Highlights
-
•
We evaluated β-glucan for alleviating ammonia stress in Tor putitora.
-
•
A decrease in transcript level of inos was observed in β-glucan-fed ammonia-challenged fish.
-
•
β-Glucan had no effect on most of the immune genes of ammonia-exposed fish.
-
•
Aquaporins 1a and 3a expression decreased in β-glucan-fed ammonia-exposed fish.
-
•
Feeding 0.75% β-glucan improved resistance to ammonia stress in T. putitora.
Keywords: Β-glucan, Ammonia, Immune genes, Anti-oxidative genes, Nitric oxide synthase, Golden mahseer
Abstract
The study investigated the effects of dietary administration of β-glucan on aquaporins and antioxidative & immune gene expression in endangered golden mahseer, Tor putitora juveniles, exposed to ammonia stress. For that, fish were fed experimental diets having 0 (control/basal), 0.25, 0.5, and 0.75% β-d-glucan for five weeks and then exposed to ammonia (10 mgL−1 total ammonia nitrogen) for 96 h. Administration of β-glucan differentially influenced the mRNA expression of aquaporins, anti-oxidative, and immune genes in ammonia-exposed fish. For instance, the transcript abundance of catalase and glutathione-s-transferase in gill varied significantly among the treatment groups, with the lowest levels in 0.75% β-glucan fed groups. At the same time, their hepatic mRNA expression was similar. Congruently, transcript abundance of inducible nitric oxide synthase considerably decreased in the β-glucan fed ammonia-challenged fish. Conversely, the relative mRNA expression of various immune genes viz., major histocompatibility complex, immunoglobulin light chain, interleukin 1-beta, toll-like receptors (tlr4 and tlr5) and complement component 3 remained largely unchanged in ammonia-exposed mahseer juveniles that were fed with graded levels of β-glucan. On the other hand, a significantly lower transcript level of aquaporins 1a and 3a was noticed in the gill of glucan-fed fish compared to ammonia-exposed fish that received the basal diet. However, branchial aquaporin 3b remained unaltered. Altogether, this study showed that dietary intake of 0.75% β-glucan improved resistance to ammonia stress to a certain degree, probably through activating anti-oxidative system and reducing brachial ammonia uptake.
1. Introduction
Fish in aquaculture are invariably subjected to a variety of environmental and procedural stressors. Among them, ammonia is the most common environmental stressor especially, in intensive culture systems with high stocking density, since it is the end product of protein catabolism and the primary excretory product of fish [1]. Furthermore, the degradation of leftover feed and nitrogenous fertilizers, along with insufficient water exchanges/filtration, causes ammonia to build up in water [2]. Generally, despite lower feeding rates, accumulation of dissolved inorganic nitrogen is higher at lower temperatures/colder months, when the density of phytoplankton, the major consumer of ammonia, is minimal [3]. Moreover, the efficiency of nitrification is also lesser at lower temperatures [4]. Golden mahseer, being a coldwater fish, is more prone to ammonia toxicity.
Ammonia stress can cause several physiological changes, including growth retardation, osmoregulatory disturbance, organ damage, depression of immune and anti-oxidative status, and eventually mortality in aquaculture species [5,6]. More specifically, at high environmental levels, excretion of ammonia is hindered and/or intake of ammonia from the environment happens, causing ammonia poisoning [1,7]. Earlier studies have indicated that elevated levels of ammonia may produce reactive oxygen species (ROS), reactive nitrogen species (RNS), and nitric oxide (NO.), imparting oxidative stress [1,7]. In catfish under hyper-ammonia stress, inducible nitric oxide synthase (inos) induction has been documented, which results in enhanced NO generation [8,9]. To counteract oxidative stress-induced damages, cells possess antioxidant defense systems, comprising catalase, superoxide dismutase, glutathione-s-transferase, etc., that act at varying rates to protect or repair such damages. Hence, changes in the antioxidative enzymes can be used as a possible biomarker of stress [10]. Immunoglobulins, cytokines and toll-like receptors are important molecules in immune function regulation [11]. It is well established that aquaporins play an essential role in water and solute/ammonia movements through membranes facilitating osmotic balance [12,13]. However, the expression patterns of aquaporins under ammonia exposure in fish remain largely unknown.
Considering these physiological impacts, there is necessary to decrease the deleterious effects of ammonia toxicity in fish. Dietary manipulation has attracted interest as a tool to mitigate stress-induced damages [14,15]. For instance, oral administration of feed supplements such as cineole [6], taurine [5,16], inositol [17], seaweeds and herbal extracts [1,18,19] were demonstrated to alleviate the adverse effects of ammonia stress in aquaculture species. β-Glucan is one of the promising immunostimulants used in aquaculture considering its immuno-stimulatory, anti-inflammatory, and anti-oxidant properties [20,21]. Recently, prolonged dietary intake of β-glucan has been proposed to mitigate the detrimental effects of stress and shown to improve the welfare of fish exposed to different stressors [22,23]. However, studies investigating the effect of dietary β-glucan supplementation on the anti-ammonia stress response are limited, particularly in the expression of aquaporins and inos genes. Therefore, in the present study, the protective role of dietary β-glucan intake against sub-lethal ammonia stress was investigated in an endangered fish golden mahseer, Tor putitora.
2. Material and method
2.1. Experimental/test diets
Four experimental diets were prepared to include 0 (basal/control), 0.25, 0.5, and 0.75% β-d-glucan. To the basal diet, 0.25, 0.5, and 0.75% of β-d-glucan was supplemented by replacing an equal amount of wheat flour (Table 1). The dietary ingredients were accurately weighed, carefully blended, steam cooked, pelletized (1.5 mm diameter), and dried. The β-d-glucan (β-1,3/1,6-glucan) purchased from M/s Kuber Impex Ltd, Madhya Pradesh, India, was extracted from Saccharomyces cerevisiae and has been found to have 70.60% purity. The experiment involved four treatment groups, viz. control (0% β-glucan), 0.25% β-glucan, 0.5% β-glucan, and 0.75% β-glucan.
Table 1.
Ingredients composition of experimental diets (% dry weight basis) fed to golden mahseer juveniles during the experimental trial.
| Ingredients | % inclusion |
|||
|---|---|---|---|---|
| Dietary treatment groups |
Control |
0.25% β-glucan | 0.5% β-glucan | 0.75% β-glucan |
| Soybean meal$ | 10.00 | 10.00 | 10.00 | 10.00 |
| Fish meala | 55.00 |
55.00 |
55.00 |
55.00 |
| Wheat flour$ | 18.14 | 17.89 | 17.64 | 17.39 |
| Fish protein hydrolysatea | 5.20 | 5.20 | 5.20 | 5.20 |
| Soy lecithinb | 0.50 | 0.50 | 0.50 | 0.50 |
| Egg albumin powderb | 1.00 | 1.00 | 1.00 | 1.00 |
| Fish oila |
4.00 | 4.00 | 4.00 | 4.00 |
| Vegetable oil# | 4.00 | 4.00 | 4.00 | 4.00 |
| Vitamin pre-mixc |
1.00 | 1.00 | 1.00 | 1.00 |
| Mineral pre-mixc | 1.00 | 1.00 | 1.00 | 1.00 |
| L-ascorbyl phosphateb | 0.03 | 0.03 | 0.03 | 0.03 |
| α-tocopheryl acetateb | 0.03 | 0.03 | 0.03 | 0.03 |
| Betaine hydrochlorideb | 0.10 | 0.10 | 0.10 | 0.10 |
| β-glucan powder (70.60% purity)* | 0.00 | 0.25 | 0.50 | 0.75 |
| Proximate analyses [% dry matter basis] | ||||
| Moisture | 4.86 | 5.04 | 5.20 | 4.63 |
| Dry matter | 95.14 | 94.96 | 94.80 | 95.37 |
| Crude protein |
44.37 |
44.03 | 43.54 | 44.12 |
| Crude lipid | 13.93 | 14.57 | 14.31 | 14.05 |
| Ash | 13.40 | 13.14 | 13.38 | 12.86 |
Janatha Fishmeal and Oil Products, Udupi, Karnataka, India.
