Abstract
BACKGROUND:
Stem cell-based therapies have been developed to treat various types of wounds. Human adipose-derived stem cells (hADSCs) are used to treat skin wounds owing to their outstanding angiogenic potential. Although recent studies have suggested that stem cell spheroids may help wound healing, their cell viability and retention rate in the wound area require improvement to enhance their therapeutic efficacy.
METHODS:
We developed a core–shell structured spheroid with hADSCs in the core and human dermal fibroblasts (hDFs) in the outer part of the spheroid. The core–shell structure was formed by continuous centrifugation and spheroid incubation. After optimizing the method for inducing uniform-sized core–shell spheroids, cell viability, cell proliferation, migration, and therapeutic efficacy were evaluated and compared to those of conventional spheroids.
RESULTS:
Cell proliferation, migration, and involucrin expression were evaluated in keratinocytes. Tubular assays in human umbilical vein endothelial cells were used to confirm the improved skin regeneration and angiogenic efficacy of core–shell spheroids. Core–shell spheroids exhibited exceptional cell viability under hypoxic cell culture conditions that mimicked the microenvironment of the wound area.
CONCLUSION:
The improvement in retention rate, survival rate, and angiogenic growth factors secretion from core–shell spheroids may contribute to the increased therapeutic efficacy of stem cell treatment for skin wounds.
Keywords: Angiogenesis, Core–shell, Hetero-cell type
Introduction
Injecting adipose derived stem cells (ADSCs) into wound sites can improve wound healing [1]. ADSC secrete paracrine factors that can up-regulate re-epithelialization and angiogenesis in cutaneous wounds [2]. For example, vascular endothelial growth factor (VEGF), a representative paracrine factor released from ADSC, is essential for wound healing owing to its anti-apoptotic and angiogenic properties [3]. Easy growth, simple separation, and few side effects are features of ADSC that can be considered as additional factors for wound healing. ADSC spheroids have been developed to improve the advantages of transplanting ADSCs. The ADSC spheroids injected into the wound showed higher survival and retention rates than the ADSCs single cell suspension [4–7]. Spheroids typically have diameters of several hundred micrometers. As a result, ADSCs located in the center of a spheroid are exposed to hypoxic conditions [8]. A controlled mild hypoxic condition inside the spheroid encourages ADSCs to communicate more with their surrounding cells, which promotes increased proliferation and viability.
The co-culture of human ADSCs (hADSCs) and human dermal fibroblasts (hDFs) has been reported to improve cell viability and growth factor release, thereby facilitating wound healing [9, 10]. Co-transplantation of hADSCs and hDFs enhances extracellular matrix deposition, which is critical for wound closure [11] and reduces inflammation after wounding [12, 13]. Fibroblasts are the most prevalent and important type of cell for wound healing [14]. The spheroids using fibroblast are known to increase the expression of angiogenic factors such as hepatocyte growth factor (HGF) and VEGF [15]. Furthermore, fibroblasts can be obtained quickly from various sources and doubled within short period than other representative adult stem cells [16]. Therefore, we fabricated a hetero-cell type spheroid with a core–shell strategy to maximize the advantages of both hADSCs and hDFs co-transplantation in terms of enhancing cell viability, retention rate, and therapeutic cell to cell signaling pathways (Fig. 1). The core–shell structured spheroid had hADSCs in the core and hDFs located in the shell. We found that using hetero-cell type and core–shell structure strategies increased gene expression and secretion of representative angiogenic growth factors. These increments fortified wound healing effect [17]. We further confirmed that the core–shell structure strategy strengthens cell-to-cell connections and increases gene expression of cell adhesion proteins, allowing injected spheroids to remain in the wound for longer [18]. In addition, under hypoxic environments, the viability, and the secretion of the angiogenic growth factors were increased in our core–shell spheroids compared to other groups. Taken together, our hetero-cell type core–shell spheroids may be used in new approaches to enhance the wound healing effects using stem cells.
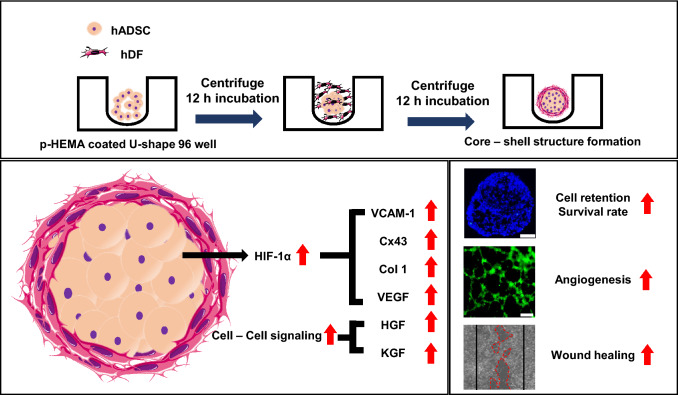
Fig. 1.
