Abstract
Cortical interneurons can be categorized into distinct populations based on multiple modalities, including molecular signatures and morpho-electrical (M/E) properties. Recently, many transcriptomic signatures based on single-cell RNA-seq have been identified in cortical interneurons. However, whether different interneuron populations defined by transcriptomic signature expressions correspond to distinct M/E subtypes is still unknown. Here, we applied the Patch-PCR approach to simultaneously obtain the M/E properties and messenger RNA (mRNA) expression of >600 interneurons in layer V of the mouse somatosensory cortex (S1). Subsequently, we identified 11 M/E subtypes, 9 neurochemical cell populations (NCs), and 20 transcriptomic cell populations (TCs) in this cortical lamina. Further analysis revealed that cells in many NCs and TCs comprised several M/E types and were difficult to clearly distinguish morpho-electrically. A similar analysis of layer V interneurons of mouse primary visual cortex (V1) and motor cortex (M1) gave results largely comparable to S1. Comparison between S1, V1, and M1 suggested that, compared to V1, S1 interneurons were morpho-electrically more similar to M1. Our study reveals the presence of substantial M/E variations in cortical interneuron populations defined by molecular expression.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12264-022-00983-x.
Keywords: Cortical interneuron, Cell population, Transcriptomic signature, Morpho-electrical properties, Correlation analysis, Brain area
Introduction
Cortical interneurons can be categorized into distinct populations based on multiple criteria, such as soma location, dendritic and/or axonal morphology, intrinsic electrophysiology, and gene expression [1–4]. Based on molecular marker expression, cortical interneurons have been divided into three major subclasses [expressing parvalbumin (Pvalb), somatostatin (Sst), or serotonin 3A receptor (Htr3aR)] with distinct morpho-electrical M/E properties [5]. However, these three major subclasses each contain multiple diverse subtypes that are considered to have more specific functions in neural circuitry [6, 7]. Thus, the identification of more specific interneuron subtypes with characteristic gene expression profiles and distinguishable M/E features is a critical step toward a more mechanistic understanding of how cortical interneurons participate in neural processing. Moreover, different studies or labs have applied their criteria to identify interneuron subtypes, which makes their mutual results hard to interpret. To this end, obtaining multimodal information at the single-cell level is needed but is still in its infancy due to technical constraints. Indeed, there is a growing consensus that an efficient classification of cortical interneurons requires the integration of multiple modalities [8–12].
The recent application of single-cell RNA sequencing (scRNAseq) to neuronal classification has identified many transcriptomic cell types, greatly contributing to our understanding of the diversity of cortical interneurons [13, 14]. Moreover, many transcriptomic signatures (or marker genes) have been selected to indicate distinct cell types, such as Rspo2 for Pvalb subtypes and Myh8 for Sst subtypes. Previous studies have shown that Lamp5-Lhx6 cells correspond to deep-layer neurogliaform cells (but are recognized as chandelier cells in another study [15]) and the expression of Hpse and Chodl distinguish non-Martinotti cells and long-projecting interneurons, respectively [13, 16, 17]. Based on these reports, it is optimistic to think that these transcriptomic signatures provide genetic access to label specific interneuron subtypes [18]. However, it is still unclear to what extent different cell populations defined by expression profiles of these putative transcriptomic signatures represent functionally distinct interneuron subtypes (e.g., M/E subtypes). To tackle this problem, we combined a modified single-cell RT-PCR procedure with the patch-clamp recording (Patch-PCR) to simultaneously obtain the M/E properties as well as mRNA expression (e.g., transcriptomic signatures and neurochemical markers) in individual cortical interneurons, followed by cell population identification and multimodal correlation analysis.
Arealization and layered structures are structural hallmarks and functional substrates of the cerebral cortex. Growing evidence suggests that there are substantial variations in gene expression pattern, cell type distribution, and neuronal wiring specificity between different cortical layers and brain areas [7, 19, 20]. Recent Patch-seq studies in M1 and V1 have identified many transcriptomic interneuron subtypes by pooling together cells from all layers [10, 11], overlooking the potentially unique correlations within specific layers. Meanwhile, it is unclear to what extent transcriptomic signatures from scRNAseq are correlated to the M/E properties in S1. In the canonical cortical circuit model, layer V (L5) is considered an output hub that sends long-range projections to a variety of other cortical and subcortical structures. Since projection neurons in L5 strategically recruit nearby interneurons to finely control their firing patterns, it is important to profile the diversity of cortical interneurons in specific layers. Therefore, we focused on L5 interneurons in S1 to provide a comprehensive dataset, facilitating a multimodal correlation analysis of these interneurons and a systematic comparison with L5 interneurons of M1 and V1 using recently-published Patch-seq datasets [10, 11].
Here, by applying the developed Patch-PCR technique, we collected M/E properties and molecular marker expression profiles (i.e., neurochemical markers and recently-identified transcriptomic signatures) in L5 interneurons of S1, aiming to investigate the correlation of molecularly-defined cell populations with M/E properties. To begin with, an integrated classification procedure based on extracted morphological and electrophysiological features yielded 11 major M/E subtypes. Then, we defined interneuron cell populations based on either the combinatorial expression of neurochemical markers (NCs) or the high expression of specific transcriptomic signatures (TCs). Further correlation analysis revealed that most NCs and TCs comprise several M/E types, and it is hard to clearly distinguish different TCs based on extracted M/E properties. Using model-based prediction analysis, we found that selected neurochemical marker combinations and the expression levels of specific transcriptomic signatures were significantly associated with the prediction of certain M/E types. Further analysis of L5 interneurons from V1 and M1 using recently-published Patch-seq data obtained results similar to S1. Finally, a direct comparison of axonal projection patterns and firing behaviors of TCs shared between S1, M1, and V1 indicated that, compared to V1, L5 interneurons of S1 appeared morpho-electrically more similar to M1. Overall, we demonstrated that cortical interneuron cell populations defined by molecular marker expression contain substantial heterogeneity in M/E properties and hardly represent distinct M/E subtypes.
Materials and Methods
Mice
All procedures were approved by the Animal Care and Use Committee of the Shanghai Medical College of Fudan University and were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All mice, which were originally obtained from the Jackson Laboratory or gifted from other labs, were maintained following the Guidelines for the Care and Use of Laboratory Animals at Fudan University (FE21173). Mice of both sexes with an average age of 28 postnatal days (P25–P30) were used. Mice with a C57B/L6 background were used unless otherwise noted. Mice were group-housed with a 12/12 h light/dark cycle and with food and water provided ad libitum. The mouse strains used were as follows: Gad2-ires-Cre (RRID: IMSR_JAX:013044), tdTomato reporter line Ai14 (RRID: IMSR_JAX:007914), Htr3a-EGFP (CD-1 background, RRID: MMRRC_000273-UNC). The number of pups per litter was kept at 4–6 to obtain comparable body weights at the time of experiments.
Slice Preparation
Slices were prepared in accordance with previous studies [21]. All solutions were prepared with Diethyl pyrocarbonate (DEPC)-treated autoclaved water to minimize RNase contamination. All glassware was baked at 250°C for at least 2 h to inactivate RNases. RNase ZAP (Thermo Fisher Scientific, Waltham, USA; cat. no. AM9780) was routinely used during the whole preparation process, including gloves, bench, spatulas, stir bars, counters, and anything else that may have come into contact with the reagents or solutions. Dedicated chemical reagents and stock solutions were used whenever possible to reduce cross-contamination.
Electrophysiological Recording and Cytosol Aspiration
Technical details of brain slice electrophysiological recording and several tricks for successful cytosolic aspiration after patch recording can be found in a previously-published Patch-seq protocol [22]. Glass electrodes (3–5 MΩ) were filled with an internal solution prepared according to a previous protocol with some modifications [22]. Components of the internal solution were as follows (in mmol/L): 93 K-gluconate, 12 KCl, 10 HEPES, 0.2 EGTA, 4 Mg-ATP, 0.3 Na-GTP, 10 Na2-phosphocreatine, 20 µg/mL glycogen, and 0.5% biocytin; pH adjusted to 7.25 with RNase-free 1.0 mol/L KOH using a dedicated pH meter. The osmolarity was adjusted to ~260 mOSM using RNase-free water. An RNase inhibitor (RRI; Clontech, Mountain View, USA; cat. no. 2313A, 1:80) was added to inactivate endogenous RNases on the day of use. A small volume of internal solution (~0.5 µL) was added using a customized 1-mL syringe with a 0.2-µm syringe filter and a tapered pipette tip. The apparatus used for patch-clamp recording and cell content collection was wiped carefully with RNase ZAP and then cleaned with RNase-free 75% EtOH and DEPC-treated water. Special attention was paid to the circulation tube, stage, capillary glass, pipette holder, micropipette puller (P-97, Sutter Instrument, Novato, USA), positive-pressure device (custom-made apparatus used to eject the pipette contents into the PCR tube after aspirating the cell contents), and the ice-cold container used to store pre-filled PCR tubes and 1-mL syringe with internal solution.
