Abstract
The increase in the types and complexity of diseases has led to significant advances in diagnostic techniques and the availability of effective therapies. Recent studies have focused on the role of mitochondrial dysfunction in the pathogenesis of cardiovascular diseases (CVDs). Mitochondria are important organelles in cells that generate energy. Besides the production of adenosine triphosphate (ATP), the energy currency of cells, mitochondria are also involved in thermogenesis, control of intracellular calcium ions (Ca2+), apoptosis, regulation of reactive oxygen species (ROS), and inflammation. Mitochondrial dysfunction has been implicated in several diseases including cancer, diabetes, some genetic diseases, and neurogenerative and metabolic diseases. Furthermore, the cardiomyocytes of the heart are rich in mitochondria due to the large energy requirement for optimal cardiac function. One of the main causes of cardiac tissue injuries is believed to be mitochondrial dysfunction, which occurs via complicated pathways which have not yet been completely elucidated. There are various types of mitochondrial dysfunction including mitochondrial morphological change, unbalanced levels of substances to maintain mitochondria, mitochondrial damage by drugs, and mitochondrial deletion and synthesis errors. Most of mitochondrial dysfunctions are linked with symptoms and diseases, thus we focus on parts of mitochondrial dysfunction about fission and fusion in cardiomyocytes, and ways to understand the mechanism of cardiomyocyte damage by detecting oxygen consumption levels in the mitochondria.
Keywords: Mitochondrial dysfunction, Oxygen consumption rate, Cardiomyocytes, Mitochondrial fission
Introduction
Mitochondria play an important role in maintaining cardiac homeostasis by providing energy to the cardiomyocytes as well as controlling the key intracellular pathways. The cardiac tissue responds to long-term hemodynamic load through the initiation of hypertrophic remodeling. If hypertrophy is not counteracted it will eventually lead to organ failure. Cardiac hypertrophic adaptations are complex and involve multiple cellular events and mitochondrial dysfunction has been indicated as a potential and important player in the development of cardiac hypertrophy. Additionally, substantial evidence shows that a significant portion of mitochondrial processes, necessary for normal cardiomyocyte physiology, are impacted by these hypertrophic changes. Cardiac hypertrophy at the cellular level is related to altered mitochondrial morphology and function [1] Recent evidence indicates that morphological changes in mitochondria affect the metabolic state of the organelle.
Mitochondrial membrane potential (MMP) plays a very important role in the energy storage process during oxidative phosphorylation and is a marker of mitochondrial activity and contributes to the functioning of the mitochondria [2]. Disruption of the membrane potential and fluctuations from the normal range are known to have a major impact on cell survival. Membrane potential is also associated with mitophagy [3], the process of removal of damaged, mutated, or abnormal mitochondria by microautophagy or macroautophagy to maintain mitochondrial homeostasis [4]. Mitophagy has an essential role in cardiovascular homeostasis as it efficiently removes dysfunctional mitochondria that accumulate in cells [5].
Other factors that affect mitochondrial maintenance are mitochondrial fission and fusion, which play an important role in disease-related processes such as cell death and autophagy [6]. Specifically, mitochondrial fission is significantly associated with mitophagy. Some studies have verified that the overexpression of dynamin-related protein 1 (DRP1), which is involved in mitophagy, causes excessive fission in mitochondria [7].
Cardiomyocytes have many mitochondria to generate large amounts of energy so, when the mitochondria undergo malfunction, critical heart diseases occur [8]. Ischemic heart disease (IHD) is a leading cause of mortality worldwide. An inadequate supply of blood and nutrients to the heart muscle in IHD can result in damage to the cardiomyocytes and lead to acute myocardial infarction [9] [10]. At the cellular level, a decrease in mitochondrial function associated with the impairment of the mitochondrial respiratory chain and ATP synthesis leads to increased oxidative stress, resulting in damage to the mitochondrial structure. This emphasizes the role of mitochondrial dysfunction in the pathophysiology of cardiovascular diseases (CVDs) [11].
Mitochondrial dysfunctions contributes to diabetes through insulin resistance in insulin-related tissues and this lead to increases metabolic dysregulation in cardiovascular tissue [12]. Mitochondrial dysfunction is common in patients with heart failure (HF) and/or insulin resistance [13]. Various types of mitochondrial dysfunction in cardiac cells have been investigated to date, and this has led to the development of several techniques to identify the changes in the cardiac cells. To better understand mitochondrial dysfunction in cardiomyocytes, it is necessary to organize the types of dysfunctions and detection methods. Therefore, in this review, we summarize the features of mitochondrial dysfunction in cardiac cells and methods to detect them.
