Abstract
Objective:
Multiple organizations recommend an annual digital anal rectal examination (DARE) for people at highest risk for anal cancer. We assessed DARE utilization among sexual minority men and transgender women.
Methods:
Community-recruited and asymptomatic individuals from a mid-sized U.S. city were enrolled into the Prevent Anal Cancer Self-Swab Study, a longitudinal clinical trial of anal cancer screening. Self-reported data from the baseline survey were used to assess utilization of DARE in the last year and during the lifetime. Adjusted odds ratios (aOR) and confidence intervals (CI) for factors associated with each outcome were determined using multivariable logistic regression.
Results:
Among 241 participants, median age was 46 years (interquartile range, 33–57 years), 27.0% were living with HIV, and 24.5% reported a prior diagnosis of anal warts. A total of 13.7% (95% CI 9.4%−18.0%) of individuals reported a DARE in the prior year while 53.9% (95% CI 47.7%−60.2%) reported a DARE during the lifetime. The following were associated with a DARE in the prior year: increasing age (aOR 1.04, 95% CI 1.01–1.08 for each additional year), any prior anal cytology (aOR 2.62, 95% CI 1.19–5.80, compared to no prior test or no knowledge of a test), and preferred receptive position during anal sex (aOR 4.93, 95% CI 1.17–20.86 compared to insertive)
Conclusion:
Despite guidelines recommending an annual DARE, it was uncommonly reported. There is an urgent need to understand barriers to conducting DARE among individuals most vulnerable to anal cancer and their health care providers.
Keywords: Anal neoplasms, Digital anal rectal examination, Digital rectal examination, Mass screening, Sexual and gender minorities, Utilization, HIV, Human papillomavirus, Anal sex, Condyloma
Précis
While annual digital anal rectal examinations are recommended for anal cancer detection among sexual minority men, only 13.7% reported the procedure in the prior year.
INTRODUCTION
Squamous cell carcinoma of the anus (SCCA) is a rare malignancy overall, with an incidence of 1.8/100,000 person-years (py). Incidence is much higher in sexual minority men (SMM) with HIV (85/100,000py) and HIV-negative SMM (19/100,000py) with strong variation by age.1 Given substantially elevated risk in people living with HIV and SMM, a growing number of organizations recommend some type of anal cancer screening including anal cytology (to screen for precancer), high-resolution anoscopy-directed biopsy (to diagnose precancer and cancer), and a digital anal rectal examination (DARE) to detect early invasive SCCA.2,3 DARE is a mildly invasive procedure that involves a health care provider (HCP) viewing and palpating the perianus and palpating the anal canal for masses.4 DARE can detect masses as small as 3 mm in diameter;5 however, the mean tumor size for this condition at diagnosis is > 30 mm.6
The use of DARE could lead to earlier detection of SCCA, which, in turn, can lead to a reduction in SCCA morbidity and mortality;7 thus, DARE has been recommended by state and national government agencies and international societies for the detection of early invasive disease in people living with HIV (PLH).2,8–11 For example, the New York State Department of Health has recommended an annual DARE for PLH since 2007.3 Most recently in 2021, the Centers for Disease Control and Prevention (CDC) also recommended that, in addition to PLH, HIV-negative SMM with any history of receptive anal sex receive an annual DARE.2 However, in the most recent U.S. data from 2011, clinics receiving Ryan White funding conducted an anorectal examination on only 23% of PLH.12 Similarly suboptimal use of DARE among PLH has been observed in Australia and Canada.13,14
By World Health Organization criteria, most, but not all, conditions for a DARE public health screening program have been satisfied. Notably, a lack of robust sensitivity and specificity data for DARE to detect invasive anal cancer may contribute to low uptake;15 however, most diagnosed SCCAs have been visible at the perianus or palpable,6,7 adverse events associated with DARE are rare,16 and acceptability is high among SMM.16,17
High-resolution anoscopy (HRA)-directed biopsies, which can diagnose invasive SCCA and non-invasive precancers, is now known to reduce morbidity and mortality; however, suitable biomarkers and screening algorithms to optimize referral to HRA have yet to be identified18 while HRA infrastructure is inadequate even in large coastal cities in the U.S. given equipment cost and difficulty in learning the procedure.19,20 Until HRA infrastructure improves, DARE will be the only widely available HCP tool for detecting SSCA.
Our objective was to estimate the prevalence of DARE in the prior year among SMM and transgender women living in a Midwestern US city between January 2020 and August 2022. In addition, we assessed factors associated with a DARE in the prior year and any DARE during the lifetime.