Single-Cell cDNA Library Construction, Quality Check, and Molecular Marker Detection
mRNA species in single-cell harvests (~4.3 µL) were subsequently converted into cDNA using a Smart-seq2-based protocol as described previously [22]. Full-length cDNA was amplified using KAPA HiFi HotStart ReadyMix (2×, KK2602, Roche, Basel, Switzerland). Bead purification of PCR products was performed using AMPure XP (A63881, Beckman Coulter, Brea, USA) according to the manufacturer’s instructions. Specifically, the ratio of beads/PCR products was set to 0.7 based on a previous study [23]. We checked the concentrations and size distributions of partial cDNA samples (n = 203) on an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, USA; cat. no. G2938C) using an Agilent High Sensitivity DNA Kit (Agilent Technologies, cat. no. 5067-4626) according to the manufacturer’s instructions. A good cDNA sample showed a prominent peak spanning a 1,500–2,000 base-pair (bp) region with very few short (<500 bp) fragments. We used two parameters to quantitatively describe the quality of cDNA samples: the average length and total yield of cDNA fragments in the range of 400–9,000 bp. A cut-off threshold was set to 1,300 bp in average length and 1 ng in total yield based on previous criteria [8] (Fig. S1A). In addition, quantitative PCR (qPCR) amplification of the housekeeping gene GAPDH was applied in these quality-checked cDNA samples as previously described [23]. The typical Ct value of a good cDNA sample was 16–18 (Fig. S1C). Samples with Ct values of GAPDH >19 were typically excluded from further analysis.
Neurochemical markers were examined using PCR and transcriptomic signatures by qPCR. Two different sets of transcriptomic signatures were detected separately via qPCR (Fig. 1H) according to the expression of three primary interneuron markers (Pvalb, Sst, and Htr3aR) in the first-round PCR detection (Fig. 1G). In some cases, qPCR was also applied to the detection of neurochemical markers to further confirm the PCR results. Primers used in this study were mainly obtained from previous papers with the rest newly designed using Primer-BLAST (National Center for Biotechnology Information, Bethesda, USA) (Tables S1 and S2). Dilutions of the cDNA library (100 pg as template) generated from total RNA extraction were applied to test the efficiency of each primer. All primers yielded clear bands at the appropriate positions in the gel. PCR products were collected and sequenced to check for nonspecific amplification of cDNA. Sequences of amplification products highly matched their reference sequences. The components of the sc-PCR reaction (10 μL) were as follows: 1 μL cDNA template (10× dilution of original cDNA amplification solution), 0.3 μmol/L primers, 5 μL 2× PCR Buffer for KOD FX Neo (Toyobo, Kita-ku, Japan), 2 μL deoxynucleotide (dNTPs) (2 mmol/L), and 1.4 μL autoclaved water. The PCR conditions were 60°C annealing temperature with a 30-s elongation time using 35 cycles. The components of the sc-qPCR reaction (10 μL) were as follows: 5 μL 2× PowerUpTM SYBRTM Green Master Mix (Applied Biosystems, Waltham, USA), 0.3 μL primers, 1 μL cDNA template (5× dilution of purified cDNA solution), and 3.4 μL nuclease-free water. The thermal cycle conditions for qPCR were selected according to the manufacturer’s instructions (Tm <60°C, 40 cycles). The melting curves of all amplification products showed a single peak. The fluorescence intensity threshold of the amplification curve of the same batch was automatically determined. The Ct values of those genes not amplified after 40 cycles were set at 40.
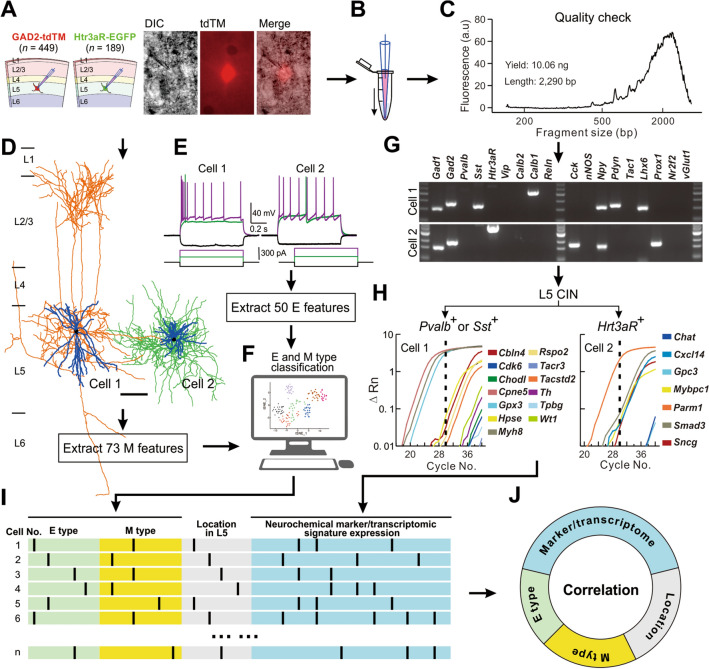
Fig. 1.
Multimodal data acquisition using Patch-PCR. A Cartoons and images showing patch-clamp recording of L5 interneurons in S1 that are fluorescently labeled in Gad2-tdTomato (n = 449 from 69 mice) and Htr3aR-EGFP mouse lines (n = 189 from 27 mice). B, C Aspiration of cell contents by a glass electrode (B), and quality check of single-cell cDNA libraries using the Agilent 2100 bioanalyzer (C). Total yield (ng) and the average length of cDNA fragments (bp) were used to quantitatively estimate the quality of single-cell cDNA libraries. D Biocytin filling and morphological reconstruction of two L5 interneurons showing differential axonal projection patterns (blue, dendrites; orange, axon in cell 1; green, axon in cell 2; scale bar, 100 µm). E Intrinsic electrophysiological properties of the same cells as in D showing typical firing patterns in response to long square-wave current injections. F Extraction of morphological and electrophysiological features followed by M- and E-type classification. G PCR detection of commonly-used interneuron markers from the same cells as in D. Gad1/2 and vGlut1 served as positive and negative controls, respectively. A 100-bp DNA ladder was used for reference. H qPCR examination of transcriptomic signatures from the same cells as in D. Two sets of genes were tested separately in different cells according to the expression of primary neurochemical markers (Pvalb/Sst or Htr3aR). I, J Illustration of multimodal data collection and correlation analysis.
Electrophysiological Analysis
The features used to describe electrophysiological properties were mainly taken from previous studies [12, 24]. We enlarged our electrophysiological feature set by defining some new features based on previous descriptions, such as “hump” properties [25, 26]. To facilitate consistent comparisons between cells, we developed a standard analysis pipeline covering a wide range of electrophysiological properties. The selection of individual sweeps for feature calculation was kept consistent across cells to avoid analysis bias. A full list of electrophysiological features and their definitions are summarized in Table S3.
Morphological Analysis
Biocytin-filled neurons were stained as previously described [1]. Fluorophore-conjugated Streptavidin (1:500, #016–160–084, Jackson ImmunoResearch, West Grove, USA; RRID: AB_2337244) was used to visualize neuronal processes. Prepared slices were imaged under a confocal microscope (FV-3000; Olympus, Tokyo, Japan). Series of Z-stacks (scanning size was set to 1024 × 1024 with an interval of 1 µm along the Z-axis) covering all visible processes of target cells were obtained, with at least 5% overlap between neighboring Z-stacks. These individual Z-stacks were then stitched automatically or semi-automatically using Xuvstitch software (Imaris, Bitplane, Belfast, United Kingdom) and exported to a composite 2D file. Series TIFFs (tag image file format) exported from the composite 2D file through Imaris software were used for later manual reconstruction using Neurolucida software (MicroBrightField, Williston, USA).
