement or reduction of heparan sulfate storage.[2, 5, 6] While there are clinical trials for MPS III subtypes A and B, many have failed or been discontinued.[7] Reasons for discontinuation often stem not only from lack of efficacy, but also from perceived small market size, difficulty in designing a trial with a small patient population, lack of clear clinical end-points and challenges demonstrating statistical power and efficacy sufficient for FDA approval in a short time frame.[8–11] Thus, the potential risks to the company performing the trial have historically been seen as incommensurate with the potential financial return, yet trial funding trends in the last few decades have shifted this narrative and demonstrated potential market viability.[12, 13]
Clinical trial costs have historically been a barrier to initiating or continuing clinical trials for rare childhood diseases, considering the risk of failure, and can make investment unappealing.[8–11] Government initiatives in the United States (US), such as the FDA Office of Office of Orphan Products Development, and the NIH Office of Rare Diseases have been formed out the recognition that collectively, these diseases carry great societal burden, and society benefits from public funding to stimulate research and development with the intention of creating marketable products eventually. Despite the perspective that researching orphan diseases is expensive and generally not viable in the market, we found few or no peer-reviewed investigations into what the actual cost of most rare diseases are, particularly MPS III.
Thus, we aimed to estimate the economic burden of MPS III including: (1) the patient intangible health losses expressed through monetary estimates of disability-adjusted life years (DALYs), (2) the caregiver intangible health losses (DALYs), (3) economic burden of disease when considering lost productivity of caregivers and (4) estimated direct costs, both medical and non-medical.
Methods
Model Overview and Assumptions
Projected burden of disease estimates were calculated using an incidence-based approach with a model accounting for comorbidity[14] on a multistage scheme for MPS III patients born from 1992–2053 in the US (Figure 1), presuming only excess comorbidities associated with MPS III (Supplementary Table 1). A total of 1,073 MPS III patients were simulated with a lifelong time horizon. In the same timeframe, 2,146 parents with a time horizon of 25-year after onset of child’s symptoms. Burden of disease considered direct costs, DALYs in the patient due to illness, DALYs in both parents due to mental health, and lost productivity due to caregiver burden. The value of labor for parents was set to $75,424 annually ($52,378 in wages, $23,046 in social fees, averaged between male and female) based on the US Census and Bureau of Labor Statistics 2019 and it was presumed that the equivalent of one full-time person worth of labor would be lost fulltime during the symptomatic phase of the disease until death of the patient. This value was chosen because MPS III patients are typically wheelchair bound in later stages of the disease and require more caregiving than many other lysosomal storage diseases.
Figure 1. Concept Diagram of Simulated Patient.
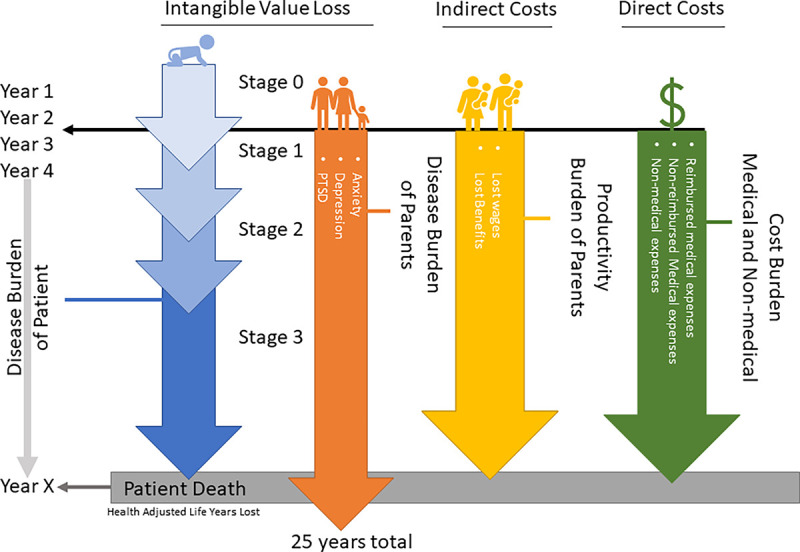
A conceptual example of a single MPS III patient is shown for the purposes of illustration. Patient burden was calculated by estimated direct costs and intangibles (DALYs) from onset of symptoms, which included comorbidity for each stage of the disease and expected age of death (Year X). The first 2.5 years of life are presumed normal, with no change in disease burden. Caregiver burden included indirect costs (productivity loss) and intangibles (DALYs) due to mental health complications. Economic burden of direct costs, indirect costs, and intangible value loss (DALYs) for the patient and parents were assigned to each respective year chronologically so discounting rates for the dollar amount could be applied appropriately.
