Abstract
Left ventricular outflow tract obstruction represents a life-threatening complication in patients undergoing transcatheter mitral valve replacement. Interventional approaches to prevent left ventricular outflow tract obstruction are complex and require exceptional procedural expertise. We demonstrate feasibility and safety of first-in-human device-mediated mechanical laceration of the anterior mitral valve leaflet prior to transapical transcatheter mitral valve replacement. (Level of Difficulty: Advanced.)
Key Words: leaflet laceration, LVOT obstruction, transcatheter mitral valve replacement
Central Illustration
Transcatheter mitral valve replacement (TMVR) using dedicated devices is an emerging option for patients with mitral regurgitation (MR).1 However, widespread adoption of TMVR has been hindered by high rates of screening failures, primarily due to the risk for significant left ventricular outflow tract obstruction (LVOT-O).2 Techniques to prevent LVOT-O targeting the septal myocardium or anterior mitral valve leaflet (AML) are technically demanding, time consuming, or associated with relevant additional risk.3
Learning Objectives
-
•
To apprehend advantages and disadvantages of techniques to prevent LVOT-O in patients undergoing TMVR.
-
•
To understand the potential role of device-mediated mechanical laceration of the AML as a safe and effective alternative to prevent LVOT-O in at-risk patients undergoing TMVR.
The aim of this first-in-human case report is to demonstrate the safe and effective use of a dedicated transcatheter leaflet-splitting device to lacerate the AML, thereby preventing LVOT-O in a patient at risk with severe primary MR undergoing TMVR.
History of Presentation
An 80-year-old female patient was repeatedly admitted to the emergency department for acute heart failure with peripheral edema and NYHA functional class III and IV dyspnea. Transthoracic echocardiography (TTE) revealed severe primary MR, concomitant severe secondary tricuspid regurgitation, and elevated pulmonary artery pressure. Biventricular function was preserved. Transesophageal echocardiography (TEE) revealed eccentric MR, likely due to postendocarditic destruction of the posterior mitral valve leaflet (PML) (Video 1) and demonstrated circumferential mitral annular calcification extending into the PML. Detailed echocardiographic baseline parameters are summarized in Table 1.
Table 1.
Echocardiographic Baseline Parameters
| LV ejection fraction, % | 64.1 |
| Stroke volume index, mL/m2 | 40.8 |
| Interventricular septal thickness, mm | 13.6 |
| LVEDV, mL | 136.9 |
| LVESV, mL | 49.1 |
| LA volume, mL | 122.4 |
| EROA, cm2 | 0.90 |
| Regurgitant volume, mL | 107.6 |
| PASP, mm Hg | 68.0 |
| TAPSE, mm | 19.0 |
EROA = effective regurgitant orifice area; LA = left atrial; LV = left ventricular; LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume; PASP = pulmonary artery systolic pressure; TAPSE = tricuspid annular plane systolic excursion.
Past Medical History
The patient had a history of breast cancer and granulomatosis with polyangiitis affecting the kidneys, joints, and paranasal sinus. In addition, she was on dialysis for renal failure related to granulomatosis with polyangiitis and had persistent atrial fibrillation. A previous diagnosis of mitral valve endocarditis had been managed with antibiotic therapy 3 years previously.
Differential Diagnosis
Echocardiography strongly supported the hypothesis of severe primary MR as a leading cause of recurrent congestive heart failure admissions. TEE did not show vegetations suggestive of infective endocarditis, and blood cultures were negative.
Investigations
After heart team assessment, indication for treatment was confirmed, and the patient was considered at prohibitive risk for open heart surgery. Moreover, mitral valve anatomy, in particular pronounced leaflet calcification and postendocarditis destruction of the PML (Video 2), made this patient unsuitable for transcatheter edge-to-edge repair, with a low likelihood of effective treatment. As a result, the patient was screened for the transapical tether-based Tendyne TMVR system (Abbott Structural Heart).