Based on the literature and available analysis toolkits (for example, Neurolucida Explorer and L-Measure), a set of morphological features was created, which included widely used shape features (e.g., branch order and total branch length), and new features such as the laminar distribution of axons and nodes. Morphological measurements (including raw data) were extracted using Neurolucida Explorer, based on which morphological features were further analyzed. Morphological features involving differences in the Z-dimension were not included in our morphological analysis due to the shrinkage in the Z-dimension of the brain slices after processing. As the geometry and projection patterns of axons are decisive in distinguishing the morphological subtypes of interneurons [1, 27], we mainly focused our analysis on axonal properties, although some dendritic features were also included in our analysis. A full list of morphometric features and their definitions is available in Table S4.
Classification of M Types and E Types and Validation of M/E Types
The overall workflow for M- and E-type classification involved: (1) establishing a training set using a representative subset of samples, (2) feature selection, (3) classification model training, (4) prediction of the unlabeled test set using selected models, (5) visualization of all samples in a low-dimensional space, and (6) comparison with unsupervised clustering results. To obtain an optimized morphological and electrophysiological feature set for supervised model training, we applied a custom-made feature-selection pipeline. Highly-correlated features were initially identified and removed by the GGally package with a threshold set at 0.85. Then, an optimal set of features was identified by a random forest-based recursive feature-elimination function implemented in the Caret package. We examined the distribution patterns of individual selected features among different M and E types using the featurePlot function implemented in the same package. These plots provided information about which features were likely to be important to predict certain M and E types (Fig. S3). The training, optimization, and prediction accuracy estimation of different supervised models was done using the trainControl, train, and predict functions implemented in the Caret package. Six widely-used algorithms were initially selected to train models: support vector machine (SVM), random forest (RF), neuralnet (NN), naïve Bayes (NB), k-nearest neighbor (KNN), and gradient boosting machines (GBM). After comparing the prediction accuracies of different models in the training data sets (data not shown), we selected four well-performing models for later prediction: SVM, RF, KNN, and NN (Fig. S2A). We used these models to predict their M- or E-type labels in the testing sets using the same set of morphological or electrophysiological features (Fig. S2A). Each sample was given four same or different M- or E-type labels. To ensure a reliable prediction of M and E types, only samples with consensus predictions (at least 3 models having the same prediction) were labeled accordingly, and the remaining samples with inconsistent labels were excluded from further analysis (~9% of all predicted samples, Fig. S2A).
For the integrated classification of M/E clusters, an unsupervised hierarchical cluster analysis employing Ward’s method was applied based on combined morphological and electrophysiological features. The cut-off for significant clusters was 60% of the maximum linkage distance [28], based on which 9 clusters were identified. Cluster stability was then evaluated by subsampling analysis [29]. Random subsampling containing 90% of each cluster was generated 500 times, and the subsamples were used for hierarchical cluster analysis with the number of clusters set as 9. The Jaccard similarities of all obtained clusters were >0.5 (0.7889 ± 0.1468, Fig. S10A). The significance of the unsupervised clustering was further validated by comparison with the fully-randomized dataset using silhouette analysis [30]. A significant reduction of average silhouette values of randomized databases was found compared to the average silhouette values of the original clustering (0.002 ± 0.004 of randomized datasets and 0.1100 of the original dataset, P <0.001, one sample t-test), indicating that the original clustering was “meaningful”.
NC and TC Identification
Using unsupervised hierarchical clustering (R package “pHeatmap”), NCs and TCs were defined based on the binary expression (ON/OFF) of neurochemical markers [3, 31] and quantitative expression profiles of transcriptomic signatures [13], respectively. In total, 13 interneuron markers were used for NC group classification. As secondary markers, Tac1 and Pdyn were included in our PCR detection (Fig. 1G) and correlated strongly with Pvalb and Sst respectively. We attributed cells co-expressing Pvalb and Sst to the Pvalb or Sst subclass based on which the second marker was expressed [32]. Cells co-expressing Pvalb and Sst but with no matching secondary marker or more than two primary markers were excluded from NC classification. Cluster stability was then evaluated by subsampling analysis as described above. Most clusters had an average Jaccard similarity >0.5 (0.6500 ± 0.1932, Fig. S10B).
Original Ct values in qPCR experiments were transformed into 40-Ct. Data were then normalized by transcriptomic signatures before clustering analysis. To capture the prominent transcriptomic features within each TC, representative transcriptomic signatures were identified based on their normalized expressions exceeding 1 (i.e., 1× SD) (Fig. S7). Overall, 17 out of 20 TC groups had distinguishable transcriptomic signatures (Fig. 4A). Cluster stability was then evaluated by subsampling analysis as above. The Jaccard similarity of all clusters was higher than (or close to) 0.5 [medial ganglionic eminence (MGE): 0.6767 ± 0.1535; caudal ganglionic eminence (CGE): 0.6763 ± 0.1871; Fig. S10C].
Fig. 4.
Morpho-electric correlates of TCs. A Putative TCs generated by unsupervised hierarchical clustering based on expression profiles of transcriptomic signatures. MGE (n = 395) and CGE (n = 173) cells (defined by the expression of Pvalb/Sst and Htr3aR) were separately clustered using different sets of transcriptomic signatures. Representative transcriptomic signatures for identified TC groups were found except for TCs 3, 7, and 19. Colors indicate normalized expression levels of individual transcriptomic signatures. B UMAP plots showing the distribution of individual TCs based on combined M/E features (metric = Euclidean, n_neighbors = 5, n_epochs = 200; red boxes, MGE-related TCs; blue boxes, CGE-related TCs. TCs with a sample size <5 (TC 20) are not shown. Points colored as in (A). C Comparison of TCs with M/E types. D, E Confusion matrices for classifying cells into different TCs in the MGE (D) and CGE (E) using the SVM classifier (stratified 20-fold cross-validation, C = 1.34, Radial Based Function, gamma = 0) based on combined M/E features. TCs with at least 5 cells were included in the analysis.
MB-MDR Analysis
Generally, the model-based multifactor dimensionality reduction (MB-MDR) approach consists of three steps, which are executed by two main functions (mbmdr and mbmdr.PermTest) embedded in the mbmdr package. A detailed description of these steps and related results can be found in the original paper [33]. The transcription factors Lhx6 and Prox1 were not included in the MB-MDR analysis because they were universally expressed in MGE- and CGE-related M/E types. For a given M/E type, MB-MDR analysis reported multiple statistically significant (adjusted P-value <0.01) neurochemical marker combinations. We next used Matthews correlation coefficient (MCC) scores [34] to evaluate the performance of these combinations in predicting target M/E type. A higher MCC score indicates a better prediction of the target M/E type. Since higher MCC scores were obtained using the fourth order compared to the other two orders (Fig. 3D), we focused our results on the fourth-order MB-MDR. To obtain a characteristic expression of neurochemical markers for each M/E type, we counted the occurrences of all neurochemical markers (expressed or non-expressed) present in the top 10 combinations (ranked by MCC score). We reasoned that certain neurochemical markers were important for predicting a specific M/E type if they were present in many reported combinations. Based on this assumption, neurochemical markers with a probability >50% are shown in Fig. 3E.
Fig. 3.
Correlations between neurochemical markers and M/E types. A Hierarchical clustering of NCs based on the binary expression of neurochemical markers (n = 506; red, positive expression; blue, negative expression). Different clusters are named by NCs followed by representative neurochemical markers. B Chord diagram showing the correspondence between NCs and M/E types. Weak connections (defined as <10% in each M/E type) are represented by light-gray edges. C Percentage of M/E types in each NC. D Comparison of MCC scores between different orders. Data are presented as the mean ± SEM, NS, no significant difference (P >0.05), *P <0.05, **P <0.01, ***P <0.005, ****P <0.0001. One-way ANOVA was performed separately in each M/E type. Detailed data and exact sample numbers are presented in Table S7. E Characteristic neurochemical marker expression for each M/E type identified through MB-MDR analysis. Expressed and non-expressed neurochemical markers are displayed above and below the middle horizontal line, respectively.
High-Dimension Data Visualization
For the t-distributed stochastic neighbor embedding (t-SNE) visualization of M/E types, we used 123 extracted morphological and electrophysiological features and all 220 cells that had M/E-type labels. All features were standardized across this set of cells. We used the Rtsne function (R package Rtsne) to compute the coordinates followed by a ggplot function (R package ggplot2) to plot. The parameters used in the Rtsne function were as follows: perplexity = 25, theta = 0.1, pca = F. For the t-SNE visualization of M/E clusters obtained from unsupervised hierarchical clustering, we used the same set of morphological and electrophysiological features and all 281 cells that had values for all 123 features. All features were standardized across this set of cells. The parameters used in the Rtsne function in this case were as follows: perplexity = 30, theta = 0.15, pca = F.
