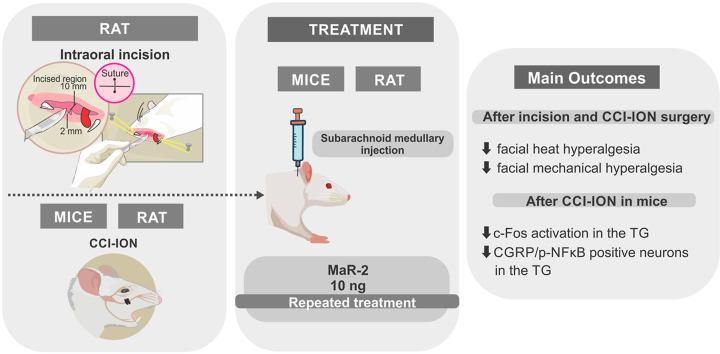
Abstract
Pain is a common symptom associated with disorders involving the orofacial structures. Most acute orofacial painful conditions are easily recognized, but the pharmacological treatment may be limited by the adverse events of current available drugs and/or patients’ characteristics. In addition, chronic orofacial pain conditions represent clinical challenges both, in terms of diagnostic and treatment. There is growing evidence that specialized pro-resolution lipid mediators (SPMs) present potent analgesic effects, in addition to their well characterized role in the resolution of inflammation. Maresins (MaR-1 and MaR-2) were the last described members of this family, and MaR-2 analgesic action has not yet been reported. Herein the effect of MaR-2 in different orofacial pain models was investigated. MaR-2 (1 or 10 ng) was always delivered via medullary subarachnoid injection, which corresponds to the intrathecal treatment. A single injection of MaR-2 caused a significant reduction of phases I and II of the orofacial formalin test in rats. Repeated injections of MaR-2 prevented the development of facial heat and mechanical hyperalgesia in a model of post-operative pain in rats. In a model of trigeminal neuropathic pain (CCI-ION), repeated MaR-2 injections reversed facial heat and mechanical hyperalgesia in rats and mice. CCI-ION increased c-Fos positive neurons and CGRP+ activated (nuclear pNFkB) neurons in the trigeminal ganglion (TG), which were restored to sham levels by MaR-2 repeated treatment. In conclusion, MaR-2 showed potent and long-lasting analgesic effects in inflammatory and neuropathic pain of orofacial origin and the inhibition of CGRP-positive neurons in the TG may account for MaR-2 action.
Keywords: Specialized pro-resolution lipids, Rats, Mice, Formalin test, Intraoral incision, Chronic constriction injury of the infraorbital nerve, Hyperalgesia, c-Fos, CGRP
Graphical abstract
Highlights
-
•
MaR-2 reduces formalin-induced nociceptive behavior.
-
•
MaR-2 prevents heat and mechanical hyperalgesia in the postoperative pain model.
-
•
MaR-2 reverses heat and mechanical hyperalgesia in a neuropathic pain model.
-
•
MaR-2 reduces c-Fos activation in the trigeminal ganglion.
-
•
MaR-2 reduces the activation of CGRP+ neurons in the trigeminal ganglion.
1. Introduction
Orofacial pain encompasses a variety of acute and chronic pain conditions that affect structures innervated by the trigeminal nerve, including the teeth and their supporting structures, the oral mucosa, masticatory muscles, and the temporomandibular joint. Due to the location and function of orofacial structures, this region is highly susceptible to trauma, inflammation, infection, and surgeries (Benoliel et al., 2011). Most acute orofacial pain conditions are well managed with current available pharmacological options, but others, including trigeminal neuropathic pain and temporomandibular disorders present clinical challenges (Chichorro et al., 2017). In some cases, disruption of acute resolution processing may lead to uncontrolled inflammation and orofacial pain chronification (Serhan and Chiang, 2013).
In the past years, it has been proposed that the resolution of inflammation is an active process, mediated by specialized pro-resolution lipid mediators (SPMs) (Chiang and Serhan, 2017; Serhan, 2017; Serhan et al., 2015). In addition to promoting the resolution of the inflammatory process, there is increasing evidence of the analgesic effects of these SPMs in a variety of acute and chronic pain models in rats and mice (Dubuisson and Dennis, 1977). Although, there is substantial knowledge about some SPMs, for example in the case of resolvins (Luo et al., 2019; Park et al., 2011; Xu et al., 2010, 2013; Zhang et al., 2020), only recently the anti-inflammatory and analgesic potential of maresins commenced to be explored. Maresins are docosahexaenoic acid (DHA) derivatives produced by macrophages, currently represented by Maresin-1 (MaR-1) and Maresin-2 (MaR-2). MaR has proved to be a potent resolution mediator, stopping polymorphonuclear infiltration and stimulating efferocytosis by macrophages (Fattori et al., 2019; Serhan et al., 2012).
In addition, there are some reports of potential analgesic actions of MaR-1. It has been shown that MaR-1 attenuates capsaicin and formalin-induced nociceptive responses, as well as, reduces heat and mechanical hyperalgesia in inflammatory and neuropathic pain models (Fattori et al., 2019; Serhan et al., 2012). Some mechanisms involved in analgesic effects of MaR-1 include reduction of calcitonin gene-related peptide (CGRP) release by dorsal root ganglion (DRG) neurons and the number of leukocytes near the CGRP-positive fibers, decrease in the levels of pro-inflammatory cytokines and inhibition of TRPV1-currents in DGR neurons (Fattori et al., 2019; Liang et al., 2021; Serhan et al., 2012). There is also evidence that MaR-1 provides analgesic effect in the trigeminal system. MaR-1 blocked capsaicin-evoked TRPV1 currents in trigeminal ganglion (TG) neurons and abolished synaptic plasticity in the subnucleus caudalis (Sp5C) induced by temporomandibular joint inflammation (Park, 2015). Despite the potent and promising analgesic effects of MaR-1, to our knowledge, only one study has investigated the biological anti-inflammatory activity of MaR-2 (Deng et al., 2014) and there are no studies addressing the effects of MaR-2 in trigeminal pain. MaR-1 and MaR-2 have similar chemical structures and in vitro anti-inflammatory effects, which suggest that MaR-2 may also present analgesic effects (Deng et al., 2014; Serhan et al., 2009). In light of these considerations, this study aimed to screen the antinociceptive effect of MaR-2 by using different models of orofacial pain, including the formalin test, a postoperative and a trigeminal neuropathic pain model. In the latter, we extended the investigation of the analgesic effect in two rodents’ species, rats and mice. Finally, the ability of MaR-2 to reduce neuronal activation in the TG was assessed to shed some light on potential mechanisms associated with its analgesic effect.
2. Material and methods
2.1. Animals
The experiments were carried out on male Rattus norvegicus Wistar rats weighing between 200 and 250 g, and Swiss mus musculus mice weighing between 20 and 25 g, supplied by the colony of Federal University of Paraná, and maintained in 4 (rats) or 10 (mice) per cage (41 × 32 × 16.5 cm). The experiments were carried out between 9 a.m. and 5 p.m., after at least two days of previous acclimatation of the animals in the laboratory. The animals remained under controlled conditions of temperature (between 22 ± 1 °C) and light (12 h light/dark cycle, lights on at 07:00h a.m.) with free access to food and water, with the wood shavings changed on alternate days. All protocols used were approved by the Ethics Committee for the Use of Animals in the Biological Sciences Sector of the Federal University of Paraná (CEUA/BIO-UFPR; approval Nº 1118) and followed the Brazilian guidelines of CONCEA (National Council of Control of Animal Experimentation). All efforts were made to minimize the number of animals used and their suffering.
2.2. Drugs and solutions
MaR-2 (Cayman Chemical, Michigan, USA) was initially diluted in 99.8% absolute ethyl alcohol (Commercial Neon, São Paulo, Brazil) and subsequently diluted in 0.9% sodium chloride sterile solution (Equiplex Pharmaceutical Industry, Goiania, Brazil) up to the desired concentrations (2 ng/μL). The dose was chosen according to a previous study that demonstrated the analgesic effect of MaR-1 (Fattori et al., 2019). The CGRP receptor antagonist (CGRP 8–37 fragment, Sigma-Aldrich, USA) was diluted in 0.9% sodium chloride sterile solution up to the desired concentration. The dose was chosen based on previous studies (Nagoshi et al., 2002; Sun et al., 2016). Formalin (Alphatec, Paraná, Brazil) was diluted in 0.9% sodium chloride sterile solution (Equilex Pharmaceutical Industry, Goiania, Brazil). The anesthetics used were isoflurane (BioChimico, Rio de Janeiro, Brazil), ketamine hydrochloride (Syntec, São Paulo, Brazil) and xylazine hydrochloride (Syntec, São Paulo, Brazil).
