Abstract
Background:
This pilot study explored the feasibility and acceptability of implementing text-based assessments of oral chemotherapy adherence in adolescents and young adults (AYA) with leukemia.
Methods:
AYA prescribed maintenance 6-mercaptopurine (6MP) received daily text message surveys and utilized an electronic pill bottle for 28 days. Text surveys assessed 6MP adherence and contextual associates (eg, mood). Feasibility was defined by recruitment/retention rates, survey completion rates, cost, and technical issues. After the 28-day period, AYA completed an acceptability survey. Secondary analyses compared text survey and electronic pill bottle adherence rates, and explored the daily associations between contextual factors and 6MP nonadherence.
Results:
Eighteen AYA enrolled (M age = 18, range 15–22) and completed study procedures (100% recruitment and retention rates). Adherence survey completion rates were high (M = 88.9%), the technology cost was $204.00, and there were few technical issues. AYA reported high satisfaction with the surveys and perceived them as a helpful medication reminder. While not significantly correlated, survey and electronic pill bottle adherence data converged on the majority of days (>90%). Exploratory analyses showed that AYA were more likely to miss a dose of 6MP on weekends (OR = 2.33, P = .048) and on days when their adherence motivation (OR = 0.28, P = .047) and negative affect (OR = 3.92, P = .02) worsened from their own typical functioning.
Conclusions:
For AYA with leukemia, daily text-based surveys are a feasible and acceptable method for delivering medication adherence assessments, and may operate as a short-term intervention. To develop personalized mobile health interventions, findings also highlighted the need to study time-varying predictors of 6MP nonadherence.
Keywords: 6-mercaptopurine, acute lymphoblastic leukemia, adherence, adolescent, mobile health, young adult
1 ǀ. INTRODUCTION
Treatment nonadherence is pervasive, particularly among adolescents and young adults (AYA), and results in devastating health consequences and significant health care costs.1–4 AYA with acute lymphoblastic leukemia (ALL) are an exemplar cancer population for piloting new adherence assessment strategies.5 During the maintenance phase, AYA with ALL must take daily oral chemotherapy called 6-mercaptopurine (6MP) for 2–3 years to prevent relapse. In one trial (COG-AALL03N1), nearly 50% of AYA demonstrate 6MP adherence rates below a 95% critical level for relapse prevention.1 Routine assessment of treatment adherence is one of 15 evidence-based standards designed to promote comprehensive psychosocial care to children with cancer.6,7 Yet, regular and valid adherence assessments are rarely obtained in practice.7 Several logistical and systems barriers impede regular adherence assessment, including lack of time and competing priorities during the clinical encounter, limited use of validated measures, and poor understanding of the real-world determinants of nonadherence.7–13 To ultimately improve uptake of the Adherence Standard and promote understanding of proximal adherence barriers, this study sought to determine the acceptability and feasibility of implementing text-based assessments of daily 6MP adherence in AYA with ALL.
Obtaining regular and valid adherence assessments is difficult to achieve, in part, because there is no ideal adherence assessment tool. A study of oncologists, psychosocial leaders, and administrators from 144 pediatric oncology programs reported that the most common method of assessing adherence was directly asking the patient in clinic.9 In contrast, there was limited use of standardized self-reported and objective measures, which are less prone to social desirability and recall biases.8,14 Standardized measures are often difficult to implement in busy oncology settings; questionnaires are lengthy and reliant on the accuracy of patient/family retrospective reporting, and electronic adherence monitors are costly, cumbersome, and difficult to interpret.8,11,12 Metabolite concentration profiles provide information regarding 6MP adherence prior to the blood draw, but do not provide contextualized data about daily adherence patterns.15
Mobile technology is a scalable solution for addressing this critical standards-to-practice gap, especially for AYA who are native digital users and have high ownership of mobile devices (~95% own a smartphone).16,17 Mobile devices facilitate ecological momentary assessments (EMA) or repeated surveys of behaviors (eg, medication adherence) in real time,18 often via text messaging. EMA also allow for brief assessments of other contextual factors that may proximally influence adherence (eg, physical symptoms), thus providing key opportunities to understand real-time adherence behaviors in context.19–22 Compared to other assessments, EMA is inexpensive, less vulnerable to memory decay, and potentially feasible to implement on a larger scale across pediatric oncology clinics. In the research context, determining the acceptability and feasibility of EMA is a necessary first step toward identifying the time-varying factors that impact 6MP adherence. The absence of this information stalls the development of interventions that deliver personalized adherence support at the right moment.
EMA has shown high feasibility across studies of AYA with other chronic health conditions23,24 and utility in measuring the temporal determinants of health behaviors, including a few studies that examined nonadherence to other treatment regimens.25–29 In other pediatric populations, compliance to daily EMA surveys sent for 3+ weeks is high (M = 75%),24 and initial studies have shown convergence between EMA, objective measures (eg, blood glucose meters),30 and retrospective self-report questionnaires.27 However, AYA with ALL may have unique perspectives on receiving daily surveys about cancer at a time when they are transitioning to less frequent medical follow up, self-managing chemotherapies from home, and returning to normal. Moreover, past EMA studies have largely neglected implementation considerations that are particularly relevant for practice (eg, limited reporting of logistical/technical challenges, delivering surveys to a study phone vs personal phone, not sharing data with providers).23
EMA is a promising tool for identifying real-time 6MP nonadherence and temporal contextual antecedents and possibly, improving implementation of the Adherence Standard. Prior to clinical deployment, this pilot aimed to explore the feasibility and acceptability of using daily text message surveys (EMA) to measure 6MP adherence, as well as other proximal contexts relevant to daily adherence (eg, physical and emotional symptoms, adherence motivation, family-based stressors, location/social company) in AYA with ALL receiving maintenance treatment. Feasibility was defined by recruitment and retention rates, an average EMA survey completion rate of ≥75%, cost, and technical glitches. In a secondary aim, we identified the initial convergent validity of EMA of 6MP adherence with an electronic adherence monitor by examining the degree of correlation and daily agreement between these two measurements. We hypothesized that EMA data would converge with the electronic pill bottle at both the aggregate and daily levels. To guide future intervention development, daily associations between EMA of contextual factors and electronically monitored 6MP nonadherence were also explored.
