Abstract
Background
The impacts of socioeconomic status (SES) on COVID-19-related changes in cancer prevention behavior have not been thoroughly investigated. We conducted a cohort study to examine the effects of SES on changes in cancer prevention behaviors during the COVID-19 pandemic.
Methods
We invited adult participants from previous studies conducted at Ohio State University to participate in a study assessing the impact of COVID-19 on various behaviors. Post-COVID-19 cancer prevention behaviors, including physical activity, daily intake of fruits and vegetables, alcohol and tobacco consumption, and qualitative changes in post-COVID-19 behaviors relative to pre-COVID levels, were used to construct a prevention behavior change index that captures the adherence status and COVID-related changes in each behavior, with higher index scores indicating desirable changes in prevention behaviors. Participants were classified into low, middle, or high SES based on household income, education, and employment status. Adjusted regression models were used to examine the effects of SES on changes in cancer prevention behaviors during the COVID-19 pandemic.
Results
The study included 6,136 eligible participants. The average age was 57 years, 67% were women, 89% were non-Hispanic Whites, and 33% lived in non-metro counties. Relative to participants with high SES, those with low SES had a 24% [adjusted relative ratio, aRR = 0.76 (95%CI 0.72–0.80)], 11% [aRR = 0.89 (95%CI 0.86–0.92)], and 5% [aRR = 0.95 (95%CI 0.93–0.96)], lower desirable changes in prevention behaviors for physical activity, fruit and vegetable intake, and tobacco use, respectively. Low SES had a higher desirable change in alcohol consumption prevention behaviors, 16% [aRR = 1.16 (95%CI 1.13–1.19)] relative to high SES. The adjusted odds of an overall poor change in prevention behavior were adjusted odds ratio (aOR) 1.55 (95%CI 1.27 to 1.89) and aOR 1.40 (95%CI 1.19 to 1.66), respectively, higher for those with low and middle SES relative to those with high SES.
Conclusion
The adverse impacts of COVID-19 on cancer prevention behaviors were seen most in those with lower SES. Public health efforts are currently needed to promote cancer prevention behaviors, especially amongst lower SES adults.
1. Introduction
The COVID-19 pandemic caused dramatic changes in activities of daily living. Since its onset, measures to control the pandemic have included quarantine orders, stay-at-home mandates, social distancing policies, and closures of schools and non-essential businesses [1]. Attributed to these changes, studies report shifts in individuals’ adherence to cancer prevention behaviors such as eating nutritious diets, consuming alcohol, and engaging in physical activity [2–5]. Yet, the pandemic [6] and its mitigation measures have inequitably harmed disadvantaged socioeconomic groups [7–9], with much less known about the differential impact of the pandemic on maintaining adherence to cancer prevention behaviors across the socioeconomic gradient.
Modifiable risk factors play a critical role in the development of cancer. Evidence indicates that cancer morbidity and mortality can be reduced by engaging in physical activity, consuming nutritious foods, and avoiding tobacco use and alcohol intake [10–15]. Accordingly, several organizations proposed recommendations to promote behaviors that, separately and in combination [16–18], could prevent new cancer diagnoses and deaths [19, 20]. These behaviors include quitting or never smoking, consuming alcohol in moderation (one or fewer drinks daily for women and two or fewer drinks daily for men), eating at least five non-starchy fruits and vegetables daily, and maintaining a weekly physical activity of at least 75 vigorous-intensity or 150 moderate-intensity minutes [10–15, 21, 22].
Adherence to cancer prevention behaviors is conditioned by multilevel factors that span the individual, social, economic, and physical environments [23]. Evidence indicates that socioeconomic status is inversely associated with adherence to cancer prevention behaviors. As such, individuals with a lower socioeconomic status show a higher prevalence of poor diet, physical inactivity, and tobacco use [24–26]. Alcohol consumption is higher among men of lower socioeconomic status [26, 27]. These differences reflect the fact that people with fewer resources experience more financial, structural, and personal obstacles to practicing cancer prevention behaviors [28].
As a result of COVID-19, multilevel factors known to influence the adherence to cancer prevention behaviors [29] have been altered [30–34] and could exacerbate existing disparities in the adherence to recommended cancer prevention behaviors. Our objective was to examine the impact of the COVID-19 pandemic on cancer prevention behaviors and the differential effects of socioeconomic status on changes in cancer prevention behavior throughout the pandemic. Understanding how the COVID-19 pandemic affected cancer prevention behaviors among people across the socioeconomic gradient is vital to inform cancer control strategies. Changes in cancer prevention behaviors at the population level can have long-term consequences in reducing cancer morbidity and mortality.
2. Materials and methods
This study was part of an NCI-funded initiative conducted in conjunction with 16 other NCI-designated Cancer Centers—the IC-4 (Impact of COVID-19 on the Cancer Continuum Consortium) (S1 Methods). The Institutional Review Board of The Ohio State University (OSU) approved this study in June 2020. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
2.1. Theoretical framework
This study was based on the Health Belief Model (HBM) [35, 36]. According to the HBM, the change in health behaviors depends on a series of health beliefs, including 1) perceived susceptibility to exposure to COVID-19, 2) perceived severity of the consequences of contracting COVID-19 (e.g. hospitalization or death), 3) perceived benefits of the effectiveness of the proposed COVID-19 prevention measures, 4) perceived barriers to executing the proposed prevention measures, 5) cues to the proposed prevention actions, and 6) self-efficacy in the person’s ability to successfully perform COVID-19 prevention measures.
2.2. Setting
Participants from Ohio and Indiana who agreed to be re-contacted from previous studies conducted at the OSU Comprehensive Cancer Center (OSUCCC) were asked to participate in this study (S1 Methods). Ohio and Indiana mandated statewide stay-at-home orders on March 23 and March 24, 2020, respectively. Both states initiated a gradual reopening on 1 May 2020.
2.3. Sample selection
Eligible participants were adults 18 years or older who consented to participate in the study. To ensure the inclusion of the most vulnerable, underserved, and minority populations, we sought to recruit healthy adult volunteers, cancer patients, cancer survivors, and survivors’ caregivers in our catchment area in Ohio and Indiana. This was achieved by employing two recruitment strategies. First, we identified and reached out to individuals who had previously participated in OSU studies and consented to be contacted for future research projects. In addition, we invited all identified cancer patients and survivors to nominate their primary caregivers to participate in the study. Second, we utilized our community partners and the OSUCCC Pelotonia listservs to recruit participants to further enhance the representativeness of our study sample and ensure the inclusion of minority and underserved communities.
2.4. Data collection
We used several data collection methods, including web, telephone, and mailed surveys. Respondents with valid emails received an initial survey invitation email and three reminders seven days apart. All participants were initially selected using an eligibility form to confirm their current Ohio or Indiana residence before conducting the survey. Participants were able to save the web survey and resume it at a later time. Those who partially completed the web survey received an email reminder one week after they had last accessed the survey. A trained interviewer contacted participants without an email address and those with invalid emails on file by phone. Participants who were initially reached by phone were offered the option to complete the survey over the phone or online. For those who requested a mailed survey, we sent a cover letter and a paper survey with a self-addressed stamped return envelope. For non-English-speaking participants, a bilingual staff member administered the survey in the appropriate language. Participants were offered a $10 gift card upon completion of the survey. Data were collected and managed using the Research Electronic Data Capture (REDCap) secure web-based application hosted at OSU. Data were collected from June 19, 2020, through November 30, 2020.