Himedia Laboratories, Mumbai, India.
Procured from local market.
Prepared manually and all components from Himedia Ltd Mumbai, India.
Fortune Edible Oils and Foods, Adani Wilmar Limited, Ahmedabad, India.
β-glucan powder, sourced from Saccharomyces cerevisiae, was procured from M/s Kuber Impex Ltd, Indore, India. The active β−1,3/1,6 glucan content in the powder was 70%.
Mineral mixture (g/kg diet in cellulose): calcium carbonate (40% Ca) 0.5 g, magnesium oxide (60% Mg) 1.24 g, ferric citrate 0.2 g, potassium iodide (75% I) 4 mg, zinc sulfate (36% Zn) 0.4 g, copper sulfate (25% Cu) 0.3 g, manganese sulfate (33% Mn) 0.3 g, dibasic calcium phosphate (23% Ca, 18% P) 2 g, cobalt sulfate 2 mg, potassium hydrogen phosphate (28% K; 22% P) 3 g sodium selenite (30% Se) 3 mg, Sodium chloride 0.4 g.
Vitamin mixture (mg/kg diet in cellulose): DL-α tocopherol acetate 100 mg, retinyl acetate 0.75 mg DL cholecalciferol 6 mg, thiamin 10 mg, riboflavin 15 mg, pyridoxine 10 mg, vit. B 0.05 mg, nicotinic acid 10 mg, folic acid 0.2 mg, inositol 500 mg, biotin 0.1 mg, calcium panthotenate 20 mg, Cyanocobalamine 0.02 mg, choline chloride 1000 mg.
2.2. Experimental design and feeding of fish
The study was carried out at the golden mahseer hatchery facility of ICAR-DCFR, Bhimtal, in a flow-through system. For this, 144 golden mahseer, Tor putitora (Hamilton, 1822) juveniles were assigned into four treatment groups at random in triplicates. Twelve mahseer juveniles (av. weight 6.33 ± 0.1 g) were placed in every replicate rectangular fiber-reinforced plastic (FRP) tray (46.0 cm x 46.0 cm x 19.5 cm; length, width, and depth) with a meshed-bottom sheet for water movement. Three such replicate trays were kept in an FRP trough (partially submerged; 220.0 cm x 50.0 cm x 40.0 cm) for each treatment group. Prior to the trial, fish were acclimated in the experimental unit for 30 days on the basal diet.
Fish in each treatment group were fed with their respective diet ad libitum for five weeks post acclimation. Any uneaten feed and fecal waste was siphoned every morning. A moderate water flow (7–10 L/hour) was maintained in each trough, and aerators were used for maintaining dissolved oxygen (DO). The quality of water was checked on a regular basis (pH: 7.3−7.8; temperature: 22.1–23.4 °C; DO: 5.8–6.4 mg/L; total hardness: 124–139 mg/L; alkalinity: 73–86 mg/L; ammonia: <0.01 mg/L and nitrite: <0.1 mg/L). A multi-parameter water testing system was used to keep track of temperature, DO, and pH (HQ40d, Hach, USA). While, commercially available kits were used to measure alkalinity, total hardness, ammonia, and nitrite (HiMedia, India). After five weeks of feeding, the fish were batch weighed and weight gain percentage was calculated using the formula
2.3. Ammonia challenge
After the feeding trial, the water flow in experimental units/troughs was stopped, and golden mahseer juveniles in each treatment (16 juveniles each, in duplicates) were exposed to ammonia-nitrogen (total ammonium nitrogen (TAN) concentration of 10 mg L−1) for 96 h. The group that received the control diet (0% β-glucan) was split into two groups: one served as control, and the other was exposed to ammonia. A stock solution of NH4Cl (1000 mg/L) was used as a source of TAN, and an appropriate volume of the freshly made stock solution was added to get the desired concentration in the experimental units. Throughout the ammonia challenge trial, each tank's total water volume was kept at 300 L. Continuous aeration was ensured to provide the optimum DO, and feeding was stopped during the challenge period. Every morning, the fecal matter was removed by siphoning to reduce ammonia buildup, and around 75% water of the units was replenished, and TAN levels were adjusted by adding the required volume of NH4Cl stock solution. Further, the TAN levels were measured frequently [24] to avoid differences between nominal and actual TAN concentrations in the units. After 96 h of ammonia exposure, fish were sampled for gene expression study.
2.4. Sampling
After 96 h of ammonia exposure, six juveniles (n = 6 each) from different treatment groups were randomly collected, anaesthetized (50 µL clove oil/L of water), and euthanized. The fish were then dissected out aseptically, and tissues samples (liver and gill) were collected and quickly frozen in liquid nitrogen for further gene expression study.
2.5. Quantitative expression of various genes
TRIzol reagent (Invitrogen, USA) was used to extract total RNA (as per the manufacturer's recommendations). A spectrophotometer (Biotek, VT, USA) and agarose gel electrophoresis were used to evaluate the amount and quality of the extracted RNA, respectively. The PrimeScript First-Strand cDNA synthesis kit was then used to reverse transcribe the RNA and create complementary DNA (cDNA) (Takara, USA). Real-time qPCR was conducted using the synthesized cDNA as a template. The relative expression levels of targeted genes namely catalase (cat), superoxide dismutase (sod), glutathione-s-transferase (gst), inducible nitric oxide synthase (inos), toll-like receptor 4 (tlr4), toll-like receptor 5 (tlr5), interleukin-1β (il-1β), complement factor 3 (c3), immunoglobulin light chain (igl), major histocompatibility complex 1 (mch1), and aquaporins (aqp namely aquaporins 1a: aqp1a; aquaporins 3a: aqp3a; and aquaporins 3b: aqp3b) were evaluated in a qPCR machine (Applied Biosystem, USA) using SYBRPremix Ex TaqII (Takara, USA) and gene-specific primers (designed according to the method described by Thornton and Basu [25], using Primer 3.0 (http://primer3.ut.ee/) software, Table 2) under standardized reaction conditions [21]. Then, utilizing 18S ribosomal RNA (18 s) as the reference gene, the relative expression (fold-change) of the various genes was assessed using Pfaffl's [26] mathematical model.
Table 2.
