Summary
Radiation is commonly used in the treatment of many cancers. However, its effects on anti-tumor immune responses are incompletely understood. Here, we present a detailed immunological analysis of two tumors from a patient with multiple non-small cell lung cancer metastases to the brain. One tumor was resected without treatment; the second was irradiated to a total dose of 30 Gy and resected following further progression. Comprehensive single-cell analysis reveals a substantially reduced immune cell fraction in the irradiated tumor, including the depletion of tissue-resident macrophages and infiltration of pro-inflammatory monocytes. Despite the presence of similar somatic mutations in both tumors, radiation is associated with the depletion of exhausted, tumor-resident T cell clones and their replacement by circulating clones unlikely to contribute to tumor-specific immunity. These results provide insight into the local effects of radiation on anti-tumor immunity and raise important considerations for the combination of radiation and immunotherapy.
Keywords: cancer immunotherapy, T cells, CD8+ T cells, CD4+ T cells, lung cancer, radiation, stereotactic radiosurgery, brain metastasis, metastasis, single-cell RNA sequencing
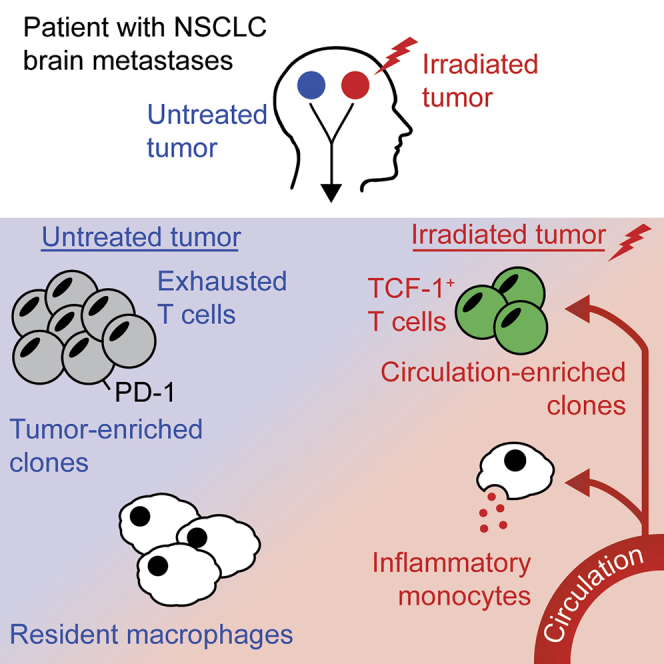
Graphical abstract
Highlights
-
•
Detailed analysis of a matched pair of untreated and irradiated NSCLC metastases
-
•
Radiation is associated with reduced overall immune infiltration
-
•
Pro-inflammatory monocytes infiltrate the treated tumor
-
•
Tumor-enriched T cell clones are depleted in the irradiated lesion
The effects of radiation on the tumor immune microenvironment are unclear. Hudson et al. report a detailed characterization of an autologous pair of non-small cell lung cancer brain metastases. Radiation is associated with extensive immune remodeling, including infiltration of inflammatory monocytes and depletion of tumor-enriched CD8+ T cells.
Introduction
Promoting and generating anti-tumor immunity is an emerging and important strategy to treat cancer. Immune checkpoint blockade (ICB), which restricts the function of inhibitory signaling molecules expressed by exhausted T cells, is now a mainstay in the treatment of many cancer types.1 However, the poor response of many patients to ICB has spurred the search for combination therapies to improve their efficacy. In many cases, this involves targeting multiple immune checkpoint receptors present on T cells, such as PD-1 and CTLA-4.2 Another active area of investigation is the combination of ICB with radiation therapy.3
Radiation has been employed to directly kill cancer cells for over a century. With the recent advent of cancer immunotherapy, radiation has also been studied for the ability to promote anti-tumor immunity and thus synergize with ICB.4,5 However, the differential effects of radiation and ICB within the tumor microenvironment (TME) pose a paradox for such strategies6: immune cells—particularly lymphocytes—are highly sensitive to death by radiation, yet immunotherapies rely on the survival and expansion of these same cells.7,8 Thus, in order to design optimal therapies combining ICB and radiation, it is critical to determine the effects of radiation on tumor-specific T cell responses.
Here, we report a study of two brain metastases resected from a single patient with non-small cell lung cancer (NSCLC). One tumor was resected without treatment, and the second tumor was resected following stereotactic radiosurgery (SRS), which delivers a high dose of conformal radiation to the tumor or surgical resection cavity.9 We show that, despite similar mutational profiles between the two tumors, radiation therapy is associated with dramatic changes in the tumor immune microenvironment. The irradiated tumor was infiltrated by fewer immune cells in total, with higher frequencies of interleukin 1β (IL-1β)-expressing monocytes. T cell infiltration was dramatically lower in the irradiated sample. Because these are autologous samples, we can directly compare immune receptor sequences between the two tumors, providing insight into the antigen specificity of T cells in the untreated and irradiated tumors. While the T cells in the irradiated tumor had a more functional phenotype, radiation was associated with the depletion of tumor-enriched clones and increased infiltration of circulating clones, indicating the likely ablation of tumor-specific T cells and their replacement by circulating bystander clones.
Results
Case history
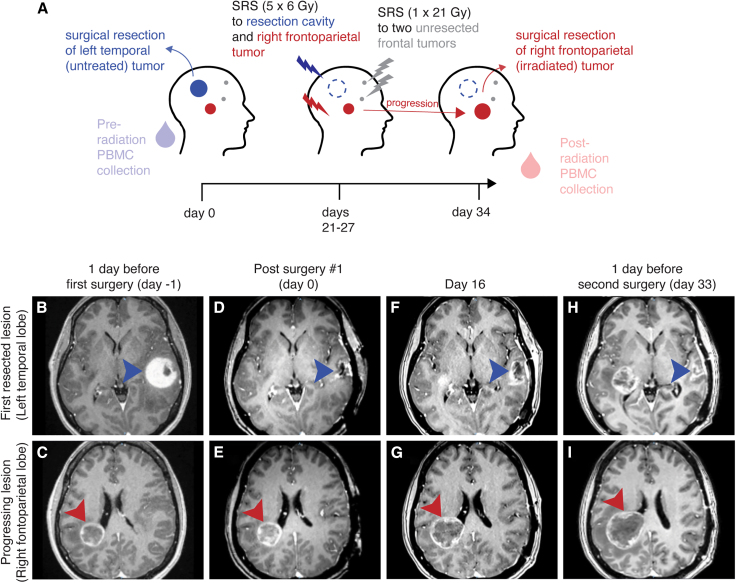
A patient with NSCLC was treated at Emory University Hospital for multiple brain metastases. One large left temporal tumor was surgically resected without additional therapy (termed the untreated tumor sample), and SRS to the resection cavity and three other tumors was performed 21–27 days post-surgery (Figure 1A). The resection cavity and a right frontoparietal lobe metastasis (diameter 3 cm) each received 30 Gy in 5 fractions of 6 Gy each (Figure S1A). The two other lesions, one in the right frontal lobe and the other in the left frontal lobe, each measuring 0.5 cm in diameter, were each treated to 21 Gy in a single fraction (Figures S1B–S1D).