Mechanism of mitochondrial dysfunction and its effects on cardiomyocytes
Mitophagy (PINK1/Parkin mediated pathway)
Autophagy in the heart is very important in aging, ischemia/reperfusion (I/R) injury, HF, genetic cardiomyopathy, and diabetic cardiomyopathy. Also, autophagy is important in cardiotoxic conditions caused by anticancer drugs such as doxorubicin [14].
Selective elimination of organelles via autophagy is termed organellophagy [15]. Mitophagy is a type of organellophagy—a cellular process that selectively removes the aged and damaged mitochondria via the specific sequestration and engulfment of mitochondria for subsequent lysosomal degradation. It plays a pivotal role in reinstating cellular homeostasis in normal physiology and conditions of stress. Mitophagy not only removes damaged mitochondria but also plays a role in the maternal inheritance of mitochondrial DNA through the removal of sperm-derived mitochondria.
Mitophagy also mediates the removal of mitochondria from developing erythrocytes [16]. Reticulocytes are the organelles which include ribosomes and mitochondria. And mitophagy is intermediated to eliminate those mitochondria during the transformation from reticulocyte to mature erythrocytes. Hence mitophagy is a necessary function for erythrocyte development. Mitophagy also targets mitochondria damaged by toxic chemicals or stimuli such as mtDNA, mtRNA damage, ROS, etc. [17], sequesters them in double-membrane vesicles [16] and finally delivers them to lysosomes for degradation [17]. This process removes depolarized or damaged mitochondria that can no longer produce energy efficiently, and enables the synthesis of new mitochondria [18], thus maintaining cellular homeostasis [15].
In the recent study, RhoA, the GTPase expressed in various types of cells are found to promote Parkin-mediated mitophagy in the heart. The study established RhoA knock-out mouse model and evaluated cardiac functions according to the existence of Parkin [19]. In addition, another study examined SIRT-1 pathway in Sema3A-overexpressed condition [20]. In this Sema3A overexpressed mouse model, mitophagy advanced protective effects against cell apoptosis and ROS induction in cardiomyocytes. I/R injury can be alleviated by increasing mitophagy by inducing the expression of HSPB8 through overexpression of DUSP12 in myocardiac tissue and H9C2 cells [21].
In mammalian cells, PTEN-induced putative kinase-1 (PINK1), a serine/threonine kinase (mitochondrial membrane kinase) [22], and parkin, an E3 ubiquitin ligase, are biomarkers of mitochondrial function. If they are damaged or misrecognized, mitophagy is initiated. The PINK1 gene has a transmembrane domain (TM), a serine/threonine kinase domain, and a mitochondrial targeting sequence (MTS) in the N-terminus [23]. In the case of normal mitochondria, MTS translocates partially across the mitochondrial membrane via the translocase of the outer membrane [1] and the translocase of the inner membrane (TIM) while maintaining membrane potential [24]. MTS entering the mitochondrial matrix is cleaved by mitochondria processing peptidase (MPP). The cut segments located in the mitochondrial inner membrane are processed by presenilin associated rhomboid like (PARL), a serine protease. In normal mitochondria, parkin exists in an inactive state in the plasma due to the rapid degradation of PINK1 [25], thus protecting the mitochondria from PINK1/parkin-mediated mitophagy [26]. On the other hand, when mitochondria are damaged (depolarization serves as a trigger that induces mitophagy) [18], PINK1 accumulates in the mitochondrial outer membrane by TOM and outer membrane localization signal (OMS). Mitofusin 2 (MFN2) and mitofusin 1 (MFN1) can directly control the mitophagy. Genetic delete of MFN2 in cardiac myocytes cause inhibition of mitophagy, which means importance about role of MFN2 [27]. When the mitochondria are in a stable state, they phosphorylate MFN2 [22] and recruit parkin to mitochondria [28], and activate the action of E3 ubiquitin ligases UBR1, UBR2, and UBR4 [22, 29, 30]. Then, parkin ubiquitinates outer mitochondrial membrane proteins to trigger selective autophagy [31]. The excessive accumulation of PINK1 in the outer membrane overexpressed or further aggravated by inhibiting PINK1 cleavage, which ultimately activates mitophagy [32].
Another study in a mouse model showed that cardiac-specific loss of autophagy induces cardiomyopathy [33]. Cardiac-specific deficiency of autophagy-related 5 (ATG5), an essential protein for autophagy, causes abnormal enlargement of the heart, left ventricular dilatation, and contractile dysfunction. This means that autophagy is essential for maintaining the overall size, structure, and function of the heart. In another study, it has been confirmed that in mice deficient in ATG5, the size of the left ventricle increased, and the heart was overworked. Also, irregularities in the cardiac sarcomere and damage to the mitochondria were found through ultrastructural analysis [34]. In a study by Wang et al., mice with AMP-activated protein kinase (AMPK) α2 mutation were used to identify the decrease in mitophagy in the heart, and it was observed that transverse aortic constriction-induced HF was aggravated [22]. This confirms that among the isoforms of AMPKα, AMPKα2 promotes the phosphorylation of Ser495 of PINK1, and is essential for mitophagy, which can slow down the progression of HF.
