Background:
The Amplatzer Amulet (AA) and Watchman devices (WD) are the 2 most frequently used devices for percutaneous LAA closure globally.
Objective:
To evaluate the safety and clinical outcomes associated with these 2 devices in patients undergoing percutaneous LAA closure.
Method:
We systematically searched all electronic databases from inception until February 21, 2023. The primary endpoint was procedure related complications. Secondary endpoints were device related thrombus, stroke, cardiovascular mortality, peri device leak, systemic embolism, and all-cause mortality.
Results:
A total of 3 randomized clinical trials with 2150 patients were included in this meta-analysis. The mean age was 75 and 76 years in the Amplatzer group and in the Watchman group, respectively. The odds of procedure-related complications (OR, 1.80 [95% CI: 1.21–2.67], P < .001) were significantly higher among patients with AA compared to the WD. However, the odds of all-cause mortality (OR, 0.75 (95% CI: 0.49–1.16), P = .20), stroke (OR, 0.79 [95% CI: 0.47–1.34], P = .39), systemic/pulmonary embolism (OR, 1.34 [95% CI: 0.30–6.04], P = .70), and major bleeding (OR, 1.10 [95% CI: 0.83–1.48], P = .50) were comparable between the two devices. The odds of device related thrombus (OR, 0.72 [95% CI: 0.46–1.14], P = .17) was comparable between both the group of patients, however the incidence of peri device leak was significantly lower in AA group (OR, 0.41 [95% CI: 0.26–0.66], P < .001) compared with WD group of patients.
Conclusion:
The AA was not superior to the Watchman device in terms of safety and efficacy. However, the Amulet occluder was associated with a higher incidence of procedure-related complications, and lower peri device leak.
Keywords: Amplatzer Amulet, LAAC, outcomes, watchman device
Highlights.
The Amplatzer Amulet and Watchman devices are the two most frequently used devices for percutaneous LAA closure globally, however with very limited literature and conflicting results till date.
This meta-analysis of randomized controlled trials shows that The Amplatzer Amulet was non-superior to the Watchman device in terms of mortality and safety outcomes. However, the Amulet occluder was associated with a higher incidence of procedure-related complications, and lower peri device leak compared with watchman device.
Further larger trials with longer follow up duration might shed more light towards the devices related outcomes such as mortality, and stroke.
1. Introduction
The LAA is a trabecular pouch that physiologically acts as a decompression system during high left atrial pressures.[1] However, in individuals with atrial fibrillation, this embryonic remnant encourages blood stasis, triggering the coagulation cascade and leading to the formation of thrombus.[2] There is a 5-fold increase in the incidence of thromboembolic events in individuals with non-valvular atrial fibrillation.[2] Treatment with oral anticoagulation mitigates the risk by approximately 30%.[2] Nonetheless, these oral anticoagulants are not without drawbacks, including lifelong administration and increased bleeding risk.[3]
There have been significant evolutions since 1949, when the left auricular appendix was first surgically resected in 2 rheumatic heart disease patients with recurrent thrombi,[4] which in turn inspired the concept of the modern devices used today. Several LAA closure systems developed in recent times include the Amplatzer Cardiac Plug and Amulet devices (St. Jude Medical-Abbott, St. Paul, MN) and the Watchman Left Atrial Appendage Closure Device (Boston Scientific, Marlborough, MA).[5] The LAA closure was devised as a measure to prevent thromboembolic events in selected candidates, including individuals with clearly established oral anticoagulation adherence problems as well as individuals with contraindications to oral anticoagulation. Despite their wide adoption globally, there is limited data comparing the efficacy and outcomes of both devices. Hence, we sought to conduct a meta-analysis to evaluate the clinical outcomes associated with both devices.
2. Methods
This study was carried out in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) 2020 checklist,[6] and was performed according to established methods, as described previously.[7,8]
2.1. Outcomes
The primary endpoint of interest for this meta-analysis was procedure related complications. The secondary endpoints of interest were stroke, systemic/pulmonary embolism, major bleeding, cardiovascular mortality, and all-cause mortality.