Figure 1.
Case history of a patient with two resected brain metastases
(A) A patient presented with four NSCLC metastases. A large left temporal tumor was surgically resected. The resection cavity (dark blue) and a right frontoparietal lesion (dark red) were treated with 5 × 6 Gy stereotactic radiosurgery (SRS). Two smaller frontal lesions were treated with 1 × 21 Gy. The right frontoparietal tumor progressed throughout treatment and was resected 34 days after the initial surgery. Blood was collected and PBMCs isolated at the time of the initial (pre-radiation) and second (post-radiation) surgeries.
(B–I) T1 post-contrast magnetic resonance imaging (MRI) throughout the course of treatment. Arrowheads point to the untreated (dark blue) or irradiated (dark red) tumors.
The right frontoparietal tumor progressed through radiation and was surgically resected 34 days after the first operation (termed the irradiated tumor sample). The patient was prescribed dexamethasone (10 mg bolus +4 mg/6 h) 2 days prior to the first surgery, tapered, and restarted with an identical regimen 3 days prior to the second surgery. No other systemic treatment was administered. Tumor and peripheral blood mononuclear cells (PBMCs) were collected at the time of each surgery (termed the pre-radiation PBMCs and post-radiation PBMCs, respectively).
Lower immune infiltration in the irradiated tumor
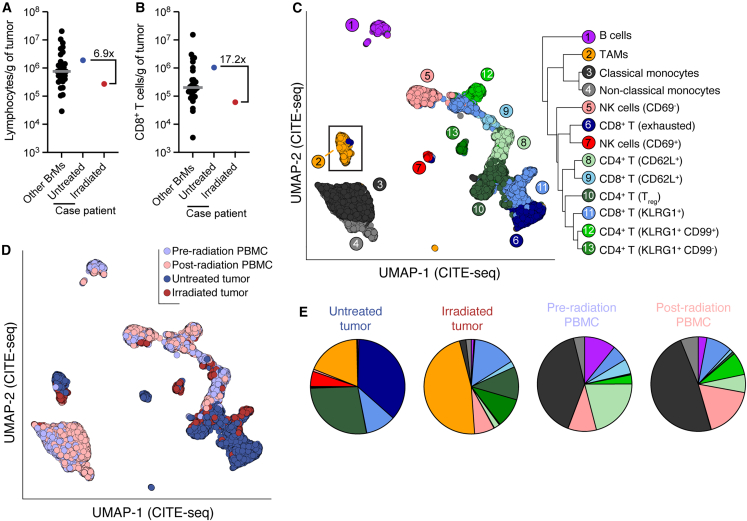
We quantified the immune infiltrate of each tumor by flow cytometry: the untreated tumor was infiltrated by 1.9 × 106 lymphocytes and 1 × 106 CD8+ T cells per gram of tissue; the irradiated tumor was infiltrated by 2.7 × 105 and 6.1 × 104 lymphocytes and CD8+ T cells per gram (Figures 2A and 2B). The untreated tumor ranked in the upper quartile of 31 immunotherapy- and radiation-naive brain metastasis samples in our previously reported cohort.10 The irradiated tumor was in the lower quintile of all brain metastases (Figures 2A and 2B) and contained the lowest CD8+ T cell infiltration among our previously reported cohort of 14 lung metastases to the brain.10 To identify radiation-associated changes in immune infiltrate in a comprehensive manner, we performed RNA sequencing, T cell receptor (TCR) sequencing, and cellular indexing of transcriptomes and epitopes (CITE) sequencing on single CD45+ cells isolated from the untreated tumor, irradiated tumor, and pre-radiation and post-radiation PBMCs (Figure S2). Our CITE-seq panel included 137 antibodies specific for 130 distinct cell antigens.
Figure 2.
Reduced and altered immune cell infiltration in the irradiated tumor
(A and B) Flow cytometric quantification of total lymphocytic (A) and CD8+ (B) T cell infiltration into the autologous untreated and irradiated tumors compared with brain metastases from 30 other individuals.10 Gray bars indicate medians. BrMs, brain metastases.
(C) Uniform manifold approximation and projection (UMAP) and clustering of 45,947 cells sequenced from circulating and tumor-infiltrating CD45+ cells. Protein expression data (CITE-seq) was used for clustering and projection.
(D) UMAP with cells colored by tissue of origin.
(E) Frequency of clusters among CD45+ cells in each sample.
Clustering on CITE-seq data identified thirteen broad CD45+ cell types with varying distributions between tumor-infiltrating and circulating cells (Figures 2C–2E; Table S1). Notably, CD8+ T cell frequency among CD45+ cells was reduced from 47% in the untreated tumor to 17% in the irradiated tumor (Figure 2E), in line with flow cytometry results (55%–22%; Figures 2A and 2B). The frequency of tumor-associated macrophages (TAMs) among CD45+ cells was 2.5-fold higher in the irradiated tumor (Figure 2E). In contrast to these drastic changes between the untreated and irradiated tumors, circulating CD45+ subsets were broadly similar before and after treatment, indicating a localized—as opposed to systemic—remodeling of the anti-tumor immune response by radiation (Figure 2E).