For the morpho-electric UMAP (Uniform Manifold Approximation and Projection) plot of TCs, we used combined morphological and electrophysiological features and cells that had both TC assignments and all values of these 123 features. All features were standardized across this set of cells. We used the umap function (R package umap) to calculate the coordinates (n_neighbors = 5, n_epochs = 200, mid_dist = 0.2, method = “naive”) followed by ggplot function to plot. TC groups with a sample size <5 are not shown. MGE and CGE samples were visualized separately to obtain a finer distribution pattern.
SVM Prediction of TC Groups
We trained SVM classifiers (Python, Delaware, USA; scikit-learn package) using the Gaussian kernel radial basis function (gamma: 0.01, C: 1.07). The SVM algorithm was used due to the limited number of cells in most TCs and the outnumbered features included. A confusion matrix was used to show the fraction of cells from each T-type that was classified into each TC group. Only TCs with at least 5 cells were used.
Logistic Regression Analysis
Binary logistic regression was applied as previously reported [35].
Analysis of Patch-seq Data
In V1, we obtained 1,159 cells with transcriptomic data, of which 139 had morphological data. In M1, we obtained 470 cells with transcriptomic data, of which 154 had morphological data. We downloaded morphological reconstructions (in SWC format) for further feature extraction using Neurolucida Explorer [transformed into action script communication file (ASC) format using NLMorphology software]. Electrophysiological features were automatically extracted using codes written in Python. The original counts per million (cpm) data were first transformed by Log2 to get a Gaussian-like distribution followed by a normalization using the scale function. Cells were initially divided into the MGE and CGE parts based on the expression of subclass markers in the hierarchical clustering. In total, we got 1,000 MGE cells and 159 CGE cells in V1, 403 MGE cells and 67 CGE cells in M1. Classification of MGE cells and CGE cells was performed separately. A similar hierarchical clustering approach was used to identify TCs based on these transcriptomic datasets (see “NC and TC Identification” section) (Fig. 5A, C).
Fig. 5.
Correlation of TCs with morpho-electric properties in V1 and M1. A Hierarchical clustering dendrogram showing the classification of TCs in L5 interneurons of V1 based on expression profiles of the same set of transcriptomic signatures. MGE (left, n = 1000) and CGE (right, n = 159) cells are separately analyzed. Original transcriptomic signature expression data derived from [20]. Colors indicate expression levels of individual transcriptomic signatures [transformed by log2(cpm + 1)]. B UMAP plots show the distribution of individual TCs. Combined morphological and electrophysiological features are used in MGE cells (red boxes) while electrophysiological features are used in CGE cells (blue boxes). C As in A, but for TCs obtained from M1. MGE (left, n = 403) and CGE (right, n = 67) cells are separately analyzed. Original transcriptomic signature expression data derived from [19]. Colors indicate expression levels of individual transcriptomic signatures [transformed by log2(cpm + 1)]. D As in B, except TCs from M1. E Confusion matrices of SVM classifiers trained to predict MGE-related (left, based on combined morphological and electrophysiological features) and CGE-related (right, based on electrophysiological features) TCs of V1. Only TCs with at least 5 cells are used. F As in E, except TCs from M1. Only TCs with at least 5 cells are used. G Comparison of prediction accuracies of TCs between S1, V1, and M1. Data are presented as the mean ± SEM; NS, P >0.05; *P <0.05; **P <0.01; ***P <0.005; ****P <0.0001; one-way ANOVA followed by independent Student’s t-test. Detailed data and exact sample numbers are presented in Table S8.
To compare the percentages of transcriptomic signatures expressed in the four major interneurons subclasses (Fig. S9), we did not take the Log2(cpm + 1) >0 as a criterion since the Smart-seq2 used in Gouwens et al., 2020 [11] and Scala et al., 2021 [10] is so sensitive that it could potentially detect rare contaminating mRNAs [36]. Instead, we manually set the threshold (>4 or 5) of each transcriptomic signature by taking both the distribution pattern of original data points and the clustering output into account (Fig. 5A, C).
Neuronal BLAST (NBLAST) Scores
The NBLAST method (implemented in nat.nblast R package [37]) was used to assess the similarity of axon morphologies directly between a pair of cells. Briefly, the distance and local direction of nearby points within a pair of morphological structures were analyzed and compared. Since the original NBLAST algorithm was developed and validated using the morphologies of Drosophila neurons, we re-trained this algorithm using our morphology data. Similarity scores were calculated using this modified NBLAST algorithm with a range between 0 (completely dissimilar) and 1 (comparable with itself). Similarity scores in each direction between a given pair of morphologies were averaged to obtain a symmetrized matrix.
Correlation of Neuronal Firing
Pearson correlation was used to indirectly reflect the similarity of firing behaviors between cells. To enable an objective comparison between cells, a representative trace was used with approximately half of the maximum firing frequency. As the stimulus duration in different studies was different (1,000 ms in V1, 800 ms in S1, and 600 ms in M1), we used the first 600-ms response for further analysis. We generated a firing histogram by cutting the response into 20-ms bins and counting the number of action potentials in each bin. To assess the similarity of firing patterns between a given pair of TCs, correlations between the individual cell firing histogram of one TC and the average histogram of the other TC were calculated.
Quantification and Statistical Analysis
No statistical methods were used to predetermine sample sizes, but the sizes in our study were comparable to previous publications [11, 38]. Since the identical experimental condition was used for all acquired data, no randomization was applied during data collection. Data collection and analysis were not performed blind to the experimenters as there was a single experimental condition for all acquired data. Significance testing between more than two groups was accomplished using ordinary one-way analysis of variance (ANOVA) or the Kruskal-Wallis test depending on the distributions of data points. Normal t-tests were used to determine whether a significant difference in averages existed between the two groups. A normality check of the data distribution was applied routinely before statistical analysis. Statistical analysis was performed using R and GraphPad Prism 8 (GraphPad Software, San Diego, USA). All data are presented as the mean ± SEM unless otherwise stated. P <0.05 was considered statistically significant.
Results
Establishment and Validation of Patch-PCR
In this study, Patch-PCR was used to simultaneously obtain electrophysiological and morphological properties as well as the mRNA expression profiles of individual interneurons whose somata were located in L5 of S1 (Fig. 1). The S1 region in ex vivo brain slices is readily identified by the barrel-like structure (layer IV) under low magnification (10×). We used Gad2-Cre;Ai14 transgenic mice to fluorescently label cortical interneurons [39]. Then, labeled interneurons in L5 of S1 were carefully selected for patch-clamp recording under high magnification (60×). We located the soma of each recorded interneuron by post hoc biocytin staining and those occasionally outside L5 were not included in our analysis. Due to the sparse representation of CGE-derived Htr3aR+ interneurons in the deep cortical layers [7], Htr3aR-EGFP knock-in mice were used to exclusively target this subpopulation in L5 [40]. Healthy-looking cells were patched and a series of hyperpolarizing and depolarizing current injections were applied to record the electrophysiological responses (Fig. 1E), followed by biocytin filling for later morphological recovery and reconstruction (Fig. 1D), as well as mRNA extraction (Fig. 1B) for later PCR detection of targeted genes. M types and E types were identified based on the extracted morphological and electrophysiological features (Fig. 1F).
To avoid competing reactions among different primers using traditional multiplex PCR [41], a non-specific preamplification of the first-strand full-length cDNA was performed (single-cell cDNA library generation) [22], followed by the amplification of targeted genes using standard PCR for neurochemical markers (Fig. 1G) and qPCR for transcriptomic signatures (Fig. 1H). Of note, given that the transcriptomic cell types originating from the MGE, (Pvalb, and Sst subclasses) and the CGE (Htr3aR subclass) mainly expressed non-overlapping transcriptomic signatures, cells expressing Pvalb/Sst (MGE origin) and Htr3aR (CGE origin) were separately examined with two different sets of transcriptomic signatures (Fig. 1H). To facilitate the correlation analysis between different modalities (Fig. 1J), we created a barcode database for each cell to record these raw multi-dimensional metadata (Fig. 1I).
