Introduction
Hirschsprung disease (HD) is a congenital disorder of intestinal motility characterized by the absence of enteric neurons at the end of the bowel [1]. HD affects 1 in 5,000 live-born infants and 90% of patients are diagnosed within the first year of life [2]. Adult HD patients present with symptoms that begin in adulthood or childhood due to failure of diagnosis [1, 3]. Patients with inflammatory bowel disease (IBD) are considered to be more susceptible to HD in infancy than those without IBD, with a relative risk ranging from 24 to 40 times higher [4]. However, the rare co-morbidity of IBD and adult HD presenting after childhood is prone to missed diagnosis. Here, we report a case in which a patient was diagnosed with stricturing Crohn’s disease (CD) at the first visit, and pathological examination after surgery revealed adult HD complicated by CD.
Case report
In January 2020, a 29-year-old male presented to the gastroenterology department of Xiangya Hospital with diarrhea five or six times per day with watery or bloody stools, abdominal pain, bloating, nausea, and vomiting since 1 month prior. He was diagnosed with colonic CD at 21 years old due to severe diarrhea with watery or bloody stools, abdominal pain, and an anal fistula. To control inflammation, he was initially treated with prednisone 40 mg/day by mouth to induce remission and then with mesalamine 2 g/day for maintenance. The patient did not accept advanced therapy for individual reasons, although his symptoms subsequently recurred. Laboratory testing revealed an increased white blood cell count (10.1 × 109/L) and procalcitonin level (2.69 ng/mL). Stool examination showed 15–20 white blood cells and 15–20 red blood cells per high-power field, without pathogens. The levels of inflammatory markers were highly elevated, with a C-reactive protein level of 47.3 mg/L, an erythrocyte sedimentation rate of 65 mm/h, a tumor necrosis factor-α level of 10.6 pg/mL, an interleukin-6 level of 41.1 pg/mL, and a D-dimer level of 2.31 mg/L. An Epstein‒Barr virus DNA level of 526.1 IU/mL (whole-blood specimens) was shown and the T-SPOT. Tuberculosis test was positive (the spot numbers of the ESAT-6 and CFP-10 wells were both >50). Tests to detect Cytomegalovirus DNA, hepatitis B virus surface antigen, hepatitis C virus antibody, and Clostridium difficile (glutamate dehydrogenase and tcdA/B) were all negative. Colonoscopy showed longitudinal ulcers, a cobblestone pattern in the mucosa, and a sigmoid colon stricture (Figure 1A). Computed tomography enterography (CTE) showed uneven segmented colonic wall thickening from the transverse colon to the rectum and a suspicious jejunal–sigmoid fistula. Considering the past medical history of CD, clinical symptoms, and laboratory examinations, the patient was diagnosed with stricturing CD, secondary intestinal fistula, intestinal infection, and latent tuberculosis infection (tuberculosis lesions were not found). He was treated with antibiotics (levofloxacin 0.5 g/day and metronidazole 1 g/day) and enteral nutrition by tube feeding. Considering the subsequent need for biologics and immunosuppressants, the patient was given anti-tuberculosis treatment (isoniazid, rifampin, and ethambutol) for 3 months.