Radiation-associated changes in tumor-infiltrating myeloid cells
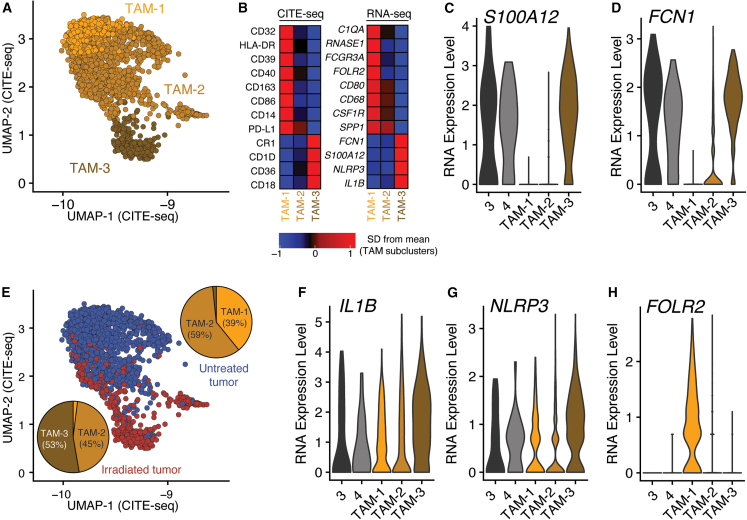
Given the higher frequency of TAMs in the irradiated tumor, we performed a detailed analysis to determine phenotypic differences between the two tumor samples. Microglial markers SALL1, TMEM119, and P2RY12 were minimally expressed among tumor-infiltrating cells, suggesting that microglia did not highly infiltrate the brain metastases in this patient (Figure S3). We performed subclustering of TAMs based on gene expression and found three subsets: TAM-1, TAM-2, and TAM-3 (Figure 3A; Table S2). TAM-1s expressed high protein levels of major histocompatibility complex (MHC) class II, CD40, and CD86, suggesting a role in promoting anti-tumor adaptive immune responses (Figure 3B). TAM-3s more closely resembled monocytes, with expression of FCN1, a marker of classical monocytes,11 and S100A12, a gene expressed in classical monocytes and downregulated during monocyte-to-macrophage differentiation12 (Figures 3C and 3D). TAM-2s appeared to be an intermediate population with shared characteristics with both the TAM-1 and TAM-3 subclusters.
Figure 3.
Distinct tumor-associated macrophage (TAM) populations infiltrate an irradiated and untreated brain metastasis
(A) UMAP projection of TAM subclusters (see inset in Figure 2C).
(B) Relative protein (left) and gene (right) expression of selected markers among TAM subclusters.
(C and D) Expression of monocyte markers S100A12 and FCN1 among monocyte clusters (3 and 4) and TAM subclusters (TAM-1, TAM-2, and TAM-3).
(E) UMAP projection of tumor-infiltrating TAMs, colored by tumor of origin. Pie charts show composition of TAMs in each tumor.
(F and G) Expression of monocyte activation-associated genes IL1B, which encodes IL-1β, and NLRP3, which encodes a key member of the inflammasome.
(H) Expression of FOLR2, which encodes the folate receptor β.
While TAM-2s were present in both tumors, TAM-1s were predominately found in the untreated tumor and TAM-3s in the irradiated tumor (Figure 3E). These data are consistent with pro-inflammatory monocyte infiltration of tumors after radiation13 and also indicate a loss of tumor-resident MHC II+, FOLR2+ macrophages that efficiently prime effector CD8+ T cells and are associated with tumor control and patient survival14,15 (Figures 3F–3H).
Radiation-associated phenotypic and clonotypic alterations in tumor-infiltrating T cells
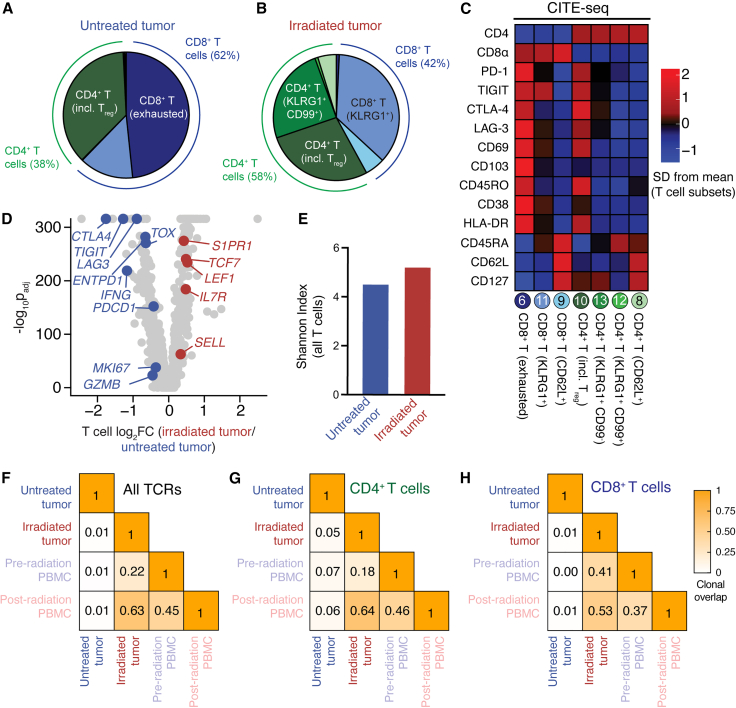
T cell phenotype was also strikingly different between the irradiated and untreated tumors. In the untreated tumor, half of all T cells belonged to the exhausted CD8+ T cell cluster (Figures 2E, 4A, and 4B), characterized by expression of residence and checkpoint molecules such as PD-1, TIGIT, CTLA-4, LAG-3, CD69, and CD103 (Figure 4C). Exhausted CD8+ T cells were virtually absent in the irradiated tumor (Figure 4B), a striking contrast from our previous study of 31 radiation-naive brain metastases.10 Overall, T cells in the irradiated tumor had a more memory- or progenitor-like phenotype compared with the untreated tumor, with higher expression of genes encoding TCF-1 (TCF7), CD127 (IL7R), and L-selectin/CD62L (SELL; Figure 4D).
Figure 4.
Dramatic differences in the phenotype and antigen specificity of tumor-infiltrating T cells in the untreated and irradiated tumors
(A and B) Frequency of each T cell cluster among tumor-infiltrating T cells in the (A) untreated and (B) irradiated tumors.
(C) Selected CITE-seq (protein) relative expression differences between T cell clusters.
(D) Volcano plot showing gene expression differences between total T cells from the irradiated and untreated tumors.
(E) Diversity of all T cell clones in the untreated and irradiated tumors as calculated by the Shannon diversity index.
(F–H) Clonal overlap (Morisita-Horn index) of all T cell receptor sequences between samples (F), those expressed in CD4+ T cells (G), or in CD8+ T cells (H). All T cells with a detected T cell receptor β (TCRβ) gene were used in TCR analyses.
The study of autologous samples allowed us to directly compare TCR sequences between the untreated and irradiated tumors. CD4+ and CD8+ T cells in the untreated tumor expressed a more clonal, extremely private TCR repertoire that was unshared with circulating T cells (Figures 4E–4H). Virtually no clonal overlap was detectable between the untreated tumor and circulating T cells from either time point (Figures 4F–4H). In contrast, high TCR overlap was present between the irradiated tumor and circulating T cells, particularly with those clones circulating at the time of the second surgical resection (Figures 4F–4H). This finding suggests near-total depletion of tumor-resident T cells by radiation and their subsequent replacement with circulating T cell clones. Given the exhausted phenotype of T cells in the untreated tumor and the low frequency of tumor-specific CD8+ T cells in the blood of patients with immunotherapy-naive cancer,16,17 it is likely that most of these newly infiltrating T cells after radiation are not tumor specific. This interpretation is consistent with higher gene expression of effector- and division-associated genes (GZMB, IFNG, MKI67) in the untreated tumor and low expression of activation markers such as HLA-DR and CD38 in the irradiated tumor (Figures 4C and 4D). The lack of very early, TCR-driven activation markers such as CD69 and CD25 in the irradiated tumor also argues against nascent antigen-specific T cell activation in the untreated tumor (Figures S4A and S4B). Thus, despite their more functional phenotypic appearance (Figure 4D), CD8+ T cells in the irradiated tumor may not contribute meaningfully to tumor-specific immunity.
