Background:
The prevalence of nonalcoholic fatty liver disease (NAFLD) is rising rapidly in the world. Our aim is to investigate the efficacy and safety of statins in the treatment of NAFLD.
Methods:
This study was conducted by searching The National Library of Medicine, Cochrane Library, China National Knowledge Infrastructure, Web of Science, and Wanfang Data Knowledge Service Platform databases. Literature data are expressed as mean difference (MD) and 95% confidence intervals (CIs) or relative risk and 95% CI. For I2 > 50% trials, random effect model is used for statistical analysis, otherwise fixed effect model is used.
Results:
Fourteen studies are selected for this meta-analysis, which includes totally 534 patients in the treatment group and 527 patients in the control group. As a result, 5 studies show that the total effective rate of the treatment group is 17% higher than that of the control group (Z = 2.11, relative risk = 1.17, 95% CI: [1.01–1.35]). Twelve studies show that alanine aminotransferase levels of the experimental group are lower than that of the control group (Z = 2.63, P = .009, MD = −5.53, 95% CI: [−9.64 to −1.41]). Eleven studies show that aspartate transaminase levels of the experimental group are lower than that of the control group (Z = 2.01, P = .04, MD = −3.43, 95% CI: [−6.77 to −0.08]). Six studies show that alkaline phosphatase levels of the experimental group are lower than that of the control group (Z = 0.79, P = .43, MD = −3.46, 95% CI: [−12.08 to 5.16]). Eight studies show that gamma-glutamyl transpeptidase levels of the experimental group are lower than that of the control group (Z = 2.04, P = .04, MD = −4.05, 95% CI: [−7.96 to −0.15]). Thirteen studies show that triglyceride levels of the experimental group are lower than that of the control group (Z = 4.15, P < .0001, MD = −0.94, 95% CI: [−1.39 to −0.50]). Eleven studies show that the total cholesterol levels of the experimental group are lower than that of the control group (Z = 5.42, P < .00001, MD = −1.51, 95% CI: [−2.05 to −0.96]). Seven studies show that low-density lipoprotein-cholesterol levels of the experimental group are lower than that of the control group (Z = 5.00, P < .00001, MD = −0.85, 95% CI: [−1.18 to −0.52]).
Conclusion:
Statins can significantly reduce liver biochemical indicators in patients with NAFLD.
Keywords: meta-analysis, NAFLD, NASH, statins, systematic review
1. Introduction
Fatty liver diseases are divided into alcoholic fatty liver disease and nonalcoholic fatty liver disease (NAFLD). Fatty liver with alcohol intake >60 g/d (420 g/w) for men or >40 g/d (280 g/w) for women belongs to the category of AFLD. Fatty liver with alcohol intake of <20 g/d (140 g/w) for men or <10 g/d (70 g/w) for women is 1 diagnostic standard of NAFLD.[1] NAFLD is the most prevalent chronic liver disease.[2] It comprises NAFL, nonalcoholic steatohepatitis (NASH), advanced fibrosis, and cirrhosis. NAFL is a benign condition and NASH is its aggressive form.[3] The early symptoms of NAFLD are not obvious, and the disease progress is slow.[4] When it progresses to cirrhosis, NAFLD may rapidly cause hepatocellular cancer (HCC) or liver transplantation.[5] As a dynamic process, the etiology of NAFLD is complexly affected by many factors.[6]
Considering the development of NAFLD pathogenesis, the “multiple-hit theory has gradually replaced the “double-hit theory.”[7] The first attack refers to vulnerable liver cells that come from an accumulation of fatty acids and triglycerides (TGs).[8] The second attack is the inflammatory cascade in hepatocytes. It may promote the occurrence and development of liver fibrosis.[9] Afterward, the third strike is to produce liver fibrosis during the hepatocytes’ repair. The final strike is to cause microcirculation disturbance, ischemia, and hepatocyte necrosis. Then hepatic lobular reconstruction and cirrhosis may come up.[10] Liver fat accumulation, caused by obesity and insulin resistance (IR), represents an important “first hit.”[11] Epidemiological studies show that type 2 diabetes mellitus and obesity are major risk factors for NAFLD.[12] The liver fat accumulation of NAFLD could originate from the IR, hyperinsulinemia, or excessive lipid availability.[13] IR enhances free fatty acids processed by the accumulation of adipose tissue in the liver. Hyperinsulinemia reduces the oxidation reaction of fatty acids in the liver. It increases the esterification reaction of free fatty acids, then may aggravate NAFLD in terms of the accumulation of TGs.[14] Improvement of IR can relieve metabolic syndrome, type 2 diabetes mellitus, and related complications. Meanwhile, reducing liver fat deposition is important. It is necessary to reduce the occurrence of cirrhosis, HCC, and its complications.[15]
Statins belong to the family of reductase inhibitors of hydroxymethylglutaryl coenzyme A (CoA). Statins prevent the conversion of hydroxymethylglutaryl CoA to methyldihydroxyvaleric acid, and then it reduces hepatic fibrosis or hepatocyte steatosis through a series of fat metabolism changes.[16] Based on an antioxidant function, statins can increase nitric oxide bioavailability, improve endothelial cell function, and affect hepatocyte fatty acid synthesis.[17] Statins also can improve the hepatic response to injury stimuli, regulate the bile acid pool size, reduce cholesterol levels, and inhibit the activation of cholesterol-modified receptors.[18]
The relationship between statins with abnormal lipid metabolism in NAFLD patients is not unambiguous, so we conducted a systematic review and meta-analysis of statins in the treatment of NAFLD.