2.5. Survey development
The survey elements (S1 Table) were finalized in conjunction with other members of the IC-4 [37]. The survey included individual behaviors related to mitigation of COVID-19 transmission, challenges related to social distancing, self/family isolation, stress, and health behaviors highly relevant to cancer and other chronic diseases. Questions also assessed perceived stigma associated with COVID-19 with respect to different population groups and covariates, such as health literacy and mental health, suspected of moderating these influences.
2.6. Study measures
2.6.1. Exposure
The primary exposure was individual-level SES. Respondents were asked to report their highest grade or level of school completed, their combined annual household income, and their current employment status (S2 Table). We used the responses to these three questions to develop an aggregate SES score. The participants’ responses to questions about total household income and highest attained education level were scored between 0 and 3 and between 0 and 2 for the self-reported employment status. The aggregate SES score was then used to classify participants into one of three groups: low SES [lower quartile (SES scores 0 to 4)], middle SES (SES scores 5 and 6), or high SES [upper quartile (SES scores 7 and 8)].
2.6.2. Outcomes
Our primary outcome was the post-COVID-19 change in cancer prevention behaviors relative to pre-COVID-19 levels. Participants were asked to report current (i.e., post-COVID-19 pandemic) cancer prevention behaviors, including the number of days per week of physical activity of at least moderate intensity, the total daily frequency of fruit and vegetable intake, alcohol consumption, the number of binge drinking days of five or more alcoholic beverages on the same occasion, and tobacco use. We classified participants as adherent and non-adherent on each post-COVID-19 cancer prevention behavior using established cancer prevention recommendations [19, 20]. Additionally, participants described qualitative changes in each of the self-reported post-COVID-19 cancer behaviors relative to the pre-COVID-19 levels (i.e., same, more, or less]. We combined participants’ adherence statuses on each prevention behavior with the post-COVID-19 behavior changes to construct a two-dimensional 6-point cancer prevention behavior change (CPBC) score for each of the four cancer prevention behaviors (i.e., 24-point CPBC score). The resulting score captured the adherence status and COVID-related changes in each behavior, with higher CPBC scores indicating desirable changes in prevention behavior. The aggregate CPBC score was used to rank participants’ prevention behavior changes during the COVID-19 pandemic into one of four quartiles: poor (0 to 13), average (14 to 15), good (16 to 18), and excellent (≥18) (S3 Table).
2.7. Statistical analysis
Overall and stratified characteristics were summarized using descriptive statistics, including means and standard deviations (SD) for continuous variables and frequencies and proportions for categorical variables. Differences between participants with low, middle, and high SES were compared using analysis of variance (ANOVA) for continuous variables and χ2 or Fisher’s exact tests for categorical variables. We used modified Poisson regression models [38] to examine the adjusted associations, on the relative ratios (aRR) scale, between SES and each of the cancer prevention behaviors, using the 6-point CPBC score. We constructed adjusted multinomial logistic regression models with excellent aggregate cancer prevention behavior change as the reference outcome to assess the association between SES and COVID-19 related changes in cancer prevention behaviors. All statistical analyzes were conducted using SAS v9.4, with two-tail tests and a significance level of 0.05.
2.8. Sensitivity analysis
In a sensitivity analysis, all missing values were imputed using multiple imputations by chained equations to create 10 imputed data sets [39]. The imputation by chained equations approach utilizes a flexible variable-by-variable multivariable imputation model to address missing data for datasets with complex data structures. As such, we used logistic regression-based imputation models to impute binary and ordinal variables, the discriminant function to impute nominal variables, and a regression-based approach with predictive mean matching to impute continuous variables. The parameter estimates obtained from each imputed data set were combined using the Rubin method [40].
3. Results
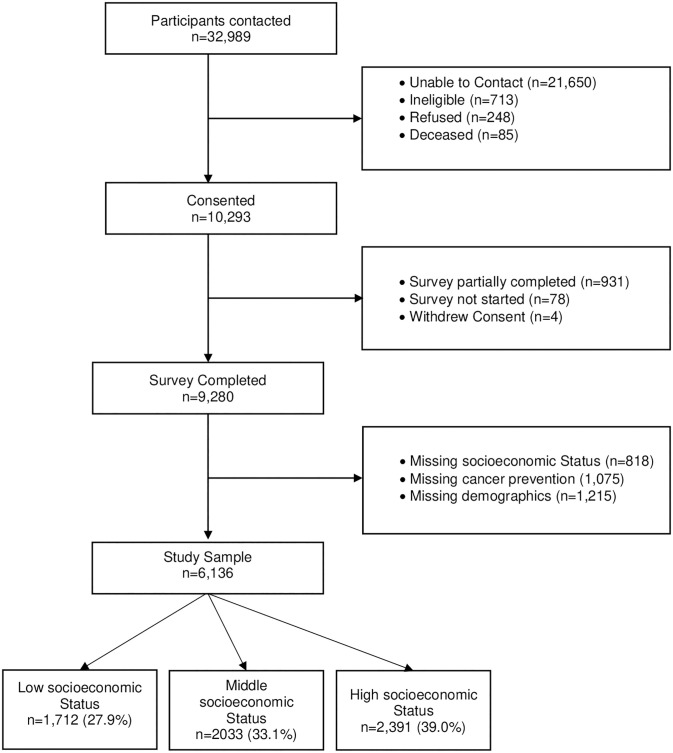
The study sample included 6,136 eligible participants (Fig 1). The overall and SES stratified characteristics of the survey participants are described in Table 1. The sample mean age (SD) was 56.8 (13.2) years, 67.1% were female, 88.6% were non-Hispanic White and 32.5% lived in non-metro counties. An estimated 75% of the participants were married or lived as married and 57.5% were enrolled in private insurance. Higher SES was significantly associated with younger age, higher probability of being a male, identified as White non-Hispanic, being married/living as married, having private insurance, and residing in a non-rural county.
Fig 1. Study schema.
Table 1. Characteristics of survey participants by socioeconomic status (n = 6,136).