Schematic illustration of the core–shell spheroid formation process and the molecular mechanisms enhancing cell retention, cell survival, and secretion of growth factors that increase the wound healing efficacy of cells. Abbreviations; p-HEMA, poly-hydroxyethyl methacrylate; VCAM-1, vascular cell adhesion protein 1; Cx43, connexin 43; Col 1, collagen 1; VEGF, vascular endothelial growth factor; HGF, hepatocyte growth factor; KGF, keratinocyte growth factor
Materials and methods
Hetero-cell type core–shell spheroids
hADSCs and hDFs were purchased from Lonza (Basel, Switzerland) and cultured in Dulbecco's modified Eagle's medium (Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% (v/v) fetal bovine serum (Gibco BRL) and 1% (v/v) penicillin/streptomycin (Gibco BRL). The cells were incubated at 37 °C with 5% CO2. The medium was changed every two days. Cells within six passages were used for the experiments. Control and core–shell spheroids were formed in p-HEMA coated U-shaped 96-well plates (SPL Life Sciences, Pocheon, South Korea). Control spheroids were loaded into each well at 30,000 cells/well and centrifuged (1,300 × g for 5 min). Following centrifugation, the spheroids were incubated for 24 h in a 5% CO2 incubator at 37 °C and served as a control group for the core–shell spheroids. To form core–shell spheroids, hADSCs were loaded into each well and centrifuged (1,300 × g, 5 min, 15,000 cells/well) [19]. After centrifugation, hADSCs were incubated for 12 h in a 5% CO2 incubator at 37 °C. Then, hDFs were loaded onto the formed hADSC spheroids and centrifuged again (1,300 × g, 5 min, additional 15,000 cells/well). After the second centrifugation, core–shell spheroids were incubated additionally for 12 h in 5% CO2 incubator at 37 °C.
Spheroid characterization
The spheroids formed using 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Sigma-Aldrich, St. Louis, MO, USA) stained hADSCs and 3,3′-dihexyloxacarbocyanine iodide (DiO; Sigma-Aldrich)-stained hDFs were fixed in 4% (v/v) paraformaldehyde in water for 1 h, dehydrated with 20% sucrose, and embedded in optimal cutting temperature (OCT) compound (SciGen Scientific, Gardenas, CA, USA). The spheroids were then cut into 10 µm sections. DiI- and DiO- stained spheroids were evaluated using fluorescence microscopy (DMi8; Leica, Tokyo, Japan). The size of the spheroids was observed using optical microscopy (CKX53, Olympus, Tokyo, Japan), and the captured images were calculated using Photoshop CC (Adobe Systems, San Jose, CA, USA).
Quantitative real-time reverse transcription- polymerase chain reaction (qRT-PCR)
Expression levels of mRNAs from core–shell spheroids were analyzed using qRT-PCR. Total RNA was extracted from the samples using 1 mL TRIzol reagent (Life Technologies, Inc., Carlsbad, CA, USA) and 200 µL chloroform. Lysed samples were centrifuged at 12,000 rpm for 10 min at 4 °C. The RNA pellet was washed with 75% (v/v) ethanol in water then dried. After drying, the samples were dissolved in RNase-free water. For qRT-PCR, the SsoAdvanced Universal SYBR Green Supermix kit (Bio-Rad, Hercules, CA, USA) and CFX ConnectTM Real-Time PCR Detection System (Bio-Rad) were used.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
To evaluate cell viability, the spheroids were fixed in 4% (v/v) paraformaldehyde in water for 1 h, dehydrated with 20% sucrose, and embedded in OCT compound (SciGen Scientific). The spheroids were then cut into 10 µm sections. To evaluate the apoptotic activity of each spheroid, the sections were subjected to a TUNEL assay using an ApopTag1 fluorescent in situ apoptosis detection kit (Millipore, Bedford, MA, USA) according to the manufacturer's instructions. After 4′, 6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA, USA) staining, the TUNEL assay was performed using fluorescence microscopy (Leica).
Conditioned medium (CM)
The spheroids were transferred to p-HEMA coated 6-well culture plates (20 spheroids/well) and incubated with 2 mL serum-free DMEM (Gibco BRL) supplemented with 1% (v/v) PS (Gibco BRL) for 48 h to collect CM for further analyses.
Enzyme-linked immunosorbent assay (ELISA)
Concentrations of angiogenic growth factors in the CM obtained from the spheroids samples were determined using ELISA kits for human VEGF, HGF, and keratinocyte growth factor (KGF) (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol.
Migration assay
Keratinocyte migration assays were performed using 24-well plates. Briefly, 2 × 104 cells were added to each well and scraped 24 h after seeding. The number of cells that migrated into the scraped area was calculated using brightfield microscopy connected to an optical microscopy (Olympus CKX53). The cell migration area in the captured images was calculated using Photoshop CC (Adobe Systems).
Effects of CM on the proliferation and involucrin expression levels in normal human epithelial keratinocytes (NHEKs)
NHEKs were seeded in 24-well plates (2 × 104 cells/well) for the cell counting kit (CCK)-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) assay and in 6-well plates (1 × 105 cells/well) for immunocytochemistry. After 24 h, the cells were treated with a mixture of NHEK cultured media and CM derived from spheroids (3:1, v/v). The NHEKs were then cultured for 24 and 72 h. subsequently, the CCK-8 assay for NHEK proliferation was performed. After 48 h of CM treatment, immunocytochemistry for involucrin (ab53112, 1:500; Abcam, Cambridge, MA, USA) was performed. Involucrin-positive fluorescence signals were visualized using fluorescein isothiocyanate-conjugated secondary antibodies (Jackson ImmunoResearch Inc., West Grove, PA, USA). The cells were counterstained with DAPI and phalloidin and examined under a fluorescence microscope (Leica).