2. Methods
2.1. Article search strategy
All published articles were searched, from the earliest to January 2023, on the National Library of Medicine, Cochrane Library, China National Knowledge Infrastructure, Wanfang Data Knowledge Service Platform databases, and Web of Science. There was no limitation to languages.
2.2. Inclusive criteria
Randomized controlled trials were selected.
The patients included in the literature meet the diagnostic criteria of NAFLD.
The control group received standard treatment, whereas the experimental group received a certain dose of statins and standard treatment.
Outcome indicators are total effective rate, weight, alanine aminotransferase (ALT), aspartate transaminase (AST), body mass index, gamma-glutamyl transpeptidase (GGT), low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol, TG, total cholesterol (TC), and alkaline phosphatase (AP). For any abnormal sign or symptom, an adverse event has to be discussed.
2.3. Exclusion criteria
Repeated references.
Animal research or cell research.
Literature that does not meet the requirements of this study.
The design is not rigorous, such as the diagnosis and efficacy evaluation standards are not standardized, the sample data is not clear, and so on.
2.4. Quality evaluation and data extraction
The risk of bias was evaluated according to the Cochrane system evaluation tool. The evaluation content mainly includes 6 aspects: random allocation method; hidden allocation scheme; selective reporting of outcomes; blind method; incomplete outcome data; other potential sources of bias. According to the above 6 items, the included studies were evaluated for high risk of bias, low risk of bias, and unknown risk of bias.
2.5. Statistical analysis
Revman 5.3 software (Stata edition SE-16.0, Stata Corporation, College Station, TX) provided by Cochrane Collaboration Network is used for the meta-analysis. Data processing includes the heterogeneity test, the meta-analysis, and the publication bias analysis. For the included studies, the Q-statistic method is used for a statistical heterogeneity analysis. Heterogeneity test indicates that there is homogeneity among multiple similar studies (P > .10, I2 ≤ 50%), and then the fixed effect model is used. If studies have heterogeneity (P < .10, I2 ≥ 50%), the random effect model is used. For binary variables, the odds ratio and its 95% confidence interval (CI) are used. For continuous variables, mean difference (MD) is used. The standardized MD and its 95% CI are used for the statistical inference. P < .05 is considered to be statistically significant. Publication bias is analyzed by a funnel plot.
3. Results
3.1. Study selection
We identified 3911 trials, and there were 2976 records left after removing duplicates. According to the inclusion criteria, unqualified 2962 records were excluded. Finally, 14 eligible articles are included in the meta-analysis (Fig. 1).
Figure 1.
PRISMA 2009 flow diagram.
3.2. Study characteristics and quality
The principal characteristics of the 14 trials are summarized in Table 1. Qualities are assessed according to Cochrane (Table 2). Most studies have low risk for all items, so the included studies are quality.
Table 1.
Basic information included in the literature.
| Author | Year | Age | M/F | Duration (wk) | Treatment | Control | Patient/diagnosis | Evaluation indicator | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Statins | Cases | Measures | |||||||
| Joy[19] | 2017 | T: 56.7 ± 9.9 | 5/7 | 24 | 6 | Sitagliptin | 6 | Placebo | NASH/ | ALT, AST, weight, TG, |
| C: 54.7 ± 9.8 | 100 mg/d | Liver biopsy | AP, BMI, GGT, HDL-C | |||||||
| LDL-C | ||||||||||
| Nelson[20] | 2009 | T: 52.6 ± 8.6 | 11/5 | 48 | 10 | Simvastatin | 6 | Placebo | NASH/ | ALT, AST, BMI, AP, |
| C: 52.5 ± 13.0 | 40 mg/d | Liver biopsy | TG, TC, LDL | |||||||
| Athyros[21] | 2006 | T: 59 ± 13 | 80/43 | 54 | 61 | Atorvastatin | 62 | Fenofibrate | NAFLD/ | Weight, ALT, AST, |