| Demographics | All (n = 6,136) | Socioeconomic Status* | P-value | ||
|---|---|---|---|---|---|
| Low (n = 1,712) | Middle (n = 2,033) | High (n = 2,391) | |||
| All (%) | |||||
| Age, years | < .0001§ | ||||
| Mean (SD) | 56.8 (13.2) | 58.3 (14.3) | 57.6 (13.0) | 54.9 (12.4) | |
| Median (25th, 75th) | 58.0 (48.0 to 67.0) | 61.0 (49.0, 69.0) | 59.0 (50.0, 67.0) | 55.0 (47.0, 64.0) | |
| Age, years | < .0001† | ||||
| 18–34 | 452 (7.4) | 156 (9.1) | 131 (6.4) | 165 (6.9) | |
| 35–49 | 1217 (19.8) | 273 (16) | 351 (17.3) | 593 (24.8) | |
| 50–64 | 2543 (41.4) | 603 (35.2) | 870 (42.8) | 1070 (44.8) | |
| 65+ | 1924 (31.4) | 680 (39.7) | 681 (33.5) | 563 (23.6) | |
| Sex | 0.003† | ||||
| Male | 2018 (32.9) | 494 (28.9) | 676 (33.3) | 848 (35.5) | |
| Female | 4118 (67.1) | 1218 (71.1) | 1357 (66.8) | 1543 (64.5) | |
| Race Ethnicity | < .0001† | ||||
| White, Non-Hispanic | 5434 (88.6) | 1438 (84) | 1811 (89.1) | 2185 (91.4) | |
| Black, Non-Hispanic | 326 (5.3) | 151 (8.8) | 104 (5.1) | 71 (3) | |
| Hispanic | 127 (2.1) | 41 (2.4) | 44 (2.2) | 42 (1.8) | |
| Other‡ | 249 (4.1) | 82 (4.8) | 74 (3.6) | 93 (3.9) | |
| Marital Status | < .0001† | ||||
| Single, Never Married | 556 (9.0) | 237 (13.8) | 173 (8.5) | 146 (6.1) | |
| Married/Living as Married | 4604 (75.0) | 986 (57.6) | 1597 (78.6) | 2021 (84.5) | |
| Widowed, Separated or Divorced | 976 (15.9) | 489 (28.6) | 263 (12.9) | 224 (9.4) | |
| Health Insurance | < .0001† | ||||
| None | 182 (3.0) | 106 (6.2) | 63 (3.1) | 13 (0.5) | |
| Public Insurance | 795 (13.0) | 507 (29.6) | 200 (9.8) | 88 (3.7) | |
| Private Insurance | 3528 (57.5) | 500 (29.2) | 1191 (58.6) | 1837 (76.8) | |
| Public & Private Insurance | 1631 (26.6) | 599 (35.0) | 579 (28.5) | 453 (19.0) | |
| State | < .0001† | ||||
| Indiana | 233 (3.8) | 94 (42.3) | 68 (35.0) | 71 (23.2) | |
| Ohio | 4145 (96.2) | 1618 (57.7) | 1965 (65.0) | 2320 (76.8) | |
| Region of Residence | < .0001† | ||||
| Rural | 1991 (32.5) | 724 (42.3) | 712 (35.0) | 555 (23.2) | |
| Metro | 4145 (67.6) | 988 (57.7) | 1321 (65.0) | 1836 (76.8) | |
* Including education, household income, and occupational status
† Chi-Square tests from the association between each demographic variable and socioeconomic status
‡ Including participants who self-identified with more than one racial group
§ One-way ANOVA
The impacts of the COVID-19 pandemic on cancer prevention behaviors are outlined in Table 2. An estimated 42.3% of participants were physically inactive or had less physical activity post-COVID-19. Less fruit and vegetable intake post-COVID-19 was reported by 10.5% of participants. Alcohol consumption increased post the COVID-19 pandemic in 15.2% of participants with 21.2% of those who reported alcohol consumption pre-COVID-19 reporting binge drinking for at least one day in the past 30 days. More tobacco use post-COVID-19 was reported by 1.5% of participants.
Table 2. Cancer prevention behavior changes post the COVID-19 pandemic by socioeconomic status (n = 6,136).
| Cancer Prevention Behavior‡ | All (n = 6,136) % (95% CI) |
Socioeconomic Status* | P-value† | ||
|---|---|---|---|---|---|
| Low (n = 1,712) | Middle (n = 2,033) | High (n = 2,391) | |||
| % (95% CI) | % (95% CI) | % (95% CI) | |||
| Cancer Prevention Behavior Change § | < .0001 | ||||
| Poor | 29.8 (28.6–30.9) | 31.8 (29.6–34.1) | 30.6 (28.6–32.6) | 27.6 (25.8–29.4) | |
| Average | 23.8 (22.8–24.9) | 26.9 (24.8–29.0) | 23.6 (21.7–25.5) | 21.9 (20.3–23.6) | |
| Good | 22.0 (21.0–23.0) | 20.0 (18.1–22.0) | 22.5 (20.7–24.4) | 23.0 (21.3–24.7) | |
| Excellent | 24.4 (23.3–25.5) | 21.3 (19.4–23.3) | 23.4 (21.5–25.3) | 27.5 (25.7–29.4) | |
| Physical Activity | < .0001 | ||||
| Not Physical Activity | 14.0 (13.2–14.9) | 22.9 (20.9–25.0) | 13.3 (11.9–14.9) | 8.2 (7.2–9.4) | |
| Less Physical Activity | 28.3 (27.1–29.4) | 27.3 (25.2–29.5) | 29.2 (27.3–31.3) | 28.1 (26.3–30.0) | |
| Same Physical Activity | 38.0 (36.8–39.2) | 37.7 (35.4–40.0) | 40.4 (38.2–42.6) | 36.1 (34.2–38.1) | |
| More Physically Active | 19.8 (18.8–20.8) | 12.1 (10.6–13.7) | 17.1 (15.5–18.8) | 27.5 (25.7–29.4) | |
| Fruit and Vegetable Intake | < .0001 | ||||
| No Fruit or Vegetable Intake | 3.7 (3.2–4.2) | 5.7 (4.6–6.9) | 3.5 (2.7–4.4) | 2.4 (1.8–3.1) | |
| Less Intake | 10.5 (9.7–11.3) | 12.9 (11.4–14.6) | 10.3 (9.0–11.7) | 8.8 (7.7–10.0) | |
| Same Intake | 73.2 (72.0–74.3) | 69.2 (66.9–71.3) | 74.9 (73.0–76.8) | 74.5 (72.7–76.3) | |
| More Intake | 12.7 (11.9–13.6) | 12.3 (10.8–13.9) | 11.3 (9.9–12.7) | 14.3 (12.9–15.7) | |
| Alcohol Consumption | < .0001 | ||||
| No Alcohol Intake | 38.8 (37.6–40.0) | 57.6 (55.2–60.0) | 36.8 (34.7–38.9) | 27.1 (25.3–28.9) | |
| Less Intake | 6.4 (5.8–7.1) | 4.3 (3.4–5.3) | 7.6 (6.5–8.8) | 6.9 (6.0–8.0) | |
| Same Intake | 39.6 (38.3–40.8) | 30.1 (27.9–32.3) | 42.6 (40.4–44.7) | 43.8 (41.8–45.9) | |
| More Intake | 15.2 (14.3–16.2) | 8.1 (6.8–9.5) | 13.1 (11.7–14.6) | 22.2 (20.5–23.9) | |
| Binge Alcohol Drinking || | 0.586 | ||||
| Yes | 21.4 (20.1–22.7) | 21.1 (18.2–24.2) | 22.3 (20.1–24.7) | 20.8 (18.9–22.8) | |
| No | 78.6 (77.3–79.9) | 78.9 (75.8–81.8) | 77.7 (75.3–79.3) | 79.2 (77.2–81.1) | |
| Tobacco Use | < .0001 | ||||
| No Tobacco Use | 89.6 (88.8–90.3) | 82.8 (80.9–84.5) | 90.4 (89.0–91.7) | 93.8 (92.7–94.7) | |
| Less Intake | 1.5 (1.2–1.8) | 3.0 (2.3–4.0) | 1.3 (0.8–1.9) | 0.5 (0.2–0.8) | |
| Same Intake | 6.0 (5.4–6.6) | 9.1 (7.8–10.6) | 5.4 (4.5–6.5) | 4.1 (3.4–5.0) | |
| More Intake | 1.5 (1.2–1.8) | 5.1 (4.1–6.2) | 2.9 (2.2–3.7) | 1.6 (1.2–2.2) | |
* Included measures for education, household income, and occupational status
† Chi-Square Tests from the association between each cancer prevention behavior and socioeconomic status
‡ Compared to the pre- COVID-19 pandemic levels
§ Classification is based on the quartiles of a 24-point post-COVID-19 cancer prevention behavior change score that included physical activity (0–6 points), fruit and vegetable intake (0–6 points), alcohol consumption (0–6 points), and tobacco use (0–6 points). A higher score quartile indicates better prevention behavior change. Score quartiles cutoff points were poor (0 to 13), average (14 to 15), good (16 to 18), and excellent (≥18)
||At least one day during the past 30 days with 5 or more alcoholic drinks _on the same occasion amongst participants with alcohol consumption (n = 3,755)
CI = Confidence Interval
Cancer prevention behavior changes post the COVID-19 pandemic were significantly associated with SES (Table 2). The proportion of participants in the excellent cancer prevention behavior quartile increased significantly with higher SES [low SES vs. high SES; 21.3% vs. 27.5%; P-value < .001]. Relative to pre-COVID-19 levels, higher SES was significantly associated with increases in the post-COVID-19 prevalence of more physical activity [low SES vs. high SES; 12.1% vs. 27.5%; P-value < .001], higher fruit and vegetable intake [low SES vs. high SES; 12.3% vs. 14.3%; P-value < .001], and more alcohol consumption [low SES vs. high SES; 8.1% vs. 22.2%; P-value < .001]. Higher SES was also associated with lower tobacco use [low SES vs. high SES; 3.0% vs. 0.5%; P-value < .001].
