Abstract
Some anthropogenic pollutants, such as heavy metals and nanoparticles (NPs), are widely distributed and a major threat to environmental safety and public health. In particular, lead (Pb), cadmium (Cd), chromium (Cr), arsenic (As), and mercury (Hg) have systemic toxicity even at extremely low concentrations, so they are listed as priority metals in relation to their significant public health burden. Aluminum (Al) is also toxic to multiple organs and is linked to Alzheimer’s disease. As the utilization of many metal nanoparticles (MNPs) gradually gain traction in industrial and medical applications, they are increasingly being investigated to address potential toxicity by impairing certain biological barriers. The dominant toxic mechanism of these metals and MNPs is the induction of oxidative stress, which subsequently triggers lipid peroxidation, protein modification, and DNA damage. Notably, a growing body of research has revealed the linkage between dysregulated autophagy and some diseases, including neurodegenerative diseases and cancers. Among them, some metals or metal mixtures can act as environmental stimuli and disturb basal autophagic activity, which has an underlying adverse health effect. Some studies also revealed that specific autophagy inhibitors or activators could modify the abnormal autophagic flux attributed to continuous exposure to metals. In this review, we have gathered recent data about the contribution of the autophagy/mitophagy mediated toxic effects and focused on the involvement of some key regulatory factors of autophagic signaling during exposure to selected metals, metal mixtures, as well as MNPs in the real world. Besides this, we summarized the potential significance of interactions between autophagy and excessive reactive oxygen species (ROS)-mediated oxidative damage in the regulation of cell survival response to metals/NPs. A critical view is given on the application of autophagy activators/inhibitors to modulate the systematic toxicity of various metals/MNPs.
Keywords: Metals, Nanoparticles, Toxicity, Autophagy
Introduction
Heavy metals are non-biodegradable with toxic effects on some microorganisms, plants, animals, and human bodies [1]. Inorganic arsenic (iAs) ranks first in the list of toxic hazards listed by the U.S. Environmental Protection Agency, and Pb ranks second [2]. Heavy metals mainly come from the chemical industry, agriculture, urban areas, and environmental emergencies. With the widespread use of metals and their NPs, people are increasingly exposed to heavy metals such as Pb, Hg, As, Cd, Cr, and Al in a variety of ways and opportunities.
Nowadays, metal pollution has become a crucial exogenous cause affecting public health that can induce acute, chronic, and long-term health hazards in exposed people. Prolonged exposure to Cr can lead to ailments such as bronchitis, dermatitis, and lung cancer [3]. In addition, Al has multiple organ toxicities, which can bring about maladies including liver and kidney diseases, pancreatitis, myocarditis, enteritis, anemia, Alzheimer’s disease, and dementia [4]. In most cases, humans are essentially exposed to a whole range of environmental metals simultaneously instead of one single metal. The study on the hazards of metal mixture exposures is of greater public health significance, partly due to the combined toxicity promoted by the common cellular regulatory pathways [5].
Of note, metal nanoparticles (MNPs) have shown great potential in various applications worldwide, however, some toxic MNPs are considered detrimental to human health in real-life [6]. In vitro and in vivo studies have shown that some MNPs are immunotoxic, reproductively toxic, neurotoxic, and developmental toxic [7, 8]. The main mechanisms by which MNPs exert toxicity are binding to the cell surface directly to disrupt the cell membrane, releasing poisonous metal ions, and inducing the production of ROS [9]. It is generally agreed that elevated ROS levels play a prominent part in the mechanism of toxicity in MNPs [10]. While triggering oxidative stress is among the common mechanisms of NPs, cellular autophagy is rather unique and specific in response to MNPs exposure [11].
The autophagic processes are complicated and involve the removal of damaged organelles and cell wastes and the decomposition of non-critical components to provide energy in a low-energy state. Autophagy in mammalian cells can be active at both a basal level and under stressful conditions [12]. At present, accumulating studies have identified autophagy as being widely involved in diverse physiological and pathogenetic processes underlying neurodegenerative diseases [13], cancers [14], and metabolic diseases [15]. Moreover, current studies have found that abnormalities in autophagic function are also strongly associated with metal toxicity [16, 17], and the regulation of autophagy has been proven to modify metal-induced toxicity [18, 19]. As the research discovered that Cr(III) activated sphingomyelin phosphodiesterase 2 (SMPD2) to induce autophagosome formation [20]. Cd could block autophagic flux to induce hepatocyte injury via breaking the integration of autophagosomes and lysosomes. Hepatocyte injury was aggravated after the addition of the autophagy inhibitor chloroquine (CQ) to block autophagic flux [21]. At present, autophagy has been considered as a potential mechanism in metal toxicity alongside the maintenance of cellular metabolic homeostasis. Herein, we focus on the toxic effects induced by single/mixed metals and NPs contact, followed by a summary of the changes in autophagy levels with their exposure, along with the effects of autophagy regulation in certain adverse reactions induced by them.
Overview of autophagy
Autophagy, also known as type II programmed cell death, refers to the normal dynamic life process in which cells are degraded by lysosomes, which selectively remove damaged, aged, or surplus biological macromolecules or organelles and release free small molecules for cell recycling. Notably, autophagy mediates cell death and diverse diseases in the case of different kinds of metal insults [16, 22].
The key regulatory factors of autophagy
The key factors and pathways implicated in the molecular machinery of autophagy are as follows: (1) Target of rapamycin/Mammalian target of rapamycin (TOR/mTOR): TOR is widely involved in physiological activities and essential for cell growth and metabolism. As a core molecule in the regulation of autophagy, it is a key protein in the control of autophagy, sensing various signals of cellular changes and enhancing or decreasing the level of autophagy occurrence. The unc-51-like autophagy activating kinase 1 (ULK1) complex is composed of ULK1 itself, autophagy-related gene 13 (Atg13), FIP200, and autophagy-related gene 101 (Atg101) [23]. It is instrumental in recruiting autophagy proteins to initiate autophagy formation [24]. It was noted that mTOR phosphorylates ULK1 to inhibit autophagy when it is active [25]. (2) Phosphatidylinositol 3-kinase/protein kinase-B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway: PI3K1 phosphorylates Phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3), and Akt inhibits the tuberous sclerosis complex 1/tuberous sclerosis complex 2 (TSC1/TSC2) complex after being activated by PIP3, thus activating mTOR and inhibiting autophagy [26]. (3) AMP-activated protein kinase (AMPK) is a vital energy receptor that modulates cellular metabolism and maintains energy homeostasis. A decrease in intracellular ATP levels potentiates the phosphorylation and activation of AMPK by live kinase B1 (LKB1), which then inhibits mTOR complex 1 (mTORC1) activity via the phosphorylation and activation of TSC2 or by conjugation to RAPTOR, a key subunit of mTORC1 [27, 28]. AMPK can also promote autophagy directly by phosphorylating the vacuolar protein sorting 34 (VPS34) complex, or indirectly by regulating transcription factors (such as forkhead box O3 (FOXO3), transcription factor EB (TFEB), and bromodomain-containing protein 4 (BRD4)) [25, 29]. (4) Tumor suppressor p53 (p53) is a crucial regulator during autophagy. It was found that p53 in the nucleus can increase autophagy by activating selective regulatory factors upstream of mTOR, while p53 in the cytoplasm can inhibit autophagy [30–32]. (5) TFEB is in charge of autophagy and lysosomal gene regulation and has a pivotal position within autophagy-lysosome biogenesis [33]. Mitofusin 2 (MFN2) is a mitochondrial fusion protein that strengthens autophagosomes formation and promotes the fusion of autophagosomes and lysosomes [34]. In addition, damage-regulated autophagy modulator 1 (DRAM1), Rab7, and lysosome-associated membrane protein 2 (LAMP2) exert critical functions to regulate autophagosomes and lysosomes fusion [34–36].
The regulatory process of autophagy
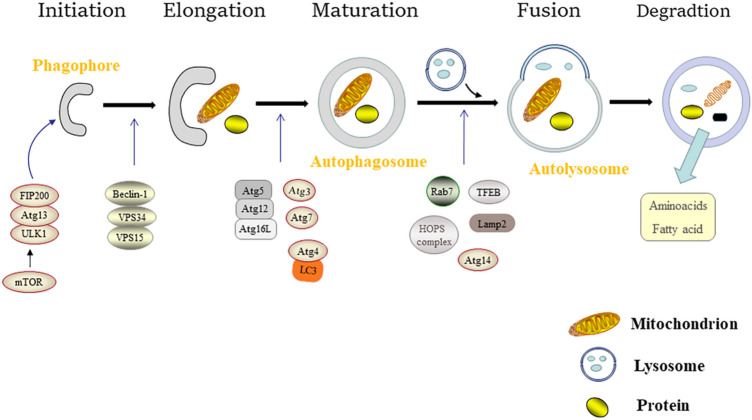
Cellular autophagy is a dynamic process that includes the generation of autophagosomes, autophagosomes and lysosomes integration, and autophagosomes decomposition, which involves the formation and fusion of membranes. Especially, each procedure is regulated by relevant regulatory factors. Figure 1 depicts a journey from phagophores to autolysosomes, including activation [37–40] and elongation [37, 38, 41, 42] of the phagophores, maturation of autophagosomes [41, 43], fusion of autophagosomes and lysosomes [43–45], as well as degradation of the autolysosomes.
Fig. 1.
A schematic diagram illustrates how ULK1-Atg13-FIP200, Beclin-1-VPS34-VPS15, Atg5-Atg12-Atg16L, the HOPS complex, mTOR, LC3, Rab7, TFEB, etc. interact during autophagy and their effects on the journey from the formation to the degradation of autophagosomes
Toxicity of Cd and autophagy dysfunction
At present, the main ways of exposure to Cd are occupational exposure, environmental Cd pollution, tobacco consumption, food, etc. [46]. Cd, a class I carcinogen in humans [47], is mainly toxic to liver and kidney, and exposure to Cd may cause damage to the liver’s free radicals and lipid metabolism [48], as well as disruption of renal tubular function [49]. Cd is reproductively toxic, interfering with the synthesis and secretion of reproductive hormones [50], damaging the blood-testis barrier (BTB), and affecting sperm count and viability [51]. Cd can also act on vascular endothelial cells and is an essential external risk factor for atherosclerosis and hypertension [52]. Besides, Cd is immunotoxic in that its exposure can lead to alterations in the number, maturation, and function of T cells [53].
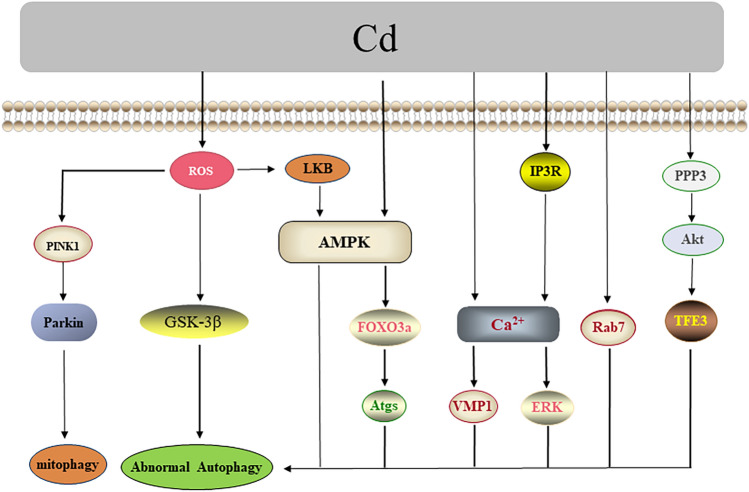
The molecular mechanisms of Cd toxicity are primarily oxidative stress, genetic expression abnormalities, signaling pathways abnormalities caused by calcium (Ca2+) disorder, and the dynamic modulation of enzyme activity [54–57]. As research progressed, autophagy was also identified as being involved in the toxicity of Cd. On the other hand, the FOXO family also has a crucial role in autophagy. It showed that AMPK could phosphorylate the Ser588 site of FOXO3a and Cd exposure could phosphorylate the Thr172 site of the AMPK protein, thus inducing mesenchymal stem cells’ (MSCs’) death by AMPK/FOXO3a-mediated autophagy [58]. Moreover, activation of transcription factor binding to IGHM enhancer 3 (TFE3) could promote autophagosome-lysosome fusion, protein phosphatase 3 (PPP3)/calcineurin could negatively regulate phosphorylation of Akt, a decrease of Akt activity led to dephosphorylation of TFE3 at the Ser565 site in the cytoplasm, and Cd exposure increased the activity of PPP3/calcineurin, thus inducing MSCs’ death by Akt/TFE3-mediated autophagy [59]. It was also found that Cd exposure might also inhibit autophagosome-lysosome fusion by reducing the expression of Rab7 so as to exacerbate hepatotoxicity [21]. Cd-induced ROS could act as the upstream signal of the PTEN-induced putative kinase1 (PINK1)/Parkin pathway to mediate mitophagy in mice brains [60]; it could also activate the LKB1-AMPK signaling pathway to induce autophagy in mouse skin epidermal JB6 cells [61]. Autophagy was also induced by activating glycogen synthase kinase-3β (GSK-3β) in MES-13 mesangial cells [62]. Cd exposure could increase intracellular Ca2+ through IP3R of the endoplasmic reticulum (ER) and later induce autophagy mediated by Ca2+/ERK in MES-13 cells [63]. In addition, Cd exposure activated vacuole membrane protein 1 (VMP1) by increasing intracellular Ca2+ level, and VMP1 induced protein expression of autophagy markers p62 and LC-3II and subsequent apoptotic cell death in Ramos B cells as well as mouse spleen apoptotic damage [64]. Besides, Cd was shown to induce neuroprotective autophagy by activating the PI3K/Beclin-1/B cell lymphoma-2 (Bcl-2) signaling pathway in rat cerebral cortical neurons and PC 12 cells [65, 66]. In summary, the processes of autophagy regulation after exposure to Cd are shown in Fig. 2.