2.2. Search strategy
We conducted a systematic literature search across the following databases: PubMed, Embase, and Scopus. Predefined MeSH terms were used by applying the BOOLEAN (“AND” and “OR”) logic. The following search terms were used: “Left atrial appendage closure” AND “Amplatzer Amulet” OR “Amulet device” AND “Watchman device” OR “Watchman FLX” AND “randomized control trials” AND “Outcomes.” The search was performed from inception until February 21, 2023, without any language or date restrictions. All the studies were carefully screened and exported to Endnote 2020 library (Clarivate Analytics, USA). The detailed search strategy has been provided in Table S1, Supplemental Digital Content, http://links.lww.com/MD/J217. Two reviewers (V.J. and S.P.A.) reviewed the studies based on the title and abstract. A third author (A.B.S.) arbitrated discrepancies regarding the inclusion of studies.
2.3. Eligibility criteria
2.3.1. Inclusion criteria.
Studies were sought to be eligible if patients are of ≥18 years of age,
Studies including intervention and control groups where the intervention group employed patients with an Amplatzer Amulet (AA) device, while the placebo/control group comprised patients with a watchman device (WD).
Studies were required to report at least one of the desired outcomes, and
Study designs including randomized controlled trials only.
2.3.2. Exclusion criteria.
Studies on animals, abstracts, editorials, commentaries, systematic reviews, single patient case studies, letters, observational, comparative studies,
Studies with insufficient data were excluded.
Studies, where a single arm was presented without comparators and with non-compliant outcomes, were also excluded.
2.4. Data extraction, quality assessment, and statistical analysis
Data of the eligible selected studies, such as demographic, comorbidities, risk factors, and outcomes of both groups, were extracted into a shared spreadsheet by 2 authors (V.J. and A.B.). Two investigators (V.J. and P.S.) independently appraised the potential risk of bias using Cochrane Collaboration’s tool for assessing the risk of bias in randomized controlled trials.[9] We then classified studies of low, moderate, or high quality based on the scores after evaluation. Baseline continuous variables were summarized as mean (SD), whereas dichotomous variables were described as frequencies or percentages. A conventional, two-arm meta-analysis for primary and secondary outcomes was performed by adopting the Dersimonian and Laird random-effects model for the study variations. Outcomes were reported as pooled odds ratio (OR) and their corresponding 95% confidence interval (95% CI). Statistical significance was met if 95% CI did not cross the numeric “1” and the two-tailed P value was less than .05. The heterogeneity among studies was assessed using the Higgins I-squared (I2) statistical model, with I2 values < 75% considered mild-moderate and ≥ 75% considered high.[10,11] All statistical work, including analyses and graphical illustrations, was conducted using STATA (version 17.0, StataCorp).[12]
3. Results
3.1. Study selection
The preliminary database search using the pre-specified keywords yielded 453 articles, of which 168 studies were excluded after removing duplicates. Two hundred fifty-five studies were further excluded from the initial post title and abstract screening based on the inclusion and exclusion criteria and comparison arm (AA and WD). The full-text review was conducted for the remaining thirty articles identified during the search period for screening. Twenty-seven studies were excluded as they either had unmatching target populations, were not primary research articles or case reports, or lacked a comparison arm. Hence, 3 studies that met the eligibility criteria were included in our study (Table 1).[13–15] The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram is depicted in Figure S1, Supplemental Digital Content, http://links.lww.com/MD/J218.
Table 1.