MATERIALS AND METHODS
Study Design and Protection of Human Subjects
Individuals with and without HIV, aged ≥ 25 years, were recruited into the Prevent Anal Cancer Self-Swab study in Milwaukee, Wisconsin, a metropolitan statistical area with a population of 1.6 million people. Details of the study protocol have been published.21 In brief, this is a prospective, randomized, two-arm clinical study to evaluate engagement with annual home-based vs clinic-based sampling of anal canal exfoliated cells for HPV DNA-based testing. The current analysis used data from the baseline survey. All participants consented to the study in writing and all study activities were approved by the institution’s human research protections committee.
Recruitment primarily occurred through social media, in addition to flyers, clinics, and word-of-mouth. Materials encouraged individuals to enroll in an anal cancer screening study. Study enrollment began January 9, 2020, was suspended due to the coronavirus disease 2019 pandemic on March 14, 2020, and then resumed on November 3, 2020. Enrollment ended August 31, 2022. Individuals were excluded if they reported a prior diagnosis of anal cancer, the use of clopidogrel, warfarin, apixaban or another anticoagulant (other than aspirin or non-steroidal anti-inflammatory drugs), or a diagnosis of hemophilia, cirrhosis with bleeding varices, or thrombocytopenia.
Data Collection
The eligibility survey, consenting session, and baseline survey were usually conducted online without face-to-face contact with participants who were not reimbursed for completing the baseline survey. DARE was described in the baseline survey as follows: “During a digital anal rectal exam (DARE), a doctor or nurse puts a finger into your anus to check for problems. Sometimes it is called a digital rectal exam or a DRE.” Participants were then asked if they had ever had a DARE (Yes/No/I don’t know) and, for those answering “Yes” to ever having a DARE, the survey asked when the last DARE occurred (“within the last year,” “1–2 years ago,” “2–5 years ago,” “more than 5 years ago,” and “I don’t know”).
Individuals were asked about HIV status in the baseline survey. Those reporting HIV-negative status, or not knowing their status, were tested for HIV (Determine™ HIV-1/2 AG/AB Combo, Scarborough, Maine, USA; Elecsys® HIV Duo, Roche Diagnostics, Indianapolis, Indiana, USA) at the initial clinical encounter although one person did not answer the HIV question and then did not attend the initial clinical encounter and thus their HIV status is missing.
Analysis
Of 253 individuals who consented, 241 completed the baseline survey including questions about their history of DARE. Individuals who reported “I don’t know” to ever having a DARE were grouped for analysis with persons reporting no history of a DARE. For the timing of the last DARE, individuals reporting a DARE not “within the last year” were grouped together with persons who reported not knowing when the last DARE occurred. For any prior anal cytology, individuals answering “I don’t know” were grouped with persons reporting no history of anal cytology. Participants were also asked if they were afraid to have anal cancer screening for fear of a bad result with responses on a 4-point Likert scale. For analysis, the “strongly disagree” and “disagree” responses were combined as were “strongly agree” and “agree” responses.
The proportion of persons reporting DARE in the last year and its association with categorical exposures was assessed with χ2 and Fisher’s Exact tests, while ordinal exposures were assessed with Cochran-Armitage trend tests. A sensitivity analysis restricted the dataset to individuals taking the baseline survey from July 24, 2021 to August 31, 2022 (n=107) to assess the potential for a temporal change in DARE prevalence after CDC guidelines were published July 23, 2021. The analysis was not restricted by age given a lack of consensus on when screening should begin in this population.22 An alpha standard of 0.05 was used in all statistical tests and all p-values were two-sided. This ancillary analysis of the baseline data did not have a priori power calculations.
Odds ratios were estimated for the association between exposures and timing of the last DARE and ever having a DARE using univariate and multivariable logistic regression. We note that a history of a DARE during the lifetime was common (53.9%, Supplementary Table 1) and might call for estimation of prevalence ratios rather than odds ratios;23 however, to allow better comparison of point estimates between the two outcomes, only odds ratios were estimated for both. A Likelihood Ratio test with a p-value of < 0.20 between each exposure and outcome was used to identify exposures to include in multivariable modeling. Using manual backward elimination, exposures with a p-value of > 0.05 were removed from the multivariable model until all remaining exposures had a p-value of < 0.05. Age was considered a confounder and was retained in all models. Adjusted and unadjusted odds ratios were reported with 95% confidence intervals (CI). Firth’s bias correction was used in all regression models to reduce bias associated with cells with no data.24 This multivariable analysis protocol was used for both outcomes.
Since study enrollment occurred both before and during the coronavirus disease 2019 pandemic, the association between enrollment date and each outcome was assessed. Enrollment date was not associated with either outcome (Fisher’s Exact p=0.59 for a DARE within the last year and χ2 p=0.44 for any DARE history).
Analyses were conducted using SAS 9.4 TS Level 1M6 (SAS Institute, Cary, North Carolina, USA).