Fig. 2.
Cd-induced autophagy. Cd-induced ROS activates the PINK1/Parkin and LKB1/AMPK pathways along with GSK-3β, mediating mitophagy and cellular autophagy. The increase in Ca2+ induced by Cd can act as an upstream signal to regulate ERK and VMP1, which in turn induce autophagy. Additionally, Cd mediates autophagic cell death through the PPP3/Akt/TFE3 and AMPK/FOXO3a pathways. Cd may also inhibit autophagy by suppressing Rab7
Toxicity of Cr and autophagy dysfunction
Cr is widely used in industry nowadays, and occupational exposure potentially cause allergic dermatitis and chronic lung diseases. An investigation of occupational workers showed that occupational exposure to Cr(VI) increased the risk of lung cancer [67]. In addition to occupational exposure, humans may be exposed to Cr through food and water that contain Cr [68]. An experimental study indicated that Cr(VI) exposure may induce renal tubular necrosis [69]. The accumulation of Cr in the body could damage the liver and immune system [70]. It was found that Cr(VI) could not only enter the reproductive system of male rats through the BTB and interfere with the normal development of sperm [71], but also damage the tissue structure of the ovaries and thus reduce the fertility of female rats [72].
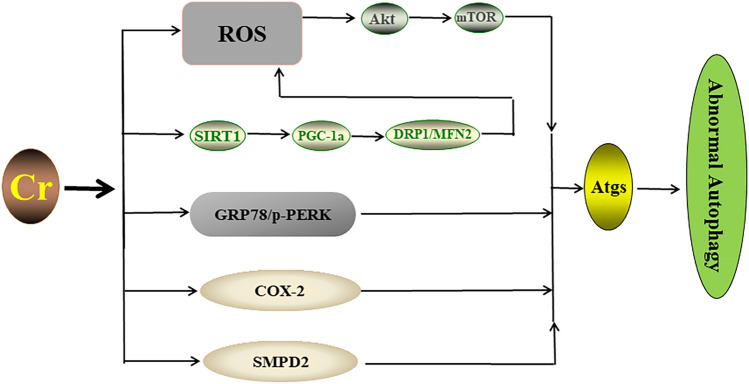
As a principal mechanism of Cr toxicity, ROS-induced oxidative stress was initiated together with lipid peroxidation, DNA damage [73–75], abnormal apoptosis [76], and mitochondrial dysfunction [77]. In addition, the imbalance of intracellular Ca2+ homeostasis was also involved in Cr(VI)-induced liver injury [78, 79]. Autophagy is also a potential mechanism involved in Cr toxicity, while ceramide may be the component lipid of the autophagosome membrane. Furthermore, it was found that Cr(III) can activate sphingomyelin phosphodiesterase 2 (SMPD2) to increase the ceramide level in HK2 cells and induce autophagy [20]. Cr(VI) exposure modified the expression profile of motion-related protein 1 (DRP1) and mitofusin 2 (MFN2) by inhibiting the silent information-regulated transcription factor 1/peroxisome proliferator-activated receptor gamma coactivator 1-alpha (SIRT1/PGC-1a) pathway. This imbalance of mitochondrial dynamics subsequently promoted excessive ROS production and the induction of autophagy-related proteins (Beclin-1, Atg5, and Atg4B) in a dose-dependent manner, along with renal tubular pathologies [80]. In addition, Cr(VI) exposure was shown to initiate autophagy mediated by the ROS/Akt/mTOR signaling pathway to attenuate apoptosis in L-02 hepatocytes [81]. Exposure to Cr(VI) increased the level of cyclooxygenase-2 (COX-2), which affected the expression of autophagy proteins (p62, LC3-II, and Beclin-1), thus reducing the viability of LMH cells [82]. Moreover, the levels of endoplasmic reticulum stress (ER stress) related proteins such as glucose-regulating protein 78 (GRP78) and phosphorylated protein kinase RNA-like ER kinase (p-PERK) were enhanced after Cr(VI) exposure, and thus ER stress was triggered to induce autophagy in A549 cells [83]. In particular, Cr(VI) could destroy structural complementarity and alter the mitochondrial function in DF-1 cells and initiate mitophagy [84]. Together, these studies provide the selective pathways by which Cr exposure triggers the abnormal processes of autophagy or mitophagy (Fig. 3).
Fig. 3.
The selective ways Cr triggers alterations in autophagy. Cr regulates the mitochondrial proteins DRP1 and MFN2 via the SIRT1/PGC-1a pathway, leading to mitochondrial dysfunction and excessive ROS production, which in turn inhibits the Akt/mTOR pathway. ER stress induced by Cr via the GRP78/p-PERK pathway can further induce autophagy. In addition, Cr can also activate SMPD2 or increase COX-2 to regulate autophagy
Toxicity of Pb and autophagy dysfunction
Pb has a long half-life and remains ubiquitous in the environment. Apart from occupational exposure, humans may be exposed to Pb in many substances, including paint, cosmetics, and Pb-containing vehicle exhaust. Pb is a systemic toxicant, with cardiac and renal toxicity following both acute and chronic Pb exposure [85]. Notably, Pb is neurotoxic. It was found that Pb altered the onset of nerve development by targeting the neural cell adhesion molecule (NCAM) [86]. Pb also promoted synapse dysfunction and cognitive impairment in terms of reduced growth of neuronal dendrites and dendritic spines, decreased the number of synapses, and increased synaptic gaps [87, 88]. Pb could reduce the number of neurons, and alter the differentiation process of neural stem cells [89, 90]. Oxidative stress and ER stress may be causative factors for neurodegenerative injury caused by Pb exposure [91]. Other studies indicated that Pb can exert toxicity to the liver, kidney, and brain by impairing mitochondrial structure and modifying enzyme activity in the electronic respiratory transmission chain and ATP synthesis [92–95]. Besides, Pb was found to be embryotoxic, and long-term exposure with high level could also damage immune function.
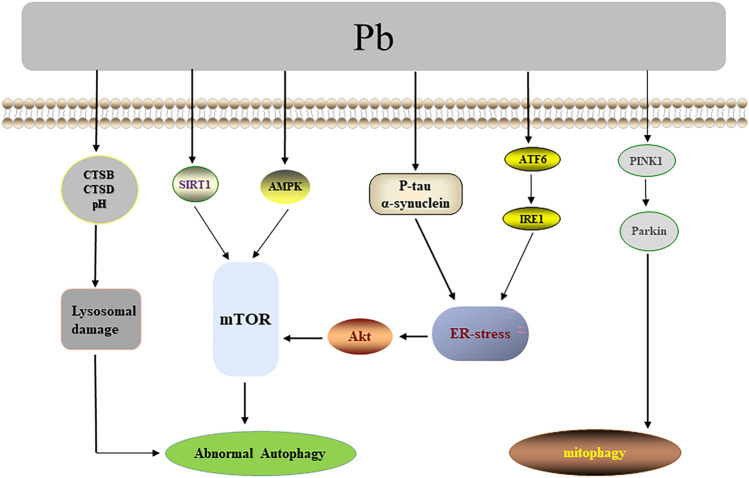
Other than the toxicity mechanisms mentioned above, the contribution of autophagy to the toxicity of Pb has also been widely noted. It was observed that Pb induced abnormal hyperphosphorylation of tau and aggregation of a-synuclein in the hippocampus of Sprague-Dawley rats (SD rats), which caused ER stress, promoted autophagy and apoptosis via inhibiting the Akt/mTOR pathway, and thus impaired the learning and memorizing abilities of SD rats [96]. Pb also induced mitophagy in HEK293 cells and male Kunming mice renal cortex through PINK1/Parkin pathway [97] and promoted the activating transcription factor 6 (ATF6)/inositol-requiring enzyme 1 (IRE1) signaling pathway to trigger a subsequent ER stress response [98]. In addition, Pb mediated hepatocyte injury and renal cytotoxicity via the SIRT1/mTOR [99] as well as AMPK-mTOR pathways [100], respectively. Several studies indicated that Pb exposure disrupted lysosomal function by altering lysosomal pH and cathepsin B/D (CTSB/CTSD), contributing to autophagosome accumulation, which in turn damaged renal tubular cells in rats [101]. Pb could also hamper autophagosome and lysosome fusion, significantly reducing the number or size of lysosomes and resulting in the injury of autophagy in neural cells [102]. Pb additionally disrupted intercellular communication through the activation of autophagy and induced apoptosis in cardiomyocytes [103]. To summarize, the processes of autophagy/mitophagy regulation following Pb exposure are shown in Fig. 4.
Fig. 4.
Pb-associated regulation of autophagy/mitophagy. Abnormal hyperphosphorylation of Tau, aggregation of a-synuclein, and ATF6/IRE1 are involved in Pb-induced ER stress, thereby inhibiting the Akt/mTOR pathway and activating autophagy. Pb may regulate autophagy through the SIRT1/mTOR, AMPK-mTOR, and PINK1/Parkin pathways. Besides, Pb exposure disrupts lysosomal function by altering lysosomal pH and CTSB/CTSD, contributing to autophagosome accumulation
Toxicity of As and autophagy dysfunction
Food, drinking water, air pollution as well as occupational environment exposure are typical sources of As exposure [104]. As occupies different priority list of hazardous substances because of its unique carcinogenic properties and extensive organ toxicity [105]. Due to its bioaccumulation tendency, chronic exposure to As is a potential risk factor for the development of cancer (skin, lung, and urinary bladder) [104]. As exposure is also linked with reproductive risk by inducing spermatogenic cell apoptosis and decreasing sperm quantity [106]. Also, a diverse degree of nephrotoxic [107] and neurotoxic [108] effects is linked with environmental As exposure.
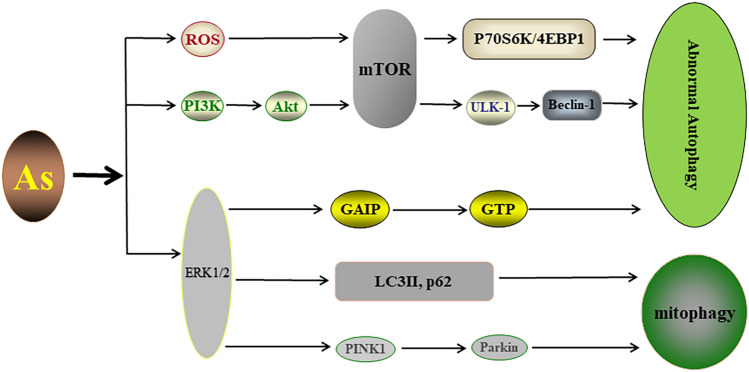
Many studies have provided insights into the cellular and molecular mechanisms of As toxicity, which involve intracellular stresses including oxidative stress [109], DNA damage [110], and epigenetic changes [111]. Furthermore, emerging evidence suggested a contribution of stress responses, autophagy, and mitophagy, to various accumulated toxicity induced by As exposure. The inhibition of mTOR upon As exposure might be mediated by ROS [112], while As exposure could damage mitochondria and produce excessive ROS [113]. As also induced phosphorylation of ERK1/2 and activated ERK1/2 phosphorylated Galpha-interacting protein (GAIP), which increased the rate of GTP hydrolysis and controlled the lysosomal-autophagic pathway [114]. Moreover, the ERK1/2 signaling pathway activated by As exposure also increased the levels of LC3II and p62, along with triggering the PINK1/Parkin pathway to mediate mitophagy, which reduced the apoptosis rate of L-02 cells [115]. Notably, As exposure triggered autophagy via the PI3K/Akt/mTOR pathway in the brains of mice, accompanied by lesions in brain tissue [116]. Studies also showed that subchronic As exposure induced mouse ovotoxicity accompanied by autophagy activation via mTOR/ULK-1/Beclin-1 signaling [117]. Intriguingly, As exposure inhibited mTOR and its downstream markers, the phosphorylation of p70S6K (p-p70S6K) and 4E-binding protein 1 (p-4EBP1), by which As promoted autophagy, accounted largely for reducing the transformation and tumorigenicity of BEAS-2B cells [118]. Altogether, the processes of autophagy/mitophagy regulation in response to As exposure are shown in Fig. 5.
Fig. 5.
The autophagy/mitophagy pathways in response to As exposure. ROS/mTOR and PI3K/Akt/mTOR pathways may be involved in As-mediated autophagy as upstream signals. Activation of ERK by As can modulate the lysosomal-autophagic pathway via regulation of GAIP. Moreover, the ERK1/2 signaling pathway activated by As exposure also increased the levels of LC3II and p62, along with triggering the PINK1/Parkin pathway
Toxicity of Hg/MeHg and autophagy dysfunction
The main sources of Hg are the incineration of fossil fuels and medical waste, the discharge of Hg-containing wastewater from industrial enterprises, and the use of Hg-containing pesticides. Hg is also known as a neurotoxic metal. It has been found that Hg poisoning may exert its neurotoxic effect by increasing the content of the messenger molecule NO in brain tissue and inhibiting the activity of Na/K-ATPase [119]. Kidney is the main target organ of Hg. Hg exposure can damage kidney cells and cause nephrotic syndrome and renal tubular injury. Methylmercury (MeHg) can also affect gonad development and is reproductively toxic and embryotoxic [120].