2 ǀ. METHODS
2.1 ǀ. Participants
Participants were AYA with ALL at the Children’s Hospital of Philadelphia. Inclusion criteria were: (a) between the ages of 15 and 25 (guided by the NCI,31 adjusted to represent the younger age of patients treated at our center), (b) diagnosed with ALL, (c) prescribed daily 6MP, (d) completed at least 1 month of maintenance chemotherapy to allow participants to establish an initial 6MP routine, and (e) proficient in English. AYA were excluded if they had significant cognitive impairments that would interfere with participation. Participants were screened for eligibility by reviewing charts and clinic schedules in the electronic health record. Eighteen AYA were offered participation and all agreed to participate.
2.2 ǀ. Text message platform
Twilio is a third party communication platform that integrates with research electronic data capture (REDCap)32 to automatically send survey questions and responses to and from a REDCap database to a participant’s mobile phone via a text message. Participants text back their response to each survey question, then their response is stored in the REDCap database. At our institution, REDCap is free to utilize for research and clinical care.
2.3 ǀ. Procedures
AYA were recruited in-person in the outpatient oncology clinic for this institutional review board-approved study by a research staff member with <20% devoted effort on the project. After informed consent/assent, AYA completed a brief demographic survey via REDCap (on an iPad), then were provided with an electronic pill bottle for storing their 6MP (medication event monitoring system [MEMS Track-Cap]). They were asked about the typical timing of their 6MP to tailor the timing of certain surveys (Figure 1). For 28 days (the approximate time between monthly clinic visits), participants received up to 14 survey questions per day. If a participant did not start the survey questions within an hour after it was sent, one reminder was sent. Following the 28-day period, AYA completed an acceptability survey via REDCap (on an iPad during the next clinic visit), returned the MEMS, and were compensated up to $100 ($2/day for completing all EMA questions, $20 for using and returning the MEMS, $10 for completing the acceptability questionnaire, bonus $14 for completing all tasks). We provided a summary of each participant’s adherence data (MEMS and EMA) to their oncology provider via an email and a note in the health record.
FIGURE 1.
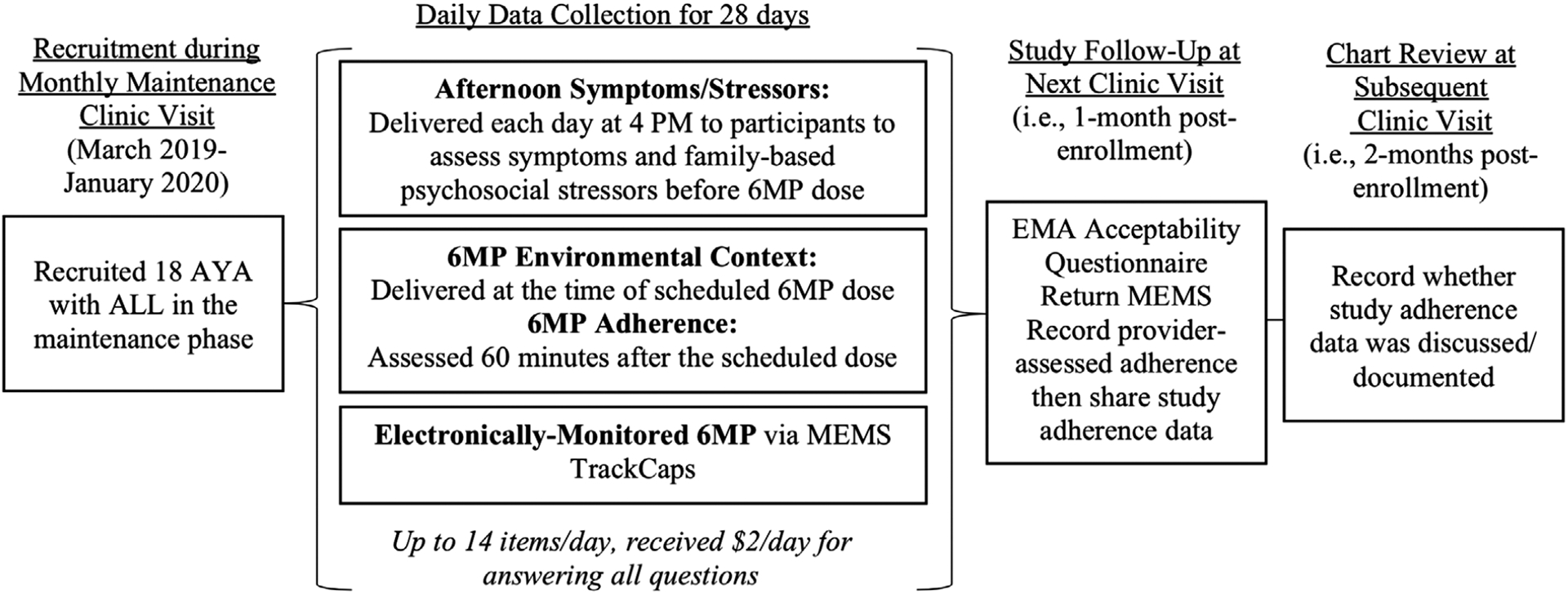
Study design
2.4 ǀ. Measures
2.4.1 ǀ. Demographic/disease information
AYA provided data on their age, sex, gender, race/ethnicity, and school/work status. Health records were reviewed to collect disease information including cycle of maintenance therapy, months since diagnosis, trial enrollment, health insurance (categorized as private or public), and notes about 6MP adherence in: (a) the clinic visit immediately following the 28-day study period (prior to sharing study adherence data), and (b) the subsequent clinic visit 1 month later (after sharing study data; Figure 1).