RESULTS
Median age of consented participants was 46 years (interquartile range, 33–57 years) and 65.6%, 19.1%, and 12.9% identified as non-Hispanic white, non-Hispanic Black, or Hispanic (table 1). The large majority had health insurance including Medicare, Medicaid, or Ryan White care (93.4%). A total of 27.0% were PLH, 20.3% reported receiving ≥1 dose of HPV vaccine and 24.1% reported prior anal cytology. Almost one-quarter of individuals (24.5%) reported receiving a prior diagnosis of anal warts.
Table 1.
Characteristics of participants and time since the last digital anal rectal examination, Milwaukee, Wisconsin, USA 2020–2022, The Prevent Anal Cancer Self-Swab Study, n (%)
| Characteristic | Total n=241 (column %) | ≤1 year n=33 (row %) | >1 year, never, or don’t know n=208(row %) |
|---|---|---|---|
| Age, years median (IQR)** | 46 (33–57) | 57 (44–62) | 44 (33–56) |
| Age, years (categorical)**a | |||
| 25–34 | 75 (31.1) | 4 (12.1) | 71 (34.1) |
| 35–44 | 42 (17.4) | 5 (15.2) | 37 (17.8) |
| 45–54 | 46 (19.1) | 7 (21.2) | 39 (18.8) |
| 55–81 | 78 (32.4) | 17 (51.5) | 61 (29.3) |
| Gender identity b | |||
| Man | 227 (94.2) | 32 (97.0) | 195 (93.8) |
| Transgender, non-binary, or other | 14 (5.8) | 1 (3.0) | 13 (6.3) |
| Race/Ethnicity b | |||
| White, non-Hispanic | 158 (65.6) | 24 (72.7) | 134 (64.4) |
| Black, non-Hispanic | 46 (19.1) | 5 (15.2) | 41 (19.7) |
| Hispanic | 31 (12.9) | 4 (12.1) | 27 (13.0) |
| Otherc | 5 (2.1) | 0 (0.0) | 5 (2.4) |
| Missing | 1 (0.4) | 0 (0.0) | 1 (0.5) |
| Sexual orientation b | |||
| Gay | 198 (82.2) | 30 (90.9) | 168 (80.8) |
| Bisexual | 29 (12.0) | 3 (9.1) | 26 (12.5) |
| Otherd | 13 (5.4) | 0 (0.0) | 13 (6.3) |
| Missing | 1 (0.4) | 0 (0.0) | 1 (0.5) |
| Have health insurance b | |||
| No | 14 (5.8) | 2 (6.1) | 12 (5.8) |
| Yes | 225 (93.4) | 31 (93.9) | 194 (93.3) |
| Missing | 2 (0.8) | 0 (0.0) | 2 (1.0) |
| HIV status | |||
| Positive | 65 (27.0) | 9 (27.3) | 56 (26.9) |
| Negative | 175 (72.6) | 24 (72.7) | 151 (72.6) |
| Missing | 1 (0.4) | 0 (0.0) | 1 (0.5) |
| Had HPV vaccination | |||
| No | 134 (55.6) | 21 (63.6) | 113 (54.3) |
| Yes | 49 (20.3) | 5 (15.2) | 44 (21.2) |
| Don’t know | 57 (23.7) | 7 (21.2) | 50 (24.0) |
| Missing | 1 (0.4) | 0 (0.0) | 1 (0.5) |
| Ever had anal cytology ** | |||
| No/Don’t know | 183 (75.9) | 18 (54.6) | 165 (79.3) |
| Yes | 58 (24.1) | 15 (45.5) | 43 (20.7) |
| Position during anal sex | |||
| Always or mostly insertive | 47 (19.5) | 2 (6.1) | 45 (21.6) |
| More or less versatile | 111 (46.1) | 16 (48.5) | 95 (45.7) |
| Always or mostly receptive | 72 (29.9) | 14 (42.4) | 58 (27.9) |
| Never engaged in anal sex | 8 (3.3) | 0 (0.0) | 8 (3.9) |
| Missing | 3 (1.2) | 1 (3.0) | 2 (1.0) |
| Ever had an anal wart diagnosis * | |||
| No | 180 (74.7) | 19 (57.6) | 161 (77.4) |
| Yes | 59 (24.5) | 14 (42.4) | 45 (21.6) |
| Missing | 2 (0.8) | 0 (0.0) | 2 (1.0) |
| Afraid to have anal cancer screening for fear of a bad result * b | |||
| Strongly Disagree/Disagree | 209 (86.7) | 32 (97.0) | 177 (85.1) |
| Strongly Agree/Agree | 31 (12.9) | 0 (0.0) | 31 (14.9) |
| Missing | 1 (0.4) | 1 (3.0) | 0 (0.0) |
p<0.05;
p<0.01;
p<0.001;
All hypothesis tests are Pearson chi square unless otherwise noted; calculation of p-values does not include missing data.
Cochran-Armitage Test for Trend
Fisher’s Exact Test
Other includes Asian, American Indian or Alaskan Native, Other, and I don’t know.
