Abstract
As malaria transmission declines, the need to monitor the heterogeneity of malaria risk at finer scales becomes critical to guide community-based targeted interventions. Although routine health facility (HF) data can provide epidemiological evidence at high spatial and temporal resolution, its incomplete nature of information can result in lower administrative units without empirical data. To overcome geographic sparsity of data and its representativeness, geo-spatial models can leverage routine information to predict risk in un-represented areas as well as estimate uncertainty of predictions. Here, a Bayesian spatio-temporal model was applied on malaria test positivity rate (TPR) data for the period 2017–2019 to predict risks at the ward level, the lowest decision-making unit in mainland Tanzania. To quantify the associated uncertainty, the probability of malaria TPR exceeding programmatic threshold was estimated. Results showed a marked spatial heterogeneity in malaria TPR across wards. 17.7 million people resided in areas where malaria TPR was high (≥ 30; 90% certainty) in the North-West and South-East parts of Tanzania. Approximately 11.7 million people lived in areas where malaria TPR was very low (< 5%; 90% certainty). HF data can be used to identify different epidemiological strata and guide malaria interventions at micro-planning units in Tanzania. These data, however, are imperfect in many settings in Africa and often require application of geo-spatial modelling techniques for estimation.
Subject terms: Malaria, Statistics
Introduction
The importance of targeting interventions through adequate malaria planning and informed decision making has been emphasized by the recently launched World Health Organization (WHO) High Burden High Impact initiative (HBHI)1. This initiative encourages national malaria control programs (NMCPs) across Africa to use local, routine and survey data to stratify malaria risk at the national and sub-national levels and accordingly define appropriate targets for their malaria strategic plans1. To date, national stratification using available routine data from health information systems has been conducted in several African countries including Burkina Faso2, Eritrea3, Ghana4,5, Kenya6,7, Madagascar8–10, Malawi11, Mali12, Namibia13,14, Rwanda15, South Africa16, Swaziland17, Tanzania18,19, Uganda20, Zambia21,22 and Zimbabwe23 with most utilizing incidence as a metric of malaria measure. The sources of data used by NMCPs for national stratification vary between countries and is dependent on the availability, access and quality of information24,25.
In recent years, the launch of the WHO test and treat policy26 along with investments to digitize the health management information system (HMIS) under the electronic district health information software (DHIS2) has resulted in gradual improvements in the quality and completeness of routine data from health facilities (HFs). Routine data offers a source of data that is temporally and spatially much more comprehensive than parasite prevalence from periodic household surveys. They provide real-time and spatially granular information which is essential for effective monitoring and timely planning of interventions.
Most NMCPs in many countries have some form of stratified maps of malaria risk based on aggregating routine data, climatic stratification, or parasite prevalence27,28. These stratification maps are usually produced at the higher administrative levels (macro)—or lower administrative levels (micro). Recent malaria guidelines advocate for the use of routine data for monitoring and evaluation at country levels and demonstrate its utility as part of donor requests for monitoring progress29. However, at the micro-planning units, limitations of routine HF data including its availability and geographic and temporal representativeness, can limit its utility. These factors contribute to uncertainty in estimates generated from these data and has over the years hindered its direct use for decision making. For example, at the micro-levels, not all areas have HFs resulting in long commuting distance for communities to reach the nearest HF. Thus, the estimation of disease indicators for these communities is not straight forward without application of appropriate spatial modelling techniques. Routine data from communities in areas with HFs may have additional deficiencies such as reporting completeness30. Conducting disease specific micro-stratification is important for understanding heterogeneity of disease risk. The ability to stratify malaria risk at a finer level will lead to even better spatially targeted responses aligned to the HBHI concept. This becomes increasingly beneficial in areas moving towards lower transmission risk to quantify the levels of heterogeneity and support elimination efforts.
For empirical routine data to provide accurate malaria estimates, all community fever cases should ideally reach HFs, be tested and accurately captured within the DHIS224. However, this is often not the case. Routine data do not account for factors such as treatment seeking rates, health utilization behaviors, the underlying heterogeneous distribution of the population and the differing testing rates between transmission settings. All of these, can potentially under/over-estimate malaria risk16,24. In the absence of complete and perfect empirical data, statistical modelling techniques represents a practical way to close these gaps and obtain best estimates for all settings. Spatio-temporal models have been extensively used for various diseases24,31–33 and are based on the principles that data are spatially correlated and observations in adjacent areas will be more similar than observations that are farther away, thereby smoothing risk in space and time according to a neighborhood structure34. The models allow to efficiently handle incomplete or missing data, account for potential biases21,24,35 and are also useful for understanding the associated levels of uncertainty in the data.
Mainland Tanzania has formally adopted macro-stratification as part of its National Malaria Strategic Plan (NMSP) 2021–202536 aimed at providing tailored combinations of interventions according to council level epidemiological risk18,19,36. Multiple metrics have been previously used to provide a simplified risk-strata per council based on survey data from school children38 and routine data from DHIS218. To further account for the intra-council heterogeneity and support decentralized planning, the stratification was extended to the ward level to develop a micro-stratification risk map using aggregated routine data as highlighted in previously published work36,37. The routine metrics utilized in this micro-stratification approach37 included annual parasite incidence (API), test positivity rate (TPR) confirmed with malaria Rapid Diagnostic Test (mRDT), and test positivity rates from antenatal care clinics (ANC TPR). Furthermore, inclusion of data was limited to HFs with a minimum of 50% completeness of reporting. The use of empirical routine data in this micro-stratification approach however, did not adjust for the existing spatial and temporal gaps nor the related uncertainties, thereby resulting in an incomplete ward-level stratification where 5% of all the wards had no HFs and thus no stratification could be conducted here37.
Here, we used Bayesian conditional auto-regressive (CAR) spatio-temporal modelling techniques to leverage all the available routine data collected over 36 months from all reporting HFs across wards in mainland Tanzania. The aim was to improve previous micro-stratification efforts in mainland Tanzania37. In this study, we focused on the mRDT TPR, a widely used malaria metric reported by routine health systems6,39–49. Malaria TPR has been shown to be significantly associated with malaria incidence and a strong predictor of malaria transmission43–45. It offers a more consistent and acceptable case definition since it provides a clearer denominator and does not require information on HF catchment population that remains largely undefined44,50.
