Abstract
In the context of hypertrophic cardiomyopathy (HCM), a ventricular septal defect (VSD) is a rare finding. We present the case of a large spontaneously closed muscular VSD in a patient with HCM. We describe the role of cardiovascular magnetic resonance in the assessment of a VSD and its differential diagnosis in HCM. (Level of Difficulty: Advanced.)
Key Words: cardiovascular magnetic resonance, hypertrophic cardiomyopathy, ventricular septal defect
Central Illustration
History of Presentation
A 57-year-old man with a history of hypertrophic cardiomyopathy (HCM) was referred to our department for follow-up cardiovascular magnetic resonance (CMR). The patient was initially diagnosed with HCM at 41 years of age. A genetic work-up identified an MYBPC3 mutation (c.2429G>A, p.[Arg810His], interpreted as likely pathogenic) as the underlying cause of his cardiomyopathy. In early childhood, he was also diagnosed with a muscular ventricular septal defect (VSD), which was never considered for surgical treatment, as intermittent echocardiographic follow-up studies failed to show a significant interventricular shunt. Previous CMR studies at another center identified a muscular defect in the ventricular septum that was interpreted as a deep crypt in the context of the already diagnosed HCM. Except for previous episodes of atrial fibrillation, the patient was asymptomatic from a cardiac perspective and used to play squash 3 or 4 times a week.
Learning Objectives
-
•
To evaluate the different mechanisms of spontaneous closure of a muscular VSD in an adult patient.
-
•
To differentiate the various causes of myocardial defects commonly observed in patients with HCM.
-
•
To obtain flow imaging by CMR to assess net shunt across the VSD in a patient with HCM.
Medical History
At 49 years of age, the patient was diagnosed with a leiomyosarcoma of the descending aorta that required total surgical resection and radiotherapy. He developed no recurrence thereafter.
Differential Diagnosis
As a differential diagnosis, myocardial invaginations such as crypts, clefts, crevices, and recesses have been commonly reported in patients with HCM.1, 2, 3 Although they might sometimes bear a certain similarity, they are distinctively different entities compared with a closed muscular VSD. Per definition, crypts never have, compared with a VSD, a transmural opening into the right ventricle (RV). This patient’s historic echocardiographic reports, from childhood, long before the onset of HCM, indeed described the presence of a muscular VSD.
Management
The patient remained under oral anticoagulation for atrial fibrillation.
Investigations
A CMR scan was performed USING a 3-T Vida scanner (Siemens) with a standardized protocol.4 The left ventricular (LV) cavity was small, with a low normal ejection fraction (59%) and systolic obliteration of the mid and apical ventricular cavity. There were no regional wall motion abnormalities at rest. There was asymmetrical hypertrophy of the basal to mid septum (basal anteroseptum, 20 mm; mid inferoseptum, 17 mm vs 8 mm lateral wall at the same level), mid anterior wall (17 mm), and apical segments (11-14 mm; LV mass index 77 g/m2). No mitral systolic anterior motion or LV outflow tract obstruction was detected at rest. As additional findings, there were 2 deep and broad myocardial crypts in the basal inferior wall (Figure 1) and hypertrophic papillary muscles. The RV had normal volumes and ejection fraction. In addition, there was a patent large (16 × 14 mm) muscular VSD in the basal and mid septum, which had been spontaneously closed by an unusual mechanism. The VSD was isolated from the RV by a fibromuscular band on the RV side attached to the basal and apical septum (Videos 1 and 2), and there was therefore no net systemic-to-pulmonary shunt.
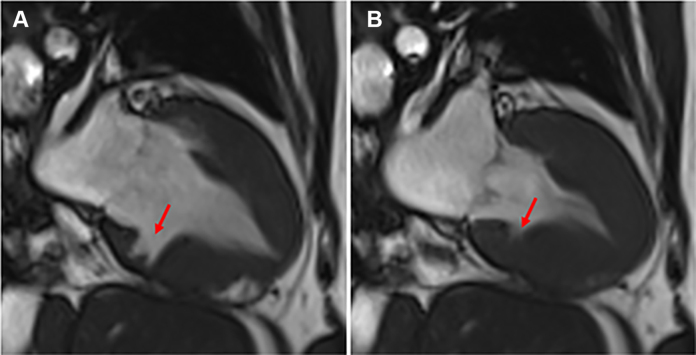
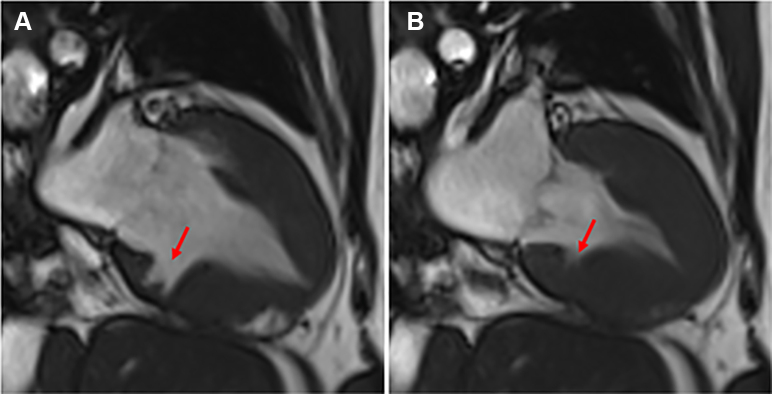
Figure 1.
