Abstract
Objective
To demonstrate, by a cost-effectiveness analysis, the efficiency of mechanical thrombectomy (MT) versus medical management (MM) in patients with a low Alberta Stroke Program Early CT Score (ASPECTS) from the RESCUE Study.
Methods
A cost-effectiveness model was designed to project both direct medical costs and quality-adjusted life-years (QALYs) of MT versus MM in eight European countries (Spain, UK, France, Italy, Belgium, Germany, Sweden, and the Netherlands). Our model was created based on previously published health-economic data in those countries. Procedure costs, acute, mid-term, and long-term care costs were projected based on expected modified Rankin Scale (mRS) scores as reported in the RESCUE-Japan LIMIT trial.
Results
MT was found to be a cost-effective option in eight different countries across Europe (Spain, Italy, UK, France, Belgium, Germany, the Netherlands, and Sweden). with a lifetime incremental cost-effectiveness ratio varying from US$2 875 to US$11 202/QALY depending on the country. A cost-effectiveness acceptability curve showed 100% acceptability of MT at the willingness to pay (WTP) of US$40 000 for the eight countries.
Conclusions
MT is efficient versus MM alone for patients with low ASPECTS in eight countries across Europe. Patients with a large ischemic core could be treated with MT because it is both clinically beneficial and economically sustainable.
Keywords: Stroke, Thrombectomy, Economics
WHAT IS ALREADY KNOWN ON THIS TOPIC
Current guidelines from the European Stroke Organisation–European Society for Minimally Invasive Neurological Therapy recommend mechanical thrombectomy (MT) only in patients with Alberta Stroke Program Early CT Score (ASPECTS) ≥6. The efficiency of MT in patients with large established infarcts is yet to be defined.
WHAT THIS STUDY ADDS
This cost-effectiveness model demonstrates that MT is cost-effective for patients with acute ischemic stroke with a large ischemic core (defined as ASPECTS 3–5), compared with medical management over a lifetime horizon and healthcare perspective.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
MT in patients with acute ischemic stroke with a large ischemic core provides both clinical and economic benefits. Resource allocation and budgetary analyses should be followed to establish the implications of treating a larger population at a national and local level.
Introduction
Stroke is the third most frequent cause of death in Europe and the fourth cause of premature death. Around the world, approximately 15 million people each year will have stroke, resulting in an estimated 5.5 million deaths.1 One in six people worldwide will experience a stroke in their lifetime, and about 87% of all strokes are ischemic.1 Of patients who survive, approximately 50% have some type of disability, with 26% dependent on others for daily living and 20% requiring institutional care.2 Due to the likelihood of an increased incidence of cardiovascular diseases, optimizing stroke care is essential and requires efficient patient triage, transport, treatment, and funding.
The Alberta Stroke Program Early CT Score (ASPECTS) is the most established scale to evaluate brain parenchyma before any intervention. It is used in all hospitals because of its availability through the use of non-contrast CT and its interobserver concordance. In accordance with European guidelines, patients with an ASPECTS ≥6 should receive mechanical thrombectomy (MT) treatment, whereas patients with an ASPECTS <6 may be treated with medical management (MM) alone.3
Last year, the RESCUE-Japan LIMIT trial was published, a randomized clinical trial focusing on patients with a low ASPECTS and comparing MT with MM. Results showed that a good clinical outcome measured by a modified Rankin Scale (mRS) score 0–2 was achieved twice as often with MT as with MM alone (14% vs 7.8%, respectively).
Such results are lower than those from the HERMES meta-analysis which pooled patient-level data, demonstrating the additional benefit of MT in reducing disability for patients with large vessel anterior circulation ischemic stroke and an ASPECTS ≥6, irrespective of patient characteristics, geographic location, and eligibility for IV tissue plasminogen activator (IV-tPA; 46% for MT and 26% for MM).4
Adoption of MT for the treatment of large vessel occlusion (LVO) has raised the question of the cost for payers, emphasizing the need to assess the impact on the overall healthcare budget. Several studies have shown that MT is cost-effective and results in reduced disability and more quality-adjusted life-years QALYs).5–8 In particular, MT has found to be highly cost-effective across Europe.9
However, these health-economic results are published based on patients with a good initial ASPECTS. Current approaches to endovascular treatment of LVO ischemic stroke tend to increase the indications for such treatment, aiming to treat a larger number of patients who might benefit from this technique.