| C: 61 ± 12 | 20 mg/d | +RT | Liver biopsy and | GGT, AP, TG, TC, | ||||||
| +Fenofibrate | ultrasonography | LDL-C, HDL-C | ||||||||
| +RT | ||||||||||
| Alam[22] | 2018 | T: 41.7 ± 9.1 | 12/28 | 48 | 20 | Sitagliptin | 20 | RT | NASH/ | ALT, AST, GGT, BMI, |
| C: 35.5 ± 6.9 | 100 mg/d | Liver biopsy | Weight, TG, LDL, | |||||||
| +RT | HDL, AP | |||||||||
| Samy[23] | 2011 | 46.6 ± 5.56 | 22/28 | 32 | 25 | Atorvastatin | 25 | RT | NAFLD/ | TG, TC, LDL, HDL |
| 40 mg/d + RT | Liver biopsy and | |||||||||
| ultrasonography | ||||||||||
| Smits[24] | 2016 | T: 61.5 ± 1.7 | 27/7 | 12 | 17 | Sitagliptin | 17 | Placebo | NAFLD/ | HbA1c, Weight, BMI, |
| C: 65.8 ± 1.4 | 100 mg/d | Liver biopsy | ALT, AST, AP, GGT | |||||||
| Peng[25] | 2013 | T: 48.63 ± 5.24 | 45/35 | 24 | 40 | Simvastatin | 40 | RT | NAFLD/ | Total effective rate |
| C: 48.37 ± 6.03 | 40 mg/d + RT | Liver biopsy and | TG, TC, | |||||||
| Ultrasonography | ||||||||||
| Xu[26] | 2015 | 35–64 | 65/25 | 12 | 45 | Lovastatin | 45 | RT | NAFLD/ | ALT, GGT, TG, TC, |
| 20 mg/d + RT | Ultrasonography | Total effective rate | ||||||||
| You[27] | 2011 | 24–64 | 86/29 | 16 | 57 | Atorvastatin | 58 | RT | NAFLD/ | TG, TC, LDL-C, HDL-C |
| 20 mg/d + RT | Ultrasonography | Total effective rate | ||||||||
| Bi[28] | 2016 | T: 58.47 ± 2.51 | 66/34 | 24 | 50 | Atorvastatin | 50 | RT | NAFLD/ | ALT, AST, TG, TC, |
| C: 58.54 ± 2.46 | 20 mg/d + RT | Ultrasonography | LDL-C, HDL-C, BMI, | |||||||
| HbA1c | ||||||||||
| Qin[29] | 2013 | T: 51.50 ± 10.20 | 89/29 | 24 | 61 | Atorvastatin | 57 | RT | NAFLD/ | BMI, ALT, AST, FPG, |
| C: 52.20 ± 9.60 | 20 mg/d + RT | Ultrasonography | TG, TC, LDL-C, HDL-C | |||||||
| Lai[30] | 2012 | T: 48.71 ± 11.02 | 58/2 | 36 | 30 | Simvastatin | 30 | Placebo | NAFLD/ | ALT, AST, GGT |
| C: 48.59 ± 11.97 | 10 mg/d | Ultrasonography | total effective rate | |||||||
| Jiang[31] | 2018 | T: 44.62 ± 8.14 | 51/37 | 12 | 44 | Fluvastatin | 44 | RT | NAFLD/ | ALT, AST, TC, TG, BMI, |
| C: 43.64 ± 7.84 | 40 mg/d + RT | Ultrasonography | Total effective rate, LDL-C, HDL-C | |||||||
| Li[32] | 2011 | 21–60 | 48/32 | 12 | 40 | Atorvastatin | 40 | RT | NAFLD/ | ALT, AST, GGT, TG, |
| 10 mg/d + RT | Ultrasonography | TC, LDL-C, HDL-C | ||||||||
ALT = alanine aminotransferase, AP = alkaline phosphatase, AST = aspartate transaminase, BMI = body mass index, GGT = gamma-glutamyl transpeptidase, HbA1c = glycated hemoglobin, HDL-C = high-density lipoprotein-cholesterol, LDL-C = low-density lipoprotein-cholesterol, NAFLD = nonalcoholic fatty liver disease, NASH = nonalcoholic steatohepatitis, TC = total cholesterol, TG = triglyceride.
Table 2.
Quality evaluation of included literatures.
| Included studies | Random allocation | Allocation concealment | Double-blind method | Evaluation of blindness | Data integrity | Selective report | Others |
|---|---|---|---|---|---|---|---|
| Joy 2017 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
| Nelson 2009 | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk | Low risk |
| Athyros 2006 | Unclear | Low risk | High risk | Unclear | Unclear | Unclear | Unclear |
| Alam 2018 | Low risk | Unclear | Unclear | Low risk | Unclear | Low risk | Unclear |
| Samy 2011 | Unclear | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Smits 2016 | Unclear | Unclear | Unclear | Low risk | Low risk | Low risk | Unclear |
| Peng 2013 | Low risk | Low risk | Low risk | Unclear | Low risk | Low risk | Unclear |
| Xu 2015 | Unclear | Unclear | Low risk | Unclear | Low risk | Unclear | Unclear |
| You 2011 | Unclear | Unclear | Unclear | Unclear | Low risk | Unclear | Unclear |
| Bi 2016 | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk | Unclear |
| Qin 2013 | Unclear | Low risk | Unclear | Unclear | Low risk | Unclear | Unclear |
| Lai 2012 | Unclear | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Jiang 2018 | Unclear | Unclear | Unclear | Unclear | Low risk | Unclear | Unclear |
| Li 2011 | Unclear | Unclear | Unclear | Low risk | Low risk | Unclear | Low risk |
