Abstract
Cell-based therapies have been used as promising treatments for several untreatable diseases. However, cell-based therapies have side effects such as tumorigenesis and immune responses. To overcome these side effects, therapeutic effects of exosomes have been researched as replacements for cell-based therapies. In addition, exosomes reduced the risk that can be induced by cell-based therapies. Exosomes contain biomolecules such as proteins, lipids, and nucleic acids that play an essential role in cell–cell and cell–matrix interactions during biological processes. Since the introduction of exosomes, those have been proven perpetually as one of the most effective and therapeutic methods for incurable diseases. Much research has been conducted to enhance the properties of exosomes, including immune regulation, tissue repair, and regeneration. However, yield rate of exosomes is the critical obstacle that should be overcome for practical cell-free therapy. Three-dimensional (3D) culture methods are introduced as a breakthrough to get higher production yields of exosomes. For example, hanging drop and microwell were well known 3D culture methods and easy to use without invasiveness. However, these methods have limitation in mass production of exosomes. Therefore, a scaffold, spinner flask, and fiber bioreactor were introduced for mass production of exosomes isolated from various cell types. Furthermore, exosomes treatments derived from 3D cultured cells showed enhanced cell proliferation, angiogenesis, and immunosuppressive properties. This review provides therapeutic applications of exosomes using 3D culture methods.
Keywords: Cell culture method, Cell-free therapy, Exosome, Three-dimensional culture method
Introduction
Most cells secrete extracellular vesicles (EVs), these secreted EVs play an important role in intercellular communication [1]. Exosomes are part of EVs and are wrapped with a bi-layered lipid membrane with a size distribution of approximately 30–150 nm in diameter, which can penetrate biological membranes owing to their high biocompatibility and small size [2, 3]. Exosomes are enriched in non-specific proteins such as tetraspanin proteins, endosomal sorting complex required for transport proteins, heat shock proteins, actin, and flotillin [3, 4]. In addition, exosomes contain specific proteins based on donor cells. For example, CD4 + T cells secrete major histocompatibility complexes I and II through exosomes [3]. In addition to proteins, several microRNAs (miRNAs) have been identified to be present in exosomes [5]. With these varying contents, exosomes are secreted into the extracellular space and regulate cell–cell communication, cell differentiation, and proliferation, angiogenesis, stress response, and immune response [5, 6].
Exosomes from various cell types have been treated as a novel approach for regenerative methods instead of cell-based therapy. Zhang et al. reported that human umbilical cord mesenchymal stem cell (UC-MSC)-derived exosomes significantly promoted re-epithelialization in an in vivo rat skin burn model, enhancing wound healing [7]. Long et al. reported that bone marrow mesenchymal stem cell (BM-MSC)-derived exosomes alleviated chronic hippocampal dysfunction induced by status epilepticus with reduced proinflammatory cytokines [8].
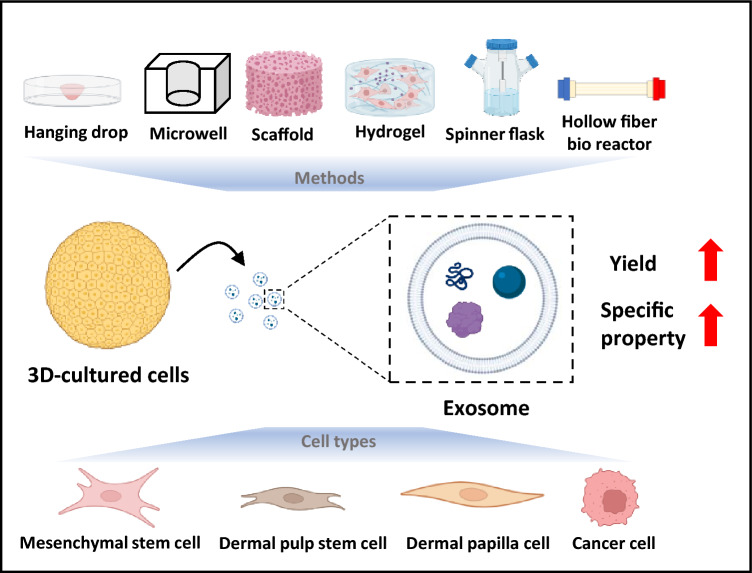
Undoubtably, exosomes have therapeutic potential for incurable diseases. However, exosome production yield (the amount of exosomes produced per cell and time) is low (1–4 μg protein/day, 106 cells) compared to the need for exosomes (100–500 μg per patient) required to perform clinical trials [9, 10]. To overcome this obstacle, three-dimensional (3D) culture methods have been investigated instead of traditional two-dimensional (2D) culture methods for the extraction of exosomes [11]. 3D culture methods have been devised to more accurately mimic cell–cell and cell–matrix interactions in tissues [12]. Although hanging drop is regarded as a standard method for 3D culture systems, new approaches have been devised to overcome limitations such as the limited size of spheroids, insufficient environment for adhesion, and distribution imbalance of nutrients and oxygen in the hanging drop method [13–16]. Various 3D culture methods, including microarray wells, hydrogels, scaffolds, spinner flasks, and hollow fiber bioreactors, are used to produce exosomes with elevated production yield, higher similarity to native tissues, and upregulated gene expression. These are remarkable substitutes for traditional 2D cultures for exosome extraction (Fig. 1) [11, 17, 18].
Fig. 1.
Schematic illustration of exosome production using various three-dimensional (3D)-culture methods and cell types. 3D culture systems, such as hanging drop, microarray, scaffold etc. achieved enhanced features of exosomes, including higher production rate, higher cell proliferation, angiogenesis, and immunosuppressive properties. (biorender.com was used to create this figure)
To understand the prospective features of exosomes from 3D culture methods, we reviewed the pros and cons of various existing 3D culture methods. The effects of exosomes on different 3D culture methods are also briefly discussed. Cell types that can be used as regenerative medicines are described in relation to target diseases. This is followed by the enhanced production yield and therapeutic effects of exosomes by 3D culture. Finally, we present the recent perspectives of treatment with 3D culture exosomes. This review can provide a preview of the pathway for development of 3D culture methods and applications for enhanced exosome treatments. In addition, we provide information on the factors that are upregulated in exosomes based on their cell types and describe how we can use these improved exosomes as regenerative medicine to treat incurable diseases.
3D culture methods for exosome production
To increase production yield of exosomes, several 3D culture methods have been researched. As previously explained, the 3D culture method increases cell–cell and cell–matrix interactions [19] which enhanced exosome production yield and therapeutic effects of exosomes [20]. In addition, some 3D cultured cells can produce exosomes in serum-free medium [21]. Considering these advantages, 3D culture is an effective method for extracting exosomes. Although 3D cultures using a hanging drop method or a microwell array have been studied, various other 3D culture methods have been devised to overcome the limitations of hanging drop and microwell array methods and provide several additional effects of exosomes acquired from these 3D cultured cells [22]. In addition, methods using spinner flask and fiber bioreactor have been studied for the mass production of exosomes and accurate exosome acquisition.
Hanging drop method
The hanging drop method is a 3D culture method that uses a mechanism to gather the cells in the center of a droplet and then the cells aggregate spontaneously (Fig. 2A). The hanging drop method has the advantages in forming spheroids without using any scaffolds or other supportive structures. Also hanging drop method is relatively simple and easy to be performed and cost-effective for forming spheroid compared to other conventional methods. However, hanging drop method also have several disadvantages compared to other 3D cell culture methods. For example, hanging drop method has a relatively low throughput due to limited production (a spheroid per single droplet). This means that it can be a time-consuming method to generate a large number of spheroids for high-throughput production [23]. Therefore, hanging drop method need further development in terms of mass production. Additionally, limited production yield of the hanging drop method is directly related to difficulty in exosome extraction and inability in culture medium exchange [24]. Despite these properties, there were several studies that produced exosomes using the hanging drop method. Conventional hanging drop methods for producing 3D spheroids have been studied to enhance the therapeutic properties and to promote anti-inflammatory effects [25]. However, the hanging drop method has also been used for exosome production recently due to the benefits of 3D culture. Kim et al. formed MSC derived 3D spheroids using the hanging drop method with droplets of 2.5 × 104 MSCs in 35 μl medium for 72 h. Exosome production yield of 3D spheroids formed using the hanging drop method was approximately twofold higher than that of the conventional 2D cultured cells [26]. This study explains the mechanism of enhanced exosome production yield by hypoxic conditions in the central area of the spheroids. On the other hand, Giusti et al. formed cancer 3D spheroids using hanging drop methods to mimic in vivo tumor features [27].