2.3. Subarachnoid medullary injection
MaR-2 or saline was administered via medullary subarachnoid injection to reach the subnucleus caudalis (Sp5C), according to the procedure described by Fischer et al., 2005) (Fischer et al., 2005). Initially, the injection region was trichotomized and after anesthesia by isoflurane inhalation, a hypodermic needle (13 × 0,45 mm) connected by a polyethylene catheter to a 25 μL Hamilton syringe was inserted between the occipital bone and the C1 vertebra up to 4 mm and slightly tilted towards the skull. After administration of 5 μL of MaR-2 solution (2 ng/μL), the needle was slowly removed, and the animals were monitored until they recover from anesthesia. Animals that presented motor impairment were excluded from the experiments.
2.4. Nociceptive models
2.4.1. Orofacial formalin test
The test was performed as previously describe by our group (Chichorro et al., 2004). Before the test, rats were maintained individually in the observation boxes for an acclimatization period of about 30 min. Briefly, animals were injected subcutaneously with 50 μL of 2.5% formalin or vehicle (50 μL of sterile saline) into the upper lip and were returned to the observation cages. The facial grooming time (i.e., time spent rubbing the injected area with its forepaws) was recorded at 3-min intervals over a period of 30 min. The first and second phases of the facial formalin response were considered 0–3 min and 12–30 min after injection, respectively, whereas the period between 3 and 12 min was considered the quiescent phase.
2.4.2. Postoperative pain model
This model consisted in an intraoral incision as previously described (Araya et al., 2020b; Urata et al., 2015). The animals were anesthetized with a mixture of ketamine and xylazine (50 and 10 mg/kg, respectively, i.p.) and an incision was made on the right side of the intra oral mucosa (about 2 mm deep and 1 mm long). The incision was sutured with a 4.0 silk thread. The sham group was subjected to the same procedures, excepted for the incision.
2.4.3. Trigeminal neuropathic pain model
The chronic constriction injury of the infraorbital nerve (CCI-ION) was performed according to the method previously proposed (Vos et al., 1994) with minor modifications (Chichorro, 2006). The animals were anesthetized with a mixture of ketamine and xylazine (50 and 10 mg/kg, respectively, i.p.) followed by asepsis of the face with iodized alcohol. After anesthesia was established, an incision was made in the skin below the right eye and about 3 mm after the insertion of the vibrissae to expose the rostral portion of the infraorbital nerve. The infraorbital nerve was dissected from the adjacent tissues and, two loose ligatures were placed, approximately 2 mm apart, with 4.0 sterile silk thread (Johnson & Johnson, São Paulo, Brazil) around the nerve bundle. The tissues were sutured with the same thread. The animals in the false-operated group (Sham) underwent the same surgical procedure, but the nerve bundle was not constricted. At the end of the surgery, the animals were kept in a warm room until their complete recovery from anesthesia.
2.5. Behavioral tests
2.5.1. Assessment of heat hyperalgesia
Facial heat hyperalgesia was assessed in rats and mice using the same protocol, as previously described (Araya et al., 2020a, 2020b; Luiz et al., 2015). The animals were contained with one hand of the experimenter and with the other, a heat source of approximately 50 °C was positioned at about 1 cm from the insertion area of the vibrissae, on the right side of the face. The time for each animal to exhibit reactions to vigorously move the head away from the heat source or to show rapid and consecutive movements of the vibrissae was recorded. A cut-off time of 20 s has been stipulated to avoid tissue damage (Supplementary video 1).
Supplementary video related to this article can be found at https://doi.org/10.1016/j.crneur.2023.100093
The following is the supplementary data related to this article:
1
2.5.2. Assessment of mechanical hyperalgesia
Facial mechanical hyperalgesia in rats was assessed as previously described (Chichorro et al., 2006). The animals were left to habituate for at least 2 h in the observation boxes, which was followed by application of 8 Von Frey filaments in increasing order (Semmes-Weinstein monofilaments, Stoelting, USA, 0.04; 0.07; 0.16; 0, 4, 1.0, 2.0, 4.0 and 8.0 g) in the region innervated by the infraorbital, on the side ipsilateral to the surgery. Each filament was applied 3 consecutive times with an interval of 30 s between each application. The animals' response threshold to mechanical stimulation was considered to be the filament that evoked twice behaviors such as rapid head removal, facial grooming, and attack/escape reactions. Only animals that obtained a threshold of response to mechanical stimulation equal to or greater than 8 g before any other procedure (i.e. baseline measure) were included in the experiments.
In mice, facial mechanical hyperalgesia was assessed as previously described (Luiz et al., 2015). For the evaluation of mechanical hyperalgesia in mice, the animals were habituated for 2 h individually in clear acrylic boxes (9 × 7 × 11 cm) to allow access to the forehead. The test consisted of applying the von Frey filament (0.04 g) 10 consecutive times at intervals of ∼30 s in the periorbital region. Attack/escape or head withdrawal reactions were considered positive responses to facial stimulation (Supplementary video 2). Only mice showing mean baseline positive response frequencies over 25% for the 10 applications were used in subsequent experiments.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.crneur.2023.100093
The following is the supplementary data related to this article:
2
2.6. Immunofluorescence assay
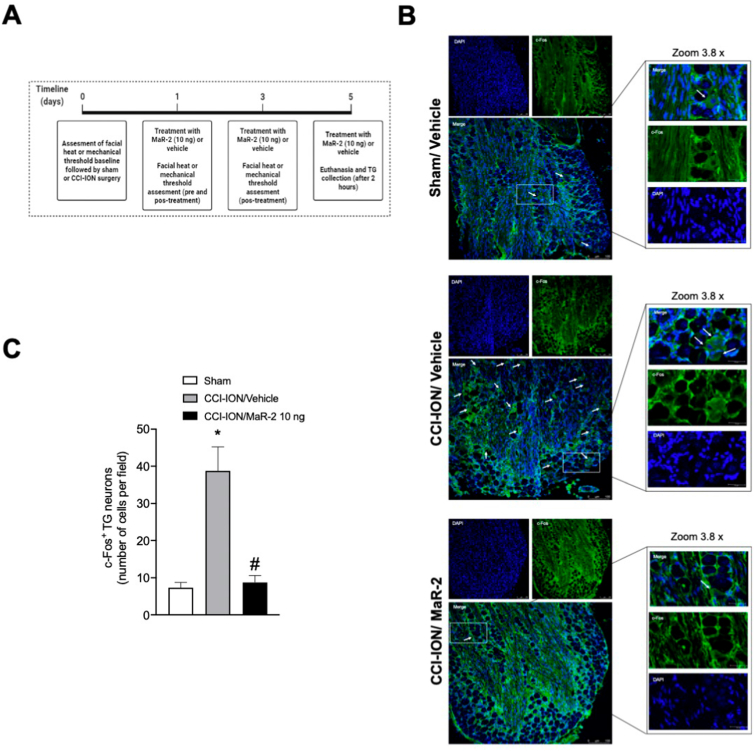
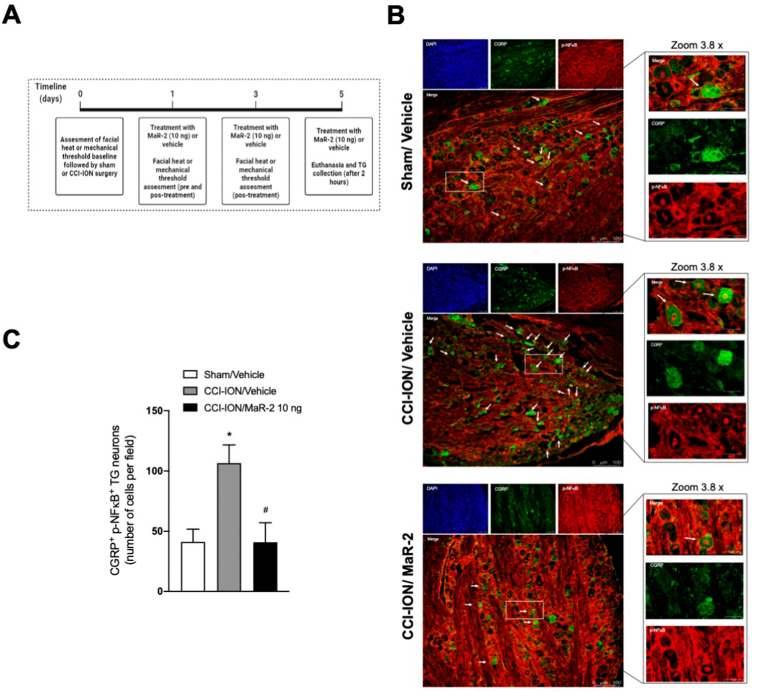
Mice subjected to CCI-ION or sham surgery were euthanized for collection of the ipsilateral trigeminal ganglion on day 5 after surgery. The samples were kept in 4% PFA for 24 h and then transferred to a 30% sucrose solution for 72 h before being included in the ideal cut-off reagent (Tissue-Tek, OCT Compound, Sakura Finetek, Torrence, CA, USA). The TG was embedded in OCT, and the sections (15 μm) processed for immunofluorescence. For staining of c-Fos positive cells, it was used a c-Fos Monoclonal Primary Antibody (1:500, cat #MA1-21190, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) and an Alexa Fluor 488 secondary antibody (1:500, cat # A-11001, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). The nuclei were contrasted with DAPI (4 ′, 6-diamidino2-phenylindol [1: 1000, cat #D1306, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA]). The CGRP+ fibers were stained with anti-CGRP primary antibody (1:500, cat #C8198, Sigma-Aldrich, Darmstadt, Germany) followed by incubation with an Alexa Fluor 488 goat anti-rabbit secondary antibody (1:500, cat #A11008, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA]). The phospho-NFκB+ fibers were stained with phospho-NFκB p65 primary antibody A-8 (1: 200, cat #SC-166748, Santa Cruz Biotechnology Inc, Dallas, Texas, USA) followed by incubation with an Alexa Fluor 647 goat anti-mouse (1:500, cat #115-605-003, Jackson ImunoResearch, West Grove, PA, USA). The images and analyzes were performed using a confocal microscope (TCS SP8, Leica Microsystems, Mannheim, Germany). The results are expressed as number of positive cells, quantified using the LAS X Life Science software platform (Leica Microsystems, Mannheim, Germany).