Shared mutational and transcriptional profiles between the untreated and irradiated tumors
The striking differences in TCR repertoires between the untreated and irradiated tumors indicate the recognition of distinct sets of antigens by the infiltrating T cells in each tumor. To determine the somatic mutations and gene expression profiles of each tumor, we performed mRNA sequencing and whole-genome sequencing (WGS) on both tumors as well as PBMCs collected from each time point.
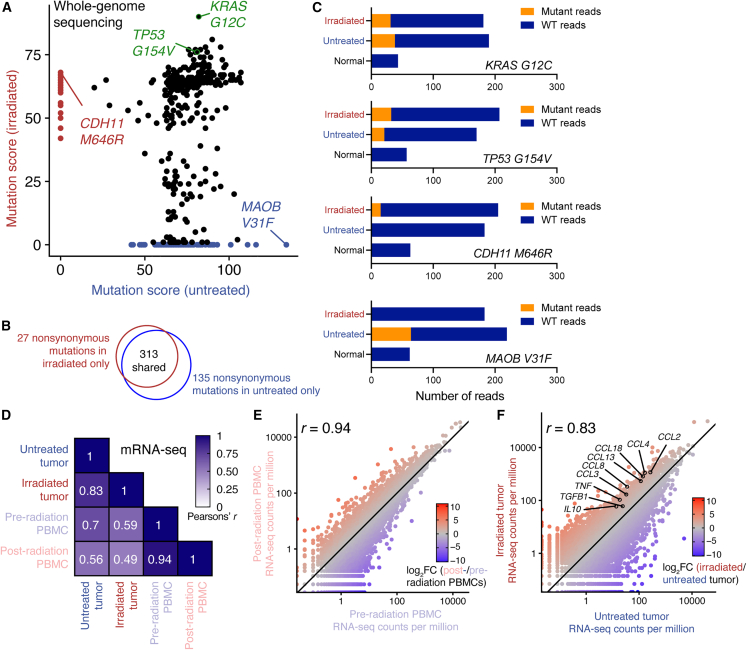
WGS was performed to depths of 167× and 179× on the untreated and irradiated tumors, respectively, and to 44× and 50× on the pre- and post-radiation PBMC samples, respectively. 475 single-nucleotide variations that caused non-synonymous changes in protein sequence were identified; 313 of these (66%) were shared between the tumors, including likely driver mutations TP53 G115V and KRAS G12C (Figures 5A–5C). No EGFR mutations were detected in either tumor.
Figure 5.
The untreated and irradiated tumors exhibit similar genetic and transcriptomic profiles
Whole-genome and mRNA sequencing were performed on both tumor samples.
(A) Mutation quality scores in the untreated and irradiated tumors for each of 475 non-synonymous single-nucleotide variants. A higher-quality score indicates a greater probability for the mutated allele to be present at a significantly different frequency in the tumor and normal (PBMC) samples.
(B) Venn diagram showing overlap in the two samples’ non-synonymous mutational profiles.
(C) Example frequencies of shared (KRAS G12C, TP53 G154V) and unshared (CDH11 M646R, MAOB V31F) somatic mutations in the tumor and normal samples.
(D) Correlation of gene expression (measured by mRNA sequencing) between each sequenced sample.
(E) Correlation of gene expression between pre- and post-radiation PBMCs.
(F) Correlation of gene expression between the untreated and irradiated tumors. Selected cytokine genes are shown; see also Figure S5.
In (E) and (F), a slope of 1 is indicated with a solid line. mRNA was isolated from lymphocyte-depleted tumors (see STAR Methods).
mRNA sequencing revealed a largely similar transcriptional profile (r = 0.94) between pre- and post-radiation PBMCs (Figures 5D and 5E). The untreated and irradiated tumors also exhibited broadly similar gene expression (r = 0.83; Figure 5F), but differences were evident in gene set enrichment analysis and cytokine gene expression (Figures 5F and S5; Table S3). Notably, human leukocyte antigen (HLA) gene expression, which encodes MHC molecules, was similar or slightly higher in the irradiated tumor, arguing against impaired antigen presentation as a basis for impaired T cell responses in the irradiated tumor (Figure S6). Further, monocyte chemoattractant protein-1 (CCL2) was highly upregulated in the irradiated tumor, forming a potential basis for its high level of monocyte infiltration (Figure S6).
Discussion
In this study, we report a detailed analysis of an irradiated and untreated brain metastasis from a single patient. This study has several important caveats, including its limited sample size, the potential confounding effects of pre-existing tumor heterogeneity, and the unusual clinical course of the patient. These are discussed in a separate section below. Nonetheless, these results raise important considerations for combined treatment with immunotherapy and radiation.
In our study, radiotherapy was associated with decreased immune infiltration between the two case patient lesions as well as when compared with 30 other patients with radiation- and immunotherapy-naive brain metastasis.10 The ultimate outcome was a large, radiation-associated remodeling of tumor-infiltrating macrophages, T cells, and even natural killer (NK) cells (Figure 2E). The most notable difference in myeloid immune infiltrate between the two tumors was the higher frequency of monocyte-like cells in the irradiated tumor. These cells expressed high levels of monocyte marker genes found in circulating monocytes and also high levels of IL1B, indicating an activated phenotype. This infiltration of irradiated tumors by monocytes has been reported in pre-clinical models and is mediated by CCL213,18; notably, we also find higher levels CCL2 mRNA in the irradiated tumor. In pre-clinical models, CCL2 blockade in combination with radiation delayed tumor growth and decreased tumor proliferation and vascularity.13,18 These results highlight the potential of TAM-modulating therapies targeting the CCL2/CCR2 axis or other pathways.19,20
The T cell infiltrate following radiation superficially appeared more phenotypically functional with a more diverse TCR repertoire (in line with previous studies21), lower expression of PD-1, and higher expression of TCF-1. However, we find that this is likely explained by infiltration of circulating T cells that are enriched for non-tumor-specific antigen reactivity and are unlikely to contribute to tumor-specific immunity.16,17 Further, markers of nascent, antigen-specific activation such as CD69 and CD25 were not expressed among tumor-infiltrating T cells in the irradiated tumor, arguing against their priming in the irradiated tumor at this time interval after radiation.
