Abstract
Objective
Longer sleep duration in infancy supports cognitive and affective functioning – likely through effects on brain development. From childhood through old age, there is evidence for a close link between sleep and brain volume. However, little is known about the association between sleep duration and brain volume in infancy, a developmental period of unprecedented brain maturation. This study aimed to close this gap by assessing sleep duration across the first year of life and gray and white matter volume at 12-mo age.
Method
Infant sleep duration trajectories across the first year of life were based on maternal reports at 1, 3, 6, 9, and 12 months of age. Infant specific trajectories were generated by running a logarithmic regression for each infant and residualizing the resulting slopes for their intercept. Structural magnetic resonance imaging (MRI) scans were acquired at 12-mo age. Gray and white matter volume estimates were residualized for intracranial volume and age at scan.
Results
Data to calculate sleep trajectories was available for 112 infants. Overall, sleep duration decreased over the course of the first year of life and was best described by a logarithmic function. Of these infants, data on brain volume was available for 45 infants at 12-mo age. Infants whose sleep duration decreased less during the first year of life relative to their intercept had, on average, greater white matter volume (β = .36, p = .02). Furthermore, average sleep duration across the first year of life, and sleep duration specifically at 6 and 9 months were positively associated with white matter volume. Sleep duration during the first year of life was not significantly associated with gray matter volume at 12-mo age.
Conclusion
Sufficient sleep duration may benefit infant white matter development – possibly by supporting myelination. The fact that sleep duration was not associated with gray matter volume is in line with preclinical studies suggesting that sleep may be crucial for the balance between synaptogenesis and synaptic pruning but not necessarily relate to a net increase in gray matter volume. Supporting sleep during periods of rapid brain development and intervening in case of sleep problems may have long-term benefits for cognitive function and mental health.
Keywords: Sleep, Infancy, Brain development, Gray matter volume, White matter volume
Highlights
-
•
Sleep duration (SD) decreases over the first year of life.
-
•
SD trajectories are associated with white matter volume at 12 months.
-
•
SD during the first year is not associated with gray matter volume at 12 months.
1. Introduction
Sleep is a cornerstone of early development. Across species, most of infancy is spent asleep with significant interindividual variability in how much infants sleep (Galland et al., 2012; Kayser and Biron, 2016; Paavonen et al., 2020). Longer sleep duration in infancy is prospectively related to better cognitive development (Smithson et al., 2018; Taveras et al., 2017) and emotion regulation (Hysing et al., 2016; Morales-Muñoz et al., 2020; Williams et al., 2017). Given the trajectory of increasing brain mass, metabolic expenditure for brain growth and repair, and average sleep duration at different ages, it has been suggested that the primary function of early sleep is neuroplasticity but shifts to neural repair at approximately 2–3 years (Cao et al., 2020). This could imply that insufficient sleep in infancy is associated with detrimental consequences for brain development. However, thus far, in humans there is limited understanding of how variation in infant sleep relates to early brain development. In this study, we characterized the association between sleep duration across the first year of life and gray and white matter volume at one year of age.
Across different age groups there is evidence for a close link between sleep and brain integrity and function. In childhood, sleep disturbances are associated with smaller gray matter volumes (Kocevska et al., 2017) and shorter sleep duration is associated with smaller hippocampus volumes (Taki et al., 2012). In a large study including 37.000 adult participants, it has been shown that individuals who slept between 6 and 8 h had larger gray matter volumes in several brain regions including the orbitofrontal cortex, the hippocampus, and the cerebellum and fewer white matter hyperintensities compared to individuals who slept more or less than that (Tai et al., 2022). In older adults, sleep fragmentation is related to lower total gray matter volume (Lim et al., 2016) and lower sleep quality is associated with variation in white matter microstructure in frontal-subcortical tracts (Sexton et al., 2017). This is in line with rodent studies which show that experimental sleep deprivation affects brain micro-structure (Bellesi et al., 2018; Campbell et al., 2002; Guzman-Marin et al., 2008; Guzmán-Marín et al., 2003; Jones et al., 2019, 2021; Raven et al., 2019; Yang et al., 2014).
Most of these studies were conducted in either adolescent or adult animals. Only a few studies directly compare the role of sleep in different age groups and suggest that sleep affects the adolescent and the adult brain differently (Acosta-peña et al., 2015; Maret et al., 2012). Insufficient sleep may be more detrimental at ages when the rate of maturational change in the brain is at its peak (Li et al., 2017). While adolescence likely represents one such period, infancy is the postnatal period associated with the most significant changes in the developing brain (Knickmeyer et al., 2008). A small number of animal studies have investigated the effects of sleep deprivation in early development and provided evidence for sleep contributing to the process of early neural fine-tuning driven by experience. Disrupting rapid eye movement (REM) sleep for example interferes with neural plasticity in young cats (Bridi et al., 2015) and less efficient pruning and long-term strengthening of new synapses after a motor-learning task in young mice (Li et al., 2017). Furthermore, disrupting the circadian rhythm immediately after birth harms neuron morphology in the amygdala, hippocampus and prefrontal cortex (Ameen et al., 2022).
The far-reaching consequences of sleep deprivation in infancy for brain development likely stem from the increased susceptibility of the brain during this period of rapid developmental change. The first year of life is marked by doubling of total brain volume (Knickmeyer et al., 2008), increase in surface area by almost 80% (Li et al., 2013; Lyall et al., 2015) as well as peak in the rate of white matter myelination (Dubois et al., 2014; Girault et al., 2019). These spurts in infant brain development may be supported by long sleep durations and impaired by low sleep quantity. Infants spend on average more time asleep than awake and more time asleep than children and adults – a pattern that holds true across species (Kayser and Biron, 2016). Over the first 12 months of life, sleep duration decreases. In the first two months, infants sleep on average 14.6 h. Average sleep duration decreases to an average of 12.9 h at 6 months and seems to level off with 12 months-olds sleeping on average 12.9 h implying a non-linear trajectory (Galland et al., 2012). At the same time, sleep problems are most prevalent during infancy (Williamson et al., 2019) and a particularly great degree of variability in sleep quality can be observed during this developmental period (Iglowstein et al., 2003; Paavonen et al., 2020). In sum, sleep in infancy has the potential to play a significant role in brain development because a) the brain is changing rapidly during the first year of life, and b) fundamental processes of brain development benefit from high sleep quality and quantity. Because there is significant variation in sleep duration between infants, this may be one important aspect explaining interindividual variability in brain developmental trajectories.