2.7. Experimental protocols
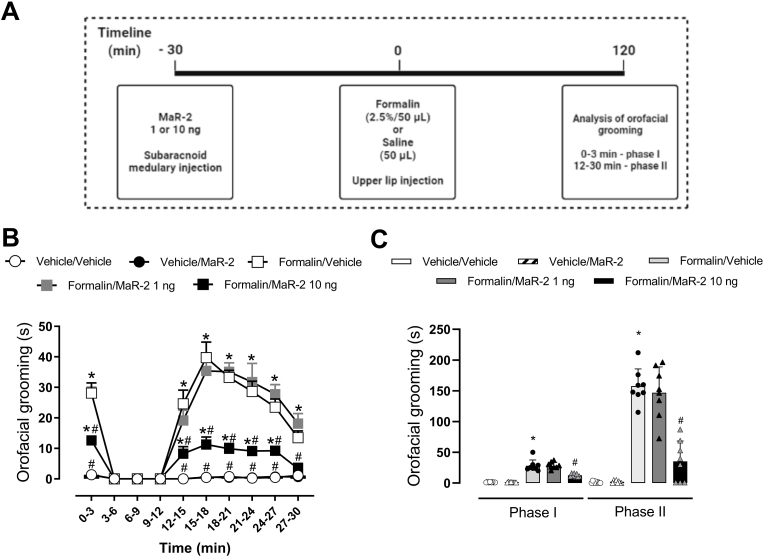
In the formalin test, MaR-2 (1 or 10 ng) or vehicle (equivalent volume) were administered via medullary subarachnoid injection 30 min before Formalin (2.5%/50 μL) or vehicle (50 μL) injection into the upper lip of rats and the grooming response was recorded during 30 min.
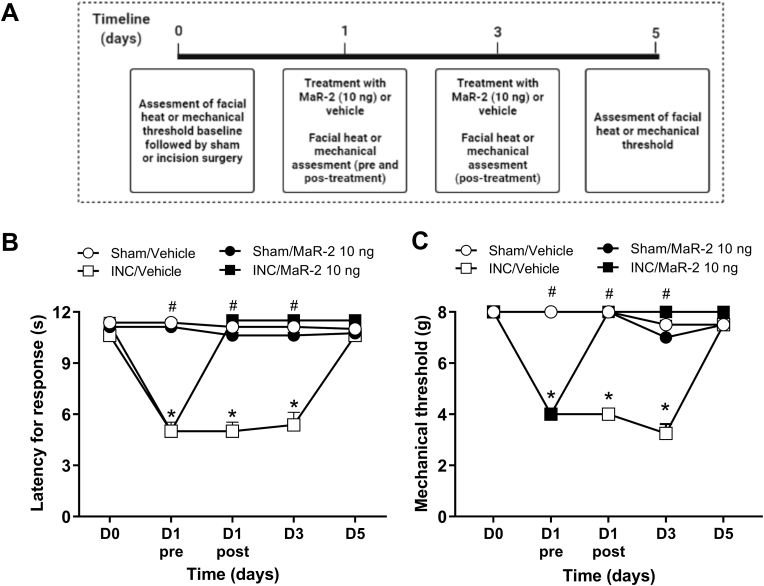
In the postoperative orofacial pain model, the animals' baseline responsiveness to heat and mechanical stimuli were assessed (day 0), and on day 1 after the intraoral incision surgery, treatment with MaR-2 (10 ng) was initiated. The treatment was repeated on day 3 and the evaluation of hyperalgesia was performed on postoperative days 3 and 5. The days of test were determined according to the time course of the development of hyperalgesia in this model, as already published by our group (Araya et al., 2020a).
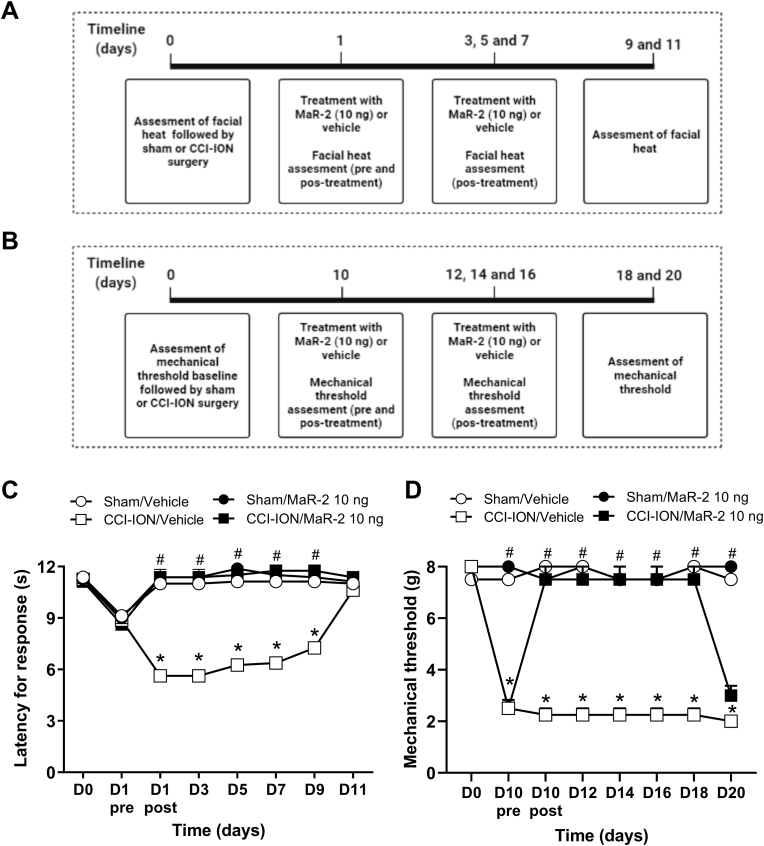
In the CCI-ION model in rats and mice, the animals' baseline response to heat was evaluated on day 0, and on day 1 after surgery, treatment with MaR-2 (10 ng) was initiated. The treatment was repeated on days 03, 05, and 07, and the assessment of heat hyperalgesia was performed on day 01 after nerve injury, before and 1 h after the treatments, and on days 03, 05, 07, 09, and 11 after CCI-ION or sham surgery, 1 h after the treatments. Likewise, the animals' baseline responsiveness to mechanical stimuli was assessed on day 0, and treatment was initiated on day 10 after nerve injury, being repeated on days 12, 14, and 16. The assessment of mechanical hyperalgesia was performed on day 10 after nerve injury, before and 1 h after treatment, and on days 12, 14, 16, 18, and 20 after CCI-ION or sham surgery, 1 h after the treatments.