Quality checks and a series of control experiments were performed to confirm the validity of the Patch-PCR procedure. We randomly selected 203 of 639 samples to check cDNA quality using a bioanalyzer (Fig. 1C), and found that 86.2% had cDNA libraries with average length >1,300 bp and total yield >1.0 ng (Fig. S1A, B). Moreover, we found that 10%–80% Ct values of housekeeping and positive control genes were distributed in relatively narrow ranges (GAPDH: 16–19, Gad1: 19–22, Gad2: 19–23, Fig. S1C), indicating that the total amount of mRNA extracted from patched interneurons was overall comparable. To further confirm the PCR results, we assessed the protein expression of the corresponding genes of the same patched interneurons using post hoc immunostaining in some cases (Fig. S1D). Vesicular glutamate transporter 1 (VGluT1) mRNA, which is always detected in harvested pyramidal cells, was detected in <10% of harvested interneurons, indicating that the false-positive signal from surrounding cells or tissues was minimal during the mRNA-collecting stage (Fig. S1E, F). Technical repetition of PCR detection was also carried out to confirm the reliability of our data. Overall, these data suggested that Patch-PCR as established here is technically reliable and sensitive for detecting sets of genes in single cells while keeping their morphological and electrophysiological properties available. We collected 639 interneuron samples in L5 of S1 with intrinsic properties and gene expression data, of which 386 with sufficient morphological complexity (i.e., ~80% of cells had total axon lengths >8,000 µm) were included in our analysis (Fig. S1G, H).
An Integrated Classification of M/E Types Using Morphological and Electrophysiological Features
To quantitatively characterize the M/E properties, we extracted 50 electrophysiological and 73 morphological features for each cell. Combining widely-used interneuron taxonomy with an automatic classification pipeline [1, 31, 42, 43] (Fig. S2A), we defined 7 morphological subtypes (M types) and 7 electrophysiological subtypes (E types) in this specific cortical lamina (Fig. 2A, B). When visualized in UMAP plots, most M-type and E-type cells had a fairly cohesive and largely non-overlapping distribution (Fig. S2B, F). To further confirm whether these M types or E types represent morphologically and electrophysiologically distinct subtypes, we applied an alternative unsupervised cluster analysis to the same datasets (Fig. S2C, G). In general, subtypes derived from automatic classification showed remarkable correspondence to clusters obtained from hierarchical clustering (Fig. S2E, I). Moreover, these M and E types covered all the major morphological and electrophysiological subtypes defined in the literature, and correspondence between them was found (see Table S5 for a detailed description) [1, 2, 12]. For example, cells in M type 1 and 2 exhibit typical fanning-out and T-shaped Martinotti cell (MC) morphologies, respectively [27]. Of note, chandelier cells, which are mainly located in L2 and L6 [19], were not successfully recovered in this study, consistent with a previous study in adult mouse V1 [1]. Next, we investigated to what extent M types and E types are correlated by intersectionally overlaying them. There was a complicated correspondence between M and E types, although interneurons within the same M type tended to have 1–2 dominant E types, and vice versa (Fig. 2C).
Fig. 2.
Identification of M/E types. A Example reconstruction of 7 morphological subtypes (M-types). Soma depths were normalized by the cortical thickness in each slice. Dashed horizontal lines, approximate layer boundaries identified by DAPI staining (see Fig. S2 for further details). Dark blue, dendrites; other colors, axons; scale bar, 100 µm. See Table S5 for correspondence between M types and well-known morphological interneuron subtypes. B Example traces of 7 electrophysiological subtypes (E types) showing typical firing patterns in response to an 800-ms square-wave stimulus (black, the hyperpolarization trace obtained with the smallest current stimulation; red, the first depolarization trace that elicited at least one action potential, green. the depolarization trace showing an intermediate firing rate; see Table S5 for correspondence between E types and well-known electrophysiological interneuron subtypes). C Chord diagram showing the correspondence between M types and E types. M- and E-type combinations with relatively strong connections are considered major M/E types (colored edges, defined as >5% of total samples). The remaining weak connections are represented by light-gray edges. D t-SNE embedding of 11 M/E types identified in C using extracted M/E features. Points were colored as in (C). E Percentages of cells with M/E-type labels. F Relative proportions of different M/E types.
To obtain M/E types that reflected the major morpho-electric properties of interneurons in this region, we combined M and E types and took the 11 main combinations as M/E types (>5% of the total) [2] (Fig. 2C). We then visualized these major M/E types by projecting their morpho-electric features using t-SNE and found little overlap between them (Fig. 2D). As confirmation, we partitioned the original M/E dataset into 9 clusters (C1–C9) using an unsupervised hierarchical clustering method (Fig. S4A, B). We found a remarkable correspondence between M/E types and clusters (Fig. S4C). As these 11 types represented morpho-electrically well-defined major interneuron subtypes, we focused on them in the following correlation analysis. Overall, these M/E types covered ~80% of all samples with variable percentages of each type (Fig. 2E, F). We also examined the somatic distributions of M/E types in this specific layer and found a sub-laminar preference for some types (Fig. S5A, B). For example, M/E types 4, 5, and 7 were preferentially located in the lower region, while M/E types 3 and 11 occupied the upper region of L5.
Correlations Between Neurochemical Markers and M/E Types
Interneuron-specific neurochemical markers have been widely used as a proxy for distinct cortical interneuron subtypes [3, 31]. However, accumulating evidence suggests that a single marker in most cases targets several phenotypic subtypes [1, 6, 27, 44]. Although the intersection and subtraction of neurochemical markers are expected to improve targeting specificity [38], it remains unclear to what extent the combinatorial expression of neurochemical markers corresponds to specific cortical interneuron subtypes defined by M/E properties.
Since neurochemical markers usually define interneuron subtypes in an ON/OFF manner and substantial co-expression exists between them [3, 39] (Fig. S6A, B), a hierarchical clustering method was applied to identify cell populations based on the binary expression patterns of 13 neurochemical markers (Fig. 1G). We obtained 9 neurochemical clusters with distinguishable patterns (Fig. 3A). We next analyzed the correlation between NCs and M/E types and found that there appeared to be a one-to-several correspondence in most cases, and vice versa (Fig. 3B, C). Further analysis showed that NCs roughly appeared to correspond to sets of different M/E types in a way consistent with broad interneuron subclasses (Pvalb, Sst, and Vip) (Fig. 3C). Although a one-to-one correspondence between NCs and M/E types was not found in most cases, certain NCs correlated with specific M/E types. For example, 75.0% of cells in NC 7 and 88.6% of cells in NC 9 corresponded to M/E type 2 and M/E type 9, respectively (Fig. 3C).
Although the above analysis revealed a complicated correlation between NCs and M/E types in L5 of S1, it was still unclear whether there exist combinations of neurochemical markers associated with a given M/E type. Here, we applied the MB-MDR analysis [33] to explore this question. In the current application scenario, for a given M/E type, MB-MDR reported multiple statistically significant combinations with a certain number of neurochemical markers (i.e., order in MB-MDR). Then, we used an MCC score to estimate the predictive performance of selected combinations (ranked by the P-values reported in MB-MDR) for a particular M/E type [34]. Subsequently, we compared the MCC scores between different orders (order range: 2–4) of each M/E type, and found that the scores in the high-order group were significantly higher than those in the low-order group, accompanied by a decrease of within-group variation as the order increased (Fig. 3D). These results indicated that combinatorial expression of a greater number of neurochemical markers improves the precision of cell targeting [38, 45]. After further analysis of our MB-MDR results, we found that each M/E type had a characteristic neurochemical marker expression pattern (Fig. 3E), some of which were meaningful. For example, 74.2% and 75.0% of cells in M/E types 4 and 5 (both types had a non-MC morphology) were Sst+/Calb1−, consistent with the previous result that Calb1-negative Sst interneurons usually have a non-MC morphology in neocortex [6, 27].
Correlation Between Transcriptomic Signatures and Morpho-Electric Properties
Recent scRNAseq had identified many transcriptomic subtypes and discriminative transcriptomic signatures in cortical interneurons [13, 14]. However, it was still unclear whether interneuronal populations that strongly express specific transcriptomic signatures correspond to distinct M/E subtypes. Here, we set out to investigate the correlation of previously identified transcriptomic signatures [13] with the M/E properties of interneurons in L5 of S1. Since these transcriptomic signatures came from scRNAseq and were frequently co-expressed with each other (Fig. S6C–F), we applied an unsupervised algorithm (hierarchical clustering) to define TCs based on their quantitative expression profiles (Figs 1H and 4A). Overall, we identified 20 TCs (12 for the MGE and 8 for the CGE), of which 17 strongly expressed specific transcriptomic signatures (e.g., Rspo2 in TC 1 and Chat in TC 19) (Figs 4A and S7). Of note, different from previous transcriptomic cell types defined by the whole transcriptomic profiles [10, 11, 13], TCs identified here were derived from expression profiles of a predefined set of transcriptomic signatures [13]. A further examination of the somatic locations revealed a sub-laminar preference for some TCs (Fig. S5C). For example, cells in TC 5_Th, TC 8_Gpx3, and TC 10_Chodl were preferentially located in the lower region, while cells in TC 4_Hpse and TC 6_Cbln4 occupied the upper region in L5.