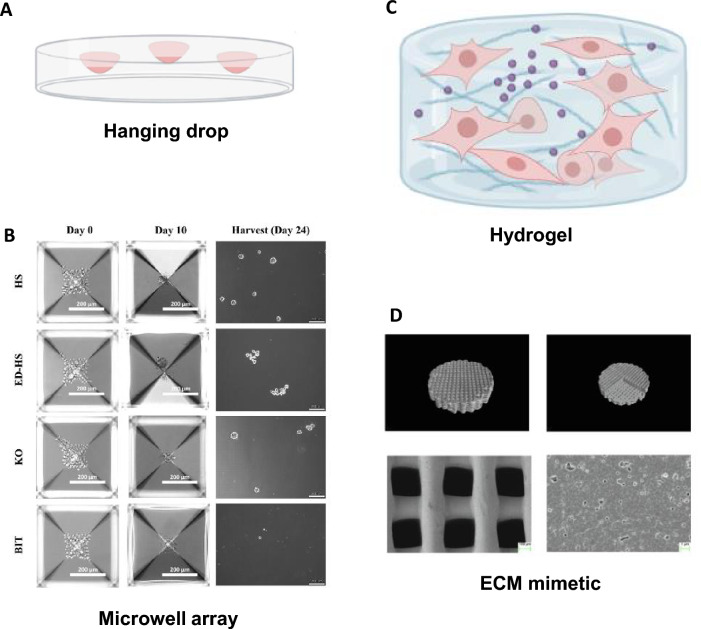
Fig. 2.
Diverse 3D culture methods for exosome production. A Schematic figure of spheroids using the hanging drop method. (biorender.com was used to create this figure) B Microwell array method. Reprinted with permission from Wiley [29]. C Schematic figure of hydrogel-based exosome production. (biorender.com was used to create this figure) D Micro-CT images of the 3D scaffold. Reprinted with permission from Elsevier [37]
Microwell array
As previously described, the hanging-drop method is difficult to handle. In addition, it has limitations in regulating the spheroid size. However, microwell arrays are suitable for generating spheroids of the desired size and shape (Fig. 2B). In a microwell array, cells are seeded into an array of small wells (usually ranging from 100 to 500 µm in diameter) that could be coated with a material that promotes self-assembling or growth of cells. In addition, the shape of microwell can be designed to squares, cylinders, hexagons, and etc. as researcher desired [28]. Using a microwell array for the production of 3D spheroids is easier than using the hanging drop method and generates a large number of 3D spheroids due to continuous patterns of microwells [14]. Using these advantages of microwell array, Faruqu et al. formed 3D cultured dental pulp pluripotent-like stem cells using a microwell array that secrete exosomes under optimal serum-free conditions for easy exosome extraction [29]. Furthermore, Faruqu et al. also reported that 3D cultured UC-MSCs using a microwell array under serum-free condition secreted exosomes enhancing fibroblast migration and proliferation [21].
Scaffold
The hanging drop method and microwell arrays are representative method for 3D cell culture. However, they have insufficient microenvironments for providing large adhesion area. 3D cultured spheroids with inadequate adhesion area can cause cell necrosis or apoptosis in the central part of the spheroids [15]. Scaffolds can provide a microenvironment for enhancing cell adhesion that mimics the in vivo conditions. Compared to 2D cultures, exosomes produced from 3D cultured MSCs exhibited improvement in tissue regeneration activity [30]. Spheroids of a certain size are more vulnerable to cellular and physiological gradients, but scaffold-based 3D culture methods could enhance cell–matrix interactions by encouraging cells to be resistant to cell and physiological gradients. In addition, exosome production by scaffold-loaded 3D cultured cells was reported to be enhanced [31]. Various types of scaffolds have been applied to exosome production, including hydrogels and extracellular matrix (ECM) mimetics.
Hydrogel
Hydrogels are water-swollen and crosslinked polymer networks that can be tailored to mimic the properties of the ECM (Fig. 2C). Hydrogel-based 3D cultures provide abundant adhesion sites and an optimizable ECM for the cells. Among the hydrogels that can be applied to 3D culture, photocrosslinkable chitosan hydrogel increases cell adhesion and provides an appropriate cell culture environment [32]. Hydrogels used as a 3D culture method positively influenced the characterization and biofunction of cell-derived exosomes due to the optimized 3D microenvironment. For example, Yu et al. devised a self-assembling collagen hydrogen that can be used to culture microscale 3D models. They mixed freeze-dried rat tail collagen 1 and human periodontal ligament stem cell suspension before being injected into premade molds to form collagen hydrogels. 3D cultured human periodontal ligament stem cells in the hydrogel upregulated the expression of osteogenesis related genes and proteins. In addition, their devised collagen hydrogel enhanced exosome production yield by 2.5-fold and protein content by 2.9-fold. [17]. Han et al. reported that a microneedle array patch using mesenchymal stem cell (MSC)-loaded gelatin methacryloyl (GelMA)-based hydrogel improved the cell retention and survival rate, sustained the release of exosomes, and improved the function of exosomes that were derived from 3D cultured cells [33]. It has been also reported that GelMA-based hydrogel provided the biological niches to cultured MSCs. Therefore, MSCs loaded on GelMA-based hydrogel showed increment in cell stemness and exosome production yield [34].
ECM mimetics
ECM mimetics are synthetic materials designed to mimic the structure and function of the ECM. For example, nanofibrous scaffolds that mimic the ECM can be produced by electrospinning polymer blends such as polylactic acid and polycaprolactone. The fabricated scaffold can be functionalized by attaching functional ligands to enhance exosome production yield. These materials can be used to promote cell attachment and differentiation, as well as enhance exosome production yield [35]. In addition, synthesized scaffolds can have unique compositions and structural patterns for certain purposes. For example, mesh-like scaffolds enhance cell adhesion, proliferation, and the homogenous distribution of transplanted cells [36]. Several studies have been conducted using the above advantages of ECM mimetics. Gao et al. fabricated a hydroxyapatite scaffold that was 3D-printed (Fig. 2D). The scaffold was a 3D cylinder with 200 μm pores. Exosomes released from scaffold-based 3D cultured cells showed higher expression of tetraspanins (CD9 and CD81) and endosomal pathway proteins (TSG101) than exosomes released from 2D cultured cells [37]. Yang et al. used a 3D graphene scaffold synthesized by chemical vapor deposition. Synthesized 3D graphene scaffold exhibited continuous and porous structure with 100 to 300 μm pore size. The 3D graphene scaffold had electrical conductivity as well as microscale topographic features and a porous structure. Therefore, secreted exosomes exhibited enhanced therapeutic effects on Alzheimer’s disease mice with their memory and cognitive deficits [38].
Spinner flask
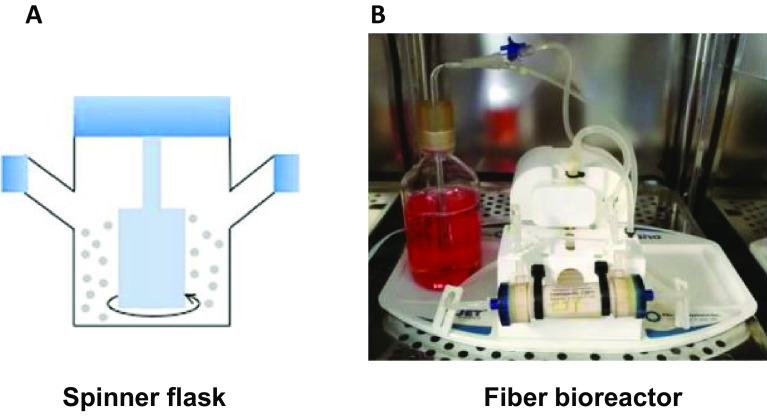
A spinner flask is a 3D cell culture system that uses rotation to allow cells to aggregate to a supporting matrix. The spinner flask stirs the medium of the flask to obtain a uniform distribution of nutrients and oxygen by two side caps for gas exchange (Fig. 3A). High cell culture yield and the possibility of mass culture are the advantages of this culture system. However, shear stress generated by spinning can cause damage to cultured cells [16]. In addition, serum is required for spheroid formation in the spinner flask, but for exosome isolation, cells should be cultured in serum-free medium. In this point, the feature of the spinner flask that total medium exchange is difficult can be a disadvantage for exosome production. Nevertheless, Harazti et al. cultured MSCs in a spinner flask using support matrix beads for aggregation. Isolated exosomes from spinner flask cultured MSCs showed higher siRNA delivery efficiency to neurons than exosomes from 2D cultured MSCs [11].