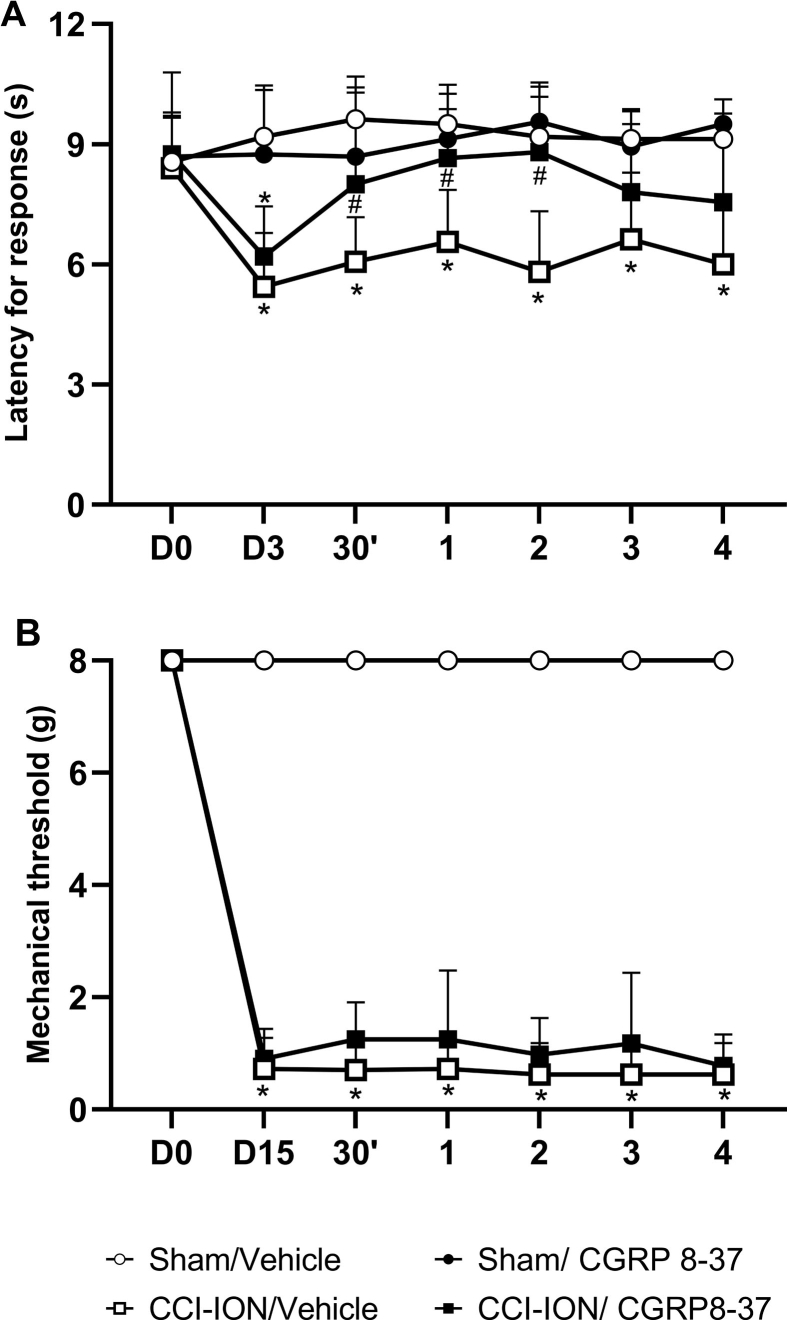
An independent group of rats was subjected to sham or CCI-ION surgery after heat or mechanical baseline assessment. On day 3 after surgery, they received a single subarachnoid medullary injection of CGRP 8–37 (5 μM) or the corresponding vehicle, and the latency to the heat response was evaluated at 30 min and at 1 h-intervals up to 4 h. Likewise, facial mechanical threshold was assessed on day 15 after sham or CCI-ION surgery followed by rats’ treatment with CGRP 8–37 (5 μmol) or the corresponding vehicle by a single subarachnoid medullary injection. Mechanical hyperalgesias was assessed at 30 min and at 1 h-intervals up to 4 h after the treatments.
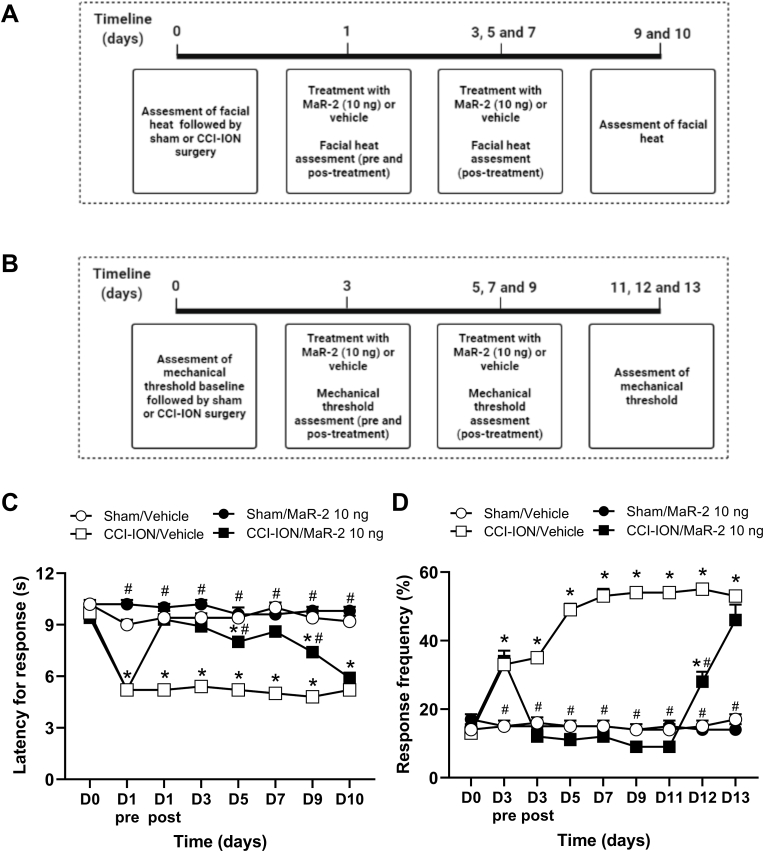
In mice, the baseline mechanical threshold was assessed on day 0, and treatment was initiated on day 3 after CCI-ION or sham surgery, being repeated on days 5, 7, and 9. Evaluation of mechanical hyperalgesia was performed on day 3 after nerve injury, before and 1 h after the treatments, and on days 05, 07, 09, 11, 12 and 13 after CCI-ION or sham surgery, 1 h after the treatments. Independent groups of rats and mice were used for each stimulus. The days of test were determined according to the time course of the development of heat and mechanical hyperalgesia in rats and mice in this model, as already published by our group (Araya et al., 2020b; Fattori et al., 2020). An independent group of mice were used for tissue collection, but only after confirmation that mice subjected to CCI-ION developed facial hyperalgesia and MaR-2 was able to reduce it. For these analyses, mice received repeated subarachnoid medullary injections of MaR-2 (10 ng) or vehicle on days 1, 3, and 5 after sham or CCI-ION surgery, and 2 h after the last treatment, they were euthanized for brief collection of the TG and Sp5C. The schematic representation of each experimental protocol is depicted above the corresponding figure.
2.8. Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) for groups of 8 animals. One-way ANOVA followed by the post hoc of Bonferroni (Fig. 1, Fig. 5, Fig. 6B) or two-way ANOVA with repeated measures followed by Bonferroni post hoc test was used to determine differences among experimental groups (Fig. 1, Fig. 2, Fig. 3, Fig. 4D and Supplementary Fig. 1). The level of significance was set at P < 0.05. All the analyses were carried out using GraphPad Prism software (version 8, San Diego, CA, USA).
Fig. 1.
MaR-2 reduces formalin-induced nociceptive behavior. (A) Timeline of the experimental procedures. (B) MaR-2 (1 or 10 ng) or vehicle (equivalent volume) were administered via medullary subarachnoid injection 30 min before formalin (2.5%/50 μL) or vehicle (50 μL) injection into the upper lip of rats and the grooming response was recorded for 30 min. (C) Represent the effect of MaR-2 (1 or 10 ng) in the Phase I (0–3 min) and Phase II (12–30 min) of formalin-induced response, respectively. Data are expressed as mean ± SEM (n = 8), two-way ANOVA followed by Bonferroni's post-hoc test (B) and one-way ANOVA followed by Bonferroni's post-hoc test (C). *P ≤ 0.05 when compared to the Vehicle/Vehicle group and #P ≤ 0.05 when compared to the Vehicle/Formalin group, two-way ANOVA with repeated measures followed by Bonferroni post-hoc test.