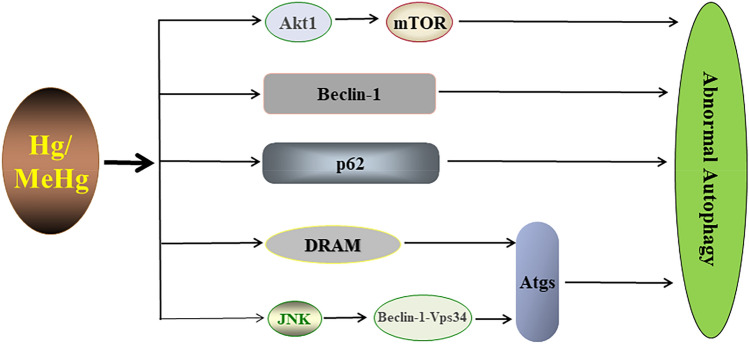
Metal Hg can cause membrane damage, promote oxidative stress, affect enzyme activity, and damage DNA [121–123]. In particular, MeHg has been proven to have significant systemic toxicity caused by excessive oxidative stress, impairing mitochondrial function, modifying epigenetics, and disrupting Ca2+ homeostasis [124–126]. Notably, many studies have revealed a close interplay between autophagy and apoptosis in response to Hg/MeHg. The research showed that low concentration of Hg (5 µM HgCl2) exposure induced autophagy and mediated hepatocyte death in rats through Atg5-Atg12 covalent coupling, which was regulated by damage-regulated autophagy modulator (DRAM) in a p53-dependent manner [127]. The study also found that Hg exposure disrupted the structure and function of lysosomes and initiated the autophagic death of HuH-7 cells [128]. MeHg induced autophagy in human neural stem cells by inhibiting the Akt1/mTOR signaling pathway [129]. P62 is an autophagic adaptor protein that links autophagy to the kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2 (Keap1/Nrf2) pathway. After MeHg exposure, the expression of p62 was enhanced and could competitively combine Keapl with Nrf2, resulting in the degradation of Keapl by autophagy. Interestingly, p62 could also induce Nrf2 activation again, thus developing a positive feedback circuit [130]. MeHg exposure markedly decreased Beclin-1 level, posing a concern for pituitary hemorrhage, dilation, etc. [131]. In addition, MeHg promoted the formation of the Beclin-1-Vps34 complex and increased the independent autophagy of mTOR by activating JNK, which mediated the death of neuronal cells [132]. Collectively, the processes of autophagy regulation associated with Hg/MeHg exposure are shown in Fig. 6.
Fig. 6.
Regulation of autophagy by Hg. Hg inhibits Akt/mTOR and activates Beclin-1, p62, DRAM, and JNK. JNK activation promotes the formation of Beclin-1-Vps34 complex, which in turn induces autophagy
Toxicity of Al and autophagy dysfunction
Al is widespread in nature and available to humans through food, medications, and occupational exposure [133]. Research suggests that Al exposure can cause liver damage, which correlates with oxidative stress [134] and inflammatory reactions [135]. Al can induce testicular dysfunction in rats by inhibiting enzyme activity [136]. In addition, oxidative stress induced by Al exposure can damage bones [137], inhibit renal function [138], induce immunotoxicity [139], and injure glands [4]. It has also been found that AlCl3 may induce hypertension as a result of interfering with the function of the erythrocyte membrane [140]. What’s more, Al causes aggregation and precipitation of amyloid β-protein (Aβ) in the brain [141], disrupts Ca2+ homeostasis in the body [142], affects enzyme activity [143], and promotes oxidative stress [144], all of which can contribute to learning and memory impairment.
The toxic mechanisms of Al mainly include oxidative stress, lipid peroxidation [145], pro-inflammatory reaction [146], DNA damage [147], protein degeneration [148], and the modulation of enzyme activity [149]. More interestingly, the toxicity of Al is affected by autophagy as well. Research revealed that Al exposure induced not only vacuolar degeneration and necrosis in liver tissue [150] but also damaged synaptic plasticity in rat hippocampal neurons via PI3K/Akt/mTOR pathway [151]. It also showed that exposure to Al increased the mRNA expression of Atg3, Atg5, and Atg9, which could be responsible for autophagosome formation and activated the autophagic process in MC3T3-E1 cells, consequently decreasing the rate of apoptosis [152]. Furthermore, Al increased the expression of Beclin-1 and raised the ratio of LC3II/LC3I, which activated autophagy and led to apoptosis in astrocytes [153].
Toxicity of metal mixtures and autophagy dysfunction
In real life, people are easily exposed to metal mixtures, and the mechanisms of their toxic effects are complex, so the studies of metal mixture toxicity and mechanisms are of great health importance. It was found that the co-exposure of As, Cd, and Pb can increase the level of Pb in some areas of the brain, and the co-exposure of Pb and Cd may damage the blood-brain barrier (BBB) [154]. Copper (Cu), iron (Fe) and Ca were proven to inhibit the absorption of Pb in the gastrointestinal tract. Manganese (Mn) induced Pb retention in the brains of rats and damaged cognitive function in these rats [155]. Exposure to a mixture of Pb, As, and Mn caused more pronounced disorders of heme synthesis than exposure to one metal alone [156]. Pb, As, and Cd have a synergistic effect on neurodevelopmental toxicity, and their mixtures can affect the function of the glia and neurons. The mixed exposure to As, Cd, Pb, Cr, and Hg damaged the structure along with the function of the liver, kidney, and lung, as well as the reproductive organ of male mice [157].
The expression proficiency of autophagy proteins (Beclin-1, Atg7, p62, Atg5, and LC3II) was significantly higher in the livers of rats with combined Cd and Pb exposure than in rats exposed to Cd or Pb alone. Besides, liver tissue vacuolation was more severe in the presence of both Cd and Pb [158]. Many studies have found that autophagic pathways or lysosomal function [102] can be affected by metal exposure. However, these are mainly about a single metal [22], and studies related to changes in autophagy levels upon metal mixture exposures are still limited. Based on the effect of single metal exposure on autophagy, we can consider the role of autophagy in metal mixture toxicity as a future research direction.
Toxicity of nanoparticles (NPs) and autophagy dysfunction
Studies have found that TiO2 NPs can easily enter the body through inhalation and cross the BBB to accumulate in the brain, especially in the cortex and hippocampus. Exposure to TiO2 NPs can lead to activation of microglia and inflammatory signaling pathways, production of ROS, and cell death, resulting in neuritis and brain damage [159]. Exposure to TiO2 NPs during pregnancy reduced the proliferation of hippocampal cells in the offspring of rats and impaired their learning and memory [160]. It was observed that the toxic effects of TiO2 NPs were also related to the age of rats, with juvenile rats being more hepatotoxic than adult rats [161]. Other studies suggested that CuO NPs restricted the growth of duckweed, including reduced leaf number, shorter root length, lower photochemical efficiency, chlorophyll content, dry weight, etc. [162]. In addition to causing mortality in Daphnia magna, CuO NPs also transmitted toxicity to aquatic organisms via the dietary chain [163]. Besides, after 30 days of treatment with 50 nm Al2O3 NPs at the concentrations of 25, 50, and 75 mg/kg, significant alterations in the ultrastructure and function of mitochondria in mice were observed, which impaired spatial learning and memory [164].
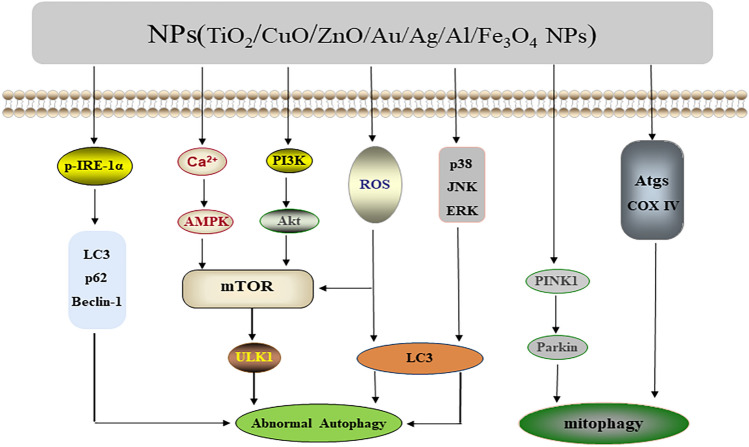
NPs have been extensively studied for their possible mechanisms in cell toxicity, and they play many different roles in modulating cell fate. The formation of ROS is a considerable focus of NPs toxicity. High concentrations of ROS can destroy cellular components, resulting in cell death and the development of disease [165]. Notably, the effect of autophagy on the toxicity of NPs has also attracted increasing attention. For instance, the accumulation of ROS induced by NPs interfered with lysosomal hydrolases and induced autophagy [166]. It was found that ROS caused by acute exposure to ZnO NPs can act as an upstream signal to upregulate the expression of LC3A and thus induce autophagic death of immune cells [167]. Fe3O4 NPs could lead to the death of A549 cells by promoting ROS generation as well as mTOR-mediated autophagy [168]. The ROS generated by TiO2 NPs exposure not only disrupted the lysosomal function of AGS cells and blocked the autophagic flux [169], but also triggered an inflammatory response in RAW264.7 cells [170] and the antioxidant damage response in rat astrocytes [171]. CuO NPs-induced ROS upregulated the level of autophagy and thus attenuated the apoptosis of MCF7 cells [172]. Furthermore, TiO2 NPs exposure affected the function of the placenta through PINK1/Parkin-mediated mitophagy [173] and miRNA-regulated autophagy [174], yet it also mediated autophagy through p-IRE-1α-mediated ER stress [175] and MAPK signaling pathway-related molecules ERK, JNK, and p38 [176], which had toxic effects on lung cells and mesenchymal stem cells, respectively. Increased levels of autophagy mediated by CuO NPs resulted in the death of A549 cells [177]. AuNPs could alkalize lysosomes and block fusion with autophagosomes [178]. AlNPs affected the expression levels of LC3II, Beclin-1, p62, and COX IV, which caused autophagy and induced learning and memory impairment in mice [164]. ZnO NPs enhanced autophagy by inhibiting PI3K/Akt/mTOR, thus inducing apoptosis [179]. AgNPs were found to mediate autophagy to protect SH-SY5Y cells from apoptosis through the Ca2+/CaMKKβ/AMPK/mTOR pathway [180]; they also induced lysosomal membrane permeability and disrupted the autophagic-lysosomal pathway, which subsequently increased the death of HepG2 cells [181]. It also found that Fe3O4 NPs could disturb lysosomal function and induce autophagosome accumulation in MCF-7 cells via modulating the phosphorylation profiles of mTOR and ULK1 [182]. Together, the regulatory pathways of autophagy/mitophagy related to NPs exposure are illustrated in Fig. 7.
Fig. 7.
Signaling of autophagy/mitophagy mediated by NPs. ZnO NPs, Fe3O4 NPs, TiO2 NPs, and CuO NPs are able to mediate autophagy via ROS. ZnO NPs inhibit the PI3K/Akt/mTOR pathway. TiO2 NPs activate the PINK1/Parkin pathway and also mediate autophagy through p-IRE-1α-mediated ER stress and MAPK signaling pathway-related molecules ERK, JNK, and p38. AgNPs and Fe3O4 NPs regulate autophagy through the Ca2+/CaMKKβ/AMPK/mTOR and mTOR/ ULK1 pathways, respectively. AlNPs and AuNPs can affect the expression of LC3II, p62, and other autophagy proteins to further modulate autophagy
Pathological changes associated with metals/NPs-mediated regulation of autophagy
Collectively, we have briefly reviewed the mechanisms underlying the toxicity of metals and NPs as well as a description of the changes in the autophagic pathways that contributed to cytotoxicity. To further understand how autophagy functions in metals/NPs-mediated toxicity, we summarized the pathological changes associated with metals/NPs-mediated autophagy regulation (Table 1).
Table 1.
The pathological changes associated with metals/NPs-mediated autophagy regulation
| Metals/NPs | Autophagic activity | Toxicity of Metal/NPs | Pathological changes | References |
|---|---|---|---|---|
| Cd | Inhibition | Increase |
Hepatic steatosis Glomerular atrophy |
[183] [184] |
| Activition | Increase |
Renal tubular dilatation Hepatic lobular injury Renal cortex injury |
[185] [186] [187] |
|
| Cr | Activition | Increase |
Renal tubular rupture Damage of liver mitochondria Cardiomyocyte necrosis Blurred boundaries of glomerula |
[80] [188] [189] [190] |
| Pb | Inhibition | Increase | Structural changes of the spleen, ferritin deposits | [191] |
| Activition | Increase | Hippocampus damage | [192] | |
| As | Inhibition | Increase | Skin tumorigenesis | [193] |
| Activition | Increase |
Islet cell hypertrophy Glomerular atrophy Aortic injuries Mitochondrial damage in jejunal cells Testicular tissue damage Purkinje cell layer damage Hepatic steatosis Liver fibrosis |
[194] [195] [196] [197] [198] [199] [200] [201] |
|
| Hg | Inhibition | Increase | Tubular necrosis, interstitial hyperemia, and inflammatory cell infiltration | [202] |
| Activition | Increase | Spleen damage | [203] | |
| Al | Activition | Increase | Femoral damage | [204] |
| Activition | Decrease |
Testicular damage Liver inflammatory injury |
[205] [206] |
|
| NPs | Activition | Increase | Neurovascular toxicity | [207] |
| Activition | Decrease | Liver damage | [208] |
Activation/Inhibition represents that the autophagy is activated or inhibited by the metals/NPs. Increase/Decrease represents that the autophagic activity enhances or attenuates the toxicity of the metals/NPs.
