KCNH2, also known as the human ether-a-go-go related gene or hERG, encodes the Kv11.1 channel proteins that conduct the rapidly activating delayed rectifier K+ current (IKr) in the heart. IKr plays a primary role in ventricular repolarization, and a decrease or loss in IKr is associated with an increased risk for the deadly cardiac arrhythmias (Sanguinetti et al., 1995). A major goal in cardiac safety assessment is to identify mechanisms that modify IKr, ventricular repolarization, and arrhythmogenic risk. To understand how IKr can be modified, one can think of IKr as being equal to the number of Kv11.1 channels in the membrane (M), the open probability of the Kv11.1 channels (Po), and the amplitude of the single Kv11.1 channel current (i) (Hille, 2001).
Po and i reflect the biophysical properties of Kv11.1 channels in the cell membrane. In contrast, M reflects several different cytoplasmic processes, including Kv11.1 channel trafficking, production, and degradation. The kinetics of the cytoplasmic processes that regulate M are orders of magnitude slower than the biophysical properties that regulate Po and i (Figure). As a result, modifying Po and i have immediate impacts on IKr and ventricular repolarization, whereas modifying the cytoplasmic processes that regulate M take minutes, hours, or even days to fully impact IKr, ventricular repolarization, and arrhythmia susceptibility.
Figure.
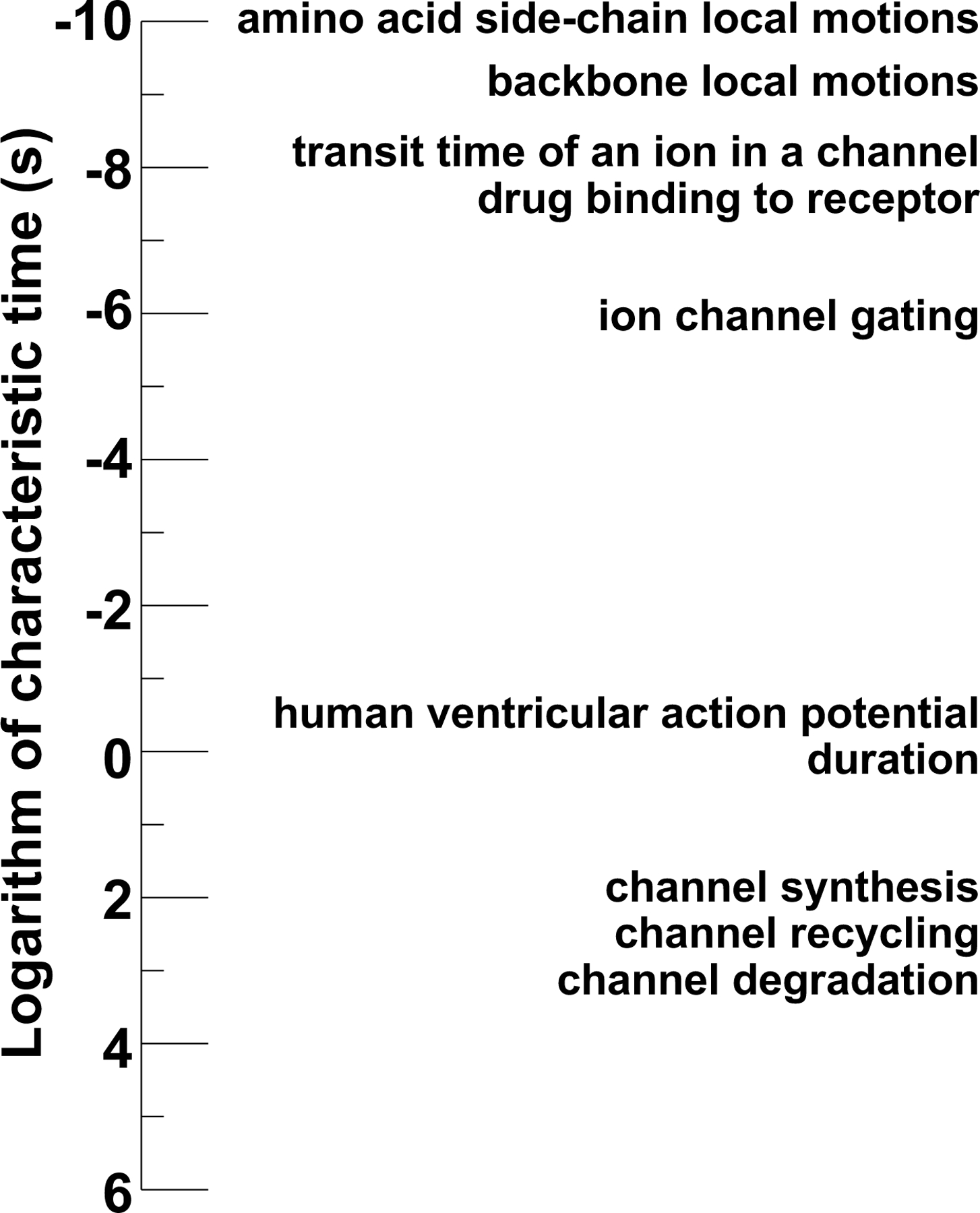
Logarithmic of characteristic time for different ion channel protein processes and the human ventricular action potential.