3.3. Meta-analysis of outcome
3.3.1. Total effective rate.
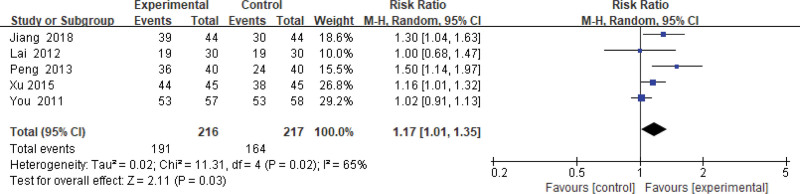
Four hundred thirty-three patients of 5 articles are included in this assessment (216 in the experimental group and 217 in the control group). There is heterogeneity (P = .02, I2 = 0.65), so the random effect model is used. It shows that the difference is statistically significant (Fig. 2). The effective rate of the experimental group is 17% higher than that of the control group (relative risk = 1.17, 95% CI: [1.01–1.35]).
Figure 2.
Changes in the total effective rate of the experimental group compared with the control group.
3.3.2. Weight.
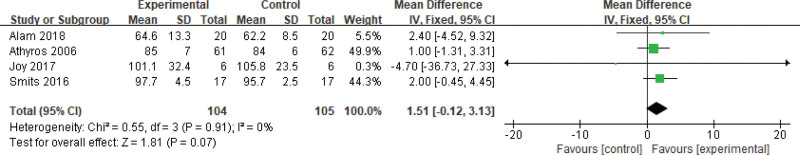
Two hundred nine patients of 5 articles are included in this assessment (104 in the experimental group and 105 in the control group). Because there is no heterogeneity (P = .91, I2 = 0%), a fixed-effects model is conducted. It shows that the difference is not statistically significant (Fig. 3).
Figure 3.
The experimental group compared with the control group in weight changes after treatment.
3.3.3. Body mass index.
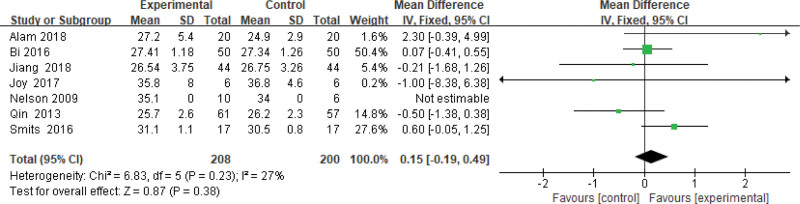
Four hundred eight patients of 7 articles are included in this assessment (208 in the experimental group and 200 in the control group). There is small heterogeneity (P = .23, I2 = 0.27), so a fixed-effects model is conducted. It shows that the difference is not statistically significant (Fig. 4).
Figure 4.
The experimental group compared with the control group in BMI changes after treatment. BMI = body mass index.
3.3.4. ALT level.
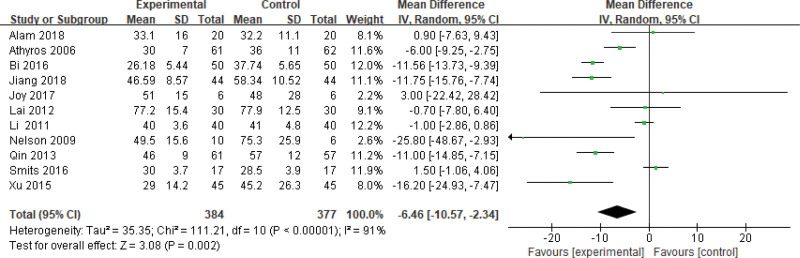
Seven hundred sixty-one patients of 12 articles are included in this assessment (384 in the experimental group and 377 in the control group). There is a large heterogeneity (P < .00001, I2 = 0.91), so the random effect model is used. It shows that the difference is statistically significant (Fig. 5). The ALT level of the experimental group is lower than that of the control group (Z = 3.08, P = .002, MD = −6.46, 95% CI: [−10.57 to −2.34]).
Figure 5.
The experimental group compared with the control group in ALT changes after treatment. ALT = alanine aminotransferase.
3.3.5. AST level.
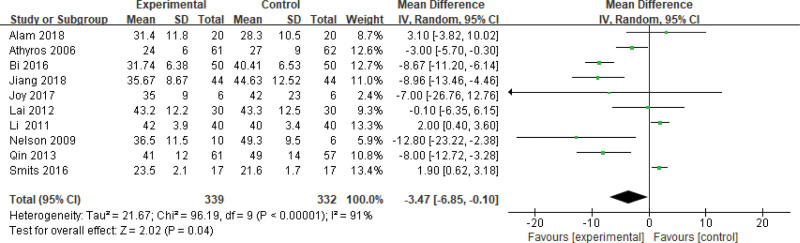
Six hundred seventy-one patients of 11 articles are included in this assessment (339 in the experimental group and 332 in the control group). There is a large heterogeneity (P < .00001, I2 = 0.91), so the random effect model is used. It shows that the difference is statistically significant (Fig. 6). The AST level of the experimental group is lower than that of the control group (Z = 2.02, P = .04, MD = −3.47, 95% CI: [−6.85 to −0.10]).
Figure 6.
The experimental group compared with the control group in AST changes after treatment. AST = aspartate transaminase.
