Abstract
Key Clinical Message
When managing patients with differentiated thyroid cancers (DTC) and lytic bone lesions, physicians should consider etiologies other than DTC bony metastases when there is no biochemical and functional radiographic evidence of extensive DTC burden.
Abstract
Systemic mastocytosis (SM) is a clonal expansion of mast cells associated with an increased risk of solid malignancies. There is no known association between systemic mastocytosis and thyroid cancer. We report a young woman who presented with cervical lymphadenopathy, palpable thyroid nodule, and lytic bone lesions who was diagnosed with papillary thyroid cancer (PTC). The patient's post‐surgical thyroglobulin was lower than expected for metastatic thyroid cancer, and the lytic bone lesions did not demonstrate uptake of I123. Upon further evaluation, the patient was found to have SM. We report a case of co‐occurrence of PTC and SM.
Keywords: mastocytosis, simultaneous diagnosis, thyroid cancer
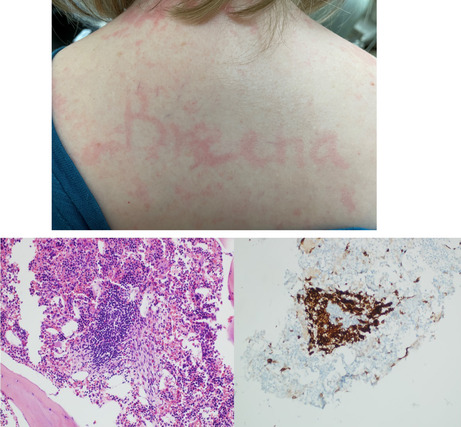
Dermographism was demonstrated when writing on the skin. Histology of the pelvic lytic lesions showed atypical appearing mast cells consistent with systemic mastocytosis.
1. INTRODUCTION
Papillary thyroid cancer (PTC) accounts for 80%–85% of differentiated thyroid cancer (DTC) and carries a favorable 10‐year survival rate of over 95%. 1 , 2 DTC is found to metastasize to the bone in about 4% of cases and historically lowers the 10‐year survival rate to ~40%. 3 Osseus metastases from thyroid cancer are more common with follicular or medullary thyroid cancer, whereas PTC represents 1.4%–7% of all osseus metastases from thyroid cancer. 3 Systemic mastocytosis (SM), a heterogenous disease caused by malignant proliferation of mast cells, will involve large osteolytic lesions in the axial skeleton in 50%–70% of cases. 4 , 5 , 6 Interestingly, SM is associated with an increased risk for solid cancers, particularly melanoma and non‐melanoma skin cancers. 5 There is no established increased risk of thyroid cancer in patients with SM.
2. CASE REPORT
A 43‐year‐old woman with no significant past medical history presented with cervical lymphadenopathy for 3 weeks. She denied any fevers, night sweats, flushing, or skin rash. CT scan of the neck showed multiple bilateral enlarged cervical lymph nodes, the largest being 3.3 cm, with bilateral thyroid nodules with calcifications. Imaging also revealed numerous lytic lesions in her spine and pelvis (Figure 1). On physical examination, she had palpable cervical lymph nodes with a hard left‐sided palpable thyroid nodule. She had tenderness to palpation on the thoracic spine. There was no skin rash, but dermographism was demonstrated (Figure 2). Initial laboratory tests were unremarkable, including thyroid function tests, serum calcium of 9.8 mg/dL (ref 8.6–10.2), and normal alkaline phosphatase of 71 U/L (ref 35–104).
FIGURE 1.
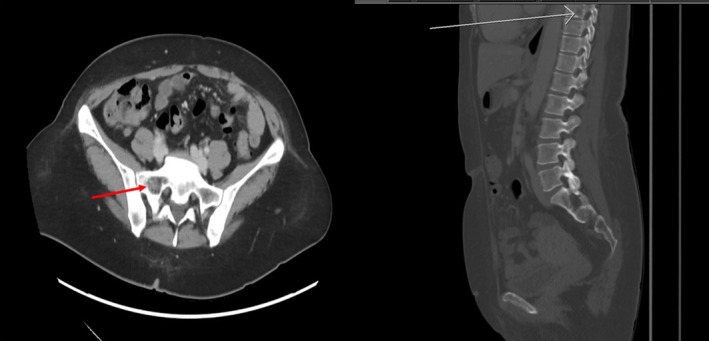
Computer tomography scan showing enlarged cervical lymphadenopathy bilaterally with the largest being 3.3 cm with bilateral thyroid nodules with calcifications present. In addition, there are multiple lytic lesions present in the spine and pelvis.
FIGURE 2.