Results
Routine data coverage and description
A total of 7878 HFs offering laboratory services and performing testing with mRDTs were included in the analysis for the reporting period 2017–2019 (Table 1). During this period, a total of 228,717 facility monthly reports were received resulting in an overall reporting rate of 80.7% across 93.7% wards. Dispensary, laboratories and clinics represented most of all the HFs (85.7%), followed by health centers (10.8%) and hospitals (3.5%) (Supplementary Fig. S1). Of the total malaria tests performed by mRDT (n = 56,546,468) in the period of analysis, 15,454,915 (27.3%) were positive for malaria, showing a marked variation in the crude malaria TPR from 0.0 to 82.5% across all wards. The number of HFs per ward widely ranged with higher number of HFs found in urban wards compared to rural wards. A large number of wards consisted of only one (27.9%) or two (29.4%) HFs. On the whole, 6.3% of wards had no HFs or non-reporting HFs, corresponding to 4% of the total population.
Table 1.
The coverage and completeness of malaria Test Positivity Rates (TPR) across wards in mainland Tanzania from 2017 to 2019.
| # of Health Facilities Performing mRDT Testing | # of Wards | % of Wards | Population Residing (%) | Facility Reporting Rates (%) | mRDT Confirmed Malaria Cases | Total Tested with mRDT | Average TPR (%) (Min–Max) | ||
|---|---|---|---|---|---|---|---|---|---|
| Urban | Mixed | Rural | |||||||
| 0 | 208 | 35.1 | 12.0 | 52.9 | 2,094,992 (4%) | - | - | - | - |
| 1 | 924 | 12.2 | 7.7 | 80.1 | 10,887,759 (20%) | 86.1 | 2,906,562 | 9,019,681 | 32.2 (0.0–82.5) |
| 2 | 974 | 7.5 | 7.8 | 84.7 | 13,249,794 (25%) | 84.9 | 4,997,646 | 14,366,228 | 34.8 (0.0–79.9) |
| 3 | 594 | 11.8 | 14.0 | 74.2 | 9,534,633 (18%) | 83.2 | 3,379,948 | 10,604,367 | 31.9 (0.3–81.3) |
| 4 | 287 | 15.7 | 17.8 | 66.6 | 5,987,459 (11%) | 80.1 | 2,057,873 | 7,126,957 | 28.9 (0.6–71.1) |
| 5 | 155 | 28.4 | 21.9 | 49.7 | 3,730,785 (7%) | 76.7 | 1,005,588 | 4,925,657 | 20.4 (0.7–63.6) |
| 6 | 64 | 31.3 | 18.8 | 50.0 | 1,797,811 (3%) | 73.6 | 433,992 | 2,280,514 | 19.0 (0.8–69.9) |
| 7 | 40 | 65.0 | 20.0 | 15.0 | 2,094,947 (4%) | 70.2 | 165,987 | 2,206,765 | 7.5 (0.7–52.1) |
| 8 | 27 | 66.7 | 18.5 | 14.8 | 1,426,109 (3%) | 71.1 | 178,763 | 1,838,587 | 9.7 (0.6–43.3) |
| 9 | 12 | 66.7 | 25.0 | 8.3 | 614,121 (1%) | 64.1 | 163,956 | 979,849 | 16.7 (3.7–51.5) |
| 10 + | 26 | 92.3 | 7.7 | 0.0 | 2,301,807 (4%) | 65.7 | 164,600 | 3,197,863 | 5.1 (0.7–28.2) |
| 7878 | 3311 | 15.5 | 11.2 | 73.3 | 53,720,216 | 80.6 | 15,454,915 | 56,546,468 | 27.3 (0.0–82.5) |
Model selection
Assessment of the coefficients of the predictors selected from the covariates selection procedure (Supplementary Fig. S3) showed that Enhanced Vegetation Index (EVI) (Coefficient: 0.078; Standard Error: 0.002), Night Time Lights (NTL) (− 0.043; 0.002) and Temperature Suitability Index (TSI) (0.150; 0.004) were significant predictors of malaria TPR and were therefore included in the analysis.
Comparison of the Deviance Information Criteria (DIC) values between the three model specifications showed that model C had the lowest DIC value (304,069.5) when compared to model A (306,978.1) and model B (307,065.9) (Supplementary Table S1). Improving the model complexity improved the model goodness of fit and thereby Model C was selected and implemented. Model validation statistics were computed to validate the model performance and are summarized in Supplementary Table S1. The MAE of the selected Model C was computed to be 0.04 suggesting good model precision, the RMSE was 0.06 suggesting low bias and the R2 was 0.91 suggesting a good predictive performance of the model.
Table 2 presents the posterior parameters for the selected model C. EVI (Posterior mean; confidence interval—0.236; 0.231–0.241) and TSI (0.579; 0.511–0.647) were positively associated with malaria TPR indicating that vegetation index and temperatures are favorable for increasing the risk of transmission. As expected, NTL (− 0.300; − 0.371 to − 0.229) showed a negative correlation to the malaria risk implying areas in rural settings are more prone to malaria risk. All the model parameters were significant at the 95% credible interval.
Table 2.
Posterior model parameter estimates.
| Parameter | Posterior Mean (95% CI) (Log odds scale) |
|---|---|
| Intercept | –1.594 (–1.692 to –1.495) |
| EVI | 0.236 (0.231–0.241) |
| NTL | –0.300 (–0.371 to –0.229) |
| TSI | 0.579 (0.511–0.647) |
Heterogeneity of predicted malaria TPR at ward level
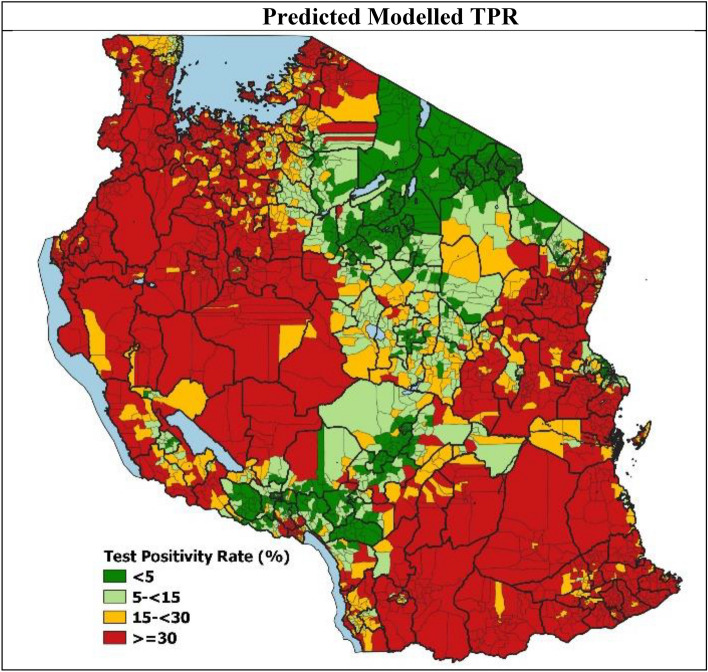
The heterogeneity in the final modelled malaria TPR risk (Fig. 1) is evident across the country with higher transmission levels seen in the North-West and South-East parts of the country, whilst lower transmission levels are seen in the central corridor running from the North-East to South-West parts of the country. At the national level, the predicted mean malaria TPR for the period of analysis was 25.6% (95% credible interval 23.9–27.6) with heterogeneity across the wards ranging from 0.2% (0.1–0.4) to 81.4% (80.9–81.9%).