Fig. 3.
Diverse mass 3D culture methods for exosome production. A Spinner flask-based 3D culture of cells and identification of exosome. Reprinted with permission from Elsevier [11]. B Photograph of the hollow fiber bioreactor-based 3D culture system. Reprinted with permission from BMC [18]
Fiber bioreactor
The hollow fiber bioreactor is a 3D cell culture system that contains multiple hollow semi-permeable fiber membranes (Fig. 3B). For 3D cell culture, cells are seeded in intra- or extra-fiber spaces, with diameters of approximately 200 μm. Hollow-fiber bioreactors have the advantages of mass culture and high cell culture yield. Another advantage is that nutrients and oxygen can be automatically injected through a sealed tube to reduce labor. Gas and nutrient exchange occur through a semipermeable fiber membrane. Therefore, the media flow in one direction for the clearance of waste from the culture [39]. Because mass production is possible by connecting numerous hollow fibers to one bioreactor, it is suitable for automated mass exosome production. These characteristics of the hollow fiber bioreactor improved exosome production efficiency. Yan et al. compared the exosome production yield of MSCs cultured in a hollow-fiber bioreactor to conventional 2D culture MSCs. The yield of exosomes from bioreactor-cultured 3D MSCs was 7.5-fold higher than that of exosomes from 2D cultured MSCs [40]. Cao et al. also reported a higher exosome production yield from 3D MSCs cultured in a hollow-fiber bioreactor. The yield of 3D-exosomes was 19.4-fold higher compared to that of 2D-exosomes. Furthermore, the concentration of 3D-exosomes in the harvested supernatants was 15.5-fold higher compared to that of 2D-exosomes [18].
Others
In some cases, exosome production in 3D culture was conducted by stacking fibroblast sheets. Fibroblasts express more ECMs compared to other cell types and were cultured with ascorbic acid for a long time to form a fibroblast sheet. By stacking these cell sheets, a 3D culture environment was formed. Exosomes from scaffold-free 3D cultured fibroblasts promoted ECM synthesis and secretion, when the exosomes internalized into the surrounding cells [41]. In another case, there was an exosome production method from 3D cultured cells that used an ultralow adhesion material. Hu et al. fabricated 3D fibroblast spheroids using an ultra-low attachment flask [42]. In addition, Kim et al. prepared 3D MSC spheroids by using poly(2-hydroxyethyl methacrylate) (poly-HEMA)-coated plates. Spheroids with a smaller diameter produced more exosomes than those with a larger diameter. Compared with the hanging drop method, the poly-HEMA method tended to produce several small spheroids at the same cell seeding density. Therefore, the exosome production yield was higher when using the poly-HEMA method than when using the hanging drop method [26].
Exosomes from 3D spheroids of diverse cell types
Mesenchymal stem cells (MSCs)
MSCs are adult stem cells that can self-renew and differentiate into various cell types, such as adipocytes, chondrocytes, and osteoblasts. Generally, MSCs show positive surface markers CD73, CD90, and CD105 and lack of expression of surface markers CD14, CD34, CD45, CD19, CD79a, and HLA-DR [43]. Traditionally, bone-marrow have been used as a major source of MSCs isolation for more than 20 years [44]. However, BM-MSCs were extracted through an invasive and painful procedure. Due to this obstacle, readily available and non- or less-invasive sources such as adipose-tissue, umbilical cord, and dental tissue were studied for MSCs isolation sources [45, 46]. Although they are MSCs with similar morphology and surface markers, their differentiation potentials are different. For example, BM-MSCs show advantages with osteogenesis and chondrogenesis compared to adipose derived-MSCs (ADSCs) [47, 48].
MSCs are mostly used cell therapy method because of their capacity of secreting trophic factors related to critical cell functions such as cell–cell interactions. Many previous studies have shown that paracrine mechanisms can mediate tissue repairment and regeneration. Exosomes are considered as a major driver of these therapeutic effects with paracrine factors secreted from MSCs [49]. BM-MSCs derived exosomes have paracrine factors related to lung protection, insulin-like growth factor-1 (IGF-1), anti-apoptotic effects, miR-22, and promote treatment for stroke via promoting neurite remodeling and functional recovery [50–52]. However, as described in differences of differentiation potential of MSCs based on their sources, characters related to each MSC were slightly different. Katsuda et al. reported an enzyme related with degradation of beta-amyloid contained fourfold more in ADSCs exosomes than BM-MSCs exosomes [9]. Also, Del Fattore reported BM- and UC-MSCs exosomes prohibit proliferation and induce apoptosis of cancer cells, U87MG glioblastoma cells, but AD-MSC exosomes promote growth of U87MG glioblastoma cells without affecting cell viability [53]. Finally, Lopez-Verrilli et al. described differences between growth of dorsal root ganglia and neurite treated with MSCs from various sources [54, 55]. To fostering usages of these exosomes with various characteristics from different sources, exosomes were studied for enhancing their therapeutic effects and production yield with 3D culture.
Bone-marrow mesenchymal stem cells (BM-MSCs)
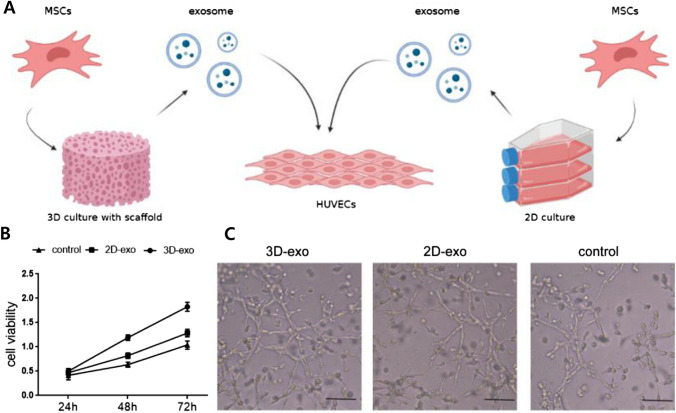
BM-MSCs are multipotent adult stem cells that are extracted from the bone marrow. They can differentiate into osteoblasts, chondrocytes, adipocytes and myocytes. Also, they played an essential role in mediating the immune system and hematopoietic stem cell activity [55]. Treated exosomes extracted from BM-MSCs enhance osteogenesis, angiogenesis, and bone regeneration [17, 56]. Gao et al. compared application of 2D and scaffold-based 3D cultured BM-MSCs exosomes on HUVECs proliferation (Fig. 4A). 3D culture exosomes have higher expression levels of tetraspanins (CD9 and CD81), vascular endothelial growth factor (VEGF), and high mobility group box 1 protein than 2D culture exosomes and show enhanced HUVECs proliferation (Fig. 4B). Human umbilical vein endothelial cells (HUVECs) treated with 3D culture exosomes show enhanced tube formation (Fig. 4C) [37, 57]. Dinh et al. used a pulmonary fibrosis (PF) model and injected 3D MSC-derived exosomes. The results showed an enhanced inspiratory capacity and respiratory compliance. In addition, miRNAs hsa-let-7a-5p and hsa-let-7f-5p were upregulated [58].
Fig. 4.
A Schemes for enhancing proliferation of HUVECs through 3D cultured BM-MSC exosomes and 2D cultured exosomes (biorender.com was used to create this figure). B CCK-8 assay of the HUVECs C Light microscopy images of the tube formation assay and the number of tubes formed by HUVECs. Reprinted with permission from Elsevier, 2021 [37]
Umbilical cord mesenchymal stem cells (UC-MSCs)
UC-MSCs are similar to BM-MSCs but can be extracted from readily prepared sources. And secretomes of UC-MSCs and BM-MSCs are slightly different in inflammation related cytokines, IL-5, IL-7, IL-10, IL-12, IL-13, and VEGF [59]. In traditional 2D cell cultures, UC-MSCs have a short doubling time (~ 4 days) compared to BM-MSCs (~ 7 days). In addition, the yield of exosomes derived from UC-MSCs was four times more than that of exosomes derived from BM-MSCs and adipose derived-MSCs. Based on these advantages, UC-MSCs have been used to optimize exosome production [11].