Meier and colleagues recognized this issue and developed a model to calculate how changes in the cytoplasmic processes that regulate the number of Kv11.1 channels in the membrane (M), IKr, and ventricular repolarization (Meier et al., 2023). To do this, they integrate their model with the O’Hara-Rudy (ORd) ventricular action potential (AP) model (O’Hara et al., 2011). They apply the integrated model to predict how conditions that alter the biophysical properties and number of Kv11.1 channels in the membrane impact IKr and the ventricular AP waveform. This Perspective provides a primer for how Meier and colleagues modeled the cytoplasmic processes that regulate channel number, and it highlights their important contribution in understanding time-dependent changes in arrhythmogenic risk.
Meier and colleagues introduce a two-state kinetic model for the trafficking of an ion channel to the membrane, where the variable represents the number of channels in the membrane and the number in the cytoplasm. The number of channels in the membrane increases with membrane insertion and decreases with internalization.
The number of ion channels inserted in the membrane over time is proportional to the number of ion channels in the cytoplasm, with the proportionality constant equal to the forward trafficking rate times the time interval. Similarly, the number, where is the internalization rate.
After dividing through by, one obtains an equation for the rate of change in the number of ion channels at the cell surface.
Using similar reasoning, one can write an equation for the rate of change of the number of ion channels internalized in the cytoplasm.
is the ion channel production rate (number of channels per unit time) and is the degradation rate of the ion channel.
To simulate the trafficking of individual channels, one can treat a transition rate as a probability. For example, gives the probability that an ion channel comes into existence during a time interval. So roughly, during a time step by generating a uniform random number between zero and one (like rolling dice), if, a channel comes into existence. Once a channel exists in the cytoplasm, one determines the probability the channel will be inserted in the membrane as:
The probability that the channel will be degraded:
And the probability that the channel stays in cytoplasm:
One continues in this fashion to simulate a channel in the membrane.
Meier and colleagues use stochastic simulations to determine the rate constants for Kv11.1 channels by optimizing agreement with experimental results for Kv11.1 channel trafficking. Since they can use the same model structure for deterministic simulations, they are able to integrate the model with a Markovian model of IKr and the ORd AP model. Importantly, this integration allows Meier and colleagues to simulate how drugs that block IKr and affect Kv11.1 channel trafficking impact IKr and ventricular repolarization. In addition, they model the impact that changes in temperature and extracellular [K+] have on IKr and the ventricular AP waveform (Zhao et al., 2016). The simulations underscore the importance for understanding how the slow changes in the number of Kv11.1 channels in the membrane can be a critical determinant for identifying potentially arrhythmogenic changes in IKr and ventricular repolarization. For example, simulating a fever predicted an initial increase in the amplitude of IKr, but continuing the simulation for 24-hours predicted a decrease in IKr due to a reduction in Kv11.1 channels in the membrane. Similarly, simulating hypokalemia initially predicted a relatively small decrease in IKr, but after a week, the simulation predicted a larger decrease in IKr that resulted in arrhythmogenic changes in the ventricular AP waveform.
In conclusion, Meier and colleagues illustrate the importance of modeling cytoplasmic processes that regulate the number of ion channels in the membrane when predicting the risk for arrhythmias. A strength and limitation are the model’s simplicity and its sole focus on Kv11.1 channels. All the cardiac ion channels are regulated by dynamic cytoplasmic processes, which are also modulated by various physiological factors (temperature, ionic conditions, and drugs). These limitations reflect the need for more experimental data on the cytoplasmic processes that regulate the trafficking of Kv11.1 and the other cardiac ion channels. Despite these limitations, the work represents a timely and significant contribution.
Supplementary Material
Funding
This work was supported by National Heart Lung and Blood Institute grants R01HL153042 and R01HL141343.
Footnotes
Competing interest statement
None.
References
- Hille B (2001). Ion Channels of Excitable Membranes Sinauer Associates, Inc. [Google Scholar]
- Meier S, Grundland A, Dobrev D, Volders PGA & Heijman J. (2023). In silico analysis of the dynamic regulation of cardiac electrophysiology by K(v) 11.1 ion-channel trafficking. J Physiol [DOI] [PMC free article] [PubMed]
- O’Hara T, Virag L, Varro A & Rudy Y. (2011). Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput Biol 7, e1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Jiang C, Curran ME & Keating MT. (1995). A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81, 299–307. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang T, Guo J, Yang T, Li W, Koichopolos J, Lamothe SM, Kang Y, Ma A & Zhang S. (2016). Febrile temperature facilitates hERG/IKr degradation through an altered K(+) dependence. Heart Rhythm 13, 2004–2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