Fig. 5.
MaR-2 reduces c-Fos activation in the trigeminal ganglion of mice subjected to CCI-ION. (A) Timeline of the experimental procedures. (B) Representative images of the TG of sham rats treated with vehicle, and CCI-ION rats treated with vehicle or MaR-2 immunostained for DAPI (blue) and c-Fos (green). The lower panels show co-localization (DAPI+cFos), which is indicated by the arrows. (C) Quantification of c-Fos positive cells in the TG of sham rats treated with vehicle, and CCI-ION rats treated with vehicle or MaR-2. Data are expressed as mean ± SEM of c-Fos positive cells (C) (n = 6). *P ≤ 0.05 when compared to the Sham/Vehicle group and #P ≤ 0.05 when compared to the CCI-ION/Vehicle group, ordinary one-way ANOVA. (TG, trigeminal ganglion; CCI-ION, chronic constriction injury of the infraorbital nerve). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
MaR-2 reduces expression of CGRP activated neurons in the trigeminal ganglion of mice subjected to CCI-ION. (A) Timeline of the experimental procedures. (B) Representative images of the TG of sham rats treated with vehicle, and CCI-ION rats treated with vehicle or MaR-2 immunostained for DAPI (blue), CGRP (green) and p-NFκB (red). The lower panels co-localization (CGRP+p-NFκB), which is indicated by the arrows. (C) Quantification of CGRP+p-NFκB positive cells in the TG of sham rats treated with vehicle, and CCI-ION rats treated with vehicle or MaR-2. Data are expressed as mean ± SEM CGRP+p-NFκB positive cells (C) (n = 6). *P ≤ 0.05 when compared to the Sham/Vehicle group and #P ≤ 0.05 when compared to the CCI-ION/Vehicle group, ordinary one-way ANOVA. (TG, trigeminal ganglion; CCI-ION, chronic constriction injury of the infraorbital nerve). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
MaR-2 reverses heat and mechanical hyperalgesia in the postoperative orofacial pain model in rats. (A) Timeline of the experimental procedures. (B, C) Repeated injection of MaR-2 (10 ng) reversed heat and mechanical hyperalgesia, respectively. Data are expressed as mean ± SEM (n = 8). *P ≤ 0.05 when compared to the Sham/Vehicle group and #P ≤ 0.05 when compared to the INC/Vehicle group, two-way ANOVA with repeated measures followed by Bonferroni post-hoc test. (INC, incision surgery).
Fig. 3.
MaR-2 reverses heat and mechanical hyperalgesia in a trigeminal neuropathic pain model in rats. (A, B) Timeline of the experimental procedures. (C) MaR-2 (10 ng) repeated treatment reversed facial heat hyperalgesia in CCI-ION rats. (D) MaR-2 (10 ng) repeated treatment reversed facial mechanical hyperalgesia in CCI-ION rats. Data are expressed as mean ± SEM (n = 8). *P ≤ 0.05 when compared to the Sham/Vehicle group and #P ≤ 0.05 when compared to the CCI-ION/Vehicle group, two-way ANOVA with repeated measures followed by Bonferroni's post-hoc test. (CCI-ION, chronic constriction injury of the infraorbital nerve).
Fig. 4.
MaR-2 reverses heat and mechanical hyperalgesia in a trigeminal neuropathic pain model in mice. (A, B) Timeline of the experimental procedures. (C) MaR-2 (10 ng) repeated treatment reversed facial heat hyperalgesia in CCI-ION mice. (D) MaR-2 (10 ng) repeated treatment reversed facial mechanical hyperalgesia in CCI-ION mice. Data are expressed as mean ± SEM (n = 8). *P ≤ 0.05 when compared to the Sham/Vehicle group and #P ≤ 0.05 when compared to the CCI-ION/Vehicle group, two-way ANOVA with repeated measures followed by Bonferroni's post-hoc test. (CCI-ION, chronic constriction injury of the infraorbital nerve).
3. Results
3.1. MaR-2 reduces formalin-induced nociceptive behavior
Two-way ANOVA with repeated measures showed statistical difference in the treatment (F (4, 35) = 104.5, P < 0.0001) and time (F (3.628, 127.0) = 66.44, P < 0.0001) factors. Also, an interaction among these factors was observed (F (36, 315) = 15.21, P < 0.0001). As can be seen in Fig. 1B, post-hoc test showed that formalin (2.5%, 50 μL) subcutaneous injection induced a biphasic nociceptive response, which was significantly different from vehicle-injected rats (P < 0.0001). The response was divided in phase I (0–3 min) and phase II (12–30 min), as illustrated in Fig. 1C. One-way ANOVA with repeated measures showed that the facial grooming response evoked by formalin was significantly different from the control group in phase I (F (4, 35) = 57, P < 0.0001) and phase II (F (4, 35) = 66, P < 0.0001). A single subarachnoid injection of MaR-2 at 1 ng failed to change the amount of time animals spent performing the facial grooming behavior (P > 0.9999). On the other hand, MaR-2 at 10 ng caused a significant reduction of phase I and phase II (P < 0.0001) of formalin-induced nociceptive responses. In addition, MaR-2 at the highest doses did not change the grooming behavior time of control rats (i.e., vehicle-injected rats, (P = 0.1616).
3.2. MaR-2 prevents heat and mechanical hyperalgesia development in the postoperative orofacial pain model in rats
As shown in Fig. 2, two-way ANOVA with repeated measures showed statistical difference in the heat latency for response, on the treatment (F (3, 28) = 128.5, P < 0.0001) and time (F (2.215, 62.02) = 233.0, P < 0.0001) factors. Also, an interaction among these factors was observed (F (12, 112) = 153.0, P < 0.0001). In the postoperative pain model due to intraoral incision, there is a decrease in the response latency of the animal after the surgery (Fig. 2B) compared to the control group, indicating heat hyperalgesia (P < 0.0001).
In the mechanical threshold, two-way ANOVA with repeated measures showed statistical difference on the treatment (F (3, 28) = 61.83, P < 0.0001) and time (F (1.976, 55.33) = 37.89, P < 0.0001) factors. An interaction among these factors was observed (F (12, 112) = 26,57, P < 0.0001). Intraoral incision surgery caused a decrease in the mechanical threshold, indicating the presence of mechanical hyperalgesia (Fig. 2 C, P < 0.0001). Both heat and mechanical hyperalgesia started on day 1 and persisted until day 3 after surgery.
Repeated administrations of MaR-2 on postoperative days 1 and 3 (10 ng) reversed heat and mechanical hyperalgesia (Fig. 2B and C, P < 0.0001). Sham animals that received repeated subarachnoid medullary injections of MaR-2 did not show changes in the latency to heat response (Fig. 2B, P = 0.2573) or in the mechanical threshold through the evaluation period (Fig. 2C, P > 0.9999).
3.3. MaR-2 reverses heat and mechanical hyperalgesia in a trigeminal neuropathic pain model in rats
As depicted in Fig. 3, two-way ANOVA with repeated measures showed statistical difference in the latency for response to heat on the treatment (F (3, 28) = 321.3, P < 0.0001) and time (F (7, 196) = 45.86, P < 0.0001) factors. An interaction among these factors was observed (F (21, 196) = 37.19, P < 0.0001). After CCI-ION in rats, there was a decrease in the latency (Fig. 3C), indicating heat hyperalgesia (P < 0.0001). Subarachnoid medullary injection of MaR-2 (10 ng) on alternate days, starting on day 1 up to day 7, prevented the development of heat hyperalgesia (Fig. 3C, P < 0.0001).
In the mechanical threshold, two-way ANOVA with repeated measures showed statistical difference on the treatment (F (3, 28) = 175.8, P < 0.0001) and time (F (4.305, 120.5) = 28.03, P < 0.0001) factors. Also, an interaction among these factors was observed (F (21, 196) = 19.79, P < 0.0001). CCI-ION in rats caused a decrease in the mechanical threshold (Fig. 3D), indicating mechanical hyperalgesia (P < 0.0001). Likewise, Mar-2 treatment (10 ng, once a day, on days 10, 12, 14 and 16) reversed the mechanical hyperalgesia (Fig. 3D, P < 0.0001). The effect of the last injection of MaR-2 was still detected on day 18 after surgery (Fig. 3D, P < 0.0001), but on day 20 there was no significance difference between CCI-ION rats injected with vehicle or MaR-2 (Fig. 3D, P > 0.9999). Sham animals that received repeated subarachnoid medullary injections of MaR-2 did not show changes in the latency to heat response (Fig. 3C, P > 0.9999) or in the mechanical threshold (Fig. 3D, P > 0.9999) through the evaluation period.