3D culture exosomes show enhanced production yield and therapeutic efficacy of UC-MSCs than 2D culture exosome [11, 18, 38, 40]. Haraszti et al. have shown that exosomes extracted from spinner flask-based 3D culture were 20-fold higher production yield than exosomes from 2D culture. With the tangential flow filtration (TFF) isolation method, the yield of exosomes from 3D culture was 120-fold higher than that of exosomes derived from 2D culture. And these 3D cultured UC-MSC exosomes were enriched with ribonucleoproteins, collagen, and secreted proteins. In addition, neurons internalized more 3D culture exosomes than 2D culture exosomes [11]. Yang et al. showed that exosomes extracted from scaffold 3D culture contain higher levels of proteins related to the elimination of Aβ deposition in the brain, such as neprilysin, insulin-degrading enzyme, and heat shock protein 70, which can lower the risk of Alzheimer’s disease (AD) [60], than exosomes from 2D culture. In addition, AD mice injected with exosomes from 3D culture showed lower levels of tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and heme oxygenase-1 (HO-1), which can alleviate the inflammatory response and oxidative stress leading to the reduction of Aβ accumulation compared to the 2D culture exosome-injected group [38, 61]. For enhanced wound healing effect, Camões et al. reported spinner flask 3D cultured UC-MSC exosomes. When compared to 2D cultured UC-MSC exosomes, both contained proteins related to the inflammatory response, cell migration, hemostasis, and homing. 3D culture exosomes exclusively contained hemostasis proteins such as CAPZB, CDC42, H3A, H3F3A/B, HIST2, LMAN1, PLAT, PLAU, PLAUR, PPIB, RAC1, and SERPINB2 [62, 63]; inflammation-related proteins such as CD151, PDGFRB, PLAU, RAC1, and TGFβ [64, 65]; and proteins involved in the formation of ECM structures such as CD44, FN1, ITGB1, PDCD6IP, PLAUR, and VCAN [66–68]. Through enriched proteins related to the wound healing process, 3D culture exosomes showed enhanced wound healing effects on wound sites compared to 2D culture exosomes. In addition, fibroblast viability was promoted by 3D culture exosome treatment [66].
Amnion-derived mesenchymal stem cells (AMSCs)
The amniotic membrane of the human placenta is the source of AMSCs. AMSCs are easy to obtain without contamination and have fewer ethically related problems compared to other MSCs [69]. AMSCs shows higher level of integrin alpha-4 (ITGA4) and CD49 compared to BM-MSCs [70]. In addition, these cells have a low risk of tumor formation because there is no telomerase expression, which is otherwise considered a side effect of MSC transplantation [71, 72].
Owing to their high accessibility and safety, AMSCs have been studied as a therapeutic method. Treated AMSCs induce angiogenesis and re-epithelialization in the wound area [73]. Shu et al. reported that when AMSCs were injected in experimental autoimmune encephalomyelitis mice, the expression of neuron-repair factors such as nerve growth factor, ciliary neurotrophic factor, and brain-derived neurotrophic factor was promoted, and the production of proinflammatory cytokines such as interferon gamma, TNF-α, IL-1β, and interleukin-17 was decreased in the spleen and central nervous system [72]. Miceli et al. reported enhanced in vitro capillary-like structure formation of HUVECs and immunosuppressive potential when ultra-low-attachment culture dish-based 3D culture AMSC exosomes were treated. However, there were no significant differences between 2 and 3D cultured AMSC exosomes [69].
Dental pulp stem cells (DPSCs)
DPSCs are extracted from dental pulp tissue of human teeth. DPSCs have fibroblast-like morphology similar to BM-MSCs but are smaller than BM-MSCs. DPSCs show similar immunophenotypes and osteogenic, adipogenic, and chondrogenic potential compared with BM-MSCs [74]. However, DPSCs have a short doubling time (~ 2 days) compared to BM-MSCs doubling time (~ 3 days). Also, DPSCs have higher potential for osteogenesis and lower potential for adipogenesis as demonstrated by their upregulated osteogenic differentiation factors and runt-related transcription factor 2 and downregulated adipogenic differentiation factors and peroxisome proliferator-activated receptor gamma [74, 75].
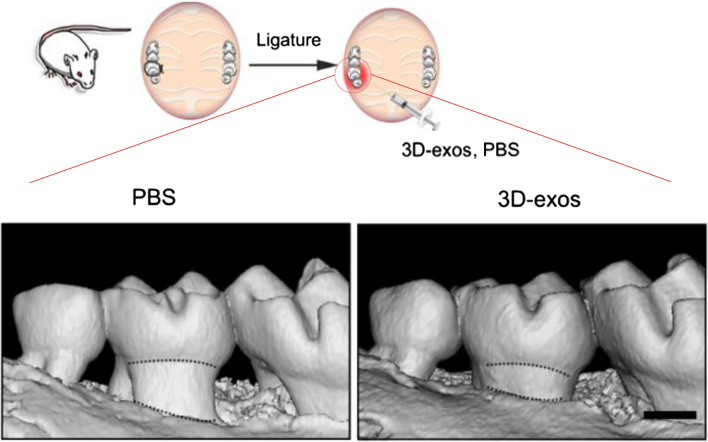
DPSCs were studied to treat periodontitis by lowering proinflammatory reaction. Shen et al. reported that DPSC-derived exosomes show effective therapeutic results in periodontitis, inducing the conversion of macrophages convert from a proinflammatory phenotype to an anti-inflammatory phenotype [76, 77]. Zhang et al. reported that with ultra-low-attachment culture dish-based 3D cultured DPSCs, the yield of exosomes was sixfold higher compared to that of 2D cultured DPSCs. In addition, after injection of exosomes from 2 and 3D culture into the periodontitis model of a ligature-induced periodontitis mouse model and a dextran sulfate sodium-induced colitis mouse model, 3D culture exosomes showed lower levels of osteoclasts and downregulated expression of TNF-α, IL-1β, and interleukin 6 compared to 2D culture exosomes and PBS only groups [78, 79] Zhang et al. also reported that 3D cultured DPSC exosomes lowered the number of Th17 cells and increased the number of Tregs compared with 2D cultured DPSC exosomes and PBS only groups. Periodontitis and colitis can be alleviated through the balance between Th17 cells and Tregs as shown in Fig. 5. [78, 80, 81].
Fig. 5.
Micro-CT images to show 3D reconstructions of maxillae of the PBS- and 3D exosome treated groups (scale bar = 500 μm). Reprinted with permission from Springer Nature, 2021 [78]
Dermal papilla cells (DP cells)
Dermal papilla cells (DP cells) are located at the base of hair follicles in the dermis layer of the skin. They play a crucial role in hair follicle formation, growth, and cycling by regulating the development and differentiation of surrounding epithelial cells. Researchers have attempted to determine the hair follicle cycle to help people suffering from hair loss. Based on these efforts, the promotion of follicles in the resting phase (telogen) to the active phase (anagen) is of interest [82]. Regulating DP cells can transform follicles from telogen to anagen phase [83, 84]. However, Higgins et al. reported 2D cultured DP cells have no therapeutic effect on hair follicle induction. Nevertheless, de novo hair follicles were induced in 3D cultured DP cells [85]. Through this approach, researchers have tried to use expressed secretomes and EVs from 3D cultured DP cells and obtained positive results for the regeneration of hair follicles [86, 87].
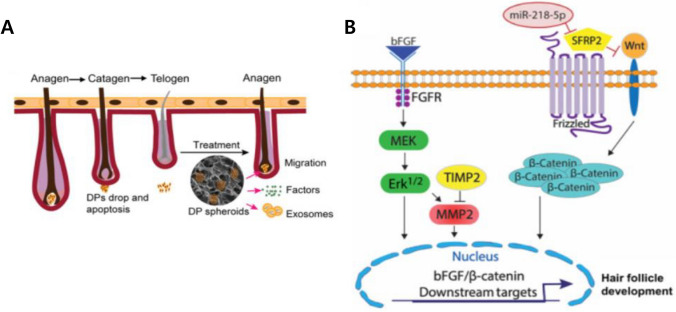
Exosome from DP cells was also studied to make hair regrowth. Hu et al. reported that ultralow attachment culture dish-based spheroids of DP cells showed enhanced expression of CD133 and β-catenin [88]. CD133 is expressed in the early stages of the anagen phase. CD133 induces the ECM produced by DP cells called versican, which can be detected during the late anagen stage [89]. In addition, β-catenin that induces stem cells differentiate into follicular keratinocytes, is an essential factor for sustaining the function of hair follicles [90]. Minoxidil, a well-known treatment for hair loss, enhances the production of Ki67 only; however, 2D cultured DP cell exosomes and an ultra-low-attachment culture dish-based 3D culture exosomes showed higher expression of CD133 and β-catenin with Ki67. With these upregulated factors, in vivo hair regrowth in C57BL/6 mice and the anagen phase of hair follicles were both accelerated (Fig. 6A) [88]. In 3D cultured DP cell exosomes, miR-218-5p were up-regulated 25-fold more than 2D DP cells exosome. miR-218-5p were shown that can stimulate the Wingless-related integration site signaling pathway, which is important for the hair growth cycle as shown in Fig. 6B [88, 91].