Baseline characteristic of included studies arranged in form of (AA/WD).
| Variables | Galea et al SWISS-APERO (2021) | Lakkireddy et al Amulet IDE (2021) | Mansour et al (2022) |
|---|---|---|---|
| Study type | RCT | RCT | RCT/prospective |
| Total patient (AA/WD) | 111/110 | 934/934 | 26/25 |
| Inclusion criteria | Nonvalvular AF and clinical indication for LAAC with CHA2DS2Vasc score > 2 and HASBLED score > 3 | Nonvalvular AF and at increased risk of stroke with CHA2DS2Vasc score > 3 | patient who underwent LAAO |
| Male, % | 71.2/70 | 58.8/61.3 | 20/19 |
| Age, yr | 76.5/77.3 | 75/75.1 | 75/76 |
| Arterial HTN, % | 90/87 | NR | 21/17 |
| DM, % | 21.6/30.9 | NR | 5/6 |
| CHD, % | 35.1/37.3 | NR | NR |
| CVD/stroke, % | 40.5/38.2 | 18/19.9 | 12/12 |
| MI | 9/12.7 | 14.6/15.8 | NR |
| HAS-BLED score, mean | 3.1 ± 0.8/3.2 ± 1.0 | 3.2 ± 1.0/3.3 ± 1.0 | 4.1/4.2 |
| CHA2DS2Vasc score, mean ± SD | 4.2 ± 1.4 | 4.5 ± 1.3 | 3.9 |
| 4.4 ± 1.4 | 4.7 ± 1.4 | 3.9 | |
| Procedure success | 96.40/97.30 | 96/94.5 | NR |
| Device-related thrombus, n (%) | 2 (2.1)/5 (5.5) | 30 (3.3)/40 (4.5) | 0 (0)/1 (4) |
| Follow-up (median) | 45 d | 18 mo | 12 mo |
AA = Amplatzer Amulet, HTN = hypertension, WD = watchman device.
3.2. Baseline characteristics of included studies
Three randomized controlled trials with a total of 2150 patients were included in our analysis. The mean age of patients among AA and WD were 75 years and 76 years, respectively. 61% of patients in the AA group were male, while 63% were in the WD group. Past medical history among AA and WD includes myocardial infarction (14% vs 15.46%), previous cerebrovascular events (21% vs 22.42%), Arterial hypertension (78.83% vs 79.25%), Diabetes Mellitus (21.16% vs 29.62%), Chronic Kidney Disease (5.1% vs 5.92%). The mean CHA2DS2-Vasc score (4.2 vs 4.34) and mean HAS-BLED score (3.46 vs 3.56) were comparable between both groups (Table 1).
3.3. Risk of bias assessment
Overall, the included studies had a low risk of bias. The details of the quality assessment are presented in Figure S2, Supplemental Digital Content, http://links.lww.com/MD/J219.
3.4. Meta-analysis of primary and secondary endpoints
The odds of procedure-related complications (OR, 1.80 [95% CI: 1.21–2.67], P < .001) were significantly higher among patients with AA compared to WD with no heterogeneity detected across studies (I2 = 0%) (Fig. 1).
Figure 1.
Forest plot of primary outcome: procedure related complications. CI = confidence intervals, OR = odd ratio.