Perhaps most importantly, we find that radiation depleted tumor-resident T cells; this has important implications for the sequencing of radiation therapy and checkpoint blockade. An early burst of proliferating circulating CD8+ T cells is associated with clinical response to PD-1 pathway blockade; however, circulating activated CD8+ T cells eventually return to baseline levels following the presumed migration of these activated cells to tumor or other inflamed sites.22 Thus, administration of radiation after checkpoint blockade may deplete recently arrived effector cells generated by PD-1 blockade or other immunotherapies. This is consistent with previous results showing that patients with melanoma brain metastasis receiving SRS during or before ipilimumab demonstrated better overall survival and local control than those receiving SRS after ipilimumab.23 Conversely, given our observed infiltration of circulating T cell clones into the irradiated tumor, administration of PD-1 pathway blockade with or immediately following radiation may improve tumor-specific infiltration into irradiated tumors. This latter hypothesis is consistent with studies demonstrating benefit of radiation concomitant with or prior to checkpoint blockade.4,24,25,26
Limitations of the study
Our study is subject to two key limitations. First, since our samples are from two separate tumors, we cannot completely distinguish between pre-existing tumor heterogeneity and the effects of radiation. Thus, it is possible that the different immune profiles of the two studied tumors reflect pre-existing differences in the size, location, or immunologic infiltrate of the respective tumors. However, we consider this unlikely for several reasons. First, our observation of monocyte infiltration and elevated CCL2 expression in the irradiated tumor is consistent with pre-clinical models that exhibit CCL2-mediated recruitment of monocytes following radiation.13,18 Second, we have previously shown that brain-metastasis-infiltrating T cells express a resident phenotype and a distinct TCR repertoire that is highly dissimilar from circulating clones,10 consistent with studies from other tumor types.16,17,27 Third, a previous study of multiple metastases resected from multiple patients showed high levels of TCR overlap between different tumors.28 Finally, and perhaps most convincingly, the irradiated lesion is remarkable for its low infiltration and lack of exhausted CD8+ T cells when compared with our larger cohort of previously reported brain metastasis samples.10 Thus, while the observed differences could potentially predate the radiation therapy, the reduced T cell number and increased TCR overlap with peripheral blood in the treated tumor are consistent with radiation-induced changes.
Second, we only analyze two tumors from a single patient with an unusual clinical course. Local progression after SRS from brain metastases is uncommon,29,30,31 particularly in the short time frame reported here,32 and it is possible that the TP53/KRAS co-mutation in these tumors contributed to radioresistance.33 Moreover, the optimal dose and fractionation for large brain metastases not amenable to single-fraction SRS has not been established.34 Our institutional standard is 30 Gy in 5 fractions for lesions 3 cm and larger, but given the heterogeneous approaches to SRS dosing, our findings may not be broadly generalizable to all dose and fractionation schedules of SRS. Moreover, this study captures the TME at a single time point after radiation therapy. Further remodeling of the brain metastasis immune microenvironment with time after radiation is likely.
Additionally, given the analysis of a single patient, our results may or may not be applicable to a broader patient population. Larger studies of radiation-naive and irradiated brain metastases are underway.35 However, even in studies with large numbers of patients, a lack of patient-matched samples and/or TCR profiling risks confounding intrinsic radioresistance of pre-existing tumor-infiltrating T cells with post-radiation infiltration of circulating cells. Our study including these advantages is consistent with a number of studies showing T cell death at doses much lower than those given here,7,36,37,38 including a study of patient-matched biopsies finding ∼90% decreases in infiltrating CD8+ T cells following radiation of cervical cancer at doses of 20–30 Gy.39 Thus, our data are consistent in supporting near-total depletion of brain-metastasis-infiltrating T cells at the treatment dose, subject to the confounding effect of pre-existing tumor heterogeneity discussed above.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| TotalSeq-C Human Universal Cocktail, V1.0 | Biolegend | Cat# 399905; RRID# AB_2876728 |
| Anti-CD45 PE | Miltenyi | Cat# 130-117-281 |
| Anti-CD4 APC | Miltenyi | Cat# 130-113-222 |
| Anti-CD8α BV510 | Biolegend | Cat# 301048; RRID #AB_2561942 |
| Chemicals, peptides, and recombinant proteins | ||
| Zombie NIR Fixable Viability dye | Biolegend | Cat# 423106 |
| Percoll | Sigma | Cat# P1644 |
| CountBright Absolute Counting Beads | Fisher | C36950 |
| Lymphocyte separation medium | Corning | Cat# 25-072-CV |
| Deposited data | ||
| Pre-processed whole-genome and single-cell sequencing | This paper | Mendeley Data: https://doi.org/10.17632/kdmdc8pkjt.1 |
| Software and algorithms | ||
| Cell Ranger, version 6 | 10x Genomics | https://support.10xgenomics.com/single-cell-gene-expression/software/pipelines/latest/what-is-cell-ranger |
| Seurat | Hao et al.40 | CRAN |
| FlowJo | BDflow | https://www.flowjo.com/ |
| Burrows-Wheeler Aligner | Li & Durbin41 | https://github.com/lh3/bwa |
| Manta | Illumina | https://github.com/Illumina/manta |
| Strelka | Illumina | https://github.com/Illumina/strelka |
| STAR Aligner | Dobin et al.42 | https://github.com/alexdobin/STAR |
| edgeR | Robinson et al.43 | Bioconductor |
| ggplot2 | https://ggplot2.tidyverse.org | CRAN |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Lisa Sudmeier (lisa.jane.sudmeier@emory.edu).
Materials availability
This study did not generate new reagents.
Experimental model and subject details
Human patients
Tumor tissue and peripheral blood were collected from immunotherapy-naïve patients undergoing resection of brain metastases at Emory University Hospital. Informed consent was obtained, with experiments carried out with the approval of the Emory University Institutional Review Board under protocols IRB00045732, IRB00095411, and STUDY00001995. All consenting patients were included in sample collection unless insufficient tissue was available. We recently published10 a detailed characterization of immune infiltrate in brain metastases from this cohort (Sudmeier et al., Cell Reports Medicine 2022). The samples in this study were collected as part of ongoing sample collection as described in Sudmeier et al.; however, we did not report data from the irradiated metastasis and matched PBMCs in that study.
Blood was collected in lithium heparin tubes at the time of surgery, and blood and tumor tissue were stored at 4°C until samples were processed, typically in less than 1 h. The patient was immunotherapy-naïve but received dexamethasone (10 mg bolus +4 mg/6 h) two days prior to the first surgery and three days prior to the second surgery.