We obtained the forecasted birth rate in the US, as calculated by the Institute for Health Metrics and Evaluation (IHME), through GHDx the dataset “Global Fertility, Mortality, Migration, and Population Forecasts 2017–2100.”[15] For years 1992–2022, CDC Vital Statistics was used since the live-birth rate is known for these years. Due to the unpredicted decline in US fertility rate 2020–2022 compared to the IHME models, this analysis makes the presumption that US fertility rates will normalize to IHME predictions by 2025. The natural sex ratio of male to female live births of 105 to 100 was used for male and female estimate stratifications (UN World Population Prospects 2022). A live-birth prevalence of MPS III in the US of 0.27 per 100,000 live births was used, which was derived from National MPS Society between 1995–2005.[16]
Disability Adjusted Life Year Modeling: Patients
Health-adjusted life expectancy (HALE) at birth for the US was presumed 65.2 for males and 67.0 for females based on the World Health Organization (WHO) World Health Statistics 2022,[17] and takes into account expected disability throughout life in the general population. Due to clinical similarity between subtypes,[18] our multi-stage model used the presumption that the average disease course will manifest similarly between subtypes in terms of stage onsets.[1, 4] Disability weight values were derived from the Global Burden of Disease Study 2010.[19] The presumed occurrence of each MPS III subtype was estimated based on the natural rate of each subtype observed in France, the United Kingdom, Greece, and Australia cumulatively: 64.2% MPS IIIA; 19.8% MPS IIIB; 9.9% MPS IIIC; and 6.2% MPS IIID (Figure 2a).[20, 21] This rate was chosen due to the availability of quality data in these studies, the fact they are developed nations and the demographic similarities between these nations and the United States.
Figure 2. Annual Undiscounted Economic Burden of MPS III in the US.
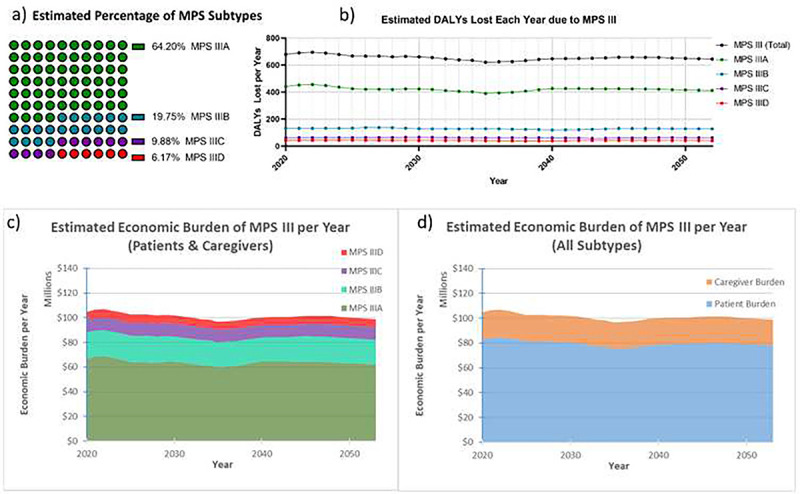
Total undiscounted DALYs within the US are shown above (a) from year 2020–2053, and are based on the IHME projections for the US birthrate, with adjustments from 2020–2025 to account for unpredicted decline in birthrate. These estimates are broken down by MPS III subtype, and presume subtype distribution close to natural rates previously observed in the literature (b). Year-over-year economic burden is shown (c and d) subcategorized by MPS III subtype (c) and patient (DALYs, direct costs) versus caregiver (DALYs, lost wages and social benefit loss) burden (d). The above figures presume a DALY value of $114,339 in the US, which is the status quo of US healthcare 1996–2016.
Multi-stage Comorbidity Disability Weight Modeling
The average non-adjusted life expectancy for each subtype of MPS III was used: MPS IIIA 15.22 years; MPS IIIB 18.91 years; MPS IIIC 23.43 years.[22] Average life expectancy of MPS IIID was not available, so the weighted average of every other subtype was used (16.86 years) -- this was close to the average lifespan of two reported MPS IIID cases (14 years).[21] The comorbidity calculations for MPS III emulated a three-stage natural history previously described.[1, 4, 22] For stage 1 (onset age 1–4), the presumed average age of onset was the midpoint of the range of onset (2.5 years old), similar with stage 2 (4.0 years old) and stage 3 (11.5 years old). The first 2.5 years of life (Stage 0) were considered healthy for this model,[23, 24] and the final years of life were presumed stage 3 until death. The cumulative disability weight of Stage 0 was 0; Stage 1 was 0.149;[1, 3, 4, 25–28] Stage 2 was 0.357;[1, 4, 25, 27–31] and Stage 3 was 0.68[1, 4, 26–36] (Supplementary Table 1).
Disability Adjusted Life Year Modeling: Caregivers
Caregiver DALYs were factored into caregiver economic burden (Supplementary Table 2). For major depressive disorder (MDD) an odds ratio (OR) of 2.90 for mothers and 2.42 for fathers was used.[37] Post-traumatic stress disorder (PTSD) prevalence in parents of MPS III children was presumed to be 26.9% in mothers, and 15.8% in fathers. These statistics reference a retrospective study of MPS III parents in the Netherlands,[37] which was the only available public study on the prevalence of depression, anxiety, and PTSD in caregivers of MPS III patients. We utilized a simple and widely used logistical regression model to obtain estimated risk ratio (eRR)[38] instead of using the OR, which can overestimate the risk,[39–41] and US baseline prevalence of these mental health disorders. The baseline expected US prevalence of clinical depression and general anxiety disorder were presumed to be 10.4% and 19.0% for mothers, and 5.5% and 11.9% in fathers.[42, 43] The baseline expected prevalence of PTSD was presumed to be 5.2% in mothers and 1.8% in fathers based on the National Comorbidity Survey.[44] Because there is very limited data on how long depression, anxiety and PTSD persist in parents of MPS III children, the presumed length of disease was approximated to 25 years after onset of symptoms, regardless of MPS III subtype, which is about 4–12 years after the death of the child. This is a conservative estimate considering the long-term health consequences from the death of a child in middle-age lasts on average 18.05 years after the death of the child.[45] In our model’s case, it also prevents a paradox of longer child lifespan leading to more simulated years lived with depression, anxiety and PTSD.