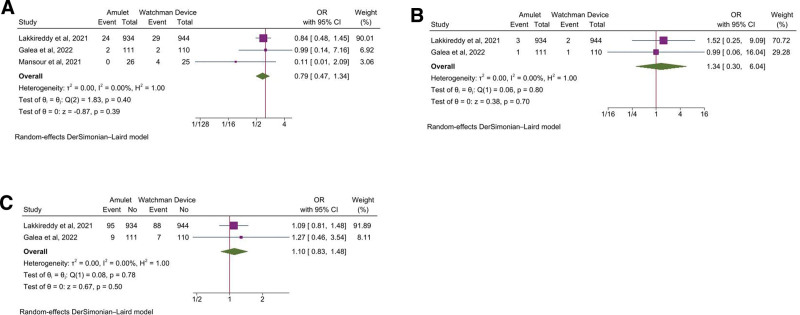
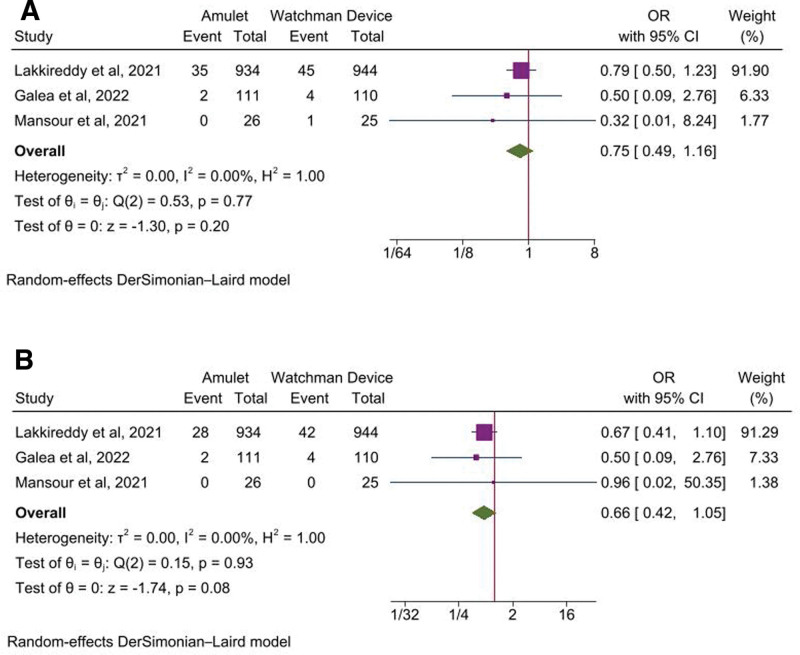
Among the secondary endpoints, the odds of stroke (OR, 0.79 [95% CI: 0.47–1.34], P = .39, I2 = 0%), systemic or pulmonary embolism (OR, 1.34 [95% CI: 0.30–6.04], P = .70, I2 = 0%), major bleeding (OR, 1.10 [95% CI: 0.83–1.48], P = .50, I2 = 0%) (Fig. 2A–C), all-cause mortality (OR, 0.75 [95% CI: 0.49–1.16], P = .20, I2 = 0%), and cardiovascular mortality (OR, 0.66 [95% CI: 0.42–1.05], P = .08, I2 = 0%) were comparable between the 2 devices (Fig. 3A and B).
Figure 2.
Forest plot of secondary outcomes: A) Stroke, B) Systemic/pulmonary embolism, C) Major bleeding. CI = confidence intervals, OR = odd ratio.
Figure 3.
Forest plot of secondary outcomes: A) All-cause mortality, B) Cardiovascular Mortality. CI = confidence intervals, OR = odd ratio.
3.5. Device related complications
The incidence of device related thrombus (OR, 0.72 [95% CI: 0.46–1.14], P = .17, I2 = 0%) was comparable between both the group of patients, however the incidence of peri-device leaks was significantly lower in AA group (OR, 0.41 [95% CI: 0.26–0.66], P < .001) compared with WD group of patients with no heterogeneity detected across studies (I2 = 0%) (Fig. 4A and B).
Figure 4.
Forest plot of secondary outcomes: A) Device related thrombus, B) Peri-device leak. CI = confidence intervals, OR = odd ratio.
4. Discussion
In this systematic review and meta-analysis, the 2 most frequently used and approved LAA closure devices worldwide were compared: the AA left atrial appendage occluder device, and the Watchman left atrial appendage closure device. Overall, it was found that both the devices fared similarly in terms of the endpoints investigated, except that the patients receiving AA devices had higher odds of procedure-related complications and lower incidence of peri-device leaks compared to those receiving the WDs.