Modulation of autophagy and the toxicity of metals/NPs
As mentioned above, activation or inhibition of autophagy is involved in the toxicity induced by metals/NPs; therefore, it is of interest that autophagic modulation by chemicals may modify the toxicity of metals/NPs. We can focus on changes in harmful effects to make a beneficial autophagy regulation by using autophagy inhibitors or activators to avoid or lessen negative biological effects. It was found that Cd exposure induced autophagy in mouse spleen tissue and that the autophagy inhibitor CQ was effective in reducing Cd-induced apoptosis in spleen and immune cells [64]. Additionally, autophagy inhibitor 3-methyladenine (3-MA) exacerbated Cd-induced germ cell apoptosis; while autophagy inducer rapamycin diminished Cd-induced germ cell apoptosis [209]. Chloroquine diphosphate (CDP) can disrupt the structure and function of lysosomes and alter the process of autophagy-lysosome fusion. Treatment with 3-MA and CDP inhibited autophagy in hepatocytes induced by Cr(VI) exposure and therefore reduced apoptosis [81], while treatment with rapamycin relieved hepatocyte injury [18]. Specially, inhibition of autophagy with 3-MA exacerbated Pb-induced renal tubular and osteogenic apoptosis as well as cardiac fibroblast death. While activation of AMPK significantly augmented cellular autophagic activity and reduced Pb-induced renal tubular apoptosis [210–212]. Treatment with 3-MA reduced As-induced cytotoxicity, while treatment with rapamycin reduced the viability of cells in the As-treated group [213]. MeHg-induced neurotoxicity could be reduced to some extent by autophagy. Study also confirmed that treatment with 3-MA/CQ or rapamycin increased or reduced MeHg-induced apoptosis, respectively [214]. Al exposure led to apoptosis and cognitive impairment in MC3T3-E1 cells and zebrafish, individually, and the use of rapamycin reduced the apoptosis rate of MC3T3-E1 cells and improved learning ability in zebrafish after AlCl3 exposure [152, 215]. Moreover, it was also found that high doses of Al may lead to apoptosis through the Beclin-1-dependent autophagic signaling pathway, and 3-MA reduced Al-induced apoptosis [153]. AgNPs could induce protective autophagy, and the addition of CQ to inhibit autophagy significantly enhanced the cytotoxicity of AgNPs [180].
Perspectives
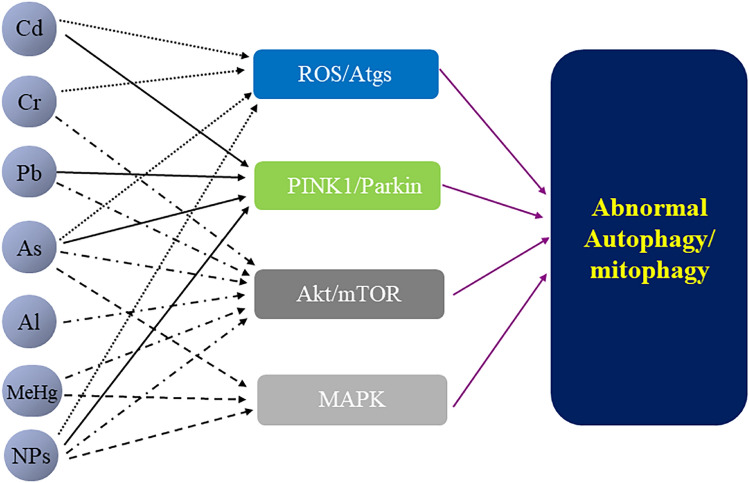
In this review, we addressed the general toxicity of both single metal and metal mixtures as well as NPs, mainly focusing on the role of autophagic regulation in the toxicity of metals/NPs. At first, we described studies on a single metal affecting autophagy, and it was clear that different metals may influence autophagy in the same pathway. Cd, Cr, As, and NPs individually regulate autophagy through the induction of ROS. Cd, Pb, As, and NPs respectively regulate mitophagy via the PINK1/Parkin pathway. Cr, Pb, As, MeHg, Al, and NPs separately regulate autophagy through the Akt/mTOR pathway. Metals (As and MeHg) and NPs regulate autophagy by the MAPK pathway, respectively (e.g., Fig. 8). However, little is known about whether exposure to metal mixtures regulates autophagy via ROS or otherwise. Because exposure to metal mixtures is closer to the real-life situation, exploring the toxicity of metal mixture exposures from the perspective of autophagy can greatly contribute to the well-being of the public. Therefore, this review presented a new perspective for further studies on the toxicity of metal mixture exposures. Meanwhile, NPs are widely used in various fields, and their potential hazards are becoming increasingly prominent. This review also provided an overview of nanotoxicity from the perspective of autophagy, where it was observed that NPs could regulate autophagy via multiple modalities and induce different biological effects. Consequently, the role of autophagy regulation should also draw extensive attention in future nanotoxicity studies. In view of this, we may be able to reduce or eliminate the adverse health effects of metals/NPs through optimization, substitution, or intervention (e.g., intervention using autophagic chemomodulators or targets of metals/NPs action) based on our understanding of their material properties, exposure pathways, uptake and metabolism, and toxic effects.
Fig. 8.
The common pathways of autophagy regulation by metals/NPs. The regulatory process involves the activation of Akt/mTOR, PINK1/Parkin, and MAPK signaling pathways, as well as the further regulation of autophagy proteins by ROS. ROS induced by metals (Cd, Cr, and As) and NPs may be implicated in multiple modes of autophagy regulation. Metals (Cd, Pb, and As) and NPs mediate mitophagy via PINK1/Parkin. Akt/mTOR can be regulated by ROS and ER stress, and metals (Cr, Pb, As, MeHg, and Al) and NPs mediate Akt/mTOR-induced autophagy. MAPK signaling pathway-related ERK, JNK, and p38 act as upstream signals to regulate LC3, Beclin-1, and p62. Metals (As and MeHg) and NPs regulate autophagy by the MAPK pathway
Acknowledgements
This review was supported by the National Natural Science Foundation of China (81202173), the Key Teachers Training Plan of Henan Province (2018GGJS007) and the Technological Projects Foundation for Key R&D and Promotion in Henan Province (192102310047) and the Young Teachers Training Program of Zhengzhou University (2016-40).
Author contributions
All authors have contributed to this work.
Funding
This review was supported by the National Natural Science Foundation of China (81202173), the Key Teachers Training Plan of Henan Province (2018GGJS007) and the Technological Projects Foundation for Key R&D and Promotion in Henan Province (192102310047) and the Young Teachers Training Program of Zhengzhou University (2016-40).
Data availability
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rahman Z, Singh VP. The relative impact of toxic heavy metals (THMs) (arsenic (as), cadmium (cd), chromium (cr)(VI), mercury (hg), and lead (pb)) on the total environment: an overview. Environ Monit Assess. 2019;191(7):419. doi: 10.1007/s10661-019-7528-7. [DOI] [PubMed] [Google Scholar]
- 2.Wang M, Hossain F, Sulaiman R, Ren X. Exposure to inorganic arsenic and lead and autism spectrum disorder in children: a systematic review and meta-analysis. Chem Res Toxicol. 2019;32(10):1904–1919. doi: 10.1021/acs.chemrestox.9b00134. [DOI] [PubMed] [Google Scholar]
- 3.Al OM, Yang F, Massey IY. Exposure routes and health effects of heavy metals on children. Biometals. 2019;32(4):563–573. doi: 10.1007/s10534-019-00193-5. [DOI] [PubMed] [Google Scholar]
- 4.Igbokwe IO, Igwenagu E, Igbokwe NA. Aluminium toxicosis: a review of toxic actions and effects. Interdiscip Toxicol. 2019;12(2):45–70. doi: 10.2478/intox-2019-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res Int. 2016;23(9):8244–8259. doi: 10.1007/s11356-016-6333-x. [DOI] [PubMed] [Google Scholar]
- 6.Attarilar S, Yang J, Ebrahimi M, Wang Q, Liu J, Tang Y, et al. The toxicity phenomenon and the related occurrence in metal and metal oxide nanoparticles: a brief review from the biomedical perspective. Front Bioeng Biotechnol. 2020;8:822. doi: 10.3389/fbioe.2020.00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahadar H, Maqbool F, Niaz K, Abdollahi M. Toxicity of nanoparticles and an overview of current experimental models. Iran Biomed J. 2016;20(1):1–11. doi: 10.7508/ibj.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh S. Zinc oxide nanoparticles impacts: cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol Mech Methods. 2019;29(4):300–311. doi: 10.1080/15376516.2018.1553221. [DOI] [PubMed] [Google Scholar]
- 9.Buchman JT, Hudson-Smith NV, Landy KM, Haynes CL. Understanding nanoparticle toxicity mechanisms to inform redesign strategies to reduce environmental impact. Acc Chem Res. 2019;52(6):1632–1642. doi: 10.1021/acs.accounts.9b00053. [DOI] [PubMed] [Google Scholar]
- 10.Mortezaee K, Najafi M, Samadian H, Barabadi H, Azarnezhad A, Ahmadi A. Redox interactions and genotoxicity of metal-based nanoparticles: a comprehensive review. Chem Biol Interact. 2019;312:108814. doi: 10.1016/j.cbi.2019.108814. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Ju D. The role of Autophagy in nanoparticles-induced toxicity and its related cellular and molecular mechanisms. Adv Exp Med Biol. 2018;1048:71–84. doi: 10.1007/978-3-319-72041-8_5. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Zhou R, Ma Z. Autophagy and energy metabolism. Adv Exp Med Biol. 2019;1206:329–357. doi: 10.1007/978-981-15-0602-4_16. [DOI] [PubMed] [Google Scholar]
- 13.Reich N, Holscher C. Acylated Ghrelin as a multi-targeted therapy for Alzheimer’s and Parkinson’s disease. Front Neurosci. 2020;14:614828. doi: 10.3389/fnins.2020.614828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floriano BF, Carvalho T, Lopes TZ, Takahashi L, Rahal P, Tedesco AC, et al. Effect of berberine nanoemulsion photodynamic therapy on cervical carcinoma cell line. Photodiagnosis Photodyn Ther. 2021;33:102174. doi: 10.1016/j.pdpdt.2020.102174. [DOI] [PubMed] [Google Scholar]
- 15.Lin Z, Miao J, Zhang T, He M, Zhou X, Zhang H, et al. D-mannose suppresses osteoarthritis development in vivo and delays IL-1beta-induced degeneration in vitro by enhancing autophagy activated via the AMPK pathway. Biomed Pharmacother. 2021;135:111199. doi: 10.1016/j.biopha.2020.111199. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Miah M, Culbreth M, Aschner M. Autophagy in neurodegenerative diseases and metal neurotoxicity. Neurochem Res. 2016;41(1–2):409–422. doi: 10.1007/s11064-016-1844-x. [DOI] [PubMed] [Google Scholar]
- 17.Saran U, Tyagi A, Chandrasekaran B, Ankem MK, Damodaran C. The role of autophagy in metal-induced urogenital carcinogenesis. Semin Cancer Biol. 2021;76:247–257. doi: 10.1016/j.semcancer.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y, Xiao F, Luo L, Zhong C. Activation of autophagy protects against ROS-mediated mitochondria-dependent apoptosis in L-02 hepatocytes induced by cr(VI) Cell Physiol Biochem. 2014;33(3):705–716. doi: 10.1159/000358646. [DOI] [PubMed] [Google Scholar]
- 19.Bai L, Liu R, Wang R, Xin Y, Wu Z, Ba Y, et al. Attenuation of Pb-induced Aβ generation and autophagic dysfunction via activation of SIRT1: neuroprotective properties of resveratrol. Ecotox Environ Safe. 2021;222:112511. doi: 10.1016/j.ecoenv.2021.112511. [DOI] [PubMed] [Google Scholar]
- 20.Yang CL, Chiou SH, Tai WC, Joseph NA, Chow KC. Trivalent chromium induces autophagy by activating sphingomyelin phosphodiesterase 2 and increasing cellular ceramide levels in renal HK2 cells. Mol Carcinog. 2017;56(11):2424–2433. doi: 10.1002/mc.22689. [DOI] [PubMed] [Google Scholar]
- 21.Zou H, Wang T, Yuan J, Sun J, Yuan Y, Gu J, et al. Cadmium-induced cytotoxicity in mouse liver cells is associated with the disruption of autophagic flux via inhibiting the fusion of autophagosomes and lysosomes. Toxicol Lett. 2020;321:32–43. doi: 10.1016/j.toxlet.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee S, Sarkar S, Bhattacharya S. Toxic metals and autophagy. Chem Res Toxicol. 2014;27(11):1887–1900. doi: 10.1021/tx500264s. [DOI] [PubMed] [Google Scholar]
- 23.Zachari M, Ganley IG. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017;61(6):585–596. doi: 10.1042/EBC20170021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin MG, Hurley JH. Structure and function of the ULK1 complex in autophagy. Curr Opin Cell Biol. 2016;39:61–68. doi: 10.1016/j.ceb.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polivka JJ, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142(2):164–175. doi: 10.1016/j.pharmthera.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Chen Y. AMPK and Autophagy. Adv Exp Med Biol. 2019;1206:85–108. doi: 10.1007/978-981-15-0602-4_4. [DOI] [PubMed] [Google Scholar]