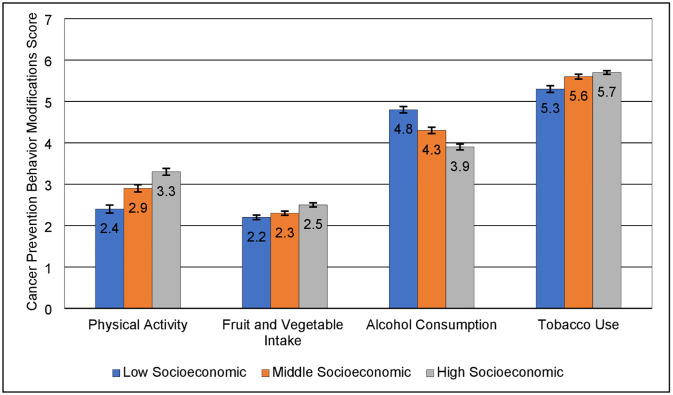
The average CPBC score increased with higher SES for physical activity, fruit and vegetable intake, and tobacco use, indicating higher desirable cancer prevention behavior changes post-COVID-19 among participants with higher SES (Fig 2). However, the mean CPBC score for alcohol consumption was higher in the low vs. high SES (4.8 vs. 3.9), indicating lower desirable post-COVID-19 behavior changes among participants in the high SES. Table 3 describes the adjusted RR for the effects of SES on the CPBC score for each cancer prevention behavior. Relative to participants with high SES, those with low SES had a 24% [aRR = 0.76 (95% CI: 0.72–0.80)], 11% [aRR = 0.89 (95% CI: 0.86–0.92)], and 5% [aRR = 0.95 (95% CI: 0.93–0.96)], lower CPBC score for physical activity, fruit and vegetable intake, and tobacco use, respectively. However, low SES was associated with a better CPBC score for alcohol consumption, 16% [aRR = 1.16 (95% CI: 1.13–1.19)] relative to high SES. Similar trends were observed for the middle vs. high SES associations (Table 3).
Fig 2. Mean post COVID-19 cancer prevention behavior change scores by socioeconomic status.
The score includes measures of physical activity (0–6), fruit and vegetable intake (0–6), alcohol consumption (0–6), and tobacco use (0–6) compared to levels before the COVID-19 Pandemic. A higher score indicates better prevention behavior.
Table 3. Adjusted relative ratios (aRR) for factors associated with higher* cancer prevention behavior change score post the COVID-19 pandemic (n = 6,136).
| Factor | More Physical Activity | More Fruit & Vegetable Intake | Less Alcohol Consumption | Less Tobacco Use |
|---|---|---|---|---|
| aRR (95% CI) | aRR (95% CI) | aRR (95% CI) | aRR (95% CI) | |
| Age, years | ||||
| 18–34 | Ref. | Ref. | Ref. | Ref. |
| 35–49 | 0.94 (0.87–1.01) | 0.98 (0.92–1.04) | 1.10 (1.03–1.17) | 1.00 (0.96–1.04) |
| 50–64 | 0.96 (0.89–1.03) | 0.99 (0.94–1.05) | 1.23 (1.16–1.30) | 1.04 (1.00–1.07) |
| 65+ | 1.02 (0.93–1.11) | 1.03 (0.96–1.10) | 1.26 (1.18–1.34) | 1.16 (1.11–1.20) |
| Sex | ||||
| Male | Ref. | Ref. | Ref. | Ref. |
| Female | 0.98 (0.94–1.01) | 1.11 (1.08–1.14) | 1.05 (1.03–1.08) | 1.06 (1.04–1.08) |
| Race Ethnicity | ||||
| White, non-Hispanic | Ref. | Ref. | Ref. | Ref. |
| Black, non-Hispanic | 0.85 (0.77–0.93) | 1.04 (0.98–1.11) | 1.04 (1.00–1.09) | 1.01 (0.97–1.04) |
| Hispanic | 1.08 (0.96–1.21) | 1.10 (1.00–1.21) | 1.04 (0.96–1.12) | 1.02 (0.97–1.07) |
| Other† | 1.04 (0.95–1.14) | 1.11 (1.03–1.19) | 1.14 (1.08–1.19) | 1.03 (0.99–1.06) |
| Marital status | ||||
| Single, Never Married | Ref. | Ref. | Ref. | Ref. |
| Married/Living as Married | 0.99 (0.93–1.06) | 1.00 (0.95–1.06) | 0.95 (0.91–0.99) | 1.03 (1.00–1.06) |
| Widowed, Separated or Divorced | 0.98 (0.90–1.06) | 0.96 (0.90–1.02) | 0.94 (0.90–0.98) | 1.01 (0.98–1.04) |
| Health Insurance | ||||
| Public & Private Insurance | Ref. | Ref. | Ref. | Ref. |
| None | 1.11 (0.98–1.27) | 1.03 (0.93–1.14) | 1.07 (1.00–1.14) | 0.97 (0.91–1.03) |
| Public Insurance | 0.97 (0.90–1.05) | 0.97 (0.93–1.02) | 1.00 (0.97–1.04) | 0.96 (0.94–0.99) |
| Private Insurance | 1.09 (1.02–1.17) | 1.02 (0.97–1.07) | 0.93 (0.89–0.96) | 1.07 (1.04–1.10) |
| State | ||||
| Ohio | Ref. | Ref. | Ref. | Ref. |
| Indiana | 0.96 (0.87–1.07) | 0.99 (0.92–1.07) | 1.02 (0.97–1.07) | 1.01 (0.98–1.04) |
| Region of Residence | ||||
| Metro | Ref. | Ref. | Ref. | Ref. |
| Rural | 1.00 (0.96–1.05) | 0.97 (0.94–1.00) | 1.02 (1.00–1.05) | 0.99 (0.98–1.01) |
| Socioeconomic Status ‡ | ||||
| High | Ref. | Ref. | Ref. | Ref. |
| Middle | 0.89 (0.85–0.92) | 0.92 (0.89–0.94) | 1.05 (1.02–1.07) | 0.98 (0.97–0.99) |
| Low | 0.76 (0.72–0.80) | 0.89 (0.86–0.92) | 1.16 (1.13–1.19) | 0.95 (0.93–0.96) |
* Passion regression with robust error variance with each 6-point cancer prevention behavior change score as outcome adjusted for age, sex, race-ethnicity, marital status, state, region of residence, health insurance, and socioeconomic status. A higher score indicates better change in cancer prevention behavior post the COVID-19 pandemic
† Including participants who self-identified with more than one racial group
‡ Included measures for education, household income, and occupational status; RR = Relative Ratio; CI = Confidence Interval
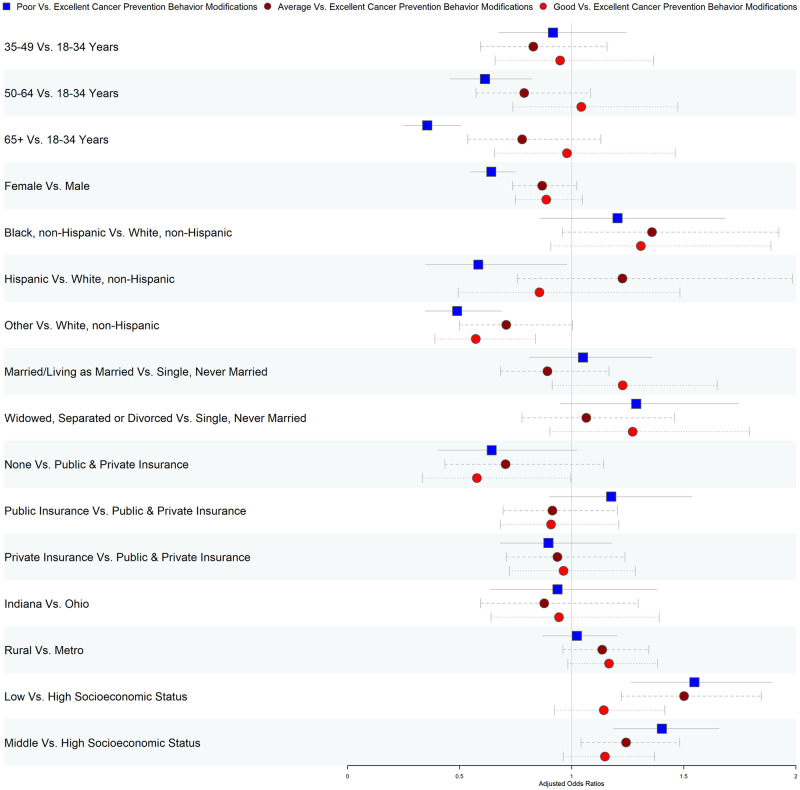
Fig 3 and S4 Table denotes the effects of SES on the overall cancer prevention behavior changes post the COVID-19 pandemic. Relative to participants with excellent CPBC post-COVID-19, the adjusted odds of poor CPBC for low SES were adjusted odds ratio (aOR) 1.55 (95% CI: 1.27–1.89) and aOR 1.40 (95% CI: 1.19–1.66) for middle SES, respectively compared with high SES. Compared to those with high SES, low and middle SES participants had higher odds of average CPBC relative to excellent CPBC. However, SES status was not a significant predictor for the good vs. excellent CPBC association. Results from the sensitivity analysis using multiple imputations were consistent with our main findings (S5 and S6 Tables).
Fig 3. Adjusted* odds ratios for factors associated with overall cancer prevention behavior changes post the COVID-19 pandemic (n = 6,136).
*Multinomial logistic regression models adjusting for age, sex, race-ethnicity, marital status, state, region of residence, health insurance, and socioeconomic status.