3.3.6. GGT level.
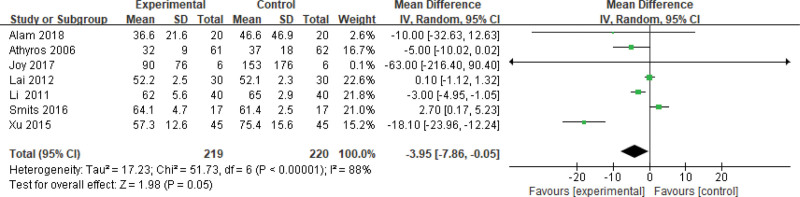
Four hundred thirty-nine patients of 8 articles are included in this assessment (219 in the experimental group and 220 in the control group). There is a large heterogeneity (P < .00001, I2 = 0.88), so the random effect model is used. It shows that the difference is statistically significant (Fig. 7). The GGT level of the experimental group is lower than that of the control group (Z = 1.98, P = .05, MD = −3.95, 95% CI: [−7.86 to −0.05]).
Figure 7.
The experimental group compared with the control group in GGT changes after treatment. GGT = gamma-glutamyl transpeptidase.
3.3.7. AP level.
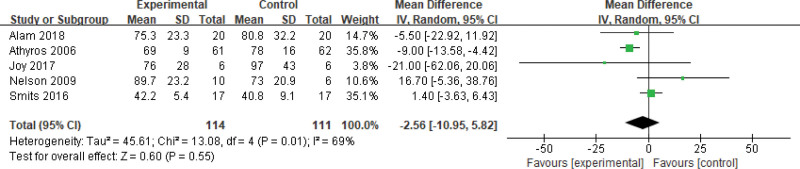
Two hundred twenty-five patients of 6 articles are included in this assessment (114 in the experimental group and 111 in the control group). There is a large heterogeneity (P = .01, I2 = 0.69), so the random effect model is used. It shows that the difference is statistically significant (Fig. 8). The AP level of the experimental group is lower than that of the control group (Z = 0.60, P = .55, MD = −2.56, 95% CI: [−10.95 to 5.82]).
Figure 8.
The experimental group compared with the control group in AP changes after treatment. AP = alkaline phosphatase.
3.3.8. TG level.
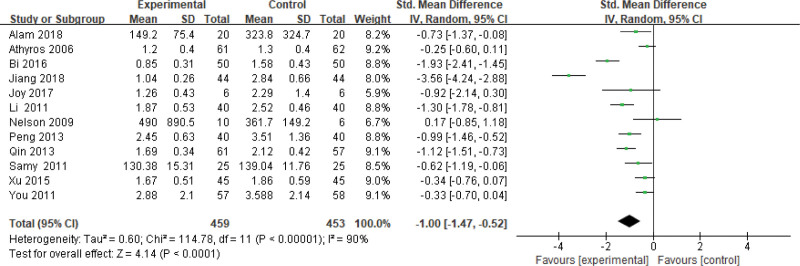
Nine hundred twelve patients of 13 articles are included in this assessment (459 in the experimental group and 453 in the control group). There is a large heterogeneity (P < .00001, I2 = 0.90), so the random effect model is used. It shows that the difference is statistically significant (Fig. 9). The TG level of the experimental group is lower than that of the control group (Z = 4.14, P < .0001, MD = −1.00, 95% CI: [−1.47 to −0.52]).
Figure 9.
The experimental group compared with the control group in TG changes after treatment. TG = triglyceride.
3.3.9. TC level.
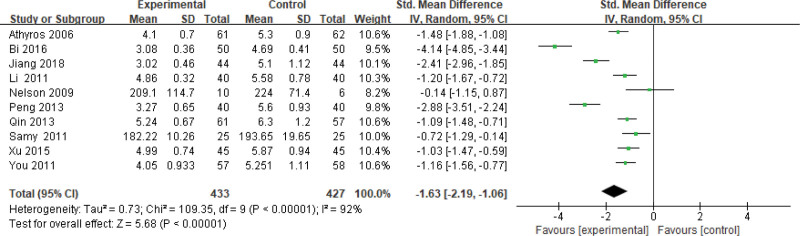
Eight hundred sixty patients of 11 articles are included in this assessment (433 in the experimental group and 427 in the control group). There is a large heterogeneity (P < .00001, I2 = 0.92), so the random effect model is used. It shows that the difference is statistically significant (Fig. 10). The TC level of the experimental group is lower than that of the control group (Z = 5.68, P < .00001, MD = −1.63, 95% CI: [−2.19 to −1.06]).
Figure 10.
The experimental group compared with the control group in TC changes after treatment. TC = total cholesterol.