Figure 1.
Predicted malaria Test Positivity Rates (TPR) in mainland Tanzania.
Following classification of the estimated malaria TPR values into risk strata using the NMCP defined thresholds (Supplementary Table S2), 1348 (40.7%) wards were assigned to high transmission risk strata, 583 (17.6%) wards to moderate transmission, 633 (19.1%) wards to low transmission, whilst 747 (22.6%) wards to the very low transmission strata. The average estimated malaria TPR distribution per risk stratum is summarized in Table 3.
Table 3.
Distribution of wards by transmission strata.
| Malaria TPR Risk Strata | # of Wards (%) | # of Population Residing (%) | Average Predicted Malaria TPR (Credible Interval %) |
|---|---|---|---|
| Very Low (< 5%) | 747 (22.6%) | 13,795,566 (25.7%) | 2.5 (1.9–3.3) |
| Low (5- < 15%) | 633 (19.1%) | 11,967,597 (22.3%) | 9.1 (8.0–10.7) |
| Moderate (15- < 30%) | 583 (17.6%) | 8,894,349 (16.6%) | 22.2 (20.5–24.5) |
| High (≥ 30%) | 1348 (40.7%) | 19,062,704 (35.5%) | 47.5 (44.9–50.4) |
| 3311 (100%) | 53,720,216 (100%) | 25.6 (23.9–27.6) |
Interpreting uncertainty in malaria TPR at the ward level
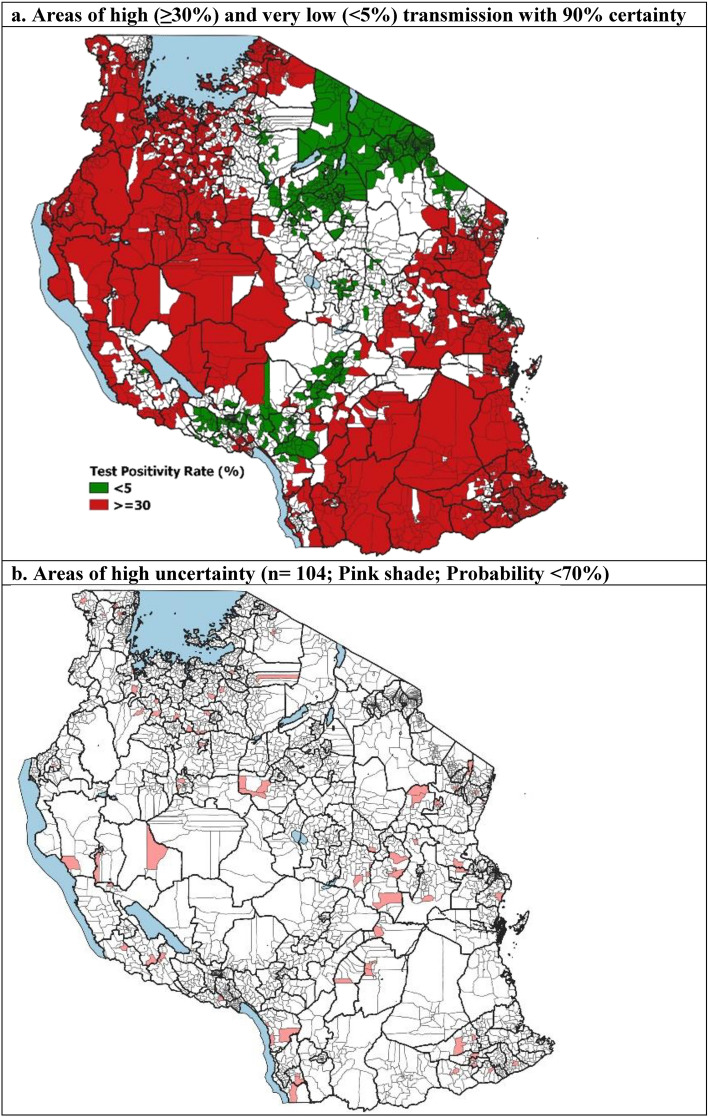
The model exceedance and non-exceedance probabilities provided some level of confidence in the assigned risk strata to allow NMCPs and the council health teams to efficiently plan targeted interventions at the micro levels. This is particularly useful in the extreme high and very low transmission risk areas where the largest transition in intervention packages from control to elimination strategies are observed36.
A malaria TPR of ≥ 30% is the threshold set by the NMCP to denote areas with high transmission and that qualify for the most intensive control interventions to reduce transmission. Approximately 17.7 million people (33%) were estimated to reside in 1227 wards with a probability of ≥ 90% which shows a high transmission risk. The majority of this population was located predominantly in the North-West and South-East of the country. Another 11.7 million people (22%) resided in 662 wards with very low transmission risk of < 5% and were found largely in the North-East councils (Fig. 2a). These indicate areas where elimination strategies such as strengthening surveillance systems should be considered36. Approximatively 1.2 million people resided in 104 wards where the assigned risk strata had large levels of uncertainty (probability < 70%) (Fig. 2b).
Figure 2.
Exceedance and non-exceedance probability of predicted malaria Test Positivity Rates (TPR).