4. Discussion
This study is novel in its investigation of individuals’ adherence to cancer-prevention behaviors during the COVID-19 pandemic in a sample of adults from Ohio and Indiana. Our findings reveal a differential impact of COVID-19 on the adherence to cancer prevention behaviors according to socioeconomic status, exacerbating existing inequities. The adverse impacts of COVID-19 on cancer prevention behaviors were seen primarily in those with a lower socioeconomic status. Compared to pre-COVID levels, individuals with low SES were more likely to report less physical activity, less fruits and vegetables consumption, and increased tobacco use. On the contrary, individuals with higher socioeconomic status were more likely to report even better levels of cancer prevention behaviors than before the pandemic, with the exception to alcohol consumption.
Our study builds on the limited but growing literature on the differential impacts of the COVID-19 pandemic on health-promoting behaviors across socioeconomic groups. Consistent with our findings, previous studies on the early effects of the pandemic’s lockdowns on physical activity reported widening inequalities in physical activity levels between those of low and high socioeconomic status [3, 41]. In contrast, another study that evaluated the pandemic’s disruption on a broader array of healthy behaviors (sleep, diet quality, physical activity, frequency of alcohol consumption, and frequency of snacking), observed both positive and negative changes in lifestyle behaviors, irrespective of socioeconomic status [2].
A broad literature explores how socioeconomic status shapes motivations for healthy behavior and offers insights into why we observed that COVID-19 exacerbated socioeconomic disparities in adherence to cancer prevention behaviors [28]. Motives for healthy behavior focus on stress, perceived lower benefits, class distinctions, and knowledge of risk. The literature suggests that higher socioeconomic groups face less stress, where stress might encourage coping through unhealthy behavior, and gain more longevity benefits from healthy behavior. Additionally, higher socioeconomic groups accrue prestige by setting themselves apart with healthy behavior, and by adopting healthier behaviors because they have greater knowledge of the risks of unhealthy behavior [28]. Given the relevance to COVID-19, we consider the role that stress may play as well as and the perceived benefit of healthy behaviors.
Related to stress, in 2020, low-wage workers lost jobs at five times the rate of middle-wage workers [7], and people who lost their jobs were more likely to experience material and financial hardships and food insecurity [9, 42, 43]. Low-income adults were nearly twice as likely to report major negative mental health impacts, such as serious psychological distress [44], as compared to high-income adults [8]. This could have unequal implications on coping behaviors and addiction to substances like alcohol and tobacco [45–47]. Though it is interesting to note that financial crises have been associated with reduced substance use overall, possibly because individuals have less money to spend on harmful substances [48, 49].
The limited or uncertain benefit of adhering to cancer prevention behaviors could alternatively explain our results. Throughout the pandemic, people of lower socioeconomic status were more likely to work in essential jobs that did not allow them to work from home. This includes but is not limited to warehouse and grocery workers, bus drivers, and those in other forms of public transport. Based on the literature [28], perhaps their increased exposure to the virus reduced the perceived benefits of adhering to cancer prevention behaviors.
While individual motives and behaviors may be influenced by socioeconomic status, addressing disparities in cancer prevention behaviors requires a multi-level approach. As the effects of COVID-19 continue to unfold, ongoing research and multi-level strategies are needed to promote cancer prevention behaviors equitably. Expanding health insurance coverage may be one such strategy, where evidence shows that Medicaid and Medicaid expansion is linked to increased financial security and reduced poverty rates [50–53]. The COVID-19 pandemic also spurred new funding and programs to address health-related social needs. Researchers have and could continue to evaluate the impact of policy changes made during COVID-19 to identify strategies for reducing disparities in cancer prevention behaviors. Preliminary evidence suggests that value-based payments may be a potential way to pay for services that could support cancer prevention behaviors, including food supports [54]. Similarly, hospitals and health systems could increase their use of patient navigators or adopt cross-sector collaboration software to optimize referrals to community resources. Access to these technologies would enable clinicians to refer their patients to local community resources that could help them overcome barriers to cancer prevention behaviors. Finally, as noted by many others, it is critical to engage individuals and communities who have historically been excluded from policy design and implementation to ensure solutions reflect their priorities [54].