3.3.10. LDL-C level.
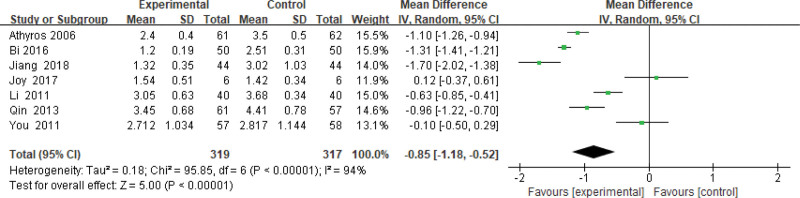
Six hundred thirty-six patients of 7 articles are included in this assessment (319 in the experimental group and 317 in the control group). There is a large heterogeneity (P < .00001, I2 = 0.94), so the random effect model is used. It shows that the difference is statistically significant (Fig. 11). The LDL-C level of the experimental group is lower than that of the control group (Z = 5.00, P < .00001, MD = −0.85, 95% CI: [−1.18 to −0.52]).
Figure 11.
The experimental group compared with the control group in LDL-C changes after treatment. LDL-C = low-density lipoprotein-cholesterol.
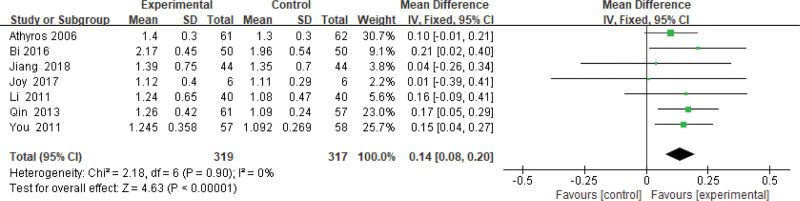
3.3.11. High density lipoprotein-cholesterol level.
Six hundred thirty-six patients of 7 articles are included in this assessment (319 in the experimental group and 317 in the control group). There is no heterogeneity (P = .90, I2 = 0%), so the fixed-effects model is used. It shows that the difference is not statistically significant (Fig. 12).
Figure 12.
The experimental group compared with the control group in HDL-C changes after treatment. HDL-C = high-density lipoprotein-cholesterol.
3.3.12. Adverse reactions.
Some patients have adverse events (4 patients for muscle weakness or pain, 9 patients for constipation, 15 patients for mild gastrointestinal reactions, 5 patients for abdominal pain, and 7 patients for abdominal distension). After treatment, all did not affect the following research. No serious adverse reaction was reported.
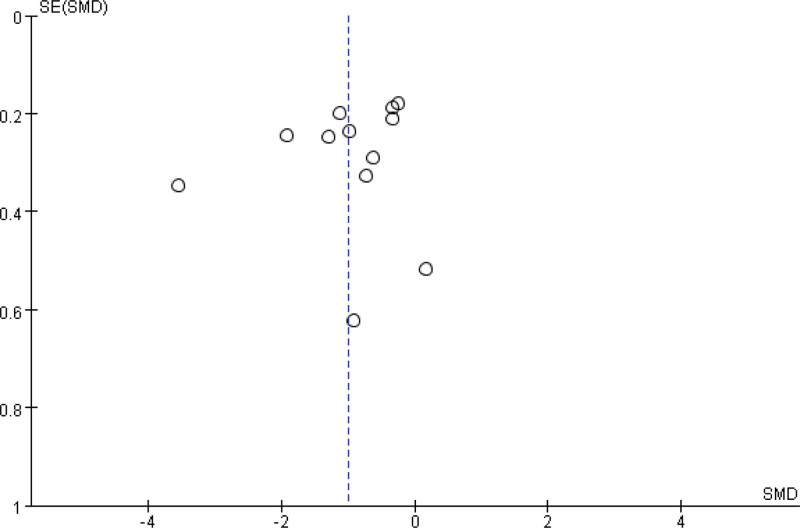
3.3.13. Publication bias.
A funnel plot is applied to evaluate the publication bias of all 14 studies, and the bias is mild (Fig. 13).
Figure 13.
Funnel plot.
4. Discussion
4.1. Efficacy analysis
Results demonstrate that statins have significant therapeutic effects on NAFLD. Our study shows that excessive fat deposition plays a key role in hepatocyte cytolysis and intrahepatic cholestasis. It is also reported that excessive fat deposition in hepatocytes leads to hepatocyte dysfunction and liver tissue injury in NAFLD patients.[33] In this research, the result shows that liver echo gradually returned to normal. It indicates a correlation between biochemical betterment and improvement of liver echogenicity and/or liver fibrosis, which is the parameter most influential on the prognosis in NAFLD/NASH patients, after statins’ treatment. The index of liver fibrosis decreased in the results. Considering the known pharmacological mechanism, statins may involve in all stages of NAFLD. Because of an antioxidative stress function, statins can reduce collagenase activity and oxidized LDL in plaque lipids.[34] The decreasing endothelin function of statins improves IR[35]; then hepatic steatosis alleviates.[36] Statins elevate the β-oxidative activity of fatty acid B2 while activating the oxidase activity of fatty acyl CoA.[37] As a consequence, shrinkage of plasma-free fat suppresses the inflammatory response.[38] Statins can alleviate collagen deposition, inhibit the formation of lipid peroxides, and then inhibit the progression of liver fibrosis.[39]
4.2. Limitations
Although the design is reasonable, our study has some limitations. Firstly, some studies may have unclear details in the randomized block, the randomization concealment, or the blinding method. All may lead to bias. Secondly, considering the characteristics of NAFLD, a long-term follow-up is needed. Finally, the total count of literatures and samples is small. Statins in the NAFLD management need further large-sample, multicenter, and quality randomized controlled trials.