Fig. 6.
A Schemes illustrating how DP spheroids are used as a treatment for transition of the hair cycle from telogen to anagen via the migration and secretion of exosomes. B Schemes illustrating how miR-218-5p abundant 3D exosome can enhance hair follicle development through up-regulating β-catenin. Reprinted with permission from Springer Nature, 2020 [88]
Lung spheroid cells (LSCs)
Cell therapy has been studied for lung diseases such as chronic obstructive pulmonary disease and idiopathic PF [92, 93]. However, conventionally used cell therapy method with MSCs have little benefit in lung regeneration [94, 95]. Instead, LSCs showed therapeutic benefits in ameliorating PF and lung regeneration [96, 97]. LSCs express CD90, CD105, KRT5, aquaporin 5 (AQP5), and CCSP for lung mesenchymal markers and epithelial markers [98, 99].
Exosome can deliver effective therapeutic factors from LSCs to disease experimental model. Dinh et al. reported the LSC exosome-treated group showed increased AQP5 and von Willebrand factor-positive cells and decreased α-smooth muscle actin-positive cells, representing alleviated alveolar epithelia and vascular injury and decreased collagen deposition with fibrotic response [58, 100, 101]. In addition, SMAD3 and monocyte chemoattractant protein-1 were lower in the LSC exosome-treated group, which can lower the risk of fibrosis [102, 103]. In in vivo PF model, inspiratory capacity, respiratory compliance, and resistance were improved with LSC exosome treatment [58]. For miRNAs, hsa-miR-99a-5p, hsa-miR-100-5p, and antifibrotic miR-30a-3p were upregulated in LSC exosomes [58]. There was no comparison with 2D cultured LSC exosomes, but when considering the therapeutic results related to LSCs, the application of LSC exosomes needs further investigation.
Cancer cells
2D cultured cancer cell lines have been used to determine the mechanisms of cancer growth and development. However, 2D culture cells are not sufficient to describe native tumors [104, 105]. To improve cell–cell and cell–matrix interactions in traditional 2D culture, 3D cultures considered for a more accurate understanding of the native tumor environment and their mechanisms [104–107]. Tu et al. reported nonadherent culture wells-based 3D culture exosome isolation and its useful adaptation to mimic the progression of cancer in the pancreatic cell line, PANC-1. Compared to 2D culture exosomes, the production of 3D culture exosomes is approximately threefold higher [108]. For miRNAs in PANC-1, miR-1246, miR-21, miR-17-5p, and miR-196a had higher levels in 3D culture exosomes than in 2D culture exosomes, while the expression of miR-4454 was higher in 2D culture exosomes [108]. The former miRNA groups that are abundant in 3D culture exosomes are related to carcinogenesis, proliferation, and inhibition of apoptosis in various cancer types [109–112]. However, miR-4454 is related to cancer metastasis [113]. Also, glypican-1 was upregulated in 2D culture exosomes and 3D culture exosomes compared to conventional 2D culture [108]. Naseri et al. reported the maturation of dendritic cells (DCs) induced by cancer stem cells (CSCs) for T-cell proliferation and CSCs-directed cytotoxicity. The expression of DCs for IL-12 to IL-10 ratios in the hanging drop-based 3D cultured CSCs exosome-treated groups was upregulated. In addition, T-cell proliferation by DCs was significantly higher in 3D cultured CSCs exosomes [114]. Table 1 summarizes the above exosome related studies using 3D culture methods.
Table 1.
Types of 3D cultrue methods and cells for exosome production
| Cell lines | 3D culture Method | Outcome & effect of exosomes | Ref | |
|---|---|---|---|---|
| Cell type | Origin | |||
| Bone marrow MSC | Human | Hanging drop | Production of exosomes (twofold) ↑ | [26] |
| Rat | GelMA hydrogel |
Production of exosomes ↑ Anti-inflammation ↑ Neuroprotection ↑ Fibrotic formation ↓ |
[33] | |
| Mouse | GelMA hydrogel |
Production of exosomes ↑ Stemness marker ↑ Anti-inflammation ↑ Targeting to ischemic area ↑ |
[34] | |
| Human | 3D hydroxyapatite scaffold |
HUVEC tube formation ↑ HUVEC angiogenesis ↑ HUVEC proliferation ↑ |
[37] | |
| Umbilical cord MSC | Human | The Aggrewell™ plate |
Fibroblast migration ↑ Fibroblast proliferation ↑ |
[21] |
| Human | 3D graphene scaffold |
In vivo spatial learning ↑ Aβ accumulation ↓ Inflammation ↓ Oxidative stress ↓ |
[38] | |
| Human | Spinner flask |
Production of exosomes (20-fold) ↑ Delivery of siRNA to Neuron ↑ |
[11] | |
| Human | Hollow fiber bioreactor |
Production of exosomes (19.4-fold) ↑ Renoprotection effect ↑ Anti-inflammatory ↑ |
[18] | |
| Human | Hollow fiber bioreactor |
Production of exosomes (7.5-fold) ↑ Chondrocyte proliferation ↑ Chondrocyte migration ↑ Chondrocyte matrix synthesis ↑ Chondrocytes stability ↑ Chondrocyte apoptosis ↓ |
[40] | |
| Human | Spinner vessel | Wound healing ↑ | [66] | |
| Dental pulp stem cell | Human | The Aggrewell™ plate | Nanog ↑ | [29] |
| Human | Ultra-low-attachment culture dish |
Production of exosomes (6.2-fold) ↑ Anti-inflammation ↑ Periodontitis, colitis ↓ |
[78] | |
| Amnion stem cell | Human | Ultra-low-attachment culture dish |
Migration ↑ Tubulogenesis ↑ Angiogenesis ↑ Immunoflamation ↓ |
[69] |
| Dermal papilla cell | Mouse | Ultra-low-attachment culture dish |
WNT/β-catenin pathway ↑ Migration ↑ Hair growth ↑ |
[88] |
| Lung spheroid cell | Human | - |
Inspiratory capacity ↑ Respiratory compliance ↑ Respiratory resistance ↑ Fibrosis ↓ |
[58] |
| Cancer spheroid cell | Human | 3D integumentary organ system |
Production of exosomes (threefold) ↑ GPC-1 protein ↑ miR-4454 ↓ |
[108] |
| Human | Hanging drop | In vivo cancer characteristic ↑ | [27] | |
| Periodontal ligament stem cell | Human | Collagen hydrogel |
Production of exosomes ↑ Osteogenesis ↑ BM-MSC migration ↑ Bone defect ↓ |
[17] |
| Fibroblast | Human | Ultra-low-attachment culture dish |
Collagen biosynthesis ↑ Inflammation ↓ |
[42] |
Conclusion and future perspective
Exosomes have been used in many recent medical studies and have shown promising results. However, owing to the limitations of the exosome production methods, their use must be optimized using various techniques. For this purpose, several techniques have been researched, such as 3D culture and genetic engineering of cells. The genetic engineering method is also used to increase the production or effectiveness of exosomes, but the 3D culture method is less invasive, making it advantageous compared to genetic engineering [115]. 3D culture methods attempt to enhance the specific properties and production yield of exosomes by mimicking the characteristics and interactions of the cell niches in the body.
3D cell culture methods can be classified into several categories, including hanging drops, microwell arrays, scaffolds, hydrogels, spinner flasks, and hollow fiber bioreactors. These methods increase the production efficiency of exosomes and encourage them to exhibit specific properties. Each method has advantages and disadvantages in terms of production efficiency, labor requirements, and functional properties. For example, the method of using scaffolds could have a specific functional ligand in addition to being able to create a 3D cell structure. Whereas the hanging drop method requires a lot of effort from researchers, which is not suitable for mass culture of cells and mass production of exosomes. Diverse cell types are used for exosome production using 3D culture methods. Three types of MSCs, DPSCs, DP cells, LSCs, and cancer cells were the main cell types studied for the production and effects of exosomes. Exosomes produced by 3D culture of these cell types showed enhanced production yield and specific properties, such as enhanced therapeutic effect, immunomodulatory effect, and induction of differentiation of the target cells. For example, as previously explained, 3D cultured DPSC exosomes could be used as treatments for periodontitis and colitis through modulating the balance of Th17 cells and Treg cells. In addition, exosomes extracted from scaffold 3D culture could be applied to treatment of Alzheimer’s disease through higher levels of protein release related to the elimination of Aβ deposition in the brain.