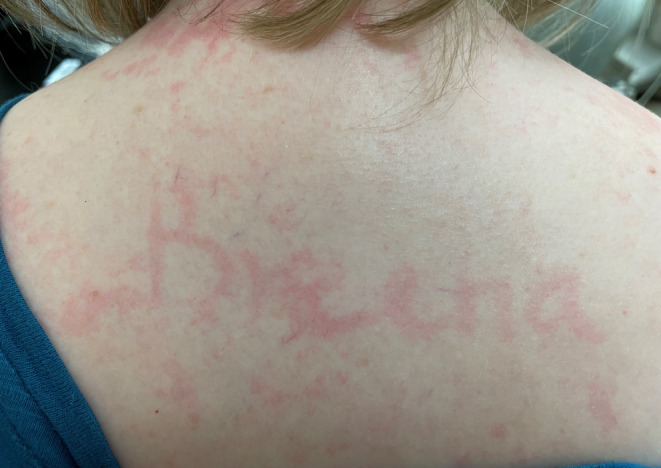
Dermographism was demonstrated when writing on the skin.
Neck ultrasound revealed a dominant 2.1‐cm left hypoechoic thyroid nodule with microcalcifications and cervical lymph nodes. Fine‐needle aspiration of the thyroid nodule and lymph nodes each confirmed PTC. Patient underwent total thyroidectomy with bilateral central and lateral neck dissection. The specimen demonstrated classic PTC—multiple foci with the largest 4.8 cm in diameter, extensive angioinvasion, and 27 out of 46 cervical lymph nodes positive for PTC (pT3N1bM0). Histology showed papillary architecture with high nuclear to cytoplasm ratio, nuclear overlap, nuclear grooves, and chromatin clearing (Figure 3). Post‐surgical serum thyroglobulin was 17.0 ng/mL with undetectable thyroglobulin antibodies. Surprisingly, I123 whole body scan via recombinant human thyrotropin (rhTSH) stimulation showed no metabolic activity in axial skeleton (Figure 4). The stimulated thyroglobulin level peaked at 22.30 ng/mL.
FIGURE 3.
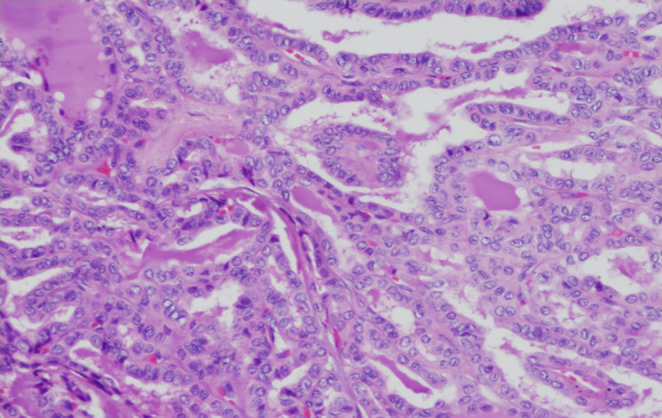
Histology of the resected thyroid gland showing papillary architecture with high nuclear to cytoplasm ratio, nuclear overlap, nuclear grooves, and chromatin clearing.
FIGURE 4.
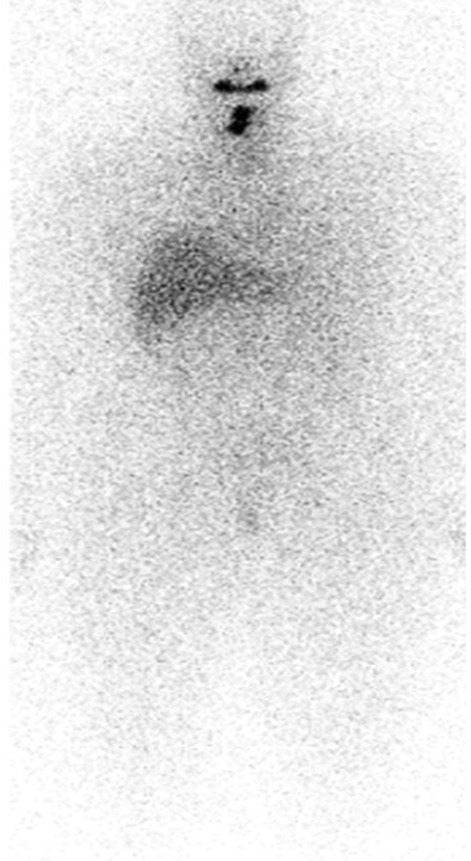
I‐123 whole body scan showing no I‐123 avid distant metastasis.
To evaluate her numerous osteolytic lesions, CT‐guided bone marrow biopsy of the pelvic lytic lesion was performed. The biopsy confirmed systemic mastocytosis with dense aggregates of >25 spindled and atypical mast cells, expression of CD117 and CD25, and KITD816V mutation (Figure 5). Serum tryptase was 52.0 mcg/L (normal 2.2–13.2). She did not have a hematologic neoplasm associated with SM. Baseline DXA showed normal bone density for age (Z‐score‐1.3 in spine).