Lastly, it was found that rapamycin, an immunosuppressive antibiotic, induces autophagy by inhibiting the mechanistic target of rapamycin (mTOR) in both HL-1 cells (a mouse cardiac muscle cell line) and AC16 cells which are human cardiomyocytes. Also, it induces mitophagy and promotes mitochondrial elimination. It was confirmed that rapamycin improved mitochondrial function, as determined by the MMP and cellular respiration analysis, in antimycin A (AMA) treated cardiomyocytes. AMA is a drug that increases mitochondrial superoxide generation, damages mitochondria, decreases MMP, and depresses cellular respiration [35]. These studies appear to suggest that cardiac function can be improved by regulating the factors affecting mitochondrial mitophagy in mice and human cardiomyocytes.
Mitochondrial fission and fusion
DRP1 of the mitochondrial fission/fusion process is a member of the dynamin superfamily of guanosine triphosphatases (GTPases), encoded by human dynamin 1 like (DNM1L) [36], and is an important regulator of the mitochondrial fission process. Mitochondrial recruitment of DRP1 is the critical step to initiate mitochondrial fission and mitophagy [37]. In a recent study, Klf4 aggravated the myocardial I/R through controlling the expression of ROCK1 by inducing DRP1 mediated mitochondrial fission [38]. In addition, S89, a small molecule agonist targeting endogenous MFN1, enhanced mitochondrial fusion in the I/R mouse heart [39]. DRP1 is downregulated, mitochondria are elongated, and mitochondrial fission and mitophagy are inhibited, causing mitochondrial dysfunction, thereby promoting cardiac dysfunction and increasing susceptibility to I/R [40]. In mammalian cells, membrane adapters such as mitochondrial fission 1 protein (Fis1), mitochondrial fission factor (Mff), and mitochondrial dynamics proteins of 49, 51 kDa (MiD49, and MiD51) gather DRP1 on the mitochondrial surface. The role of Fis1 and Mff in mitochondrial fission was demonstrated by confirming mitochondrial elongation and fission in Fis1-null and Mff-null mouse embryonic fibroblasts [41]. The expression of DRP1 induces fission by contracting mitochondria through GTP binding and hydrolysis around the mitochondrial outer membrane. Although mitochondrial fission is essential for cellular homeostasis, it is often abnormally activated in CVDs [42].
In a normal state, mitochondrial fusion occurs by binding mitochondria through MFN1/2 in the mitochondrial outer membrane [36]. Subsequently, the fusion-active long-optic atrophy 1 (L-OPA1) isoform and the fusion-inactive short-optic atrophy1 (S-OPA1) isoform maintain a balanced state. However, YME1L [43], a mitochondrial ATP-dependent metalloprotease, cooperates with metalloendopeptidase OMA1 to cleave L-OPA1 and generate S-OPA1. The accumulation of S-OPA1 leads to an imbalance between the L-form and the S-forms, increasing mitochondrial cleavage and leading to energy loss [44]. Also, mitochondrial fission and fusion interact in such a way that a decrease in one causes an increase in the other [42].
In some studies of mitochondrial fission and fusion, it has been verified that mitochondrial fission progressed with mitochondrial respiration disorders, and fatal dilated cardiomyopathy (DCMP) occurred when MFN1/2 was ablated using Cre-mediated excisions in murine hearts in vivo [45].
Assessment of mitochondrial oxygen consumption rate (OCR)
The primary function of mitochondria is to produce the energy necessary for cells by oxidative phosphorylation. ATP, the energy-carrying molecule, is generated through mitochondrial respiration. The most appropriate way to measure the overall efficiency of mitochondrial energy production is to measure the mitochondrial OCR [46, 47].
Before the twenty-first century, OCR was classically measured using Clark electrodes. However, the use of the electrodes is limited to suspensions and high-yield whole cells and is also a time-consuming process [48]. Recently, the efficient evaluation of OCR has been made possible using bioenergetic techniques, and there have been a significant number of studies conducted using OCR [49]. In several studies, OCR measurements were made using a Seahorse XFe analyzer (Agilent) [46, 50–52]. This machine measures glycolysis by analyzing the extracellular acidification rate (ECAR) and measures mitochondrial oxidative phosphorylation on the basis of the OCR, through real-time and live cell analysis.