Tubular formation assay
Endothelial cell tube formation was assessed using an angiogenesis assay kit (ab204726, Abcam), according to the manufacturer’s instructions. Human umbilical vein endothelial cells (HUVECs) were seeded onto an extracellular matrix gel (2 × 104 cells/well) in 100 µL of HUVEC culture medium and CM derived from spheroids (3:1, v/v). and was incubated for 4 h at 37 °C. Following incubation, HUVECs were stained with staining dye for 30 min at 37 °C and observed under a fluorescence microscope (IX71, Olympus) [20].
Statistical analysis
All data are presented as the mean ± standard deviation and were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). One-way analysis of variance and unpaired Student’s t-test were used to determine the statistical significance. Differences with *p < 0.05, **p < 0.001, #p < 0.05, and ##p < 0.001 were considered significant.
Results
Characterization of core–shell spheroids
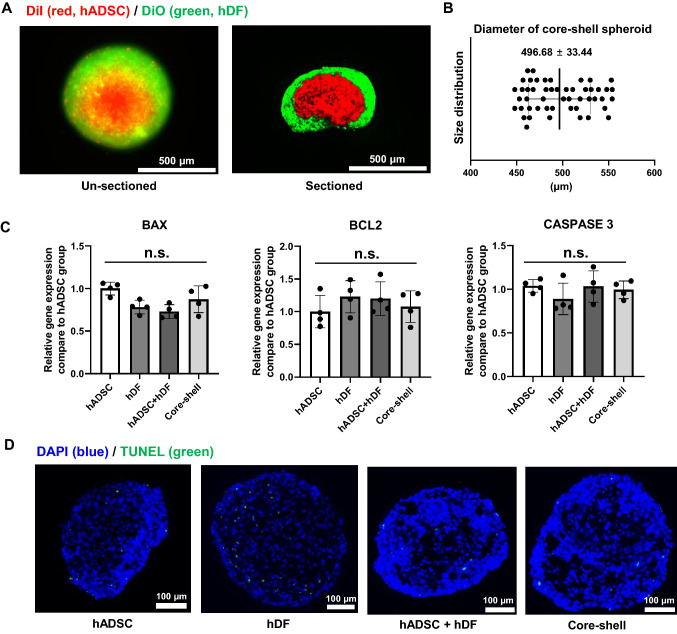
To check the formation of a hetero cell type core–shell spheroid, we first observed the structure and viability of the core–shell spheroid and compared it to that of spheroids of a single cell type. The sectioned figure (Fig. 2A, right) clearly indicates the core–shell structural formation. The size distribution of the core–shell spheroid was observed. The average diameter was 496.68 ± 33.44 μm (Fig. 2B) [21]. The size was determined by the number of cells. Previous study has shown that 3 × 104 cells had the highest healing effect [8]. We checked the viability of core–shell spheroids and compared them to single type spheroids (hADSC only and hDF only), and randomly mixed hADSC + hDF spheroids. The levels of apoptosis-related genes, such as B cell lymphoma-2 (BCL2), Bcl-2-associated X (BAX), and caspase-3, were analyzed using qRT-PCR (Fig. 2C) [22]. No significant differences were found when comparing the core–shell group to the other groups. TUNEL assays results also showed no significant differences in apoptosis between the groups (Fig. 2D) [23].
Fig. 2.
Characterization of core–shell spheroid. A Representative fluorescence image showing the structure of core–shell spheroid using DiI (red, hADSC) and DiO (green, hDF). Scale bar = 500 μm. B Size distribution of core–shell spheroids (n = 50). C Relative mRNA expression showing apoptotic activity in terms of BAX, BCL2, and caspase 3 levels from different types of spheroids (n = 4, n.s. not significant). D Representative images of TUNEL-stained (blue, nucleus; green, apoptotic cell) spheroid sections (Scale bars = 100 μm)
Enhanced expression levels of angiogenic genes and proteins
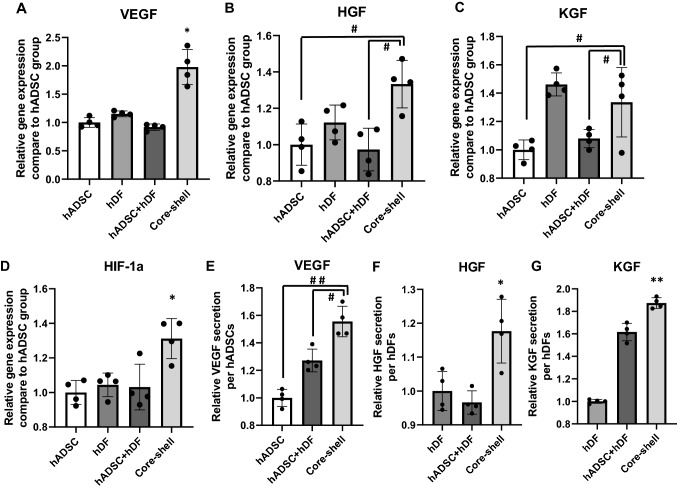
The effects of hetero-cell type core–shell spheroid structures on the expression levels of VEGF, HGF, KGF, and hypoxia-inducible factor-1 alpha (HIF-1α) genes were examined via qRT-PCR (Fig. 3A–D). The core–shell group showed significantly upregulated HIF-1α gene expression than other groups along with the enhancement of VEGF, HGF, and KGF gene expression [24, 25]. The VEGF gene expression in the core–shell group was approximately twofold higher than that in other groups. The gene expression of HGF and KGF in the core–shell group was also 1.3-fold higher than that in randomly mixed hADSC + hDF group. Angiogenic growth factor secretion as evaluated by ELISA (Fig. 3E–G) showed that the amount of growth factors in CM was approximately 1.5-fold higher for VEGF, 1.2-fold higher for HGF, and 1.8-fold higher for KGF in the hetero-cell type core–shell spheroid group as compared to that in other groups.