Comparison of the risk strata estimated from the model with the empirical estimates of malaria TPR (which did not account for uncertainty) showed 7.4% of the total wards to be misclassified. Amongst these, 68 wards (2.2%) in the low strata were found to be misclassified to the very low risk strata by the empirical malaria TPR. Another 32 wards (1.0%) in the high risk strata were found to be misclassified to the moderate risk strata. These represent areas where the largest impact of misclassification would likely be observed due to the significant differences in the intervention strategies in these strata.
Discussion
In this work, a Bayesian spatio-temporal modelling framework was used to leverage routine information from HFs and provide robust estimates of malaria risk at ward level. The model allowed to smoothen the risk and fill the spatial and temporal gaps in routine data, handle the associated uncertainty in a robust manner and account for any spatial and temporal dependencies in the data. The analysis highlighted the sub-council level spatial heterogeneities in malaria TPR with higher transmission particularly seen in the North-West and South-East parts of the country. These areas have traditionally been shown to have similar patterns of higher prevalence18,38,51–53. Factors potentially contributing to resilience in changes to the risk could be due to the geographic location, climatic factors and socio-economic factors amongst many.
As countries begin to transition towards lower malaria transmission, the need to monitor the increasing heterogeneities at finer scales and inform appropriate tailored strategies becomes critical. HF data represents an essential source of local information describing the dynamics of the malaria situation with a high level of resolution in time and space. Understanding their structure and representativeness can be useful to replace modelled prevalence estimates derived from sparse cross-sectional surveys that is widely considered as the current gold standard. Nevertheless, at the local administrative levels, incomplete HF reporting or non-reporting HFs create varying degrees of spatial and temporal data gaps. Moreover, as observed in this analysis, about 57.3% wards had only 1 or 2 reporting HFs, thereby contributing to a higher level of uncertainty.
The modelling framework used here allowed for a more robust estimation of malaria TPRs by borrowing information from neighboring wards, rather than relying only on limited information from one single ward. In addition to adjusting for the missing information, the approach provides measures of uncertainty that are required to make relevant policy decisions. Previous work done in mainland Tanzania36,37 used combinations of empirical routine data to develop a micro-stratification risk map, but that approach did not consider the uncertainty in transmission risk for the population at risk. This is important to allow NMCPs to understand the fidelity of estimates, understand progress made towards achieved targets and more confidently transition malaria strategies. The current paper builds on this by providing a more robust estimate of risk. By presenting the risk in terms of exceedance and non-exceedance probabilities, the developed model allows programs to also identify areas with high uncertainty in their assigned risk (Probability < 70%). These areas are likely within wards in which there is a natural level of heterogeneity such as major altitudinal changes, natural swamps or man-made agricultural areas. Importantly, these would need to be differentiated from wards with poor HF reporting performances, or those with small numbers of patients tested at a HF resulting in larger uncertainty in actual estimates.
The current approach taken in this paper may be applied to other sub-Saharan African (SSA) countries that are facing challenges with incomplete and missing routine information at the higher spatial scales. In such places, particularly those moving towards lower transmission of risk, the use of real-time routine information becomes important to allow continuous analysis of the existing local heterogeneity. Using statistical models can be valuable to address some of these existing data issues. Nevertheless, continued efforts to strengthen routine surveillance systems must remain a country priority to help guide local evidence-based planning and implementation.
This study has some limitations. The approach uses routine data that are only representative of the population who seeks care and are laboratory tested. It therefore does not capture the variations in testing rates, infections within the communities that do not reach the facility, or those that are asymptomatic. The unavailability of treatment seeking information at ward level limited the analysis to account for this important factor. Using a combination of metrics from both routine and survey sources could further improve the estimates. Future work may look into leveraging information from both sources to better understand the relationship between the data sources and how well they reflect the different components of the transmission system. Establishing this relationship would also be important to better develop thresholds used for defining risk categories. To date, cut-offs used for defining malaria risk are mainly based on pragmatic, plausible criteria but not linked to likely biological/ epidemiological impacts of specific interventions. There is also a need to consider other layers of malaria-related information to further increase the value of malaria TPR for decision making and provide a more holistic approach to inform malaria policies sub-nationally.
The CAR modelling approach used aggregated estimates per ward and thereby assumed the ward administrative boundaries to represent the catchment population for HFs within wards. This can have several implications. Firstly, it did not account for differing facility utilization behaviors and population movements across neighboring ward borders. Many factors can drive patient choices such as the size of HFs, distance, perceptions and costs24. Using geo-statistical methods to account for the geo-spatial location of HFs as well as incorporating information on behaviors driving facility usage can better inform the risk estimates. Secondly, the use of aggregated data can mask underlying data quality issues thereby limiting the understanding of the true nature of data54,55. Finally, the use of aggregated data poses the challenge of the modifiable areal unit problem (MAUP) which is a common geographical statistical problem. This occurs when results are affected by variability introduced through aggregating data or due to changes in the polygon shape used in the analysis56. In this work, data were aggregated to the ward level for providing estimates at a resolution that is programmatically meaningful for micro-stratification.
The use of the complex analytical methodologies for dealing with incomplete data demands analytical skills largely beyond the capacity of most NMCPs. Hence, it is important that such methods remain within local research institutions with the required know-how for annual monitoring. Increased usage of maps for local decision making by NMCPs was recently shown to be associated with factors such as knowledge and understanding of the data sources and their limitations, and also trust and perceived ownership of the data28. Therefore, capacitating NMCPs to establish a high-quality surveillance system and to interpret the data after an appropriate analytical process represents a sustainable way of promoting data use for decision making24.
Conclusion
This work demonstrated the potential of routine HF data to identify different epidemiological strata and thereby providing the malaria program with an evidence base to guide malaria interventions at micro-planning units in Tanzania. These data, however, are imperfect in many settings in Africa and often require application of geo-spatial modelling techniques for estimation. These techniques allow for filling the existing spatial and temporal data gaps, accounting for statistical uncertainty, and leveraging this rich source of information for optimizing micro-planning of interventions.
Methods
Geographical scope and context
Mainland Tanzania is organized into multiple administrative levels. The country has 26 administrative regions, divided into 184 councils. The councils represent the main administrative level responsible for resource allocation and tailoring interventions as per the national guidelines. Councils are further divided into wards, which serve as the lowest resources allocation and disease reporting unit. A total of 3311 wards have been defined according to the 2012 national census for mainland Tanzania (Supplementary Fig. S6). There is a range from 2 to 43 wards per council, depending on the size of the council, altitudinal variation and population density.