We first asked how consistent are the M/E properties of cells in the same TC. We used UMAP plots to visualize the distribution patterns of individual TCs in a 2D M/E space (MGE- and CGE-related TCs were analyzed separately) (Fig. 4B). We found that cells in many TCs were in scattered locations on the UMAP plots (Fig. 4B), indicating that the existence of non-trivial variations in M/E properties. Nevertheless, a few particular TCs indeed had fairly tight distributions in the UMAP plot (e.g., TC2_Myh8 and TC18_Gpc3), suggesting that cells in these TCs exhibit consistent M/E properties.
We next investigated whether cells mapped to different TCs could be distinguished in terms of their M/E properties. We compared the M/E type assignments to the TCs to assess the correspondence between them (Fig. 4C). Certain TCs, such as TC 2_Myh8 and TC 6_Cbln4, were found mainly in a single M/E type. For many other TCs, however, cells were distributed across several M/E types. In addition, we examined how well TCs could be predicted from M/E features by training SVM classifiers. The TC classifiers had an overall prediction accuracy of 31.3% in MGE-related TCs and 35.8% in CGE-related TCs, and the prediction accuracy varied considerably across TCs (Fig. 4D, E). Together, these data suggest that, although cells mapped to certain TCs exhibited distinguishable M/E phenotypes, many other TCs were difficult to be distinguished using these M/E properties.
Last, we investigated whether the expression levels of transcriptomic signatures are related to the prediction of M/E types. We first examined the average expression levels of transcriptomic signatures in different M/E types, and found high expressions of some transcriptomic signatures in specific M/E types, such as Cbln4 in M/E type 3 and Chat in M/E type 11 (Fig. S8A). To further identify their potential relevance, we used binomial logistic regression to determine whether the association between M/E types and the expression levels of transcriptomic signatures was statistically significant. Overall, 9 out of 11 M/E types were found to correlate with specific transcriptomic signatures (P <0.001) (Fig. S8B). For example, M/E types 2, 3, 4, 9, 10, and 11 were significantly and positively correlated with the expression levels of Myh8, Cbln4/Hpse, Gpx3, Parm1, Gpc3, and Chat, respectively (Fig. S8B, C).
Morpho-Electric Characterization of Transcriptomic Signatures in V1 and M1
Growing numbers of studies have suggested that cortical interneurons between brain areas exhibit large diversity regarding their cell type composition, gene expression patterns, and functional properties [16, 20, 46]. Two recent studies sampling interneurons from the entire V1 and M1 using Patch-seq provided datasets to directly investigate the correlation between transcriptomic signatures and M/E properties [10, 11], facilitating a direct comparison between different brain areas. To begin with, we divided the L5 interneurons of V1 and M1 into different TCs using the gene expression data of the same set of transcriptomic signatures as in S1 (Fig. 5A, C). We further compared the proportions of transcriptomic signatures expressed in major interneuron subclasses (Pvalb, Sst, Vip, and non-Vip) between these two areas (Fig. S9). Although the proportions of most transcriptomic signatures in a given subclass were comparable between these two areas (e.g., Cbln4, Cpne5, and Gpx3), significant differences were also found in some cases (Fig. S9). For example, there was a significantly higher proportion of cells expressing Chodl in the Sst subclass of V1 compared to M1, and Tpbg was the opposite.
Next, we investigated how consistent the M/E properties of cells within a given TC are in V1 and M1. Like the previous analysis in S1, we first visualized the distributions of individual TCs via the UMAP plot that was constructed based on extracted M/E features (Fig. 5B, D). Since the number of L5 interneurons that were fully reconstructed in CGE samples was small in these two studies (16 in V1 and 27 in M1), electrophysiological data was used to generate UMAP plots of CGE samples. Examining the locations of MGE and CGE samples demonstrated that, at the subclass level, most L5 interneurons of V1 and M1 occupied one of the four major domains, corresponding to the well-known Pvalb, Sst, Vip, and Non-Vip (Lamp5 in previous literature) subclasses (Fig. 5B, D). L5 interneurons of V1 and M1 in most TCs were typically found in scattered locations on the UMAP plot (Fig. 5B, D), similar to the results in S1.
We then explored whether cells mapped to different TCs could be distinguished in terms of their M/E properties. Like the previous analysis in S1, we trained SVM classifiers on M/E features to predict different TCs (Fig. 5E, F). As noted above, electrophysiological features were used for the prediction of TCs in the CGE. The overall prediction accuracy of V1 was 47.7% in the MGE and 47.1% in the CGE (Fig. 5E), both higher than those in S1. However, TC classifiers in M1 had a prediction accuracy of 33.9% in the MGE and 35.8% in the CGE (Fig. 5F), both similar to those in S1. Since prediction accuracy varied considerably across TC groups, we next compared the average prediction accuracies between S1, V1, and M1. We found that the average prediction accuracy of TCs in V1 was significantly higher than that of S1 (Fig. 5G). Of note, there was also a trend that the average prediction accuracy of TC groups in V1 was higher than that in M1, although this trend did not reach statistical significance (Fig. 5G). As transcriptomic signatures in this study were derived from V1 [13], this higher prediction accuracy in V1 suggests that M/E correlates of transcriptomic signatures have brain-area specificity.
Morpho-Electric Comparisons of TCs Between S1, V1, and M1
The previous analysis investigated the correlations between transcriptomic signatures and M/E properties in S1, V1, and M1. It remained unclear whether there is a difference in the M/E properties of the same TCs between these brain areas. To facilitate direct comparison, we choose those TCs that strongly expressed the same transcriptomic signatures among the three brain areas. Based on this consideration, 4 TCs, Rspo2, Hpse, Cbln4, and Gpx3, were included for later comparison. We examined the morphological reconstructions and electrophysiological response trajectories of representative cells from these TCs in S1, V1, and M1 (Fig. 6A). Example morphologies are shown aligned to the average cortical template in each brain area, accompanied by averaged axon depth distributions. To further determine how similar the M/E properties of these 4 TCs between S1 and the other two areas were, we assessed the similarity of overall axonal morphologies and firing patterns between cells of the same TCs among different areas. Using a modified NBLAST algorithm [37], we measured the pairwise similarity of axonal morphologies between individual cells from these three areas. We found that the similarity in axonal morphology between S1 and M1 in Hpse, Cbln4, and Gpx3, but not Rspo2, was significantly higher than that between S1 and V1 (Fig. 6B). To facilitate a comparison of firing patterns, we measured the distributions of action potentials in representative traces and analyzed the correlation between cells, and found that firing pattern similarity between S1 and M1 in all 4 TCs was significantly higher than that between S1 and V1 (Fig. 6C). Together, these data suggest that functionally relevant M/E properties of L5 interneurons in S1 are more similar to those in more-proximal M1 than more-distal V1 (Fig. 6D).
Fig. 6.
Morpho-electric comparison between S1, V1, and M1. A Exemplary morphology and corresponding electrophysiology of 4 TCs from S1, V1, and M1. Axons are colored by TCs, and dendrites are shown in black. Morphology scale bar, 100 µm. Electrophysiology scale bar: left, 50 mV; right, 100 pA; lower, 400 ms. Axon depth histograms calculated from all reconstructions of the TCs are shown to the right. Histograms are shown as the mean (lines) ± SEM (shaded regions). B Axon morphological similarity between TCs from S1, V1, and M1. Detailed data and exact sample numbers are presented in Table S9. C Correlation of firing patterns between TCs from S1, V1, and M1. Detailed data and exact sample numbers are presented in Table S9. D Schematic showing that TCs of S1 are both morphologically and electrophysiologically more similar to those of more-proximal M1 compared to more-distal V1. Data are presented as the mean ± SEM; NS, P >0.05; *P <0.05; **P <0.01; ***P <0.005; ****P <0.0001; Student’s t-test (B, C).