4.3. Application prospects
Some studies found that insulin and sulfonylureas can increase the risk of NAFLD, whereas statin combination can alleviate this side effect.[40] NAFLD may progress to HCC, and it is an important cause of liver transplantation.[41] Doctors found that low-dose pravastatin or cerivastatin has a significant therapeutic effect on hyperlipidemia after liver transplantation and the safety is acceptable.[42] Statins may improve symptoms, suppress NAFLD development, and even prevent HCC or liver transplantation. The clinical applications of statins are worth further investigation.
5. Conclusion
Statins can significantly reduce liver biochemical indicators in patients with NAFLD.
Acknowledgments
The authors thank Dr Gengxin Wan for assistance with data extraction.
Author contributions
Conceptualization: Wenli Zhao, Ye Zhao, Yan Zhao.
Data curation: Haiyan Zhou.
Formal analysis: Haiyan Zhou, Maeda Toshiyoshi.
Funding acquisition: Haiyan Zhou.
Methodology: Haiyan Zhou, Wenli Zhao, Yan Zhao.
Project administration: Ye Zhao.
Resources: Maeda Toshiyoshi.
Software: Yan Zhao.
Supervision: Ye Zhao, Yan Zhao.
Validation: Yan Zhao.
Writing – original draft: Haiyan Zhou, Wenli Zhao.
Writing – review & editing: Haiyan Zhou, Wenli Zhao, Ye Zhao, Yan Zhao.
Abbreviations:
- ALT
- alanine aminotransferase
- AP
- alkaline phosphatase
- AST
- aspartate transaminase
- CI
- confidence intervals
- CoA
- coenzyme A
- GGT
- gamma-glutamyl transpeptidase
- HCC
- hepatocellular cancer
- IR
- insulin resistance
- LDL-C
- low-density lipoprotein-cholesterol
- MD
- mean difference
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- TC
- total cholesterol
- TG
- triglyceride
This project was supported by the 2022 Scientific Research Project of Hunan Provincial Education Department (22C1372).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Zhou H, Toshiyoshi M, Zhao W, Zhao Y, Zhao Y. Statins on nonalcoholic fatty liver disease: A systematic review and meta-analysis of 14 RCTs. Medicine 2023;102:26(e33981).
Contributor Information
Haiyan Zhou, Email: 2283945668@qq.com.
Maeda Toshiyoshi,, Email: mar31880@gmail.com.
Wenli Zhao, Email: 820761907@qq.com.
Yan Zhao,, Email: 820761907@qq.com.
References
- [1].Johnston MP, Patel J, Byrne CD. Causes of mortality in non-alcoholic fatty liver disease (NAFLD) and alcohol related fatty liver disease (AFLD). Curr Pharm Des. 2020;26:1079–92. [DOI] [PubMed] [Google Scholar]
- [2].Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018;53:362–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim SR, Kim KI. [An overview of NAFLD/NASH in Japan]. Yakugaku Zasshi. 2016;136:565–72. [DOI] [PubMed] [Google Scholar]
- [4].Sheka AC, Adeyi O, Thompson J, et al. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323:1175–83. [DOI] [PubMed] [Google Scholar]
- [5].Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152:1090–1099.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Parthasarathy G, Revelo X, Malhi H. Pathogenesis of nonalcoholic steatohepatitis: an overview. Hepatol Commun. 2020;4:478–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fang YL, Chen H, Wang CL, et al. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: from “two hit theory” to “multiple hit model”. World J Gastroenterol. 2018;24:2974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038–48. [DOI] [PubMed] [Google Scholar]
- [9].Doulberis M, Kotronis G, Gialamprinou D, et al. Non-alcoholic fatty liver disease: an update with special focus on the role of gut microbiota. Metabolism. 2017;71:182–97. [DOI] [PubMed] [Google Scholar]
- [10].Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2013;14:20704–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Noureddin M, Sanyal AJ. Pathogenesis of NASH: the impact of multiple pathways. Curr Hepatol Rep. 2018;17:350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Utzschneider KM, Van de Lagemaat A, Faulenbach MV, et al. Insulin resistance is the best predictor of the metabolic syndrome in subjects with a first-degree relative with type 2 diabetes. Obesity (Silver Spring). 2010;18:1781–7. [DOI] [PubMed] [Google Scholar]
- [13].Roden M. Mechanisms of disease: hepatic steatosis in type 2 diabetes--pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab. 2006;2:335–48. [DOI] [PubMed] [Google Scholar]
- [14].Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–9. [DOI] [PubMed] [Google Scholar]
- [15].Chitturi S, Wong VW, Chan WK, et al. The Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017-Part 2: management and special groups. J Gastroenterol Hepatol. 2018;33:86–98. [DOI] [PubMed] [Google Scholar]