Method details
Tissue and PBMC processing
Four samples were studied here (Figure 1A): an untreated tumor, an irradiated tumor, and matched PBMCs from the time of each resection (“pre-radiation PBMCs” and “post-radiation PBMCs”). Brain metastasis tissues and matched PBMCs were collected at the time of surgery and processed as previously described.10 Briefly, tumors were weighed, cut into pieces, and digested with an enzymatic cocktail for 1 h at 37°C. A single-cell suspension was generated using a cell strainer, and cells were pelleted and washed in PBS with 2% fetal bovine serum (FBS). A 44%/67% Percoll gradient was used to separate white blood cells, and the interface was collected and washed following centrifugation of the gradient. PBMCs were isolated by underlaying whole blood with Corning Lymphocyte Separation Medium and centrifuging for 20 min. The interface was collected and washed in PBS with 2% FBS.
To quantify immune infiltrate, a small volume of freshly-isolated tumor-infiltrating cells and PBMCs were incubated with CountBright counting beads, stained with fluorescently-labeled anti-CD45 and anti-CD8 antibodies, and analyzed on a BD LSR II cytometer to determine absolute numbers of lymphocytes and CD8+ cells. The remainder of cells were resuspended in FBS with 10% DMSO, frozen to −80 °C at a cooling rate of 1°C/min, and stored in liquid nitrogen until use in single-cell sequencing experiments.
Single cell sequencing
Aliquots of cryopreserved cells from each sample were simultaneously and rapidly thawed, washed twice in RPMI with 10% FBS, and counted. Cells were stained with Zombie NIR viability dye (BioLegend), anti-CD45 PE, anti-CD4 APC, anti-CD8α BV510, and the TotalSeq-C Universal Cocktail v1.0 (BioLegend) in PBS with 2% FBS and 2 mM EDTA. To avoid competition of fluorescent antibodies with CITE-sequencing antibodies, non-competing clones were used. For CD8α, clone RPA-T8 was used for fluorescent labeling and SK1 for CITE-seq; for CD45, clone REA1023 was used for fluorescent labeling and HI30 for CITE-seq; for CD4, clone REA623 for fluorescent labeling and RPA-T4 for CITE-seq. The TotalSeq-C Universal Cocktail v1.0 was added at 1 test per 6 x 105 cells. After staining, cells were washed in PBS with 2% FBS and 2 mM EDTA.
Following staining, cells were sorted on a BD FACS Aria II into RPMI with 10% FBS. We sorted live, CD45+ cells from each sample. To further characterize T cell responses in the tumors, we also sorted CD45+ cells positive for CD4 or CD8 for separate single-cell sequencing captures. This resulted in six total captures performed on a 10x Genomics Chromium controller in the Emory Yerkes Nonhuman Primate (NHP) Genomics Core: one capture of CD45+ cells from each PBMC and tumor sample, and one capture each of CD45+ cells positive for CD4 or CD8 from each tumor sample. Gene expression, CITE-sequencing, and T cell receptor (TCR) libraries were generated from each sample by the Emory Yerkes NHP Genomics Core. An overview of this experiment is shown in Figure S2A.
Single-cell sequencing alignment and quality control
Single-cell gene expression and CITE-seq data were aligned and mapped with Cell Ranger version 6 (10x Genomics). T cell receptor sequences were called with cellranger vdj. As described previously,10 TCRβ sequences were used to identify T cell clonotypes. T cells sharing the same CDR3 amino acid sequence and using the same TRBV and TRBJ gene family were considered to belong to the same clonotype. 61,823 cells were sequenced. To exclude dead, doublet, and poorly-sequenced cells, cells with <0.8% or >10% of reads originating from mitochondrial genes, <800 total genes detected or <2,500 or >20,000 number of RNA molecules sequenced were excluded from further analysis. 45,947 cells met these quality control criteria. Final quality control data are shown in Figure S2B.
Whole genome sequencing
For whole genome sequencing (WGS), normal DNA was isolated from PBMCs with a Qiagen AllPrep kit using the manufacturer’s instructions. Tumor DNA was isolated in an identical manner from the lymphocyte-depleted fraction of the tumor after the Percoll gradient. WGS was performed by Novogene. Normal samples were sequenced to a depth of 43.8x and 49.5x at the first and second timepoint, respectively. Tumor samples were sequenced to 166.5x and 178.7x depth for the untreated and irradiated tumor, respectively.
Sequencing reads were aligned with the Burrows-Wheeler Aligner.41 Manta and Strelka were used to call indels and single nucleotide variants relative to pre-radiation PBMCs.44,45 Annovar was used to annotate output.46
mRNA-sequencing
For mRNA-sequencing, RNA was isolated with the Qiagen AllPrep kit simultaneously with DNA isolation as described above. RNA was submitted to the Emory Yerkes NHP Genomics Core for paired-end sequencing. Reads were aligned to the human genome (GRCh38) and assigned to genes with the STAR aligner.42 Count data were analyzed in R with the edgeR package.43
Quantification and statistical analysis
Single-cell analysis
Gene expression and antibody capture (CITE-seq) data were analyzed with Seurat v4.40 Gene expression/RNA-sequencing data were normalized and scaled with the SCTransform command. Antibody capture data were normalized using the centered log ratio (CLR) transformation and subsequently scaled. UMAP projection47 and clustering were performed with antibody capture data after removing isotype controls. 11 principal components, 200 neighbors, and a minimum distance of 0.1 were used for nearest-neighbor graph construction, clustering (resolution = 0.4), and UMAP projection.
For calculation of cluster frequencies among tumor-infiltrating or circulating CD45+ cells, only CD45+ captures were used (Figure 2E). TAMs were subclustered using the FindSubCluster command in Seurat with a resolution of 0.4 and using the original Louvain algorithm for modularity optimization. Differential gene expression was calculated in all cases using the FindMarkers command in Seurat. TCR diversity was quantified with the Shannon index calculated by the diversity command in the vegan package.48 TCR overlap was quantified by the Morisita-Horn index. Plots were made with Seurat or ggplot2.49
mRNA-sequencing
Count data were normalized to counts per million with the cpm function in edgeR. Given the availability of only one sample per condition, differential expression analysis between the two tumor samples was performed using the exactTest function and tagwise dispersion from the PBMC samples. Gene set enrichment analysis was performed with the fgsea package using log10FDR∗sign(fold change) as the ranking statistic.50 Results – particularly p values – should be interpreted with caution given the availability of one tumor in each group.
Acknowledgments
We thank Pauline Hamon, Ewelina Sobierajska, Andreas Wieland, and Rafi Ahmed for helpful discussions and the Emory Yerkes Nonhuman Primate Genomics Core for assistance with single-cell and mRNA sequencing experiments. This research was supported by R00AI153736, P51OD011132, S10OD026799, and P50CA217691 from the National Institutes of Health (NIH), a Nell W. and William Simpson Elkin Fellowship from Emory University to L.J.S., and a first-time tenure-track award (RR220069) from the Cancer Prevention and Research Institute of Texas (CPRIT) to W.H.H. W.H.H. is a CPRIT Scholar in Cancer Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author contributions
J.J.O. performed surgical resection of brain metastases. W.H.H. and L.J.S. designed the study, performed experiments, and interpreted data. W.H.H. prepared the manuscript, with editing from J.J.O. and L.J.S.