Therefore, we decided to create a model to study the health-economic impact beyond broadening the indication for MT, including patients with low ASPECTS from around Europe.
Material and methods
Study population
The model included patients with an acute ischemic stroke due to LVO of the anterior circulation and non-contrast CT ASPECTS <6.
Clinical inputs
The Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism–Japan Large Ischemic Core Trial (RESCUE-Japan LIMIT) was an open-label, parallel-group, randomized clinical trial conducted in 45 hospitals in Japan.10 A total of 203 patients were included, 101 patients were treated with MT and 102 with MM (IV-tPA alone). The percentage of patients with an mRS score of 0 to 3 at 90 days was 31.0% in the MT group and 12.7% in the MM group. Details of mRS scores are presented in the supplementary material (online supplemental table S1).
jnis-2022-019849supp001.pdf (2.6MB, pdf)
Model structure
A short-term decision tree (90 days) and a lifetime (20 years) state transition Markov model were designed to project direct medical costs and effectiveness of treating patients with MT versus MM for patients with acute ischemic stroke with a low ASPECTS. The model was created using Microsoft Excel version 2205. The short-term decision tree assessed the cost and clinical efficacy.
Distribution of patients with low ASPECTS was based on a decision tree model of expected 3-month post-treatment mRS score from the RESCUE-Limit Japan trial.10 mRS is a standard functional assessment on a 7-point scale from 0 (no disability) to 6 (death). However, the space between ordinal levels is not equal, and disability, and therefore cost, related to higher mRS score is not linear.11 During the acute phase, it was assumed that the patients were at no risk of recurrent stroke. A lifetime Markov model was created to estimate the transitions in clinical outcomes and associated post-stroke costs with 3-month cycles. In the Markov model (mid-term from 90 days to 1 year, and long-term phase after 1 year), tunnel states were used to consider the probabilities of recurrence, which depend on the time spent in a particular state. During each cycle, a patient might remain in the same health state, have a recurrent stroke, or die. The assumption is that a mRS score can change only after a recurrent stroke and in one direction, towards a poorer health state.
A patient who had a recurrent stroke, transitioned back to the first 3-monthly cycle after the 90-day mRS score was taken and rejoined the Markov model in the first 3–6 month cycle or died. All the recurrent strokes with a mRS score >2 were managed with usual care and no further MT. A half cycle correction was applied to consider that transitions can occur at any point during the cycle. Cost and outcomes were discounted annually at 3%.
Long-term analysis was carried out using a long-term Markov state transition model, which includes the quarterly recurrence risk of stroke,12 13 death from any other cause based on the mRS score after the stroke,11 and the transition from one mRS state to another14 (online supplemental figure S1).
Mortality was captured both by the specific mortality rates from national statistical databases in the eight countries and from the HR of dying from a specific mRS score. All long-term analysis inputs are presented in table 1.
Table 1.