- [16].Torres-Peña JD, Martín-Piedra L, Fuentes-Jiménez F. Statins in non-alcoholic steatohepatitis. Front Cardiovasc Med. 2021;8:777131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ahsan F, Oliveri F, Goud HK, et al. Pleiotropic effects of statins in the light of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cureus. 2020;12:e10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Park HS, Jang JE, Ko MS, et al. Statins increase mitochondrial and peroxisomal fatty acid oxidation in the liver and prevent non-alcoholic steatohepatitis in mice. Diabetes Metab J. 2016;40:376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Joy TR, McKenzie CA, Tirona RG, et al. Sitagliptin in patients with non-alcoholic steatohepatitis: a randomized, placebo-controlled trial. World J Gastroenterol. 2017;23:141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nelson A, Torres DM, Morgan AE, et al. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomized placebo-controlled trial. J Clin Gastroenterol. 2009;43:990–4. [DOI] [PubMed] [Google Scholar]
- [21].Athyros VG, Mikhailidis DP, Didangelos TP, et al. Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: a randomised study. Curr Med Res Opin. 2006;22:873–83. [DOI] [PubMed] [Google Scholar]
- [22].Alam S, Ghosh J, Mustafa G, et al. Effect of sitagliptin on hepatic histological activity and fibrosis of nonalcoholic steatohepatitis patients: a 1-year randomized control trial. Hepat Med. 2018;10:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Samy W, Hassanian MA. Paraoxonase-1 activity, malondialdehyde and glutathione peroxidase in non-alcoholic fatty liver disease and the effect of atorvastatin. Arab J Gastroenterol. 2011;12:80–5. [DOI] [PubMed] [Google Scholar]
- [24].Smits MM, Tonneijck L, Muskiet MH, et al. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo-controlled trial. Diabetologia. 2016;59:2588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Peng D, Lu J, Xiang S. Efficacy of Simvastatin in the treatment of nonalcoholic fatty liver disease and observation on changes of blood lipid. China Medical Herald. 2013;10:79–81. [Google Scholar]
- [26].Xu Y. Application and observation curative effect of lovastatin in the treatment of nonalcoholic steatohepatitis. Healthy People. 2015;9:8–9. [Google Scholar]
- [27].You Y, Chen W, Kong X. Curative effect of atorvastatin on nonalcoholic fatty liver disease. Mil Med J South China. 2011;25:494–7. [Google Scholar]
- [28].Bi J. Effect of atorvastatin on type 2 diabetes mellitus complicated with nonalcoholic fatty liver disease. Diabetes New World. 2016;19:31–3. [Google Scholar]
- [29].Feng M, Qin M, Liang K, et al. Therapeutic efficacy of atorvastatin in treatment of non-alcoholic fatty liver disease in patients with type II diabetes mellitus. J Clin Hepatol. 2013;29:512–5. [Google Scholar]
- [30].Dai H, Lai S, Wu T. Clinical studies of vitamin E, simvastatin in the treatment of nonalcoholic fatty liver disease. Chin Foreign Med Res. 2012;10:27–8. [Google Scholar]
- [31].Jiang L, Chen G, Chen W, et al. Clinical efficacy of fluvastatin in the treatment of nonalcoholic fatty liver disease and its effect on serum inflammatory factors, liver steatosis and fibrosis. Chin J Integr Tradit West Med Digestion. 2018;26:43–7. [Google Scholar]
- [32].Li Y, Lei H. The curative effect of atorvastatin in nonalcoholic fatty liver disease. Clin Med. 2011;31:23–4. [Google Scholar]
- [33].Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158:1851–64. [DOI] [PubMed] [Google Scholar]
- [34].Paradies G, Paradies V, Ruggiero FM, et al. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:14205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kitade H, Chen G, Ni Y, et al. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients. 2017;9:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gracia-Sancho J, García-Calderó H, Hide D, et al. Simvastatin maintains function and viability of steatotic rat livers procured for transplantation. J Hepatol. 2013;58:1140–6. [DOI] [PubMed] [Google Scholar]
- [37].Tziomalos K. Lipid-lowering agents in the management of nonalcoholic fatty liver disease. World J Hepatol. 2014;6:738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nascimbeni F, Pellegrini E, Lugari S, et al. Statins and nonalcoholic fatty liver disease in the era of precision medicine: more friends than foes. Atherosclerosis. 2019;284:66–74. [DOI] [PubMed] [Google Scholar]
- [39].Janicko M, Drazilova S, Pella D, et al. Pleiotropic effects of statins in the diseases of the liver. World J Gastroenterol. 2016;22:6201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Argo CK, Loria P, Caldwell SH, et al. Statins in liver disease: a molehill, an iceberg, or neither? Hepatology. 2008;48:662–9. [DOI] [PubMed] [Google Scholar]
- [41].Nascimbeni F, Aron-Wisnewsky J, Pais R, et al. Statins, antidiabetic medications and liver histology in patients with diabetes with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zachoval R, Gerbes AL, Schwandt P, et al. Short-term effects of statin therapy in patients with hyperlipoproteinemia after liver transplantation: results of a randomized cross-over trial. J Hepatol. 2001;35:86–91. [DOI] [PubMed] [Google Scholar]