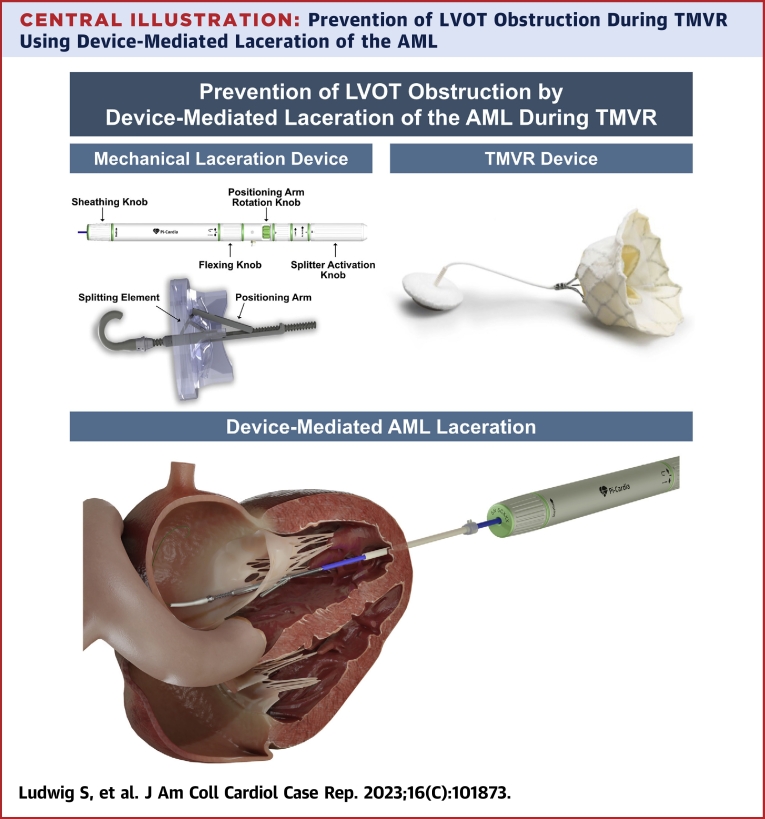
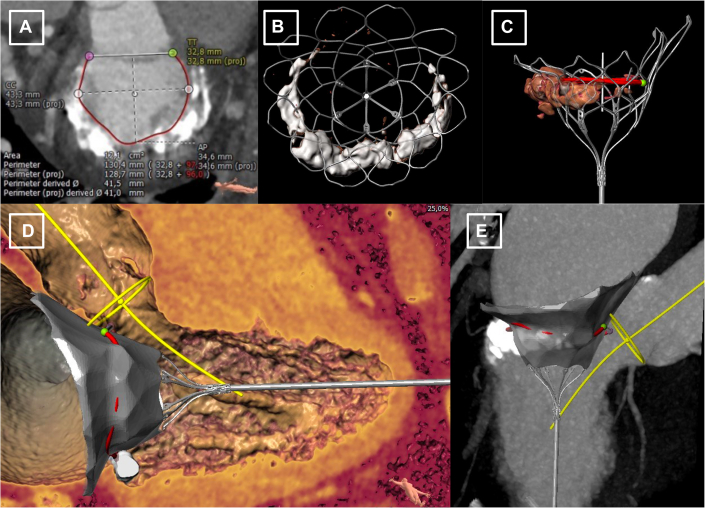
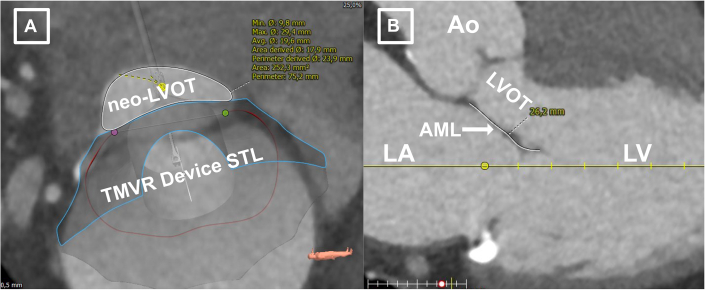
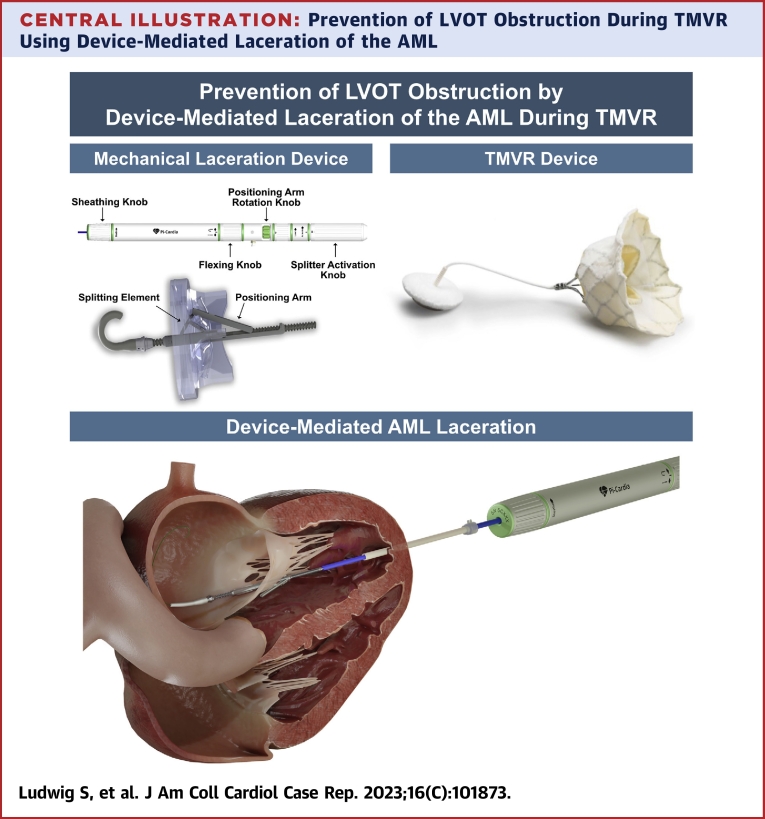
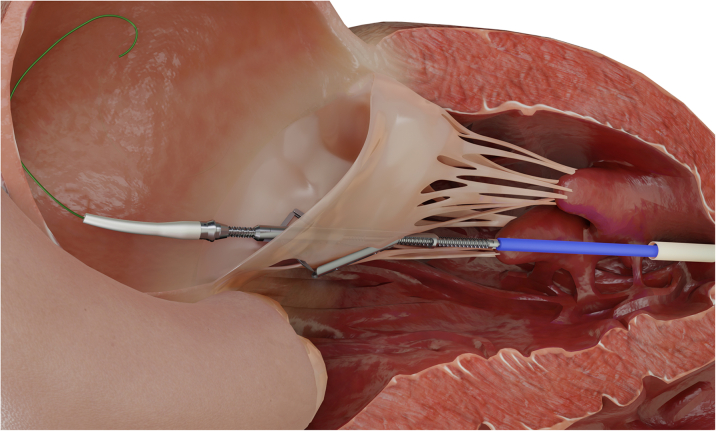
Following standardized computed tomographic segmentation and virtual device implantation, the patient was determined eligible to receive a low-profile 35M transcatheter heart valve (THV) (Figure 1). However, because of anticipated risk for LVOT-O with a borderline cross-sectional neo–left ventricular outflow tract (LVOT) area (252.3 mm2 in end-systole) (Figure 2A) and an elongated AML (26.2 mm) (Figure 2B), the decision was made for compassionate use of a dedicated mechanical transcatheter leaflet-splitting device (ShortCut, Pi-Cardia) for AML laceration (Central Illustration).