Fig. 3.
Enhanced expression of representative angiogenic growth factors via the upregulation of HIF-1α expression. Relative mRNA expression levels of A VEGF, B HGF, C KGF, and D HIF-1 α (n = 4, *p < 0.05 compared to other groups, and #p < 0.05 between two groups). Relative protein secretion of E VEGF/hADSCs, F HGF/hDF, and G KGF/hDF in different types of spheroids cultured for 48 h, as evaluated using ELISA (n = 4, #p < 0.05 between two groups, ##p < 0.001 between two groups, *p < 0.05 vs. other groups, and **p < 0.001 vs. other groups)
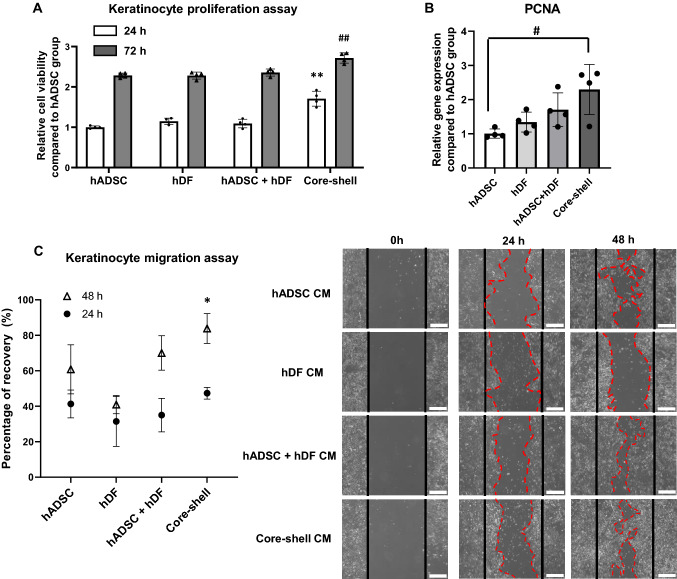
Effects of CM derived from core–shell spheroids on the proliferation and migration of NHEKs
NHEK proliferation was assessed after 24 and 72 h of spheroid CM treatment (Fig. 4A). NHEK proliferation increased significantly in the core–shell group after 24 and 72 h compared to that in the other groups. qRT-PCR gene expression data also showed prominent proliferating cell nuclear antigen expression in the core–shell group compared to that in other groups (Fig. 4B). The migration ability of CM-treated NHEKs was also evaluated. The recovered area after the initial scratch increased significantly in the core–shell groups after 48 h compared to that in the other groups (Fig. 4C). 24 h after the initial scratch, the recovered area (cell migrated area) observed in the core–shell group and other groups did not show any statistical difference. However, a significant statistical difference was detected in the core–shell group compared to other groups at 48 h after the initial scratch.
Fig. 4.
Response of NHEKs to conditioned medium (CM) derived from different types of spheroids. NHEK viability following treatment with CM was measured using the A CCK-8 assay kit (n = 4, **p < 0.001 compared to other 24 h groups, ##p < 0.001 compared to other 48 h groups) and B relative mRNA expression levels of the proliferating PCNA in NHEKs (n = 4, #p < 0.05 between two groups). C Quantification of CM-induced NHEK migration (left), (n = 4, *p < 0.05 compared to other 48 h groups) and representative images of 24 and 48 h NHEK migration (right). The red line indicates the border of migrated NHEKs (Scale bar = 100 μm)
Wound healing potential of core–shell spheroid
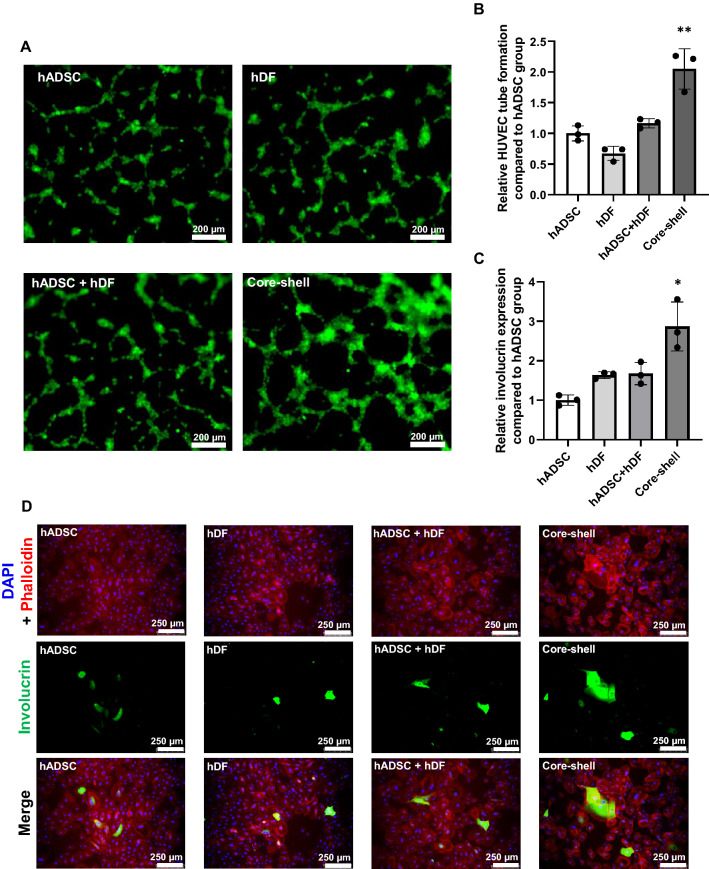