The aim of this study was to investigate trajectories of sleep duration across the first year of life and the association with gray matter volume (GMV) and white matter volume (WMV) at 12-mo age. Mothers reported at 1, 3, 6, 9, and 12 months about their infants’ sleep duration. Because sleep is highly dynamic, trajectories differ between infants, and long-lasting sleep problems may have more severe consequences than more temporally isolated sleep problems (Smithson et al., 2018), we chose to characterize the trajectories of sleep across the first year of life. Most studies on infant sleep and development have only assessed sleep once or twice over the course of the first year (Bernier et al., 2010, 2013; Friedrich et al., 2017; Gibson et al., 2012; Mäkelä et al., 2020; Morales-Muñoz et al., 2020; Scher, 2005) and thus provide little insight into how changes in sleep duration over time affect development. One study with more frequent sleep assessments has shown that infants may start with similar sleep durations but may have different developmental trajectories over the following months (Smithson et al., 2018). A single measurement of sleep duration may thus be insufficient to represent infant sleep. Structural MRI scans were performed at 12-mo age. Because the decrease in sleep duration during the first year of life may not be linear, we also explored quadratic and logarithmic functions to characterize sleep trajectories. We hypothesized that the trajectory of sleep duration during the first year of life would be associated with GMV and WMV at 12-mo age. No previous study has investigated the association between trajectories of sleep duration and brain development. On the one hand, a decline in sleep duration is part of normative development (Galland et al., 2012). On the other hand, longer sleep durations have generally been associated with better developmental outcomes (Smithson et al., 2018; Taveras et al., 2017). Therefore, we expect that a smaller decrease in sleep duration relative to their intercept will be associated with greater GMV and WMV. In addition, we explored whether this was driven by higher average sleep across the first year of life and whether sleep duration at specific time points (1, 3, 6, 9, and 12 months) would be associated with GMV and WMV.
2. Methods
2.1. Study design and population
Mother-child dyads for the study were drawn from a prospective, longitudinal study at the University of California, Irvine, Development, Health and Disease Research Program. Participants were recruited during early pregnancy and represent a socio-demographically diverse convenience sample. All included infants were singletons, had no known cord, placental, or uterine anomalies, or fetal congenital malformations. Infant sleep duration was assessed through maternal reports over the course of the first year of life at 1-, 3-, 6-, 9-, and 12-mo age. At 12-mo age, structural MRI scans were performed. This study was approved by the university's IRB. Pregnant women and parents on behalf of their infants provided written informed consent. N = 147 children participated in the study from which the participants for these analyses are drawn. From this sample, data on sleep trajectories was available for 112 infants. Of those, 45 were successfully scanned at 12-mo age (Table 1 for descriptive statistics). Infants without sleep data did not differ in sociodemographic characteristics (household income, maternal education, race and ethnicity) from infants for whom this data was not available, and infants who were successfully scanned did not differ in sociodemographic characteristics from infants for whom MRI data could not be successfully obtained (ps > .05).
Table 1.
Descriptive statistics (N = 45, except birth weight percentile N = 44).
| N (%)/M(SD) | |
|---|---|
| Infant sex (female) | 18 (40%) |
| Gestational age at birth (weeks) | 39.2 (1.36) |
| Birth weight percentile | 54.68 (27.91) |
| SES index (education and income combined; 5-point scale) | 2.98 (0.95) |
| Age at MRI scan (days) | 377.09 (16.58) |
| GMV (cm3) | 631.27 (60.58) |
| WMV (cm3) | 268.69 (28.22) |
| ICV (cm3) | 1051.22 (102.53) |
Note. SES = socioeconomic status; WMV = white matter volume; GMV = gray matter volume. ICV = intracranial volume.
2.2. Measures
2.2.1. Infant sleep
Maternal perceptions of infant sleep over the previous two weeks were assessed using the Brief Infant Sleep Questionnaire (BISQ) (Sadeh, 2004). The BISQ consists of 13 items assessing for instance, day (7 a.m.–7 p.m.) and night (7 p.m.–7 a.m.) sleep duration, night awakenings, where and how the infant falls asleep. In this paper, only information on day and night sleep duration was included. Test-retest reliability for sleep duration obtained with the BISQ is high (r = .82 for sleep duration at night and r = 0.89 for sleep duration during the day) and sleep duration at night is correlated with actigraphy and daily sleep log estimates (Sadeh, 2004). Sleep duration was reported in hours and minutes but for the purpose of the analyses transformed into minutes. To calculate the total sleep duration, day (7 a.m.–7 p.m.) and night (7 p.m.–7 a.m.) sleep duration were summed. Data was excluded if either day or night sleep duration was missing which was the case for 15 reports. Outliers (i.e., z > ± 3.29) were adjusted per time point (Tabachnick et al., 2001) by adding or subtracting the difference between the last two acceptable values to the last acceptable value. Only infants with sleep assessments for at least three time points were included in the analyses. As a result, 112 out of 142 infants were included in the analyses. At 1-mo age, 96 reports were available, at 3-mo age 94, at 6-mo age 103, at 9-mo age 72, and at 12-mo 87 reports (Table 2).
Table 2.
Means, standard deviation and correlations for reported sleep duration and estimates based on broken stick model.
| N | M(SD) sleep duration | M(SD) sleep duration estimated by broken stick model | Correlation (p) | |
|---|---|---|---|---|
| 1 month | 96 | 15:28 (3:13) | 15:20 (2:46) | .78 (<.001) |
| 3 months | 94 | 13:47 (2:39) | 13:53 (2:02) | .98 (<.001) |
| 6 months | 103 | 13:06 (2:16) | 13:12 (1:42) | .96 (<.001) |
| 9 months | 72 | 12:26 (2:08) | 12:19 (1:32) | .95 (<.001) |
| 12 months | 87 | 12:49 (1:54) | 12:40 (1:25) | .92 (<.001) |
Note. M and SD reported in hours:minutes. N for broken stick estimates is always N = 112 since missing data is imputed. Ns for reported sleep durations and correlations are reported in the table.
2.2.2. Gray and white matter volume
Infants were scanned during natural sleep in a Siemens 3T scanner (TIM Trio, Siemens Medical System Inc., Germany) at UCI Health Nikken Imaging Center. Successful scans that passed quality control were obtained in N = 52 infants. In N = 45 of these infants sleep data was available. Prior to the scan, infants were fitted with hearing protection. The protocol included T1-weighted images which were obtained using a three-dimensional (3D) magnetization prepared rapid gradient echo (MP-RAGE) sequence (TR 2400 ms; TE 3.16 ms; TI 1200 ms; Flip Angle 8°; 6:18 min) and T2-weighted images were obtained with a turbo spin echo sequence (TR 3200 ms; TE1 13 ms; TE2 135 ms; Flip Angle 180°; 4:18 min). The spatial resolution was a 1 × 1 × 1 mm voxel for T1-weighted images and 1 × 1 × 1 mm voxel with 0.5 mm interslice gap for T2-weighted images. A multi-atlas based iterative expectation maximization segmentation algorithm was used for the tissue segmentation – identifying gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) (Cherel et al., 2015). The sum of these three tissue volumes represents the intracranial volume (ICV). GMV and WMV were residualized for ICV and age at scan and used in the statistical analyses.
2.2.3. Covariates
Length of gestation, birth weight and socio-economic status (SES) were considered as potential confounders because pre-term birth, low birth weight, and low SES have been shown to be associated with infant sleep and brain development (Brito and Noble, 2014; Luijk et al., 2019; MacKinnon et al., 2020; Padilla et al., 2015; Pascoe et al., 2019; Pesonen et al., 2009). Infant sex is related to brain volume (Knickmeyer et al., 2008). Gestational age at birth, infant sex, and birth weight were abstracted from the delivery medical records. Birth weight percentile based on infant sex and gestational age was calculated using international standards from an international prospective population-based study (Fernandes et al., 2020). Maternal SES was calculated by averaging maternal educational level and household income. Educational level was based on a 5-point scale from (1) less than high school to (5) advanced degree. Household income was recoded into a 5-point scale based on categories from ≤15,000$ to ≥100,000$. Infant sex and gestational age at birth were included as covariates in all analyses in the first step. SES and birth weight percentile were ultimately not included as covariates because they were not significantly associated with any of the sleep parameters or with GMV or WMV (Table 3).