- 30.Maiuri MC, Galluzzi L, Morselli E, Kepp O, Malik SA, Kroemer G. Autophagy regulation by p53. Curr Opin Cell Biol. 2010;22(2):181–185. doi: 10.1016/j.ceb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 31.White E. Autophagy and p53. Cold Spring Harb Perspect Med. 2016;6(4):a26120. doi: 10.1101/cshperspect.a026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Jiang Z, Ma H, Ning L, Chen H, Li L, et al. Volatile oil of Acori graminei rhizoma-induced apoptosis and autophagy are dependent on p53 status in human glioma cells. Sci Rep. 2016;6:21148. doi: 10.1038/srep21148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J, Zhang XQ, Zhang Z. Transcription factor EB agonists from natural products for treating human diseases with impaired autophagy-lysosome pathway. Chin Med. 2020;15(1):123. doi: 10.1186/s13020-020-00402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C, Qin H, Tan J, Hu Z, Zeng L. The role of ubiquitin-proteasome pathway and autophagy-lysosome pathway in cerebral ischemia. Oxid Med Cell Longev. 2020;2020:5457049. doi: 10.1155/2020/5457049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babuta M, Furi I, Bala S, Bukong TN, Lowe P, Catalano D, et al. Dysregulated autophagy and lysosome function are linked to exosome production by micro-RNA 155 in alcoholic liver disease. Hepatology. 2019;70(6):2123–2141. doi: 10.1002/hep.30766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mcewan DG, Popovic D, Gubas A, Terawaki S, Suzuki H, Stadel D, et al. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol Cell. 2015;57(1):39–54. doi: 10.1016/j.molcel.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Tan CC, Yu JT, Tan MS, Jiang T, Zhu XC, Tan L. Autophagy in aging and neurodegenerative diseases: implications for pathogenesis and therapy. Neurobiol Aging. 2014;35(5):941–957. doi: 10.1016/j.neurobiolaging.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol. 2010;12(8):747–757. doi: 10.1038/ncb2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatakeyama S. TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem Sci. 2017;42(4):297–311. doi: 10.1016/j.tibs.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Kang R, Zeh HJ, Lotze MT, Tang D. The beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18(4):571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22(2):124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarkar S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans. 2013;41(5):1103–1130. doi: 10.1042/BST20130134. [DOI] [PubMed] [Google Scholar]
- 43.Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176(1–2):11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diao J, Liu R, Rong Y, Zhao M, Zhang J, Lai Y, et al. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature. 2015;520(7548):563–566. doi: 10.1038/nature14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Branca J, Morucci G, Pacini A. Cadmium-induced neurotoxicity: still much ado. Neural Regen Res. 2018;13(11):1879–1882. doi: 10.4103/1673-5374.239434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Y, Costa M. Metals and molecular carcinogenesis. Carcinogenesis. 2020;41(9):1161–1172. doi: 10.1093/carcin/bgaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abu-El-Zahab H, Hamza RZ, Montaser MM, El-Mahdi MM, Al-Harthi WA. Antioxidant, antiapoptotic, antigenotoxic, and hepatic ameliorative effects of L-carnitine and selenium on cadmium-induced hepatotoxicity and alterations in liver cell structure in male mice. Ecotoxicol Environ Saf. 2019;173:419–428. doi: 10.1016/j.ecoenv.2019.02.041. [DOI] [PubMed] [Google Scholar]
- 49.Nanayakkara S, Komiya T, Ratnatunga N, Senevirathna ST, Harada KH, Hitomi T, et al. Tubulointerstitial damage as the major pathological lesion in endemic chronic kidney disease among farmers in north central province of Sri Lanka. Environ Health Prev Med. 2012;17(3):213–221. doi: 10.1007/s12199-011-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar S, Sharma A. Cadmium toxicity: effects on human reproduction and fertility. Rev Environ Health. 2019;34(4):327–338. doi: 10.1515/reveh-2019-0016. [DOI] [PubMed] [Google Scholar]
- 51.Venditti M, Ben RM, Romano MZ, Messaoudi I, Reiter RJ, Minucci S. Evidence of melatonin ameliorative effects on the blood-testis barrier and sperm quality alterations induced by cadmium in the rat testis. Ecotoxicol Environ Saf. 2021;226:112878. doi: 10.1016/j.ecoenv.2021.112878. [DOI] [PubMed] [Google Scholar]
- 52.Chen H, Lu Y, Cao Z, Ma Q, Pi H, Fang Y, et al. Cadmium induces NLRP3 inflammasome-dependent pyroptosis in vascular endothelial cells. Toxicol Lett. 2016;246:7–16. doi: 10.1016/j.toxlet.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 53.Ebrahimi M, Khalili N, Razi S, Keshavarz-Fathi M, Khalili N, Rezaei N. Effects of lead and cadmium on the immune system and cancer progression. J Environ Health Sci Eng. 2020;18(1):335–343. doi: 10.1007/s40201-020-00455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Son J, Lee SE, Park BS, Jung J, Park HS, Bang JY, et al. Biomarker discovery and proteomic evaluation of cadmium toxicity on a collembolan species, Paronychiurus kimi (Lee) Proteomics. 2011;11(11):2294–2307. doi: 10.1002/pmic.200900690. [DOI] [PubMed] [Google Scholar]
- 55.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283(2–3):65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Faiz H, Boghossian M, Martin G, Baverel G, Ferrier B, Conjard-Duplany A. Cadmium chloride inhibits lactate gluconeogenesis in mouse renal proximal tubules: an in vitro metabolomic approach with (13)C NMR. Toxicol Lett. 2015;238(3):45–52. doi: 10.1016/j.toxlet.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 57.Choong G, Liu Y, Templeton DM. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem Biol Interact. 2014;211:54–65. doi: 10.1016/j.cbi.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Yang M, Pi H, Li M, Xu S, Zhang L, Xie J, et al. From the cover: autophagy induction contributes to cadmium toxicity in mesenchymal stem cells via AMPK/FOXO3a/BECN1 signaling. Toxicol Sci. 2016;154(1):101–114. doi: 10.1093/toxsci/kfw144. [DOI] [PubMed] [Google Scholar]
- 59.Pi H, Li M, Zou L, Yang M, Deng P, Fan T, et al. AKT inhibition-mediated dephosphorylation of TFE3 promotes overactive autophagy independent of MTORC1 in cadmium-exposed bone mesenchymal stem cells. Autophagy. 2019;15(4):565–582. doi: 10.1080/15548627.2018.1531198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei X, Qi Y, Zhang X, Gu X, Cai H, Yang J, et al. ROS act as an upstream signal to mediate cadmium-induced mitophagy in mouse brain. Neurotoxicology. 2015;46:19–24. doi: 10.1016/j.neuro.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 61.Son YO, Wang X, Hitron JA, Zhang Z, Cheng S, Budhraja A, et al. Cadmium induces autophagy through ROS-dependent activation of the LKB1-AMPK signaling in skin epidermal cells. Toxicol Appl Pharmacol. 2011;255(3):287–296. doi: 10.1016/j.taap.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang SH, Shih YL, Kuo TC, Ko WC, Shih CM. Cadmium toxicity toward autophagy through ROS-activated GSK-3beta in mesangial cells. Toxicol Sci. 2009;108(1):124–131. doi: 10.1093/toxsci/kfn266. [DOI] [PubMed] [Google Scholar]
- 63.Wang SH, Shih YL, Ko WC, Wei YH, Shih CM. Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cell Mol Life Sci. 2008;65(22):3640–3652. doi: 10.1007/s00018-008-8383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gu J, Wang Y, Liu Y, Shi M, Yin L, Hou Y, et al. Inhibition of autophagy alleviates cadmium-induced mouse spleen and human B cells apoptosis. Toxicol Sci. 2019;170(1):109–122. doi: 10.1093/toxsci/kfz089. [DOI] [PubMed] [Google Scholar]
- 65.Wang QW, Wang Y, Wang T, Zhang KB, Jiang CY, Hu FF, et al. Cadmium-induced autophagy promotes survival of rat cerebral cortical neurons by activating class III phosphoinositide 3-kinase/beclin-1/B-cell lymphoma 2 signaling pathways. Mol Med Rep. 2015;12(2):2912–2918. doi: 10.3892/mmr.2015.3755. [DOI] [PubMed] [Google Scholar]
- 66.Wang Q, Zhu J, Zhang K, Jiang C, Wang Y, Yuan Y, et al. Induction of cytoprotective autophagy in PC-12 cells by cadmium. Biochem Biophys Res Commun. 2013;438(1):186–192. doi: 10.1016/j.bbrc.2013.07.050. [DOI] [PubMed] [Google Scholar]
- 67.Nasirzadeh N, Mohammadian Y, Dehgan G. Health risk assessment of occupational exposure to hexavalent chromium in iranian workplaces: a meta-analysis study. Biol Trace Elem Res. 2022;200(4):1551–1560. doi: 10.1007/s12011-021-02789-w. [DOI] [PubMed] [Google Scholar]
- 68.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Exp Suppl. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Velma V, Tchounwou PB. Chromium-induced biochemical, genotoxic and histopathologic effects in liver and kidney of goldfish, carassius auratus. Mutat Res. 2010;698(1–2):43–51. doi: 10.1016/j.mrgentox.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chakraborty R, Renu K, Eladl MA, El-Sherbiny M, Elsherbini D, Mirza AK, et al. Mechanism of chromium-induced toxicity in lungs, liver, and kidney and their ameliorative agents. Biomed Pharmacother. 2022;151:113119. doi: 10.1016/j.biopha.2022.113119. [DOI] [PubMed] [Google Scholar]
- 71.Marouani N, Tebourbi O, Mahjoub S, Yacoubi MT, Sakly M, Benkhalifa M, et al. Effects of hexavalent chromium on reproductive functions of male adult rats. Reprod Biol. 2012;12(2):119–133. doi: 10.1016/s1642-431x(12)60081-3. [DOI] [PubMed] [Google Scholar]
- 72.Samuel JB, Stanley JA, Sekar P, Princess RA, Sebastian MS, Aruldhas MM. Persistent hexavalent chromium exposure impaired the pubertal development and ovarian histoarchitecture in wistar rat offspring. Environ Toxicol. 2014;29(7):814–828. doi: 10.1002/tox.21810. [DOI] [PubMed] [Google Scholar]
- 73.El-Demerdash FM, El-Sayed RA, Abdel-Daim MM. Rosmarinus officinalis essential oil modulates renal toxicity and oxidative stress induced by potassium dichromate in rats. J Trace Elem Med Biol. 2021;67:126791. doi: 10.1016/j.jtemb.2021.126791. [DOI] [PubMed] [Google Scholar]
- 74.Marouani N, Tebourbi O, Mokni M, Yacoubi MT, Sakly M, Benkhalifa M, et al. Hexavalent chromium-induced apoptosis in rat uterus: involvement of oxidative stress. Arch Environ Occup Health. 2015;70(4):189–195. doi: 10.1080/19338244.2013.828673. [DOI] [PubMed] [Google Scholar]
- 75.Madejczyk MS, Baer CE, Dennis WE, Minarchick VC, Leonard SS, Jackson DA, et al. Temporal changes in rat liver gene expression after acute cadmium and chromium exposure. PLoS One. 2015;10(5):e127327. doi: 10.1371/journal.pone.0127327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan Y, Ming Z, Gong-Hua H, Lan G, Lu D, Peng L, et al. Cr(VI) induces the decrease of ATP level and the increase of apoptosis rate mediated by ROS or VDAC1 in L-02 hepatocytes. Environ Toxicol Pharmacol. 2012;34(2):579–587. doi: 10.1016/j.etap.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 77.Zhong X, de Cassia DSES, Zhong C. Mitochondrial biogenesis in response to chromium (VI) toxicity in human liver cells. Int J Mol Sci. 2017;18:1877. doi: 10.3390/ijms18091877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yi X, Zhang Y, Zhong C, Zhong X, Xiao F. The role of STIM1 in the cr(vi)-induced [Ca(2+)]i increase and cell injury in L-02 hepatocytes. Metallomics. 2016;8(12):1273–1282. doi: 10.1039/c6mt00204h. [DOI] [PubMed] [Google Scholar]
- 79.Yi X, Xiao F, Zhong X, Duan Y, Liu K, Zhong C. A ca(2+) chelator ameliorates chromium (VI)-induced hepatocyte L-02 injury via down-regulation of voltage-dependent anion channel 1 (VDAC1) expression. Environ Toxicol Pharmacol. 2017;49:27–33. doi: 10.1016/j.etap.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 80.Zheng X, Li S, Li J, Lv Y, Wang X, Wu P, et al. Hexavalent chromium induces renal apoptosis and autophagy via disordering the balance of mitochondrial dynamics in rats. Ecotoxicol Environ Saf. 2020;204:111061. doi: 10.1016/j.ecoenv.2020.111061. [DOI] [PubMed] [Google Scholar]
- 81.Liang Q, Xiao Y, Liu K, Zhong C, Zeng M, Xiao F. Cr(VI)-induced autophagy protects L-02 hepatocytes from apoptosis through the ROS-AKT-mTOR pathway. Cell Physiol Biochem. 2018;51(4):1863–1878. doi: 10.1159/000495713. [DOI] [PubMed] [Google Scholar]