Real-time qPCR primers.
| Primers | Sequence (5′−3′) | Ta (°C) |
Amplification efficiency (%) | Amplicon size (bp) | Accession no. |
|---|---|---|---|---|---|
| cat | F- CCGATGAGGGCAACTGGGAT R-TGAGAGTGGATGAAGGACGGAA |
60 | 106.9% | 91 | MG821473.1 |
| sod1 | F- GGCACCGTTCATTTCGAGCA R-TGATGCAGCCGTTTGTGTTGT |
60 | 101.3% | 130 | KY569539.1 |
| gst | F-AGAGGGAAGATGGAGTCGGT R-AACCAAAGGCACCTGCTGAAA |
60 | 101.9% | 138 | XM_026224781.1 |
| inos | F-AGGTGGCAGAGAGATGAACGAA R-AGGAGGCTTTGTGAGGGTGG |
60 | 103.8% | 95 | HQ589354.1 |
| il1β | F-CAACCTGTGTGCCTGGGAAT R-CTCGTTCGGGTCATCGGCTTT |
60 | 107.5% | 135 | MN193586.1 |
| tlr4 | F-GCGGGACTTTCAAGCAGGGA R-AGCGACACCAGGCACTATCAA |
60 | 107.6% | 119 | LC441112.1 |
| tlr5 | F- CTGATCCTCAGGACTGGCAC R- GTTCCGTTGTGACTGCAACC |
60 | 104.6% | 109 | XP_016373368 |
| mhc1 | F- GTTTTGCCCTGGTGTTCCAC R- CTTCCTCGTCTCCAGTCACG |
60 | 99.9% | 220 | AFO38429 |
| c3 | F-AGCAGGAGGTGGAAGGGACA R-CAGGTTTGGGACACTCAGGCA |
60 | 106.9% | 113 | MN531579.2 |
| igl | F- GTGTGTGGCCAGTAAGGGAT R- ACCGGGACTCAGATTGACAC |
60 | 109.1% | 94 | BAB90985 |
| aqp1a | F-GCGAGCGGTATCGTGTATGG R-AGCTGGAAGGTAGCGAAGAGT |
60 | 97.1 | 116 | LC069004.1 |
| aqp3a | F-GGCAAAGGAAGACATCAGGACC R-CCACAACCAAACATCACCAGGA |
60 | 97.8 | 154 | LC069008.1 |
| aqp3b | F-CCCAGTTGATTCTAAGCGGAGG R-GATTCCCAATGTAGCGGCGAA |
60 | 101.2 | 83 | LC069010.2 |
| 18s | F-ATTGACGGAAGGGCACCACC R-CAGACAAATCGCTCCACCAACT |
60 | 102.47% | 166 | SRX2442156 |
2.6. Statistical analysis
The data were subjected to a one-way analysis of variance using the statistical program SPSS (Version 19.0), then Tukey's post-hoc multiple comparisons. A probability value of less than 0.05 was used to determine statistical significance. The data is presented as a mean with standard error (SE).
2.7. Ethical statement
The Institutional Animal Ethics Committee at the ICAR-DCFR, Bhimtal, Nainital, Uttarakhand, provided the guidelines for all of the experimental and sampling techniques used in this study.
3. Results
3.1. Growth of mahseer juveniles
Dietary intake of β-glucan for five weeks had no significant influence on the weight gain (%) of golden mahseer compared to those fed on the basal diet (Fig. 1). Although there was no statistically significant difference, the fish fed β-glucan (0.25–0.75%) reported a somewhat greater mean weight gain percentage than the control group.
Fig. 1.
Effect of dietary β-glucan supplementation on growth performance of golden mahseer juveniles. Data are expressed as Mean ± SE, n = 36.
3.2. Differential expression of genes encoding anti-oxidative enzymes and inducible nitric oxide synthase (inos) of golden mahseer juveniles exposed to ammonia
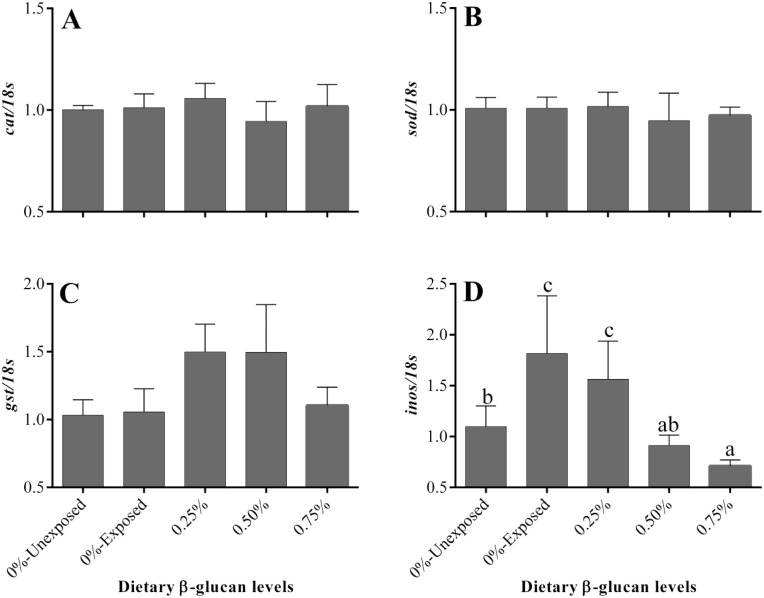
Feeding β-glucan differentially modulated the mRNA levels of anti-oxidative genes in gill and liver tissues of ammonia-exposed golden mahseer juveniles. In gills, the abundance of antioxidative (catalase, superoxide dismutase, and glutathione-s-transferase) genes was significantly higher in ammonia-challenged fish that received a control diet, and β-glucan intake down-regulated their expression in different magnitudes (Fig. 2). For instance, the transcript abundance of branchial catalase was lowest in fish that received 0.75% β-glucan, while all the β-glucan fed fish irrespective of their intake levels recorded significantly the lowest mRNA levels of gst in the gill. On the other hand, the hepatic expression of all the studied antioxidative (cat, sod, and gst) genes were not found to vary among the various dietary groups exposed to ammonia (Fig. 3). Likewise, in the liver, transcript levels of inos were significantly higher in control and 0.25% β-glucan fed groups after 96 h of ammonia challenge. Higher intake of β-glucan (0.5 and 0.75%) reduced the abundance of hepatic inos (Fig. 3).
Fig. 2.
Effect of dietary β-glucan supplementation on the branchial expression of genes encoding anti-oxidative enzymes of golden mahseer juveniles exposed to ammonia. Different superscripts (a, b) above the bars in each panel, if any, indicate significant difference (p < 0.05). Data are expressed as Mean ± SE, n = 6.
Fig. 3.
Effect of dietary β-glucan supplementation on the hepatic expression of genes encoding anti-oxidative enzymes and inducible nitric oxide synthase (inos) of golden mahseer juveniles exposed to ammonia. Different superscripts (a, b, c) above the bars in each panel, if any, indicate significant difference (p < 0.05). Data are expressed as Mean ± SE, n = 6.