Figure 1.
Pre-procedural Computed Tomography Planning for Transcatheter Mitral Valve Replacement
Contrast-enhanced multislice computed tomography segmentation of the mitral annulus (A) and virtual implantation of a low-profile 35M valve (B to E).
Figure 2.
Elevated Risk for LVOT Obstruction Following TMVR
(A) Predicted neo–left ventricular outflow tract (LVOT) area after virtual valve implantation at end-systole. (B) Elongated anterior mitral valve leaflet (AML). Ao = aorta; LA = left atrium; LV = left ventricle; STL = STereoLithography; TMVR = transcatheter mitral valve replacement.
Central Illustration.
Prevention of LVOT Obstruction During TMVR Using Device-Mediated Laceration of the AML
AML = anterior mitral leaflet; LVOT = left ventricular outflow tract; TMVR = transcatheter mitral valve replacement.
Management
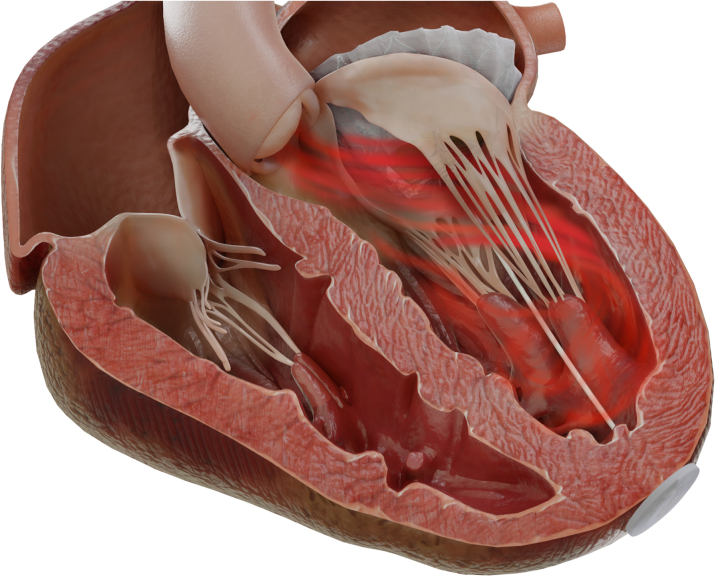
The procedure was performed under general anesthesia using 3-dimensional transesophageal echocardiographic and fluoroscopic guidance and using a cerebral embolic protection device (Sentinel, Boston Scientific). Following standard transapical access, “balloon flossing” from the left atrium to the left ventricular apex to exclude entanglement in the mitral subvalvular apparatus was performed using a Swan-Ganz catheter. An 18-F DrySeal Flex Introducer Sheath (W.L. Gore & Associates) was advanced across the mitral annular plane, followed by insertion of the ShortCut catheter. Axial alignment was verified using standard X-plane TEE and fluoroscopy. In the second step, the AML was engaged in the central A2 segment and lacerated from base to free edge by controlled retraction of the catheter (Figure 3). Engagement and laceration of the AML were clearly visible by distension of the AML toward the apex with subsequent sudden detachment from the catheter. The resultant AML split was identifiable on 2-dimensional and 3-dimensional TEE (Videos 3 and 4, Figure 4). Subsequently, THV implantation was successfully performed in a standard fashion using a 36-F delivery catheter (Figure 5). Intraprocedural LVOT-O was closely monitored by using color Doppler on TEE to determine LVOT flow characteristics, TEE-derived LVOT gradient, and invasively measured peak-to-peak gradient and by monitoring acute hemodynamic changes. Final echocardiography showed no valvular or paravalvular regurgitation and no relevant flow acceleration in the LVOT (Video 5). Invasively derived peak-to-peak LVOT gradient was 5 mm Hg (mean gradient 7 mm Hg). The total procedure time was 88 minutes, after which the patient was extubated promptly and transferred to intensive care unit.