Simulation Process
For each year from 1992 onward, the US live-birth prevalence of MPS III was multiplied by the total number of live births for the respective year, creating a cohort. This cohort was tracked, and years lived in disability (YLD) were assigned to each respective year of their life, until their age of expiration, where years life lost (YLL) were assigned. For example, MPS IIIA 2020 cohort (presumed average birthdate is June) was tracked through each year, with YLD due to disease starting in 2023 (2.5y) with the disability weight of Stage 1, transitioning to Stage 2 halfway through 2024 (4y), and to Stage 3 in 2033 (11.5y), which continues until time of death in 2035 (15.22y), when YLL is applied to the year that cohort dies. A cohort of MPS IIIA born in 2021 follows this same pattern and the cumulative DALYs in each year are added together for each respective year and so on. A separate simulation can be applied to parents of these children to derive DALYs (YLD in parents due to anxiety, depression, and PTSD), and another to determine indirect cost in USD 2023 due to lost wages. DALYs were valued in USD 2023 according to the methods described, and a 3% discount rate was applied.
The cumulative burden, valued in USD 2023, of one single case of each MPS III subtype was simulated separately to answer the question of how much burden is placed on a single family. The methods were the same, and the discount rate begins at the point of birth for each of these. Intangible burden (DALYs), out-of-pocket expenses (both indirect costs and medical expenses), and reimbursed medical expenses (medical costs accrued, but paid by insurance) were stratified separately for illustration.
Disease Burden Estimates
A monetary value per each DALY of $114,339 was used, based on a comprehensive study investigating cost-effectiveness across all US healthcare services from 1996–2016.[46] We used this as our primary analysis since it represents status quo for US healthcare services. A 3% annual discount rate was used accruing from 2023 onward[47]. We also conducted a sensitivity analysis with similar parameters using upper limit of $150,000 and a lower limit of $69,499, since this is reported as an international cost-effectiveness threshold for very-high income countries.[48] Direct costs were taken from the EveryLife Foundation 2019 survey of rare diseases “Lysosomal Storage Disease” ICD-10 category for medical expenses, non-medical expenses and out-of-pocket medical expenses.[49] This presumed that MPS III cost burden will approximate to the average for all lysosomal storage diseases. For all dollar values, a one-time 17% inflation factor was applied to convert USD 2019 to USD 2023 (Bureau of Labor Statistics, 2023).
Results
DALYs per MPS III Birth with Current Standard of Care
The multistage model used for this analysis demonstrated an average DALY loss of 55.80, 54.46, 53.10 and 55.08, for a male child born in the US with MPS IIIA, MPS IIIB, MPS IIIC and MPS IIID, respectively. The DALY loss for a female MPS III child born in the US was 58.18, 57.06, 55.70 and 57.68, respectively (Supplementary Table 3). The YLL in all subtypes was 4–9 times greater than YLD. Caretaker disease burden (YLD) for each child born with MPS III, regardless of child sex at birth, was 2.08 DALYs for the child’s father and 4.40 DALYs for the child’s mother (Supplementary Table 3).
Estimated Economic Burden of MPS III in the United States
After applying the IHME projections for US birthrate and presuming 0.27 MPS III cases per 100,000 live-birth in the US, a year-over-year estimate of the total number of undiscounted DALYs was generated using a dynamic cohort starting at those born in 1992 and ending with those born in 2053. Based on the US birthrate estimates and incidence of MPS III, there are an estimated average of 11 MPS III births each year, and due to the presumed declining US birth rate, this eventually reaches 10 annual MPS III births by 2053. Based on the weighted average lifespan of MPS III patients, it was also derived that the estimated total US population of MPS III patients may be at a steady state of around 185–195 patients from 2020–2053, with a slow decline that correlates with a declining US birthrate. This timeframe was the focus of the analysis (Figure 2b). The total estimated undiscounted societal economic burden on an annual basis were also extrapolated and visualized by subtype (Figure 2c) and patient economic burden compared to caregiver economic burden (Figure 2d).
Burden of MPS III Cases 2023–2043
The analysis performed for all MPS subtypes demonstrated a cumulative discounted societal burden of $2.05 billion between 2023–2043 alone, with an upper limit of $2.47 billion and a lower limit of $1.27 billion. In total, 80.5% of this amount was from MPS III patient burden, and 19.5% representing caregiver burden. By subtype, burden composition was similar between subtypes: MPS IIIA 15.94% caregiver burden (Figure 3a); MPS IIIB 17.54% caregiver burden (Figure 3b); MPS IIIC 19.55% caregiver burden (Figure 3c); MPS IIID 16.49% caregiver burden (Figure 3d). Total burden from 2023–2043 by subtype was $1.25 billion for MPS IIIA, $430 million for MPS IIIB, $220 million for MPS IIIC and $131 million for MPS IIID. The total discounted economic burden caused for each individual birth, is $8.11 million for MPS IIIA; $8.19 million for MPS IIIB; $8.00 million for MPS IIIC; and $8.29 million for MPS IIID (Figure 3f). This was calculated by simulating a single hypothetical patient, discounting from year of birth, and taking into account caregiver burden. Economic burden composition was similar regardless of year, as this analysis presumes no improvement in standard of care for MPS III.