Table 3.
Correlations (r(p)) of variables of interest with covariates (N = 45, except birth weight percentile N = 44).
| Infant sex | Gestational age at birth | SES | Birth weight percentile | |
|---|---|---|---|---|
| GMV (res.) | −.03 (.85) | .22 (.16) | .16 (.29) | .02 (.91) |
| WMV (res.) | −.15 (.34) | .13 (.39) | .08 (.61) | .08 (.60) |
| Intercept | −.05 (.77) | −.08 (.62) | .01 (.94) | −.15 (.32) |
| Slope (res.) | −.05 (.76) | .17 (.27) | −.01 (.93) | .20 (.20) |
| Average sleep | −.07 (.64) | .06 (.68) | .05 (.74) | .08 (.59) |
| Sleep at 1-mo | −.13 (.38) | −.12 (43) | .01 (.93) | .04 (.78) |
| Sleep at 3-mos | −.07 (.65) | −.01 (.93) | .07 (.63) | −.07 (.66) |
| Sleep at 6-mos | −.08 (.58) | .10 (.51) | −.02 (.90) | .20 (.21) |
| Sleep at 9-mos | −.01 (.96) | .21 (.17) | .11 (.47) | .27 (.08) |
| Sleep at 12-mos | .11 (.48) | .24 (.11) | .03 (.83) | −.08 (.62) |
Note. WMV = white matter volume; GMV = gray matter volume; Slope was residualised for intercept, WMV and GMV were residualised for intracranial volume and age at scan.
2.3. Statistical analysis
All analyses with the exception of the broken stick method were performed in SPSS (IBM Corp, 2020). For the broken stick method, we used the ‘brokenstick’ package (Van Buuren, 2022) in R Statistical Software (version 4.0.2; R Core Team, 2020). Figures were created using the package ‘ggplot2’ (Wickham, 2016).
2.3.1. Characterization of sleep trajectories across the first year of life
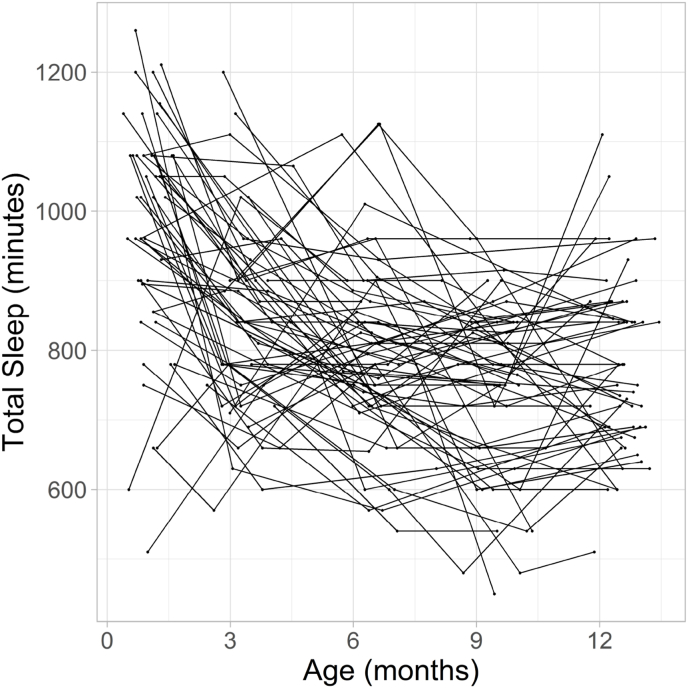
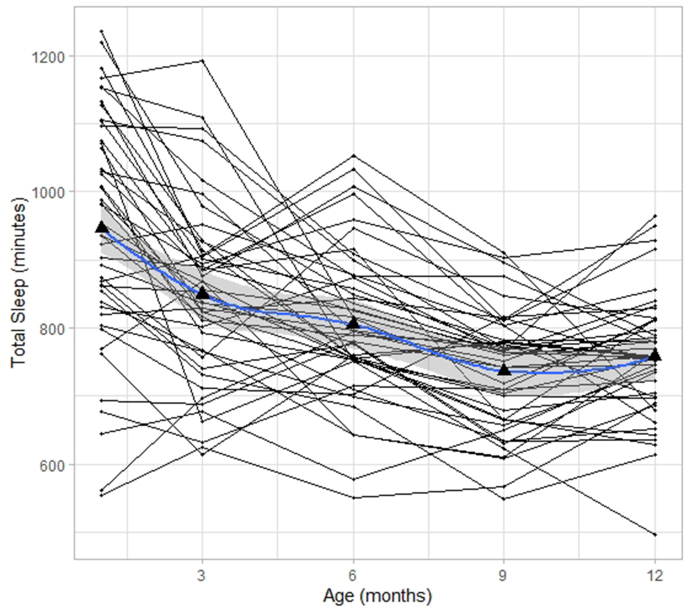
Fig. 1 displays the trajectories for sleep duration for all infants with sleep data for at least three time points. This figure illustrates that sleep duration and trajectories across the first year of life varied across infants. In Table 2, average sleep duration at each time point is reported. On average, sleep duration decreases from 1 month to 12 months. For our primary analyses, we were interested in characterizing the developmental trajectory across the first year of life. Since visual inspection suggests that sleep trajectories did not always follow a linear slope, we ran three mixed models with the five measurement points nested within subjects to determine which slope function to use in the main regression analyses. In all models, an autoregressive covariance structure was chosen as a plausible model for longitudinal data. A restricted maximum likelihood approach was used. Three functions were examined: linear, quadratic, and logarithmic. All models included a random intercept and the fixed and random effect for age. Random effects were allowed to correlate using an unstructured covariance structure. For the linear model, age was not transformed. This resulted in a model fit of BIC = 5776.13. For the quadratic model, age was centered and both the lower order linear age term and age squared were included (both fixed and random) (Faraway, 2002). This resulted in a model fit of BIC = 5749.61. For the logarithmic model, the natural logarithm of age was included. This resulted in a model fit of BIC = 5732.82. Thus, the model fit for the logarithmic function was best and was used for the main regression analyses (Seltman, 2012) to address the specific hypotheses. Infant specific intercepts and slopes were generated by running a logarithmic regression for each infant. Intercept and slope were highly, negatively correlated (r(110) = −0.89, p < .001) indicating that infants with higher intercepts (longer sleep duration in newborns) showed a steeper decrease in sleep duration across the first year of life. Since this resulted in multicollinearity in the following analyses, slope was residualized for intercept before using it as a predictor for GMV and WMV. Since most infants exhibit a negative slope, for most infants a negative values represent infants with a steeper decline in sleep duration than would be predicted from the intercept.
Fig. 1.
Spaghetti plot for sleep duration (in minutes) across the first year of life.