Procedural related complications comprises of acute implantation success rate, serious complication, device embolization, air embolization, Thrombus formation, pericardial effusion, procedural related death and access related compilations.[13–15]
In 2015, FDA granted approval to the WD for its demonstration of non-inferiority to anticoagulant therapy in preventing strokes in response to the PROTECT AF and PREVAIL trials.[16–18] A single seal-mechanism of the WD, however was considered incomplete because of the variable and complex structure of the left atrial appendage. Thus, sowing a seed for a dual-seal mechanism device with a lobe and a disc connected by a central waist that could overcome the limitations of anatomical diversity and provide a tight seal in the LAA ostium along with a reduction in the risk of leakage.[13] After the first head-to-head comparison of the AA versus the WD showed the non-inferiority of the Amulet, it gained its popularity. In 2021, FDA approved the use of AA Left Atrial Appendage Occluder for nonvalvular atrial fibrillation patients with a risk of ischemic stroke and not candidates for anticoagulant therapy.[19]
Similarly, a recent analysis studying procedural and short-term follow-up outcomes of Amulet and Watchman FLX, showed a significantly higher incidence of periprocedural complication in the amulet device.[20] The introduction of the second-generation Watchman FLX device has minimized its previous limitations in terms of device stability, safety, and appendage occlusion with 2 rows of anchors, the ability for fully recapture and non-traumatic distal end. In addition, the open architecture with an 18-strut design fits snugly at the left atrial appendage ostium, resulting in the reduction of significant peridevice leakage.[21] This revised design might be the driving force behind the results of this meta-analysis. Among the procedural complications, pericardial effusion and device embolization were common. Such inflictions are mostly incidental, and operator based.[13,22] The experience of the operator performing the LAAC procedure thus plays a pivotal role in preventing procedural complications.[18]
Significantly, a study in canine model of the anatomical impact of devices portrayed more complete endothelization of the WD compared with the amplatzer device following 28 days. This was hypothesized to result from the Amplatzer disk’s extension beyond the LAA orifice margin, which may alert surrounding structures and delay healing.[23] Nevertheless, the overall efficacy of the device in stroke prevention wasn’t hampered and subsequent studies of the effect of peridevice leakage due to incomplete LAA closure found no significant increase in long-term adverse effects.[24]
4.1. Strength and limitation
The strength of this paper lies in compilation of studies with head-to-head comparison of amulet and WDs till date. Only randomized controlled trials were included to bolster the current evidence and minimize the risk of bias. In terms of limitations, we found that some trials included both the 1st generation watchman (Watchman 2.5) as well as 2nd generation WD (Watchman FLX), which might temper the results like significant lower incidence of peridevice leak in AA compared to WDs.[14] Nevertheless, a recent study by Fukuda et al delineated no significant differences in the 2 devices in terms of periprocedural complications.[25] In addition, while acknowledging the limited number of RCTs and participants, results of meta-analysis had minimal-to-low heterogeneity across studies, further strengthening the robustness of results. Nevertheless, further large, randomized trials should be encouraged and aimed at studying the use of these LAA devices in order to provide more conclusive evidence.
5. Conclusion
The AA was not superior to the Watchman device in terms of safety and efficacy. However, the Amulet occluder was associated with a higher incidence of procedure-related complications, and lower peri device leak. Further large-randomized trials are warranted to support these findings at longer follow up.
Author contributions
Conceptualization: Vikash Jaiswal, Monodeep Biswas.
Data curation: Vikash Jaiswal, Helen Huang.
Formal analysis: Vikash Jaiswal, Song Peng Ang.
Investigation: Vikash Jaiswal, Song Peng Ang, Helen Huang, Mohammed Ghanim.
Methodology: Vikash Jaiswal.
Project administration: Vikash Jaiswal, Mohammed Ghanim.
Resources: Mohammed Ghanim.
Software: Helen Huang.
Supervision: Vikash Jaiswal, M Chadi Alraies, Monodeep Biswas.
Validation: Vikash Jaiswal, Abhigan babu Shrestha, Zarghoona Wajid, Evbayekha Osas Endurance, David Song, M Chadi Alraies, Monodeep Biswas.