2.4.2 ǀ. EMA surveys
See Table 1 for a complete list of all EMA questions, response choices, and use in past studies. Each afternoon at 4 PM, participants received nine survey questions assessing physical symptoms (pain, fatigue, nausea) and psychosocial functioning (affect, adherence motivation, family-based stressors). Tailored to the timing of each participant’s typical 6MP dose, AYA were sent two questions assessing their current location (categorized as home vs other) and social company (family vs not). One hour after the typical 6MP dosing time, AYA received two questions asking whether they took 6MP and at what time. If participants indicated “Not yet, but I plan to take it soon,” a follow-up question was sent 1 h later.
TABLE 1.
Ecological momentary assessment (EMA) survey questions
| Variable | Timing | Items | Description |
|---|---|---|---|
| Physical symptoms | 4 PM |
|
3 Items assessing intensity of current pain, fatigue, and nausea, on a scale of 0 (none) to 10 (worst); adapted from two adult oncology EMA studies46,47 |
| Emotional symptoms | 4 PM |
|
2 Items assessing degree of positive affect and negative affect, on a scale of 0 (not at all) to 3 (extremely); adapted from a physical activity EMA study24 |
| Adherence motivation | 4 PM |
|
1 Item assessing motivation to take 6MP, on a scale of 0 (not motivated) to 4 (extremely motivated); adapted from EMA study of medication adherence in adults with HIV48 |
| Family stressors | 4 PM |
In the past 24 h, have you…
|
3 Adapted items from the Hassles Scale for Children,49 assessing whether or not the AYA experienced a disagreement/misunderstanding with parents, ease with talking to parents, and loneliness; adapted from EMA study of adolescents with asthma27 |
| Location/social company | During typical dosing time |
|
2 Items assessing where the AYA was (home, school, car, outdoors, restaurant, store, someone else’s house, gym, someplace else) and who they were with (alone, mom/dad, sister(s)/brother(s), other family, friend(s), classmate(s), someone else); adapted from EMA studies of diabetes adherence,50 physical activity,24 and asthma symptoms27 |
| 6MP adherence and timing | 60 min after typical dosing time |
|
2 Items assessing whether or not the AYA took 6MP and, if so, at what time. If participant indicated “Not yet, but I plan to take it soon,” received 1 additional question 1 h later; adapted from AYA diabetes EMA study50 |
2.4.3 ǀ. Electronic adherence monitor
Each participant was provided with a MEMS TrackCap, an electronic adherence monitor,33 to assess daily 6MP adherence over the 28-day period. This method of electronic adherence monitoring has been validated in pediatric cancer with 6MP metabolic profiles15 and shown consistent accuracy in independent testing.34 MEMS TrackCaps provide dates and times that a bottle containing 6MP was opened. The accompanying medAmigo software displays time-stamped adherence data for each day. Participants were instructed to place 6MP in the MEMS within 24 h of enrollment, use the MEMS for the full duration of the study (rather than a pillbox or pharmacy bottle), and not to open the bottle unless they were taking 6MP at that time. For each day, 6MP adherence was classified as 1 (missed dose) or 0 (took dose).
2.4.4 ǀ. Feasibility/acceptability
Informed by established standards for measuring acceptability/feasibility,35 feasibility metrics included: (a) percentage of EMA surveys completed, (b) recruitment and retention rates, (c) technology cost, and (d) technical issues. A staff member monitored the REDCap database one to two times per week for any errors with survey scheduling/delivery. AYA completed a 17-item acceptability questionnaire that evaluated self-reported ease, burden, and value of EMA, including 16 multiple choice items (eg, “I felt comfortable with the questions on the text message surveys” on a scale of “Not at All” to “Extremely”) and one open-ended item (“Were there aspects of the study that you thought were especially good or bad? If so, what were they?”). Items were adopted from a prior mobile health study with AYA cancer survivors36 and consistent with iterative mobile health development frameworks.37,38 We reviewed each participant’s health record to determine whether shared study adherence data were discussed during a subsequent oncology visit.
2.5 ǀ. Data analytic plan
Quantitative feasibility/acceptability data were summarized using descriptive statistics and frequencies/percentages. Qualitative responses on the open-ended acceptability item were analyzed for themes using directed content analysis techniques.39 Two authors (Alexandra M. Psihogios, Annisa Ahmed) independently coded all data and then reached agreement on the codes/emerging themes through discussion. We examined convergence between the MEMS and EMA adherence data in two ways. First, bivariate correlations compared aggregate adherence data across the 28 days from the EMA surveys, MEMS, and provider assessments from the electronic health record (% correct doses taken across 28 days). Second, to compare adherence reports at the day level, we calculated the percentage agreement/discrepancy of daily EMA adherence data compared to MEMS data.
For exploratory analyses, mixed-effect models were employed using SAS PROC GLIMMIX to examine whether EMA of contextual factors predicted the binary adherence outcome. We employed MEMS data for the 6MP adherence outcome because: (a) it has been previously validated, (b) contained more variability than EMA, and (c) allowed analysis into whether skipping the EMA adherence question was associated with a missed dose. However, three participants did not consistently utilize their MEMS correctly, and we utilized their EMA adherence data to resolve any discrepancies. Any EMA survey that was completed after the participant took 6MP that day were excluded to ensure that contextual predictors were assessed prior to 6MP adherence (typically, within a few hours). Separate mixed-effect models were constructed for different predictors. Each model included the predictor as the fixed effect and a random intercept for each patient to account for between-person variability. We first examined the binary predictor of weekday versus weekend (Friday through Sunday). For the remaining time-varying contextual variables, we decomposed the between-subject and within-subject effects by creating two predictors from the original score: (a) the individual mean across all time points, and (b) the deviation of the daily score from the individual mean.
2.6 ǀ. Sample size justification
Our prior mobile health work has demonstrated that pilot samples as small as n = 10 lead to meaningful changes to the intervention, technology, and protocols.40 For exploratory analyses, an approximate estimate of power was based on G * Power.41 A sample size of 18 is sufficient to detect small (.20) effects across 28 days using repeated measures analysis of variance and a correlation of .5 among repeated measures, with >95% power and a P-value of <.05.