2.3.2. Sleep trajectories across the first year of life and brain volume at 12-mo age
Linear regression analyses were conducted to investigate the association of the sleep parameters with GMV and WMV (residualized for ICV and age at scan) at 12 months, independently. In the first step, only infant sex and gestational age at birth were included as covariates. For the two main analyses, we regressed slope (residualized for the intercept) in the second step onto GMV and WMV at 12 months. The Bonferroni-corrected p-value was .025.
2.3.3. Average sleep duration and sleep duration at individual time points across the first year of life and brain volume at 12-mo age
In additional exploratory regression analyses, we tested whether GMV and WMV at 12 months are associated with overall sleep duration across the first year of life (represented by the average of the sleep duration measures from the five time points at 1, 3, 6, 9, and 12 months) and with sleep duration at each assessment time point (1, 3, 6, 9, and 12 months). Both approaches face two challenges: missing data and irregularity in the exact age at assessment. Since sleep duration decreases on average from 1 month to 12 months, results would be biased by missing data when calculating the average. Missing data would also create a problem when testing the association between sleep duration at individual time points and brain volumes because each analysis would be conducted on a slightly different dataset with different sample sizes, which would impede comparison across time points. Furthermore, as illustrated in Fig. S1, age at measurement varies around the intended measurement time points of 1, 3, 6, 9, and 12 months. The broken stick method was developed to deal with such timing irregularities as well as with missing data (Buuren, 2020). This method predicts sleep for uniform time points for all participants even if there is irregularity and imputes data for missing time points by fitting straight lines onto observed data by using linear mixed models. We tested two models on the data set of 112 infants: one fitting data with two lines to three time points (1, 6, and 12 months) and one fitting data with four lines and five time points (1, 3, 6, 9, and 12 months). The first model with three time points had an R2 = 0.81 while the model with five time points had an R2 = 0.94 indicating that it fit the observed data better. Therefore, sleep estimates were based on the latter model. Fig. S2 displays the trajectories based on the broke stick estimates. In Table 1, we show the means and correlations between the reported sleep durations and the sleep durations estimated with the broken stick method. Overall, correlations between reported and estimated data were significant at p < .001.
3. Results
3.1. Descriptive statistics
Data on sleep trajectories as well as GMV and WMV was available in N = 45 infants. Descriptive data on sleep duration and covariates are presented in Table 1, Table 2
3.2. Main analyses
All regression models were adjusted for infant sex and gestational age at birth in the first step, however, these covariates were not significant in any of the models (ps > .25), which is likely due to the fact that GMV and WMV had been residualized for ICV and boys had significantly larger ICVs than girls (t(43) = 4.87, p < .001).
3.2.1. Sleep during the first year of life and GMV
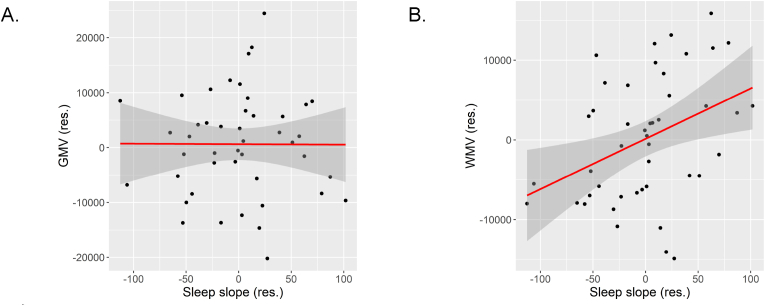
The results of the regression analyses with GMV as the dependent variable are presented in Table 4. In the primary analysis, associations of infant-specific slopes based on a logarithmic function with GMV were investigated. Slope was residualized for intercept to avoid multicollinearity and entered in the following step. Slope was not associated with GMV, either (β = −.04, p = .78, Fig. 2A). In addition, associations of average sleep duration across the first year of life and sleep duration at 1, 3, 6, 9, and 12 months were tested in separate exploratory regression analyses. Sleep durations were modelled using the broken stick method (see 2.3.1). As reported in Table 4, none of these sleep measures were significantly associated with GMV (ps > .49).
Table 4.
Linear regression results for gray matter volume (residualized for intracranial volume and age at scan).
| Independent Variable | B (SE) | β | R2 | R2 change | p |
|---|---|---|---|---|---|
| Sleep slope (res.) | −8.42 (30.51) | −.04 | .05 | .00 | .78 |
| Average sleep | −6.05 (16.23) | −.06 | .05 | .00 | .71 |
| Sleep at 1-mo | 2.56 (8.56) | .05 | .05 | .00 | .77 |
| Sleep at 3-mos | −7.66 (10.99) | −.11 | .06 | .01 | .49 |
| Sleep at 6-mos | −8.50 (13.06) | −.10 | .06 | .01 | .52 |
| Sleep at 9-mos | .0.50 (16.71) | .01 | .05 | .00 | .98 |
| Sleep at 12-mos | −10.70 (16.41) | −.10 | .06 | .01 | .52 |
Note. All models were adjusted for infant sex and gestational age at birth. Each row represents an independent model.
Fig. 2.
Scatter plot for the association between the slope of sleep duration across the first year of life (residualized for intercept) with A. gray matter volume (residualized for intracranial volume and age at scan) and B. white matter volume (residualized for intracranial volume and age at scan).
3.2.2. Sleep during the first year of life and WMV
Following the same steps as for GMV, infant-specific slopes based on a logarithmic function were regressed onto WMV. In the next step, slope (residualized for intercept) was added. Slope was significantly associated with WMV (β = .37, p = .02; see Table 5 and Fig. 2B). Since slope was residualized for intercept, this suggests infants whose sleep duration decreased less during the first year of life relative to their intercept had, on average, larger WMV. The final model explained 17% of the variance in WMV – 13% were explained by the decline in sleep duration over the first year of life. Similarly, exploratory regression analyses showed that average sleep across the first year of life was significantly associated with WMV (β = .38, p = .01) with infants who on average slept more during the first year of life having greater WMV. Sleep duration at 6 and at 9 months was also significantly associated with WMV (β = 0.31, p = .04 and β = 0.39, p = .009, respectively). The associations of sleep duration at 1, 3, and 12 months were in the same direction but were not significant (ps > .07).
Table 5.
Linear regression results for white matter volume (residualized for intracranial volume and age at scan).
| Independent Variable | B (SE) | β | R2 | R2 change | p |
|---|---|---|---|---|---|
| Sleep slope (res.) | 60.38 (23.84) | .37 | .17 | .13 | .02 |
| Average sleep | 33.66 (12.59) | .38 | .18 | .14 | .01 |
| Sleep at 1-mo | 12.89 (6.91) | .28 | .11 | .08 | .07 |
| Sleep at 3-mos | 14.42 (9.00) | .24 | .09 | .06 | .12 |
| Sleep at 6-mos | 22.34 (10.45) | .31 | .13 | .10 | .04 |
| Sleep at 9-mos | 35.14 (12.91) | .39 | .19 | .15 | .009 |
| Sleep at 12-mos | 24.02 (13.33) | .28 | .11 | .07 | .08 |
Note. All models were adjusted for infant sex and gestational age at birth. Each row represents an independent model.