Routine health facility data processing
Data from 7878 (93%) reporting HFs across 3103 (93.7%) wards in mainland Tanzania were used to assemble malaria TPR data (Supplementary Fig. S1). The remaining wards (6.3%) did not have reporting HFs. Aggregated routine data (see data aggregation description below) from the laboratory register representing all ages were obtained from HMIS/DHIS2 for 36 months (2017–2019). DHIS2 is an open source, web-based software platform for reporting, analysis, and dissemination of health data. It captures information from both the private (26%) and public (74%) HFs, and can be accessed by officials working in the health sector through registered credentials. Each month, HFs provide paper-based monthly summary reports with data that are entered into DHIS2.
Monthly laboratory testing reporting tools were introduced in HFs in October 2015 to capture: (1) the total number of malaria tests performed by blood slides and mRDT across all age groups, and (2) the number of positive malaria cases. The reporting rates have gradually improved from 49.6% in 2016 to 87.7% in 2019. mRDTs were introduced in mainland Tanzania in 2009 in several rolled-out phases before country wide scale up was achieved in 201357. Currently, mRDTs are the most widely-used diagnostic method for malaria (88% of total tests performed), with only a small proportion of facilities, mainly private facilities, still using microscopy.
The indicators extracted were used to compute the mRDT TPR, defined as the proportion of the number of malaria laboratory confirmed cases (numerator) amongst the total number of mRDTs performed (denominator)..
Data cleaning and geocoding
In this analysis, the HMIS data consisted of monthly laboratory reports of all patients tested with mRDT and reported by all public and private HFs with available geo-coordinates. These facilities represented 92.7% (N = 7878) of all HFs offering laboratory testing and those captured in the DHIS2. The remaining 7.3% HFs did not submit any monthly laboratory reports across the entire period of analysis and were therefore excluded. No information was available on whether they simply did not report, or whether they did not test. In Tanzania, only HFs offering laboratory testing services are expected to submit the monthly laboratory reports. However, this information is not clearly demarcated in the current master HF list and therefore understanding the exact proportion of HFs that were missing in the DHIS2 was not possible.
All reports were first checked for duplicate submissions for the same month by the same HF and duplicates were removed. As the DHIS2 database in Tanzania is unable to record zero values, these are marked blank. Hence, to distinguish zero values from missing values, it was assumed that missing values of otherwise complete reports were true zeros. To ensure the correct allocation of HFs to their respective wards, the geographical coordinates of the reporting HFs were obtained from the master registry HF list of Tanzania58 and linked to the DHIS2 data using the unique HF identifier code. The national ward shapefile was then used to allocate the HFs to their respective wards (Supplementary Fig. S1).
Data aggregation and classification
The HMIS monthly data were aggregated for the whole year in order to align with the NMSP development which has cycles of three years, and we therefore provided average risk estimates for the period 2017–2019. This resulted in a total of 9214 space–time data points that were included in the analysis.
The classification of routine metrics into malaria risk categories has been previously defined in the country using prevalence survey data from school children as a gold standard. This classification was guided by a set of criteria ensuring the minimization of misallocation of councils belonging to the higher strata to the lower strata, which would have led to the largest changes in the optimal intervention packages36 (Supplementary Table S2). We classified the estimated malaria TPR values into risk strata using the national criteria of risk as follows: < 5% as very low transmission; 5– < 15% as low transmission, 15– < 30% as moderate transmission and ≥ 30% as high transmission (Supplementary Table S2).
Environmental and ecological covariates
A set of biologically plausible covariates known to affect malaria risks were considered for the geo-spatial modelling34,59. The data were extracted from open source remote sensing platforms. The covariates included precipitation60, EVI61, TSI62, NTL63 water vapor64 and the average HF reporting rates within a ward (Supplementary Text S1). The covariates were standardized using the observed mean and standard deviation.
A covariate selection procedure was performed in order to select a parsimonious minimal set of covariates59,65. The malaria TPR data series were matched to the covariates and a non-spatial generalized linear regression model was applied using the bestglm package in R66. This approach selected the best combination of the covariates based on the lowest value of the Bayesian Information Criteria (BIC). TSI, NTL and EVI were among the selected covariates as predictors (Supplementary Text S1).
Model specification
A Bayesian Besag-York-Mollié 2 Model (BYM2)67 was used to model the spatial and temporal distribution of malaria TPR at the ward level adjusting for the selected covariates. The model combined the data and prior knowledge to produce posterior probability distributions and predict smoothed malaria TPR estimates thereby filling the missing values for wards with no facility data. The model was used to estimate malaria TPR at the administrative level of the ward and accounted for prediction uncertainty across wards with incomplete data or no reporting facilities (Supplementary Text S2).
Let represent total number of positive malaria cases at the ward , in year , and the total people tested for malaria at ward j in year k. The malaria test positivity rate (TPR) given the selected covariates was modelled using a binomial likelihood:
where the link with the chosen environmental and ecological covariates is made through a regression model based on a linear predictor defined as:
with the intercept, is a set of selected covariates; are the corresponding regression parameters; corresponds to the CAR structured spatial random effect that smoothens the data according to a neighbourhood structure. The CAR model was applied to a symmetric spatial neighborhood matrix structure , developed at the ward level. defines a neighborhood structure across all the wards of the country (Supplementary Fig. S4), where each element connects the wards and , i.e., = 1 if wards share a common boundary and = 0 otherwise; corresponds to the unstructured exchangeable component using independent and identically distributed (i.i.d) random effect and is the temporal random effect specified using i.i.d zero-mean normally distributed random effect.
In order to test the goodness of fit, CAR models with different specifications of the spatio-temporal structures were implemented (Supplementary Table S1). Model A did not have a spatial random effect component, model B had a spatial random effect component and model C was run with a spatial and temporal random effect structure (Supplementary Table S1). The model goodness of fit was evaluated using the DIC and the best model was selected and used for subsequent analyses. The model was estimated using Integrated Nested Laplace Approximation (INLA)68–70 (Supplementary Text S2).