3 ǀ. RESULTS
3.1 ǀ. Feasibility/acceptability
Of those approached, 100% of AYA with ALL taking 6MP (N = 18) enrolled in the study, received EMA, returned the MEMS, and completed the acceptability measure (100% retention; see Table 2 for demographics). All owned their own smartphone with texting plan. The Twilio system cost $204.00 total to sustain over 12 months (less than a penny per text plus $10.00–30.00/month to maintain three phone numbers). Technology glitches were minor (eg, 2 survey days for two participants failed to trigger due to system errors related to the daylight savings time change) and easily resolved by the REDCap team. AYA completed an average of 88.9% of the daily adherence surveys (SD = 16.7, range 39.3–100.0%), and 79.5% of the surveys assessing contextual associates (SD = 23.3, range 25.3–100.0%). Average daily EMA completion rates were relatively stable (Figure 2). There were two outliers with lower completion rates (≥2 SDs from the mean); one reported that this was due to a broken phone.
TABLE 2.
Participant demographic and treatment information
| Variable | n (%) | Mean (SD) | Range |
|---|---|---|---|
| Current age | 18 (100.0) | 17.94 (2.31) | 15.00–22.00 |
| Age at diagnosis | 18 (100.0) | 15.89 (2.54) | 9.00–20.00 |
| Sex (female) | 4 (22.2) | - | - |
| Ethnicity (Hispanic) | 5 (27.8) | - | - |
| Race | |||
| White/Caucasian | 12 (66.7) | - | - |
| Black/African American | 2 (11.1) | - | - |
| Asian | 1 (5.6) | - | - |
| Other | 3 (16.7) | - | - |
| Primary insurance type (public) | 7 (38.9) | - | - |
| Took 6MP in the evening (8–11 PM) | 16 (88.9) | - | - |
| Treated on a clinical trial | 3 (16.7) | - | - |
| Months since diagnosis | 18 (100.0) | 20.41 (8.52) | 11.03–37.80 |
| Cycle of maintenance | 18 (100.0) | 4.67 (3.27) | 1.00–11.00 |
| Number of missed 6MP doses (MEMS) | 15 (83.3) | 2.60 (3.09) | 0.00–10.00 |
| Number of missed 6MP doses (EMA) | 18 (100) | 0.89 (1.64) | 0.00–7.00 |
| Number of missed 6MP doses (provider) | 16 (88.9) | 0.63 (0.96) | 0.00–3.00 |
FIGURE 2.
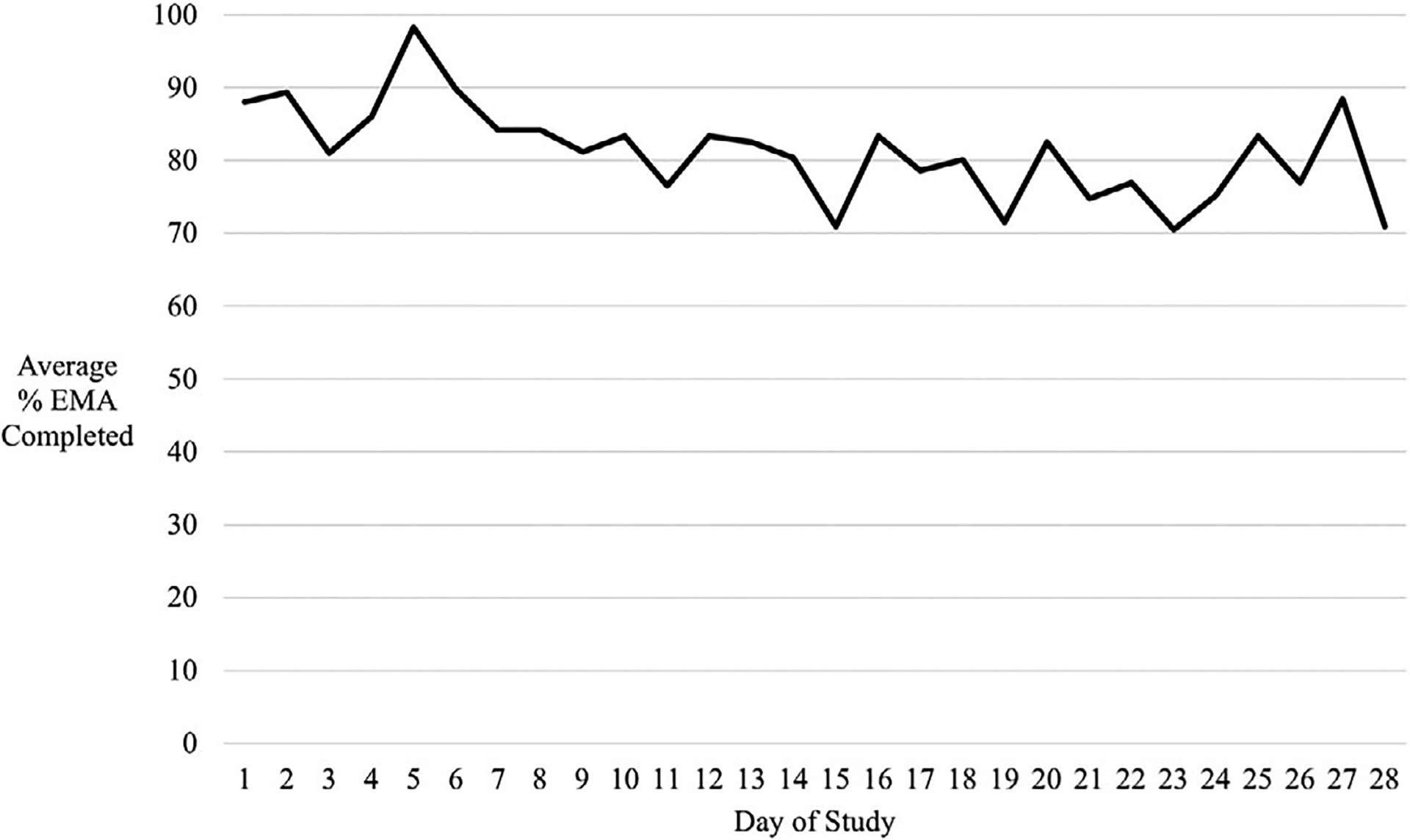
Average percentage ecological momentary assessment (% EMA) survey questions completed across 28 days
3.1.1 ǀ. Quantitative survey
AYA generally viewed the text surveys favorably, with more than half endorsing that they were “A lot” or “Completely” comfortable with the questions, and the questions made them more aware of taking 6MP (Table 3). Most AYA reported few technical glitches and that it was easy to find the time to answer the surveys, although they felt they received “A bit too many.” At least half reported that they were at least “Somewhat” interested in sharing information with their oncology team via text messaging, and that the surveys made them feel more motivated and independent with taking 6MP. Few participants (n = 7) found the MEMS as helpful. Ten AYA (55.6%) reported that they forgot to take 6MP, but then were reminded to take it from the adherence survey; five indicated that this occurred one-two times and five reported that this occurred three-five times.