Figure 3. US Societal Burden of MPS III from 2023–2043.
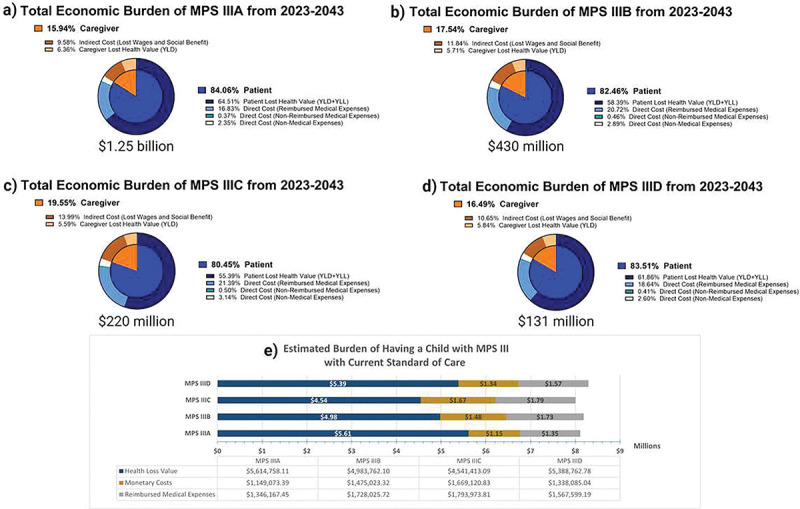
The above graphs visualize the presumed disparities between MPS III subtypes regarding discounted burden components (a-d). Overall, all subtypes had between 15.94–19.55% of their burden composition associated caregivers. When parsing out differences between intangible value lost, reimbursed medical expenses and monetary costs for parents (d), all subtypes cost families between $1.15m-$1.67m in monetary costs (lost productivity, non-reimbursed medical costs, and non-medical costs), and displace between $4.54m-$5.61m value in health loss (both patient and parents). An estimated $1.35m-$1.79m in reimbursed medical expenses were also accrued.
For reference, the dynamic present value of damages each subtype of MPS III up until a given year between 2023–2053 is plotted for MPS IIIA (Figure 4a), MPS IIIB (Figure 4b), MPS IIIC (Figure 4c), MPS IIID (Figure 4d). Lower-limit and upper-limit DALY valuations are provided, with the US average DALY cost shown in middle. Cumulative burden for all subtypes from 2023 to a given year until 2053 are graphed in Figure 4e with an increased y-axis range. This analysis used burden of disease from 2023–2043 as shown. The total breakdown of 2023–2043 burden composition by direct cost, indirect cost and intangible burden is shown (Figure 4f), with 68.2% of burden representing intangible value loss ($1.39 billion). Out of this intangible category, 80.7% was from YLL in the patients, 14.6% was YLD in the patients, and the remaining 4.7% was YLD in the caregivers. Approximately 21.2% of MPS III burden 2023–2043 ($432 million) were due to direct costs, with 86.0% of direct costs being reimbursed medical expenses, 12.1% non-medical expenses and 1.9% out-of-pocket medical expenses. Indirect costs, which were the value of labor lost in caregivers, represented 10.6% ($216 million) of MPS III burden 2023–2043.
Figure 4. Cumulative Societal Burden of MPS III 2023–2043.
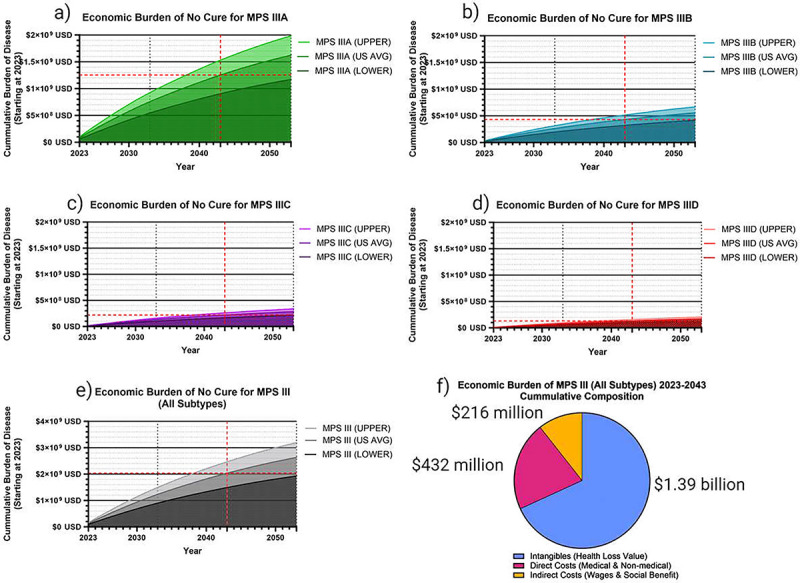
The following figures demonstrate the cumulative societal burden of not finding a cure for MPS III by subtype (a-d), starting in 2023 as a zero point, and cumulatively increasing from there. The total cumulative burden for all subtype is also shown (c). The average US healthcare value per DALY of $114,339 was used as the primary analysis, with an upper limit of $150,000 (ICER) and a lower limit of $69,499 was used. An inflation factor of 17% was used to convert to USD 2023 (a-e), and a standard 3% discount rate was used. The cumulative burden composition in this analysis for all MPS III subtypes was 21.2% direct costs, 10.6% indirect costs, and 68.2% intangible value loss (f).