Utilizing the contents of exosomes can be an option for further improving exosome treatment efficacy. This modification can be achieved by overexpressing certain proteins or miRNAs in the cells that produce exosomes. The overexpression of proteins or RNAs induces exosomes to deliver more proteins or RNAs. In addition to genetic modification, it is possible to induce the overexpression of specific proteins or genes through various external stimuli, such as electric stimuli and organic/inorganic nanoparticles [116, 117]. These methods can be linked to the 3D culture methods to further improve the effect of exosomes. Until recently, the effects of 3D spheroids’ exosomes in human research were not extensively studied. Therefore, further clinical studies are needed to prove the therapeutic effects of exosomes.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF), and the Ministry of Science and ICT (NRF-2019R1C1C1007384, NRF-2020M2D9A3094171, and NRF-2021R1A4A1032782). This research was also supported by a Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Health & Welfare; project Number: 21A0102L1-12). Industrial Technology Alchemist Program funded by Ministry of Trade, Industry, and Energy (MOTIE) of Korea (20018560)
Declarations
Conflict of interest
The authors declare no financial conflicts of interest.
Ethical statement
No experiments involving animals or humans were conducted in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dong-Hyun Lee and Dae Won Yun are co-first authors and contributed equally to this work.
Contributor Information
Suk Ho Bhang, Email: sukhobhang@skku.edu.
Sang Hyoun Choi, Email: shchoi@kirams.re.kr.
References
- 1.Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017;26:617–631. doi: 10.1089/scd.2016.0349. [DOI] [PubMed] [Google Scholar]
- 2.Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17:170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19:47. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:1–14. doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hade MD, Suire CN, Suo Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021;10:1959. doi: 10.3390/cells10081959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem cells. 2015;33:2158–2168. doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- 8.Long Q, Upadhya D, Hattiangady B, Kim DK, An SY, Shuai B, et al. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci U S A. 2017;114:E3536–E3545. doi: 10.1073/pnas.1703920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013;3:1197. doi: 10.1038/srep01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haraszti RA, Miller R, Stoppato M, Sere YY, Coles A, Didiot MC, et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther. 2018;26:2838–2847. doi: 10.1016/j.ymthe.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Shri M, Agrawal H, Rani P, Singh D, Onteru SK. Hanging drop, a best three-dimensional (3D) culture method for primary buffalo and sheep hepatocytes. Sci Rep. 2017;7:1203. doi: 10.1038/s41598-017-01355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gidrol X, Fouqué B, Ghenim L, Haguet V, Picollet-D’hahan N, Schaack B. 2D and 3D cell microarrays in pharmacology. Curr Opinion Pharmacol. 2009;9:664–668. doi: 10.1016/j.coph.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Todorov L, VadeBoncouer T. Etiology and use of the “hanging drop” technique: A review. Pain Res Treat. 2014;2014:146750. doi: 10.1155/2014/146750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foglietta F, Canaparo R, Muccioli G, Terreno E, Serpe L. Methodological aspects and pharmacological applications of three-dimensional cancer cell cultures and organoids. Life Sci. 2020;254:117784. doi: 10.1016/j.lfs.2020.117784. [DOI] [PubMed] [Google Scholar]
- 17.Yu W, Li S, Guan X, Zhang N, Xie X, Zhang K, et al. Higher yield and enhanced therapeutic effects of exosomes derived from MSCs in hydrogel-assisted 3D culture system for bone regeneration. Biomater Adv. 2022;133:112646. doi: 10.1016/j.msec.2022.112646. [DOI] [PubMed] [Google Scholar]
- 18.Cao J, Wang B, Tang T, Lv L, Ding Z, Li Z, et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res Ther. 2020;11:206. doi: 10.1186/s13287-020-01719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thippabhotla S, Zhong C, He M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci Rep. 2019;9:13012. doi: 10.1038/s41598-019-49671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei Y, Schaffer DV. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc Natl Acad Sci. 2013;110:E5039–E5048. doi: 10.1073/pnas.1309408110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faruqu FN, Liam-Or R, Zhou S, Nip R, Al-Jamal KT. Defined serum-free three-dimensional culture of umbilical cord-derived mesenchymal stem cells yields exosomes that promote fibroblast proliferation and migration in vitro. FASEB J. 2021;35:e21206. doi: 10.1096/fj.202001768RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moshksayan K, Kashaninejad N, Warkiani ME, Lock JG, Moghadas H, Firoozabadi B, et al. Spheroids-on-a-chip: Recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sens Actuators B Chem. 2018;263:151–176. [Google Scholar]
- 23.Ryu NE, Lee SH, Park H. Spheroid culture system methods and applications for mesenchymal stem cells. Cells. 2019;8:1620. doi: 10.3390/cells8121620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Groot T, Veserat K, Berthier E, Beebe D, Theberge A. Surface-tension driven open microfluidic platform for hanging droplet culture. Lab Chip. 2016;16:334–344. doi: 10.1039/c5lc01353d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Timmins NE, Nielsen LK. Generation of multicellular tumor spheroids by the hanging-drop method. In: Hauser H, Fussenegger M, editors. Tissue engineering. Totowa: Springer; 2007. pp. 141–151. [DOI] [PubMed] [Google Scholar]
- 26.Kim M, Yun HW, Park DY, Choi BH, Min BH. Three-dimensional spheroid culture increases exosome secretion from mesenchymal stem cells. Tissue Eng Regen Med. 2018;15:427–436. doi: 10.1007/s13770-018-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giusti I, Poppa G, D’Ascenzo S, Esposito L, Vitale AR, Calvisi G, et al. Cancer three-dimensional spheroids mimic in vivo tumor features, displaying “inner” extracellular vesicles and vasculogenic mimicry. Int J Mol Sci. 2022;23:11782. doi: 10.3390/ijms231911782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manzoor AA, Romita L, Hwang DK. A review on microwell and microfluidic geometric array fabrication techniques and its potential applications in cellular studies. Can J Chem Eng. 2021;99:61–96. [Google Scholar]
- 29.Faruqu FN, Zhou S, Sami N, Gheidari F, Lu H, Al-Jamal KT. Three-dimensional culture of dental pulp pluripotent-like stem cells (DPPSCs) enhances Nanog expression and provides a serum-free condition for exosome isolation. FASEB BioAdv. 2020;2:419–433. doi: 10.1096/fba.2020-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang R, Wang J, Chen H, Shi X, Wang X, Zhu Y, et al. The topography of fibrous scaffolds modulates the paracrine function of Ad-MSCs in the regeneration of skin tissues. Biomater Sci. 2019;7:4248–4259. doi: 10.1039/c9bm00939f. [DOI] [PubMed] [Google Scholar]
- 31.Su N, Gao P-L, Wang K, Wang J-Y, Zhong Y, Luo Y. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials. 2017;141:74–85. doi: 10.1016/j.biomaterials.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda J, Khademhosseini A, Yeo Y, Yang X, Yeh J, Eng G, et al. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials. 2006;27:5259–5267. doi: 10.1016/j.biomaterials.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 33.Han M, Yang H, Lu X, Li Y, Liu Z, Li F, et al. Three-dimensional-cultured MSC-derived exosome-hydrogel hybrid microneedle array patch for spinal cord repair. Nano Lett. 2022;22:6391–6401. doi: 10.1021/acs.nanolett.2c02259. [DOI] [PubMed] [Google Scholar]
- 34.Han M, Zhang Z, Liu Z, Liu Y, Zhao H, Wang B, et al. Three-dimensional-cultured MSC-derived exosome with hydrogel for cerebral ischemia repair. Biomater Adv. 2023;149:213396. doi: 10.1016/j.bioadv.2023.213396. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Holzwarth JM, Ma PX. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol Biosci. 2012;12:911–919. doi: 10.1002/mabi.201100466. [DOI] [PubMed] [Google Scholar]
- 36.Xie Y, Kawazoe N, Yang Y, Chen G. Preparation of mesh-like collagen scaffolds for tissue engineering. Mater Adv. 2022;3:1556–1564. [Google Scholar]