Other includes Queer, Heterosexual, Other, and I don’t know
IQR indicates interquartile range; HPV, human papillomavirus
Only 13.7% (95% CI 9.4%−18.0%) of individuals reported receiving a DARE in the prior year and it was associated with increasing age (test for trend, p=0.003). For example, 5.3% of individuals aged 25–34 years reported a DARE in the prior year compared with 21.8% of those aged 55–81 years (table 1). Of all persons aged 45 years and older, 19.4% reported a DARE in the prior year (data not shown).
There was no difference by HIV status with 13.9% and 13.7% of PLH and HIV-negative individuals reporting a DARE in the prior year (p=0.98). When stratified by age group, HIV status continued to be unassociated with a DARE in the prior year (Figure 1). Of those with HIV aged ≥ 35 years of age, 14.8% reported a DARE in the last year (data not shown). A higher proportion of non-Hispanic white (15.2%) than non-Hispanic Black (10.9%) or Hispanic (12.9%) individuals reported a DARE although it was not significant (Fisher’s Exact, p=0.91). When prevalence was assessed for the 13-month period after the CDC DARE recommendation (July 24, 2021 – August 31, 2022), 16.1% and 17.1% of PLH and HIV-negative individuals, respectively, reported a DARE in the prior year (p=0.90).
FIGURE 1.
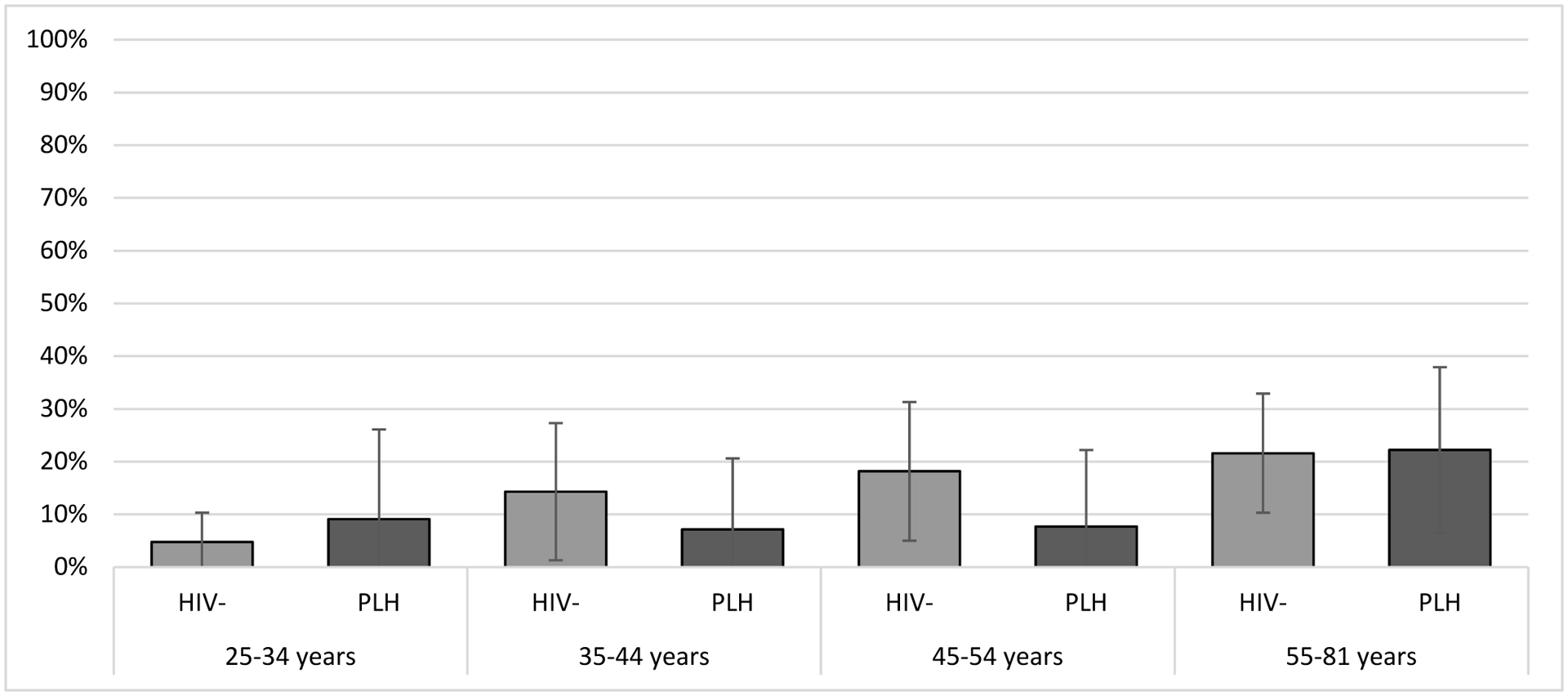
Prevalence of a digital anal rectal examination in the previous year, stratified by HIV status and age in years, Milwaukee, Wisconsin, 2020–2022, The Prevent Anal Cancer Self-Swab Study.