TABLE 3.
EMA acceptability ratings
| Item (mean) N = 18 | Not at all/A littlen (%) | Somewhatn (%) | A lot/Completelyn (%) |
|---|---|---|---|
| Surveys had manytechnical glitchesa (0.56) | 15 (83.3) | 3 (16.7) | 0 (0) |
| Comfortable with the questions on the surveys (3.11) | 1 (5.6) | 4 (22.2) | 13 (72.2) |
| Hard to find the time to answer surveysa (1.11) | 13 (72.2) | 2 (11.1) | 3 (16.7) |
| Surveys increased awareness of taking 6MP (2.78) | 0 (0) | 8 (44.4) | 10 (55.6) |
| Surveys were helpful (2.44) | 2 (11.1) | 9 (50.0) | 7 (38.9) |
| Interested in text messaging with oncology team (2.22) | 4 (22.2) | 8 (44.4) | 6 (33.3) |
| Surveys made me feel more motivated to take 6MP (2.17) | 5 (27.8) | 6 (33.3) | 7 (38.9) |
| Surveys increased my independence with taking 6MP (2.11) | 5 (27.8) | 5 (27.8) | 8 (44.4) |
| Surveys increased awareness of my physical health (1.72) | 9 (50.0) | 5 (27.8) | 4 (22.2) |
| Surveys increased awareness of my mood/stressors (1.67) | 7 (38.9) | 7 (38.9) | 4 (22.2) |
| Looked forward to taking the surveys (1.67) | 8 (44.4) | 7 (38.9) | 3 (16.7) |
| Special pill bottle made me feel more motivated to take 6MP (1.56) | 11 (61.1) | 2 (11.1) | 5 (27.8) |
| Just the right amount | A bit too many | Way too many | |
| What do you think about the number of text message surveys? | 7 (38.9) | 10 (55.6) | 1 (5.6) |
Note. Acceptability items on scale of 0 (Not at all) to 4 (Completely).
Lower scores are more favorable ratings.
3.1.2 ǀ. Qualitative feedback
Thirteen participants provided a response to the open-ended acceptability item. We identified three themes: (a) the adherence survey question served as a medication reminder (endorsed by n = 7, 53.8%), (b) the other EMA questions and MEMS were less helpful and more burdensome (n = 5, 33.3%), and (c) matching the text adherence survey to the typical dose timing promoted adherence (n = 3, 23.0%). After sharing study adherence data, three providers documented in the health record that they discussed the data with their patient at a subsequent maintenance visit (all three patients had missed ≥1 6MP dose). One provider further assessed adherence barriers and involved social work for support. Another documented that it was more difficult for the patient to remember to take 6MP since the text surveys stopped. The last discussed discrepancies between MEMS and EMA adherence assessments.
3.2 ǀ. Comparing adherence reports
Among the 15 participants who utilized the MEMS correctly each day, the average number of missed 6MP doses was 2.60 (SD = 3.09, range 0–10, 90.7% adherent; Table 1). Self-reported EMA adherence data (n = 18) indicated an average of 0.89 missed doses (SD = 1.64, range 0–7, 96.8% adherent). For the 16 matched providers who assessed and documented on 6MP adherence for the same month, prior to sharing study data with them, the average number of missed doses was lower (M = 0.63, SD = 0.96, range 0–3, 97.8% adherent).
Aggregate adherence data from MEMS, EMA, and provider assessments were not significantly correlated (r = −.25 to −.02, P > .05). When comparing MEMS and EMA adherence at the day level (n = 15 AYA with 351 days of complete data), there was congruence of adherence the majority of days (n = 322 days, 91.7%). For 23 days (6.6%), MEMS indicated a missed dose, whereas EMA indicated that the dose was taken. Both measurement tools agreed with nonadherence for 4 days (1.1%). For 2 days, EMA indicated a missed dose and MEMS indicated that the dose was taken (0.6%). The relationship between answering the EMA adherence question and electronically monitored 6MP adherence approached statistical significance (Table 4). When an individual’s average EMA completion rate improved by 20%, the odds of missing 6MP decreased by 53% (OR = 0.47, 95% CI 0.22–1.0, P = .051).
TABLE 4.