4. Discussion
The aim of the present study was to investigate sleep duration trajectories across the first year of life and the association with GMV and WMV at one year of age. We found that on average, sleep duration, measured in 3-mo intervals, decreased from one month to 12 months age. Overall, this pattern of change was better described by a logarithmic model rather than a linear or quadratic one. Sleep duration at each time point as well as its trajectory over the first year of life varied between infants. The trajectory of sleep duration across the first year of life was associated with greater WMV but not GMV.
Infants whose sleep duration decreased more across the first year of life relative to their intercept tended to have smaller WMV at 12-mo age. This may be due to a slower decrease in sleep duration across the first year of life or the overall greater amount of time slept as the average sleep duration was also associated with WMV. While these explanations cannot be uncoupled in this study, the latter explanation was supported by the association between average sleep duration and WMV. This observed association between sleep and WMV was not driven by concurrent sleep – sleep at 12 months was not significantly associated with WMV. However, there were specific time points that were associated with WMV. We found that longer sleep duration specifically at 6 and 9 months age was significantly associated with smaller WMV. It is possible that between 6 and 9 months, the brain is particularly susceptible for variations in sleep durations. However, sleep at 6 and 9 months was not independent of sleep at the other time points. Infants who had longer sleep durations at 6 and 9 months were likely to have longer sleep durations at the other time points as well. Moreover, while the associations between WMV and the other time points were not significant, they were in the same direction and this study may have lacked the statistical power to detect significant associations at the other time points. Thus, it is not clear whether sleep problems at only one time point would be sufficient to affect WMV at 12 months. However, it is likely that chronic sleep problems may have negative consequences for the maturing brain (Bellesi et al., 2018; Smithson et al., 2018). The first year of life is marked by a sharp increase in WMV (Deoni et al., 2012). White matter primarily consists of axons and the number of axons, the caliber of axons, and the myelination of axons contribute to the total WMV (Paus, 2010). Postnatal development is characterized by elimination of axons and thus increases in WMV during the first year of life are more likely attributed to increases in axonal caliber and the thickness of the myelin sheath (Paus, 2010). Preclinical studies have provided evidence for sleep affecting myelination (de Vivo and Bellesi, 2019). In adolescent mice, chronic sleep loss was associated with reduced myelin thickness (Bellesi et al., 2018) and sleep deprivation in rodents has been shown to be associated with a decrease in the transcription of proteins linked to myelination (de Vivo and Bellesi, 2019). The thickness of the myelin sheath determines the speed of conduction together with axonal diameter and intermodal length and spacing (Monje, 2018). Consequently, myelination is fundamental for the timing of transmission within neural circuits and can have profound consequences for perception, motor control and complex cognitive processes (Salzer and Zalc, 2016). Because myelin responds to environmental stimulation and neural activity (Forbes and Gallo, 2017; E. M. Gibson et al., 2014), it has been postulated as an important marker of neural plasticity (Monje, 2018).
Contrary to our expectations, sleep duration during the first year of life was not associated with GMV. This is in contrast to observations in children and adults, in whom sleep duration is associated with GMV (Kocevska et al., 2017; Tai et al., 2022). Infancy is a period marked by profound changes in gray matter, with gray matter more than doubling in volume during the first year (Knickmeyer et al., 2008), driven in part by rapid synaptogenesis (Kostović et al., 2019). The overproduction of synapses is counterbalanced by selective pruning (Huttenlocher et al., 1982; Huttenlocher and Dabholkar, 1997). Interestingly, rodent studies suggest that sleep may be crucial in shaping infant gray matter development, which may not necessarily be reflected by a net gain in GMV because sleep is particularly important for maintaining an appropriate balance in the number of dendritic spines early in development through the elimination of dendritic spines (Li et al., 2017; Maret et al., 2012; Yang and Gan, 2012). In adolescent mice, it has been shown that during sleep pruning of dendritic spines dominates over dendrite formation, while the opposite was true during wakefulness (Maret et al., 2012). Brain development is not only affected by sleep but also by experiences during wakefulness. The formation and elimination of spines is likely guided by experiences during wakefulness. Li et al. (2017) suggest that REM sleep plays a role in the consolidation of motor learning because dendritic spines are selectively pruned during REM sleep but at the same time leaning-induced spines are strengthened. Thus, sleep may moderate the effect of environmental enrichment on gray matter development. Nevertheless, typical study designs with human infants may have neither the spatial nor the temporal resolution to detect how sleep balances synaptic gains and losses and consolidates learning experiences by selectively preserving the right synapses.
The findings of this study suggest that improving infant sleep may be beneficial for infant brain development, and if replicated, could help inform public policy and/or guide intervention strategies centered on the importance of early sleep patterns. Infant sleep interventions generally focus on sleep hygiene and safety (Carrow et al., 2020; Hall et al., 2015). For instance, teaching parents about aspects of sleep ecology including established bedtime routine, regular sleep and wake-up times, noisiness, and light exposure in the bedroom, may improve sleep duration (Sadeh et al., 2009). Factors like maternal stress, prenatal tobacco and alcohol exposure, and neighborhood deprivation have been associated with infant sleep and brain development that could provide promising intervention targets (Andre et al., 2020; Brito and Noble, 2014; Grimes et al., 2019; MacKinnon et al., 2020; Moog et al., 2021; Morales-Muñoz et al., 2018; Pesonen et al., 2009). Interventions on a larger scale such as improving air-quality and reducing light and noise pollution could have far-reaching effects (Johnson et al., 2018; Liu et al., 2020).
The current study used maternal reports of sleep but parents may overestimate sleep duration (Dayyat et al., 2011). Future studies should replicate these findings using a more objective method such as actigraphy (So et al., 2005). Nevertheless, average sleep duration at each time point was in line with sleep durations reported in a meta-analysis (Galland et al., 2012). Exploring aspects of sleep quality such as sleep fragmentation in addition to duration may also offer further insights into the association between sleep and brain development.
This study was correlational and does not allow drawing conclusions regarding the direction of these associations, i.e., whether variation in sleep duration induces changes in the brain or whether certain brain phenotypes are the cause for poorer sleep. However, compelling evidence from rodent studies suggests that depriving animals of sleep may causally affect brain micro-structure and impair neural plasticity. Sleep deprivation causes alterations in dendritic spine formation and density, less long-term potentiation, reductions in myelin thickness as well as neurogenesis (Bellesi et al., 2018; Campbell et al., 2002; Guzman-Marin et al., 2008; Guzmán-Marín et al., 2003; Jones et al., 2019, 2021; Raven et al., 2019; Yang et al., 2014). Concurrent assessments of brain volume with measures of sleep duration in future studies would allow conducting lagged analyses and shedding light on the direction of associations between sleep and brain development in infancy. Future studies may also explore white matter changes in greater depth by using methods such as diffusion tensor imaging and multi-parameter mapping to measure myelination (Zhang et al., 2020).
To summarize, we here report an association between sleep duration and WMV in infancy. Because sleep duration varied greatly across the first year of life and across infants, optimizing sleep during infancy may hold promise for interventions to support early brain development.
Funding
This work was supported by US PHS (NIH) grants R01 HD-060628, R01 MH-091351, UH3 OD-023349, U54HD079124-05, the National Institute of Child Health and Human Development grant K99 HD100593, ERC grant ERC-Stg 639766, and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project number 327654276 - SFB1315/2 subproject B04).