In univariate logistic regression, increasing age (OR 1.05 95% CI 1.02–1.08, for each additional year) and prior anal cytology (OR 3.19 95% CI 1.49–6.80, compared to no/don’t know about prior anal cytology) were associated with receiving a DARE in the last year (Table 1). Individuals who always or mostly took the receptive position in anal sex had higher odds of reporting a DARE in the last year (OR 4.51 95% CI 1.10–18.50) compared to individuals who always or mostly took the insertive position. Individuals who had a prior anal wart diagnosis had more than double the odds of receiving a DARE in the last year (OR 2.64 95% CI 1.23–5.64).
In multivariable regression, factors associated with having received a DARE in the last year were increasing age (aOR 1.04 95% CI 1.01–1.08, for each additional year), having had prior anal cytology (aOR 2.62 95%CI 1.19–5.80 compared to those without prior cytology or no knowledge of prior cytology) and taking the receptive anal sex position always or mostly (Table 2). Compared to individuals who preferred insertive anal sex, individuals who preferred receptive anal sex had almost 5 times higher odds of having a DARE in the last year (aOR 4.93 95% CI 1.17–20.86).
Table 2:
Factors associated with participants who received a digital anal rectal examination in the last year, Milwaukee, Wisconsin, USA 2020–2022, univariate and multivariable analysis
| OR (95% CI) | aORa (95% CI) | |
|---|---|---|
| Age, years (continuous)b | 1.05 (1.02–1.08) | 1.04 (1.01–1.08) |
| Sexual orientation | ||
| Gay | 1.0 | - |
| Bisexual | 0.73 (0.22–2.42) | - |
| Otherc | 0.21 (0.01–3.93) | - |
| Ever had anal cytology | ||
| No/Don’t know | 1.0 | 1.0 |
| Yes | 3.19 (1.49–6.80) | 2.62 (1.19–5.80) |
| Position during anal sex | ||
| Always or mostly insertive | 1.0 | 1.0 |
| More or less versatile | 3.15 (0.78–12.62) | 3.67 (0.90–14.98) |
| Always or mostly receptive | 4.51 (1.10–18.50) | 4.93 (1.17–20.86) |
| Never engaged in anal sex | 1.07 (0.04–28.67) | 1.77 (0.06–51.09) |
| Ever had an anal wart diagnosis | ||
| No | 1.0 | - |
| Yes | 2.64 (1.23–5.64) | - |
Note, confidence intervals in bold do not include unity
Variables are adjusted for each other.
The odds ratio for age reflects increased odds for each one year increment.
Other includes Queer, Heterosexual, Other, and I don’t know
A total of 53.9% (95% CI 47.7%−60.2%) of individuals reported ever having a DARE (Supplemental table 1). Individuals who reported ever having a DARE had a median age of 53 years while those who reported having no prior DARE, or no knowledge of a prior DARE, had a median age of 36 years. In univariate analysis, age, race/ethnicity, sexual orientation, HPV vaccination, ever having anal cytology and a diagnosis of anal warts were associated with a history of a DARE. In multivariable analysis, ever having a DARE was associated with increasing age (aOR 1.07 95% CI 1.04–1.09, for each additional year) and a prior diagnosis of anal warts (aOR 2.89 95% CI 1.44–5.80, compared with no diagnosis) (Supplemental table 2).
DISCUSSION
Only 13.7% of SMM and trans women reported having a DARE in the prior year. This particularly vulnerable population may be benefiting little from recommendations to use DARE for anal cancer detection. While HIV confers a higher risk for SCCA, PLH were no more likely to report DARE in the last year than HIV-negative individuals. While SCCA presents at a median age of 47.4 years and 57.8 years for individuals with and without HIV, respectively,25 only 18.7% of individuals 45 to 54 years of age reported a DARE in the last year.
To our knowledge there are no data on DARE prevalence from a community-recruited sample of SMM and transgender women. A Canadian study estimated DARE prevalence in a clinic-recruited sample of mostly SMM with HIV (84%) observing a lifetime DARE prevalence of 70%.14 This estimate is higher than what we observed (53.9%) possibly due to recruitment methods or higher DARE utilization in Canada. The study also reported higher odds of receiving a DARE among white men than African, Caribbean and Black men or Asian men14 which is consistent with our observations of a lower prevalence of DARE in non-Hispanic Black men compared with non-Hispanic white men although the difference was not significant.