- 82.Liu K, Chen P, Lu J, Zhu Y, Xu Y, Liu Y, et al. Protective effect of purple tomato anthocyanidin on chromium(VI)-induced autophagy in LMH cells by inhibiting endoplasmic reticulum stress. Biol Trace Elem Res. 2020;194(2):570–580. doi: 10.1007/s12011-019-01795-3. [DOI] [PubMed] [Google Scholar]
- 83.Ge H, Li Z, Jiang L, Li Q, Geng C, Yao X, et al. Cr (VI) induces crosstalk between apoptosis and autophagy through endoplasmic reticulum stress in A549cells. Chem Biol Interact. 2019;298:35–42. doi: 10.1016/j.cbi.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 84.Xu Y, Wang X, Geng N, Zhu Y, Zhang S, Liu Y, et al. Mitophagy is involved in chromium (VI)-induced mitochondria damage in DF-1 cells. Ecotoxicol Environ Saf. 2020;194:110414. doi: 10.1016/j.ecoenv.2020.110414. [DOI] [PubMed] [Google Scholar]
- 85.Mitra P, Sharma S, Purohit P, Sharma P. Clinical and molecular aspects of lead toxicity: an update. Crit Rev Clin Lab Sci. 2017;54(7–8):506–528. doi: 10.1080/10408363.2017.1408562. [DOI] [PubMed] [Google Scholar]
- 86.Hu Q, Fu H, Ren T, Wang S, Zhou W, Song H, et al. Maternal low-level lead exposure reduces the expression of PSA-NCAM and the activity of sialyltransferase in the hippocampi of neonatal rat pups. Neurotoxicology. 2008;29(4):675–681. doi: 10.1016/j.neuro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 87.Wang T, Guan RL, Liu MC, Shen XF, Chen JY, Zhao MG, et al. Lead exposure impairs hippocampus related learning and memory by altering synaptic plasticity and morphology during juvenile period. Mol Neurobiol. 2016;53(6):3740–3752. doi: 10.1007/s12035-015-9312-1. [DOI] [PubMed] [Google Scholar]
- 88.Tang M, Luo L, Zhu D, Wang M, Luo Y, Wang H, et al. Muscarinic cholinergic modulation of synaptic transmission and plasticity in rat hippocampus following chronic lead exposure. Naunyn Schmiedebergs Arch Pharmacol. 2009;379(1):37–45. doi: 10.1007/s00210-008-0344-1. [DOI] [PubMed] [Google Scholar]
- 89.Sun L, Zou Y, Su P, Xue C, Wang D, Zhao F, et al. Lead exposure induced neural stem cells death via notch signaling pathway and gut-brain axis. Oxid Med Cell Longev. 2022;2022:7676872. doi: 10.1155/2022/7676872. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Nam SM, Chang BJ, Kim JH, Nahm SS, Lee JH. Ascorbic acid ameliorates lead-induced apoptosis in the cerebellar cortex of developing rats. Brain Res. 2018;1686:10–18. doi: 10.1016/j.brainres.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 91.Feng C, Liu S, Zhou F, Gao Y, Li Y, Du G, et al. Oxidative stress in the neurodegenerative brain following lifetime exposure to lead in rats: changes in lifespan profiles. Toxicology. 2019;411:101–109. doi: 10.1016/j.tox.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 92.Wang L, Li J, Li J, Liu Z. Effects of lead and/or cadmium on the oxidative damage of rat kidney cortex mitochondria. Biol Trace Elem Res. 2010;137(1):69–78. doi: 10.1007/s12011-009-8560-1. [DOI] [PubMed] [Google Scholar]
- 93.Maiti AK, Saha NC, More SS, Panigrahi AK, Paul G. Neuroprotective efficacy of mitochondrial antioxidant MitoQ in suppressing peroxynitrite-mediated mitochondrial dysfunction inflicted by lead toxicity in the rat brain. Neurotox Res. 2017;31(3):358–372. doi: 10.1007/s12640-016-9692-7. [DOI] [PubMed] [Google Scholar]
- 94.Lalith KV, Muralidhara Ameliorative effects of ferulic acid against lead acetate-induced oxidative stress, mitochondrial dysfunctions and toxicity in prepubertal rat brain. Neurochem Res. 2014;39(12):2501–2515. doi: 10.1007/s11064-014-1451-7. [DOI] [PubMed] [Google Scholar]
- 95.Yuan G, Dai S, Yin Z, Lu H, Jia R, Xu J, et al. Sub-chronic lead and cadmium co-induce apoptosis protein expression in liver and kidney of rats. Int J Clin Exp Pathol. 2014;7(6):2905–2914. [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang J, Cai T, Zhao F, Yao T, Chen Y, Liu X, et al. The role of alpha-synuclein and tau hyperphosphorylation-mediated autophagy and apoptosis in lead-induced learning and memory injury. Int J Biol Sci. 2012;8(7):935–944. doi: 10.7150/ijbs.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gu X, Qi Y, Feng Z, Ma L, Gao K, Zhang Y. Lead (pb) induced ATM-dependent mitophagy via PINK1/Parkin pathway. Toxicol Lett. 2018;291:92–100. doi: 10.1016/j.toxlet.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 98.Gao K, Zhang C, Tian Y, Naeem S, Zhang Y, Qi Y. The role of endoplasmic reticulum stress in lead (Pb)-induced mitophagy of HEK293 cells. Toxicol Ind Health. 2020;36(12):1002–1009. doi: 10.1177/0748233720971882. [DOI] [PubMed] [Google Scholar]
- 99.Zhao Y, Mao A, Zhang R, Guan S, Lu J. SIRT1/mTOR pathway-mediated autophagy dysregulation promotes Pb-induced hepatic lipid accumulation in HepG2 cells. Environ Toxicol. 2022;37(3):549–563. doi: 10.1002/tox.23420. [DOI] [PubMed] [Google Scholar]
- 100.Song X, Li Z, Liu F, Wang Z, Wang L. Restoration of autophagy by puerarin in lead-exposed primary rat proximal tubular cells via regulating AMPK-mTOR signaling. J Biochem Mol Toxicol. 2017 doi: 10.1002/jbt.21869. [DOI] [PubMed] [Google Scholar]
- 101.Song XB, Liu G, Liu F, Yan ZG, Wang ZY, Liu ZP, et al. Autophagy blockade and lysosomal membrane permeabilization contribute to lead-induced nephrotoxicity in primary rat proximal tubular cells. Cell Death Dis. 2017;8(6):e2863. doi: 10.1038/cddis.2017.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gu X, Han M, Du Y, Wu Y, Xu Y, Zhou X, et al. Pb disrupts autophagic flux through inhibiting the formation and activity of lysosomes in neural cells. Toxicol in Vitro. 2019;55:43–50. doi: 10.1016/j.tiv.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 103.Li C, Shi L, Peng C, Yu G, Zhang Y, Du Z. Lead-induced cardiomyocytes apoptosis by inhibiting gap junction intercellular communication via autophagy activation. Chem Biol Interact. 2021;337:109331. doi: 10.1016/j.cbi.2020.109331. [DOI] [PubMed] [Google Scholar]
- 104.Khairul I, Wang QQ, Jiang YH, Wang C, Naranmandura H. Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget. 2017;8(14):23905–23926. doi: 10.18632/oncotarget.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garbinski LD, Rosen BP, Chen J. Pathways of arsenic uptake and efflux. Environ Int. 2019;126:585–597. doi: 10.1016/j.envint.2019.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang M, He Z, Yuan H, Zhu L, Guo C, Yin L, et al. DNA damage and decrease of cellular oxidase activity in piglet sertoli cells exposed to arsanilic acid. J Vet Med Sci. 2011;73(2):199–203. doi: 10.1292/jvms.10-0236. [DOI] [PubMed] [Google Scholar]
- 107.Gong X, Ivanov VN, Davidson MM, Hei TK. Tetramethylpyrazine (TMP) protects against sodium arsenite-induced nephrotoxicity by suppressing ROS production, mitochondrial dysfunction, pro-inflammatory signaling pathways and programed cell death. Arch Toxicol. 2015;89(7):1057–1070. doi: 10.1007/s00204-014-1302-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun BF, Wang QQ, Yu ZJ, Yu Y, Xiao CL, Kang CS, et al. Exercise prevents memory impairment induced by arsenic exposure in mice: implication of hippocampal BDNF and CREB. PLoS One. 2015;10(9):e137810. doi: 10.1371/journal.pone.0137810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Flora SJ. Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med. 2011;51(2):257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 110.Muenyi CS, Ljungman M, States JC. Arsenic disruption of DNA damage responses-potential role in carcinogenesis and chemotherapy. Biomolecules. 2015;5(4):2184–2193. doi: 10.3390/biom5042184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Argos M, Chen L, Jasmine F, Tong L, Pierce BL, Roy S, et al. Gene-specific differential DNA methylation and chronic arsenic exposure in an epigenome-wide association study of adults in Bangladesh. Environ Health Persp. 2015;123(1):64–71. doi: 10.1289/ehp.1307884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li ZY, Yang Y, Ming M, Liu B. Mitochondrial ROS generation for regulation of autophagic pathways in cancer. Biochem Biophys Res Commun. 2011;414(1):5–8. doi: 10.1016/j.bbrc.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 113.Partridge MA, Huang SX, Hernandez-Rosa E, Davidson MM, Hei TK. Arsenic induced mitochondrial DNA damage and altered mitochondrial oxidative function: implications for genotoxic mechanisms in mammalian cells. Cancer Res. 2007;67(11):5239–5247. doi: 10.1158/0008-5472.CAN-07-0074. [DOI] [PubMed] [Google Scholar]
- 114.Kimura A, Ishida Y, Wada T, Hisaoka T, Morikawa Y, Sugaya T, et al. The absence of interleukin-6 enhanced arsenite-induced renal injury by promoting autophagy of tubular epithelial cells with aberrant extracellular signal-regulated kinase activation. Am J Pathol. 2010;176(1):40–50. doi: 10.2353/ajpath.2010.090146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duan T, Hu T, Wu C, Yeh YT, Lu J, Zhang Q, et al. PINK1/Parkin-mediated mitophagy is involved in NaAsO2-induced apoptosis of human hepatic cells through activation of ERK signaling. Toxicol in Vitro. 2020;66:104857. doi: 10.1016/j.tiv.2020.104857. [DOI] [PubMed] [Google Scholar]
- 116.Manthari RK, Tikka C, Ommati MM, Niu R, Sun Z, Wang J, et al. Arsenic induces autophagy in developmental mouse cerebral cortex and hippocampus by inhibiting PI3K/Akt/mTOR signaling pathway: involvement of blood-brain barrier’s tight junction proteins. Arch Toxicol. 2018;92(11):3255–3275. doi: 10.1007/s00204-018-2304-y. [DOI] [PubMed] [Google Scholar]
- 117.Ommati MM, Shi X, Li H, Zamiri MJ, Farshad O, Jamshidzadeh A, et al. The mechanisms of arsenic-induced ovotoxicity, ultrastructural alterations, and autophagic related paths: an enduring developmental study in folliculogenesis of mice. Ecotoxicol Environ Saf. 2020;204:110973. doi: 10.1016/j.ecoenv.2020.110973. [DOI] [PubMed] [Google Scholar]
- 118.Zhang T, Qi Y, Liao M, Xu M, Bower KA, Frank JA, et al. Autophagy is a cell self-protective mechanism against arsenic-induced cell transformation. Toxicol Sci. 2012;130(2):298–308. doi: 10.1093/toxsci/kfs240. [DOI] [PubMed] [Google Scholar]
- 119.Chuu JJ, Liu SH, Lin-Shiau SY. Differential neurotoxic effects of methylmercury and mercuric sulfide in rats. Toxicol Lett. 2007;169(2):109–120. doi: 10.1016/j.toxlet.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 120.Weis JS. Reproductive, developmental, and neurobehavioral effects of methylmercury in fishes. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(4):212–225. doi: 10.1080/10590500903310088. [DOI] [PubMed] [Google Scholar]
- 121.Ahmad S, Tufail N, Parveen N, Mahmood R. Attenuation of hg(II)-induced cellular and DNA damage in human blood cells by uric acid. Biochem Cell Biol. 2022;100(1):45–58. doi: 10.1139/bcb-2021-0229. [DOI] [PubMed] [Google Scholar]
- 122.Wang J, Fang Z, Gao J, Sun L, Wang Y, Liu Y, et al. Comparative study of cytotoxicity, DNA damage and oxidative stress induced by heavy metals cd(II), hg(II) and cr(III) in yeast. Curr Microbiol. 2021;78(5):1856–1863. doi: 10.1007/s00284-021-02454-4. [DOI] [PubMed] [Google Scholar]
- 123.Vassallo DV, Simoes MR, Giuberti K, Azevedo BF, Ribeiro JR, Salaices M, et al. Effects of chronic exposure to mercury on angiotensin-converting enzyme activity and oxidative stress in normotensive and hypertensive rats. Arq Bras Cardiol. 2019;112(4):374–380. doi: 10.5935/abc.20180271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fujimura M, Usuki F. Cellular conditions responsible for methylmercury-mediated neurotoxicity. Int J Mol Sci. 2022 doi: 10.3390/ijms23137218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rudgalvyte M, Peltonen J, Lakso M, Wong G. Chronic MeHg exposure modifies the histone H3K4me3 epigenetic landscape in Caenorhabditis elegans. Comp Biochem Physiol C Toxicol Pharmacol. 2017;191:109–116. doi: 10.1016/j.cbpc.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 126.Pan J, Li X, Wei Y, Ni L, Xu B, Deng Y, et al. Advances on the influence of methylmercury exposure during neurodevelopment. Chem Res Toxicol. 2022;35(1):43–58. doi: 10.1021/acs.chemrestox.1c00255. [DOI] [PubMed] [Google Scholar]