Oligomycin was the first drug used to evaluate mitochondrial dysfunction. It binds to the proton channel on the mitochondrial ATP synthase enzyme, inhibiting mitochondrial ATP synthesis, proton translocation, and oxygen uptake, thereby blocking the mitochondrial electron transport chain [52]. The authors describe a protocol to assess the basic energy metabolism profiles of three cell lines, as well as key parameters of mitochondrial function, in response to the sequential addition of mitochondria-perturbing agents such as oligomycin. Carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP) is an uncoupling agent that breaks electron transport bonds. It causes proton flux, disrupting the proton gradient and damaging the MMP. Therefore, it allows the determination of the maximal respiration rate of the mitochondria [53]. AMA and rotenone, inhibit mitochondrial complex I and III, respectively [54, 55], and since they are inhibitors of ATP synthesis, the reduction in OCR after drug treatment decreases the ATP turnover rate, which ultimately results in a minimum level of mitochondrial respiration. The addition of rotenone and AMA completely blocked the mitochondrial respiratory function, resulting in non-mitochondrial respiration [56]. Mitochondrial OCR measurement is an experimental technique that can representatively identify various mitochondrial dysfunctions in the brain [50], kidney [46], embryos [57], muscle, lung, and heart [51].
Cardiotoxicity [54] is a serious side effect of anticancer agents and is one of the primary causes of drug withdrawal in cancer patients [58, 59]. This finding emphasized the need for the preclinical safety screening of these drugs. Human cardiomyocytes derived from induced pluripotent stem cells (iPSC) have the potential for use as in vivo models to study cardiotoxicity in humans, and OCR can be used to characterize mitochondrial function in these cells. The tyrosine kinase inhibitors including sorafenib, sunitinib, dasatinib, imatinib, lapatinib, and nilotinib have been reported to have cardiotoxic effects. Cardiomyocytes derived from iPSC were treated with tyrosine kinase inhibitors to confirm dose-dependent toxicity. In these cells, OCR was more affected than ATP production.
Studies have further verified mitochondrial dysfunction-mediated cardiotoxicity of tyrosine kinase inhibitors in rat cardiomyocytes H9C2 [9, 60]. To measure mitochondria in cardiomyocytes, the adenosine diphosphate (ADP) dependent mitochondrial oxygen consumption was confirmed using a fluorescence plate reader using an oxygen-sensitive probe, a classic method used before the twenty-first century [47, 61, 62]. Tyrosine kinase-induced suppression of the mitochondrial complexes was confirmed with sorafenib having the most significant effect on mitochondrial oxygen consumption among the drugs used. In another study, cardiovascular damage in obese conditions was associated with abnormal autophagy. OCR within H9C2 was measured using the Seahorse XF Cell Mito Stress Test Kit on an Agilent seahorse XFe96 extracellular flux analyzer machine. It was confirmed that the downregulation of H19 (long non-coding RNA) gene expression promotes mitophagy in H9C2 cells, resulting in cardiorespiratory dysfunction. It has been identified that H19 inhibits the mRNA translation of PINK1, essential for the mitophagy process, and prevents excessive mitophagy, leading to the alleviation of cardiac defects that occur during obesity [63].
The most common feature of the experiments examining mitochondrial function, including those confirming mitochondrial OCR, is that glucose was replaced with galactose in the medium used to maintain the cells. This was to provide conditions suitable for the experiment by reducing the net ATP production pathway through glycolysis and activating the net ATP non-production pathway through galactose metabolism during mitochondrial function confirmation experiments [64].
Conclusion
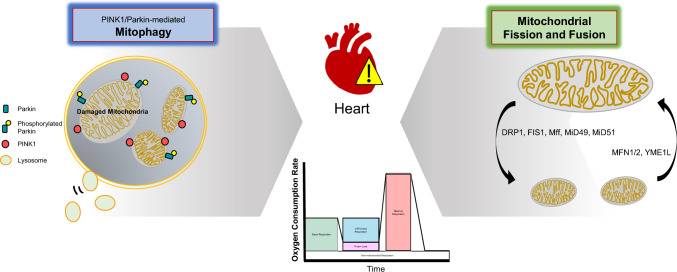
This review summarizes the mechanisms of mitochondrial dysfunction and its effects on cardiomyocytes. In addition, to confirm mitochondrial activity and function in cells, the principles and methods of measuring the OCR of mitochondria were verified. Mitophagy and mitochondrial fission/fusion regulate the number and function of mitochondria in the cells required to maintain normal physiological activities. Mitophagy, also known as mitochondria-specific autophagy, occurs through the parkin-dependent and parkin-independent pathways. The mechanism of mitophagy reviewed in this paper is a parkin-dependent pathway. This paper reviews the roles of PINK1, and MFN1/2 in mitophagy and that of DRP1, OMA1, Fis1 and Mff in mitochondrial fission and fusion. Mitophagy and mitochondrial fission/fusion affect cardiac cells and ultimately influence cardiac health. Such mitochondrial dysfunction can be typically confirmed through OCR measurement as shown in Fig. 1. The OCR method can predict the cellular pathophysiology of heart-related diseases which occur due to mitochondrial dysfunction.