Mixed-effect models predicting electronically monitored 6MP adherence
| Predictor variables | OR | 95% CI | P-value |
|---|---|---|---|
| Weekend (vs weekday) | 2.33 | 1.01–5.40 | .048* |
| Continuous predictorsa | |||
| Pain | |||
| Deviation from individual mean | 0.90 | 0.38–2.13 | .81 |
| Individual mean | 0.50 | 0.02–10.32 | .65 |
| Fatigue | |||
| Deviation from individual mean | 1.37 | 0.80–2.35 | .25 |
| Individual mean | 0.65 | 0.15–2.83 | .57 |
| Nausea | |||
| Deviation from individual mean | 0.74 | 0.39–1.40 | .36 |
| Individual mean | 3.34 | 0.51–21.88 | .21 |
| Negative affect | |||
| Deviation from individual mean | 3.92 | 1.30–11.79 | .02* |
| Individual mean | 10.46 | 0.34–319.43 | .18 |
| Positive affect | |||
| Deviation from individual mean | 1.15 | 0.32–4.13 | .83 |
| Individual mean | 1.51 | 0.06–36.49 | .80 |
| Motivation | |||
| Deviation from individual mean | 0.28 | 0.08–0.99 | .047* |
| Individual mean | 0.84 | 0.19–3.78 | .82 |
| Binary predictorsb | |||
| Disagreement with parent | |||
| Deviation from individual mean | 0.99 | 0.70–1.39 | .95 |
| Individual mean | 1.72 | 0.39–7.68 | .48 |
| Easy to talk to parents | |||
| Deviation from individual mean | 0.79 | 0.62–1.00 | .05 |
| Individual mean | 1.17 | 0.73–1.87 | .50 |
| Felt lonely | |||
| Deviation from individual mean | 1.19 | 0.89–1.59 | .23 |
| Individual mean | 1.37 | 0.72–2.61 | .34 |
| At home | |||
| Deviation from individual mean | 0.83 | 0.66–1.05 | .11 |
| Individual mean | 0.59 | 0.18–1.96 | .39 |
| With family | |||
| Deviation from individual mean | 1.17 | 0.92–1.50 | .20 |
| Individual mean | 1.44 | 0.50–4.10 | .50 |
| Completed EMA adherence survey | |||
| Deviation from individual mean | 0.98 | 0.80–1.20 | .85 |
| Individual mean | 0.47 | 0.22–1.00 | .05 |
Note. For the time-varying contextual predictors, the effect was decomposed into a between-subject effect (individual mean) and within-subject effect (deviation from individual mean).
OR of missing 6MP for every 2-unit increase in the predictor.
OR of missing 6MP for every 0.2-unit increase in the predictor (ie, 20% increase in the proportion).
significant at p<.05.
3.3 ǀ. Exploratory EMA adherence analyses
On any weekend day, AYA were 2.33 times more likely to miss 6MP compared to weekdays (95% CI 1.01–5.40, P = .048; Table 4). For time-varying contextual predictors, we found significant within-subject effects for adherence motivation and negative affect, and ease with talking to parents approached significance. When an AYA demonstrated a 2-unit increase in the deviation from their own average adherence motivation, the odds of missing 6MP that day decreased by 72% (OR = 0.28, 95% CI 0.08–0.99, P = .047). When an AYA demonstrated a 2-unit increase in the deviation from their own average negative affect, there was 3.92 greater odds of missing 6MP that day (95% CI 1.30–11.79, P = .02). On days when AYA experienced more ease with talking to their parents about their thoughts and feelings, the odds of missing 6MP decreased by 21% (OR = 0.79, 95% CI 0.62–1.00, P = .05).
4 ǀ. DISCUSSION
This study successfully delivered automated daily text message assessments (EMA) of oral chemotherapy adherence and other patient-reported symptoms/stressors in a population with known risks for nonadherence—AYA with ALL prescribed daily 6MP. We also determined that AYA were more likely to miss a dose of 6MP on weekends and on days when their adherence motivation and negative affect worsened from their typical functioning. EMA offered several unique benefits for future research and clinical implementation that other measurements did not, including the ability to feasibly assess 6MP adherence and other relevant contextual factors on a more frequent basis, high uptake and acceptability, and potentially offering one valuable short-term intervention for improving medication adherence. Electronic adherence monitors also allowed for real-time data collection, but were subject to error (three AYA did not consistently use them), expensive, and did not provide contextual data, diminishing their clinical utility.42
Delivering EMA of 6MP adherence and other proximal contexts was feasible in a research study, evidenced by all AYA owning their own phone, completion rates over the >75% threshold, relatively low cost, few observed technical glitches, ideal recruitment and retention rates, and the ability to accomplish the study with a small team. EMA completion rates were relatively stable across the 28-day period, rather than a pattern of rapid disengagement that has been observed in other populations,24 suggesting that AYA may have been willing to engage for a longer period of time. Participants reported high EMA acceptability, particularly, comfort with the questions, ease with finding the time to answer, and that the surveys helped them be more aware of taking 6MP. However, most AYA felt that there were slightly too many survey questions per day. To encourage engagement (particularly in the absence of financial incentives), future research should isolate the most predictive variables of adherence and eliminate items that do not yield meaningful information (eg, physical symptoms).
AYA perceived EMA as useful for reminding and encouraging adherence. After providing study adherence data to providers, a few utilized the data to facilitate further adherence-promoting conversations with their patients. As described in the Adherence Standard,6 assessing adherence more regularly may ultimately serve an important interventional role, potentially by way of providing a reminder, increasing accountability and self-monitoring skills, and promoting healthy habits. Notably, habit strength is positively correlated with medication adherence—if daily adherence surveys promote more automatic behavior, there may be less chance for an AYA to forget to take their medication over time.43 While medication reminders are prone to habituation,44–46 providing a two-way reminder (ie, asking whether or not the patient took the dose) may be more engaging. This represents one important intervention component to test in future trials, although likely insufficient alone; AYA nonadherence is a difficult health behavior to change and most amenable to multi-component interventions.47,48
Discrepancies are common in adherence research—multiple measurement types rarely converge.12,14,49 Contrary to our hypothesis, overall 6MP adherence data from EMA and MEMS were not correlated (nor were either reports correlated with provider assessments). However, these two measurement tools converged on the majority of days (>90%). This degree of concordance was higher compared to another study that required youth to self-report more frequently.50 The most common discrepancy was when the MEMS bottle indicated a missed dose and the EMA survey did not. Possibly, on these days, AYA responded to the survey based on their intention to take 6MP rather than their actual behavior. We also observed a trend between lower EMA completion rates and MEMS-recorded 6MP nonadherence, suggesting that some AYA may have rather skipped the adherence question altogether than endorse skipping a dose (likely due to social desirability). Providers should be aware that AYA may still underreport 6MP nonadherence through EMA, but no response may be a proxy for missing 6MP. Moreover, EMA appeared to capture more missed doses and was more consistently delivered than provider assessments in clinic.