- 127.Chatterjee S, Ray A, Mukherjee S, Agarwal S, Kundu R, Bhattacharya S. Low concentration of mercury induces autophagic cell death in rat hepatocytes. Toxicol Ind Health. 2014;30(7):611–620. doi: 10.1177/0748233712462442. [DOI] [PubMed] [Google Scholar]
- 128.Vergilio CS, Carvalho CE, Melo EJ. Mercury-induced dysfunctions in multiple organelles leading to cell death. Toxicol in Vitro. 2015;29(1):63–71. doi: 10.1016/j.tiv.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 129.Chang SH, Lee HJ, Kang B, Yu KN, Minai-Tehrani A, Lee S, et al. Methylmercury induces caspase-dependent apoptosis and autophagy in human neural stem cells. J Toxicol Sci. 2013;38(6):823–831. doi: 10.2131/jts.38.823. [DOI] [PubMed] [Google Scholar]
- 130.Unoki T, Akiyama M, Kumagai Y, Goncalves FM, Farina M, Da RJ, et al. Molecular pathways associated with methylmercury-induced Nrf2 modulation. Front Genet. 2018;9:373. doi: 10.3389/fgene.2018.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.El AH, Mohammed EA, Aboulhoda BE, Emam HY, Imam AA. Selenium protection against mercury neurotoxicity: modulation of apoptosis and autophagy in the anterior pituitary. Life Sci. 2019;231:116578. doi: 10.1016/j.lfs.2019.116578. [DOI] [PubMed] [Google Scholar]
- 132.Lin T, Ruan S, Huang D, Meng X, Li W, Wang B, et al. MeHg-induced autophagy via JNK/Vps34 complex pathway promotes autophagosome accumulation and neuronal cell death. Cell Death Dis. 2019;10(6):399. doi: 10.1038/s41419-019-1632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Willhite CC, Karyakina NA, Yokel RA, Yenugadhati N, Wisniewski TM, Arnold IM, et al. Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit Rev Toxicol. 2014;44(Suppl 4):1–80. doi: 10.3109/10408444.2014.934439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang Y, Wang H, Guo Y, Lei W, Wang J, Hu X, et al. Metal ion imbalance-related oxidative stress is involved in the mechanisms of liver injury in a rat model of chronic aluminum exposure. Biol Trace Elem Res. 2016;173(1):126–131. doi: 10.1007/s12011-016-0627-1. [DOI] [PubMed] [Google Scholar]
- 135.Mai S, He Q, Wang H, Hu X, Luo Y, Yang Y, et al. 5-lipoxygenase activation is involved in the mechanisms of chronic hepatic injury in a rat model of chronic aluminum overload exposure. Toxicol Appl Pharmacol. 2016;305:259–266. doi: 10.1016/j.taap.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 136.Sun X, Sun H, Yu K, Wang Z, Liu Y, Liu K, et al. Aluminum chloride causes the dysfunction of testes through inhibiting the ATPase enzyme activities and gonadotropin receptor expression in rats. Biol Trace Elem Res. 2018;183(2):296–304. doi: 10.1007/s12011-017-1120-1. [DOI] [PubMed] [Google Scholar]
- 137.Yang X, Yu K, Wang H, Zhang H, Bai C, Song M, et al. Bone impairment caused by AlCl3 is associated with activation of the JNK apoptotic pathway mediated by oxidative stress. Food Chem Toxicol. 2018;116(Pt B):307–314. doi: 10.1016/j.fct.2018.04.057. [DOI] [PubMed] [Google Scholar]
- 138.Liu J, Wang Q, Sun X, Yang X, Zhuang C, Xu F, et al. The toxicity of aluminum chloride on kidney of rats. Biol Trace Elem Res. 2016;173(2):339–344. doi: 10.1007/s12011-016-0648-9. [DOI] [PubMed] [Google Scholar]
- 139.Yu H, Zhang J, Ji Q, Yu K, Wang P, Song M, et al. Melatonin alleviates aluminium chloride-induced immunotoxicity by inhibiting oxidative stress and apoptosis associated with the activation of Nrf2 signaling pathway. Ecotoxicol Environ Saf. 2019;173:131–141. doi: 10.1016/j.ecoenv.2019.01.095. [DOI] [PubMed] [Google Scholar]
- 140.Zhang Q, Cao Z, Sun X, Zuang C, Huang W, Li Y. Aluminum trichloride induces hypertension and disturbs the function of erythrocyte membrane in male rats. Biol Trace Elem Res. 2016;171(1):116–123. doi: 10.1007/s12011-015-0504-3. [DOI] [PubMed] [Google Scholar]
- 141.Li C, Wang N, Zheng G, Yang L. Oral administration of resveratrol-selenium-peptide nanocomposites alleviates alzheimer’s disease-like pathogenesis by inhibiting abeta aggregation and regulating gut microbiota. ACS Appl Mater Interfaces. 2021;13(39):46406–46420. doi: 10.1021/acsami.1c14818. [DOI] [PubMed] [Google Scholar]
- 142.Wang B, Zhao J, Yu M, Meng X, Cui X, Zhao Y, et al. Disturbance of intracellular calcium homeostasis and CaMKII/CREB signaling is associated with learning and memory impairments induced by chronic aluminum exposure. Neurotox Res. 2014;26(1):52–63. doi: 10.1007/s12640-013-9451-y. [DOI] [PubMed] [Google Scholar]
- 143.Hosseini-Sharifabad A, Rabbani M, Seyed-Yousefi Y, Safavi M. Magnesium increases the protective effect of citicoline on aluminum chloride-induced cognitive impairment. Clin Psychopharmacol Neurosci. 2020;18(2):241–248. doi: 10.9758/cpn.2020.18.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li S, Zhang Q, Ding Y, Wang X, Liu P. Flavonoids ameliorate aluminum chloride-induced learning and memory impairments via suppression of apoptosis and oxidative stress in rats. J Inorg Biochem. 2020;212:111252. doi: 10.1016/j.jinorgbio.2020.111252. [DOI] [PubMed] [Google Scholar]
- 145.Exley C. Human exposure to aluminium. Environ Sci Process Impacts. 2013;15(10):1807–1816. doi: 10.1039/c3em00374d. [DOI] [PubMed] [Google Scholar]
- 146.El HA, Fennich H, Alaika O, Dakka T, Raissouni Z, Oukerraj L, et al. Reversible myocardial injury and intraventricular thrombus associated with aluminium phosphide poisoning. Case Rep Cardiol. 2017;2017:6287015. doi: 10.1155/2017/6287015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Novaes RD, Mouro V, Goncalves RV, Mendonca A, Santos EC, Fialho M, et al. Aluminum: a potentially toxic metal with dose-dependent effects on cardiac bioaccumulation, mineral distribution, DNA oxidation and microstructural remodeling. Environ Pollut. 2018;242(Pt A):814–826. doi: 10.1016/j.envpol.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 148.Mujika JI, Dalla TG, Formoso E, Grande-Aztatzi R, Grabowski SJ, Exley C, et al. Aluminum’s preferential binding site in proteins: sidechain of amino acids versus backbone interactions. J Inorg Biochem. 2018;181:111–116. doi: 10.1016/j.jinorgbio.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 149.Ohsaka Y, Nomura Y. Rat white adipocytes activate p85/p110 PI3K and induce PM GLUT4 in response to adrenoceptor agonists or aluminum fluoride. Physiol Int. 2016;103(1):35–48. doi: 10.1556/036.103.2016.1.4. [DOI] [PubMed] [Google Scholar]
- 150.Hu C, Yang J, He Q, Luo Y, Chen Z, Yang L, et al. CysLTR1 blockage ameliorates liver injury caused by aluminum-overload via PI3K/AKT/mTOR-mediated autophagy activation in vivo and in vitro. Mol Pharm. 2018;15(5):1996–2006. doi: 10.1021/acs.molpharmaceut.8b00121. [DOI] [PubMed] [Google Scholar]
- 151.Li H, Xue X, Li L, Li Y, Wang Y, Huang T, et al. Aluminum-induced synaptic plasticity impairment via PI3K-Akt-mTOR signaling pathway. Neurotox Res. 2020;37(4):996–1008. doi: 10.1007/s12640-020-00165-5. [DOI] [PubMed] [Google Scholar]
- 152.Yang X, Zhang J, Ji Q, Wang F, Song M, Li Y. Autophagy protects MC3T3-E1 cells upon aluminum-induced apoptosis. Biol Trace Elem Res. 2018;185(2):433–439. doi: 10.1007/s12011-018-1264-7. [DOI] [PubMed] [Google Scholar]
- 153.Zeng KW, Fu H, Liu GX, Wang XM. Aluminum maltolate induces primary rat astrocyte apoptosis via overactivation of the class III PI3K/Beclin 1-dependent autophagy signal. Toxicol in Vitro. 2012;26(2):215–220. doi: 10.1016/j.tiv.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 154.Andrade VM, Aschner M, Marreilha DSA. Neurotoxicity of metal mixtures. Adv Neurobiol. 2017;18:227–265. doi: 10.1007/978-3-319-60189-2_12. [DOI] [PubMed] [Google Scholar]
- 155.Pohl HR, Roney N, Abadin HG. Metal ions affecting the neurological system. Met Ions Life Sci. 2011;8:247–262. [PubMed] [Google Scholar]
- 156.Andrade V, Mateus ML, Batoreu MC, Aschner M, Marreilha DSA. Changes in rat urinary porphyrin profiles predict the magnitude of the neurotoxic effects induced by a mixture of lead, arsenic and manganese. Neurotoxicology. 2014;45:168–177. doi: 10.1016/j.neuro.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 157.Zhu H, Jia Y, Cao H, Meng F, Liu X. Biochemical and histopathological effects of subchronic oral exposure of rats to a mixture of five toxic elements. Food Chem Toxicol. 2014;71:166–175. doi: 10.1016/j.fct.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 158.Zou H, Sun J, Wu B, Yuan Y, Gu J, Bian J, et al. Effects of cadmium and/or lead on autophagy and liver injury in rats. Biol Trace Elem Res. 2020;198(1):206–215. doi: 10.1007/s12011-020-02045-7. [DOI] [PubMed] [Google Scholar]
- 159.Czajka M, Sawicki K, Sikorska K, Popek S, Kruszewski M, Kapka-Skrzypczak L. Toxicity of titanium dioxide nanoparticles in central nervous system. Toxicol in Vitro. 2015;29(5):1042–1052. doi: 10.1016/j.tiv.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 160.Mohammadipour A, Fazel A, Haghir H, Motejaded F, Rafatpanah H, Zabihi H, et al. Maternal exposure to titanium dioxide nanoparticles during pregnancy; impaired memory and decreased hippocampal cell proliferation in rat offspring. Environ Toxicol Pharmacol. 2014;37(2):617–625. doi: 10.1016/j.etap.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 161.Wang Y, Chen Z, Ba T, Pu J, Chen T, Song Y, et al. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small. 2013;9(9–10):1742–1752. doi: 10.1002/smll.201201185. [DOI] [PubMed] [Google Scholar]
- 162.Dolenc KJ. Effects of exposure to nano and bulk sized TiO2 and CuO in Lemna minor. Plant Physiol Biochem. 2017;119:43–49. doi: 10.1016/j.plaphy.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 163.Wu F, Bortvedt A, Harper BJ, Crandon LE, Harper SL. Uptake and toxicity of CuO nanoparticles to Daphnia magna varies between indirect dietary and direct waterborne exposures. Aquat Toxicol. 2017;190:78–86. doi: 10.1016/j.aquatox.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 164.Huang T, Guo W, Wang Y, Chang L, Shang N, Chen J, et al. Involvement of mitophagy in aluminum oxide nanoparticle-induced impairment of learning and memory in mice. Neurotox Res. 2021;39(2):378–391. doi: 10.1007/s12640-020-00283-0. [DOI] [PubMed] [Google Scholar]
- 165.Katerji M, Filippova M, Duerksen-Hughes P. Approaches and methods to measure oxidative stress in clinical samples: research applications in the cancer field. Oxid Med Cell Longev. 2019;2019:1279250. doi: 10.1155/2019/1279250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Halamoda KB, Chapuis BC, Guney-Ayra S, Juillerat-Jeanneret L. Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. Biochem J. 2012;441(3):813–821. doi: 10.1042/BJ20111252. [DOI] [PubMed] [Google Scholar]
- 167.Johnson BM, Fraietta JA, Gracias DT, Hope JL, Stairiker CJ, Patel PR, et al. Acute exposure to ZnO nanoparticles induces autophagic immune cell death. Nanotoxicology. 2015;9(6):737–748. doi: 10.3109/17435390.2014.974709. [DOI] [PubMed] [Google Scholar]
- 168.Khan MI, Mohammad A, Patil G, Naqvi SA, Chauhan LK, Ahmad I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials. 2012;33(5):1477–1488. doi: 10.1016/j.biomaterials.2011.10.080. [DOI] [PubMed] [Google Scholar]
- 169.Azimee S, Rahmati M, Fahimi H, Moosavi MA. TiO(2) nanoparticles enhance the chemotherapeutic effects of 5-fluorouracil in human AGS gastric cancer cells via autophagy blockade. Life Sci. 2020;248:117466. doi: 10.1016/j.lfs.2020.117466. [DOI] [PubMed] [Google Scholar]
- 170.Hu Q, Zhao F, Fan M, He C, Yang X, Huang Z, et al. The influence of titanium dioxide nanoparticles on their cellular response to macrophage cells. Comp Biochem Physiol C Toxicol Pharmacol. 2019;223:42–52. doi: 10.1016/j.cbpc.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 171.Perez-Arizti JA, Ventura-Gallegos JL, Galvan JR, Ramos-Godinez M, Colin-Val Z, Lopez-Marure R. Titanium dioxide nanoparticles promote oxidative stress, autophagy and reduce NLRP3 in primary rat astrocytes. Chem Biol Interact. 2020;317:108966. doi: 10.1016/j.cbi.2020.108966. [DOI] [PubMed] [Google Scholar]
- 172.Ahamed M, Akhtar MJ, Alhadlaq HA, Alrokayan SA. Assessment of the lung toxicity of copper oxide nanoparticles: current status. Nanomed (Lond) 2015;10(15):2365–2377. doi: 10.2217/nnm.15.72. [DOI] [PubMed] [Google Scholar]