We treated HUVECs with spheroid CMs for 4 h to investigate whether core–shell spheroids can boost angiogenesis compared to other spheroids. HUVEC tubular formation was denser in the core–shell group then in HUVECs cultured under another spheroid CM (Fig. 5A, B). In addition, epidermis regeneration ability was evaluated by qRT-PCR and immunocytochemical staining for involucrin. As determined by qRT-PCR, core–shell spheroid CM treatment to NHEKs significantly enhanced the expression of involucrin compared to that in other treatment groups (Fig. 5C). Similar results were confirmed by immunocytochemical staining (Fig. 5D). The NHEKs cultured with the CM derived from core–shell spheroid showed significantly higher involucrin signal than that of NHEKs cultured with CM derived from other spheroids.
Fig. 5.
Effect of CM retrieved from different types of spheroids on human umbilical vein endothelial cells (HUVECs) and NHEKs. A Tubular formation of HUVECs at 4 h after CM treatment. B Quantification of the tube formation in HUVEC (n = 3, **p < 0.001 compared to other groups). C Relative mRNA expression of involucrin 48 h after CM treatment (*p < 0.05 compared to other groups). D Immunocytochemistry of involucrin (green) in NHEKs stained with DAPI (nucleus, blue) and phalloidin (F-actin, red)
Enhanced cell retention and survival of core–shell spheroid in harsh environment
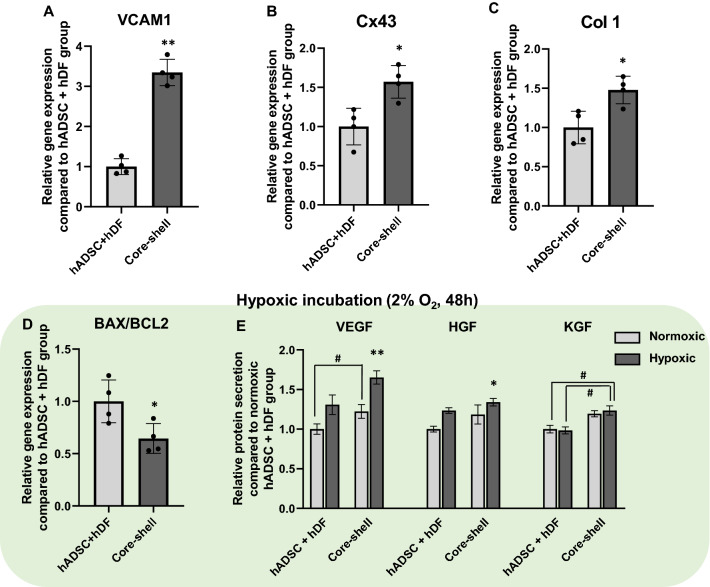
Compared to mixed hADSC + hDF spheroids, core–shell spheroids showed approximately threefold higher expression of vascular cell adhesion protein 1 (VCAM-1) (Fig. 6A). The expression of connexin 43 (Cx43) and type 1 collagen (Col1) was much higher in the core–shell group than in the hADSC + hDF group (Fig. 6B, C). The viability and angiogenic growth factor release under hypoxic incubation (2% O2, serum free medium) were also assessed. Core–shell spheroids showed a lower BAX/BCL2 ratio, indicating that they were less susceptible to apoptosis when cultured for 48 h without serum and with low oxygen levels (Fig. 6D). ELISA analysis of representative angiogenic growth factor secretion in hypoxic condition (Fig. 6E) showed that the amount of growth factors in CM was approximately 1.2-fold higher for VEGF, 1.1-fold higher for HGF, and 1.25-fold higher for KGF in the hetero-cell type core–shell spheroid group as compared to randomly mixed hADSC + hDF spheroids. In addition, VEGF and HGF secretion from core–shell spheroids in hypoxic conditions was highly elevated compared to that in normoxic conditions. The increased angiogenic growth factors release of core–shell spheroids compared to hADSC + hDF spheroids confirmed a clear increase in healing efficacy even in harsh environments.
Fig. 6.