Declaration of interests
The authors declare no competing interests.
Published: May 19, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101054.
Contributor Information
William H. Hudson, Email: william.hudson@bcm.edu.
Lisa J. Sudmeier, Email: lisa.jane.sudmeier@emory.edu.
Supplemental information
Data and code availability
-
•
The raw sequencing data cannot be deposited in a public repository due to privacy concerns. Original sequencing data is available upon request to the lead contact and with permission of the Emory University Institutional Review Board. Pre-processed whole-genome and single-cell sequencing data have instead been deposited at Mendeley Data and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
-
•
This paper does not report original code. Published software packages used in this study are listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020;11:3801. doi: 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callahan M.K., Postow M.A., Wolchok J.D. CTLA-4 and PD-1 pathway blockade: combinations in the clinic. Front. Oncol. 2014;4:385. doi: 10.3389/fonc.2014.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalbasi A., June C.H., Haas N., Vapiwala N. Radiation and immunotherapy: a synergistic combination. J. Clin. Invest. 2013;123:2756–2763. doi: 10.1172/jci69219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonia S.J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., Yokoi T., Chiappori A., Lee K.H., de Wit M., et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 5.Kelly R.J., Ajani J.A., Kuzdzal J., Zander T., Van Cutsem E., Piessen G., Mendez G., Feliciano J., Motoyama S., Lièvre A., et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl. J. Med. 2021;384:1191–1203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 6.Gough M.J., Crittenden M.R. The paradox of radiation and T cells in tumors. Neoplasia. 2022;31:100808. doi: 10.1016/j.neo.2022.100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falcke S.E., Rühle P.F., Deloch L., Fietkau R., Frey B., Gaipl U.S. Clinically relevant radiation exposure differentially impacts forms of cell death in human cells of the innate and adaptive immune system. Int. J. Mol. Sci. 2018;19:3574. doi: 10.3390/ijms19113574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura N., Kusunoki Y., Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat. Res. 1990;123:224–227. doi: 10.2307/3577549. [DOI] [PubMed] [Google Scholar]
- 9.Badiyan S.N., Regine W.F., Mehta M. Stereotactic radiosurgery for treatment of brain metastases. J. Oncol. Pract. 2016;12:703–712. doi: 10.1200/jop.2016.012922. [DOI] [PubMed] [Google Scholar]
- 10.Sudmeier L.J., Hoang K.B., Nduom E.K., Wieland A., Neill S.G., Schniederjan M.J., Ramalingam S.S., Olson J.J., Ahmed R., Hudson W.H. Distinct phenotypic states and spatial distribution of CD8+ T cell clonotypes in human brain metastases. Cell Rep. Med. 2022;3:100620. doi: 10.1016/j.xcrm.2022.100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zilionis R., Engblom C., Pfirschke C., Savova V., Zemmour D., Saatcioglu H.D., Krishnan I., Maroni G., Meyerovitz C.V., Kerwin C.M., et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50:1317–1334.e10. doi: 10.1016/j.immuni.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez F.O., Gordon S., Locati M., Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 13.Mondini M., Loyher P.-L., Hamon P., Gerbé de Thoré M., Laviron M., Berthelot K., Clémenson C., Salomon B.L., Combadière C., Deutsch E., Boissonnas A. CCR2-Dependent recruitment of Tregs and monocytes following radiotherapy is associated with TNFα-mediated resistance. Cancer Immunol. Res. 2019;7:376–387. doi: 10.1158/2326-6066.Cir-18-0633. [DOI] [PubMed] [Google Scholar]
- 14.Nalio Ramos R., Missolo-Koussou Y., Gerber-Ferder Y., Bromley C.P., Bugatti M., Núñez N.G., Tosello Boari J., Richer W., Menger L., Denizeau J., et al. Tissue-resident FOLR2+ macrophages associate with CD8+ T cell infiltration in human breast cancer. Cell. 2022;185:1189–1207.e25. doi: 10.1016/j.cell.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Wang B., Li Q., Qin L., Zhao S., Wang J., Chen X. Transition of tumor-associated macrophages from MHC class IIhi to MHC class IIlow mediates tumor progression in mice. BMC Immunol. 2011;12:43. doi: 10.1186/1471-2172-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberhardt C.S., Kissick H.T., Patel M.R., Cardenas M.A., Prokhnevska N., Obeng R.C., Nasti T.H., Griffith C.C., Im S.J., Wang X., et al. Functional HPV-specific PD-1+ stem-like CD8 T cells in head and neck cancer. Nature. 2021;597:279–284. doi: 10.1038/s41586-021-03862-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveira G., Stromhaug K., Klaeger S., Kula T., Frederick D.T., Le P.M., Forman J., Huang T., Li S., Zhang W., et al. Phenotype, specificity and avidity of antitumour CD8+ T cells in melanoma. Nature. 2021;596:119–125. doi: 10.1038/s41586-021-03704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalbasi A., Komar C., Tooker G.M., Liu M., Lee J.W., Gladney W.L., Ben-Josef E., Beatty G.L. Tumor-derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2017;23:137–148. doi: 10.1158/1078-0432.Ccr-16-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Yao W., Yuan Y., Chen P., Li B., Li J., Chu R., Song H., Xie D., Jiang X., Wang H. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157–167. doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 20.Anfray, Ummarino, Andón, Allavena Current strategies to target tumor-associated-macrophages to improve anti-tumor immune responses. Cells. 2019;9:46. doi: 10.3390/cells9010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Twyman-Saint Victor C., Rech A.J., Maity A., Rengan R., Pauken K.E., Stelekati E., Benci J.L., Xu B., Dada H., Odorizzi P.M., et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamphorst A.O., Pillai R.N., Yang S., Nasti T.H., Akondy R.S., Wieland A., Sica G.L., Yu K., Koenig L., Patel N.T., et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1–targeted therapy in lung cancer patients. Proc. Natl. Acad. Sci. USA. 2017;114:4993–4998. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiess A.P., Wolchok J.D., Barker C.A., Postow M.A., Tabar V., Huse J.T., Chan T.A., Yamada Y., Beal K. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int. J. Radiat. Oncol. Biol. Phys. 2015;92:368–375. doi: 10.1016/j.ijrobp.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian J.M., Yu J.B., Kluger H.M., Chiang V.L.S. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer. 2016;122:3051–3058. doi: 10.1002/cncr.30138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaverdian N., Lisberg A.E., Bornazyan K., Veruttipong D., Goldman J.W., Formenti S.C., Garon E.B., Lee P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903. doi: 10.1016/s1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theelen W.S.M.E., Chen D., Verma V., Hobbs B.P., Peulen H.M.U., Aerts J.G.J.V., Bahce I., Niemeijer A.L.N., Chang J.Y., de Groot P.M., et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir. Med. 2021;9:467–475. doi: 10.1016/s2213-2600(20)30391-x. [DOI] [PubMed] [Google Scholar]