Discussion
In this paper, publicly available data was analyzed, and our results suggest that MPS III will cause an estimated burden of $2.04 billion between 2023 and 2043 in the US including direct medical/non-medical costs, children’s health lost, caretaker mental health, and lost wages. Each family who bears a child afflicted with MPS III can expect to lose on average a present value between $0.89-$1.32 million in lost wages alone, $34.8-$47.6 thousand in medical out-of-pocket expenses, and $219-$304 thousand in non-medical costs. The family will also lose between $4.54 and $5.61 million present value in collective DALYs – for both patient and caregiver. This analysis also suggests that the US MPS III patient population may currently be at a steady-state of around 185 patients, which represents 0.97–1.54% of the entire estimated MPS III patient population worldwide,[50] a higher share than we were expecting given this disease is usually thought of as heavily skewed to specific countries with high carrier prevalence.[51] One important finding to our analysis is that the caregiver economic burden was relatively small in comparison to the burden to the child (~15–20% of total).
While this analysis focused on MPS III, a rare lysosomal storage disease in the US (0.27 per 100,000 live births), it is important to consider the litany of neglected rare diseases have been estimated to cost our society $966 billion per year according to the Government Accountability Office (GAO).[52] While no one can predict how soon an effective treatment can be developed before a randomized-control trial is done, understanding the value of the disease can help inform policy and funding decisions. For MPS III, the cumulative disease burden without considering direct cost is estimated to be around $100 million (USD 2023) per year for the next few decades based on our analysis. Investing in a cure would potentially be highly valuable considering the burden associated with MPS III, and the current lack of treatment or palliation. The severe burden to each family with an MPS III child is quite substantial to consider, as diseases such as diabetes have a total yearly cost of around $11,700-$13,241 when considering both direct and indirect costs,[53] despite getting substantially more research funding than MPS III. Currently in the US, there is not even newborn screening for MPS III, which by itself may be cost-effective in driving new research forward and prevent the stress of delayed diagnosis.[54–57] One of the biggest hurdles to MPS III clinical trials is simply acquiring enough patients for enrollment, and newborn screening would make this process an order of magnitude simpler.
While our analysis provides a novel perspective on the societal burden of MPS III, there are obvious limitations that should be considered. Most importantly, future improvements in general healthcare delivery cannot be easily predicted, and therefore this analysis had to presume no improvement in the standard of care in MPS III. This is likely to overestimate the intangible disease burden. For example, historically the increase in MPS III life expectancy has slightly improved, which is mostly attributable to improvement in respiratory care for pneumonia.[22] These analyses are also based on previously reported disease birth-prevalence,[16] and these may be underestimated due to missed or undiagnosed patients and cases of in utero demise. The timeline of parent anxiety, depression and PTSD is not well characterized longitudinally,[37] so our presumption of 25 years after disease onset may not reflect the true length of mental health disorders in MPS III caregivers.[45] We also presume that willingness-to-pay for one DALY averted is equivalent to the US healthcare system status quo,[46] as no contingent valuation surveys for MPS III families have been conducted, which may overestimate value of intangible value loss. This report also presumes a consistent disease birth-prevalence and a predictable birth-rate within the US. Direct cost estimates used the general ICD-10 category of “lysosomal storage disease” previously published,[49] which may not accurately reflect the direct costs for MPS III, specifically. We also estimated disability weight of MPS III by multiplicative comorbidity method, which has the possibility of overestimating YLD due to disease, however it should be noted that YLD only accounted for approximately 9% of DALYs in MPS III children. The remaining DALYs in MPS III children were due to YLL. Our analysis also did not consider future immigration/emigration from the US, which has the potential to profoundly skew the prevalence of child-bearing heterozygous disease carriers if involving high prevalence regions, such as Europe and the Middle East. The estimated indirect cost associated with caring for a child with MPS III was presumed to be the equivalent of one full-time worker because the disease is uniquely disabling, with the end-stage of the disease involving full dependency on basic activities of daily living. This relies on clinical experience with parents who have a child with MPS III, and may overestimate labor loss. We also took a conservative stance in our analysis about the value of lost productivity from the MPS III patients directly, so it is important to note that a functional cure would have implications for marginally increasing the workforce as well.
Regarding our analysis, we emphasize that the combined economic burden of over $8 million per case of MPS III born is a representation of the total potential present value of curative treatment from the point of childbirth, not a price target. While complete resolution of the disease is typically the lofty goal of any investigational treatment, future treatments of MPS III will likely recover a fraction of this burden. In the case of MPS III, if treatment plans could recover just 25% of morbidity and mortality associated with the burden of disease for MPS III, it would potentially be collectively valued at tens of millions of dollars per year based on our estimates.
Conclusions
This estimate is currently, to our knowledge, the only estimate of disease burden associated with Sanfilippo syndrome in the United States, in DALYs as well as valuation of disease burden. We also illustrate novel methods for contextualizing rare disease burden in the US. Policy makers and advocates need to consider the disease burden associated with rare diseases in the context of cumulative damages caused rather than the disease prevalence in isolation, as the latter can mask the true theoretical value of funding research in these diseases.
Supplementary Material
Strengths.