The reasons for low DARE utilization in the US are likely multifactorial and involve barriers at both patient and HCP level. An HCP recommendation for DARE is important to patients.26 But even if an HCP recommends a DARE, patients may decline due to lack of awareness of SCCA risk,26 embarrassment,27 and/or competing health issues.16 If an HCP does not recommend DARE, it may be due to a lack of knowledge about recommendations, a lack of a national consensus,13 the perceived strength of evidence for DARE,28 concern about acceptability among patients,16 discomfort with anal procedures,26,29 lack of time,28 and questions about how to conduct the exam.13
The lack of consistent DARE guidelines for HCPs Is a barrier to HCP utilization.13 For example, some organizations give a strong recommendation for DARE,10 others a moderate recommendation8 and there is inconsistency on the recommended starting age to use DARE.2,8,10 The 2021 CDC recommendations are internally inconsistent, with the section titled “HPV” using the language, “A digital anorectal examination (DARE) should be performed to detect early anal cancer”“ while the “Anal Cancer” section advises, “An annual digital anorectal examination (DARE) might be useful to detect masses…”2 Notably, the U.S. Preventive Services Task Force has not issued recommendations on anal precancer or cancer screening but is currently reviewing the question.30 A national evidence-based consensus on DARE utilization will be difficult to achieve since a clinical trial to generate evidence of DARE efficacy to reduce SCCA morbidity and mortality is likely not possible now given ethical considerations regarding a control group. More likely are recommendations involving HRA-directed biopsy to detect anal precancers. Note that DARE should accompany HRA.31 Since recommendations take time to integrate into medical practice, DARE prevalence should be measured in the future to document changes.
The strong association with increasing age in both timing of last DARE and ever having a DARE is expected in the context of cancer, whose incidence increases with age. However, the ability of DARE to detect benign conditions like anal warts and fissures, especially in younger individuals who have very little risk for SCCA,1 might be considered in a comprehensive anal health program.4
A preference for receptive anal sex had the strongest association with a DARE in the last year. Receptive anal sex increases risk for infection with human papillomavirus, the primary cause of anal cancer, which may encourage persons with a preference for receptive anal sex to request anal screening. The CDC recommendation advises that only HIV-negative men who have sex with men with a history of receptive anal sex receive an annual DARE,2 (i.e, approximately 90% of SMM).32,33 This recommendation requires provider-patient communication about anal health; however, these discussions may occur very infrequently between HCP and patient,14,34 and, thus, it seems unlikely that a patient’s preferred anal sex position is driving DARE uptake among HIV-negative SMM. Preference for receptive anal sex was associated with DARE in the last year but not with ever receiving a DARE which may be due to anal sex preferences varying over the lifetime. Finally, perhaps receptive anal sex is associated with more anal symptoms which may lead to a DARE.
Anal cytology and the diagnosis of anal warts were independently associated with a DARE in the last year and DARE during the lifetime, respectively. The association with anal cytology may be due to HCPs who are informed about anal cancer and attendant screening practices; however, cytology should not be performed if HRA is unavailable, which is when DARE is most needed. The association with anal warts may be coincident since anal symptoms may lead to the discovery of anal warts due to a DARE; however, these cross-sectional data preclude causal conclusions. The latter scenario is concerning since subclinical anal warts would not trigger additional examination that includes DARE. Three-quarters of this sample reported no anal wart history.
One primary limitation is that these DARE prevalence estimates may be subject to misclassification bias given self-reporting and similarities between DARE and DRE. For example, some individuals may report a DARE for anal cancer detection when they instead had a DRE for prostate cancer screening, in which case the actual estimates for DARE prevalence would be even lower. Also, individuals may not remember a DARE, in which case the current estimates may underestimate DARE prevalence. In addition, these prevalence estimates may not be generalizable to large cities where there may be increased anal cancer awareness; however, limited utilization of DARE has been observed in large cities.12,13 Although 32% of individuals identified as Black or Hispanic, our data are somewhat limited in understanding differences in DARE uptake by race and ethnicity due to sample size.
Conclusions
Despite recommendations, utilization of DARE is limited among individuals who are most vulnerable to anal cancer. These data are consistent with U.S. DARE utilization data from 10 years ago12 and lower than 2017 data from Canada with regard to ever having a DARE.14 Individuals with HIV were no more likely to report a DARE than those without HIV. There is an urgent need to understand barriers to DARE among SMM and trans women and among their health care providers.
Supplementary Material
ACKNOWLEDGMENTS
We thank the participants in the study, the Prevent Anal Cancer (PAC) Self-Swab Study Community Advisory Board, and the PAC Self-Swab Study Team: Bridgett Brzezinski, Maritza Pallo, Cameron Liebert, Christopher Ajala, Lisa Rein, Esmeralda Lezama-Ruiz, Dr. María E. Fernandez, Dr. Vanessa Schick, and Dr. Michael Swartz.
Role of the Financial Source
The study is possible through funding from the National Cancer Institute through the National Institutes of Health to AGN (R01CA215403) and from the Medical College of Wisconsin. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study is also supported by funding from the Cancer Prevention Research Institute of Texas to MDS (RP170668). None of these funding entities has any role in the design, collection, management, analysis, interpretation of data, writing of the report, or the decision to submit this work for publication. AGN had final responsibility for submission of this paper.