Inferior Crypt in the Left Ventricular Myocardium
Cardiovascular magnetic resonance steady-state free precession 2-chamber view demonstrating increased left ventricular wall thickness with deep and broad myocardial crypts in the basal inferior wall in diastole (A) and systole (B).
CMR phase-contrast flow mapping showed a transit flow from the LV through the VSD in systole and returning to the LV from the VSD in diastole (Figures 2 and 3). There was no evidence of shunt, as confirmed by a Qp/Qs ratio of 1:1. Qp/Qs ratio was calculated as the ratio between the through-plane flows in the main pulmonary artery (Qp) and in the ascending aorta (Qs). Additionally, serial transthoracic echocardiography was unable to identify a net shunt. There was no relevant valvular pathology or other associated congenital heart defects. The gadolinium study showed extensive patchy midwall enhancement of the hypertrophied segments (Figure 4), in keeping with HCM.
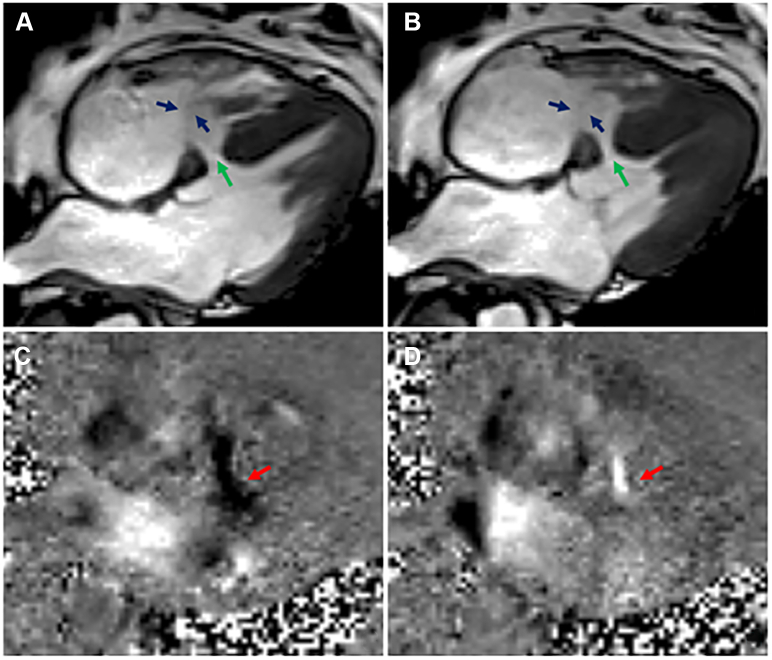
Figure 2.
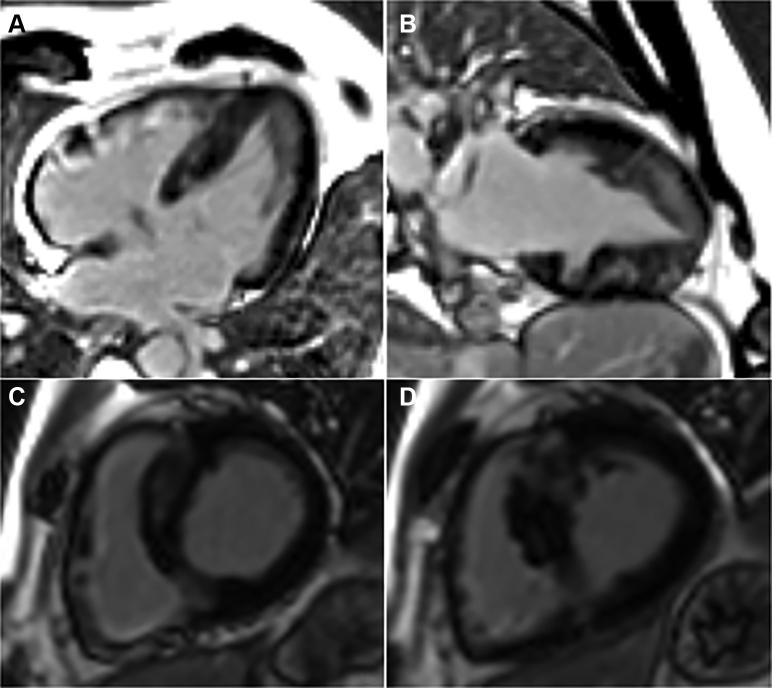
Large Muscular VSD With Persistent Flow
Cardiovascular magnetic resonance (CMR) steady-state free precession 4-chamber view in diastole (A) and systole (B) showing a large (16 × 14 mm) muscular ventricular septal defect (green arrows) in the basal and mid septum, which has been spontaneously closed by a fibromuscular band on the right ventricular side (blue arrows) attached to the basal and apical septum. CMR phase-contrast 4-chamber views showing transit flow (red arrows), forward (C) and backward (D), across the muscular interventricular septal defect (black arrows indicate the direction of flow on the image).