Exceedance probability (EP) and non-exceedance probabilities (NEP) calculated using the fitted spatio-temporal model (Supplementary Text S2) were used to quantify the likelihood of the malaria TPR estimates to be above the high (≥ 30%) or below the very low (< 5%) malaria risk thresholds. These thresholds represent the pre-defined, policy-relevant thresholds defined by the NMCP in Tanzania. Estimates obtained from the resulting model are only programmatically useful when NMCPs are able to interpret it with its underlying level of uncertainty6,71.
Model validation
To evaluate the predictive performance of the model, a subset of 10% of the dataset was held out randomly. The predictive performance of the model was estimated by computing validation statistics on the hold out data set. The mean absolute error (MAE) was computed as a measure of the absolute differences between the observed and predicted values. The root mean square error (RMSE) was computed to provide a measure of the accuracy of the individual predictions whilst the R-squared (R2) was computed to provide a measure of the proportion of variation accounted for by the model (Supplementary information Text S2).
Estimating population at risk by strata
The population for each ward was obtained from the publicly available 2012 population and housing census in Tanzania conducted by the National Bureau of Statistics72. Annual growth rates at the council level73 were applied to the ward population data to project each ward population to the period of analysis (2017–2019). These were then used to estimate the total populations residing in each of the identified malaria risk strata.
R Studio74 was used for performing analysis of the data downloaded from DHIS2. All maps were produced using the QGIS software version 3.4.1475.
Ethics approval and consent to participate
This work utilizes secondary aggregated data for analysis for which no ethics approval was required.
Supplementary Information
Acknowledgements
The authors would like to thank all the members of the NMCP of the Ministry of Health of mainland Tanzania, President's Office Regional Administration and Local Government offices, WHO country office and development partners for their participation and invaluable discussions to make this work possible. The authors also wish to thank Eda Mumo, Noel Joseph and Samuel Muchiri from the KEMRI-Welcome Trust Research Programme for providing technical input and support to make this work possible.
Abbreviations
- ANC
Antenatal care
- API
Annual parasite incidence
- BYM2
Besag-York-Mollié 2
- CAR
Conditional autoregressive
- DHIS2
District Health Information Software
- EP
Exceedance probability
- EVI
Enhanced Vegetation Index
- HBHI
High burden to high impact
- HF
Health facility
- HMIS
Health Management Information System
- INLA
Integrated Nested Laplace Approximation
- MAE
Mean absolute error
- MoH
Ministry of Health
- mRDT
Malaria rapid diagnostic testing
- NBS
National Bureau of Statistics
- NEP
Non-exceedance probability
- NMCP
National Malaria Control Programme
- NMSP
National Malaria Strategic Plan
- NTL
Night time lights
- RMSE
Root mean square error
- R2
R-squared
- TPR
Test positivity rate
- TSI
Temperature suitability index
- WHO
World Health Organization
Author contributions
S.G.T., V.A. and R.W.S. conceptualized the methodological analysis. S.G.T., F.C. and K.M. acquired the data. S.G.T. compiled the data and performed the analysis. V.A., R.W.S., E.P. and M.G. provided input on the interpretation of the analysis. S.G.T. with guidance from R.W.S., V.A., E.P., C.L. and M.G. prepared the initial draft manuscript and its finalization. V.A., R.W.S., E.P., M.G., C.L., F.M., F.C., K.M., S.A. and S.L. provided critical comments on progressive drafts. All authors reviewed and approved of the final manuscript.
Funding
Funding for SGT and FM was received from the Swiss Tropical and Public Health Institute—Towards Elimination of Malaria in Tanzania (TEMT) Project funded by the Embassy of Switzerland in Tanzania. EP is funded from the Global Fund to Fight Aids, Tuberculosis and Malaria (GFATM) and the Swiss Tropical and Public Health Institute. RWS is supported as a Wellcome Trust Principal Fellow (#212176), that also provides support to VAA. VAA was supported by a Wellcome Trust Training Fellow (# 211208). RWS and VAA are also grateful to the support of the Wellcome Trust to the Kenya Major Overseas Programme (# 203077).
Data availability
Data from routine HMIS/DHIS2 are not publicly available and were obtained by request from the NMCP of mainland Tanzania. Restrictions apply to the availability of these data and permission can be obtained with reasonable request from the Ministry of Health of mainland Tanzania.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-37669-x.
References
- 1.WHO, RBM . High Burden to High Impact: A Targeted Malaria Response. Berlin: WHO and RBM Partnership to End Malaria; 2018. [Google Scholar]
- 2.Rouamba T, Samadoulougou S, Tinto H, Alegana VA, Kirakoya-Samadoulougou F. Bayesian spatiotemporal modeling of routinely collected data to assess the effect of health programs in malaria incidence during pregnancy in Burkina Faso. Sci. Rep. 2020;10:2618. doi: 10.1038/s41598-020-58899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kifle MM, et al. Malaria risk stratification and modeling the effect of rainfall on malaria incidence in Eritrea. J. Environ. Public Health. 2019;2019:1–11. doi: 10.1155/2019/7314129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awine T, Silal SP. Accounting for regional transmission variability and the impact of malaria control interventions in Ghana: A population level mathematical modelling approach. Malar. J. 2020;19:423. doi: 10.1186/s12936-020-03496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awine T, Malm K, Peprah NY, Silal SP. Spatio-temporal heterogeneity of malaria morbidity in Ghana: Analysis of routine health facility data. PLoS ONE. 2018;13:e0191707. doi: 10.1371/journal.pone.0191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alegana VA, Suiyanka L, Macharia PM, Ikahu-Muchangi G, Snow RW. Malaria micro-stratification using routine surveillance data in Western Kenya. Malar. J. 2021;20:22. doi: 10.1186/s12936-020-03529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gething PW, et al. Information for decision making from imperfect national data: Tracking major changes in health care use in Kenya using geostatistics. BMC Med. 2007;5:37. doi: 