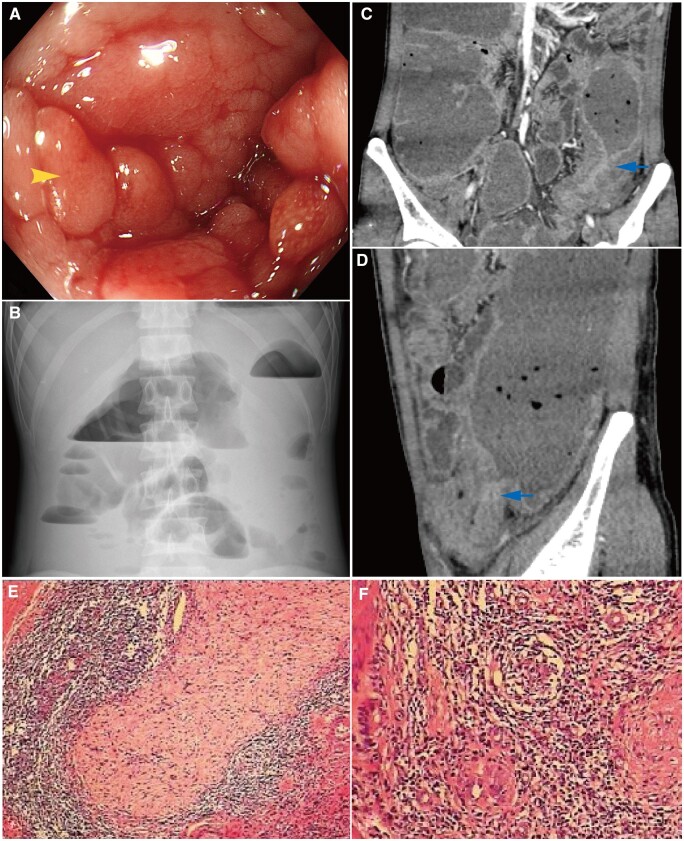
Figure 1.
Endoscopic, imaging, and histopathologic findings of the patient. (A) Longitudinal ulcers, the cobblestone pattern (the arrow) in the mucosa, and the sigmoid colon stricture (colonoscopy). (B) Intestinal obstruction (plain abdominal radiograph). (C) and (D) The location of the intestinal obstruction was proximal to the sigmoid colon (arrows) with intestinal wall thickness, luminal narrowing, and prestenotic dilation (computed tomography enterography). (E) The deficiency of myenteric and submucosal neurons (pathological examination). (F) Inflammatory cell infiltration and noncaseating granuloma (pathological examination).
In April 2020, this patient was offered emergency admission to our hospital for nausea, retching, vomiting, and the cessation of defecation and passing gas after eating an apple 1 week prior. A physical examination revealed full abdominal wall resistance, abdominal tenderness, and rebound tenderness. Plain abdominal radiograph and CTE showed an intestinal obstruction proximal to the sigmoid colon with acute peritonitis (Figure 1B–D). This man was newly diagnosed with complete intestinal obstruction, consistently with the surgical indications for subtotal colectomy. During the explorative laparotomy, surgeons reported that the colon was extensively affected with segmented strictures and a “sausage-like” appearance from the ascending colon at ∼20 cm from the ileocecal valve to the sigmoid colon. Post-operative pathological examination showed a deficiency of myenteric and submucosal neurons in the rectum and sigmoid colon, supporting the diagnosis of HD (Figure 1E). In addition, the diffuse inflammatory cell infiltration and noncaseating granuloma supported the diagnosis of CD (Figure 1F). After asking for the medical history again, it was determined that the patient had suffered from long-term recurrent constipation, bloating, and rectal tenesmus beginning at 18 years old. He reported feeding difficulty during childhood but denied a delayed first meconium. His father, mother, and grandmother all had long-term constipation, but the family history of HD and IBD was noncontributory. Given the medical history, clinical presentations, and post-operative pathology, the patient was ultimately diagnosed with adult HD complicated with CD. This patient refused to receive biologics treatment and discontinued the anti-tuberculosis treatment. After surgery, he was treated with metronidazole and long-term mesalamine 2 g/day. During follow-up, the patient’s diarrhea, abdominal pain, and constipation symptoms were relieved, and his body weight increased by 10 kg in the 2 years.
Discussion
Adult HD is defined as HD diagnosed after 10 years old that manifests as prolonged and severe constipation. Laxatives are effective for relieving symptoms temporarily, leading to the masking of symptoms [1]. The age at diagnosis of adult HD ranges from 10 to 74 years old and the average age is ∼20 years old. The male-to-female ratio is 3–5:1 [1, 5]. Almost all adult HD patients report lifelong symptoms or symptoms from early infancy, including constipation, abdominal pain, and abdominal distention [3, 6], while adult HD presenting after childhood is extremely rare [5, 7]. In this case, the patient had constipation, bloating, and rectal tenesmus beginning at 18 years old and was ultimately diagnosed with HD at 29 years old.