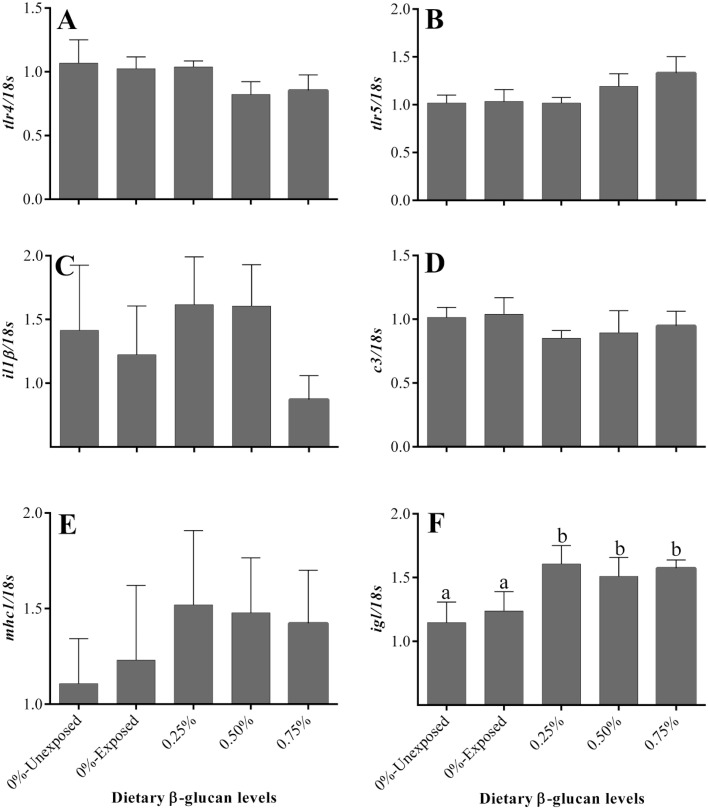
3.3. Immune genes expression in the liver of golden mahseer juveniles exposed to ammonia
To study the effect of β-glucan intake on ammonia stress tolerance, we analyzed the mRNA expression levels of several immune genes in the liver. The transcript abundance of toll-like receptors (tlr4 and tlr5), interleukin-1β (il-1β), complement factor 3 (c3), and major histocompatibility complex 1 (mhc1) were not significantly varied among the different dietary groups after 96 h of ammonia challenge (Fig. 4A-E). Conversely, the mRNA expression of igl was significantly upregulated in all the β-glucan fed groups (0.25, 0.5 and 0.75%) following the 96 h ammonia challenge (Fig. 4F). Although there was no statistically significant difference, the fish fed β-glucan (0.25–0.75%) recorded slightly greater transcript levels of mhc1 than the control group with higher inter-individual variation.
Fig. 4.
Effect of dietary β-glucan supplementation on the hepatic expression of immune genes of golden mahseer juveniles exposed to ammonia. Different superscripts (a, b) above the bars in each panel, if any, indicate significant difference (p < 0.05). Data are expressed as Mean ± SE, n = 6.
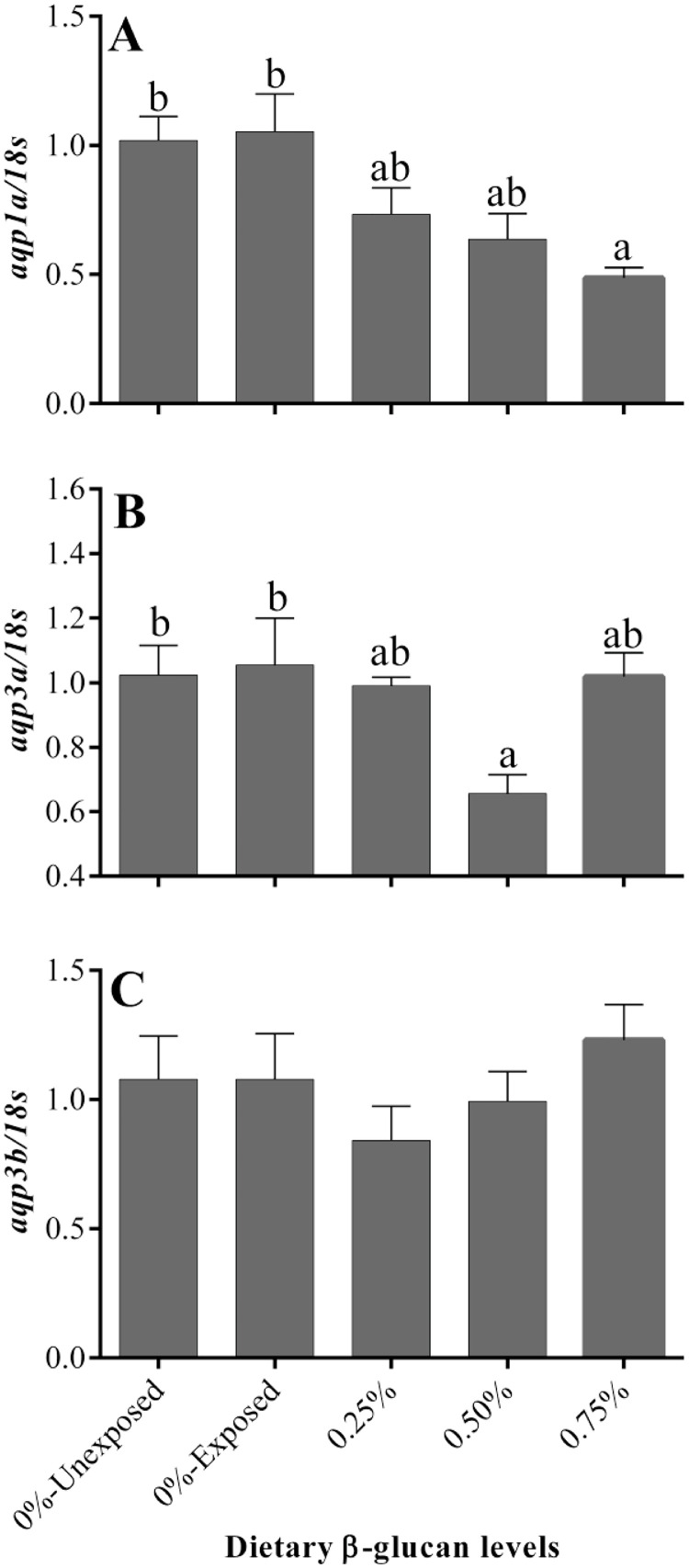
3.4. Differential expression of aquaporins in ammonia-challenged golden mahseer juveniles
β-Glucan intake differentially modulated the transcript abundance of branchial aquaporins in ammonia-exposed golden mahseer juveniles. The highest expression of branchial aquaporin 1a and 3a was noticed in ammonia-challenged fish that received the control diet, and β-glucan supplementation significantly reduced their transcript abundance (Fig. 5A and B). Among the β-glucan dietary groups, the lowest mRNA abundance of aquaporin 1a was noticed in ammonia-challenged fish fed with 0.75% β-glucan. On the other hand, similar mRNA expression of aquaporin 3b was evidenced in the gill of ammonia-challenged groups irrespective of their β-glucan intake levels (Fig. 5C).
Fig. 5.