Enhanced cell retention and survival ability of core–shell spheroids. Relative mRNA expression levels of A VCAM-1, B Cx43, and C Col 1 (n = 4, *p < 0.05 vs. hADSC + hDF group, **p < 0.05 vs. hADSC + hDF group). D Relative mRNA expression levels of BAX/BCL2 in the two types of spheroids after 48 h of hypoxic incubation (2% O2, serum-free media, n = 4, *p < 0.05 vs. hADSC + hDF group). E Relative protein secretion of VEGF, HGF, and KGF in the two types of spheroids cultured under normoxic and hypoxic conditions for 48 h (ELISA, n = 4, *p < 0.05 compared to hADSC + hDF group, **p < 0.001 compared to hADSC + hDF group, #p < 0.05 between two groups)
Discussion
Although recent studies have suggested that using stem cell spheroids can improve wound healing [26], Transplanted cells suffer irreversible ischemic damage owing to hypoxic conditions in the wound area [27], leading to eventual apoptosis. These are the main reasons for the low retention and survival rates of transplanted cells [28]. Previously, there was an effort to increase the retention rate using a hydrogel or patch, but this was weak in increasing the viability of the spheroid itself [29]. Co-transplantation of hADSCs and hDFs improves cell viability and growth factor release, which can improve wound healing efficacy [9, 10]. In addition, co-transplantation enhanced extracellular matrix deposition, which is critical for wound closure.
In this study, we devised hetero-cell type spheroids using a core–shell strategy that improved the viability and release of angiogenic growth factors in wounds under harsh conditions. Core–shell spheroids were manufactured using p-HEMA-coated U-shaped 96-well plates. The anti-fouling feature of p-HEMA hampered the attachment of the spheroids to the wall of the plates, thus enabling the secondary cells to attach to the surface of the spheroid again. As shown in Fig. 2, the core–shell spheroids showed a size range that could sufficiently induce HIF-1α upregulation [30] with no decrease in viability compared to single cell type spheroids (Fig. 2B-D). Therefore, in the inner part, similar to spheroids with a single cell type, the expression of HIF-1α was increased due to the mild hypoxic microenvironment. Previous studies have demonstrated that the environment inside stem cell spheroids enhances therapeutic paracrine secretion. In addition, core–shell spheroids showed increased expression of representative angiogenic factors via enhanced HIF-1α gene expression (Fig. 3). HIF-1α induces the upregulation of the angiogenic growth factors, such as VEGF and HGF [24, 25], along with the increment of VCAM-1 and Cx43 expression that can enhance the cell retention rate and cell to cell communication [31–33]. VEGF, an angiogenic factor, is increased. It is known to enhance angiogenesis, a crucial process required for wound healing [34]. The therapeutic efficacy of the core–shell spheroids was assessed by NHEK proliferation, migration, and involucrin expression assays. NHEKs treated with CM collected from core–shell spheroids showed significantly enhanced cell growth and increased proliferation, along with improved epidermal regeneration capacity (Fig. 4A, B and Fig. 5C). The recovered area after the initial scratch showed a dramatic enhancement in the core–shell groups compared with that in the other groups (Fig. 4C). Moreover, the enhanced angiogenic ability of the core–shell spheroids was also confirmed (Fig. 5A, B).
We also found that our core–shell spheroids showed higher cell retention and survival potential than randomly mixed spheroids. VCAM-1, Cx43, and Col 1 gene expression levels were up-regulated in the core–shell spheroid group than in the other groups (Fig. 6A-C). Upregulated HIF-1α enhances the homing ability toward wound areas, which increases the cell retention rate though the upregulation of VCAM-1 [35]. Cx43 is known to promote cell-to-cell communication and cell survival in the wound area [31]. Increased cell-to-cell communication between hADSC and hDF promotes KGF expression [10] which is important for wound healing [36]. In addition, Col 1 produces components of the extracellular matrix [37]. Therefore, up-regulation of Cx43 and Col1 enhances stem cell survival in harsh areas, such as wounds. Furthermore, core–shell spheroids presented outstanding viability and angiogenic growth factors under hypoxic conditions that mimicked the wound area (Fig. 6D) [38].
The hetero-cell type core–shell spheroid developed in this study has several advantages, such as higher retention rate, survival rate, and release of angiogenic growth factors than previously developed spheroids, resulting in enhanced therapeutic efficacy of wound treatment. The core–shell structure induced mild hypoxic conditions and up-regulated HIF-1α gene expression more efficiently than a randomly mixed structure. We found that without distinguishable structures based on the cell type, randomly mixed hetero-cell type spheroids showed lower HIF-1α expression and exhibited a low therapeutic effect. Only when the hetero-cell type spheroids were formed in a core–shell manner, the interaction between hADSCs and hDFs was increased, and therapeutic efficacy was significantly improved. Interestingly, KGF secretion was enhanced, which can upregulate keratinocyte proliferation and migration leading to fortified wound healing. Moreover, we identified the genes that were highly expressed when using this hetero-cell type core–shell spheroid and showed that they were associated with improved treatment efficacy. Therefore, hetero-cell type core–shell spheroids provide new insights into alternative techniques to enhance the therapeutic efficiency of stem cell spheroids in cell-based interventions.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF), and the Ministry of Science and ICT (NRF-2018M3A9E2023255, NRF-2019R1C1C1007384, NRF-2020M2D9A3094171, and NRF-2021R1A4A1032782). This research was also supported by a Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Health & Welfare; project Number: 21A0102L1-11).
Declarations
Conflict of interest
The authors declare no financial conflicts of interest.