Stricturing CD is one of the important clinical types of CD, involving intestinal wall thickening, luminal narrowing, prestenotic dilation, and even intestinal obstruction and perforation [8]. Stricturing CD patients should be alerted to the presence of HD when they have prolonged and severe constipation, bloating, and difficulty in defecation even if the symptoms do not begin in the neonatal period. Epidemical data indicate that individuals diagnosed with HD also experience a higher incidence rate of IBD, especially CD [4]. Genetic factors, gut microbiota alteration, and epithelial barrier regulation of the enteric nervous system and enteric glial cells are suggested in the common pathogenesis of HD and IBD [9].
There is still a question of whether IBD diagnosed post-HD has the same disease process as IBD without HD, although IBD patients with HD also successful react to classic anti-inflammatory medicines, especially biological medicines [10].
Conclusion
Stricturing CD patients with long-term severe constipation and difficulty in defecating, whether from childhood or adulthood, need to be vigilant about the co-morbidity of adult HD.
Authors’ Contributions
All authors contributed to the information collection of this case. The first draft of the manuscript was written by Y.C.; S.C. and X.L. were responsible for critical revision. All authors commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Acknowledgements
The authors would like to thank the collaboration of the Departments of Gastroenterology, Gastrointestinal Surgery, and Pathology.
Contributor Information
Yiqian Chen, Department of Gastroenterology, Xiangya Hospital, Central South University, Changsha, Hunan, P. R. China.
Xiaowei Liu, Department of Gastroenterology, Xiangya Hospital, Central South University, Changsha, Hunan, P. R. China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan, P. R. China.
Shuijiao Chen, Department of Gastroenterology, Xiangya Hospital, Central South University, Changsha, Hunan, P. R. China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan, P. R. China.
Funding
This work was supported by the Natural Science Foundation of Hunan Province [grant number 2022JJ30916], the National Natural Science Foundation of China [grant number 82270564], and the China Postdoctoral Science Foundation [grant number 2022M713521].
Conflict of Interest
None declared.
References
- 1. Doodnath R, Puri P.. A systematic review and meta-analysis of Hirschsprung’s disease presenting after childhood. Pediatr Surg Int 2010;26:1107–10. [DOI] [PubMed] [Google Scholar]
- 2. Nasr A, Sullivan KJ, Chan EW. et al. Validation of algorithms to determine incidence of Hirschsprung disease in Ontario, Canada: a population-based study using health administrative data. Clin Epidemiol 2017;9:579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miyamoto M, Egami K, Maeda S. et al. Hirschsprung’s disease in adults: report of a case and review of the literature. J Nippon Med Sch 2005;72:113–20. [DOI] [PubMed] [Google Scholar]
- 4. Bernstein CN, Kuenzig ME, Coward S. et al. Increased incidence of inflammatory bowel disease after Hirschsprung disease: a population-based cohort study. J Pediatr 2021;233:98–104.e102. [DOI] [PubMed] [Google Scholar]
- 5. Lesser PB, El-Nahas AM, Lukl P. et al. Adult-onset Hirschsprung’s disease. JAMA 1979;242:747–8. [PubMed] [Google Scholar]
- 6. Crocker NL, Messmer JM.. Adult Hirschsprung's disease. Clin Radiol 1991;44:257–9. [DOI] [PubMed] [Google Scholar]
- 7. Ponka JL, Grodsinsky C, Brush BE.. Megacolon in teen-aged and adult patients. Dis Colon Rectum 1972;15:14–22. [DOI] [PubMed] [Google Scholar]
- 8. Lee KE, Cantrell S, Shen B. et al. Post-operative prevention and monitoring of Crohn's disease recurrence. Gastroenterol Rep (Oxf) 2022;10:goac070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heuckeroth RO. Hirschsprung disease: integrating basic science and clinical medicine to improve outcomes. Nat Rev Gastroenterol Hepatol 2018;15:152–67. [DOI] [PubMed] [Google Scholar]
- 10. Sutthatarn P, Lapidus-Krol E, Smith C. et al. Hirschsprung-associated inflammatory bowel disease: a multicenter study from the APSA Hirschsprung disease interest group. J Pediatr Surg 2023;58:856–61. [DOI] [PubMed] [Google Scholar]