Effect of dietary β-glucan supplementation on the branchial expression of aquaporins of golden mahseer juveniles exposed to ammonia. Different superscripts (a, b) above the bars in each panel, if any, indicate significant difference (p < 0.05). Data are expressed as Mean ± SE, n = 6.
4. Discussion
It is known that functional feed additives and adequate nutrition stimulate fish growth and immune system and potentiate tolerance to different husbandry stressors in aquaculture. Although β-glucan has shown promise in increasing immunity and stress resistance, the results of different studies are highly variable and inconsistent depending upon the type of stressor, fish species, β-glucan source, feeding duration, dose etc. [27]. Nevertheless, little is known about its effect on the expression of water channel proteins/aquaporins and nitric oxide synthase during the ammonia challenge. Hence, in this study, we assessed the effects of dietary supplementation of β-glucan, sourced from Saccharomyces cerevisiae, on antioxidative, immune, and aquaporin gene expression in golden mahseer juveniles exposed to ammonia stress for 96 h.
In the current study, β-glucan administration had no significant effect on the weight gain (%) of golden mahseer juveniles. Our findings are in line with earlier studies on mahseer, grass carp, sea bass, and Nile tilapia, where glucan supplementation had little to no effect on growth performance [21,[28], [29], [30], [31]]. The administration of β-glucan, on the other hand, has been shown to enhance growth in a few other species [32], [33], [34], [35]. Previous studies have indicated that hyper-ammonia stress may generate free radicals such as ROS, RNS, and NO., imparting oxidative stress [1,7]. The free radicals/ROS produced under stressful conditions can be eliminated by an enzyme system consisting of superoxide dismutase (SOD), catalase, glutathione-s-transferase, etc. [10]. Hence, natural anti-oxidants or compounds with anti-oxidant characteristics have been widely used to ameliorate the deleterious effects of stressors/ROS. In this context, the anti-oxidative capacity of β-glucan is well elucidated in various animal models suggests that glucan can serve as a powerful free-radical scavenger, and that macrophages specifically phagocytose and sequester glucan through its receptors on macrophages [36,37]. In this study, the free radical scavenging potential of β-glucan might have resulted in significant down-regulation of various anti-oxidative defense genes in the gills of ammonia-challenged fish, which is consistent with earlier reports of Kayali et al. [36]. Similarly, β-glucan shown to reduce hypoxia-induced oxidative damage in yellow croaker by reducing ROS production [38]. On the contrary, significantly higher activities of antioxidative enzymes were documented in β-glucan fed tilapia and Pacific white shrimp upon ammonia stress [39,40]. The increased sensitivity of the gill to water pollutants, such as ammonia, as it is the organ that is in direct contact with the aquatic environment and the stressor, is a plausible explanation for the significant changes in the transcript abundance of branchial anti-oxidant genes compared to the liver.
Concerning inducible nitric oxide synthase (inos), ammonia exposure upregulated its hepatic expression in fish that received a basal diet. Like our observation, induction of inos, leading to increased production of NO, has been reported in catfish and freshwater prawn under ammonia stress [8,9,41,42]. They believed that induction of inos leads to higher endogenous production of NO as a protective strategy to counteract ammonia-induced oxidative stress. But, NO at higher levels, induce nitrosative stress and favors cell cycle arrest and apoptosis [43]. In the present study, glucan intake (0.5 and 0.75%) was shown to reduce NO production in ammonia-challenged golden mahseer juveniles by down-regulating the transcript abundance of inos at par with the unexposed fish, indicating a low amount of oxidative/nitrosative stress in these glucan fed groups. A similar reduction in mRNA levels of inos was recently reported in the catfish Pangasianodon hypophthalmus exposed to multiple stressors and fed with diets supplemented with zinc nanoparticles [44]. However, in-depth studies are further necessary to understand the precise molecular mechanism by which glucan down-regulates inos expression.
Studies showed that immunological markers such as cytokines, c3 proteins, toll-like receptors, etc. are sensitive to stress [45,46]. Further, exposure to environmental toxicants/pollutants known to affect the expression of immune genes following oxidative stress and the release of pro-inflammatory mediators through the toll-like receptor signaling pathway [46]. In the present study, different groups fed with graded levels of β-glucan when exposed to ammonia showed no substantial difference in the expression levels of the immune genes studied, except for the igl. This may be explained by the fact that a short duration (96 h) of sub-lethal ammonia exposure may be insufficient to influence/trigger the immune system at the molecular level. Presumably, the increased mRNA levels of igl in β-glucan fed fish after ammonia challenge may be attributable to the increased antibody production in these groups. Our results are in line with an earlier study in carp wherein the authors reported a decline in immunoglobulin production and the number of IgM secreting cells in response to stress [47]. Further, a reduction in albumin:globulin ratio was reported in stressed Labeo rohita administered with β-glucan suggesting increased immunoglobulin production [48]. Earlier studies reported that the duration of exposure to stressors (acute and chronic stress) affects the fish immune system differently [49,50]. Similarly, innate immune markers like lysozyme and total serum protein concentrations were not significantly influenced by β-glucan in Nile tilapia exposed to hypoxia for nine hours [51]. On the other hand, the significant upregulation of igl in all the β-glucan fed ammonia-challenged groups suggests that ammonia-induced stress activates immunoglobulin synthesis in golden mahseer juveniles fed with β-glucan. It has already been documented that β-glucan can influence immunoglobulins synthesis in stressed/infected fish [52,53].
Aquaporins are aqueous channel proteins that play key roles in osmoregulation by selectively permeating solute molecules such as glycerol, urea, and ammonia [54,55]. According to studies, aquaporins are responsive to ammonia stress and hence ammonia tolerance in fish is linked to aquaporin 1 and 3 [56], [57], [58], [59]. In the present study, significantly low branchial mRNA expression of aqp1a and aqp3a was evidenced in glucan-fed fish when exposed to ammonia, suggesting a reduction of ammonia uptake from the water. Similar to our findings, Ip et al. [57] observed significant reductions in aqp1aa mRNA expression in the gills and skin of ammonia-exposed Anabas testudineus as a protective strategy to reduce ammonia influx. A similar reduction of aqp3 mRNA levels was observed in several fish species when transferred from freshwater to seawater [60], [61], [62]. Conversely, few researchers reported upregulation of branchial aqp3 in ammonia-exposed fish to facilitate ammonia excretion [58,63]. In the present study, the branchial aqp3b expression remained unchanged in different ammonia-exposed groups regardless of glucan intake. The mechanism by which β-glucan regulates the expression of aquaporins in golden mahseer exposed to environmental ammonia is still unclear, and further studies are necessary.
5. Conclusion
Our study demonstrated that β-glucan supplementation minimized the effect of ammonia-induced stress in golden mahseer juveniles as shown by the low transcription of genes involved in antioxidative defense and NO production. Further, glucan intake down-regulated branchial expression of aqp1a and aqp3a suggesting reduced ammonia uptake. On the other hand, mRNA expression of most of the studied immune genes was not significantly affected by β-glucan intake in ammonia-challenged fish. Taken together, this study showed that dietary intake of β-glucan improved resistance to ammonia stress to a certain degree, probably through activating the anti-oxidative system and reducing NO production via down regulating inos.
Funding
The study was financially supported by the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India (vide reference no. BT/PR26920/AAQ/3/884/2017).