In exploratory analyses, AYA were more likely to miss a dose of 6MP on weekends and when their adherence motivation, negative affect, and communication with caregivers worsened from their own typical functioning. While these findings are hypothesis-generating, they are novel, highlight the utility of EMA for understanding how within-person fluctuations impact 6MP adherence, and offer potentially valuable tailoring variables for more personalized mobile adherence interventions. For example, just-in-time adaptive interventions (JITAI) incorporate EMA and/or sensor data to deliver tailored interventions at the right time, only when it is needed (eg, delivering a medication reminder on a weekend, when AYA are more prone to forget).51
This study demonstrated that EMA is an acceptable and feasible research method for AYA with ALL, and may have clinical utility for routinely assessing 6MP adherence and providing a short-term intervention (ie, a two-way medication reminder). Other centers seeking to implement this approach will benefit from free REDCap access and administrative support, a staff member with at least 10–20% devoted effort toward deploying and maintaining the text message assessment program, and a small fund to support Twilio text message delivery (~$200/year in this study). Findings should be interpreted within the context of study limitations, including a small study sample from a single institution (although diverse and a typical size for pilot studies) and other threats to external validity (eg, participants receiving money for completing surveys). Implementation research is needed to study the processes and strategies that would support the integration of EMA into usual clinical care. For example, reducing in-person effort by establishing a digital infrastructure that automates the initiation of surveys when 6MP is prescribed and integrates data with the electronic health record in real time. Another important area of inquiry is to test low-/no-cost strategies for maintaining AYA engagement in EMA (eg, delivering developmentally friendly rewards such as memes or quotes).52 Finally, temporal data capturing 6MP adherence in context are needed, with larger samples over a longer period of time, to develop personalized interventions that effectively address the real-world contexts where risks of nonadherence are high.19–22
ACKNOWLEDGMENTS
This research was supported by a pilot grant entitled “Real-Time Medication Adherence Assessments among Adolescents and Young Adults with Leukemia” from the Mattie Miracle Cancer Foundation. The writing of this paper was supported from a grant from the National Cancer Institute (1K08CA241335-01). The authors express their sincere gratitude to the adolescents and young adults who participated in this study. The authors would also like to thank Lara Lechtenberg, MPH for REDCap support.
Funding information
Mattie Miracle Cancer Foundation, Pilot Grant entitled: “Real-Time Medication Adherence Assessments among Adolescents and Young Adults with Leukemia”; National Cancer Institute, Grant: 1K08CA241335-01
Abbreviations:
- 6MP
6-mercaptopurine
- ALL
acute lymphoblastic leukemia
- AYA
adolescents and young adults
- EMA
ecological momentary assessment
- MEMS TrackCap
medication event monitoring system TrackCap
- REDCap
research electronic data capture
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124(15):2345–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butow P, Palmer S, Pai A, Goodenough B, Luckett T, King M. Review of adherence-related issues in adolescents and young adults with cancer. J Clin Oncol. 2010;28(32):4800–4809. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Bhatia S. Optimizing medication adherence in children with cancer. Curr Opin Pediatr. 2017;29(1):41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGrady ME, Eckman MH, O’Brien MM, Pai AL. Cost-effectiveness analysis of an adherence-promotion intervention for children with leukemia: a Markov model-based simulation. J Pediatr Psychol. 2018;43(7):758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teachey DT, Hunger SP. Acute lymphoblastic leukaemia in 2017: immunotherapy for ALL takes the world by storm. Nat Rev Clin Oncol. 2018;15:69–70. [DOI] [PubMed] [Google Scholar]
- 6.Wiener L, Kazak AE, Noll RB, Patenaude AF, Kupst MJ. Standards for the psychosocial care of children with cancer and their families: an introduction to the special issue. Pediatr Blood Cancer. 2015;62(Suppl 5):S419–S424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pai AL, McGrady ME. Assessing medication adherence as a standard of care in pediatric oncology. Pediatr Blood Cancer. 2015;62(Suppl 5):S818–S828. [DOI] [PubMed] [Google Scholar]
- 8.Duncan CL, Mentrikoski JM, Wu YP, Fredericks EM. Practice-based approach to assessing and treating non-adherence in pediatric regimens. Clin Pract Pediatr Psychol. 2014;2(3):322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scialla MA, Canter KS, Chen FF, et al. Delivery of care consistent with the psychosocial standards in pediatric cancer: current practices in the United States. Pediatr Blood Cancer. 2018;65(3):e26869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGrady ME, Brown GA, Pai AL. Medication adherence decision-making among adolescents and young adults with cancer. Eur J Oncol Nurs. 2016;20:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YP, Rohan JM, Martin S, et al. Pediatric psychologist use of adherence assessments and interventions. J Pediatr Psychol. 2013;38(6):595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landier W, Chen Y, Hageman L, et al. Comparison of self-report and electronic monitoring of 6MP intake in childhood ALL: a Children’s Oncology Group study. Blood. 2017;129(14):1919–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiener L, Canter K, Long K, Psihogios AM, Thompson AL. Pediatric psychosocial standards of care in action: research that bridges the gap from need to implementation. Psychooncology. 2020. 10.1002/pon.5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohan JM, Fukuda T, Alderfer MA, et al. Measuring medication adherence in pediatric cancer: an approach to validation. J Pediatr Psychol. 2016;42(2):232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pew Research Center. Mobile Fact Sheet. http://www.pewinternet.org/fact-sheet/mobile/. 2018. Accessed May 29, 2018.
- 17.Pew Research Center. Teens, Social Media & Technology Overview 2018. http://www.pewinternet.org/2018/05/31/teens-social-media-technology-2018/. 2018. Accessed October 8, 2018.