This study had multiple strengths. First, the survey followed a long-standing and well-established theoretical framework and included factors known to impact health disparities. Second, the survey assessed a range of recommended cancer prevention behaviors and included several measures of socioeconomic status. Finally, our novel CPBC score improves upon existing methods for measuring adherence to cancer prevention behaviors. Unlike previous measures, the CPBC score is two-dimensional and accounts for adherence and behavior changes over time, which is essential when considering long-term risk of developing cancer. Also, previous measures inadequately account for behaviors associated with the major risk factors of cancer, especially tobacco use.
Despite these strengths, we note several limitations. One limitation is the self-reported nature of the data collected, which introduces the potential of recall, social desirability, and selection bias. While not atypical for responses to survey questions about income, selection bias may be a concern because approximately one-third of participants were excluded from the main analysis of our study because they did not report household income or other study measures. However, the results from our sensitivity analysis using multiple imputations, which were consistent with our main findings, provide strong support against this concern. In addition, our study sample was not representative of the state of Ohio. Also, data collection spanned a six-month period in which the context of COVID surges and responses were not uniform and could have differentially impacted cancer prevention behaviors reported by participants. Yet, in collecting data beyond the pandemic’s early months, our study provides important, and timely insights into the longer-term impacts of the pandemic.
The current findings provide crucial insights about individuals at heightened risk during the pandemic. These results underscore the importance of formally recognizing and addressing the root causes of disparities in cancer prevention behaviors, including economic inequities, through targeted multi-level intervention strategies. It is imperative that we take action to address these disparities and ensure that all individuals have access to the resources and support they need to prevent cancer and maintain their health.
Supporting information
(DOCX)
(DOCX)
* The aggregate score was used to classify participants into low socioeconomic status (SES scores 0 to 4), middle socioeconomic status (SES scores 5 and 6), or high socioeconomic status (SES scores 7 and 8). †Including unemployed, students, homemaker, or disabled.
(DOCX)
(DOCX)
Multinomial logistic regression models adjusting for age, sex, race-ethnicity, marital status, state, region of residence, health insurance, and socioeconomic status. * Included post-COVID-19 measures of physical activity, fruit and vegetable intake, alcohol consumption, and tobacco use compared to levels before the COVID-19 Pandemic. † Including participants who self-identified with more than one racial group. ‡ Included measures for education, household income, and occupational status. OR = Odds Ratio; CI = Confidence Interval.
(DOCX)
All missing values were imputed using multiple imputations by chained equations to create ten imputed data sets. The parameter estimates obtained from each imputed data set were combined using the Rubin Method. * Passion regression with robust error variance with each 6-point cancer prevention behavior modification score as outcome adjusted for age, sex, race-ethnicity, marital status, region of residence, health insurance, and socioeconomic status. A higher score indicates better cancer prevention behavior modification post the COVID-19 Pandemic. † Including participants who self-identified with more than one racial group. ‡ Included measures for education, household income, and occupational status. RR = Relative Ratio; CI = Confidence Interval.
(DOCX)
All missing values were imputed using multiple imputations by chained equations to create ten imputed data sets. The parameter estimates obtained from each imputed data set were combined using the Rubin Method. Multinomial logistic regression models adjusting for age, sex, race-ethnicity, marital status, region of residence, health insurance, and socioeconomic status. * Include post-COVID-19 measures of physical activity, fruit and vegetable intake, alcohol consumption, and tobacco use compared to levels before the COVID-19 Pandemic. † Including participants who self-identified with more than one racial group. ‡ Included measures for education, household income, and occupational status. OR = Odds Ratio; CI = Confidence Interval.
(DOCX)
Acknowledgments
We would like to acknowelge the help and support of the Impact of COVID-19 on Behaviors across the Cancer Control Continuum in Ohio and Indiana Consortium, including Electra D. Paskett, Cecilia DeGraffinreid, Heather Hampel, and Chasity Washington.
Data Availability
This is an ongoing study. De-identified data will be available to access once the study ends. Interested researchers can request the data that support the findings of this study by contacting the office of Recruitment, Intervention and Survey Shared Resource at The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute. The procedure outlined below must be followed: 1. The researcher must submit a short proposal to the Project Publication Committee for approval. This should include the study rationale, introduction, methods, aims, data/variables, and hypothesis. 2. The Project Publication Committee will review the proposal and make suggestions and/or recommendations. 3. Once the proposal has been approved, the project statistician will start working on the analysis. The statistician and the investigators will then meet to discuss the paper and the analysis plans. The role of the statistician is to perform all analyses to ensure that methods are appropriate and statistically valid. 4. If it has been determined that the researcher will be performing the analysis (this needs to be requested in the initial proposal to the Project Publication Committee), then the researcher needs to complete the Data Distribution and Agreement Form to the Project Publication Committee. This form is a request for the specific data that the researcher needs as well as a notice of all policies and rules about using the Impact of COVID-19 data. For any data request please contact: Ms. Caroline Gault, MPH Project Manager Recruitment, Intervention and Survey Shared Resource The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute South Campus Gateway, 1590 N. High Street, Suite 525, Columbus, OH 43201 (614) 293-2452 caroline.gault@osumc.edu.
Funding Statement
This study was supported by a supplement to the Ohio State University Comprehensive Cancer Center (OSUCCC) core support grant (P30 CA016058) and the OSUCCC The Recruitment, Intervention, and Survey Shared Resource (RISSR) (P30 CA016058). The Ohio State University Center for Clinical and Translational Sciences grant support (National Center for Advancing Translational Sciences, Grant UL1TR001070) in publications related to this project. This work was supported by the National Cancer Institute (F99CA253745 to X.Z.). The National Cancer Institute P30CA016058 also supported this work.
References
- 1.Gostin LO, Wiley LF. Governmental public health powers during the COVID-19 pandemic: stay-at-home orders, business closures, and travel restrictions. Jama. 2020;323(21):2137–8. doi: 10.1001/jama.2020.5460 [DOI] [PubMed] [Google Scholar]
- 2.Mazidi M, Leeming ER, Merino J, Nguyen LH, Selvachandran S, Pujal JC, et al. Diet and lifestyle behaviour disruption related to the pandemic was varied and bidirectional among US and UK adults participating in the ZOE COVID Study. Nature Food. 2021;2(12):957–69. doi: 10.1038/s43016-021-00398-3 [DOI] [PubMed] [Google Scholar]
- 3.Hunter RF, Garcia L, de Sa TH, Zapata-Diomedi B, Millett C, Woodcock J, et al. Effect of COVID-19 response policies on walking behavior in US cities. Nature Communications. 2021;12(1):3652. doi: 10.1038/s41467-021-23937-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.González-Monroy C, Gómez-Gómez I, Olarte-Sánchez CM, Motrico E. Eating Behaviour Changes during the COVID-19 Pandemic: A Systematic Review of Longitudinal Studies. Int J Environ Res Public Health. 2021;18(21). doi: 10.3390/ijerph182111130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stockwell S, Trott M, Tully M, Shin J, Barnett Y, Butler L, et al. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: a systematic review. BMJ Open Sport & Exercise Medicine. 2021;7(1):e000960. doi: 10.1136/bmjsem-2020-000960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bambra C, Riordan R, Ford J, Matthews F. The COVID-19 pandemic and health inequalities. Journal of Epidemiology and Community Health. 2020;74(11):964. doi: 10.1136/jech-2020-214401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chetty R, Friedman JN, Hendren N, Stepner M. The economic impacts of COVID-19: Evidence from a new public database built using private sector data. national Bureau of economic research; 2020. [DOI] [PMC free article] [PubMed]
- 8.Panchal N, Kamal, R., Muñana, C., & Chidambaram, P. The Implications of COVID-19 for Mental Health and Substance Use: KFF; 2021 [https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use/.