Supplementary Fig. 1A illustrates the effect of CGRP 8–37 (5 μM/5 μL) or vehicle (5 μL), injected once into the subarachnoid medullary region, on day 3 after sham and CCI-ION surgery to assess the contribution of CGRP to facial heat hyperalgesia. Two-way ANOVA with repeated measures showed statistical difference between sham and CCI-ION groups on day 3 (F (3, 32) = 18,40, P < 0,0001). Sham operated animals treated with vehicle or CGRP 8–37 did no show statistical changes throughout the evaluation period. CCI-ION rats treated with vehicle demonstrated statistically significant reduction in the latency for heat response in all testing period compared to sham. Treatment of CCI-ION rats with CGRP 8–37 resulted in anti-hyperalgesic effect at 30 min up to 2 h compared to vehicle-treated CCI-ION rats (Interaction factor (F 18, 180) = 3.156, P < 0.0001).
Supplementary Fig. 1B illustrates the effect of CGRP 8–37 (5 μM/5 μL) or vehicle (5 μL), injected once into the subarachnoid medullary region, on day 15 after sham and CCI-ION surgery to assess the contribution of CGRP to facial mechanical hyperalgesia. Two-way ANOVA with repeated measures showed statistical difference between sham and CCI-ION groups on day 3 (F (3, 28) = 674.2; P < 0,0001). Sham operated animals treated with vehicle or CGRP 8–37 did no show statistical changes throughout the evaluation period. CCI-ION rats treated with vehicle or CGRP 8–37 demonstrated statistically significant reduction in the latency for heat response compared to the corresponding sham groups in all testing period, with no statistical difference between the treatments.
3.4. MaR-2 reverses heat and mechanical hyperalgesia in a trigeminal neuropathic pain model in mice
As evidenced in Fig. 4, two-way ANOVA with repeated measures showed statistical difference in the latency to response to heat on the treatment (F (3, 36) = 97.51, P < 0.0001) and time (F (5.206, 187.4) = 66.54, P < 0.0001) factors. An interaction among these factors was observed (F (21, 252) = 27.03, P < 0.0001). After CCI-ION in mice, there was a decrease in the latency to response to heat (Fig. 4B), indicating heat hyperalgesia (P < 0.0001). Subarachnoid medullary injection of MaR-2 (10 ng) on alternate days, starting on day 1 up to day 7, significantly reduced the heat hyperalgesia (Fig. 4B, P < 0.0001). The effect was still significant on day 9 after surgery (i.e., 48 h after the last injection, (Fig. 4B, P < 0.0001), but subsided on day 10.
In the mechanical threshold, two-way ANOVA with repeated measures showed statistical difference on the treatment (F (3, 36) = 144.9, P < 0.0001) and time (F (5.321, 191.6) = 40.79, P < 0.0001) factors. Also, an interaction among these factors was observed (F (24, 288) = 39.02, P < 0.0001). CCI-ION in mice caused a decrease in the mechanical threshold, indicating mechanical hyperalgesia (Fig. 4D, P < 0.0001). Mar-2 treatment (10 ng, once a day, on days 3, 5, 7 and 9) reversed the facial mechanical hyperalgesia (Fig. 4D, P < 0.0001). The effect of the last injection of MaR-2 was still detected on day 11 after surgery (Fig. 4D, P < 0.0001), but on days 12 and 13 there was no significant difference between CCI-ION mice injected with vehicle or MaR-2 (Fig. 4D, P > 0.9999). Sham animals that received repeated subarachnoid medullary injections of MaR-2 did not show changes in the latency to heat response (Fig. 4B, P > 0.9999) or in the mechanical threshold (Fig. 4D, P > 0.9999) through the evaluation period.
3.5. MaR-2 reduces c-Fos activation in the trigeminal ganglion of mice subjected to CCI-ION
In Fig. 5C, one-way ANOVA showed that mice subjected to CCI-ION present a significant increase in c-Fos activation in the TG compared to the sham group (F (2, 21) = 20.08, P < 0.0001). MaR-2 reduced significantly the number of c-Fos positive cells compared to samples of CCI-ION mice treated with vehicle (P < 0,0001). Fig. 5B shows representative images of c-Fos immunofluorescence in sham mice treated with vehicle and CCI-ION groups that received vehicle or MaR-2.
3.6. MaR-2 reduces the activation of CGRP+ neurons in the trigeminal ganglion of mice subjected to CCI-ION
In Fig. 6C, one-way ANOVA shows that mice subjected to CCI-ION present a significant increase in the number of TG neurons positive to CGRP and phosphorylated NFκB (p-NFκB) compared to the sham group (F (2, 15) = 7.199, P = 0.0064). CGRP was used as a marker of neurons positive to that peptide and nuclear p-NFκB was used as a surrogate of cellular activation. MaR-2 was able to reduce to sham levels the number of CGRP/p-NFκB positive neurons (P < 0.0001). Fig. 6B shows representative images of CGRP/p-NFκB in sham mice treated with vehicle and CCI-ION groups that received vehicle or MaR-2. The TG has dense fibrous tissue produced by the local resident fibroblasts (Liang et al., 2021), which enhanced the background staining of p-NFκB. Thus, only the CGRP+ cells with nuclear localization of p-NFκB were counted.
4. Discussion
The results of the present study show, for the first time, that subarachnoid medullary injection of MaR-2 results in potent antinociceptive and anti-hyperalgesic effects in different orofacial pain models. In a model of trigeminal neuropathic pain, MaR-2 caused pronounced and sustained effect against heat and mechanical hyperalgesia, both, in rats and mice. One potential mechanism involved in MaR-2 analgesic actions is the reduction of neuronal activation, more specifically CGRP-positive neurons in the TG.
The vast majority of studies that assess anti-inflammatory and analgesic actions of SPMs have used the intrathecal route and doses in the nanogram range (Fattori et al., 2020). For instance, analgesic effects of MaR-1 were reported in the dose range of 1–100 ng, injected intrathecally. However, to date, there is only one study reporting in vivo effect of MaR-2, which sowed that its intravenous injection at 1 ng in mice reduced zymosan-induced neutrophil recruitment to the peritoneal cavity and stimulated the phagocytosis of zymosan particles by human macrophages (Deng et al., 2014). Thus, we determined the doses of MaR-2 based on previous studies that showed analgesic effects of MaR-1 (Fattori et al., 2019), and used the subarachnoid medullary administration, which corresponds to the intrathecal administration (Fabbretti et al., 2006). This injection method has been used by our group to target the trigeminal nucleus complex, more specifically the Sp5C (Araya et al., 2017).
The formalin test is widely used for the screening of potentially analgesic drugs. Formalin injection in the rodents’ hind paw or upper lip induces a biphasic nociceptive response, consisting of a brief first phase, due to nociceptors activation, which is followed by a quiescent period, and a second, more prolonged phase, with inflammatory characteristics (Chichorro et al., 2004; Clavelou et al., 1989; Dubuisson and Dennis, 1977; Tjølsen et al., 1992). Herein, a single injection of MaR-2 caused a marked reduction of phase I and phase II of formalin-induced nociception. In line with this observation, intraplantar injection of MaR-1 (10 ng) reduced the nociceptive responses induced by capsaicin and formalin. Mar-1 also was shown to potently inhibit TRPV1, but not TRPA1, currents in DGR neurons, in a toxin pertussis-sensitive fashion (Serhan et al., 2012). Altogether, these results suggest that maresins are capable of blocking the activation and sensitization of nociceptors, an effect that may involve an indirect inhibitory effect on TRPV1 receptors.