This study represents, to our knowledge, the first peer-reviewed estimate of the societal burden caused by Sanfilippo syndrome using publicly available datasets.
Methods such as these can help researchers and policy makers provide valuations for rare diseases more in sync with the detriment they cause.
Limitations.
Intangible valuation in this analysis presumes willingness-to-pay for one DALY averted is on parity with the cost of the US healthcare system to avert one DALY.
DALY quantification relied on comorbidity of disease symptoms, which may differ from the true disability burden of the disease.
Direct costs rely on previous work that generalizes all lysosomal storage disorders, and will not take into account nuances of MPS III.
As with any estimate, these rely on many presumptions which may or may not apply to the current US system or in the future.
Acknowledgements
We thank the National MPS Society for feedback and support for the project.
Funding
This work was funded by NIH NINDS R01NS102624
Units of Measurement
- USD 2023
Represents United States dollar accounting for 2023 value
- DALYs
Disability adjusted life years, typically expressed as those lost due to disease
- HALE
Health adjusted life expectancy, life expectancy adjusted for health during life
- YLL
Years of life lost, the health adjusted life years lost due to mortality
- YLD
Years lived with disability, the health adjusted life years lost due to morbidity
Abbreviations and Symbols
- ADLs
Activities of daily living
- BLS
Bureau of Labor Statistics
- CDC
Center for Disease Control and Prevention (United States)
- DALY
Disability adjusted life year
- eRR
Estimated risk ratio or estimated relative risk
- FDA
Food and Drug Administration (United States)
- GAO
Government Accountability Office
- GHDx
Global health data exchange
- HALE
Health adjusted life expectancy
- ICD-10
International Statistical Classification of Diseases and Related Health Problems 10th Revision
- ICER
Institute for Clinical and Economic Review
- IHME
Institute for Health Metrics and Evaluation
- MDD
Major depressive disorder
- MPS
Mucopolysaccharidosis
- OR
Odds ratio
- PTSD
Post-traumatic stress disorder
- RR
Risk ratio or relative risk
- UN
United Nations
- US
United States
- USD
United States Dollar
- YLL
Years of life lost
- YLD
Years lived with disability
- WHO
World Health Organization
- $
US dollars
Footnotes
Competing Interests
Dr Coy D Heldermon
Stock in Lacerta, a gene therapy company.
Patent pending for an AAV gene therapy vector for Sanfilippo Type B.
Ethics Approval and Consent to Participate
Not applicable
Consent for Publication
Not applicable
Birth Rate:
CDC NVSS for past years.
https://www.cdc.gov/nchs/nvss/births.htm
IHME 2017–2100 projection for future years.
https://ghdx.healthdata.org/record/ihme-data/global-population-forecasts-2017-2100
Live-birth prevalence of MPS III[16]
MPS subtype composition estimation[20, 21]
Average wage value for a parent (2019):
https://www.census.gov/library/publications/2020/demo/p60-270.html
Composition of social fees (benefits; 2019):
https://www.bls.gov/news.release/pdf/ecec.pdf
Disability Weights[19]
Availability of Data and Material
All data utilized in the manuscript is publicly available as cited. The simulation was performed by Microsoft Excel, and the original work is available from the corresponding author upon reasonable request.
References
- 1.Valstar MJ, Ruijter GJ, van Diggelen OP, Poorthuis BJ, Wijburg FA: Sanfilippo syndrome: a mini-review. J Inherit Metab Dis 2008, 31(2):240–252. [DOI] [PubMed] [Google Scholar]
- 2.Pearse Y, Iacovino M: A Cure for Sanfilippo Syndrome? A Summary of Current Therapeutic Approaches and their Promise. Med Res Arch 2020, 8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Kamp JJ, Niermeijer MF, von Figura K, Giesberts MA: Genetic heterogeneity and clinical variability in the Sanfilippo syndrome (types A, B, and C). Clin Genet 1981, 20(2):152–160. [DOI] [PubMed] [Google Scholar]
- 4.Muschol N, Giugliani R, Jones SA, Muenzer J, Smith NJC, Whitley CB, Donnell M, Drake E, Elvidge K, Melton L et al. : Sanfilippo syndrome: consensus guidelines for clinical care. Orphanet J Rare Dis 2022, 17(1):391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seker Yilmaz B, Davison J, Jones SA, Baruteau J: Novel therapies for mucopolysaccharidosis type III. J Inherit Metab Dis 2021, 44(1):129–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashby F, Heldermon CD: The significance of triple-capsid-mutant AAV8 for treatment of Sanfilippo Syndrome Type B. Archives of Stem Cell and Therapy 2022, 3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh A, Shapiro E, Rust S, Delaney K, Parker S, Shaywitz AJ, Morte A, Bubb G, Cleary M, Bo T et al. : Recommendations on clinical trial design for treatment of Mucopolysaccharidosis Type III. Orphanet J Rare Dis 2017, 12(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rees CA, Pica N, Monuteaux MC, Bourgeois FT: Noncompletion and nonpublication of trials studying rare diseases: A cross-sectional analysis. PLoS Med 2019, 16(11):e1002966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell SA, Tudur Smith C: A comparison of interventional clinical trials in rare versus non-rare diseases: an analysis of ClinicalTrials.gov. Orphanet J Rare Dis 2014, 9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulberg AE, Bucci-Rechtweg C, Giuliano J, Jacoby D, Johnson FK, Liu Q, Marsden D, McGoohan S, Nelson R, Patel N et al. : Regulatory strategies for rare diseases under current global regulatory statutes: a discussion with stakeholders. Orphanet J Rare Dis 2019, 14(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellerio JE: The challenges of clinical trials in rare diseases. Br J Dermatol 2022, 187(4):453–454. [DOI] [PubMed] [Google Scholar]