Figure 3.
Leaflet Laceration From Base to Free Edge by Controlled Retraction of the Catheter and the Splitting Element
Figure 4.
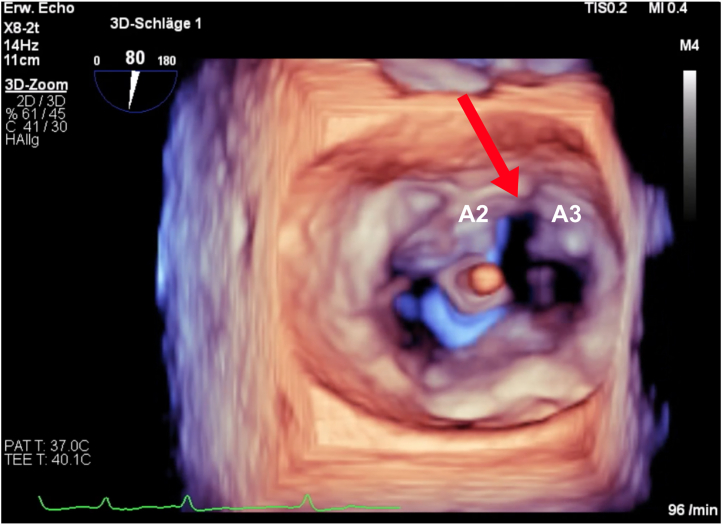
Three-Dimensional Multiplanar Reconstruction of the Mitral Valve by Transesophageal Echocardiography Showing Anterior Mitral Valve Leaflet Laceration Between A2 and A3
Figure 5.
Successful Transapical Transcatheter Mitral Valve Replacement After Leaflet Laceration and Secured Left Ventricular Outflow Tract Flow Following Leaflet Laceration
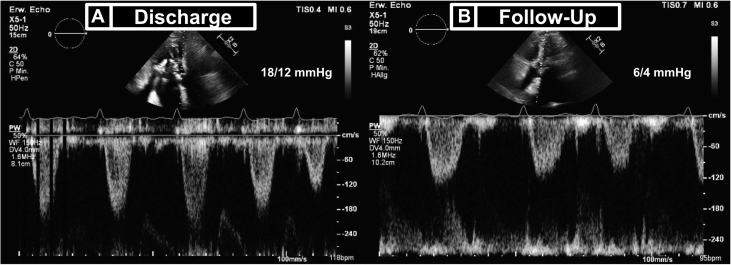
In the intensive care unit, the patient intermittently received continuous renal replacement therapy and inotropic support. Following prolonged decongestion, the patient was discharged on day 34. No other complications occurred during the in-hospital period. Discharge TTE confirmed the elimination of MR, without paravalvular leakage or clinically relevant LVOT-O (LVOT gradient 18/12 mm Hg).
Discussion
This report describes first-in-human device-mediated mechanical mitral valve leaflet laceration to prevent LVOT-O in a patient undergoing transapical TMVR. Laceration of the AML using device-mediated mechanical AML laceration was feasible, safe, and effective to ensure LVOT patency without increased gradients. Advantages of this device lie in its simple and intuitive handling and the straightforward approach for leaflet laceration.
Analogous to the transcatheter electrosurgery–based BASILICA (bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction) technique, device-mediated mechanical laceration of failed aortic bioprosthetic leaflets using the same catheter as in this report has recently been demonstrated as safe and successful in preventing coronary obstruction and is currently in trial for U.S. Food and Drug Administration approval.4,5 In this report, the catheter was used on a compassionate-use basis for intentional mechanical laceration of the AML prior to TMVR to prevent anticipated LVOT-O by anterior AML displacement. In this context, the LAMPOON (intentional percutaneous laceration of the AML to prevent outflow obstruction) technique has been adopted increasingly by experienced centers and can be considered the wire-based transcatheter electrosurgery analogue to mechanical leaflet laceration.6 In contrast to the device used in this report, LAMPOON is technically demanding and time consuming and requires extensive physician expertise and hardware.