CRediT authorship contribution statement
Alexander Ciji: Conceptualization, Investigation, Formal analysis, Writing – original draft. Priyanka H. Tripathi: Formal analysis. Anupam Pandey: Formal analysis. Md Shahbaz Akhtar: Conceptualization, Funding acquisition, Investigation, Data curation, Formal analysis, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors duly acknowledge the Director, ICAR-DCFR for facilitating all the logistics support during the study. The sincere help extended by Sh. Harish Chandra and Sh. Prem Pandey for rearing and maintenance of the experimental fish is greatly acknowledged.
Data availability
Data will be made available on request.
References
- 1.Shi Q., Wen X., Zhu D., Aweya J.J., Li S. Protective effects of Sargassum horneri against ammonia stress in juvenile black sea bream, Acanthopagrus schlegelii. J. Appl. Phycol. 2019;31:1445–1453. doi: 10.1007/s10811-018-1637-5. [DOI] [Google Scholar]
- 2.Rama S., Manjabhat S.N. Protective effect of shrimp carotenoids against ammonia stress in common carp, Cyprinus carpio. Ecotoxicol. Environ. Saf. 2014;107:207–213. doi: 10.1016/j.ecoenv.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Tucker C.S., van der Ploeg M. Seasonal changes in water quality in commercial channel catfish ponds in Mississippi. J. World Aquac. Soc. 1993;24:473–481. doi: 10.1111/j.1749-7345.1993.tb00576.x. [DOI] [Google Scholar]
- 4.Hargreaves J.A. Nitrogen biogeochemistry of aquaculture ponds-Review. Aquaculture. 1998;166:181–212. doi: 10.1016/s0044-8486(98)00298-1. [DOI] [Google Scholar]
- 5.Tan X., Sun Z., Zhu X., Ye C. Dietary supplementation with taurine improves ability to resist ammonia stress in hybrid snakehead (Channa maculatus♀ × Channa argus♂) Aquac. Res. 2018;49:3400–3410. doi: 10.1111/are.13804. [DOI] [Google Scholar]
- 6.Mirghaed A.T., Fayaz S., Hoseini S.M. Effects of dietary 1,8-cineole supplementation on serum stress and antioxidant markers of common carp (Cyprinus carpio) acutely exposed to ambient ammonia. Aquaculture. 2019;509:8–15. doi: 10.1016/j.aquaculture.2019.04.071. [DOI] [Google Scholar]
- 7.Sinha A.K., AbdElgawad H., Giblen T., Zinta G., De Rop M., Asard H., Blust R., Boeck G.D. Anti-oxidative defences are modulated differentially in three freshwater teleosts in response to ammoniainduced oxidative stress. PLoS One. 2014;9:e95319. doi: 10.1371/journal.pone.0095319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhury M.G., Saha N. Influence of environmental ammonia on the production of nitric oxide and expression of inducible nitric oxide synthase in the freshwater air-breathing catfish (Heteropneustes fossilis) Aquat. Toxicol. 2012;116–117:43–53. doi: 10.1016/j.aquatox.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Kumari S., Choudhury M.G., Saha N. Hyper-ammonia stress causes induction of inducible nitric oxide synthase gene and more production of nitric oxide in air-breathing magur catfish, Clarias magur (Hamilton) Fish Physiol. Biochem. 2019;45:907–920. doi: 10.1007/s10695-018-0593-y. [DOI] [PubMed] [Google Scholar]
- 10.Ciji A., Sahu N.P., Pal A.K., Akhtar M.S. Physiological changes in Labeo rohita during nitrite exposure: detoxification through dietary vitamin E. Comput. Biochem. Physiol. Part C. 2013;158:122–129. doi: 10.1016/j.cbpc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Wang L., Wang H., Shi W., Zhang Y., Zhu C., Pan Z., Xue C., Li J. Identification of genes and signaling pathways associated with immune response of Hemibarbus maculatus (Bleeker, 1871) to ammonia stress. Aquaculture. 2020;524 doi: 10.1016/j.aquaculture.2020.735265. [DOI] [Google Scholar]
- 12.Borgnia M., Nielsen S., Engel A., Agre P. Cellular and molecular biology of the aquaporin water channels. Annu. Rev. Biochem. Allied. Res. India. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- 13.Weihrauch D., Wilkie M.P., Walsh P.J. Ammonia and urea transporters in gills of fish and aquatic crustaceans. J. Exp. Biol. 2009;212:1716–1730. doi: 10.1242/jeb.024851. [DOI] [PubMed] [Google Scholar]
- 14.Wu X.Y., Gatlin D.M. Effects of altering dietary protein content in morning and evening feedings on growth and ammonia excretion of red drum (Sciaenops ocellatus) Aquaculture. 2014;434:33–37. doi: 10.1016/j.aquaculture.2014.07.019. [DOI] [Google Scholar]
- 15.Ciji A., Akhtar M.S. Stress management in aquaculture: a review of dietary interventions. Rev. Aquac. 2021;13:2190–2247. doi: 10.1111/raq.12565. [DOI] [Google Scholar]
- 16.Xing X., Li M., Yuan L., Song M., Ren Q., Shi G., Meng F., Wang R. The protective effects of taurine on acute ammonia toxicity in grass carp Ctenopharynodon idellus. Fish Shellfish Immunol. 2016;56:517–522. doi: 10.1016/j.fsi.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Chen S., Yu Y., Gao Y., Yin P., Tian L., Niu J., Liu Y. Exposure to acute ammonia stress influences survival, immune response and antioxidant status of pacific white shrimp (Litopenaeus vannamei) pretreated with diverse levels of inositol. Fish Shellfish Immunol. 2019;89:248–256. doi: 10.1016/j.fsi.2019.03.072. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q.Q., Liu W.B., Zhou M., Dai Y.J., Xu C., Tian H.Y., Xu W.N. Effects of berberine on the growth and immune performance in response to ammonia stress and high-fat dietary in blunt snout bream Megalobrama amblycephala. Fish Shellfish Immunol. 2016;55:165–172. doi: 10.1016/j.fsi.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Wang L., Sun Y., Xu B., Sagada G., Chen K., Xiao J., Zhang J., Shao Q. Effects of berberine supplementation in high starch diet on growth performance, antioxidative status, immune parameters and ammonia stress response of fingerling black sea bream (Acanthopagrus schlegelii) Aquaculture. 2020;527 doi: 10.1016/j.aquaculture.2020.735473. [DOI] [Google Scholar]
- 20.Meena D.K., Das P., Kumar S., Mandal S.C., Prusty A.K., Singh S.K., Akhtar M.S., Behera B.K., Kumar K., Pal A.K., Mukherjee S.C. Beta-glucan: an ideal immunostimulant in aquaculture (a review) Fish Physiol. Biochem. 2013;39:431–457. doi: 10.1007/s10695-012-9710-5. [DOI] [PubMed] [Google Scholar]
- 21.Akhtar M.S., Tripathi P.H., Pandey A., Ciji A. β-glucan modulates non-specific immune gene expression, thermal tolerance and elicits disease resistance in endangered Tor putitora fry challenged with Aeromonas salmonicida. Fish Shellfish Immunol. 2021;119:154–162. doi: 10.1016/j.fsi.2021.09.038. [DOI] [PubMed] [Google Scholar]