Ethical statement
No experiments involving animals or humans were conducted in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cooper DR, Wang C, Patel R, Trujillo A, Patel NA, Prather J, et al. Human adipose-derived stem cell conditioned media and exosomes containing MALAT1 promote human dermal fibroblast migration and ischemic wound healing. Adv Wound Care. 2018;7(9):299–308. doi: 10.1089/wound.2017.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson WM, Nesti LJ, Tuan RS. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res Ther. 2012;3:20. doi: 10.1186/scrt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilforoushzadeh MA, Khodadadi Yazdi M, Baradaran Ghavami S, Farokhimanesh S, Mohammadi Amirabad L, Zarrintaj P, et al. Mesenchymal stem cell spheroids embedded in an injectable thermosensitive hydrogel: an in situ drug formation platform for accelerated wound healing. ACS Biomater Sci Eng. 2020;6(9):5096–5109. doi: 10.1021/acsbiomaterials.0c00988. [DOI] [PubMed] [Google Scholar]
- 4.Bhang SH, Lee S, Shin J-Y, Lee T-J, Kim B-S. Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng Part A. 2012;18(19–20):2138–2147. doi: 10.1089/ten.tea.2011.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci. 2020;21(4):1306. doi: 10.3390/ijms21041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy KC, Whitehead J, Zhou D, Ho SS, Leach JK. Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. ACTA Biomater. 2017;64:176–186. doi: 10.1016/j.actbio.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu T-Y, Yu K-F, Kuo S-H, Cheng N-C, Chuang E-Y, Yu J-S. Enzyme-crosslinked gelatin hydrogel with adipose-derived stem cell spheroid facilitating wound repair in the murine burn model. Polymers. 2020;12(12):2997. doi: 10.3390/polym12122997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Im G-B, Kim S-W, Bhang SH. Fortifying the angiogenic efficacy of adipose derived stem cell spheroids using spheroid compaction. J Ind Eng Chem. 2021;93:228–236. doi: 10.1016/j.jiec.2020.09.027. [DOI] [Google Scholar]
- 9.Park S-J, Kim K-J, Kim W-U, Cho C-S. Interaction of mesenchymal stem cells with fibroblast-like synoviocytes via cadherin-11 promotes angiogenesis by enhanced secretion of placental growth factor. J Immunol. 2014;192(7):3003–3010. doi: 10.4049/jimmunol.1302177. [DOI] [PubMed] [Google Scholar]
- 10.Lu W, Yu J, Zhang Y, Ji K, Zhou Y, Li Y, et al. Mixture of fibroblasts and adipose tissue-derived stem cells can improve epidermal morphogenesis of tissue-engineered skin. Cells Tissues Organs. 2012;195(3):197–206. doi: 10.1159/000324921. [DOI] [PubMed] [Google Scholar]
- 11.Haubner F, Muschter D, Pohl F, Schreml S, Prantl L, Gassner HG. A co-culture model of fibroblasts and adipose tissue-derived stem cells reveals new insights into impaired wound healing after radiotherapy. Int J Mol Sci. 2015;16(11):25947–25958. doi: 10.3390/ijms161125935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zupan J. Mesenchymal stem/stromal cells and fibroblasts: their roles in tissue injury and regeneration, and age-related degeneration. fibroblasts—advances in inflammation. Autoimmun Cancer. 2021;2:1–25. [Google Scholar]
- 13.Kobayashi K, Suzuki T, Nomoto Y, Tada Y, Miyake M, Hazama A, et al. A tissue-engineered trachea derived from a framed collagen scaffold, gingival fibroblasts and adipose-derived stem cells. Biomaterials. 2010;31(18):4855–4863. doi: 10.1016/j.biomaterials.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Singh M, Pierpoint M, Mikos AG, Kasper FK. Chondrogenic differentiation of neonatal human dermal fibroblasts encapsulated in alginate beads with hydrostatic compression under hypoxic conditions in the presence of bone morphogenetic protein-2. J Biomed Mater Res Part A. 2011;98(3):412–424. doi: 10.1002/jbm.a.33129. [DOI] [PubMed] [Google Scholar]
- 15.Enzerink A, Rantanen V, Vaheri A. Fibroblast nemosis induces angiogenic responses of endothelial cells. Exp Cell Res. 2010;316(5):826–835. doi: 10.1016/j.yexcr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Chang Y, Li H, Guo Z. Mesenchymal stem cell-like properties in fibroblasts. Cell Physiol Biochem. 2014;34(3):703–714. doi: 10.1159/000363035. [DOI] [PubMed] [Google Scholar]
- 17.Honnegowda TM, Kumar P, Udupa EGP, Kumar S, Kumar U, Rao P. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast Aesth Res. 2015;2:243–249. doi: 10.4103/2347-9264.165438. [DOI] [Google Scholar]