Lifetime model inputs
| Parameter | Value used | Distribution | Source |
| Mean age MT and IVT (years) | 76.15 | Beta | RESCUE10 |
| General death rate of population | Country-dependent values | Not applicable | WHO |
| HR of dying mRS score 0 | 1.53 | Log-normal | Hong et al 11 |
| HR of dying mRS score 1 | 1.52 | Log-normal | Hong et al 11 |
| HR of dying mRS score 2 | 2.17 | Log-normal | Hong et al 11 |
| HR of dying mRS score 3 | 3.18 | Log-normal | Hong et al 11 |
| HR of dying mRS score 4 | 4.50 | Log-normal | Hong et al 11 |
| HR of dying mRS score 5 | 6.55 | Log-normal | Hong et al 11 |
| Quarterly recurrence risk from mRS score 0–5 (3–12 months) | 1.66% | Dirichlet | Slot et al 12 |
| Quarterly recurrence risk from mRS score 0–5 (> 12 months) | 0.51% | Dirichlet | Slot et al 12 |
| Quarterly recurrence risk after a recurrent stroke from mRS score 0–5 | 1.30% | Dirichlet | Ganesalingam et al 13 |
| Transition probability to mRS score 1 if mRS score 0 | 18.71% | Beta | Fagan et al 14 |
| Transition probability to mRS score 2 if mRS score 0 | 18.71% | Beta | Fagan et al 14 |
| Transition probability to mRS score 3 if mRS score 0 | 18.71% | Beta | Fagan et al 14 |
| Transition probability to mRS score 4 if mRS score 0 | 18.71% | Beta | Fagan et al 14 |
| Transition probability to mRS score 5 if mRS score 0 | 18.71% | Beta | Fagan et al 14 |
| Transition probability to mRS score 6 if mRS score 0 | 5.13% | Beta | Fagan et al 14 |
| Transition probability to mRS score 2 if mRS score 1 | 23.39% | Beta | Fagan et al 14 |
| Transition probability to mRS score 3 if mRS score 1 | 23.39% | Beta | Fagan et al 14 |
| Transition probability to mRS score 4 if mRS score 1 | 23.39% | Beta | Fagan et al 14 |
| Transition probability to mRS score 5 if mRS score 1 | 23.39% | Beta | Fagan et al 14 |
| Transition probability to mRS score 6 if mRS score 1 | 5.13% | Beta | Fagan et al 14 |
| Transition probability to mRS score 3 if mRS score 2 | 31.19% | Beta | Fagan et al 14 |
| Transition probability to mRS score 4 if mRS score 2 | 31.19% | Beta | Fagan et al 14 |
| Transition probability to mRS score 5 if mRS score 2 | 31.19% | Beta | Fagan et al 14 |
| Transition probability to mRS score 6 if mRS score 2 | 5.13% | Beta | Fagan et al 14 |
| Transition probability to mRS score 4 if mRS score 3 | 46.79% | Beta | Fagan et al 14 |
| Transition probability to mRS score 5 if mRS score 3 | 46.79% | Beta | Fagan et al 14 |
| Transition probability to mRS score 6 if mRS score 3 | 5.13% | Beta | Fagan et al 14 |
| Transition probability to mRS score 5 if mRS score 4 | 93.57% | Beta | Fagan et al 14 |
| Transition probability to mRS score 6 if mRS score 4 | 5.13% | Beta | Fagan et al 14 |
| Transition probability to mRS score 6 if mRS score 5 | 98.70% | Beta | Fagan et al 14 |
IVT, IV thrombolysis; mRS, modified Rankin Scale; MT, mechanical thrombectomy.
Costs
Healthcare perspective was adopted, direct medical costs were calculated using country-specific procedure, acute, mid- and long-term costs published data for each country (online supplemental table 2). Results were expressed in US dollars from 2021.
Quality of life
The measure for utilities based on mRS score categories were obtained from a prospectively validated cohort evaluating EuroQol (EQ-5D) in post-stroke patients.15 Utility scores can be found in the supplementary material (online supplemental table S3).
Cost-effectiveness analysis
Cost-effectiveness was expressed in terms of its incremental cost-effectiveness ratio (ICER), defined as the ratio of the difference in the costs between MT and MM and gain in QALYs between the treatments demonstrated by the model.12
Sensitivity analyses were performed to explore the robustness and accuracy of the model. Specifically, deterministic one-way and two-way sensitivity analyses were performed to identify and evaluate the key variables driving the model and assess the effect of uncertainties on one (two) input parameter(s) on the results.
Deterministic sensitivity analyses were performed with a 20% variation of all inputs. Probabilistic sensitivity analyses (PSAs) were conducted using 10 000 iterations of a Monte Carlo simulation by varying input parameter values from their respective distributions. Results were described using a cost-effectiveness scatterplot and cost-effectiveness acceptability curves.