- 22.Dawood M.A.O., Koshio S., Ishikawa M., Yokoyama S., El Basuini M.F., Hossain M.S., Nhu T.H., Moss A.S., Dossou S., Wei H. Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red seabream, Pagrus major. Aquac. Nutr. 2017;23:148–159. doi: 10.1111/anu.12376. [DOI] [Google Scholar]
- 23.El-Murr A.E., El Hakim Y.A., Neamat-Allah A.N.F., Baeshen M., Ali H.A. Immuneprotective, antioxidant and relative gene expression impacts of β-glucan against fipronil toxicity in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2019;94:427–433. doi: 10.1016/j.fsi.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 24.Verdouw H., Van Echteld C.J.A., Dekkers E.M.L. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 1977;12:399–402. doi: 10.1016/0043-1354(78)90107-0. [DOI] [Google Scholar]
- 25.Thornton B., Basu C. Real-time PCR (qPCR) primer design using free online software. Biochem. Mol. Biol. Educ. 2011;39:145–154. doi: 10.1002/bmb.20461. [DOI] [PubMed] [Google Scholar]
- 26.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ai Q., Mai K., Zhang L., Tan B., Zhang W., Xu W., Li H. Effects of dietary β-1, 3 glucan on innate immune response of large yellow croaker, Pseudosciaena crocea. Fish Shellfish Immunol. 2007;22:394–402. doi: 10.1016/j.fsi.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Adloo M.N., Soltanian S., Hafezieh M., Ghadimi N. Effects of long term dietary administration of β-Glucan on the growth, survival and some blood parameters of striped catfish, Pangasianodon hypophthalmus (Siluriformes: pangasiidae) Iran. J. Ichthyol. 2015;2:194–200. doi: 10.22034/iji.v2i3.75. [DOI] [Google Scholar]
- 29.Bagni M., Romano N., Finoia M.G., Abelli L., Scapigliati G., Tiscar P.G., Sarti M., Marino G. Short- and long-term effects of a dietary yeast b-glucan (Macrogard) and alginic acid (Ergosan) preparation on immune response in sea bass (Dicentrarchus labrax) Fish Shellfish Immunol. 2005;18:311–325. doi: 10.1016/j.fsi.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Lu D.L., Limbu S.M., Lv H.B., Ma Q., Chen L.Q., Zhang M.L., Du Z.Y. The comparisons in protective mechanisms and efficiencies among dietary α-lipoic acid, β-glucan and l-carnitine on Nile tilapia infected by Aeromonas hydrophila. Fish Shellfish Immunol. 2019;86:785–793. doi: 10.1016/j.fsi.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 31.Yang G., Qiu H., Yu R., Xiong L., Yan Q., Wen C., Peng M. Dietary supplementation of β-glucan, inulin and emodin modulates antioxidant response and suppresses intestinal inflammation of grass carp (Ctenopharyngodon idellus) Anim. Feed Sci. Technol. 2020;272 doi: 10.1016/j.anifeedsci.2020.114789. [DOI] [Google Scholar]
- 32.Aramli M.S., Kamangar B., Nazari R.M. Effects of dietary β-glucan on the growth and innate immune response of juvenile Persian sturgeon, Acipenser persicus. Fish Shellfish Immunol. 2015;47:606–610. doi: 10.1016/j.fsi.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Cook M.T., Hayball P.J., Hutchinson W., Nowak B.F., Hayball J.D. Administration of a commercial immunostimulant preparation, Ecoactiva as a feed supplement enhances macrophase respiratory burst and the growth rate of snapper (Pagrus auratus, Sparidae (Bloch and Schneider) in winter. Fish Shellfish Immunol. 2003;14:333–345. doi: 10.1006/fsim.2002.0441. [DOI] [PubMed] [Google Scholar]
- 34.Ji L., Sun G., Li J., Wang Y., Du Y., Li X., Liu Y. Effect of dietary β-glucan on growth, survival and regulation of immune processes in rainbow trout (Oncorhynchus mykiss) infected by Aeromonas salmonicida. Fish Shellfish Immunol. 2017;64:56–67. doi: 10.1016/j.fsi.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Misra C.K., Das B.K., Mukherjee S.C., Pattnaik P. Effect of long term administration of dietary β-glucan on immunity, growth and survival of Labeo rohita fingerlings. Aquaculture. 2006;255:82–94. doi: 10.1016/j.aquaculture.2005.12.009. [DOI] [Google Scholar]
- 36.Kayali H., Ozdag M.F., Kahraman S., Aydin A., Gonul E., Sayal A., Odabasi Z., Timurkaynak E. The antioxidant effect of b-glucan on oxidative stress status in experimental spinal cord injury in rats. Neurosurg. Rev. 2005;28:298–302. doi: 10.1007/s10143-005-0389-2. [DOI] [PubMed] [Google Scholar]
- 37.Patchen M.L., D'Alesandro M.M., Brook I., Blakely W.F., MacVittie T.J. Glucan: mechanisms involved in its “radioprotective” effect. J. Leuk. Biol. 1987;42:95–105. doi: 10.1002/jlb.42.2.95. [DOI] [PubMed] [Google Scholar]
- 38.Zeng L., Wang Y.H., Ai C.X., Zheng J.L., Wu C.W., Cai R. Effects of b-glucan on ROS production and energy metabolism in yellow croaker (Pseudosciaena crocea) under acute hypoxic stress. Fish Physiol. Biochem. 2016;42:1395–1405. doi: 10.1007/s10695-016-0227-1. [DOI] [PubMed] [Google Scholar]
- 39.Divya M., Gobi N., Iswarya A., Govindarajan M., Alharbi N.S., Kadaikunnan S., Khaled J.M., Almanaa T.N., Vaseeharan B. β-glucan extracted from eukaryotic single-celled microorganism Saccharomyces cerevisiae: dietary supplementation and enhanced ammonia stress tolerance on Oreochromis mossambicus. Microb. Pathog. 2020;139 doi: 10.1016/j.micpath.2019.103917. [DOI] [PubMed] [Google Scholar]
- 40.Wongsasak U., Chaijamrus S., Kumkhong S., Boonanuntanasarn S. Effects of dietary supplementation with β-glucan and synbiotics on immune gene expression and immune parameters under ammonia stress in Pacific white shrimp. Aquaculture. 2015;436:179–187. doi: 10.1016/j.aquaculture.2014.10.028. [DOI] [Google Scholar]
- 41.Hasan R., Koner D., Khongmawloh E., Saha N. Induction of nitric oxide synthesis: a strategy to defend against high environmental ammonia-induced oxidative stress in primary hepatocytes of air-breathing catfish, Clarias magur. J. Exp. Biol. 2020;223 doi: 10.1242/jeb.219626. [DOI] [PubMed] [Google Scholar]
- 42.Liu M., Gao Q., Sun C., Liu B., Liu X., Zhou Q., Zheng X., Xu P., Liu B. Effects of dietary tea tree oil on the growth, physiological and non-specific immunity response in the giant freshwater prawn (Macrobrachium rosenbergii) under high ammonia stress. Fish Shellfish Immunol. 2022;120:458–469. doi: 10.1016/j.fsi.2021.12.025. [DOI] [PubMed] [Google Scholar]