In the present study, the effect of MaR-2 in inflammatory pain was also demonstrated in a model of post-operative pain induced by intraoral incision. This model was proposed by Urata et al., 2015) (Urata et al., 2015) and further characterized by our group (Araya et al., 2020a, 2020b). MaR-2 (two administrations in alternate days) fully prevented the development of facial heat and mechanical inflammatory hyperalgesia induced by intraoral incision. These findings are in agreement with previous observations that MaR-1 (10 ng, intrathecal) caused a significant reduction of carrageenin-induced heat and mechanical hyperalgesia in the hind paw of mice. The same treatment also caused a significant reduction of overt pain, heat and mechanical hyperalgesia induced by Complete Freund Adjuvant (CFA) injected in the mice hindpaw (Fattori et al., 2019). Several peripheral and central mechanisms seem to contribute to MaR-1 effect, including inhibition of neutrophil and macrophage recruitment proximal to CGRP positive fibers in the paw skin, and inhibition of NFκB activation and pro-inflammatory cytokines production and glial cells activation in the spinal cord (Chiang and Serhan, 2017). The mechanisms underlying MaR-2 effects remains to be elucidated, but given the pivotal role of CGRP in the trigeminal nociceptive system, the influence of this mediator sounds an attractive starting approach. Altogether, these results indicate a potent and long-lasting analgesic effect of maresins, that differ mechanistically from current available therapies and may be useful in the management of pain of inflammatory origin.
Finally, the present results demonstrated that MaR-2 caused a marked and long-lasting reduction of heat and mechanical hyperalgesia in a model of trigeminal neuropathic pain. This finding corroborates previous observations that MaR-1 (10–100 ng, 3 intrathecal injections on consecutive days) can significantly reduce heat and mechanical hyperalgesia in a model of radicular pain in rats (Wang et al., 2020). Moreover, MaR-1 reduced vincristine-induced allodynia (i.e., a model of chemotherapy-induced neuropathic pain) when administered systemically (40 ng) to mice (Serhan et al., 2012). Since data in neuropathic pain is more limited, we extended our findings to rats and mice, and MaR-2 showed similar effects on both species. In addition, MaR-2 repeated treatment in mice reduced to sham levels the activation of TG neurons demonstrated by analysis of c-Fos positive cells. The TG is a key structure in the trigeminal nociceptive processing. In contains the neuronal cell bodies of all trigeminal afferents, which are surrounded by satellite glial cells. TG neurons express and release several neuropeptides that signal to neighboring neurons or satellite glial cells, and this cross-talk modulates sensory transmission. Also, the TG is outside the blood-brain barrier, which allows the distribution of the mediators to peripheral sites and/or to the trigeminal nucleus complex (Messlinger et al., 2020). Thus, drugs that modulated TG signaling may have an inhibitory effect in both, peripheral and central sensitization of the trigeminal nociceptive system. The precise mechanism of MaR-2 in the TG remains to be elucidated, but according to our data, it can reduce CGRP positive neurons activation. In line with this idea, herein it was shown that blockade of CGRP receptors in rats subjected to CCI-ION reduced heat hyperalgesia but failed to affect mechanical hyperalgesia. A previous study or group demonstrated that mechanical, but not heat hyperalgesia, after CCI-ION depends on facilitatory pathways from the rostral-ventromedial medulla (Nones et al., 2017). Thus, it is possible that the restricted accesses to the central nervous system of CGRP 8–37 after subarachnoid medullary injection has limited its effect. In this regard, other groups have been reported that the use of pharmacological strategies to neutralize CGRP or block its receptors in the trigeminal system alleviates heat and mechanical hypersensitivity associated to infraorbital nerve ligation in rats (Michot et al., 2015; Maegawa et al., 2021). There is also some clinical evidence that CGRP contributes to the physiopathology on trigeminal neuralgia (Zhang et al., 2020) and CGRP-targeted drugs have now been tested in this patient's population with promising results (Parascandolo et al., 2021). Moreover, in line with our observations, in the study of Fattori et al. (2019) (Fattori et al., 2019) it was demonstrated that MaR-1 markedly inhibited CGRP release induced by capsaicin, in addition to reducing CFA‐induced Nav1.8 and Trpv1 mRNA expression. Consistent up regulation of CGRP has been reported in the trigeminal ganglia, concomitantly to the detection of facial heat and mechanical hyperalgesia (Dubuisson and Dennis, 1977; Messlinger et al., 2020; Xu et al., 2010). It is noteworthy that upregulated CGRP seems not to be transported towards peripheral terminals, but rather released around the cell bodies in the TG, where it has been shown to upregulate the function and expression of transient receptor potential vallinoid-1 and adenosine triphosphate-gated purinergic ionotropic (P2X3) receptor, leading to neuronal hyperexcitability (Chiang and Serhan, 2017; Fattori et al., 2020). Altogether, these results reinforce the idea that CGRP signaling in the trigeminal ganglia contributes to peripheral sensitization of trigeminal afferents and its modulation may contribute to maresins' analgesic effect. Altogether, these results suggest that DRG or TG neurons are targeted by maresins, which may contribute to their analgesic effect. Further studies are necessary to clarify the cell types and mechanisms involved, especially in MaR-2 effects.
In conclusion, the results of the present study demonstrates that MaR-2 has a potent and long-lasting analgesic effect in inflammatory and neuropathic pain of orofacial origin. The inhibition of CGRP-positive neurons activation accounts for MaR-2 analgesic effect, but this and other mechanisms require further investigation. Given the potency of MaR-2, we suggest that, like other SPMs, it may bring several benefits in the management of acute and chronic pain conditions.
Financial support
This work was supported by grants from the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil, finance code 001); Programa de Apoio a Grupos de Excelência (PRONEX) grant supported by SETI/Araucária Foundation and MCTI/CNPq; and Paraná State Government (agreement 014/2017, protocol 46.843); and Universal CNPq (427946/2018-2). JGC and WAVJ are recipients of the CNPq Productivity fellowship. RVL, MMB and TS are recipients of PhD scholarships from CAPES. DFB are recipient of MSC scholarship from CAPES. CRF acknowledges the CNPq PDJ fellowship.
CRediT authorship contribution statement
Raphael Vieira Lopes: performed all the experiments, Formal analysis, Writing – original draft. Darciane Favero Baggio: assisted in performing the surgical procedures. Camila Rodrigues Ferraz: performed immunofluorescence assay. Mariana Marques Bertozzi: assisted in performing the surgical procedures. Telma Saraiva-Santos: performed immunofluorescence assay. Waldiceu Aparecido Verri Junior: performed manuscript, Writing – original draft, and were involved in, Conceptualization, designing the research project, Writing – review & editing, revising. Juliana Geremias Chichorro: performed manuscript, Writing – original draft, and were involved in, Conceptualization, designing the research project, Writing – review & editing, revising.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crneur.2023.100093.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
Data availability
Data will be made available on request.