- 9.Parker K, Minkin, R., & Bennett, J. Economic Fallout From COVID-19 Continues To Hit Lower-Income Americans the Hardest: Pew Research Center 2020 [https://www.pewresearch.org/social-trends/2020/09/24/economic-fallout-from-covid-19-continues-to-hit-lower-income-americans-the-hardest/.
- 10.Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of alcoholic beverages. The Lancet Oncology. 2007;8(4):292–3. doi: 10.1016/s1470-2045(07)70099-2 [DOI] [PubMed] [Google Scholar]
- 11.Islami F, Sauer AG, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and Number of Cancer Cases and Deaths Attributable to Potentially Modifiable Risk Factors in the United States. Ca-a Cancer Journal for Clinicians. 2018;68(1):31–54. doi: 10.3322/caac.21440 [DOI] [PubMed] [Google Scholar]
- 12.Song M, Giovannucci E. Preventable Incidence and Mortality of Carcinoma Associated With Lifestyle Factors Among White Adults in the United States. JAMA Oncology. 2016;2(9):1154–61. doi: 10.1001/jamaoncol.2016.0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McTiernan A, Friedenreich CM, Katzmarzyk PT, Powell KE, Macko R, Buchner D, et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med Sci Sports Exerc. 2019;51(6):1252–61. doi: 10.1249/MSS.0000000000001937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezende LFM, Sá TH, Markozannes G, Rey-López JP, Lee IM, Tsilidis KK, et al. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770000 cancer cases. Br J Sports Med. 2018;52(13):826–33. [DOI] [PubMed] [Google Scholar]
- 15.Patel AV, Friedenreich CM, Moore SC, Hayes SC, Silver JK, Campbell KL, et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med Sci Sports Exerc. 2019;51(11):2391–402. doi: 10.1249/MSS.0000000000002117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kvaavik E, Batty GD, Ursin G, Huxley R, Gale CR. Influence of individual and combined health behaviors on total and cause-specific mortality in men and women: the United Kingdom health and lifestyle survey. Archives of internal medicine. 2010;170(8):711–8. doi: 10.1001/archinternmed.2010.76 [DOI] [PubMed] [Google Scholar]
- 17.Van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. Bmj. 2008;337. doi: 10.1136/bmj.a1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen KE, Johnsen NF, Olsen A, Albieri V, Olsen LK, Dragsted LO, et al. The combined impact of adherence to five lifestyle factors on all-cause, cancer and cardiovascular mortality: a prospective cohort study among Danish men and women. British Journal of Nutrition. 2015;113(5):849–58. doi: 10.1017/S0007114515000070 [DOI] [PubMed] [Google Scholar]
- 19.Clinton SK, Giovannucci EL, Hursting SD. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J Nutr. 2020;150(4):663–71. doi: 10.1093/jn/nxz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rock CL, Thomson C, Gansler T, Gapstur SM, McCullough ML, Patel AV, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA: A Cancer Journal for Clinicians. 2020;70(4):245–71. doi: 10.3322/caac.21591 [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition ed2020.
- 22.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The physical activity guidelines for Americans. Jama. 2018;320(19):2020–8. doi: 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallis JF, Owen N, Fisher E. Ecological models of health behavior. Health behavior: Theory, research, and practice. 2015;5(43–64). [Google Scholar]
- 24.AACR Cancer Disparities Progress Reprot 2022: Achieving the bold vision of health equity for racial and ethnic minorities and other underserved populations. AACR; 2022. [DOI] [PubMed]
- 25.Akinyemiju T, Ogunsina K, Okwali M, Sakhuja S, Braithwaite D. Lifecourse socioeconomic status and cancer-related risk factors: Analysis of the WHO study on global ageing and adult health (SAGE). Int J Cancer. 2017;140(4):777–87. doi: 10.1002/ijc.30499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Islami F, Guerra CE, Minihan A, Yabroff KR, Fedewa SA, Sloan K, et al. American Cancer Society’s report on the status of cancer disparities in the United States, 2021. CA Cancer J Clin. 2022;72(2):112–43. doi: 10.3322/caac.21703 [DOI] [PubMed] [Google Scholar]
- 27.Bandi P, Minihan AK, Siegel RL, Islami F, Nargis N, Jemal A, et al. Updated Review of Major Cancer Risk Factors and Screening Test Use in the United States in 2018 and 2019, with a Focus on Smoking Cessation. Cancer Epidemiol Biomarkers Prev. 2021;30(7):1287–99. doi: 10.1158/1055-9965.EPI-20-1754 [DOI] [PubMed] [Google Scholar]
- 28.Pampel FC, Krueger PM, Denney JT. Socioeconomic Disparities in Health Behaviors. Annu Rev Sociol. 2010;36:349–70. doi: 10.1146/annurev.soc.012809.102529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emmons KM, Colditz GA. Realizing the Potential of Cancer Prevention—The Role of Implementation Science. N Engl J Med. 2017;376(10):986–90. doi: 10.1056/NEJMsb1609101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Just‐Østergaard E, Mortensen EL, Flensborg‐Madsen T. Major life events and risk of alcohol use disorders: a prospective cohort study. Addiction. 2018;113(1):25–33. doi: 10.1111/add.13947 [DOI] [PubMed] [Google Scholar]
- 31.Schäfer M, Jaeger-Erben M, Bamberg S. Life events as windows of opportunity for changing towards sustainable consumption patterns? Journal of Consumer Policy. 2012;35(1):65–84. [Google Scholar]
- 32.Sims R, Gordon S, Garcia W, Clark E, Monye D, Callender C, et al. Perceived stress and eating behaviors in a community-based sample of African Americans. Eating behaviors. 2008;9(2):137–42. doi: 10.1016/j.eatbeh.2007.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweren LJS, Larsson H, Vinke PC, Li L, Kvalvik LG, Arias-Vasquez A, et al. Diet quality, stress and common mental health problems: A cohort study of 121,008 adults. Clinical Nutrition. 2021;40(3):901–6. doi: 10.1016/j.clnu.2020.06.016 [DOI] [PubMed] [Google Scholar]
- 34.Stults-Kolehmainen MA, Sinha R. The effects of stress on physical activity and exercise. Sports medicine. 2014;44(1):81–121. doi: 10.1007/s40279-013-0090-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janz NK, Becker MH. The Health Belief Model: a decade later. Health Educ Q. 1984;11(1):1–47. doi: 10.1177/109019818401100101 [DOI] [PubMed] [Google Scholar]
- 36.Rosenstock IM. The health belief model: Explaining health behavior through expectancies. Health behavior and health education: Theory, research, and practice. The Jossey-Bass health series. Hoboken, NJ, US: Jossey-Bass/Wiley; 1990. p. 39–62. [Google Scholar]
- 37.Scarinci IC, Pandya VN, Kim YI, Bae S, Peral S, Tipre M, et al. Factors Associated with Perceived Susceptibility to COVID-19 Among Urban and Rural Adults in Alabama. J Community Health. 2021;46(5):932–41. doi: 10.1007/s10900-021-00976-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, De A. Multiple Imputation by Fully Conditional Specification for Dealing with Missing Data in a Large Epidemiologic Study. Int J Stat Med Res. 2015;4(3):287–95. doi: 10.6000/1929-6029.2015.04.03.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.RUBIN DB. Inference and missing data. Biometrika. 1976;63(3):581–92. [Google Scholar]
- 41.Dunton GF, Wang SD, Do B, Courtney J. Early effects of the COVID-19 pandemic on physical activity locations and behaviors in adults living in the United States. Preventive Medicine Reports. 2020;20:101241. doi: 10.1016/j.pmedr.2020.101241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raifman J, Bor J, Venkataramani A. Unemployment insurance and food insecurity among people who lost employment in the wake of COVID-19. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 43.Despard M.R. G-WM, Chun Y, Roll S. COVID-19 Job and Income Loss and Financial Distress: The Protective Role of Liquid Assets Washington, DC: Brookings Institute; 2020.