FIGURE 5.
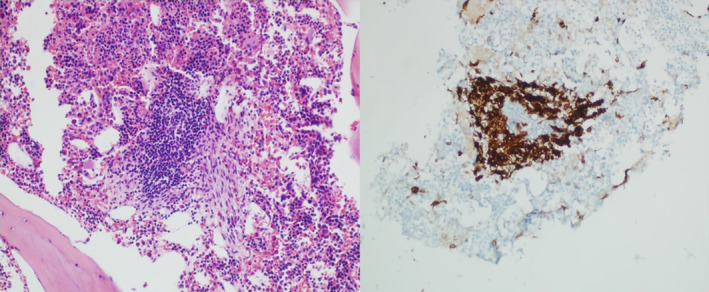
CT‐guided biopsy of the pelvic lytic lesions showing >25 atypical appearing mast cells that were immunoreactive to CD117, CD25, and tryptase consistent with a diagnosis of systemic mastocytosis.
Regarding PTC treatment, patient underwent I131 radionuclide ablation with 186.1 mCi, achieving excellent response to therapy at 12 months. Her osteolytic lesions are treated with zoledronic acid 4 mg IV every 3 months with no radiographic progression or fracture at 12 months. She is clinically doing well and has not met indication for SM immunologic therapy to date.
3. DISCUSSION
Given the morbidity and poor overall prognosis of osseus metastases from DTC, prompt diagnosis is important to guide counseling and management. In a retrospective review of 245 patients, 78% presented or developed skeletal related events (spinal cord compression, pathological fracture, need for external beam radiation, surgery, or hypercalcemia of malignancy). 7 Prophylactic measures to prevent fractures are encouraged, including surgical fixation, external beam radiation, and other local treatments. 3 In our patient, there was initial concern for osseus metastases given multiple lytic lesions on CT scan. However, her post‐surgical thyroglobulin was not significantly elevated and her I123 whole body scan showed no iodine‐avid lesions in the axial skeleton; these findings are inconsistent with metastatic DTC.
Systemic mastocytosis results from a clonal proliferation of abnormal mast cells that infiltrate extracutaneous tissues. There is a wide spectrum of disease, but SM typically presents with nonspecific clinical symptoms including flushing, nausea/vomiting, headache, wheezing, allergic reactions, and in some cases anaphylaxis. 4 , 5 , 6 The nonspecific nature of these symptoms presents a diagnostic challenge in the absence of skin involvement such as maculopapular cutaneous mastocytosis/urticaria pigmentosa, which was not seen in our patient. 4 SM is diagnosed when patients have 1 major and ≥1 minor criterion or ≥3 minor criteria according to the World Health Organization (WHO) classification (Table 1). 8
TABLE 1.
Classification of systemic mastocytosis. 12
| Subtypes | Classification of systemic mastocytosis |
|---|---|
| 1 | Indolent systemic mastocytosis |
| 2 | Smoldering systemic mastocytosis |
| 3 | Systemic mastocytosis with associated hematologic neoplasms (myeloid neoplasm, lymphoma, myeloma, chronic lymphocytic leukemia, primary amyloidosis) |
| 4 | Aggressive systemic mastocytosis |
| 5 | Mast cell leukemia |
The prevalence of bone involvement in SM is high and can manifest as focal lytic or sclerotic lesions, bony pain from marrow infiltration, or osteoporosis. Mast cells secrete many mediators that promote osteoclastic and inhibit osteoblastic function, such as histamine, heparin, tumor necrosis factor, and interleukin‐6. 9 Vertebral fractures can occur in 20% of patients with SM. 10 Antiresorptive therapy with bisphosphonates or denosumab is the current treatment of choice. 9
It is well established that patients with SM are at increased risk of developing solid cancers (Table 2). The mechanism is poorly understood but is thought to involve tumorigenic effects of mast cells. 10 , 11 , 12 In a retrospective cohort from Germany and the United States that looked at patients with mast cell activation syndrome (MCAS, an umbrella term for mast cell related symptoms including patients with SM), the most common malignancies seen were skin cancers (hazard ratio for melanoma 7.5 and for non‐melanoma skin cancer 2.5). Interestingly, the study also found a statistically significant higher prevalence of thyroid cancer in women with MCAS compared to the general population (1.39% prevalence compared to 0.215%, respectively), but no difference in men. 12 Further studies are needed to elucidate the association between thyroid cancer and systemic mastocytosis. A summary of the prevalence, clinical presentation, and treatment of SM and PTC is shown in Table 3. To our knowledge, the current case is the first published report of a patient simultaneously diagnosed with PTC and SM.