Visualization: Vikash Jaiswal, Abhigan babu Shrestha, Mohammed Ghanim, David Song, M Chadi Alraies, Monodeep Biswas.
Writing – original draft: Vikash Jaiswal, Abhigan babu Shrestha, Zarghoona Wajid, Evbayekha Osas Endurance, Fathima Shehnaz Ayoobkhan, Shazia Khan, Vamsi Garimellla, M Chadi Alraies, Monodeep Biswas.
Writing – review & editing: Vikash Jaiswal, Song Peng Ang, Abhigan babu Shrestha, Zarghoona Wajid, Evbayekha Osas Endurance, Shazia Khan, Vamsi Garimellla, Helen Huang, Mohammed Ghanim, David Song, Prachi Sharma, M Chadi Alraies, Monodeep Biswas.
Supplementary Material
Abbreviations:
- AA
- Amplatzer Amulet
- WD
- watchman device
As this is a review article whose data has been taken from a published article, ethical approval is not required.
The abstract of this study has been presented in ESC Asia 2022 conference and has been published in European Heart Journal supplement.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
Supplemental Digital Content is available for this article.
How to cite this article: Jaiswal V, Ang SP, Shrestha AB, Wajid Z, Endurance EO, Ayoobkhan FS, Khan S, Garimellla V, Huang H, Ghanim M, Song D, Sharma P, Alraies MC, Biswas M. Amplatzer amulet versus watchman device for percutaneous left atrial appendage closure: A systematic review and meta-analysis. Medicine 2023;102:26(e34185).
Contributor Information
Vikash Jaiswal, Email: vikash29jaxy@gmail.com.
Song Peng Ang, Email: hestonang23@gmail.com.
Zarghoona Wajid, Email: hj2051@wayne.edu.
Evbayekha Osas Endurance, Email: greatomri@gmail.com.
Fathima Shehnaz Ayoobkhan, Email: fshehnaz22@gmail.com.
Shazia Khan, Email: shaziakhan2306@gmail.com.
Vamsi Garimellla, Email: vcg24@med.miami.edu.
Helen Huang, Email: HelenHuang@rcsi.ie.
David Song, Email: xdavidsong@gmail.com.
Prachi Sharma, Email: drprachi1009@gmail.com.
M. Chadi Alraies, Email: alraies@hotmail.com.
Monodeep Biswas, Email: mbiswasmd@gmail.com.
References
- [1].Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82:547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67. [DOI] [PubMed] [Google Scholar]
- [3].Romero J, Perez IE, Krumerman A, et al. Left atrial appendage closure devices. Clin Med Insights Cardiol. 2014;8:45–52. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4064949/ [access date July 22, 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Madden JL. Resection of the left auricular appendix: a prophylaxis for recurrent arterial emboli. J Am Med Assoc. 1949;140:769–72. Available at: 10.1001/jama.1949.02900440011003 [access date July 22, 2022]. [DOI] [PubMed] [Google Scholar]
- [5].Holmes DR, Schwartz RS, Latus GG, et al. A history of left atrial appendage occlusion. Interv Cardiol Clin. 2018;7:143–50. [DOI] [PubMed] [Google Scholar]
- [6].Page MJ, McKenzie JE, Bossuyt PM, et al. 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jaiswal V, Ang SP, Ishak A, et al. Comparison of outcome among type 2 vs type 1 myocardial infarction: a systematic review and meta-analysis. J Invest Med. 2023;71:223–34. [DOI] [PubMed] [Google Scholar]
- [8].Jaiswal V, Ishak A, Peng Ang S, et al. Hypovitaminosis D and cardiovascular outcomes: a systematic review and meta-analysis. Int J Cardiol Heart Vasc. 2022;40:101019. Available at: https://www.sciencedirect.com/science/article/pii/S2352906722000689 [access date July 22, 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Higgins JPT, Altman DG, Gøtzsche PC, et al.; Cochrane Bias Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. Available at: https://www.bmj.com/content/343/bmj.d5928 [access date July 22, 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Borenstein M, Hedges LV, Higgins JPT, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1002/jrsm.12 [access date July 22, 2022]. [DOI] [PubMed] [Google Scholar]