- 44.Fairman RT, Weaver SR, Nyman AL, Popova L, Massey Z, Reynolds RM, et al. Disparities among smokers during the COVID-19 pandemic: Examination of COVID-19-related worries by sociodemographic factors in a U.S. Nationally representative survey. Prev Med Rep. 2022;28:101835. doi: 10.1016/j.pmedr.2022.101835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clay JM, Parker MO. Alcohol use and misuse during the COVID-19 pandemic: a potential public health crisis? Lancet Public Health. 2020;5(5):e259. doi: 10.1016/S2468-2667(20)30088-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marsden J, Darke S, Hall W, Hickman M, Holmes J, Humphreys K, et al. Mitigating and learning from the impact of COVID-19 infection on addictive disorders. Addiction. 2020;115(6):1007–10. doi: 10.1111/add.15080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volkow ND. Collision of the COVID-19 and Addiction Epidemics. Ann Intern Med. 2020;173(1):61–2. doi: 10.7326/M20-1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Goeij MCM, Suhrcke M, Toffolutti V, van de Mheen D, Schoenmakers TM, Kunst AE. How economic crises affect alcohol consumption and alcohol-related health problems: A realist systematic review. Social Science & Medicine. 2015;131:131–46. doi: 10.1016/j.socscimed.2015.02.025 [DOI] [PubMed] [Google Scholar]
- 49.Ritter A, Chalmers J. The relationship between economic conditions and substance use and harm. Drug and Alcohol Review. 2011;30(1):1–3. doi: 10.1111/j.1465-3362.2010.00282.x [DOI] [PubMed] [Google Scholar]
- 50.Estimating The Effects Of Health Insurance And Other Social Programs On Poverty Under The Affordable Care Act. Health Affairs. 2017;36(10):1828–37. doi: 10.1377/hlthaff.2017.0331 [DOI] [PubMed] [Google Scholar]
- 51.Sommers BD, Oellerich D. The poverty-reducing effect of Medicaid. J Health Econ. 2013;32(5):816–32. doi: 10.1016/j.jhealeco.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 52.Hu L, Kaestner R, Mazumder B, Miller S, Wong A. The effect of the affordable care act Medicaid expansions on financial wellbeing. Journal of Public Economics. 2018;163:99–112. doi: 10.1016/j.jpubeco.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuroki M. The effect of health insurance coverage on personal bankruptcy: evidence from the Medicaid expansion. Review of Economics of the Household. 2021;19(2):429–51. [Google Scholar]
- 54.Pandemic-Driven Health Policies To Address Social Needs And Health Equity. Health Affairs Health Policy Brief. 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
* The aggregate score was used to classify participants into low socioeconomic status (SES scores 0 to 4), middle socioeconomic status (SES scores 5 and 6), or high socioeconomic status (SES scores 7 and 8). †Including unemployed, students, homemaker, or disabled.
(DOCX)
(DOCX)
Multinomial logistic regression models adjusting for age, sex, race-ethnicity, marital status, state, region of residence, health insurance, and socioeconomic status. * Included post-COVID-19 measures of physical activity, fruit and vegetable intake, alcohol consumption, and tobacco use compared to levels before the COVID-19 Pandemic. † Including participants who self-identified with more than one racial group. ‡ Included measures for education, household income, and occupational status. OR = Odds Ratio; CI = Confidence Interval.
(DOCX)
All missing values were imputed using multiple imputations by chained equations to create ten imputed data sets. The parameter estimates obtained from each imputed data set were combined using the Rubin Method. * Passion regression with robust error variance with each 6-point cancer prevention behavior modification score as outcome adjusted for age, sex, race-ethnicity, marital status, region of residence, health insurance, and socioeconomic status. A higher score indicates better cancer prevention behavior modification post the COVID-19 Pandemic. † Including participants who self-identified with more than one racial group. ‡ Included measures for education, household income, and occupational status. RR = Relative Ratio; CI = Confidence Interval.
(DOCX)
All missing values were imputed using multiple imputations by chained equations to create ten imputed data sets. The parameter estimates obtained from each imputed data set were combined using the Rubin Method. Multinomial logistic regression models adjusting for age, sex, race-ethnicity, marital status, region of residence, health insurance, and socioeconomic status. * Include post-COVID-19 measures of physical activity, fruit and vegetable intake, alcohol consumption, and tobacco use compared to levels before the COVID-19 Pandemic. † Including participants who self-identified with more than one racial group. ‡ Included measures for education, household income, and occupational status. OR = Odds Ratio; CI = Confidence Interval.
(DOCX)
Data Availability Statement
This is an ongoing study. De-identified data will be available to access once the study ends. Interested researchers can request the data that support the findings of this study by contacting the office of Recruitment, Intervention and Survey Shared Resource at The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute. The procedure outlined below must be followed: 1. The researcher must submit a short proposal to the Project Publication Committee for approval. This should include the study rationale, introduction, methods, aims, data/variables, and hypothesis. 2. The Project Publication Committee will review the proposal and make suggestions and/or recommendations. 3. Once the proposal has been approved, the project statistician will start working on the analysis. The statistician and the investigators will then meet to discuss the paper and the analysis plans. The role of the statistician is to perform all analyses to ensure that methods are appropriate and statistically valid. 4. If it has been determined that the researcher will be performing the analysis (this needs to be requested in the initial proposal to the Project Publication Committee), then the researcher needs to complete the Data Distribution and Agreement Form to the Project Publication Committee. This form is a request for the specific data that the researcher needs as well as a notice of all policies and rules about using the Impact of COVID-19 data. For any data request please contact: Ms. Caroline Gault, MPH Project Manager Recruitment, Intervention and Survey Shared Resource The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute South Campus Gateway, 1590 N. High Street, Suite 525, Columbus, OH 43201 (614) 293-2452 caroline.gault@osumc.edu.