- 27.Caushi J.X., Zhang J., Ji Z., Vaghasia A., Zhang B., Hsiue E.H.-C., Mog B.J., Hou W., Justesen S., Blosser R., et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature. 2021;596:126–132. doi: 10.1038/s41586-021-03752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalaora S., Wolf Y., Feferman T., Barnea E., Greenstein E., Reshef D., Tirosh I., Reuben A., Patkar S., Levy R., et al. Combined analysis of antigen presentation and T-cell recognition reveals restricted immune responses in melanoma. Cancer Discov. 2018;8:1366–1375. doi: 10.1158/2159-8290.Cd-17-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gui C., Grimm J., Kleinberg L.R., Zaki P., Spoleti N., Mukherjee D., Bettegowda C., Lim M., Redmond K.J. A dose-response model of local tumor control probability after stereotactic radiosurgery for brain metastases resection cavities. Adv. Radiat. Oncol. 2020;5:840–849. doi: 10.1016/j.adro.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Azevedo Santos T.R., Tundisi C.F., Ramos H., Maia M.A.C., Pellizzon A.C.A., Silva M.L.G., Fogaroli R.C., Chen M.J., Suzuki S.H., Dias J.E.S., Jr., et al. Local control after radiosurgery for brain metastases: predictive factors and implications for clinical decision. Radiat. Oncol. 2015;10:63. doi: 10.1186/s13014-015-0367-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogelbaum M.A., Angelov L., Lee S.-Y., Li L., Barnett G.H., Suh J.H. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J. Neurosurg. 2006;104:907–912. doi: 10.3171/jns.2006.104.6.907. [DOI] [PubMed] [Google Scholar]
- 32.Churilla T.M., Chowdhury I.H., Handorf E., Collette L., Collette S., Dong Y., Alexander B.M., Kocher M., Soffietti R., Claus E.B., Weiss S.E. Comparison of local control of brain metastases with stereotactic radiosurgery vs surgical resection. JAMA Oncol. 2019;5:243–247. doi: 10.1001/jamaoncol.2018.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong T.S., Wo J.Y., Borger D.R., Yeap B.Y., McDonnell E.I., Willers H., Blaszkowsky L.S., Kwak E.L., Allen J.N., Clark J.W., et al. Phase II study of proton-based stereotactic body radiation therapy for liver metastases: importance of tumor genotype. J. Natl. Cancer Inst. 2017;109 doi: 10.1093/jnci/djx031. [DOI] [PubMed] [Google Scholar]
- 34.Masucci G.L. Hypofractionated radiation therapy for large brain metastases. Front. Oncol. 2018;8:379. doi: 10.3389/fonc.2018.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchwald Z., Jansen C., Prabhu R., Pagadala M., Chappa P., Goyal S., Zhou C., Neill S., Prokhnevska N., Cardenas M., et al. Immune niches in brain metastases contain TCF1+ stem-like T cells, are associated with disease control and are modulated by preoperative SRS. Res. Sq. 2023 doi: 10.21203/rs.3.rs-2722744/v1. [DOI] [Google Scholar]
- 36.Heylmann D., Ponath V., Kindler T., Kaina B. Comparison of DNA repair and radiosensitivity of different blood cell populations. Sci. Rep. 2021;11:2478. doi: 10.1038/s41598-021-81058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pugh J.L., Sukhina A.S., Seed T.M., Manley N.R., Sempowski G.D., van den Brink M.R.M., Smithey M.J., Nikolich-Žugich J. Histone deacetylation critically determines T cell subset radiosensitivity. J. Immunol. 2014;193:1451–1458. doi: 10.4049/jimmunol.1400434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura N., Kusunoki Y., Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat. Res. 1990;123:224–227. [PubMed] [Google Scholar]
- 39.Qinfeng S., Depu W., Xiaofeng Y., Shah W., Hongwei C., Yili W. In situ observation of the effects of local irradiation on cytotoxic and regulatory T lymphocytes in cervical cancer tissue. Radiat. Res. 2013;179:584–589. doi: 10.1667/rr3155.1. [DOI] [PubMed] [Google Scholar]
- 40.Hao Y., Hao S., Andersen-Nissen E., Mauck W.M., Zheng S., Butler A., Lee M.J., Wilk A.J., Darby C., Zager M., et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S., Scheffler K., Halpern A.L., Bekritsky M.A., Noh E., Källberg M., Chen X., Kim Y., Beyter D., Krusche P., Saunders C.T. Strelka2: fast and accurate calling of germline and somatic variants. Nat. Methods. 2018;15:591–594. doi: 10.1038/s41592-018-0051-x. [DOI] [PubMed] [Google Scholar]
- 45.Chen X., Schulz-Trieglaff O., Shaw R., Barnes B., Schlesinger F., Källberg M., Cox A.J., Kruglyak S., Saunders C.T. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics. 2016;32:1220–1222. doi: 10.1093/bioinformatics/btv710. [DOI] [PubMed] [Google Scholar]
- 46.Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McInnes L., Healy J., Saul N., Großberger L. UMAP: uniform manifold approximation and projection. J. Open Source Softw. 2018;3:861. doi: 10.21105/joss.00861. [DOI] [Google Scholar]
- 48.Oksanen J., Blanchet F.G., Friendly M., Kindt R., Legendre P., McGlinn D., Minchin P.R., O'Hara R.B., Simpson G.L., Solymos P., et al. vegan: community ecology package. 2020. https://CRAN.R-project.org/package=vegan
- 49.Wickham H. ggplot2 : elegant graphics for data analysis. Use R! 2016 doi: 10.1007/978-3-319-24277-4. [DOI] [Google Scholar]
- 50.Korotkevich G., Sukhov V., Budin N., Shpak B., Artyomov M.N., Sergushichev A. Fast gene set enrichment analysis. bioRxiv. 2021 doi: 10.1101/060012. Preprint at. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The raw sequencing data cannot be deposited in a public repository due to privacy concerns. Original sequencing data is available upon request to the lead contact and with permission of the Emory University Institutional Review Board. Pre-processed whole-genome and single-cell sequencing data have instead been deposited at Mendeley Data and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
-
•
This paper does not report original code. Published software packages used in this study are listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.