TABLE 2.
Increased prevalence of cancers associated with systemic mast cell activation syndrome (MCAS). 11
| Increased prevalence of cancers associated with MCAS |
| Melanoma |
| Breast cancer |
| Uterus cervical cancer |
| Lung cancer |
| Thyroid cancer |
| Urinary bladder cancer |
TABLE 3.
Showing prevalence, clinical presentation and treatment of systemic mastocytosis and papillary thyroid cancer. 1 , 2 , 3
| Prevalence | Age | Male/Female | Clinical presentation | Treatment | Prognosis | |
|---|---|---|---|---|---|---|
| Systemic mastocytosis | 1:10,000‐20,000 | No specific age cutoff (median age for indolent disease: 49 years old) | Equal ratio | Pruritus, flushing, abdominal cramping/diarrhea, nausea/vomiting, headache | Avoidance of triggers, treatment of comorbidities, in aggressive cases consider KIT‐inhibitors, Imatinib, Interferon alpha | Few months to normal life expectancy |
| Papillary thyroid cancer | 14.9:100,000 | Average age 48 at diagnosis | 75% female | Painless thyroid mass, cervical lymphadenopathy, voice changes | Surgical removal of thyroid, radioactive iodine administration | 5‐year survival rate 98% approximately |
AUTHOR CONTRIBUTIONS
Kevin F. Brown: Writing – original draft; writing – review and editing. Zachary W. Bloomer: Writing – original draft. Mohamed K. M. Shakir: Writing – review and editing. Matthew J. Cognetti: Writing – review and editing. Jeannie M. Muir: Supervision; writing – review and editing. Thanh D. Hoang: Conceptualization; data curation; supervision; writing – review and editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
None to declare.
ETHICS STATEMENT
The manuscript has been reviewed and approved by the IRB and Public Affairs Office.
CONSENT STATEMENT
The authors have confirmed that patient consent has been signed and collected in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or the U.S. Government.
Brown KF, Bloomer ZW, Shakir MKM, Cognetti MJ, Muir JM, Hoang TD. Simultaneous diagnosis of papillary thyroid cancer and systemic mastocytosis. Clin Case Rep. 2023;11:e7507. doi: 10.1002/ccr3.7507
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Lubitz CC, Sosa JA. The changing landscape of papillary thyroid cancer: epidemiology, management, and the implications for patients. Cancer. 2016;122(24):3754‐3759. [DOI] [PubMed] [Google Scholar]
- 2. Visciano C, Prevete N, Liotti F, Marone G. Tumor‐associated mast cells in thyroid cancer. Int J Endocrinol. 2015;2015:705169‐705168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iñiguez‐Ariza NM, Bible KC, Clarke BL. Bone metastases in thyroid cancer. J Bone Oncol. 2020;21:100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pardanani A. Systemic mastocytosis in adults: 2019 update on diagnosis, risk stratification and management. Am J Hematol. 2019;94(3):363‐377. [DOI] [PubMed] [Google Scholar]
- 5. Broesby‐Olsen S, Farkas DK, Vestergaard H, et al. Risk of solid cancer, cardiovascular disease, anaphylaxis, osteoporosis and fractures in patients with systemic mastocytosis: a nationwide population‐based study. Am J Hematol. 2016;91(11):1069‐1075. [DOI] [PubMed] [Google Scholar]
- 6. Garla VV, Chaudhary KUQ, Yaqub A. Systemic mastocytosis: a rare cause of osteoporosis. Pan Afr Med J. 2019;32:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farooki A, Leung V, Tala H, Tuttle RM. Skeletal‐related events due to bone metastases from differentiated thyroid cancer. J Clin Endocrinol Metab. 2012;97(7):2433‐2439. [DOI] [PubMed] [Google Scholar]
- 8. Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129(11):1420‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang M, Seibel MJ. Skin and bones: systemic mastocytosis and bone. Endocrinol Diabetes Metab Case Rep. 2023;2023(2):22‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Veer E, van der Goot W, de Monchy JG, Kluin‐Nelemans HC, van Doormaal JJ. High prevalence of fractures and osteoporosis in patients with indolent systemic mastocytosis. Allergy. 2012;67(3):431‐438. [DOI] [PubMed] [Google Scholar]
- 11. Hoermann G, Greiner G, Valent P. Cytokine regulation of microenvironmental cells in myeloproliferative neoplasms. Mediators Inflamm. 2015;2015:869242‐869217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molderings GJ, Zienkiewicz T, Homann J, Menzen M, Afrin LB. Risk of solid cancer in patients with mast cell activation syndrome: results from Germany and USA. F1000Res. 2017;6:1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