Fig. 1.
A schematic diagram of mitochondrial dysfunctions and OCR. The factors involved in mitophagy, and mitochondria fission/fusion are identified. In addition, mitochondrial dysfunction, including mitophagy and mitochondrial fission and fusion, has a fatal effect on mammalian cardiomyocytes, and it is very important to confirm this. In this review, the analysis of mitochondrial function affecting the heart was confirmed using OCR measurements
Abbreviation
- AMA
Antimycin A
- AMPK
AMP-activated protein kinase
- ATG5
Autophagy-related 5
- ATP
Adenosine triphosphate
- CVD
Cardiovascular disease
- DRP1
Dynamin-related protein 1
- Fis1
Mitochondrial fission 1 protein
- HF
Heart failure
- IHD
Ischemic heart disease
- iPSC
Induced pluripotent stem cells
- I/R
Ischemia/reperfusion
- L-OPA1
Long-optic atrophy 1
- Mff
Mitochondrial fission factor
- MFN2
Mitofusin 2
- MMP
Mitochondrial membrane potential
- MTS
Mitochondrial targeting sequence
- OCR
Oxygen consumption rate
- PINK1
PTEN-induced putative kinase-1
- ROS
Reactive oxygen species
- S-OPA1
Short-optic atrophy 1
- TOM
Translocase of the outer membrane
Author contributions
Conceptualization, R-EG and K-CC; writing-original draft preparation, DA.; writing-review and editing, R-EG and K-CC; supervision, K-CC; project administration, K-CC; funding acquisition, K-CC All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the Ministry of Food and Drug Safety in 2020 (20183MFDS525). In addition, this work was also supported by the Basic Research Lab Program (2022R1A4A1025557) through the National Research Foundation (NRF) of Korea, funded by the Ministry of Science and ICT.
Declarations
Conflict of interest
The authors do not have any conflicts of interest to declare.
References
- 1.Sotomayor-Flores C, Rivera-Mejias P, Vasquez-Trincado C, Lopez-Crisosto C, Morales PE, Pennanen C, Polakovicova I, Aliaga-Tobar V, Garcia L, Roa JC, Rothermel BA, Maracaja-Coutinho V, Ho-Xuan H, Meister G, Chiong M, Ocaranza MP, Corvalan AH, Parra V, Lavandero S. Angiotensin-(1–9) prevents cardiomyocyte hypertrophy by controlling mitochondrial dynamics via miR-129-3p/PKIA pathway. Cell Death Differ. 2020;27:2586–2604. doi: 10.1038/s41418-020-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Zubaidi U, Liu J, Cinar O, Robker RL, Adhikari D, Carroll J. The spatio-temporal dynamics of mitochondrial membrane potential during oocyte maturation. Mol Hum Reprod. 2019;25:695–705. doi: 10.1093/molehr/gaz055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M, Sollott SJ, Zorov DB. Mitochondrial membrane potential. Anal Biochem. 2018;552:50–59. doi: 10.1016/j.ab.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, Cuervo AM, Debnath J, Deretic V, Dikic I, Eskelinen EL, Fimia GM, Fulda S, Gewirtz DA, Green DR, Hansen M, Harper JW, Jaattela M, Johansen T, Juhasz G, Kimmelman AC, Kraft C, Ktistakis NT, Kumar S, Levine B, Lopez-Otin C, Madeo F, Martens S, Martinez J, Melendez A, Mizushima N, Munz C, Murphy LO, Penninger JM, Piacentini M, Reggiori F, Rubinsztein DC, Ryan KM, Santambrogio L, Scorrano L, Simon AK, Simon HU, Simonsen A, Tavernarakis N, Tooze SA, Yoshimori T, Yuan J, Yue Z, Zhong Q, Kroemer G. Molecular definitions of autophagy and related processes. EMBO J. 2017;36:1811–1836. doi: 10.15252/embj.201796697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonora M, Wieckowski MR, Sinclair DA, Kroemer G, Pinton P, Galluzzi L. Targeting mitochondria for cardiovascular disorders: therapeutic potential and obstacles. Nat Rev Cardiol. 2019;16:33–55. doi: 10.1038/s41569-018-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Bliek AM, Shen Q, Kawajiri S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb Perspect Biol. 2013;5:a011072. doi: 10.1101/cshperspect.a011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4:815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- 8.Pennanen C, Parra V, Lopez-Crisosto C, Morales PE, Del Campo A, Gutierrez T, Rivera-Mejias P, Kuzmicic J, Chiong M, Zorzano A, Rothermel BA, Lavandero S. Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin signaling pathway. J Cell Sci. 2014;127:2659–2671. doi: 10.1242/jcs.139394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Will Y, Dykens JA, Nadanaciva S, Hirakawa B, Jamieson J, Marroquin LD, Hynes J, Patyna S, Jessen BA. Effect of the multitargeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib, and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells. Toxicol Sci. 2008;106:153–161. doi: 10.1093/toxsci/kfn157. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandra CJA, Hernandez-Resendiz S, Crespo-Avilan GE, Lin YH, Hausenloy DJ. Mitochondria in acute myocardial infarction and cardioprotection. EBioMedicine. 2020;57:102884. doi: 10.1016/j.ebiom.2020.102884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chistiakov DA, Shkurat TP, Melnichenko AA, Grechko AV, Orekhov AN. The role of mitochondrial dysfunction in cardiovascular disease: a brief review. Ann Med. 2018;50:121–127. doi: 10.1080/07853890.2017.1417631. [DOI] [PubMed] [Google Scholar]
- 12.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102(4):401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh SR, Cleland JG, Colucci WS, Butler J, Voors AA, Anker SD, Pitt B, Pieske B, Filippatos G, Greene SJ, Gheorghiade M. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. 2017;14:238–250. doi: 10.1038/nrcardio.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sciarretta S, Maejima Y, Zablocki D, Sadoshima J. The Role of Autophagy in the Heart. Annu Rev Physiol. 2018;80:1–26. doi: 10.1146/annurev-physiol-021317-121427. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto K. Organellophagy: eliminating cellular building blocks via selective autophagy. J Cell Biol. 2014;205:435–445. doi: 10.1083/jcb.201402054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onishi M, Yamano K, Sato M, Matsuda N, Okamoto K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021;40:e104705. doi: 10.15252/embj.2020104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Killackey SA, Philpott DJ, Girardin SE. Mitophagy pathways in health and disease. J Cell Biol. 2020;219:e202004029. doi: 10.1083/jcb.202004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soh JEC, Shimizu A, Molla MR, Zankov DP, Nguyen LKC, Khan MR, Tesega WW, Chen S, Tojo M, Ito Y, Sato A, Hitosugi M, Miyagawa S, Ogita H. RhoA rescues cardiac senescence by regulating Parkin-mediated mitophagy. J Biol Chem. 2023;299:102993. doi: 10.1016/j.jbc.2023.102993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin L, Wei J, Zhu C, Hao G, Xue J, Zhu Y, Wu R. Sema3A alleviates viral myocarditis by modulating SIRT1 to regulate cardiomyocyte mitophagy. Environ Toxicol. 2023;38:1305–1317. doi: 10.1002/tox.23765. [DOI] [PubMed] [Google Scholar]
- 21.Cheng J, Ji M, Jing H, Lin H (2023) DUSP12 ameliorates myocardial ischemia-reperfusion injury through HSPB8-induced mitophagy. J Biochem Mol Toxicol 37:e23310. 10.1002/jbt.23310 [DOI] [PubMed]
- 22.Wang B, Nie J, Wu L, Hu Y, Wen Z, Dong L, Zou MH, Chen C, Wang DW. AMPKalpha2 protects against the development of heart failure by enhancing mitophagy via PINK1 phosphorylation. Circ Res. 2018;122:712–729. doi: 10.1161/CIRCRESAHA.117.312317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barazzuol L, Giamogante F, Brini M, Cali T. PINK1/Parkin mediated mitophagy, Ca(2+) signalling, and ER-mitochondria contacts in Parkinson's disease. Int J Mol Sci. 2020;21:1772. doi: 10.3390/ijms21051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim A. A panoramic overview of mitochondria and mitochondrial redox biology. Toxicol Res. 2014;30:221–234. doi: 10.5487/TR.2014.30.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eiyama A, Okamoto K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol. 2015;33:95–101. doi: 10.1016/j.ceb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen TN, Padman BS, Lazarou M. Deciphering the molecular signals of PINK1/Parkin mitophagy. Trends Cell Biol. 2016;26:733–744. doi: 10.1016/j.tcb.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Kim JS. Mitophagy: therapeutic potentials for liver disease and beyond. Toxicol Res. 2014;30:243–250. doi: 10.5487/TR.2014.30.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatti JS, Bhatti GK. Reddy PH (2017) Mitochondrial dysfunction and oxidative stress in metabolic disorders—a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. 1863;5:1066–1077. doi: 10.1016/j.bbadis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harper JW, Ordureau A, Heo JM. Building and decoding ubiquitin chains for mitophagy. Nat Rev Mol Cell Biol. 2018;19:93–108. doi: 10.1038/nrm.2017.129. [DOI] [PubMed] [Google Scholar]