- 173.Zhang Y, Xu B, Yao M, Dong T, Mao Z, Hang B, et al. Titanium dioxide nanoparticles induce proteostasis disruption and autophagy in human trophoblast cells. Chem Biol Interact. 2018;296:124–133. doi: 10.1016/j.cbi.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 174.Mao Z, Yao M, Li Y, Fu Z, Li S, Zhang L, et al. Mir-96-5p and mir-101-3p as potential intervention targets to rescue TiO(2) NP-induced autophagy and migration impairment of human trophoblastic cells. Biomater Sci. 2018;6(12):3273–3283. doi: 10.1039/c8bm00856f. [DOI] [PubMed] [Google Scholar]
- 175.Yu KN, Chang SH, Park SJ, Lim J, Lee J, Yoon TJ, et al. Titanium dioxide nanoparticles induce endoplasmic reticulum stress-mediated autophagic cell death via mitochondria-associated endoplasmic reticulum membrane disruption in normal lung cells. PLoS One. 2015;10(6):e131208. doi: 10.1371/journal.pone.0131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yu S, Mu Y, Zhang X, Li J, Lee C, Wang H. Molecular mechanisms underlying titanium dioxide nanoparticles (TiO(2)NP) induced autophagy in mesenchymal stem cells (MSC) J Toxicol Environ Health A. 2019;82(18):997–1008. doi: 10.1080/15287394.2019.1688482. [DOI] [PubMed] [Google Scholar]
- 177.Sun T, Yan Y, Zhao Y, Guo F, Jiang C. Copper oxide nanoparticles induce autophagic cell death in A549 cells. PLoS One. 2012;7(8):e43442. doi: 10.1371/journal.pone.0043442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ma X, Wu Y, Jin S, Tian Y, Zhang X, Zhao Y, et al. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano. 2011;5(11):8629–8639. doi: 10.1021/nn202155y. [DOI] [PubMed] [Google Scholar]
- 179.Roy R, Singh SK, Chauhan LK, Das M, Tripathi A, Dwivedi PD. Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition. Toxicol Lett. 2014;227(1):29–40. doi: 10.1016/j.toxlet.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 180.Li L, Li L, Zhou X, Yu Y, Li Z, Zuo D, et al. Silver nanoparticles induce protective autophagy via ca(2+)/CaMKKbeta/AMPK/mTOR pathway in SH-SY5Y cells and rat brains. Nanotoxicology. 2019;13(3):369–391. doi: 10.1080/17435390.2018.1550226. [DOI] [PubMed] [Google Scholar]
- 181.Mishra AR, Zheng J, Tang X, Goering PL. Silver nanoparticle-induced autophagic-lysosomal disruption and NLRP3-inflammasome activation in HepG2 cells is size-dependent. Toxicol Sci. 2016;150(2):473–487. doi: 10.1093/toxsci/kfw011. [DOI] [PubMed] [Google Scholar]
- 182.Zhang X, Zhang H, Liang X, Zhang J, Tao W, Zhu X, et al. Iron oxide nanoparticles induce autophagosome accumulation through multiple mechanisms: lysosome impairment, mitochondrial damage, and ER stress. Mol Pharm. 2016;13(7):2578–2587. doi: 10.1021/acs.molpharmaceut.6b00405. [DOI] [PubMed] [Google Scholar]
- 183.Niture S, Lin M, Qi Q, Moore JT, Levine KE, Fernando RA, et al. Role of autophagy in cadmium-induced hepatotoxicity and liver diseases. J Toxicol. 2021;2021:9564297. doi: 10.1155/2021/9564297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gu J, Ren Z, Zhao J, Peprah FA, Xie Y, Cheng D, et al. Calcimimetic compound NPS R-467 protects against chronic cadmium-induced mouse kidney injury by restoring autophagy process. Ecotoxicol Environ Saf. 2020;189:110052. doi: 10.1016/j.ecoenv.2019.110052. [DOI] [PubMed] [Google Scholar]
- 185.Zhao C, Yu D, He Z, Bao L, Feng L, Chen L, et al. Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells. Free Radic Biol Med. 2021;175:236–248. doi: 10.1016/j.freeradbiomed.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 186.Zou H, Wang L, Zhao J, Yuan Y, Wang T, Bian J, et al. MiR-155 promotes cadmium-induced autophagy in rat hepatocytes by suppressing Rheb expression. Ecotoxicol Environ Saf. 2021;227:112895. doi: 10.1016/j.ecoenv.2021.112895. [DOI] [PubMed] [Google Scholar]
- 187.Li JR, Ou YC, Wu CC, Wang JD, Lin SY, Wang YY, et al. Endoplasmic reticulum stress and autophagy contributed to cadmium nephrotoxicity in HK-2 cells and Sprague-Dawley rats. Food Chem Toxicol. 2020;146:111828. doi: 10.1016/j.fct.2020.111828. [DOI] [PubMed] [Google Scholar]
- 188.Zhang Y, Ma Y, Xiao Y, Lu C, Xiao F. Drp1-dependent mitochondrial fission contributes to cr(VI)-induced mitophagy and hepatotoxicity. Ecotoxicol Environ Saf. 2020;203:110928. doi: 10.1016/j.ecoenv.2020.110928. [DOI] [PubMed] [Google Scholar]
- 189.Wang Y, Wang L, Wang X, Cheng G, Xing Y, Zhang M, et al. Inflammatory injury and mitophagy in the cock heart induced by the oral administration of hexavalent chromium. Biol Trace Elem Res. 2022;200(3):1312–1320. doi: 10.1007/s12011-021-02715-0. [DOI] [PubMed] [Google Scholar]
- 190.Yin F, Yan J, Zhao Y, Guo KJ, Zhang ZL, Li AP, et al. Bone marrow mesenchymal stem cells repair cr (VI)- injured kidney by regulating mitochondria-mediated apoptosis and mitophagy mediated via the MAPK signaling pathway. Ecotoxicol Environ Saf. 2019;176:234–241. doi: 10.1016/j.ecoenv.2019.03.093. [DOI] [PubMed] [Google Scholar]
- 191.Corsetti G, Romano C, Stacchiotti A, Pasini E, Dioguardi FS. Endoplasmic reticulum stress and apoptosis triggered by sub-chronic lead exposure in mice spleen: a histopathological study. Biol Trace Elem Res. 2017;178(1):86–97. doi: 10.1007/s12011-016-0912-z. [DOI] [PubMed] [Google Scholar]
- 192.Meng H, Wang L, He J, Wang Z. The protective effect of gangliosides on lead (Pb)-induced neurotoxicity is mediated by autophagic pathways. Int J Environ Res Public Health. 2016;13(4):365. doi: 10.3390/ijerph13040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cui YH, Yang S, Wei J, Shea CR, Zhong W, Wang F, et al. Autophagy of the m(6)a mRNA demethylase FTO is impaired by low-level arsenic exposure to promote tumorigenesis. Nat Commun. 2021;12(1):2183. doi: 10.1038/s41467-021-22469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wei S, Qiu T, Yao X, Wang N, Jiang L, Jia X, et al. Arsenic induces pancreatic dysfunction and ferroptosis via mitochondrial ROS-autophagy-lysosomal pathway. J Hazard Mater. 2020;384:121390. doi: 10.1016/j.jhazmat.2019.121390. [DOI] [PubMed] [Google Scholar]
- 195.Wan F, Zhong G, Wu S, Jiang X, Liao J, Zhang X, et al. Arsenic and antimony co-induced nephrotoxicity via autophagy and pyroptosis through ROS-mediated pathway in vivo and in vitro. Ecotoxicol Environ Saf. 2021;221:112442. doi: 10.1016/j.ecoenv.2021.112442. [DOI] [PubMed] [Google Scholar]
- 196.Balarastaghi S, Barangi S, Hosseinzadeh H, Imenshahidi M, Moosavi Z, Razavi BM, et al. Melatonin improves arsenic-induced hypertension through the inactivation of the Sirt1/autophagy pathway in rat. Biomed Pharmacother. 2022;151:113135. doi: 10.1016/j.biopha.2022.113135. [DOI] [PubMed] [Google Scholar]
- 197.Wang Y, Zhao H, Shao Y, Liu J, Li J, Xing M. Interplay between elemental imbalance-related PI3K/Akt/mTOR-regulated apoptosis and autophagy in arsenic (III)-induced jejunum toxicity of chicken. Environ Sci Pollut Res Int. 2018;25(19):18662–18672. doi: 10.1007/s11356-018-2059-2. [DOI] [PubMed] [Google Scholar]
- 198.Wu S, Zhong G, Wan F, Jiang X, Tang Z, Hu T, et al. Evaluation of toxic effects induced by arsenic trioxide or/and antimony on autophagy and apoptosis in testis of adult mice. Environ Sci Pollut Res Int. 2021;28(39):54647–54660. doi: 10.1007/s11356-021-14486-1. [DOI] [PubMed] [Google Scholar]
- 199.Manthari RK, Tikka C, Ommati MM, Niu R, Sun Z, Wang J, et al. Arsenic-induced autophagy in the developing mouse cerebellum: involvement of the blood-brain barrier’s tight-junction proteins and the PI3K-Akt-mTOR signaling pathway. J Agric Food Chem. 2018;66(32):8602–8614. doi: 10.1021/acs.jafc.8b02654. [DOI] [PubMed] [Google Scholar]
- 200.Zhong G, Wan F, Wu S, Jiang X, Tang Z, Zhang X, et al. Arsenic or/and antimony induced mitophagy and apoptosis associated with metabolic abnormalities and oxidative stress in the liver of mice. Sci Total Environ. 2021;777:146082. doi: 10.1016/j.scitotenv.2021.146082. [DOI] [PubMed] [Google Scholar]
- 201.Tao Y, Qiu T, Yao X, Jiang L, Wang N, Jia X, et al. Autophagic-CTSB-inflammasome axis modulates hepatic stellate cells activation in arsenic-induced liver fibrosis. Chemosphere. 2020;242:124959. doi: 10.1016/j.chemosphere.2019.124959. [DOI] [PubMed] [Google Scholar]
- 202.Xu X, Yu Z, Han B, Li S, Sun Y, Du Y, et al. Luteolin alleviates inorganic mercury-induced kidney injury via activation of the AMPK/mTOR autophagy pathway. J Inorg Biochem. 2021;224:111583. doi: 10.1016/j.jinorgbio.2021.111583. [DOI] [PubMed] [Google Scholar]
- 203.Zhao G, Qi L, Wang Y, Li X, Li Q, Tang X, et al. Antagonizing effects of curcumin against mercury-induced autophagic death and trace elements disorder by regulating PI3K/AKT and Nrf2 pathway in the spleen. Ecotoxicol Environ Saf. 2021;222:112529. doi: 10.1016/j.ecoenv.2021.112529. [DOI] [PubMed] [Google Scholar]
- 204.Cui Y, Song M, Xiao B, Liu M, Liu P, Han Y, et al. ROS-mediated mitophagy and apoptosis are involved in aluminum-induced femoral impairment in mice. Chem Biol Interact. 2021;349:109663. doi: 10.1016/j.cbi.2021.109663. [DOI] [PubMed] [Google Scholar]
- 205.Liu M, Wang B, Cui Y, Xiao B, Liu P, Gao J, et al. PINK1/Parkin-mediated mitophagy is activated to protect against testicular damage caused by aluminum. J Inorg Biochem. 2022;232:111840. doi: 10.1016/j.jinorgbio.2022.111840. [DOI] [PubMed] [Google Scholar]
- 206.Xiao B, Cui Y, Li B, Zhang J, Zhang X, Song M, et al. ROS antagonizes the protection of parkin-mediated mitophagy against aluminum-induced liver inflammatory injury in mice. Food Chem Toxicol. 2022;165:113126. doi: 10.1016/j.fct.2022.113126. [DOI] [PubMed] [Google Scholar]
- 207.Chen L, Zhang B, Toborek M. Autophagy is involved in nanoalumina-induced cerebrovascular toxicity. Nanomedicine-Uk. 2013;9(2):212–221. doi: 10.1016/j.nano.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lee TY, Liu MS, Huang LJ, Lue SI, Lin LC, Kwan AL, et al. Bioenergetic failure correlates with autophagy and apoptosis in rat liver following silver nanoparticle intraperitoneal administration. Part Fibre Toxicol. 2013;10:40. doi: 10.1186/1743-8977-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhou GX, Zhu HL, Shi XT, Nan Y, Liu WB, Dai LM, et al. Autophagy in sertoli cell protects against environmental cadmium-induced germ cell apoptosis in mouse testes. Environ Pollut. 2021;270:116241. doi: 10.1016/j.envpol.2020.116241. [DOI] [PubMed] [Google Scholar]
- 210.Lv XH, Zhao DH, Cai SZ, Luo SY, You T, Xu BL, et al. Autophagy plays a protective role in cell death of osteoblasts exposure to lead chloride. Toxicol Lett. 2015;239(2):131–140. doi: 10.1016/j.toxlet.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 211.Guan X, Shen J, Xu Y, Feng X, Zhou R. Heme oxygenase-1 enhances autophagy by modulating the AMPK/mTORC1 signaling pathway as a renoprotective mechanism to mitigate lead-induced nephrotoxicity. Am J Transl Res. 2020;12(8):4807–4818. [PMC free article] [PubMed] [Google Scholar]
- 212.Sui L, Zhang RH, Zhang P, Yun KL, Zhang HC, Liu L, et al. Lead toxicity induces autophagy to protect against cell death through mTORC1 pathway in cardiofibroblasts. Biosci Rep. 2015 doi: 10.1042/BSR20140164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhu XX, Yao XF, Jiang LP, Geng CY, Zhong LF, Yang G, et al. Sodium arsenite induces ROS-dependent autophagic cell death in pancreatic beta-cells. Food Chem Toxicol. 2014;70:144–150. doi: 10.1016/j.fct.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 214.Yuntao F, Chenjia G, Panpan Z, Wenjun Z, Suhua W, Guangwei X, et al. Role of autophagy in methylmercury-induced neurotoxicity in rat primary astrocytes. Arch Toxicol. 2016;90(2):333–345. doi: 10.1007/s00204-014-1425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shang N, Zhang P, Wang S, Chen J, Fan R, Chen J, et al. Aluminum-induced cognitive impairment and PI3K/Akt/mTOR signaling pathway involvement in occupational aluminum eorkers. Neurotox Res. 2020;38(2):344–358. doi: 10.1007/s12640-020-00230-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.