10.1186/1741-7015-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen M, et al. Mapping malaria seasonality in Madagascar using health facility data. BMC Med. 2020;18(1):1486. doi: 10.1186/s12916-019-1486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ihantamalala FA, et al. Spatial and temporal dynamics of malaria in Madagascar. Malar. J. 2018;17:58. doi: 10.1186/s12936-018-2206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arambepola R, et al. Spatiotemporal mapping of malaria prevalence in Madagascar using routine surveillance and health survey data. Sci. Rep. 2020;10:18129. doi: 10.1038/s41598-020-75189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chirombo J, et al. Childhood malaria case incidence in Malawi between 2004 and 2017: Spatio-temporal modelling of climate and non-climate factors. Malar. J. 2020;19:5. doi: 10.1186/s12936-019-3097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cissoko M, et al. Stratification at the health district level for targeting malaria control interventions in Mali. Sci. Rep. 2022;12:8271. doi: 10.1038/s41598-022-11974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alegana V, et al. Estimation of malaria incidence in northern Namibia in 2009 using Bayesian conditional-autoregressive spatial-temporal models. Spat. Spatiotemp. Epidemiol. 2016;7:25–36. doi: 10.1016/j.sste.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alegana VA, et al. Advances in mapping malaria for elimination: Fine resolution modelling of Plasmodium falciparum incidence. Sci. Rep. 2016;6:29628. doi: 10.1038/srep29628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semakula M, Niragire F, Faes C. Bayesian spatio-temporal modeling of malaria risk in Rwanda. PLoS ONE. 2020;15:e0238504. doi: 10.1371/journal.pone.0238504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maïga A, et al. Generating statistics from health facility data: The state of routine health information systems in Eastern and Southern Africa. BMJ Glob. Health. 2019;4:e001849. doi: 10.1136/bmjgh-2019-001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sturrock HJ, et al. Fine-scale malaria risk mapping from routine aggregated case data. Malar. J. 2014;13:421. doi: 10.1186/1475-2875-13-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thawer SG, et al. Sub-national stratification of malaria risk in mainland Tanzania: A simplified assembly of survey and routine data. Malar. J. 2020;19:177. doi: 10.1186/s12936-020-03250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Runge M, et al. Simulating the council-specific impact of anti-malaria interventions: A tool to support malaria strategic planning in Tanzania. PLoS ONE. 2020;15:e0228469. doi: 10.1371/journal.pone.0228469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kigozi SP, et al. Spatial-temporal patterns of malaria incidence in Uganda using HMIS data from 2015 to 2019. BMC Public Health. 2020;20:1913. doi: 10.1186/s12889-020-10007-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett A, et al. A methodological framework for the improved use of routine health system data to evaluate national malaria control programs: Evidence from Zambia. Popul. Health Metr. 2014;12:30. doi: 10.1186/s12963-014-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lubinda J, et al. Spatio-temporal monitoring of health facility-level malaria trends in Zambia and adaptive scaling for operational intervention. Commun. Med. 2022;2:79. doi: 10.1038/s43856-022-00144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwitira I, et al. Spatial and spatio-temporal analysis of malaria cases in Zimbabwe. Infect. Dis. Poverty. 2020;9:146. doi: 10.1186/s40249-020-00764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alegana VA, Okiro EA, Snow RW. Routine data for malaria morbidity estimation in Africa: challenges and prospects. BMC Med. 2020;18:121. doi: 10.1186/s12916-020-01593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tusting LS, Bousema T, Smith DL, Drakeley C. Measuring changes in Plasmodium falciparum transmission: Precision, accuracy and costs of metrics. Adv. Parasitol. 2014;84:151–208. doi: 10.1016/B978-0-12-800099-1.00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO . T3: Test. Treat. Track. Scaling Up Diagnostic Testing, Treatment and Surveillance for Malaria. World Health Organization; 2012. [Google Scholar]
- 27.Ghilardi L, et al. How useful are malaria risk maps at the country level? Perceptions of decision-makers in Kenya, Malawi and the Democratic Republic of Congo. Malar. J. 2020;19:353. doi: 10.1186/s12936-020-03425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omumbo JA, Noor AM, Fall IS, Snow RW. How well are malaria maps used to design and finance malaria control in Africa? PLoS ONE. 2013;8:e53198. doi: 10.1371/journal.pone.0053198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO . WHO Technical Brief for Countries Preparing Malaria Funding Requests for the Global Fund (2020–2022) World Health Organization; 2020. [Google Scholar]
- 30.Rowe AK, et al. Caution is required when using health facility-based data to evaluate the health impact of malaria control efforts in Africa. Malar. J. 2009;8:209. doi: 10.1186/1475-2875-8-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott P, Wartenberg D. Spatial epidemiology: Current approaches and future challenges. Environ. Health Perspect. 2004;112:998–1006. doi: 10.1289/ehp.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iddrisu A-K, Alhassan A, Amidu N. Investigating spatio-temporal pattern of relative risk of tuberculosis in Kenya using Bayesian hierarchical approaches. J. Tuberc. Res. 2018;6:2. doi: 10.4236/jtr.2018.62017. [DOI] [Google Scholar]
- 33.Obaromi D, Ndege J, Yongsong Q. Disease mapping of tuberculosis prevalence in Eastern Cape Province, South Africa. J. Public Health. 2019;27:241–248. doi: 10.1007/s10389-018-0931-7. [DOI] [Google Scholar]
- 34.Odhiambo JN, Kalinda C, Macharia PM, Snow RW, Sartorius B. Spatial and spatio-temporal methods for mapping malaria risk: A systematic review. BMJ Glob. Health. 2020;5:e002919. doi: 10.1136/bmjgh-2020-002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sturrock HJW, et al. Mapping malaria risk in low transmission settings: challenges and opportunities. Trends Parasitol. 2016;32:635–645. doi: 10.1016/j.pt.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 36.National Malaria Control Programme (NMCP), Tanzania. Malaria Strategic Plan 2021–2025 (Ministry of Health Tanzania, 2021).