- 30.Yamano K, Youle RJ. PINK1 is degraded through the N-end rule pathway. Autophagy. 2013;9:1758–1769. doi: 10.4161/auto.24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bingol B, Sheng M. Mechanisms of mitophagy: PINK1, Parkin, USP30 and beyond. Free Radic Biol Med. 2016;100:210–222. doi: 10.1016/j.freeradbiomed.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 34.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, Nishida K, Shimizu T, Hori M, Komuro I, Takuji Shirasawa TS, Mizushima N, Otsu K. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 35.Dutta D, Xu J, Kim JS, Dunn WA, Jr, Leeuwenburgh C. Upregulated autophagy protects cardiomyocytes from oxidative stress-induced toxicity. Autophagy. 2013;9:328–344. doi: 10.4161/auto.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yapa NMB, Lisnyak V, Reljic B, Ryan MT. Mitochondrial dynamics in health and disease. FEBS Lett. 2021;595:1184–1204. doi: 10.1002/1873-3468.14077. [DOI] [PubMed] [Google Scholar]
- 37.Jin JY, Wei XX, Zhi XL, Wang XH, Meng D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol Sin. 2021;42:655–664. doi: 10.1038/s41401-020-00518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Xiong Z, Jiang Y, Zhou H, Yi L, Hu Y, Zhai X, Liu J, Tian F, Chen Y. Klf4 deficiency exacerbates myocardial ischemia/reperfusion injury in mice via enhancing ROCK1/DRP1 pathway-dependent mitochondrial fission. J Mol Cell Cardiol. 2023;174:115–132. doi: 10.1016/j.yjmcc.2022.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Guo Y, Zhang H, Yan C, Shen B, Zhang Y, Guo X, Sun S, Yu F, Yan J, Liu R, Zhang Q, Zhang D, Liu H, Liu Y, Zhang Y, Li W, Qin J, Lv H, Wang Z, Yuan Y, Yang JF, Zhong YT, Gao S, Zhou B, Liu L, Kong D, Hao X, Hu J, Chen Q. Publisher Correction: Small molecule agonist of mitochondrial fusion repairs mitochondrial dysfunction. Nat Chem Biol. 2023;19:530. doi: 10.1038/s41589-023-01294-6. [DOI] [PubMed] [Google Scholar]
- 40.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res. 2015;116:264–278. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 41.Loson OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24:659–667. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quiles JM, Gustafsson AB. The role of mitochondrial fission in cardiovascular health and disease. Nat Rev Cardiol. 2022;19:723–736. doi: 10.1038/s41569-022-00703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rainbolt TK, Lebeau J, Puchades C, Wiseman RL. Reciprocal Degradation of YME1L and OMA1 Adapts Mitochondrial Proteolytic Activity during Stress. Cell Rep. 2016;14:2041–2049. doi: 10.1016/j.celrep.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilkerson R, De La Torre P, St Vallier S. Mitochondrial OMA1 and OPA1 as gatekeepers of organellar structure/function and cellular stress response. Front Cell Dev Biol. 2021;9:626117. doi: 10.3389/fcell.2021.626117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Liu Y, Dorn GW., 2nd Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109:1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu X, Ma Y, Liu Y, Wan Q. Measurement of mitochondrial respiration in adherent cells by Seahorse XF96 Cell Mito Stress Test. STAR Protoc. 2021;2:100245. doi: 10.1016/j.xpro.2020.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hynes J, Marroquin LD, Ogurtsov VI, Christiansen KN, Stevens GJ, Papkovsky DB, Will Y. Investigation of drug-induced mitochondrial toxicity using fluorescence-based oxygen-sensitive probes. Toxicol Sci. 2006;92:186–200. doi: 10.1093/toxsci/kfj208. [DOI] [PubMed] [Google Scholar]