Abbreviations
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- DARE
Digital anal rectal examination
- HCP
health care provider
- HRA
high-resolution anoscopy
- SCCA
squamous cell carcinoma of the anus
- SMM
sexual minority men
- PLH
persons living with HIV
Footnotes
Declaration of Interests
All authors report no conflict of interest.
References
- 1.Clifford GM, Georges D, Shiels MS, et al. A meta-analysis of anal cancer incidence by risk group: toward a unified anal cancer risk scale. Int J Cancer. 2021;148:38–47. doi: 10.1002/ijc.33185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control & Prevention. Sexually Transmitted Infections Treatment Guidelines. 2021. https://www.cdc.gov/std/treatment-guidelines/STI-Guidelines-2021.pdf
- 3.New York State Department of Health-AIDS Institute. Anal dysplasia and cancer, July 2007. Accessed October 14, 2015. http://www.hivguidelines.org/clinical-guidelines/adults/anal-dysplasia-and-cancer/
- 4.Hillman RJ, Berry-Lawhorn JM, Ong JJ, et al. International Anal Neoplasia Society guidelines for the practice of digital anal rectal examination. J Low Genit Tract Dis. 2019;23:138–146. doi: 10.1097/lgt.0000000000000458 [DOI] [PubMed] [Google Scholar]
- 5.Nyitray AG, Hicks JT, Hwang LY, et al. A phase II clinical study to assess the feasibility of self and partner anal examinations to detect anal canal abnormalities including anal cancer. Sex Transm Infect. 2018;94:124–130. doi: 10.1136/sextrans-2017-053283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Read TR, Huson KL, Millar JL, et al. Size of anal squamous cell carcinomas at diagnosis: A retrospective case series. Int J STD AIDS. 2013;24:879–82. doi: 10.1177/0956462413486776 [DOI] [PubMed] [Google Scholar]
- 7.Berry JM, Jay N, Cranston RD, et al. Progression of anal high-grade squamous intraepithelial lesions to invasive anal cancer among HIV-infected men who have sex with men. Int J Cancer. 2014;134:1147–55. doi: 10.1002/ijc.28431 [DOI] [PubMed] [Google Scholar]
- 8.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. U.S. Department of Health and Human Services, National Insitutes of Health, Office of AIDS Research. Updated August 18, 2021. Accessed August 3, 2022. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection/human-papillomavirus-disease?view=full
- 9.European AIDS Clinical Society. EACS Guidelines version 11.0. Accessed August 13, 2022. https://eacs.sanfordguide.com/prevention-non-infectious-co-morbidities/cancer/cancer-screening-methods
- 10.New York State Department of Health-AIDS Institute. Screening for anal dysplasia and cancer in adults with HIV August 2022. Accessed August 13, 2022. http://www.hivguidelines.org/clinical-guidelines/adults/anal-dysplasia-and-cancer/
- 11.Australasian Society for HIV VHaSHM. HIV Monitoring Tool. Accessed July 13, 2022. https://www.ashm.org.au/resources/hiv-monitoring-tool/
- 12.New York State Department of Health-AIDS Institute: HIVQUAL-US. HIVQual-US Annual Data Report. Retrieved on September 10, 2016 from https://www.ehivqual.org/scripts/eHIVQUAL%202011%20Report%20-%20National.pdf. 2014. https://www.ehivqual.org/scripts/eHIVQUAL%202011%20Report%20-%20National.pdf
- 13.Ong J, Chen M, Temple-Smith M, et al. The inside story. Physicians’ views on digital ano-rectal examination for anal cancer screening of HIV positive men who have sex with men. J Med Screen. 2013;20:188–91. doi: 10.1177/0969141313515463 [DOI] [PubMed] [Google Scholar]
- 14.Gillis JL, Grennan T, Grewal R, et al. Racial disparities in anal cancer screening among men living with HIV: findings from a clinical cohort study. J Acquir Immune Defic Syndr. 2020;84:295–303. doi: 10.1097/qai.0000000000002335 [DOI] [PubMed] [Google Scholar]
- 15.Nyitray AG, D’Souza G, Stier EA, Clifford G, Chiao EY. The utility of digital anal rectal examinations in a public health screening program for anal cancer. J Low Genit Tract Dis. 2020;24:192–196. doi: 10.1097/LGT.0000000000000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong JJ, Walker S, Grulich A, et al. Incorporating digital anorectal examinations for anal cancer screening into routine HIV care for men who have sex with men living with HIV: a prospective cohort study. J Int AIDS Soc. 2018;21:e25192. doi: 10.1002/jia2.25192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis TW, Goldstone SE, Chen G. Tolerability of anal dysplasia screening. J Low Genit Tract Dis. 2013;17:404–8. doi: 10.1097/LGT.0b013e31827fb76c [DOI] [PubMed] [Google Scholar]