- 37.Thawer SG, et al. The use of routine health facility data for micro-stratification of malaria risk in mainland Tanzania. Malar. J. 2022;21:345. doi: 10.1186/s12936-022-04364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chacky F, et al. Nationwide school malaria parasitaemia survey in public primary schools, the United Republic of Tanzania. Malar. J. 2018;17:452. doi: 10.1186/s12936-018-2601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bi Y, et al. Can slide positivity rates predict malaria transmission? Malar. J. 2012;11:117. doi: 10.1186/1475-2875-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Githinji S, et al. A national health facility survey of malaria infection among febrile patients in Kenya, 2014. Malar. J. 2016;15:591. doi: 10.1186/s12936-016-1638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceesay SJ, et al. Changes in malaria indices between 1999 and 2007 in The Gambia: A retrospective analysis. Lancet. 2008;372:1545–1554. doi: 10.1016/S0140-6736(08)61654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kigozi SP, et al. Rapid shifts in the age-specific burden of malaria following successful control interventions in four regions of Uganda. Malar. J. 2020;19:128. doi: 10.1186/s12936-020-03196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kigozi SP. Malaria burden through routine reporting: Relationship between incidence and test positivity rates. Online J. Public Health Inform. 2019;11:1. doi: 10.5210/ojphi.v11i1.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen TP, et al. Use of the slide positivity rate to estimate changes in malaria incidence in a cohort of Ugandan children. Malar. J. 2009;8:213. doi: 10.1186/1475-2875-8-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyce RM, et al. Practical implications of the non-linear relationship between the test positivity rate and malaria incidence. PLoS ONE. 2016;11:e0152410. doi: 10.1371/journal.pone.0152410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamau A, et al. The relationship between facility-based malaria test positivity rate and community-based parasite prevalence. PLoS ONE. 2020;15:e0240058. doi: 10.1371/journal.pone.0240058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joshi PL, Chandra R, Bhattacharya M, Vaish HC. Validity of using slide positivity rate (SPR) in identification of high risk malarious segments in rural areas. J. Commun. Dis. 1997;29:41–45. [PubMed] [Google Scholar]
- 48.Yenew, C., Mulatu, S., & Alamneh, A. The trend of malaria cases, positivity rate, and determinant factors in the Amhara Regional State, Ethiopia: A mixed method. Case Rep. Infect. Dis. (2021). [DOI] [PMC free article] [PubMed]
- 49.Francis D, et al. Health facility-based malaria surveillance: The effects of age, area of residence and diagnostics on test positivity rates. Malar. J. 2012;11:229. doi: 10.1186/1475-2875-11-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macharia PM, Ray N, Giorgi E, Okiro EA, Snow RW. Defining service catchment areas in low-resource settings. BMJ Glob. Health. 2021;6:e006381. doi: 10.1136/bmjgh-2021-006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alegana V, et al. Plasmodium falciparum parasite prevalence in East Africa: Updating data for malaria stratification. PLOS Glob. Public Health. 2021;1:12. doi: 10.1371/journal.pgph.0000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitojo C, et al. Estimating malaria burden among pregnant women using data from antenatal care centres in Tanzania: A population-based study. Lancet Glob. Health. 2019;7:12. doi: 10.1016/S2214-109X(19)30405-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brunner NC, et al. The potential of pregnant women as a sentinel population for malaria surveillance. Malar. J. 2019;18:370. doi: 10.1186/s12936-019-2999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chilundo B, Sundby J, Aanestad M. Analysing the quality of routine malaria data in Mozambique. Malar. J. 2004;3:3. doi: 10.1186/1475-2875-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okello G, Molyneux S, Zakayo S, Gerrets R, Jones C. Producing routine malaria data: An exploration of the micro-practices and processes shaping routine malaria data quality in frontline health facilities in Kenya. Malar. J. 2019;18:420. doi: 10.1186/s12936-019-3061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Openshaw S. The Modifiable Areal Unit Problem. Geo Books; 1984. [Google Scholar]
- 57.Masanja IM, et al. Increased use of malaria rapid diagnostic tests improves targeting of anti-malarial treatment in rural Tanzania: Implications for nationwide rollout of malaria rapid diagnostic tests. Malar. J. 2012;11:221. doi: 10.1186/1475-2875-11-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.http://hfrportal.moh.go.tz/.
- 59.Weiss DJ, et al. Re-examining environmental correlates of Plasmodium falciparum malaria endemicity: A data-intensive variable selection approach. Malar. J. 2015;14:68. doi: 10.1186/s12936-015-0574-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.https://data.chc.ucsb.edu/products/CHIRPS-2.0/.
- 61.http://modis.gsfc.nasa.gov/data/.
- 62.Gething PW, et al. Modelling the global constraints of temperature on transmission of Plasmodium falciparum and P. vivax. Parasites Vectors. 2011;4:92. doi: 10.1186/1756-3305-4-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.https://neo.gsfc.nasa.gov/.
- 64.https://www.nasa.gov/nex/data.
- 65.Giorgi E, et al. Model building and assessment of the impact of covariates for disease prevalence mapping in low-resource settings: To explain and to predict. J. R. Soc Interface. 2021;18:20210104. doi: 10.1098/rsif.2021.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McLeod A, Xu C. bestglm: Best subset GLM. R-package: CRAN (2010).
- 67.Besag J, York J, Mollié A. Bayesian image restoration, with two applications in spatial statistics. Ann. Inst. Stat. Math. 1991;43:1–20. doi: 10.1007/BF00116466. [DOI] [Google Scholar]
- 68.Martins TG, Simpson D, Lindgren F, Rue H. Bayesian computing with INLA: New features. Comp. Stat. Data Anal. 2013;67:68–83. doi: 10.1016/j.csda.2013.04.014. [DOI] [Google Scholar]
- 69.Blangiardo M, Cameletti M, Baio G, Rue H. Spatial and spatio-temporal models with R-INLA. Spat Spatiotemporal Epidemiol. 2013;7:39–55. doi: 10.1016/j.sste.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J. R. Stat. Soc. Ser. B. 2009;71:319–392. doi: 10.1111/j.1467-9868.2008.00700.x. [DOI] [Google Scholar]
- 71.Giorgi E, et al. Using non-exceedance probabilities of policy-relevant malaria prevalence thresholds to identify areas of low transmission in Somalia. Malar. J. 2018;17:88. doi: 10.1186/s12936-018-2238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.National Bureau of Statistics (Tanzania), Tanzania, Office of Chief Government Statistician (Zanzibar). 2012 Population and Housing Census. (2013).
- 73.National Bureau of Statistics (Tanzania), Tanzania, Office of Chief Government Statistician (Zanzibar). 2012 Population and Housing Census. Basic Demographic and Socio-Economic Profile (2016).
- 74.Team Rs. RStudio: Integrated Development for R (RStudio, PBC, 2020). http://www.rstudio.com/
- 75.Team QGISD. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from routine HMIS/DHIS2 are not publicly available and were obtained by request from the NMCP of mainland Tanzania. Restrictions apply to the availability of these data and permission can be obtained with reasonable request from the Ministry of Health of mainland Tanzania.