- 18.Smyth JM, Stone AA. Ecological momentary assessment research in behavioral medicine. J Happiness Stud. 2003;4(1):35–52. [Google Scholar]
- 19.Spring B, Gotsis M, Paiva A, Spruijt-Metz D. Healthy apps: mobile devices for continuous monitoring and intervention. IEEE Pulse. 2013;4(6):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivera DE, Jimison HB. Systems modeling of behavior change: two illustrations from optimized interventions for improved health outcomes. IEEE Pulse. 2013;4(6):41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saranummi N, Spruijt-Metz D, Intille SS, Korhonen I, Nilsen WJ, Pavel M. Moving the science of behavioral change into the 21st century: part 2. IEEE Pulse. 2013;4(6):32–33. [DOI] [PubMed] [Google Scholar]
- 22.Spruijt-Metz D, Hekler E, Saranummi N, et al. Building new computational models to support health behavior change and maintenance: new opportunities in behavioral research. Transl Behav Med. 2015;5(3):335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heron KE, Everhart RS, McHale SM, Smyth JM. Using mobile-technology-based ecological momentary assessment (EMA) methods with youth: a systematic review and recommendations. J Pediatr Psychol. 2017;42(10):1087–1107. [DOI] [PubMed] [Google Scholar]
- 24.Wen CKF, Schneider S, Stone AA, Spruijt-Metz D. Compliance with mobile ecological momentary assessment protocols in children and adolescents: a systematic review and meta-analysis. J Med Internet Res. 2017;19(4):e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunton GF, Huh J, Leventhal AM, et al. Momentary assessment of affect, physical feeling states, and physical activity in children. Health Psychol. 2014;33(3):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulvaney SA, Ho Y-X, Cala CM, et al. Assessing adolescent asthma symptoms and adherence using mobile phones. J Med Internet Res. 2013;15(7):e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulvaney SA, Rothman RL, Dietrich MS, et al. Using mobile phones to measure adolescent diabetes adherence. Health Psychol. 2012;31(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunton G, Dzubur E, Li M, Huh J, Intille S, McConnell R. Momentary assessment of psychosocial stressors, context, and asthma symptoms in Hispanic adolescents. Behav Modif. 2016;40(1–2): 257–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulvaney SA, Vaala S, Hood KK, et al. Mobile momentary assessment and biobehavioral feedback for adolescents with type 1 diabetes: feasibility and engagement patterns. Diabetes Technol Ther. 2018;20(7):465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helgeson VS, Lopez LC, Kamarck T. Peer relationships and diabetes: retrospective and ecological momentary assessment approaches. Health Psychol. 2009;28(3):273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Cancer Institute. Adolescents and Young Adults with Cancer. https://www.cancer.gov/types/aya. Updated January 31, 2018. Accessed April 22, 2019.
- 32.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quittner AL, Modi AC, Lemanek KL, Ievers-Landis CE, Rapoff MA. Evidence-based assessment of adherence to medical treatments in pediatric psychology. J Pediatr Psychol. 2007;33(9):916–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGrady ME, Holbein CE, Smith AW, et al. An independent evaluation of the accuracy and usability of electronic adherence monitoring devices. Ann Intern Med. 2018;169(6):419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prev Med. 2009;36(5):452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz LA, Daniel LC, Henry-Moss D, et al. Feasibility and acceptability of a pilot tailored text messaging intervention for adolescents and young adults completing cancer treatment. Psychooncology. 2020;29(1):164–172. [DOI] [PubMed] [Google Scholar]
- 37.Hekler EB, Klasnja P, Riley WT, et al. Agile science: creating useful products for behavior change in the real world. Transl Behav Med. 2016;6(2):317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel S, Arya M. The BUS framework: a comprehensive tool in creating an mHealth app utilizing behavior change theories, user-centered design, and social marketing. J Mob Technol Med. 2017;6(1): 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz LA, Psihogios AM, Henry-Moss D, et al. Iterative development of a mHealth intervention for adolescent and young adult survivors of childhood cancer. Clin Pract Pediatr Psychol. 2019;7(1): 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. [DOI] [PubMed] [Google Scholar]
- 42.De Keyser HH, Ramsey R, Federico MJ. They just don’t take their medicines: reframing medication adherence in asthma from frustration to opportunity. Pediatr Pulmonol. 2020;55(3):818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badawy SM, Shah R, Beg U, Heneghan MB. Habit strength, medication adherence, and habit-based mobile health interventions across chronic medical conditions: systematic review. J Med Internet Res. 2020;22(4):e17883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS One. 2014;9(2):e88166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park LG, Howie-Esquivel J, Dracup K. A quantitative systematic review of the efficacy of mobile phone interventions to improve medication adherence. J Adv Nurs. 2014;70(9):1932–1953. [DOI] [PubMed] [Google Scholar]
- 46.Tao D, Xie L, Wang T, Wang T. A meta-analysis of the use of electronic reminders for patient adherence to medication in chronic disease care. J Telemed Telecare. 2015;21(1):3–13. [DOI] [PubMed] [Google Scholar]
- 47.Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590–611. [DOI] [PubMed] [Google Scholar]
- 48.Pai AL, McGrady M. Systematic review and meta-analysis of psychological interventions to promote treatment adherence in children, adolescents, and young adults with chronic illness. J Pediatr Psychol. 2014;39(8):918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pai AL, Drotar D, Kodish E. Correspondence between objective and subjective reports of adherence among adolescents with acute lymphoblastic leukemia. J Child Health Care. 2008;37(3):225–235. [Google Scholar]
- 50.Warnick JL, Westen SC, Albanese-O’Neill A, et al. Use of ecological momentary assessment to measure self-monitoring of blood glucose adherence in youth with type 1 diabetes. Diabetes Spectr. 2020;33(3):280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nahum-Shani I, Smith SN, Spring BJ, et al. Just-in-time adaptive interventions (JITAIs) in mobile health: key components and design principles for ongoing health behavior support. Ann Behav Med. 2018;52(6):446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rabbi M, Kotov MP, Cunningham R, et al. Toward increasing engagement in substance use data collection: development of the Substance Abuse Research Assistant app and protocol for a microrandomized trial using adolescents and emerging adults. JMIR Res Protoc. 2018;7(7):e166. [DOI] [PMC free article] [PubMed] [Google Scholar]