CRediT authorship contribution statement
Katharina Pittner: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing, Visualization. Jerod Rasmussen: Investigation, Data curation, Formal analysis, Writing – review & editing. Miranda M. Lim: Formal analysis, Writing – review & editing. John H. Gilmore: Formal analysis, Funding acquisition. Martin Styner: Formal analysis, Funding acquisition. Sonja Entringer: Resources, Supervision, Project administration, Funding acquisition, Writing – review & editing. Pathik D. Wadhwa: Resources, Supervision, Project administration, Funding acquisition, Writing – review & editing. Claudia Buss: Conceptualization, Methodology, Formal analysis, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare no conflicts of interest.
Handling Editor: Mark R. Opp
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbscr.2023.100091.
Contributor Information
Katharina Pittner, Email: katharina.pittner@charite.de.
Claudia Buss, Email: claudia.buss@charite.de.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- Acosta-peña E., Camacho-Abrego I., Melgarejo-Gutiérrez M., Flores G., Drucker-Colín R., García-García F. Sleep deprivation induces differential morphological changes in the hippocampus and prefrontal cortex in young and old rats. Synapse. 2015;69(1):15–25. doi: 10.1002/syn.21779. [DOI] [PubMed] [Google Scholar]
- Ameen R.W., Warshawski A., Fu L., Antle M.C. Early life circadian rhythm disruption alters brain and behavior in adulthood. Sci. Rep. 2022:1–13. doi: 10.1038/s41598-022-11335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre Q.R., McMorris C.A., Kar P., Ritter C., Gibbard W. Ben, Tortorelli C., Lebel C. Different brain profiles in children with prenatal alcohol exposure with or without early adverse exposures. Hum. Brain Mapp. 2020;41(15):4375–4385. doi: 10.1002/hbm.25130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellesi M., Haswell J.D., De Vivo L., Marshall W., Roseboom P.H., Tononi G., Cirelli C. Myelin modifications after chronic sleep loss in adolescent mice. Sleep. 2018;41(5):1–11. doi: 10.1093/sleep/zsy034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A., Beauchamp M.H., Bouvette-Turcot A.A., Carlson S.M., Carrier J. Sleep and cognition in preschool years: specific links to executive functioning. Child Dev. 2013;84(5):1542–1553. doi: 10.1111/cdev.12063. [DOI] [PubMed] [Google Scholar]
- Bernier A., Carlson S.M., Bordeleau S., Carrier J. Relations between physiological and cognitive regulatory systems: infant sleep regulation and subsequent executive functioning. Child Dev. 2010;81(6):1739–1752. doi: 10.1111/j.1467-8624.2010.01507.x. [DOI] [PubMed] [Google Scholar]
- Bridi M.C.D., Aton S.J., Seibt J., Renouard L., Coleman T., Frank M.G. Rapid eye movement sleep promotes cortical plasticity in the developing brain. Sci. Adv. 2015;1(6):1–9. doi: 10.1126/sciadv.1500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito N.H., Noble K.G. Socioeconomic status and structural brain development. Front. Neurosci. 2014;8(SEP):1–12. doi: 10.3389/fnins.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buuren S. Van. Broken stick model for irregular longitudinal data. J. Stat. Software. 2020;VV(Ii):1–47. doi: 10.1002/0471667196.ess7084.pub2. [DOI] [Google Scholar]
- Campbell I.G., Guinan M.J., Horowitz J.M. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J. Neurophysiol. 2002;88(2):1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- Cao J., Herman A.B., West G.B., Poe G., Savage V.M. Unraveling why we sleep: quantitative analysis reveals abrupt transition from neural reorganization to repair in early development. Sci. Adv. 2020;6(38) doi: 10.1126/sciadv.aba0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow J.N., Vladescu J.C., Reeve S.A., Kisamore A.N. Back to sleep: teaching adults to arrange safe infant sleep environments. J. Appl. Behav. Anal. 2020;53(3):1321–1336. doi: 10.1002/jaba.681. [DOI] [PubMed] [Google Scholar]
- Cherel M., Budin F., Prastawa M., Gerig G., Lee K., Buss C., Lyall A., Zaldarriaga Consing K., Styner M. Automatic tissue segmentation of neonate brain MR Images with subject-specific atlases. Med. Imag. 2015: Image Process. 2015;9413 doi: 10.1117/12.2082209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayyat E.A., Spruyt K., Molfese D.L., Gozal D. Sleep estimates in children: parental versus actigraphic assessments. Nat. Sci. Sleep. 2011;3:115–123. doi: 10.2147/NSS.S25676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vivo L., Bellesi M. The role of sleep and wakefulness in myelin plasticity. Glia. 2019;67(11):2142–2152. doi: 10.1002/glia.23667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni S.C.L., Dean D.C., O’Muircheartaigh J., Dirks H., Jerskey B.A. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage. 2012;63(3):1038–1053. doi: 10.1016/j.neuroimage.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J., Dehaene-Lambertz G., Kulikova S., Poupon C., Hüppi P.S., Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48–71. doi: 10.1016/j.neuroscience.2013.12.044. [DOI] [PubMed] [Google Scholar]
- Faraway J.J. vol. 168. University of Bath; 2002. Practical regression and anova using R. (Texts in Statistical Science Series). [DOI] [Google Scholar]
- Fernandes M., Villar J., Stein A., Staines Urias E., Garza C., Victora C.G., Barros F.C., Bertino E., Purwar M., Carvalho M., Giuliani F., Wulff K., Abubakar A.A., Kihara M., Cheikh Ismail L., Aranzeta L., Albernaz E., Kunnawar N., Di Nicola P., et al. INTERGROWTH-21st Project international INTER-NDA standards for child development at 2 years of age: an international prospective population-based study. BMJ Open. 2020;10(6) doi: 10.1136/bmjopen-2019-035258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes T.A., Gallo V. All wrapped up: environmental effects on myelination. Trends Neurosci. 2017;40(9):572–587. doi: 10.1016/j.tins.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M., Wilhelm I., Mölle M., Born J., Friederici A.D. The sleeping infant brain anticipates development. Curr. Biol. 2017;27(15):2374–2380.e3. doi: 10.1016/j.cub.2017.06.070. [DOI] [PubMed] [Google Scholar]
- Galland B.C., Taylor B.J., Elder D.E., Herbison P. Normal sleep patterns in infants and children: a systematic review of observational studies. Sleep Med. Rev. 2012;16(3):213–222. doi: 10.1016/j.smrv.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Gibson E.M., Purger D., Mount C.W., Goldstein A.K., Lin G.L., Wood L.S., Inema I., Miller S.E., Bieri G., Zuchero J.B., Barres B.A., Woo P.J., Vogel H., Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain erin. Science. 2014;344(6183) doi: 10.1126/science.1254446. 1252304–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R., Elder D., Gander P. Actigraphic sleep and developmental progress of one-year-old infants. Sleep Biol. Rhythm. 2012;10:77–83. doi: 10.1111/j.1479-8425.2011.00525.x. [DOI] [Google Scholar]