- 48.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt CA, Fisher-Wellman KH, Neufer PD. From OCR and ECAR to energy: perspectives on the design and interpretation of bioenergetics studies. J Biol Chem. 2021;297:101140. doi: 10.1016/j.jbc.2021.101140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhi L, Zhao J, Jaffe D, Chen Y, Wang N, Qin Q, Seifert EL, Li C, Zhang H. Measurement of oxygen consumption rate in acute striatal slices from adult mice. J Vis Exp. 2022;184:e63379. doi: 10.3791/63379. [DOI] [PubMed] [Google Scholar]
- 51.Iuso A, Repp B, Biagosch C, Terrile C, Prokisch H. Assessing mitochondrial bioenergetics in isolated mitochondria from various mouse tissues using seahorse XF96 analyzer. Methods Mol Biol. 2017;1567:217–230. doi: 10.1007/978-1-4939-6824-4_13. [DOI] [PubMed] [Google Scholar]
- 52.Plitzko B, Loesgen S. Measurement of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) in culture cells for assessment of the energy metabolism. Bio Protoc. 2018;8:e2850. doi: 10.21769/BioProtoc.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sekar P, Huang DY, Hsieh SL, Chang SF, Lin WW. AMPK-dependent and independent actions of P2X7 in regulation of mitochondrial and lysosomal functions in microglia. Cell Commun Signal. 2018;16:83. doi: 10.1186/s12964-018-0293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rana P, Anson B, Engle S, Will Y. Characterization of human-induced pluripotent stem cell-derived cardiomyocytes: bioenergetics and utilization in safety screening. Toxicol Sci. 2012;130:117–131. doi: 10.1093/toxsci/kfs233. [DOI] [PubMed] [Google Scholar]
- 55.Smolina N, Bruton J, Kostareva A, Sejersen T. Assaying Mitochondrial Respiration as an Indicator of Cellular Metabolism and Fitness. Methods Mol Biol. 2017;1601:79–87. doi: 10.1007/978-1-4939-6960-9_7. [DOI] [PubMed] [Google Scholar]
- 56.Yepez VA, Kremer LS, Iuso A, Gusic M, Kopajtich R, Konarikova E, Nadel A, Wachutka L, Prokisch H, Gagneur J. OCR-Stats: robust estimation and statistical testing of mitochondrial respiration activities using Seahorse XF Analyzer. PLoS One. 2018;13:e0199938. doi: 10.1371/journal.pone.0199938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morimoto N, Hashimoto S, Yamanaka M, Nakano T, Satoh M, Nakaoka Y, Iwata H, Fukui A, Morimoto Y, Shibahara H. Mitochondrial oxygen consumption rate of human embryos declines with maternal age. J Assist Reprod Genet. 2020;37:1815–1821. doi: 10.1007/s10815-020-01869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Force T, Kolaja KL. Cardiotoxicity of kinase inhibitors: the prediction and translation of preclinical models to clinical outcomes. Nat Rev Drug Discov. 2011;10:111–126. doi: 10.1038/nrd3252. [DOI] [PubMed] [Google Scholar]
- 59.Wallace KB. Adriamycin-induced interference with cardiac mitochondrial calcium homeostasis. Cardiovasc Toxicol. 2007;7:101–107. doi: 10.1007/s12012-007-0008-2. [DOI] [PubMed] [Google Scholar]
- 60.Nasci VL, Chuppa S, Griswold L, Goodreau KA, Dash RK, Kriegel AJ. miR-21-5p regulates mitochondrial respiration and lipid content in H9C2 cells. Am J Physiol Heart Circ Physiol. 2019;316:H710–H721. doi: 10.1152/ajpheart.00538.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nadanaciva S, Dykens JA, Bernal A, Capaldi RA, Will Y. Mitochondrial impairment by PPAR agonists and statins identified via immunocaptured OXPHOS complex activities and respiration. Toxicol Appl Pharmacol. 2007;223:277–287. doi: 10.1016/j.taap.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 62.Will Y, Hynes J, Ogurtsov VI, Papkovsky DB. Analysis of mitochondrial function using phosphorescent oxygen-sensitive probes. Nat Protoc. 2006;1:2563–2572. doi: 10.1038/nprot.2006.351. [DOI] [PubMed] [Google Scholar]
- 63.Wang SH, Zhu XL, Wang F, Chen SX, Chen ZT, Qiu Q, Liu WH, Wu MX, Deng BQ, Xie Y, Mai JT, Yang Y, Wang JF, Zhang HF, Chen YX. LncRNA H19 governs mitophagy and restores mitochondrial respiration in the heart through Pink1/Parkin signaling during obesity. Cell Death Dis. 2021;12:557. doi: 10.1038/s41419-021-03821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aguer C, Gambarotta D, Mailloux RJ, Moffat C, Dent R, McPherson R, Harper ME. Galactose enhances oxidative metabolism and reveals mitochondrial dysfunction in human primary muscle cells. PLoS One. 2011;6:e28536. doi: 10.1371/journal.pone.0028536. [DOI] [PMC free article] [PubMed] [Google Scholar]