- [11].Anon. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Wiley. Wiley.com. Available at: https://www.wiley.com/en-us/Cochrane+Handbook+for+Systematic+Reviews+of+Interventions%2C+2nd+Edition-p-9781119536628 [access date July 22, 2022]. [Google Scholar]
- [12].StataCorp. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC; 2021. [Google Scholar]
- [13].Lakkireddy D, Thaler D, Ellis CR, et al. Amplatzer Amulet left atrial appendage occluder versus watchman device for stroke prophylaxis (Amulet IDE): a randomized, controlled trial. Circulation. 2021;144:1543–52. Available at: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.121.057063 [access date July 4, 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Galea R, De Marco F, Meneveau N, et al. Amulet or watchman device for percutaneous left atrial appendage closure: primary results of the SWISS-APERO randomized clinical trial. Circulation. 2022;145:724–38. Available at: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.121.057859 [access date July 22, 2022]. [DOI] [PubMed] [Google Scholar]
- [15].Mansour MJ, Harnay E, Al Ayouby A, et al. One year outcome and analysis of peri-device leak of left atrial appendage occlusion devices. J Interv Card Electrophysiol. 2022;64:27–34. [DOI] [PubMed] [Google Scholar]
- [16].Holmes DR, Kar S, Price MJ, et al. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- [17].Holmes DR, Reddy VY, Turi ZG, et al.; PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–42. [DOI] [PubMed] [Google Scholar]
- [18].Reddy VY, Gibson DN, Kar S, et al.; Post-Approval U.S. Experience with left atrial appendage closure for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2017;69:253–61. [DOI] [PubMed] [Google Scholar]
- [19].Health C for D and R. Amplatzer Amulet Left Atrial Appendage Occluder – P200049. FDA 2021. Available at: https://www.fda.gov/medical-devices/recently-approved-devices/amplatzer-amulet-left-atrial-appendage-occluder-p200049 [access date July 4, 2022].
- [20].Della Rocca DG, Magnocavallo M, Gianni C, et al. Procedural and short-term follow-up outcomes of Amplatzer Amulet occluder versus Watchman FLX device: a meta-analysis. Heart Rhythm. 2022;19:1017–8. [DOI] [PubMed] [Google Scholar]
- [21].Kar S, Doshi SK, Sadhu A, et al. Primary outcome evaluation of a next-generation left atrial appendage closure device. Circulation. 2021;143:1754–62. Available at: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.120.050117 [access date July 4, 2022]. [DOI] [PubMed] [Google Scholar]
- [22].Thakkar J, Vasdeki D, Tzikas A, et al. Incidence, prevention, and management of periprocedural complications of left atrial appendage occlusion. Interventional Cardiol Clin. 2018;7:243–52. Available at: https://linkinghub.elsevier.com/retrieve/pii/S2211745817301657 [access date July 4, 2022]. [DOI] [PubMed] [Google Scholar]
- [23].Kar S, Hou D, Jones R, et al. Impact of Watchman and Amplatzer devices on left atrial appendage adjacent structures and healing response in a canine model. JACC Cardiovasc Interv. 2014;7:801–9. [DOI] [PubMed] [Google Scholar]
- [24].Viles-Gonzalez JF, Kar S, Douglas P, et al. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol. 2012;59:923–9. [DOI] [PubMed] [Google Scholar]
- [25].Fukuda N, Imamura T, Tanaka S, et al. Comparison in short-term safety and efficacy between new-generation WATCHMAN FLX and conventional WATCHMAN 2.5 for percutaneous left atrial appendage closure. J Clin Med. 2022;11:1618. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8949425/ [access date July 4, 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]