Several other transcatheter techniques have been developed to prevent LVOT-O during TMVR, including alcohol septal ablation and septal radiofrequency ablation.3 These noninvasive techniques aim to reduce septal myocardium but carry risk for complete heart block and require a 2-step approach.3,7,8 The SESAME (septal scoring along the midline endocardium) technique using transcatheter electrosurgery to lacerate the interventricular septum was recently introduced in a preclinical study.9
In general, prospective studies on mechanical leaflet laceration are necessary to provide guidance regarding choice of device and method. The transition to transseptal TMVR devices will require a dedicated endovascular mitral valve leaflet laceration system, which is currently under development by the manufacturer. Moreover, the TMVR system used in this case features fabric-covered stent cells, which may limit the effectiveness of AML laceration.
Follow-Up
At 4-month follow-up, the patient presented in stable condition with a substantial reduction of heart failure symptoms (NYHA functional class II). No further hospitalization for heart failure has occurred since TMVR. TTE confirmed normal THV function and a normalized LVOT gradient of 6/4 mm Hg (Figure 6).
Figure 6.
Discharge and Follow-Up Transthoracic Echocardiography Showing Pulsed-Wave Doppler–Derived Left Ventricular Outflow Tract Gradients
(A) Discharge and (B) follow-up transthoracic echocardiography.
Conclusions
The results of this first-in-human report suggest that the use of a mechanical leaflet laceration device may be a promising alternative to demanding interventional techniques previously established for preventing LVOT-O. However, additional studies are required to confirm safety, efficacy, and reproducibility for broader clinical use.
Funding Support and Author Disclosures
This study was supported by a personal grant from the German Heart Foundation to Dr Ludwig. A professional medical illustrator was paid by Pi-Cardia and provided Figures 3, 4, and 6. Dr Ludwig has received travel compensation from Edwards Lifesciences; has received advisory fees from Bayer; and has received speaker honoraria from Abbott. Dr Kalbacher has received personal fees from Edwards Lifesciences, Abbott Medical, and Pi-Cardia. Dr Schaefer has received speaker honoraria from Abbott. Dr Denti has received speaker honoraria from Abbott and Edwards Lifesciences; and is a consultant for Pi-Cardia, InnovHeart HRV, and Approxima. Dr Schofer has received speaking honoraria and proctor fees from Edwards Lifesciences. Dr Conradi is an advisory board member for Abbott, Medtronic, JenaValve, and MicroPort; and has received personal fees from Edwards Lifesciences, Boston Scientific, Neovasc, Highlife, Pi-Cardia, and MicroInterventions. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Preprocedural Transesophageal Echocardiography (3-Chamber View) Showing an Eccentric Mitral Regurgitant Jet Due to Postendocarditis Destruction of the Posterior Leaflet and Severe Subvalvular Calcification
Preprocedural 3-Dimensional Multiplanar Reconstruction of the Mitral Valve by Transesophageal Echocardiography Showing Annular and Leaflet Calcification and Postendocarditis Destruction of the Posterior Mitral Valve Leaflet
Periprocedural Transesophageal Echocardiography (X-Plane: Bicommissural View and 3-Chamber View) Showing Successful Splitting of the Anterior Mitral Valve Leaflet Using Mechanical Laceration
Postprocedural 3-Dimensional Multiplanar Reconstruction of the Mitral Valve by Transesophageal Echocardiography Showing Anterior Mitral Valve Leaflet Laceration Between A2 and A3
Red arrow shows split of anterior mitral valve leaflet between A2 and A3.