References
- Araya E.I., Baggio D.F., Koren L. de O., Andreatini R., Schwarting R.K.W., Zamponi G.W., Chichorro J.G. Acute orofacial pain leads to prolonged changes in behavioral and affective pain components. Pain. 2020;161:2830–2840. doi: 10.1097/j.pain.0000000000001970. [DOI] [PubMed] [Google Scholar]
- Araya E.I., Barroso A.R., Turnes J. de M., Radulski D.R., Jaganaught J.R.A., Zampronio A.R., Chichorro J.G. vol. 226. Physiol Behav [Internet] Elsevier; 2020. pp. 113–127. (Toll-like Receptor 4 (TLR4) Signaling in the Trigeminal Ganglion Mediates Facial Mechanical and Thermal Hyperalgesia in Rats). [DOI] [PubMed] [Google Scholar]
- Araya E.I., Nones C.F.M., Ferreira L.E.N., Kopruszinski C.M., Cunha JM da, Chichorro J.G. Brain Res [Internet] Elsevier B.V.; 2017. Role of Peripheral and Central TRPV1 Receptors in Facial Heat Hyperalgesia in Streptozotocin-Induced Diabetic Rats; pp. 146–155. [DOI] [PubMed] [Google Scholar]
- Benoliel R., Svensson P., Heir G., Sirois D., Zakrzewska J., Oke-Nwosu J., Torres S., Greenberg M., Klasser G., Katz J., Eliav E. Persistent orofacial muscle pain. Oral Dis. 2011;17:23–41. doi: 10.1111/j.1601-0825.2011.01790.x. [DOI] [PubMed] [Google Scholar]
- Chiang N., Serhan C.N. Mol Aspects Med [Internet] Elsevier Ltd; 2017. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. 58:114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichorro J. 2006. Neuralgia do Trigêmeo : estudo de mecanismos e avaliação da participação das endotelinas em um modelo experimental; pp. 1–74.https://repositorio.ufsc.br/handle/123456789/88606 Available from: February 8, 2022. [Google Scholar]
- Chichorro J.G., Lorenzetti B.B., Zampronio A.R. Involvement of bradykinin, cytokines, sympathetic amines and prostaglandins in formalin-induced orofacial nociception in rats. Br. J. Pharmacol. 2004;141:1175–1184. doi: 10.1038/sj.bjp.0705724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichorro J.G., Porreca F., Sessle B. Mechanisms of craniofacial pain. Cephalalgia. 2017;37:613–626. doi: 10.1177/0333102417704187. [DOI] [PubMed] [Google Scholar]
- Chichorro J.G., Zampronio A.R., Rae G.A. Endothelin ETB receptor antagonist reduces mechanical allodynia in rats with trigeminal neuropathic pain. Exp. Biol. Med. 2006;231:1136–1140. doi: 10.3181/00379727-232-2311136. [DOI] [PubMed] [Google Scholar]
- Clavelou P., Pajot J., Dallel R., Raboisson P. Application of the formalin test to the study of orofacial pain in the rat. Neurosci. Lett. 1989;103:349–353. doi: 10.1016/0304-3940(89)90125-0. [DOI] [PubMed] [Google Scholar]
- Deng B., Wang C.W., Arnardottir H.H., Li Y., Cheng C.Y.C., et al. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102362. 1-9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuisson D., Dennis S.G. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Fabbretti E., D'Arco M., Fabbro A., Simonetti M., Nistri A., Giniatullin R. Delayed upregulation of ATP P2X3 receptors of trigeminal sensory neurons by calcitonin gene-related peptide. J. Neurosci. 2006;26(23):6163–6171. doi: 10.1523/JNEUROSCI.0647-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattori V., Pinho-Ribeiro F.A., Staurengo-Ferrari L., Borghi S.M., Rossaneis A.C., Casagrande R., Verri W.A. The specialised pro-resolving lipid mediator maresin 1 reduces inflammatory pain with a long-lasting analgesic effect. Br. J. Pharmacol. 2019;176:1728–1744. doi: 10.1111/bph.14647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattori V., Zaninelli T.H., Rasquel-Oliveira F.S., Casagrande R., Verri W.A. vol. 151. Pharmacol Res [Internet] Elsevier; 2020. (Specialized Pro-resolving Lipid Mediators: A New Class of Non-immunosuppressive and Non-opioid Analgesic Drugs). 1-12. [DOI] [PubMed] [Google Scholar]
- Fischer L., Parada C.A., Tambeli C.H. A novel method for subarachnoid drug delivery in the medullary region of rats. J Neurosci Methods Elsevier. 2005;148:108–112. doi: 10.1016/j.jneumeth.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Liang H., Hu H., Shan D., Lyu J., Yan X., Wang Y., Jian F., Li X., Lai W., Long H. CGRP modulates orofacial pain through mediating neuron-glia crosstalk. J. Dent. Res. 2021;100(98–105) doi: 10.1177/0022034520950296. [DOI] [PubMed] [Google Scholar]
- Luiz A.P., Schroeder S.D., Rae G.A., Calixto J.B., Chichorro J.G. Neuroscience Elsevier Ltd; 2015. Contribution and Interaction of Kinin Receptors and Dynorphin A in a Model of Trigeminal Neuropathic Pain in Mice; pp. 189–200. 300. [DOI] [PubMed] [Google Scholar]
- Luo X., Gu Y., Tao X., Serhan C.N., Ji R.-R. Resolvin D5 inhibits neuropathic and inflammatory pain in male but not female mice: distinct actions of D-series resolvins in chemotherapy-induced peripheral neuropathy. Front. Pharmacol. 2019;10:1–9. doi: 10.3389/fphar.2019.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegawa H., Yoshikawa C., Usami N., Hanamoto H., Kudo C., Niwa H. Anti-calcitonin gene-related peptide antibody attenuates orofacial mechanical and heat hypersensitivities induced by infraorbital nerve injury. Biochem. Biophys. Res. Commun. 2021;569:147–153. doi: 10.1016/j.bbrc.2021.07.001. [DOI] [PubMed] [Google Scholar]
- Messlinger K., Balcziak L.K., Russo A.F. Cross-talk signaling in the trigeminal ganglion: role of neuropeptides and other mediators. J. Neural. Transm. 2020:431–444. doi: 10.1007/s00702-020-02161-7. [Internet] Springer Vienna; 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michot B., Kayser V., Hamon M., Bourgoin S. CGRP receptor blockade by MK-8825 alleviates allodynia in infraorbital nerve-ligated rats. Eur. J. Pain. 2015;19(2):281–290. doi: 10.1002/ejp.616. [DOI] [PubMed] [Google Scholar]
- Nagoshi Y., Kuwasako K., Ito K., Uemura T., Kato J., Kitamura K., Eto T. The calcitonin receptor-like receptor/receptor activity-modifying protein 1 heterodimer can function as a calcitonin gene-related peptide-(8-37)-sensitive adrenomedullin receptor. Eur. J. Pharmacol. 2002;450:237–243. doi: 10.1016/s0014-2999(02)02184-2. [DOI] [PubMed] [Google Scholar]
- Nones C.F.M., Claudino R.F., Ferreira L.E.N., Reis RC Dos, King T., Chichorro J.G. vol. 644. Elsevier Ireland Ltd; 2017. Descending facilitatory pain pathways mediate ongoing pain and tactile hypersensitivity in a rat model of trigeminal neuropathic pain; pp. 18–23. (Neurosci Lett). [Internet] [DOI] [PubMed] [Google Scholar]
- Parascandolo E., Levinson K., Rizzoli P., Sharon R. Efficacy of erenumab in the treatment of trigeminal neuralgia: a retrospective case series. Neurol Clin Pract. 2021;11(3):227–231. doi: 10.1212/CPJ.0000000000001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.K. Mediators Inflamm Hindawi Publishing Corporation; 2015. Maresin 1 Inhibits TRPV1 in Temporomandibular Joint-Related Trigeminal Nociceptive Neurons and TMJ Inflammation-Induced Synaptic Plasticity in the Trigeminal Nucleus; pp. 1–9. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.K., Xu Z.Z., Liu T., Lü N., Serhan C.N., Ji R.R. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J. Neurosci. 2011;31(50):18433–18438. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. Faseb. J. 2017;31:1273–1288. doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr. Opin. Pharmacol. 2013;13:632–640. doi: 10.1016/j.coph.2013.05.012. [Internet] Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Chiang N., Dalli J., Levy B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harbor Perspect. Biol. 2015;7:1–20. doi: 10.1101/cshperspect.a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Dalli J., Karamnov S., Choi A., Park C., Xu Z., Ji R., Zhu M., Petasis N.A. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. Faseb. J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Yang R., Martinod K., Kasuga K., Pillai P.S., Porter T.F., Oh S.F., Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009;206:15–23. doi: 10.1084/jem.200818801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Dodick D.W., Silberstein S., Goadsby P.J., Reuter U., Ashina M., Saper J., Cady R., Chon Y., Dietrich J., Lenz R. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016 doi: 10.1016/S1474-4422(16)00019-3. [Internet] Elsevier Ltd; 15:382–90. [DOI] [PubMed] [Google Scholar]
- Tjølsen A., Berge O.G., Hunskaar S., Rosland J.H. Hole K: the formalin test: an evaluation of the method. 1992. Pain 51:5–17. [DOI] [PubMed]
- Urata K., Shinoda M., Honda K., Lee J., Maruno M., Ito R., Gionhaku N., Iwata K. Involvement of TRPV1 and TRPA1 in incisional intraoral and extraoral pain. J. Dent. Res. 2015;94:446–454. doi: 10.1177/0022034514565645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos B.P., Strassman A.M., Maciewicz R.J. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's lnfraorbital nerve. J. Neurosci. 1994;14(5 Pt 1):2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y hao, Li Y., Wang J nan, Zhao Q xiang, Wen S., Wang S cong, Sun T. Neurochem Res. Springer US; 2020. A novel mechanism of specialized proresolving lipid mediators mitigating radicular pain: the negative interaction with NLRP3 inflammasome. [Internet] 45:1860–9. [DOI] [PubMed] [Google Scholar]
- Xu Z.Z., Berta T., Ji R.R. Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J. Neuroimmune Pharmacol. 2013;8:37–41. doi: 10.1007/s11481-012-9394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.Z., Zhang L., Liu T., Park J.Y., Berta T., Yang R., Serhan C.N., Ji R.R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lian Y., Zhang H., Xie N., Chen Y. CGRP plasma levels decrease in classical trigeminal neuralgia patients treated with botulinum toxin type A: a pilot study. Pain Med. 2020;21(8):1611–1615. doi: 10.1093/pm/pnaa028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
Data Availability Statement
Data will be made available on request.