- 18.Palefsky JM, Lee JY, Jay N, et al. Treatment of anal high-grade squamous intraepithelial lesions to prevent anal cancer. N Engl J Med. 2022;386:2273–2282. doi: 10.1056/NEJMoa2201048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richel O, Prins JM, de Vries HJ. Screening for anal cancer precursors: what is the learning curve for high-resolution anoscopy? AIDS. 2014;28:1376–7. doi: 10.1097/qad.0000000000000227 [DOI] [PubMed] [Google Scholar]
- 20.Damgacioglu HLY, Ortiz AP, Wu C, Shahmoradi Z, Shyu S, Li R, Nyitray AG, Sigel K, Clifford GM, Jay N, Colon Lopez V, Barnell GM, Chiao EY, Stier EA, Ortiz-Ortiz K, Ramos-Cartagena J, Sonawane K, Deshmukh AA. State variation in squamous cell carcinoma of the anus incidence and mortality, and association with HIV/AIDS and smoking in the United States. Journal of Clinical Oncology. In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyitray AG, Schick V, Swartz MD, et al. Rationale and design of the Prevent Anal Cancer Self-Swab Study: a protocol for a randomised clinical trial of home-based self-collection of cells for anal cancer screening. BMJ Open. 2021;11:e051118. doi: 10.1136/bmjopen-2021-051118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plotzker RE, Barnell GM, Wiley DJ, Stier EA, Jay N. Provider preferences for anal cancer prevention screening: results of the International Anal Neoplasia Society Survey. Tumour Virus Res. 2022:200235. doi: 10.1016/j.tvr.2022.200235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi:kwi188[pii] 10.1093/aje/kwi188 [DOI] [PubMed] [Google Scholar]
- 24.Firth D Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 25.D’Souza G, Wiley DJ, Li X, et al. Incidence and epidemiology of anal cancer in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2008;48:491–9. doi: 10.1097/QAI.0b013e31817aebfe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman PA, Roberts KJ, Masongsong E, Wiley DJ. Anal cancer screening: Barriers and facilitators among ethnically diverse gay, bisexual, transgender, and other men who have sex with men. J Gay Lesbian Soc Serv. 2008;20:328–353. doi: 10.1080/10538720802310733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koskan AM, Fernandez-Pineda M. Anal cancer prevention perspectives among foreign-born Latino HIV-infected gay and bisexual men. Cancer Control. 2018;25:1073274818780368. doi: 10.1177/1073274818780368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koskan AM, Brennhofer SA, Helitzer DL. Screening for anal cancer precursors among patients living with HIV in the absence of national guidelines: practitioners’ perspectives. Cancer Cause Control. 2019;30:989–996. doi: 10.1007/s10552-019-01209-8 [DOI] [PubMed] [Google Scholar]
- 29.Sowah LA, Buchwald UK, Riedel DJ, et al. Anal cancer screening in an urban HIV clinic: provider perceptions and practice. Journal of the International Association of Providers of AIDS Care. 2015;14:497–504. doi: 10.1177/2325957415601504 [DOI] [PubMed] [Google Scholar]
- 30.United States Preventive Services Task Force. Draft Research Plan, Anal Cancer: Screening. website. December 14, 2022. Accessed December 14, 2022. https://www.uspreventiveservicestaskforce.org/uspstf/document/draft-research-plan/anal-cancer-screening
- 31.Hillman RJ, Cuming T, Darragh T, et al. 2016 IANS international guidelines for practice standards in the detection of anal cancer precursors. Article. J Low Genit Tract Dis. 2016;20:283–291. doi: 10.1097/LGT.0000000000000256 [DOI] [PubMed] [Google Scholar]
- 32.Tota JE, Giuliano AR, Goldstone SE, et al. Anogenital human papillomavirus (HPV) infection, seroprevalence, and risk factors for HPV seropositivity among sexually active men enrolled in a global HPV vaccine trial. Clin Infect Dis. 2022;74:1247–1256. doi: 10.1093/cid/ciab603 [DOI] [PubMed] [Google Scholar]
- 33.Patel P, Bush T, Kojic EM, et al. Prevalence, incidence, and clearance of anal high-risk human papillomavirus Infection among HIV-Infected men in the SUN Study. Article. J Infect Dis. 2018;217:953–963. doi: 10.1093/infdis/jix607 [DOI] [PubMed] [Google Scholar]
- 34.Kutner BA, Simoni JM, Aunon FM, Creegan E, Balán IC. How stigma toward anal sexuality promotes concealment and impedes health-seeking behavior in the U.S. among cisgender men who have sex with men. Arch Sex Behav. 2021;50:1651–1663. doi: 10.1007/s10508-019-01595-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