Postprocedural Transesophageal Echocardiography Result Showing no Valvular or Paravalvular Regurgitation and No Flow Acceleration in the Left Ventricular Outflow Tract
References
- 1.Alperi A., Granada J.F., Bernier M., Dagenais F., Rodés-Cabau J. Current status and future prospects of transcatheter mitral valve replacement. J Am Coll Cardiol. 2021;77:3058–3078. doi: 10.1016/j.jacc.2021.04.051. [DOI] [PubMed] [Google Scholar]
- 2.Ali W.B., Ludwig S., Duncan A., et al. Characteristics and outcomes of patients screened for transcatheter mitral valve implantation: 1-year results from the CHOICE-MI registry. Eur J Heart Fail. 2022;24:887–898. doi: 10.1002/ejhf.2492. [DOI] [PubMed] [Google Scholar]
- 3.Lisko J., Kamioka N., Gleason P., et al. Prevention and treatment of left ventricular outflow tract obstruction after transcatheter mitral valve replacement. Interv Cardiol Clin. 2019;8:279–285. doi: 10.1016/j.iccl.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dvir D., Leon M.B., Abdel-Wahab M., et al. First-in-human dedicated leaflet splitting device for prevention of coronary obstruction in transcatheter aortic valve replacement. J Am Coll Cardiol Intv. 2023;16:94–102. doi: 10.1016/j.jcin.2022.10.050. [DOI] [PubMed] [Google Scholar]
- 5.Westermann D., Ludwig S., Kalbacher D., et al. Prevention of coronary obstruction in patients at risk undergoing transcatheter aortic valve implantation: the Hamburg BASILICA experience. Clin Res Cardiol. 2021;110:1900–1911. doi: 10.1007/s00392-021-01881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan J., Babaliaros V., Greenbaum A., et al. Anterior leaflet laceration to prevent ventricular outflow tract obstruction during transcatheter mitral valve replacement. J Am Coll Cardiol. 2019;73:2521–2534. doi: 10.1016/j.jacc.2019.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Killu A.M., Collins J.D., Eleid M.F., et al. Preemptive septal radiofrequency ablation to prevent left ventricular outflow tract obstruction with transcatheter mitral valve replacement: a case series. Circ Cardiovasc Interv. 2022;15 doi: 10.1161/CIRCINTERVENTIONS.122.012228. [DOI] [PubMed] [Google Scholar]
- 8.Deharo P., Urena M., Himbert D., et al. Bail-out alcohol septal ablation for left ventricular outflow tract obstruction after transcatheter mitral valve replacement. J Am Coll Cardiol Intv. 2016;9:e73–e76. doi: 10.1016/j.jcin.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Khan J.M., Bruce C.G., Greenbaum A.B., et al. Transcatheter myotomy to relieve left ventricular outflow tract obstruction: the septal scoring along the midline endocardium procedure in animals. Circ Cardiovasc Interv. 2022;15 doi: 10.1161/CIRCINTERVENTIONS.121.011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preprocedural Transesophageal Echocardiography (3-Chamber View) Showing an Eccentric Mitral Regurgitant Jet Due to Postendocarditis Destruction of the Posterior Leaflet and Severe Subvalvular Calcification
Preprocedural 3-Dimensional Multiplanar Reconstruction of the Mitral Valve by Transesophageal Echocardiography Showing Annular and Leaflet Calcification and Postendocarditis Destruction of the Posterior Mitral Valve Leaflet
Periprocedural Transesophageal Echocardiography (X-Plane: Bicommissural View and 3-Chamber View) Showing Successful Splitting of the Anterior Mitral Valve Leaflet Using Mechanical Laceration
Postprocedural 3-Dimensional Multiplanar Reconstruction of the Mitral Valve by Transesophageal Echocardiography Showing Anterior Mitral Valve Leaflet Laceration Between A2 and A3
Red arrow shows split of anterior mitral valve leaflet between A2 and A3.
Postprocedural Transesophageal Echocardiography Result Showing no Valvular or Paravalvular Regurgitation and No Flow Acceleration in the Left Ventricular Outflow Tract