- 12.Yates N, Hinkel J: The economics of moonshots: Value in rare disease drug development. Clin Transl Sci 2022, 15(4):809–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes DA, Poletti-Hughes J: Profitability and Market Value of Orphan Drug Companies: A Retrospective, Propensity-Matched Case-Control Study. PLoS One 2016, 11(10):e0164681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilderink HB, Plasmans MH, Snijders BE, Boshuizen HC, Poos MJ, van Gool CH: Accounting for multimorbidity can affect the estimation of the Burden of Disease: a comparison of approaches. Arch Public Health 2016, 74:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vollset SE, Goren E, Yuan CW, Cao J, Smith AE, Hsiao T, Bisignano C, Azhar GS, Castro E, Chalek J et al. : Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet 2020, 396(10258):1285–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puckett Y, Mallorga-Hernandez A, Montano AM: Epidemiology of mucopolysaccharidoses (MPS) in United States: challenges and opportunities. Orphanet J Rare Dis 2021, 16(1):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World health statistics 2022: monitoring health for the SDGs, sustainable development goals. In.: World Health Organization; 2022: 125. [Google Scholar]
- 18.Cleary MA, Wraith JE: Management of mucopolysaccharidosis type III. Arch Dis Child 1993, 69(3):403–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, Begum N, Shah R, Karyana M, Kosen S et al. : Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet 2012, 380(9859):2129–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meikle PJ, Hopwood JJ, Clague AE, Carey WF: Prevalence of lysosomal storage disorders. Jama 1999, 281(3):249–254. [DOI] [PubMed] [Google Scholar]
- 21.Arfi A, Richard M, Gandolphe C, Bonnefont-Rousselot D, Therond P, Scherman D: Neuroinflammatory and oxidative stress phenomena in MPS IIIA mouse model: the positive effect of long-term aspirin treatment. Mol Genet Metab 2011, 103(1):18–25. [DOI] [PubMed] [Google Scholar]
- 22.Lavery C, Hendriksz CJ, Jones SA: Mortality in patients with Sanfilippo syndrome. Orphanet J Rare Dis 2017, 12(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuiper GA, Meijer OLM, Langereis EJ, Wijburg FA: Failure to shorten the diagnostic delay in two ultra-orphan diseases (mucopolysaccharidosis types I and III): potential causes and implications. Orphanet J Rare Dis 2018, 13(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wijburg FA, Wegrzyn G, Burton BK, Tylki-Szymanska A: Mucopolysaccharidosis type III (Sanfilippo syndrome) and misdiagnosis of idiopathic developmental delay, attention deficit/hyperactivity disorder or autism spectrum disorder. Acta Paediatr 2013, 102(5):462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruijter GJ, Valstar MJ, van de Kamp JM, van der Helm RM, Durand S, van Diggelen OP, Wevers RA, Poorthuis BJ, Pshezhetsky AV, Wijburg FA: Clinical and genetic spectrum of Sanfilippo type C (MPS IIIC) disease in The Netherlands. Mol Genet Metab 2008, 93(2):104–111. [DOI] [PubMed] [Google Scholar]
- 26.Valstar MJ, Marchal JP, Grootenhuis M, Colland V, Wijburg FA: Cognitive development in patients with Mucopolysaccharidosis type III (Sanfilippo syndrome). Orphanet J Rare Dis 2011, 6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bianchi PM, Gaini R, Vitale S: ENT and mucopolysaccharidoses. Italian Journal of Pediatrics 2018, 44:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mesolella M, Cimmino M, Cantone E, Marino A, Cozzolino M, Della Casa R, Parenti G, Iengo M: Management of otolaryngological manifestations in mucopolysaccharidoses: our experience. Acta Otorhinolaryngol Ital 2013, 33(4):267–272. [PMC free article] [PubMed] [Google Scholar]
- 29.Escolar ML, Jones SA, Shapiro EG, Horovitz DDG, Lampe C, Amartino H: Practical management of behavioral problems in mucopolysaccharidoses disorders. Mol Genet Metab 2017, 122S:35–40. [DOI] [PubMed] [Google Scholar]
- 30.Bax MC, Colville GA: Behaviour in mucopolysaccharide disorders. Arch Dis Child 1995, 73(1):77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraser J, Wraith JE, Delatycki MB: Sleep disturbance in mucopolysaccharidosis type III (Sanfilippo syndrome): a survey of managing clinicians. Clin Genet 2002, 62(5):418–421. [DOI] [PubMed] [Google Scholar]
- 32.Heron B, Mikaeloff Y, Froissart R, Caridade G, Maire I, Caillaud C, Levade T, Chabrol B, Feillet F, Ogier H et al. : Incidence and natural history of mucopolysaccharidosis type III in France and comparison with United Kingdom and Greece. Am J Med Genet A 2011, 155A(1):58–68. [DOI] [PubMed] [Google Scholar]