- 37.Gao W, Liang T, He R, Ren J, Yao H, Wang K, et al. Exosomes from 3D culture of marrow stem cells enhances endothelial cell proliferation, migration, and angiogenesis via activation of the HMGB1/AKT pathway. Stem Cell Res. 2021;50:102122. doi: 10.1016/j.scr.2020.102122. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Zhai Y, Hao Y, Zhu Z, Cheng G. The regulatory functionality of exosomes derived from hUMSCs in 3D culture for Alzheimer's disease therapy. Small. 2020;16:1906273. doi: 10.1002/smll.201906273. [DOI] [PubMed] [Google Scholar]
- 39.Gloeckner H, Jonuleit T, Lemke H-D. Monitoring of cell viability and cell growth in a hollow-fiber bioreactor by use of the dye Alamar Blue. J Immunol Methods. 2001;252:131–138. doi: 10.1016/s0022-1759(01)00347-7. [DOI] [PubMed] [Google Scholar]
- 40.Yan L, Wu X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol Toxicol. 2020;36:165–178. doi: 10.1007/s10565-019-09504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clément V, Roy V, Paré B, Goulet CR, Deschênes LT, Berthod F, et al. Tridimensional cell culture of dermal fibroblasts promotes exosome-mediated secretion of extracellular matrix proteins. Sci Rep. 2022;12:19786. doi: 10.1038/s41598-022-23433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu S, Li Z, Cores J, Huang K, Su T, Dinh PU, et al. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano. 2019;13:11273–11282. doi: 10.1021/acsnano.9b04384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jovic D, Yu Y, Wang D, Wang K, Li H, Xu F, et al. A brief overview of global trends in MSC-based cell therapy. Stem Cell Rev Rep. 2022;18:1525–1545. doi: 10.1007/s12015-022-10369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 45.Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 46.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Afizah H, Yang Z, Hui JH, Ouyang H-W, Lee E-H. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Eng. 2007;13:659–666. doi: 10.1089/ten.2006.0118. [DOI] [PubMed] [Google Scholar]
- 48.Huang JI, Kazmi N, Durbhakula MM, Hering TM, Yoo JU, Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: A patient-matched comparison. J Orthop Res. 2005;23:1383–1389. doi: 10.1016/j.orthres.2005.03.008.1100230621. [DOI] [PubMed] [Google Scholar]
- 49.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22:772–780. doi: 10.1089/scd.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE. 2014;9:e88685. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Del Fattore A, Luciano R, Saracino R, Battafarano G, Rizzo C, Pascucci L, et al. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin Biol Ther. 2015;15:495–504. doi: 10.1517/14712598.2015.997706. [DOI] [PubMed] [Google Scholar]
- 54.Lopez-Verrilli MA, Caviedes A, Cabrera A, Sandoval S, Wyneken U, Khoury M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016;320:129–139. doi: 10.1016/j.neuroscience.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 55.Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Jiao G, Ren S, Zhang X, Li C, Wu W, et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther. 2020;11:38. doi: 10.1186/s13287-020-1562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng Y, Ke J, Cao P, Deng M, Li J, Cai H, et al. HMGB1-induced angiogenesis in perforated disc cells of human temporomandibular joint. J Cell Mol Med. 2018;22:1283–1291. doi: 10.1111/jcmm.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dinh PUC, Paudel D, Brochu H, Popowski KD, Gracieux MC, Cores J, et al. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat Commun. 2020;11:1064. doi: 10.1038/s41467-020-14344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romanov YA, Volgina N, Vtorushina V, Romanov AY, Dugina T, Kabaeva N, et al. Comparative analysis of secretome of human umbilical cord-and bone marrow-derived multipotent mesenchymal stromal cells. Bull Exp Biol Med. 2019;166:535–540. doi: 10.1007/s10517-019-04388-1. [DOI] [PubMed] [Google Scholar]
- 60.Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu Z, et al. Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer’s disease. Neurochem Res. 2018;43:2165–2177. doi: 10.1007/s11064-018-2641-5. [DOI] [PubMed] [Google Scholar]
- 61.Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018;32:654–668. doi: 10.1096/fj.201700600R. [DOI] [PubMed] [Google Scholar]
- 62.Anttonen T, Kirjavainen A, Belevich I, Laos M, Richardson WD, Jokitalo E, et al. Cdc42-dependent structural development of auditory supporting cells is required for wound healing at adulthood. Sci Rep. 2012;2:978. doi: 10.1038/srep00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kitajima Y, Luo L, Yan C, Tateishi S, Ono Y, Urata Y, et al. Potency of umbilical cord blood-and Wharton’s jelly-derived mesenchymal stem cells for scarless wound healing. Sci Rep. 2016;6:18844. doi: 10.1038/srep18844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zelman-Toister E, Bakos E, Cohen S, Zigmond E, Shezen E, Grabovsky V, et al. CD151 regulates T-cell migration in health and inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:257–267. doi: 10.1097/MIB.0000000000000621. [DOI] [PubMed] [Google Scholar]
- 65.Wang XJ, Han G, Owens P, Siddiqui Y, Li AG. Role of TGFβ-mediated inflammation in cutaneous wound healing. J Investig Dermatol Symp Proc. 2006;11:112–7. doi: 10.1038/sj.jidsymp.5650004. [DOI] [PubMed] [Google Scholar]
- 66.Camões SP, Bulut O, Yazar V, Gaspar MM, Simões S, Ferreira R, et al. 3D-MSCs A151 ODN-loaded exosomes are immunomodulatory and reveal a proteomic cargo that sustains wound resolution. J Adv Res. 2022;41:113–128. doi: 10.1016/j.jare.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo SA, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ren Y, Liu J, Xu H, Wang S, Li S, Xiang M, et al. Knockout of integrin β1 in induced pluripotent stem cells accelerates skin-wound healing by promoting cell migration in extracellular matrix. Stem Cell Res Ther. 2022;13:389. doi: 10.1186/s13287-022-03085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miceli V, Pampalone M, Vella S, Carreca AP, Amico G, Conaldi PG. Comparison of immunosuppressive and angiogenic properties of human amnion-derived mesenchymal stem cells between 2D and 3D culture systems. Stem cells Int. 2019;2019:7486279. doi: 10.1155/2019/7486279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barlow S, Brooke G, Chatterjee K, Price G, Pelekanos R, Rossetti T, et al. Comparison of human placenta-and bone marrow–derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008;17:1095–1108. doi: 10.1089/scd.2007.0154. [DOI] [PubMed] [Google Scholar]
- 71.Bilic G, Zeisberger SM, Mallik AS, Zimmermann R, Zisch AH. Comparative characterization of cultured human term amnion epithelial and mesenchymal stromal cells for application in cell therapy. Cell Transplant. 2008;17:955–968. doi: 10.3727/096368908786576507. [DOI] [PubMed] [Google Scholar]
- 72.Shu J, He X, Li H, Liu X, Qiu X, Zhou T, et al. The beneficial effect of human amnion mesenchymal cells in inhibition of inflammation and induction of neuronal repair in EAE mice. J Immunol Res. 2018;2018:5083797. doi: 10.1155/2018/5083797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tuca AC, Ertl J, Hingerl K, Pichlsberger M, Fuchs J, Wurzer P, et al. Comparison of matrigel and matriderm as a carrier for human amnion-derived mesenchymal stem cells in wound healing. Placenta. 2016;48:99–103. doi: 10.1016/j.placenta.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 74.Shen WC, Lai YC, Li LH, Liao K, Lai HC, Kao SY, et al. Methylation and PTEN activation in dental pulp mesenchymal stem cells promotes osteogenesis and reduces oncogenesis. Nat Commun. 2019;10:2226. doi: 10.1038/s41467-019-10197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alge DL, Zhou D, Adams LL, Wyss BK, Shadday MD, Woods EJ, et al. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010;4:73–81. doi: 10.1002/term.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen Z, Kuang S, Zhang Y, Yang M, Qin W, Shi X, et al. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact Mater. 2020;5:1113–1126. doi: 10.1016/j.bioactmat.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhuang Z, Yoshizawa-Smith S, Glowacki A, Maltos K, Pacheco C, Shehabeldin M, et al. Induction of M2 macrophages prevents bone loss in murine periodontitis models. J Dent Res. 2019;98:200–208. doi: 10.1177/0022034518805984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Chen J, Fu H, Kuang S, He F, Zhang M, et al. Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int J Oral Sci. 2021;13:43. doi: 10.1038/s41368-021-00150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suh JS, Lee SH, Fouladian Z, Lee JY, Kim T, Kang MK, et al. Rosuvastatin prevents the exacerbation of atherosclerosis in ligature-induced periodontal disease mouse model. Sci Rep. 2020;10:6383. doi: 10.1038/s41598-020-63350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tian J, Zhu Q, Zhang Y, Bian Q, Hong Y, Shen Z, et al. Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate experimental colitis via modulating Th1/Th17 and Treg cell responses. Front Immunol. 2020;11:598322. doi: 10.3389/fimmu.2020.598322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng Y, Dong C, Yang J, Jin Y, Zheng W, Zhou Q, et al. Exosomal microRNA-155-5p from PDLSCs regulated Th17/Treg balance by targeting sirtuin-1 in chronic periodontitis. J Cell Physiol. 2019;234:20662–20674. doi: 10.1002/jcp.28671. [DOI] [PubMed] [Google Scholar]