- 43.Thomas D.D., Ridnour L.A., Isenberg J.S., Flores-Santana W., Switzer C.H., Donzellie S., Hussain P., Vecoli C., Paolocci N., Ambs S., Colton C., Harris C., Roberts D.D., Wink D.A. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic. Biol. Med. 2008;45(1):18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar N., Kumar S., Singh A.K., Gite A., Patole P.B., Throat S.T. Exploring mitigating role of zinc nanoparticles on arsenic, ammonia and temperature stress using molecular signature in fish. J. Trace Elem. Med. Biol. 2022;74 doi: 10.1016/j.jtemb.2022.127076. [DOI] [PubMed] [Google Scholar]
- 45.Tort L. Stress and immune modulation in fish. Dev. Comput. Immunol. 2011;35:1366–1375. doi: 10.1016/j.dci.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Wei J., Zhou T., Hu Z., Li Y., Yuan H., Zhao K., Zhang H., Liu C. Effects of triclocarban on oxidative stress and innate immune response in zebrafish embryos. Chemosphere. 2018;210:93–101. doi: 10.1016/j.chemosphere.2018.06.163. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed I., Shenoy K.B. Effect of transportation stress on the humoral immunity of catla fry and fingerlings. J. Acad. Indus. Res. 2012;1:401–403. [Google Scholar]
- 48.Sahoo P.K., Mukherjee S.C. Effect of dietary -1,3 glucan on immune responses and disease resistance of healthy and aflatoxin B1-induced immunocompromised rohu (Labeo rohita Hamilton) Fish Shellfish Immunol. 2001;11:683–695. doi: 10.1006/fsim.2001.0345. [DOI] [PubMed] [Google Scholar]
- 49.Nardocci G., Navarro C., Cortés P.P., Imarai M., Montoya M., Valenzuela B., Jara P., Acuna-Castillo C., Fernandez R. Neuroendocrine mechanisms for immune system regulation during stress in fish. Fish Shellfish Immunol. 2014;40:531–538. doi: 10.1016/j.fsi.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Yarahmadi P., Miandare H.K., Fayaz S., Caipang C.M.A. Increased stocking density causes changes in expression of selected stress- and immune-related genes, humoral innate immune parameters and stress responses of rainbow trout (Oncorhynchus mykiss) Fish Shellfish Immunol. 2016;48:43–53. doi: 10.1016/j.fsi.2015.11.007. 961. [DOI] [PubMed] [Google Scholar]
- 51.de Souza F.P., de Lima E.C.S., Pandolfi V.C.F., Leite N.G., Furlan-Murari P.J., Leal C.N.S., Mainardi R.M., Suphoronski S.A., Favero L.M., Koch J.F.A., Pereira U.P., Lopera-Barrero N.M. Effect of β-glucan in water on growth performance, blood status and intestinal microbiota in tilapia under hypoxia. Aquac. Rep. 2020;17 doi: 10.1016/j.aqrep.2020.100369. [DOI] [Google Scholar]
- 52.Munir M.B., Hashim R., Nor S.A.M., Marsh T.L. Effect of dietary prebiotics and probiotics on snakehead (Channa striata) health: haematology and disease resistance parameters against Aeromonas hydrophila. Fish Shellfish Immunol. 2018;75:99–108. doi: 10.1016/j.fsi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Neamat-Allah A.N.F., Mahsoub Y.H., Mahmoud E.A. The potential benefits of dietary β-glucan against growth retardation, immunosuppression, oxidative stress and expression of related genes and susceptibility to Aeromonas hydrophila challenge in Oreochromis niloticus induced by herbicide pendimethalin. Aquac. Res. 2021;52:518–528. doi: 10.1111/are.14910. [DOI] [Google Scholar]
- 54.Ip Y.K., Chew S.F. Ammonia production, excretion, toxicity, and defense in fish: a review. Front. Physiol. 2010;1:134. doi: 10.3389/fphys.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung D., Sato J.D., Shaw J.R., Stanton B.A. Expression of aquaporin 3 in gills of the Atlantic killifish (Fundulus heteroclitus): effects of seawater acclimation. Comput. Biochem. Physiol. Part A. 2012;161:320–326. doi: 10.1016/j.cbpa.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen L.M., Zhao J., Musa-Aziz R., Pelletier M.F., Drummond I.A., Boron W.F. Cloning and characterization of a zebrafish homologue of human AQP1: a bifunctional water and gas channel. Am. J. Physiol. Regul. Integr. Comput. Physiol. 2010;299:R1163–R1174. doi: 10.1152/ajpregu.00319.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ip Y.K., Soh M.M.L., Chen X.L., Ong J.L.Y., Chng Y.R., Ching B., Wong W.P., Lam S.H., Chew S.F. Molecular characterization of branchial aquaporin 1aa and effects of seawater acclimation, emersion or ammonia exposure on its mRNA expression in the gills, gut, kidney and skin of the freshwater climbing perch, Anabas testudineus. PLoS One. 2013;8(4):e61163. doi: 10.1371/journal.pone.0061163c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kolarevic J., Takle H., Felip O., Ytteborg E., Selset R., Good C.M., Baeverfjord G., Asgard T., Terjesen B.F. Molecular and physiological responses to long-term sublethal ammonia exposure in Atlantic salmon (Salmo salar) Aquat. Toxicol. 2012;124-125:48–57. doi: 10.1016/j.aquatox.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Talbot K., Kwong R.W.M., Gilmour K.M., Perry S.F. The water channel aquaporin-1a1 facilitates movement of CO2 and ammonia in zebrafish (Danio rerio) larvae. J. Exp. Biol. 2015;218:3931–3940. doi: 10.1242/jeb.129759. [DOI] [PubMed] [Google Scholar]
- 60.Giffard-Mena I., Boulo V., Aujoulat F., Fowden H., Castille R., Charmantier G., Cramb G. Aquaporin molecular characterization in the sea-bass (Dicentrarchus labrax): the effect of salinity on AQP1 and AQP3 expression. Comput. Biochem. Physiol. Part A. 2007;148:430–444. doi: 10.1016/j.cbpa.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 61.Tipsmark C.K., Sørensen K.J., Madsen S.S. Aquaporin expression dynamics in osmoregulatory tissues of Atlantic salmon during smoltification and seawater acclimation. J. Exp. Biol. 2010;213:368–379. doi: 10.1242/jeb.034785. [DOI] [PubMed] [Google Scholar]
- 62.Tse W.K.F., Au D.W.T., Wong C.K.C. Characterization of ion channel and transporter mRNA expressions in isolated gill chloride and pavement cells of seawater acclimating eels. Biochem. Biophys. Res. Commun. 2006;346:1181–1190. doi: 10.1016/j.bbrc.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 63.Huang M., Zhang L.J., Wu M.X., Peng G.F., Zhang Y.L. Aquaporins1 and 3 in the tissues of Paramisgurnus dabryanus and their expression profiles in response to ammonia and drought. Front. Mar. Sci. 2022;9 doi: 10.3389/fmars.2022.1009679. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.