- Girault J.B., Cornea E., Goldman B.D., Knickmeyer R.C., Styner M., Gilmore J.H. White matter microstructural development and cognitive ability in the first 2 years of life. Hum. Brain Mapp. 2019;40(4):1195–1210. doi: 10.1002/hbm.24439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes M., Camerota M., Propper C.B. Neighborhood deprivation predicts infant sleep quality. Sleep Health. 2019;5(2):148–151. doi: 10.1016/j.sleh.2018.11.001.Neighborhood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Marin R., Suntsova N., Bashir T., Nienhuis R., Szymusiak R., McGinty D. Rapid eye movement sleep deprivation contributes to reduction of neurogenesis in the hippocampal dentate gyrus of the adult rat. Sleep. 2008;31(2):167–175. doi: 10.1093/sleep/31.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán-Marín R., Suntsova N., Steward D.R., Gong H., Szymusiak R., McGinty D. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J. Physiol. 2003;549(2):563–571. doi: 10.1113/jphysiol.2003.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W.A., Hutton E., Brant R.F., Collet J.P., Gregg K., Saunders R., Ipsiroglu O., Gafni A., Triolet K., Tse L., Bhagat R., Wooldridge J. A randomized controlled trial of an intervention for infants’ behavioral sleep problems. BMC Pediatr. 2015;15(1):1–12. doi: 10.1186/s12887-015-0492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher P.R., de Courten C., Garey L.J., Van der Loos H. Synaptogenesis in human visual cortex—evidence for synapse elimination during normal development. Neurosci. Lett. 1982;33:247–252. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- Huttenlocher Peter R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387(2):167–178. doi: 10.1002/(SICI)1096-9861(19971020)387:2<167::AID-CNE1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Hysing M., Sivertsen B., Garthus-Niegel S., Eberhard-Gran M. Pediatric sleep problems and social-emotional problems. A population-based study. Infant Behav. Dev. 2016;42:111–118. doi: 10.1016/j.infbeh.2015.12.005. [DOI] [PubMed] [Google Scholar]
- IBM Corp . IBM Corp; 2020. IBM SPSS Statistics for Windows. Version 27.0. [Google Scholar]
- Iglowstein I., Jenni O.G., Molinari L., Largo R.H. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111(2):302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- Johnson D.A., Billings M.E., Hale L. Environmental determinants of insufficient sleep and sleep disorders: implications for population health. Curr. Epidem. Rep. 2018:61–69. doi: 10.1007/s40471-018-0139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.E., Chau A.Q., Olson R.J., Moore C., Wickham P.T., Puranik N., Guizzetti M., Cao H., Meshul C.K., Lim M.M. Early life sleep disruption alters glutamate and dendritic spines in prefrontal cortex and impairs cognitive flexibility in prairie voles. Curr. Res. Neurobiol. 2021;2 doi: 10.1016/j.crneur.2021.100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.E., Opel R.A., Kaiser M.E., Chau A.Q., Quintana J.R., Nipper M.A., Finn D.A., Hammock E.A.D., Lim M.M. Early-life sleep disruption increases parvalbumin in primary somatosensory cortex and impairs social bonding in prairie voles. Sci. Adv. 2019;5(1):1–12. doi: 10.1126/sciadv.aav5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser M.S., Biron D. Sleep and development in genetically tractable model organisms. Genetics. 2016;203(1):21–33. doi: 10.1534/genetics.116.189589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer R.C., Gouttard S., Kang C., Evans D., Wilber K., Smith J.K., Hamer R.M., Lin W., Gerig G., Gilmore J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocevska D., Muetzel R.L., Luik A.I., Luijk M.P.C.M., Jaddoe V.W., Verhulst F.C., White T., Tiemeier H. The developmental course of sleep disturbances across childhood relates to brain morphology at age 7: the generation r study. Sleep. 2017;40(1) doi: 10.1093/sleep/zsw022. [DOI] [PubMed] [Google Scholar]
- Kostović I., Sedmak G., Judaš M. Neural histology and neurogenesis of the human fetal and infant brain. Neuroimage. 2019;188:743–773. doi: 10.1016/j.neuroimage.2018.12.043. [DOI] [PubMed] [Google Scholar]
- Li G., Nie J., Wang L., Shi F., Lin W., Gilmore J.H., Shen D. Mapping region-specific longitudinal cortical surface expansion from birth to 2 years of age. Cerebr. Cortex. 2013;23(11):2724–2733. doi: 10.1093/cercor/bhs265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Ma L., Yang G., Gan W.B. REM sleep selectively prunes and maintains new synapses in development and learning. Nat. Neurosci. 2017;20(3):427–437. doi: 10.1038/nn.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A.S.P., Fleischman D.A., Dawe R.J., Yu L., Arfanakis K., Buchman A.S., Bennett D.A. Regional neocortical Gray matter structure and sleep fragmentation in older adults. Sleep. 2016;39(1):227–235. doi: 10.5665/sleep.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wu T., Liu Q., Wu S., Chen J.C. Air pollution exposure and adverse sleep health across the life course: a systematic review. Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijk M.P.C.M., Kocevska D., Tham E.K.H., Gaudreau H., Reiss I.K.M., Duijts L., Cai S., Hillegers M.H.J., Jaddoe V.W.V., Tiemeier H., Broekman B.F.P., El Marroun H. Gestational age at birth and sleep duration in early childhood in three population-based cohorts. Sleep Med. X. 2019;1:1–6. doi: 10.1016/j.sleepx.2019.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall A.E., Shi F., Geng X., Woolson S., Li G., Wang L., Hamer R.M., Shen D., Gilmore J.H. Dynamic development of regional cortical thickness and surface area in early childhood. Cerebr. Cortex. 2015;25(8):2204–2212. doi: 10.1093/cercor/bhu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon A.L., Tomfohr-Madsen L., Tough S. Neighborhood socio-economic factors and associations with infant sleep health. Behav. Sleep Med. 2020:1–13. doi: 10.1080/15402002.2020.1778478. 00(00) [DOI] [PubMed] [Google Scholar]
- Mäkelä T.E., Peltola M.J., Saarenpää-Heikkilä O., Himanen S.L., Paunio T., Paavonen E.J., Kylliäinen A. Night awakening and its association with executive functioning across the first two years of life. Child Dev. 2020;91(4):e937–e951. doi: 10.1111/cdev.13326. [DOI] [PubMed] [Google Scholar]
- Maret S., Faraguna U., Nelson A.B., Cirelli C., Tononi G. Sleep and wake modulate spine turnover in the adolescent mouse cortex. Nat. Neurosci. 2012;14(11):1418–1420. doi: 10.1038/nn.2934.Sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M. Myelin plasticity and nervous system function. Annu. Rev. Neurosci. 2018;41:61–76. doi: 10.1146/annurev-neuro-080317-061853. [DOI] [PubMed] [Google Scholar]