- 18.Uitterdijk A, Groenendijk BC, Gorsse-Bakker C, Panasewicz A, Sneep S, Tempel D, et al. Time course of VCAM-1 expression in reperfused myocardial infarction in swine and its relation to retention of intracoronary administered bone marrow-derived mononuclear cells. PLoS ONE. 2017;12(6):e0178779. doi: 10.1371/journal.pone.0178779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breslin S, O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today. 2013;18(5–6):240–249. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Jo H, Gajendiran M, Kim K. Influence of PEG chain length on colloidal stability of mPEGylated polycation based coacersomes for therapeutic protein delivery. J Ind Eng Chem. 2020;82:234–242. doi: 10.1016/j.jiec.2019.10.018. [DOI] [Google Scholar]
- 21.Murphy KC, Hung BP, Browne-Bourne S, Zhou D, Yeung J, Genetos DC, et al. Measurement of oxygen tension within mesenchymal stem cell spheroids. J R Soc Interface. 2017;14(127):20160851. doi: 10.1098/rsif.2016.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roset R, Ortet L, Gil-Gomez G. Role of Bcl-2 family members on apoptosis: what we have learned from knock-out mice. Front Biosci-landmark. 2007;12(12):4722–4730. doi: 10.2741/2421. [DOI] [PubMed] [Google Scholar]
- 23.Loo DT. In situ detection of apoptosis by the TUNEL assay: an overview of techniques. In: Didenko V. Editor. DNA Damage Detection In Situ, Ex Vivo, and In Vivo. Methods in Molecular Biology, 2011; Vol 682. Humana Press, Totowa, NJ. 10.1007/978-1-60327-409-8_1 [DOI] [PubMed]
- 24.Dai Y, Xu M, Wang Y, Pasha Z, Li T, Ashraf M. HIF-1α induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol. 2007;42(6):1036–1044. doi: 10.1016/j.yjmcc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu F, Lin Y, Zhan T, Chen L, Guo S. HGF expression induced by HIF-1α promote the proliferation and tube formation of endothelial progenitor cells. Cell Biol Int. 2015;39(3):310–317. doi: 10.1002/cbin.10397. [DOI] [PubMed] [Google Scholar]
- 26.Santos JM, Camões SP, Filipe E, Cipriano M, Barcia RN, Filipe M, et al. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res Ther. 2015;6(1):1–19. doi: 10.1186/s13287-015-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, et al. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17(5–6):279–290. doi: 10.1159/000094140. [DOI] [PubMed] [Google Scholar]
- 28.Cai L, Dewi RE, Heilshorn SC. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv Func Mater. 2015;25(9):1344–1351. doi: 10.1002/adfm.201403631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SJ, Park J, Byun H, Park Y-W, Major LG, Lee DY, et al. Hydrogels with an embossed surface: an all-in-one platform for mass production and culture of human adipose-derived stem cell spheroids. Biomaterials. 2019;188:198–212. doi: 10.1016/j.biomaterials.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 30.Jauković A, Abadjieva D, Trivanović D, Stoyanova E, Kostadinova M, Pashova S, et al. Specificity of 3D MSC spheroids microenvironment: impact on MSC behavior and properties. Stem Cell Rev Rep. 2020;16(5):853–875. doi: 10.1007/s12015-020-10006-9. [DOI] [PubMed] [Google Scholar]
- 31.Wang D, Shen W, Zhang F, Chen M, Chen H, Cao K. Connexin43 promotes survival of mesenchymal stem cells in ischaemic heart. Cell Biol Int. 2010;34(4):415–423. doi: 10.1042/CBI20090118. [DOI] [PubMed] [Google Scholar]
- 32.Wei Y, Zhang L, Chi Y, Ren X, Gao Y, Song B, et al. High-efficient generation of VCAM-1+ mesenchymal stem cells with multidimensional superiorities in signatures and efficacy on aplastic anaemia mice. Cell Prolif. 2020;53(8):e12862. doi: 10.1111/cpr.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aomatsu E, Chosa N, Nishihira S, Sugiyama Y, Miura H, Ishisaki A. Cell-cell adhesion through N-cadherin enhances VCAM-1 expression via PDGFRβ in a ligand-independent manner in mesenchymal stem cells. Int J Mol Med. 2014;33(3):565–572. doi: 10.3892/ijmm.2013.1607. [DOI] [PubMed] [Google Scholar]
- 34.Cañedo-Dorantes L, Cañedo-Ayala M. Skin acute wound healing: a comprehensive review. Int J Inflam. 2019;2019:3706315. [DOI] [PMC free article] [PubMed]
- 35.Li H, Ge C, Zhao F, Yan M, Hu C, Jia D, et al. Hypoxia-inducible factor 1 alpha–activated angiopoietin-like protein 4 contributes to tumor metastasis via vascular cell adhesion molecule-1/integrin β1 signaling in human hepatocellular carcinoma. Hepatology. 2011;54(3):910–919. doi: 10.1002/hep.24479. [DOI] [PubMed] [Google Scholar]
- 36.Qu Y, Cao C, Wu Q, Huang A, Song Y, Li H, et al. The dual delivery of KGF and b FGF by collagen membrane to promote skin wound healing. J Tissue Eng Regen Med. 2018;12(6):1508–1518. doi: 10.1002/term.2691. [DOI] [PubMed] [Google Scholar]
- 37.Kisling A, Lust RM, Katwa LC. What is the role of peptide fragments of collagen I and IV in health and disease? Life Sci. 2019;228:30–34. doi: 10.1016/j.lfs.2019.04.042. [DOI] [PubMed] [Google Scholar]
- 38.Lin JH-C, Lou N, Kang N, Takano T, Hu F, Han X, et al. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28(3):681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]