- 82.Yang G, Chen Q, Wen D, Chen Z, Wang J, Chen G, et al. A therapeutic microneedle patch made from hair-derived keratin for promoting hair regrowth. ACS Nano. 2019;13:4354–4360. doi: 10.1021/acsnano.8b09573. [DOI] [PubMed] [Google Scholar]
- 83.Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rajendran RL, Gangadaran P, Bak SS, Oh JM, Kalimuthu S, Lee HW, et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci Rep. 2017;7:15560. doi: 10.1038/s41598-017-15505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Higgins CA, Chen JC, Cerise JE, Jahoda CA, Christiano AM. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc Natl Acad Sci. 2013;110:19679–19688. doi: 10.1073/pnas.1309970110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang H, Zhu N-X, Huang K, Cai B-Z, Zeng Y, Xu Y-M, et al. iTRAQ-based quantitative proteomic comparison of early-and late-passage human dermal papilla cell secretome in relation to inducing hair follicle regeneration. PLoS ONE. 2016;11:e0167474. doi: 10.1371/journal.pone.0167474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Y, Huang J, Chen R, Yang L, Wang J, Liu B, et al. Sustained release of dermal papilla-derived extracellular vesicles from injectable microgel promotes hair growth. Theranostics. 2020;10:1454. doi: 10.7150/thno.39566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu S, Li Z, Lutz H, Huang K, Su T, Cores J, et al. Dermal exosomes containing miR-218–5p promote hair regeneration by regulating β-catenin signaling. Sci Adv. 2020;6:eaba1685. doi: 10.1126/sciadv.aba1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ito Y, Hamazaki TS, Ohnuma K, Tamaki K, Asashima M, Okochi H. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J Investig Dermatol. 2007;127:1052–1060. doi: 10.1038/sj.jid.5700665. [DOI] [PubMed] [Google Scholar]
- 90.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 91.Zhou L, Wang H, Jing J, Yu L, Wu X, Lu Z. Morroniside regulates hair growth and cycle transition via activation of the Wnt/β-catenin signaling pathway. Sci Rep. 2018;8:13785. doi: 10.1038/s41598-018-32138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, et al. Bone marrow–derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 94.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 95.Bruscia EM, Ziegler EC, Price JE, Weiner S, Egan ME, Krause DS. Engraftment of donor-derived epithelial cells in multiple organs following bone marrow transplantation into newborn mice. Stem cells. 2006;24:2299–2308. doi: 10.1634/stemcells.2006-0166. [DOI] [PubMed] [Google Scholar]
- 96.Henry E, Cores J, Hensley MT, Anthony S, Vandergriff A, de Andrade JB, et al. Adult lung spheroid cells contain progenitor cells and mediate regeneration in rodents with bleomycin-induced pulmonary fibrosis. Stem Cells Transl Med. 2015;4:1265–1274. doi: 10.5966/sctm.2015-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cores J, Dinh P-UC, Hensley T, Adler KB, Lobo LJ, Cheng K. A pre-investigational new drug study of lung spheroid cell therapy for treating pulmonary fibrosis. Stem Cells Transl Med. 2020;9:786–798. doi: 10.1002/sctm.19-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cores J, Hensley MT, Kinlaw K, Rikard SM, Dinh P-U, Paudel D, et al. Safety and efficacy of allogeneic lung spheroid cells in a mismatched rat model of pulmonary fibrosis. Stem Cells Transl Med. 2017;6:1905–1916. doi: 10.1002/sctm.16-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dinh PU-C, Cores J, Hensley MT, Vandergriff AC, Tang J, Allen TA, et al. Derivation of therapeutic lung spheroid cells from minimally invasive transbronchial pulmonary biopsies. Respir Res. 2017;18:132. doi: 10.1186/s12931-017-0611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roach KM, Wulff H, Feghali-Bostwick C, Amrani Y, Bradding P. Increased constitutive αSMA and Smad2/3 expression in idiopathic pulmonary fibrosis myofibroblasts is KCa3. 1-dependent. Respir Res. 2014;15:155. doi: 10.1186/s12931-014-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jia L, Liu Y, Han Y, Zhou X, Wang F. Differential expression and inhibitory effects of aquaporins on the development of adenomyosis. Mol Med Rep. 2020;22:3840–3850. doi: 10.3892/mmr.2020.11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-β, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 2007;35:661–664. doi: 10.1042/BST0350661. [DOI] [PubMed] [Google Scholar]
- 103.Harrington JR. The role of MCP-1 in atherosclerosis. Stem cells. 2000;18:65–66. doi: 10.1634/stemcells.18-1-65. [DOI] [PubMed] [Google Scholar]
- 104.Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18:407–418. doi: 10.1038/s41568-018-0007-6. [DOI] [PubMed] [Google Scholar]
- 105.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 106.Ringuette-Goulet C, Bolduc S, Pouliot F. Modeling human bladder cancer. World J Urol. 2018;36:1759–1766. doi: 10.1007/s00345-018-2369-5. [DOI] [PubMed] [Google Scholar]
- 107.Sachs N, Clevers H. Organoid cultures for the analysis of cancer phenotypes. Curr Opin Genet Dev. 2014;24:68–73. doi: 10.1016/j.gde.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 108.Tu J, Luo X, Liu H, Zhang J, He M. Cancer spheroids derived exosomes reveal more molecular features relevant to progressed cancer. Biochem Biophys Rep. 2021;26:101026. doi: 10.1016/j.bbrep.2021.101026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghafouri-Fard S, Khoshbakht T, Hussen BM, Taheri M, Samadian M. a review on the role of miR-1246 in the pathoetiology of different cancers. Front Mol Biosci. 2022;8:771835. doi: 10.3389/fmolb.2021.771835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pfeffer SR, Yang CH, Pfeffer LM. The role of miR-21 in cancer. Drug Dev Res. 2015;76:270–277. doi: 10.1002/ddr.21257. [DOI] [PubMed] [Google Scholar]
- 111.Wu Q, Luo G, Yang Z, Zhu F, An Y, Shi Y, et al. miR-17-5p promotes proliferation by targeting SOCS6 in gastric cancer cells. FEBS Lett. 2014;588:2055–2062. doi: 10.1016/j.febslet.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 112.Sun M, Liu XH, Li JH, Yang JS, Zhang EB, Yin DD, et al. MiR-196a is upregulated in gastric cancer and promotes cell proliferation by downregulating p27kip1. Mol Cancer Ther. 2012;11:842–852. doi: 10.1158/1535-7163.MCT-11-1015. [DOI] [PubMed] [Google Scholar]
- 113.Wang H, Hu H, Luo Z, Liu S, Wu W, Zhu M, et al. miR-4454 up-regulated by HPV16 E6/E7 promotes invasion and migration by targeting ABHD2/NUDT21 in cervical cancer. Biosci Rep. 2020;40:BSR20200796. doi: 10.1042/BSR20200796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Naseri M, Zöller M, Hadjati J, Ghods R, Ranaei Pirmardan E, Kiani J, et al. Dendritic cells loaded with exosomes derived from cancer stem cell-enriched spheroids as a potential immunotherapeutic option. J Cell Mol Med. 2021;25:3312–3326. doi: 10.1111/jcmm.16401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ghosh S, Garg S, Ghosh S. Cell-derived exosome therapy: a novel approach to treat post-traumatic brain injury mediated neural injury. ACS Chem Neurosci. 2020;11:2045–2047. doi: 10.1021/acschemneuro.0c00368. [DOI] [PubMed] [Google Scholar]
- 116.Zhu M, Li Y, Shi J, Feng W, Nie G, Zhao Y. Exosomes as extrapulmonary signaling conveyors for nanoparticle-induced systemic immune activation. Small. 2012;8:404–412. doi: 10.1002/smll.201101708. [DOI] [PubMed] [Google Scholar]
- 117.Diaz-Armas GG, Cervantes-Gonzalez AP, Martinez-Duarte R, Perez-Gonzalez VH. Electrically driven microfluidic platforms for exosome manipulation and characterization. Electrophoresis. 2022;43:327–339. doi: 10.1002/elps.202100202. [DOI] [PubMed] [Google Scholar]