Discussion
The discovery of molecular signatures or unique markers has contributed to our understanding of the connections and functions of different populations of cortical interneurons. Recently, the application of scRNAseq has greatly advanced the classification of interneuron subtypes and identified numerous subtype-specific transcriptomic signatures [13, 14, 47]. Moreover, a recent Patch-seq study in V1 interneurons has identified many morphological/electrophysiological/transcriptomic (MET) types and found a strong correspondence between transcriptomes and M/E properties [11]. A parallel effort in M1 interneurons, however, found continuous M/E variations within and between individual transcriptomic cell types [10]. Therefore, whether these transcriptomic subtypes truly represent functionally specific cell populations remains largely unknown. Perhaps, a more specific and practical question is whether these putative transcriptomic signatures could be used to label distinct subpopulations of cortical interneuron. Here, we applied a Patch-PCR technique to simultaneously collect M/E properties and mRNA expression profiles (both neurochemical markers and recently-identified transcriptomic signatures) from individual interneurons in L5 of S1, followed by cell population identification and multimodal correlation analysis. Our analysis suggests that cells in most NCs and TCs contain substantial variations in M/E properties. To determine whether these findings are also applicable to other brain areas, we analyzed L5 interneurons of V1 and M1 using publicly-available Patch-seq data [10, 11]. Largely comparable results are obtained from both M1 and V1, suggesting that these phenotypic variations within molecularly-defined cell populations might be universal. Since we did not sample interneurons other than those in L5, whether results obtained in L5 are also applied to other cortical layers needs further experiments.
Patch-PCR has been widely used to study the correlation of molecular expression with morphological and/or electrophysiological features in cortical neurons [30, 48, 49]. With the requirement of detecting a relatively large number of molecules (~40 genes) and obtaining high-quality morphology, we made a series of pilot experiments and optimized the internal solution compositions and molecular detection methods based on the recently-developed Patch-seq technique [22]. After stringent control experiments had been conducted before, during, and after sample collecting, potential contamination from surrounding tissues or cells was minimal yet inevitable, consistent with previous studies [36, 48]. Moreover, technical repetitions were done to confirm the PCR results. Since our main purpose was to investigate the correlations between predetermined sets of molecular markers (neurochemical markers and transcriptomic signatures) and M/E properties, the Patch-PCR rather than Patch-seq technique was used, the latter being more suitable for identifying new transcriptomic cell types. Nevertheless, the use of Patch-PCR inherently restricted us to a predetermined set of molecular markers. Therefore, we cannot rule out the possibility that there are correlations between other molecular markers with M/E properties. As more specific transcriptomic signatures are identified [14], we speculate that M/E variations in these finer-defined cell populations will be reduced. An alternative consideration is to combine multiple transcriptomic signatures to define cell populations, which has been demonstrated to improve target specificity in some cases of neurochemical marker combinations [38].
This study has technical limitations exist. First, as the brain slicing process is likely to cut remote axonal branches of individual interneurons [50] and the aspiration of cell contents is detrimental to biocytin diffusion [22], it was technically challenging to recover complete the morphologies of particular cell types in L5, such as L5 MCs with long thin axons reaching to L1. To minimize this technical artifact as much as possible, we selected those neurons with sufficient axonal arborizations for further morphological analysis (Fig. S1G, H). Second, our identification of M/E types, although capturing the major M/E subtypes of interneurons in this specific region, has the risk of excluding some rare, but real, categories (~20% M- and E-type combinations with insufficient samples were excluded from further analysis). Future studies based on a much larger dataset could fill the gaps. Third, we used ~4-week-old mice to keep more interneurons in a healthy state in slices, which is crucial for high-quality mRNA harvesting. Moreover, most of the M/E properties of cortical interneurons are developmentally mature during this period [51, 52]. However, gene expression is a highly dynamic process varying substantially with development or activity. Thus, multimodal data from different developmental stages are needed to get a more comprehensive understanding of how cell populations defined by these transcriptomic signatures are correlated with M/E properties.
A recent study has shown that cells of the same transcriptomic cell type exhibit distinct M/E properties between different brain areas [20], suggesting possible functional specifications of the same molecular cell population in a specific local environment. In line with this idea, we took axonal projection patterns and firing behaviors, two functionally significant properties for morphology and electrophysiology, as an example to quantitatively compare the M/E similarity between cells of the same cell population from different brain areas. An interesting finding here is that L5 interneurons in S1 tended to be more morpho-electrically similar to those in M1 rather than V1. Noteworthy, more detailed comparisons using other features, such as connectivity, may provide additional information. Consistent with this, a recent large-scale transcriptomic investigation of cell types across the entire mouse isocortex suggested that neuron types are more similar to their nearby neighbors in the brain than they are to more distant brain cells [53].
Overall, our work provides an application framework in which to investigate the correlation of molecularly-defined cell groups with their M/E properties. We expect that future studies combining a higher-resolution transcriptomic taxonomy with other functional modalities (e.g., local connectivity, long-range projections [54], and in vivo activity) might reveal new insights into the functional meanings of transcriptomic types/signatures.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank members of the Yu lab for valuable discussions on this manuscript. We thank Dr. Zhen-Gang Yang and Dr. Lan Ma for providing transgenic mice. This study was supported by the National Key Research and Development Program of China (2021ZD0202500). The project was also supported by the National Natural Science Foundation of China (31930044 and 31725012), the Foundation of Shanghai Municipal Education Commission (2019-01-07-00-07-E00062), the Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01), the Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01), and ZJLab.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Jun-Wei Cao and Xiao-Yi Mao contributed equally to this work.
Contributor Information
Wen-Dong Xu, Email: wendongxu@fudan.edu.cn.
Yong-Chun Yu, Email: ycyu@fudan.edu.cn.
References
- 1.Jiang X, Shen S, Cadwell CR, Berens P, Sinz F, Ecker AS, et al. Principles of connectivity among morphologically defined cell types in adult neocortex. Science 2015, 350: aac9462. [DOI] [PMC free article] [PubMed]
- 2.Markram H, Muller E, Ramaswamy S, Reimann MW, Abdellah M, Sanchez CA, et al. Reconstruction and simulation of neocortical microcircuitry. Cell. 2015;163:456–492. doi: 10.1016/j.cell.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 3.Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, Yamaguchi N, et al. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex. 2011;21:1803–1817. doi: 10.1093/cercor/bhq252. [DOI] [PubMed] [Google Scholar]
- 4.Lim L, Mi D, Llorca A, Marín O. Development and functional diversification of cortical interneurons. Neuron. 2018;100:294–313. doi: 10.1016/j.neuron.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tremblay R, Lee S, Rudy B. GABAergic interneurons in the neocortex: From cellular properties to circuits. Neuron. 2016;91:260–292. doi: 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26:5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadwell CR, Palasantza A, Jiang X, Berens P, Deng Q, Yilmaz M, et al. Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat Biotechnol. 2016;34:199–203. doi: 10.1038/nbt.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuzik J, Zeisel A, Máté Z, Calvigioni D, Yanagawa Y, Szabó G, et al. Integration of electrophysiological recordings with single-cell RNA-seq data identifies neuronal subtypes. Nat Biotechnol. 2016;34:175–183. doi: 10.1038/nbt.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scala F, Kobak D, Bernabucci M, Bernaerts Y, Cadwell CR, Castro JR, et al. Phenotypic variation of transcriptomic cell types in mouse motor cortex. Nature. 2021;598:144–150. doi: 10.1038/s41586-020-2907-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gouwens NW, Sorensen SA, Baftizadeh F, Budzillo A, Lee BR, Jarsky T, et al. Integrated morphoelectric and transcriptomic classification of cortical GABAergic cells. Cell. 2020;183:935–953.e19. doi: 10.1016/j.cell.2020.