Results
Our projections indicate that MT treatment in patients with a low ASPECTS implies an incremental cost in every country we have studied. From the US$4 387 in Belgium to the US$16 468 in Sweden. This money nevertheless is able to improve QALYs in all countries and situations we have analyzed, identifying an incremental QALY at lifetime ranking from 1.30 in UK to 1.79 in France. We found an incremental cost per QALY that goes from US$2 875 in Italy to the US$11 202 from Sweden. Results are shown in table 2. In the base case, MT was found to be cost-effective at a willingness to pay (WTP) of US$40 000/QALY in the eight countries across Europe.
Table 2.
Results of cost-effectiveness analysis
| Spain | Italy | UK | Belgium | France | Germany | Sweden | The Netherlands | |
| Lifetime ICER (US$/QALY) | 4 595 | 2 875 | 6 635 | 3 004 | 6 947 | 3 933 | 11 202 | 5 595 |
| Lifetime Incremental QALY | 1.63 | 1.53 | 1.30 | 1.46 | 1.79 | 1.65 | 1.47 | 1.42 |
| Lifetime Incremental cost | 7 490 | 4 400 | 8 626 | 4 387 | 12 436 | 6 490 | 16 468 | 7 945 |
ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
Sensitivity analyses
Deterministic sensitivity analysis was conducted to assess the robustness of the results. This analysis was based on the RESCUE-LIMIT Japan trial scenario and inputs were varied by±20%. Deterministic sensitivity revealed that the model was most sensitive to variation in mean age, HR of dying with mRS score 3–5, and acute care cost with mRS score 3–5 (online supplemental figure S2).
Probabilistic sensitivity analysis for the eight countries
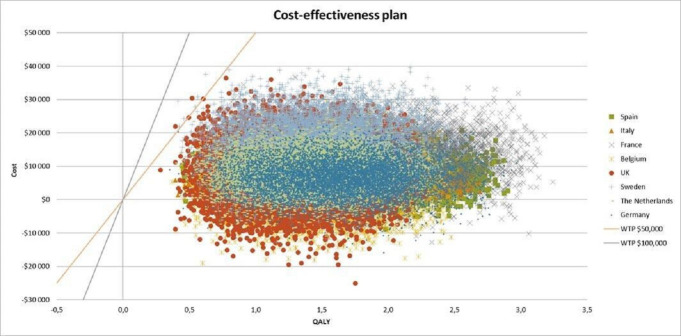
The results are shown as scatterplots of incremental costs and incremental QALYs of MT with standard medical care versus standard medical care alone for patients with low ASPECTS in the eight countries (figure 1). Each dot represents one simulation run. We conducted 10 000 simulations for each country analysis to maximize the reliability of the results. The PSA demonstrated that MT with IV-tPA has 100% probability of being cost-effective at a WTP of US$40 000/QALY in the eight countries across Europe. For Italy, the Netherlands, Germany, and Spain, the simulations demonstrated a 100% probability of being cost-effective at a WTP of US$20 000/QALY. We then studied the acceptability in each country related to the probability of being cost-effective and found a 100% probability of being cost-effective for Belgium, France, the UK, and Sweden at a WTP of US$25 000/QALY, US$30 000/QALY, US$35 000/QALY, and US$40 000/QALY, respectively (figure 2).
Figure 1.
Probabilistic sensitivity analysis for the eight countries. QALY, quality-adjusted life-years; WTP, willingness to pay.
Figure 2.