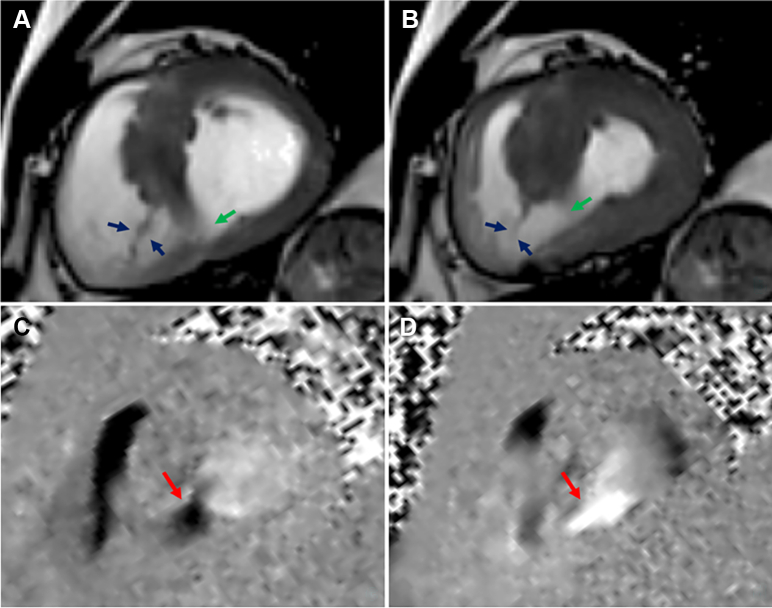
Figure 3.
Large Muscular VSD With Flow Imaging in Short Axis
Cardiovascular magnetic resonance (CMR) steady-state free precession short-axis view in diastole (A) and systole (B) showing a large (16 × 14 mm) muscular ventricular septal defect (green arrows) in the basal and mid septum, which has been spontaneously closed by a fibromuscular band on the right ventricular side (blue arrows) attached to the basal and apical septum. CMR phase-contrast short-axis views showing transit flow (red arrows), forward (C) and backward (D), across the muscular interventricular septal defect (black arrows indicating the direction of flow on the image).
Figure 4.
Late Gadolinium Enhancement Imaging
Cardiovascular magnetic resonance late gadolinium enhancement images in 4-chamber (A), 2-chamber (B), and short-axis (C, D) views demonstrating extensive patchy midwall enhancement of the hypertrophied segments.
Discussion
Overall, a muscular VSD in the presence of HCM is a rare finding in adult patients.5,6 The mechanism of this spontaneously closed and isolated muscular VSD is unusual. Generally, muscular VSDs are believed to be more likely to spontaneously close compared with perimembranous VSDs, especially if they are small in size (<5 or 6 mm).7 This most commonly occurs within the first year of life or soon after.8,9 The usual mechanism of spontaneous muscular VSD closure is muscular encroachment and superimposed fibrous tissue enclosing the whole defect or hypertrophy of the septal myocardium. In contrast, spontaneous closure of a perimembranous VSD most commonly occurs by fibrous proliferation, followed by adhesion of the septal leaflet of the tricuspid valve to the margins of the defect, with possible formation on the RV aspect of the defect of an aneurysm-like pouch that could be mistaken for an aneurysm of the membranous septum.7, 8, 9
In the present case, we hypothesize that part of the tricuspid valve apparatus, with the hypertrophied RV trabeculations, spontaneously closed the right side of the large muscular VSD, which may be related to the HCM-caused hypertrophy. The large muscular septal defect seemed to be covered by tissue incorporating the septal tricuspid valve leaflet, with associated papillary muscle and chordae, and the hypertrophied RV trabeculations (Figures 2 and 3, blue arrows). In this patient, the muscular VSD remained open from the LV, and there was transit flow (forward and backward) across the VSD without communication with the RV cavity and therefore no net shunting.