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouwens NW, Sorensen SA, Berg J, Lee C, Jarsky T, Ting J, et al. Classification of electrophysiological and morphological neuron types in the mouse visual cortex. Nat Neurosci. 2019;22:1182–1195. doi: 10.1038/s41593-019-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci. 2016;19:335–346. doi: 10.1038/nn.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tasic B, Yao Z, Graybuck LT, Smith KA, Nguyen TN, Bertagnolli D, et al. Shared and distinct transcriptomic cell types across neocortical areas. Nature. 2018;563:72–78. doi: 10.1038/s41586-018-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang ZJ, Paul A. The diversity of GABAergic neurons and neural communication elements. Nat Rev Neurosci. 2019;20:563–572. doi: 10.1038/s41583-019-0195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krienen FM, Goldman M, Zhang Q, Del Rosario RCH, Florio M, Machold R, et al. Innovations present in the primate interneuron repertoire. Nature. 2020;586:262–269. doi: 10.1038/s41586-020-2781-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naka A, Veit J, Shababo B, Chance RK, Risso D, Stafford D, et al. Complementary networks of cortical somatostatin interneurons enforce layer specific control. Elife. 2019;8:e43696. doi: 10.7554/eLife.43696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tasic B. Single cell transcriptomics in neuroscience: Cell classification and beyond. Curr Opin Neurobiol. 2018;50:242–249. doi: 10.1016/j.conb.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science. 2013;339:70–74. doi: 10.1126/science.1227622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scala F, Kobak D, Shan S, Bernaerts Y, Laturnus S, Cadwell CR, et al. Layer 4 of mouse neocortex differs in cell types and circuit organization between sensory areas. Nat Commun. 2019;10:4174. doi: 10.1038/s41467-019-12058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ting JT, Lee BR, Chong P, Soler-Llavina G, Cobbs C, Koch C, et al. Preparation of acute brain slices using an optimized N-methyl-D-glucamine protective recovery method. J Vis Exp. 2018 doi: 10.3791/53825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cadwell CR, Scala F, Li S, Livrizzi G, Shen S, Sandberg R, et al. Multimodal profiling of single-cell morphology, electrophysiology, and gene expression using Patch-seq. Nat Protoc. 2017;12:2531–2553. doi: 10.1038/nprot.2017.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Suo S, Tam PP, Han JJ, Peng G, Jing N. Spatial transcriptomic analysis of cryosectioned tissue samples with Geo-seq. Nat Protoc. 2017;12:566–580. doi: 10.1038/nprot.2017.003. [DOI] [PubMed] [Google Scholar]
- 24.Muñoz-Manchado AB, Bengtsson Gonzales C, Zeisel A, Munguba H, Bekkouche B, Skene NG, et al. Diversity of interneurons in the dorsal striatum revealed by single-cell RNA sequencing and PatchSeq. Cell Rep. 2018;24:2179–2190.e7. doi: 10.1016/j.celrep.2018.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boldog E, Bakken TE, Hodge RD, Novotny M, Aevermann BD, Baka J, et al. Transcriptomic and morphophysiological evidence for a specialized human cortical GABAergic cell type. Nat Neurosci. 2018;21:1185–1195. doi: 10.1038/s41593-018-0205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nigro MJ, Hashikawa-Yamasaki Y, Rudy B. Diversity and connectivity of layer 5 somatostatin-expressing interneurons in the mouse barrel cortex. J Neurosci. 2018;38:1622–1633. doi: 10.1523/JNEUROSCI.2415-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding C, Emmenegger V, Schaffrath K, Feldmeyer D. Layer-specific inhibitory microcircuits of layer 6 interneurons in rat prefrontal cortex. Cereb Cortex. 2021;31:32–47. doi: 10.1093/cercor/bhaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hennig C. Cluster-wise assessment of cluster stability. Comput Stat Data Anal. 2007;52:258–271. doi: 10.1016/j.csda.2006.11.025. [DOI] [Google Scholar]
- 30.Karagiannis A, Gallopin T, Dávid C, Battaglia D, Geoffroy H, Rossier J, et al. Classification of NPY-expressing neocortical interneurons. J Neurosci. 2009;29:3642–3659. doi: 10.1523/JNEUROSCI.0058-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petilla Interneuron Nomenclature Group, Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, et al. Petilla terminology: Nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci 2008, 9: 557–568. [DOI] [PMC free article] [PubMed]
- 32.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: The logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calle ML, Urrea V, Malats N, van Steen K. Mbmdr: An R package for exploring gene-gene interactions associated with binary or quantitative traits. Bioinformatics. 2010;26:2198–2199. doi: 10.1093/bioinformatics/btq352. [DOI] [PubMed] [Google Scholar]
- 34.Chicco D, Jurman G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genomics. 2020;21:6. doi: 10.1186/s12864-019-6413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi SH, Labadorf AT, Myers RH, Lunetta KL, Dupuis J, DeStefano AL. Evaluation of logistic regression models and effect of covariates for case-control study in RNA-Seq analysis. BMC Bioinformatics. 2017;18:91. doi: 10.1186/s12859-017-1498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tripathy SJ, Toker L, Bomkamp C, Mancarci BO, Belmadani M, Pavlidis P. Assessing transcriptome quality in patch-seq datasets. Front Mol Neurosci. 2018;11:363. doi: 10.3389/fnmol.2018.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa M, Manton JD, Ostrovsky AD, Prohaska S, Jefferis GSXE. NBLAST: Rapid, sensitive comparison of neuronal structure and construction of neuron family databases. Neuron. 2016;91:293–311. doi: 10.1016/j.neuron.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He M, Tucciarone J, Lee S, Nigro MJ, Kim Y, Levine JM, et al. Strategies and tools for combinatorial targeting of GABAergic neurons in mouse cerebral cortex. Neuron. 2016;91:1228–1243. doi: 10.1016/j.neuron.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vucurovic K, Gallopin T, Ferezou I, Rancillac A, Chameau P, van Hooft JA, et al. Serotonin 3A receptor subtype as an early and protracted marker of cortical interneuron subpopulations. Cereb Cortex. 2010;20:2333–2347. doi: 10.1093/cercor/bhp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weissensteiner T, Lanchbury JS. Strategy for controlling preferential amplification and avoiding false negatives in PCR typing. BioTechniques. 1996;21:1102–1108. doi: 10.2144/96216rr03. [DOI] [PubMed] [Google Scholar]
- 42.Armañanzas R, Ascoli GA. Towards the automatic classification of neurons. Trends Neurosci. 2015;38:307–318. doi: 10.1016/j.tins.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mihaljević B, Benavides-Piccione R, Bielza C, DeFelipe J, Larrañaga P. Bayesian network classifiers for categorizing cortical GABAergic interneurons. Neuroinform. 2015;13:193–208. doi: 10.1007/s12021-014-9254-1. [DOI] [PubMed] [Google Scholar]
- 44.Helm J, Akgul G, Wollmuth LP. Subgroups of parvalbumin-expressing interneurons in layers 2/3 of the visual cortex. J Neurophysiol. 2013;109:1600–1613. doi: 10.1152/jn.00782.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuman B, Machold RP, Hashikawa Y, Fuzik J, Fishell GJ, Rudy B. Four unique interneuron populations reside in neocortical layer 1. J Neurosci. 2019;39:125–139. doi: 10.1523/JNEUROSCI.1613-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim Y, Yang GR, Pradhan K, Venkataraju KU, Bota M, García del Molino LC, et al. Brain-wide maps reveal stereotyped cell-type-based cortical architecture and subcortical sexual dimorphism. Cell 2017, 171: 456–469.e22. [DOI] [PMC free article] [PubMed]
- 47.Zeisel A, Muñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, Juréus A, et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 48.Tricoire L, Pelkey KA, Erkkila BE, Jeffries BW, Yuan X, McBain CJ. A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci. 2011;31:10948–10970. doi: 10.1523/JNEUROSCI.0323-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toledo-Rodriguez M, Blumenfeld B, Wu C, Luo J, Attali B, Goodman P, et al. Correlation maps allow neuronal electrical properties to be predicted from single-cell gene expression profiles in rat neocortex. Cereb Cortex. 2004;14:1310–1327. doi: 10.1093/cercor/bhh092. [DOI] [PubMed] [Google Scholar]
- 50.Stepanyants A, Martinez LM, Ferecskó AS, Kisvárday ZF. The fractions of short- and long-range connections in the visual cortex. Proc Natl Acad Sci U S A. 2009;106:3555–3560. doi: 10.1073/pnas.0810390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan G, Yang JM, Hu XY, Li XM. Postnatal development of the electrophysiological properties of somatostatin interneurons in the anterior cingulate cortex of mice. Sci Rep. 2016;6:28137. doi: 10.1038/srep28137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang JM, Zhang J, Yu YQ, Duan S, Li XM. Postnatal development of 2 microcircuits involving fast-spiking interneurons in the mouse prefrontal cortex. Cereb Cortex. 2014;24:98–109. doi: 10.1093/cercor/bhs291. [DOI] [PubMed] [Google Scholar]
- 53.Yao Z, van Velthoven CTJ, Nguyen TN, Goldy J, Sedeno-Cortes AE, Baftizadeh F, et al. A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell. 2021;184:3222–3241.e26. doi: 10.1016/j.cell.2021.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duan Z, Li A, Gong H, Li X. A whole-brain map of long-range inputs to GABAergic interneurons in the mouse caudal forelimb area. Neurosci Bull. 2020;36:493–505. doi: 10.1007/s12264-019-00458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.