- 33.Valstar MJ, Neijs S, Bruggenwirth HT, Olmer R, Ruijter GJ, Wevers RA, van Diggelen OP, Poorthuis BJ, Halley DJ, Wijburg FA: Mucopolysaccharidosis type IIIA: clinical spectrum and genotype-phenotype correlations. Ann Neurol 2010, 68(6):876–887. [DOI] [PubMed] [Google Scholar]
- 34.Valstar MJ, Bruggenwirth HT, Olmer R, Wevers RA, Verheijen FW, Poorthuis BJ, Halley DJ, Wijburg FA: Mucopolysaccharidosis type IIIB may predominantly present with an attenuated clinical phenotype. J Inherit Metab Dis 2010, 33(6):759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilkes JA, Heldermon CD: Mucopolysaccharidosis III (Sanfilippo Syndrome)- disease presentation and experimental therapies. Pediatr Endocrinol Rev 2014, 12 Suppl 1:133–140. [PubMed] [Google Scholar]
- 36.Scarpa M, Lourenco CM, Amartino H: Epilepsy in mucopolysaccharidosis disorders. Mol Genet Metab 2017, 122S:55–61. [DOI] [PubMed] [Google Scholar]
- 37.Conijn T, Nijmeijer SCM, van Oers HA, Wijburg FA, Haverman L: Psychosocial Functioning in Parents of MPS III Patients. JIMD Rep 2019, 44:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Yu KF: What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998, 280(19):1690–1691. [DOI] [PubMed] [Google Scholar]
- 39.Knol MJ, Le Cessie S, Algra A, Vandenbroucke JP, Groenwold RH: Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. CMAJ 2012, 184(8):895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies HT, Crombie IK, Tavakoli M: When can odds ratios mislead? BMJ 1998, 316(7136):989–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mantel N, Haenszel W: Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959, 22(4):719–748. [PubMed] [Google Scholar]
- 42.Brody DJ, Pratt LA, Hughes JP: Prevalence of Depression Among Adults Aged 20 and Over: United States, 2013–2016. NCHS Data Brief 2018(303):1–8. [PubMed] [Google Scholar]
- 43.Terlizzi EP, Villarroel MA: Symptoms of Generalized Anxiety Disorder Among Adults: United States, 2019. NCHS Data Brief 2020(378):1–8. [PubMed] [Google Scholar]
- 44.Kessler RC, Berglund P, Chiu WT, Demler O, Heeringa S, Hiripi E, Jin R, Pennell BE, Walters EE, Zaslavsky A et al. : The US National Comorbidity Survey Replication (NCS-R): design and field procedures. Int J Methods Psychiatr Res 2004, 13(2):69–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers CH, Floyd FJ, Seltzer MM, Greenberg J, Hong J: Long-term effects of the death of a child on parents’ adjustment in midlife. J Fam Psychol 2008, 22(2):203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weaver MR, Joffe J, Ciarametaro M, Dubois RW, Dunn A, Singh A, Sparks GW, Stafford L, Murray CJL, Dieleman JL: Health Care Spending Effectiveness: Estimates Suggest That Spending Improved US Health From 1996 To 2016. Health Aff (Millwood) 2022, 41(7):994–1004. [DOI] [PubMed] [Google Scholar]
- 47.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA et al. : Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016, 316(10):1093–1103. [DOI] [PubMed] [Google Scholar]
- 48.Daroudi R, Akbari Sari A, Nahvijou A, Faramarzi A: Cost per DALY averted in low, middle- and high-income countries: evidence from the global burden of disease study to estimate the cost-effectiveness thresholds. Cost Eff Resour Alloc 2021, 19(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang G, Cintina I, Pariser A, Oehrlein E, Sullivan J, Kennedy A: The national economic burden of rare disease in the United States in 2019. Orphanet J Rare Dis 2022, 17(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong W, Wu S, Zhang J, Lu C, Ding Y, Meng Y: Global epidemiology of mucopolysaccharidosis type III (Sanfilippo syndrome): an updated systematic review and meta-analysis. J Pediatr Endocrinol Metab 2021, 34(10):1225–1235. [DOI] [PubMed] [Google Scholar]
- 51.Zelei T, Csetneki K, Voko Z, Siffel C: Epidemiology of Sanfilippo syndrome: results of a systematic literature review. Orphanet J Rare Dis 2018, 13(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin R: The Cost of Rare Diseases. JAMA 2021, 326(20):2000. [DOI] [PubMed] [Google Scholar]
- 53.American Diabetes A Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care 2018, 41(5):917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bodamer OA, Giugliani R, Wood T: The laboratory diagnosis of mucopolysaccharidosis III (Sanfilippo syndrome): A changing landscape. Mol Genet Metab 2014, 113(1–2):34–41. [DOI] [PubMed] [Google Scholar]
- 55.NORD: Barriers to Rare Disease Diagnosis, Care and Treatment in the US: A 30-Year Comparative Analysis. 2020.
- 56.Pollard S, Weymann D, Dunne J, Mayanloo F, Buckell J, Buchanan J, Wordsworth S, Friedman JM, Stockler-Ipsiroglu S, Dragojlovic N et al. : Toward the diagnosis of rare childhood genetic diseases: what do parents value most? Eur J Hum Genet 2021, 29(10):1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rehm HL: Time to make rare disease diagnosis accessible to all. Nat Med 2022, 28(2):241–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data utilized in the manuscript is publicly available as cited. The simulation was performed by Microsoft Excel, and the original work is available from the corresponding author upon reasonable request.