Follow-Up
The patient remains asymptomatic and displays no signs worsening exercise capacity or reduced LV function.
Conclusions
In summary, this case illustrates the spontaneous closure of a muscular VSD that displays similarities to the spontaneous closure normally seen only in perimembranous VSDs. Unique to this case is not only the site of the defect but also the various tissues involved in the spontaneous closure as well as the bidirectional flow without a net shunt. This report highlights the important role of comprehensive CMR imaging in patients with HCM, especially the role of flow imaging, which should be included in the assessment if any doubts exist about a possible shunt in a patient with known HCM.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
James L. Januzzi, Jr, MD, served as the Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Cardiovascular Magnetic Resonance Steady-State Free Precession Cine in 4-Chamber View Showing a Large Muscular Ventricular Septal Defect in the Basal and Mid Septum, Which Has Been Spontaneously Closed by a Fibromuscular Band on the Right Ventricular Side Attached to the Basal and Apical Septum
Cardiovascular Magnetic Resonance Steady-State Free Precession Cine in Short-Axis View Showing a Large Muscular Ventricular Septal Defect in the Basal and Mid Septum, Which Has Been Spontaneously Closed by a Fibromuscular Band on the Right Ventricular Side Attached to the Basal and Apical Septum
References
- 1.Cresti A., Cannarile P., Aldi E., et al. Multimodality imaging and clinical significance of congenital ventricular outpouchings: Recesses, diverticula, aneurysms, clefts, and crypts. J Cardiovasc Echogr. 2018;28(1):9–17. doi: 10.4103/jcecho.jcecho_72_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basso C., Marra M.P., Thiene G. Myocardial clefts, crypts, or crevices. Circ Cardiovasc Imag. 2014;7:217–219. doi: 10.1161/CIRCIMAGING.114.001744. [DOI] [PubMed] [Google Scholar]
- 3.Johansson B., Maceira A.M., Babu-Narayan S.V., Moon J.C., Pennell D.J., Kilner P.J. Clefts can be seen in the basal inferior wall of the left ventricle and the interventricular septum in healthy volunteers as well as patients by cardiovascular magnetic resonance. J Am Coll Cardiol. 2007;50(13):1294–1295. doi: 10.1016/j.jacc.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Kramer C.M., Barkhausen J., Bucciarelli-Ducci C., et al. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson. 2020;22(1):17. doi: 10.1186/s12968-020-00607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy S.T., Filho A.T.P., Williams R.B., Biederman R.W.W. Simultaneous hypertrophic cardiomyopathy and muscular ventricular septal defect in an adult patient. Echocardiography. 2012;29(5):E110–E111. doi: 10.1111/j.1540-8175.2011.01632.x. [DOI] [PubMed] [Google Scholar]
- 6.Zheng G., Bai J., Tang J., et al. A case of hypertrophic cardiomyopathy combined with muscular ventricular septal defect and abnormal origin of right coronary artery. BMC Cardiovasc Disord. 2019;19:16. doi: 10.1186/s12872-018-0997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins G., Disenhouse R., Keith J.D. Spontaneous closure of ventricular septal defect. Can Med Assoc J. 1969;100(16):737–743. [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X., Jinxiang L., Jinghua W., Min L., Hui X., Sirui Y. Factors influencing the spontaneous closure of ventricular septal defect in infants. Int J Clin Exp Pathol. 2015;8(5):5614–5623. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J., Jong M.K., Guileyardo J.M., Roberts W.C. A review of spontaneous closure of ventricular septal defect. Proc (Bayl Univ Med Cent) 2015;28(4):516–520. doi: 10.1080/08998280.2015.11929329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cardiovascular Magnetic Resonance Steady-State Free Precession Cine in 4-Chamber View Showing a Large Muscular Ventricular Septal Defect in the Basal and Mid Septum, Which Has Been Spontaneously Closed by a Fibromuscular Band on the Right Ventricular Side Attached to the Basal and Apical Septum
Cardiovascular Magnetic Resonance Steady-State Free Precession Cine in Short-Axis View Showing a Large Muscular Ventricular Septal Defect in the Basal and Mid Septum, Which Has Been Spontaneously Closed by a Fibromuscular Band on the Right Ventricular Side Attached to the Basal and Apical Septum