- Moog N.K., Nolvi S., Kleih T.S., Styner M., Gilmore J.H., Rasmussen J.M., Heim C.M., Entringer S., Wadhwa P.D., Buss C. Prospective association of maternal psychosocial stress in pregnancy with newborn hippocampal volume and implications for infant social-emotional development. Neurobiol. Stress. 2021;15 doi: 10.1016/j.ynstr.2021.100368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Muñoz I., Lemola S., Saarenpää-Heikkilä O., Kylliäinen A., Pölkki P., Paunio T., Broome M.R., Paavonen E.J. Parent-reported early sleep problems and internalising, externalising and dysregulation symptoms in toddlers. BMJ Paediatrics Open. 2020;4(1) doi: 10.1136/bmjpo-2019-000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Muñoz I., Nolvi S., Mäkelä T., Eskola E., Korja R., Fernandes M., Karlsson H., Paavonne E.J., Karlsson L. Sleep during infancy, inhibitory control and working memory in toddlers: findings from the FinnBrain cohort study. BMC Sleep Sci. Pract. 2020 (under revision) [Google Scholar]
- Morales-Muñoz I., Saarenpää-Heikkilä O., Kylliäinen A., Pölkki P., Porkka-Heiskanen T., Paunio T., Paavonen E.J. The effects of maternal risk factors during pregnancy on the onset of sleep difficulties in infants at 3 months old. J. Sleep Res. 2018;27(5) doi: 10.1111/jsr.12696. [DOI] [PubMed] [Google Scholar]
- Paavonen E.J., Saarenpää-Heikkilä O., Morales-Munoz I., Virta M., Häkälä N., Pölkki P., Kylliäinen A., Karlsson H., Paunio T., Karlsson L. Normal sleep development in infants: findings from two large birth cohorts. Sleep Med. 2020;69:145–154. doi: 10.1016/j.sleep.2020.01.009. [DOI] [PubMed] [Google Scholar]
- Padilla N., Alexandrou G., Blennow M., Lagercrantz H., Ådén U. Brain growth gains and losses in extremely preterm infants at term. Cerebr. Cortex. 2015;25(7):1897–1905. doi: 10.1093/cercor/bht431. [DOI] [PubMed] [Google Scholar]
- Pascoe M.J., Melzer T.R., Horwood L.J., Woodward L.J., Darlow B.A. Altered grey matter volume, perfusion and white matter integrity in very low birthweight adults. Neuroimage: Clinical. 2019;22(March) doi: 10.1016/j.nicl.2019.101780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: myelin or axon? Brain Cognit. 2010;72(1):26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Pesonen A.K., Räikkönen K., Matthews K., Heinonen K., Paavonen J.E., Lahti J., Komsi N., Lemola S., Järvenpää A.L., Kajantie E., Strandberg T. Prenatal origins of poor sleep in children. Sleep. 2009;32(8):1086–1092. doi: 10.1093/sleep/32.8.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; 2020. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Raven F., Meerlo P., Van der Zee E.A., Abel T., Havekes R. A brief period of sleep deprivation causes spine loss in the dentate gyrus of mice. Neurobiol. Learn. Mem. 2019;160(January 2018):83–90. doi: 10.1016/j.nlm.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A. A brief screening questionnaire for infant sleep problems: validation and findings for an Internet sample. Pediatrics. 2004;113(6):e570–e577. doi: 10.1542/peds.113.6.e570. [DOI] [PubMed] [Google Scholar]
- Sadeh A., Mindell J.A., Luedtke K., Wiegand B. Sleep and sleep ecology in the first 3 years: a web-based study. J. Sleep Res. 2009;18(1):60–73. doi: 10.1111/j.1365-2869.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- Salzer J.L., Zalc B. Myelination. Curr. Biol. 2016;26(20):R971–R975. doi: 10.1016/j.cub.2016.07.074. [DOI] [PubMed] [Google Scholar]
- Scher A. Infant sleep at 10 months of age as a window to cognitive development. Early Hum. Dev. 2005;81(3):289–292. doi: 10.1016/j.earlhumdev.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Seltman H.J. Experimental Design and Analysis. Carnegie Mellon University; 2012. Mixed models; pp. 357–378. [Google Scholar]
- Sexton C.E., Zsoldos E., Filippini N., Griffanti L., Winkler A., Mahmood A., Allan C.L., Topiwala A., Kyle S.D., Spiegelhalder K., Singh-Manoux A., Kivimaki M., Mackay C.E., Johansen-Berg H., Ebmeier K.P. Associations between self-reported sleep quality and white matter in community-dwelling older adults: a prospective cohort study. Hum. Brain Mapp. 2017;38(11):5465–5473. doi: 10.1002/hbm.23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithson L., Baird T., Tamana S.K., Lau A., Mariasine J., Chikuma J., Lefebvre D.L., Subbarao P., Becker A.B., Turvey S.E., Sears M.R., Beal D.S., Pei J., Mandhane P.J. Shorter sleep duration is associated with reduced cognitive development at two years of age. Sleep Med. 2018;48:131–139. doi: 10.1016/j.sleep.2018.04.005. [DOI] [PubMed] [Google Scholar]
- So K., Buckley P., Adamson T.M., Horne R.S.C. Actigraphy correctly predicts sleep behavior in infants who are younger than six months, when compared with polysomnography. Pediatr. Res. 2005;58(4):761–765. doi: 10.1203/01.PDR.0000180568.97221.56. [DOI] [PubMed] [Google Scholar]
- Tabachnick B.G., Fidell L.S., Osterlind S.J. 2001. Using Multivariate Statistics. [Google Scholar]
- Tai X.Y., Chen C., Manohar S., Husain M. Impact of sleep duration on executive function and brain structure. Commun. Biol. 2022;5(1):1–10. doi: 10.1038/s42003-022-03123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y., Hashizume H., Thyreau B., Sassa Y., Takeuchi H., Wu K., Kotozaki Y., Nouchi R., Asano M., Asano K., Fukuda H., Kawashima R. Sleep duration during weekdays affects hippocampal gray matter volume in healthy children. Neuroimage. 2012;60(1):471–475. doi: 10.1016/j.neuroimage.2011.11.072. [DOI] [PubMed] [Google Scholar]
- Taveras E.M., Rifas-Shiman S.L., Bub K.L., Gillman M.W., Oken E. Prospective study of insufficient sleep and neurobehavioral functioning among school-age children. Acad. Pediat. 2017;17(6):625–632. doi: 10.1016/j.acap.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buuren S. 2022. Broken Stick Model for Irregular Longitudinal Data. [Google Scholar]
- Wickham H. Springer-Verlag; 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- Williams K.E., Berthelsen D., Walker S., Nicholson J.M. A developmental cascade model of behavioral sleep problems and emotional and attentional self-regulation across early childhood. Behav. Sleep Med. 2017;15(1):1–21. doi: 10.1080/15402002.2015.1065410. [DOI] [PubMed] [Google Scholar]
- Williamson A.A., Mindell J.A., Hiscock H., Quach J. Child sleep behaviors and sleep problems from infancy to school-age. Sleep Med. 2019;63:5–8. doi: 10.1016/j.sleep.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Gan W.B. Sleep contributes to dendritic spine formation and elimination in the developing mouse somatosensory cortex. Dev. Neurobiol. 2012;72(11):1391–1398. doi: 10.1002/dneu.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Lai C.S.W., Cichon J., Ma L., Li W., Gan W.B. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344(6188):1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Shi J., Wei H., Han V., Zhu W., Hospital T., Science C. Neonate and infant brain development from birth to 2 years assessed using MRI-based quantitative susceptibility mapping. Neuroimage. 2020:349–360. doi: 10.1016/j.neuroimage.2018.10.031.Neonate. [DOI] [PMC free article] [PubMed] [Google Scholar]