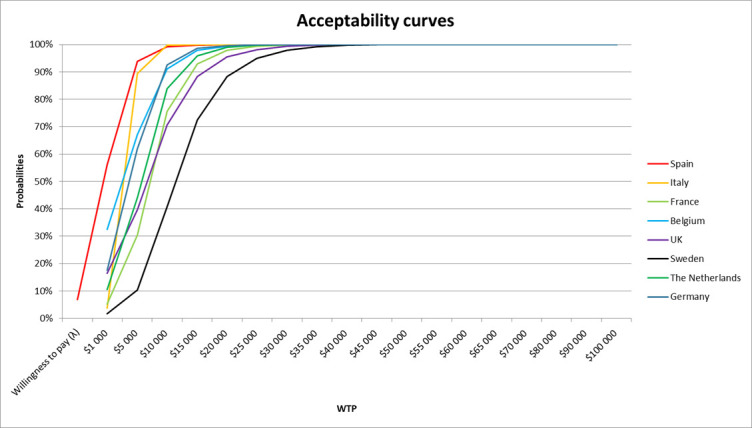
Cost-effectiveness acceptability curves for the eight countries. WTP, willingness to pay.
Discussion
In addition to immediate medical benefit, stroke therapists should consider promoting their expertise and treatments because long-term cost savings are achieved even in patients with a low ASPECTS. Treatment of stroke has vastly improved in the last 10 years; currently, we can achieve a good functional outcome (mRS score 0–2) for at least 46% of patients who present at our centers with a LVO and are treated with MT, and close to 14% for patients with a low ASPECTS.4 10 Therefore, close to 14% of the patients who meet inclusion criteria for that study and are treated with MT will be functionally independent at 3 months. As patient disability decreases, long-term cost savings increase. Outside level Ia evidence criteria (low ASPECTS, distal vessels or mRS score >1), Sanmartin et al and Sarraj et al conducted a cost study, which concluded that even in patients with low ASPECTS, MT was cost-effective in the United States.16 17 In addition, Khunte et al concluded that treating M2 occlusions was cost-effective.18
We decided to study the European population to show that economic benefits already known in a big country with a dominant private health sector may be reproducible in different countries with a public system.
Although the initial cost of stroke treatment with MT is higher than with best medical management, treatment with MT results in a higher rate of good outcomes. These costs should be considered as in investment in the community, as good clinical outcomes result in long-term savings. In a recent publication by Candio et al, 9 a complete analysis through all European countries quantifies an estimate cost savings of US$981 million in health costs and US$1.7 billion in social care costs.
As expected, the greatest costs over time are associated with patients who end up severely disabled (mRS score 4–5). MT has been shown to shift outcomes for many of these patients, compared with MM, with more patients achieving good functional outcomes. As more studies are conducted, there may be additional patients with stroke and occlusion types found eligible for MT, thereby further lowering the number of patients with a stroke with poor outcomes.
A main strength of this analysis was that the efficacy data were derived from the most recent randomized controlled trial including mRS outcomes specifically evaluated for patients with low ASPECTS in acute ischemic stroke. An additional strength was the inclusion of recurrent stroke to ensure that all clinical outcomes after a stroke were modeled.
This analysis also has several limitations, especially in relation to the cost data used in the model. In many countries both acute and long-term costs were taken from micro-costing analyses based on thrombolysis cost studies. These estimates may not be reflective of stroke care today, and costs might have been underestimated. An additional limitation was that mRS scores were based on a Japanese study since international or European country-specific data were not yet available. However, comprehensive sensitivity analyses were performed to address these limitations and the accuracy of the model remains robust within feasible ranges.
Conclusion
Our analysis suggests that treating patients with a low ASPECTS is cost-effective in eight European countries.
jnis-2022-019849supp002.pdf (511.6KB, pdf)
Footnotes
Twitter: @neuroplumber
Correction notice: Since this paper published online, the corr author address has been changed to strokenomics@stryker.com and the second author has been updated to Raffaele Scarica. The last author in this paper was also changed to Thomas Barthe.
Contributors: MM, TB, and RS devised the concept and the design of the study; All the authors contributed to the preparation of the material and data collection. The analysis was performed by TB and RS. The first draft of the manuscript was written by MM, TB, and RS, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript. MM oversaw the integrity of the entire study, including the supervision of data collection, methodology, analysis, interpretation of results, revision and edition of the final version.
Funding: The research being reported in this publication was funded by Stryker Neurovascular.
Competing interests: MM is a consultant for Stryker Neurovascular, Balt, and Cardiva.
Provenance and peer review: Not commissioned; internally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author(s) upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017;135:e146–603. 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation 2012;125:e2–220. 10.1161/CIR.0b013e31823ac046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J 2019;4:6–12. 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 5. de Andrés-Nogales F, Álvarez M, de Miquel MÁ, et al. Cost-effectiveness of mechanical thrombectomy using stent retriever after intravenous tissue plasminogen activator compared with intravenous tissue plasminogen activator alone in the treatment of acute ischaemic stroke due to large vessel occlusion in Spain. Eur Stroke J 2017;2:272–84. 10.1177/2396987317721865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campbell BCV, Mitchell PJ, Churilov L, et al. Endovascular thrombectomy for ischemic stroke increases disability-free survival, quality of life, and life expectancy and reduces cost. Front Neurol 2017;8:657. 10.3389/fneur.2017.00657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heggie R, Wu O, White P, et al. Mechanical thrombectomy in patients with acute ischemic stroke: a cost-effectiveness and value of implementation analysis. Int J Stroke 2020;15:881–98. 10.1177/1747493019879656 [DOI] [PubMed] [Google Scholar]
- 8. Health Quality Ontario . Mechanical thrombectomy in patients with acute ischemic stroke: a health technology assessment. Ont Health Technol Assess Ser 2016;16:1–79. [PMC free article] [PubMed] [Google Scholar]
- 9. Candio P, Violato M, Leal J, et al. Cost-effectiveness of mechanical thrombectomy for treatment of nonminor ischemic stroke across Europe. Stroke 2021;52:664–73. 10.1161/STROKEAHA.120.031027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshimura S, Sakai N, Yamagami H. Endovascular therapy for acute stroke with a large ischemic region. New Engl J Med 2022;386:1303–13. [DOI] [PubMed] [Google Scholar]
- 11. Hong K-S, Saver JL. Quantifying the value of stroke disability outcomes: who global burden of disease project disability weights for each level of the modified Rankin Scale. Stroke 2009;40:3828–33. 10.1161/STROKEAHA.109.561365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Slot KB, Berge E, Sandercock P, et al. Causes of death by level of dependency at 6 months after ischemic stroke in 3 large cohorts. Stroke 2009;40:1585–9. 10.1161/STROKEAHA.108.531533 [DOI] [PubMed] [Google Scholar]
- 13. Ganesalingam J, Pizzo E, Morris S, et al. Cost-utility analysis of mechanical thrombectomy using stent retrievers in acute ischemic stroke. Stroke 2015;46:2591–8. 10.1161/STROKEAHA.115.009396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fagan SC, Morgenstern LB, Petitta A, et al. Cost-effectiveness of tissue plasminogen activator for acute ischemic stroke. NINDS rt-PA Stroke Study Group. Neurology 1998;50:883–90. 10.1212/wnl.50.4.883 [DOI] [PubMed] [Google Scholar]
- 15. Rivero-Arias O, Ouellet M, Gray A, et al. Mapping the modified Rankin Scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Making 2010;30:341–54. 10.1177/0272989X09349961 [DOI] [PubMed] [Google Scholar]
- 16. Sarraj A, Pizzo E, Lobotesis K. Endovascular thrombectomy in patients with large core ischemic stroke: a cost-effectiveness analysis from the select study. J Neurointerv Surg 2020;13:875–82. [DOI] [PubMed] [Google Scholar]
- 17. Sanmartin MX, Katz JM, Wang J, et al. Cost-effectiveness of endovascular thrombectomy in acute stroke patients with large ischemic core. J Neurointerv Surg 2022:jnis-2022-019460. 10.1136/jnis-2022-019460 [DOI] [PubMed] [Google Scholar]
- 18. Khunte M, Wu X, Payabvash S. Cost-Effectiveness of endovascular thrombectomy in patients with acute stroke and M2 occlusion. J Neurointerv Surg 2021;13:784–9. 10.1136/neurintsurg-2020-016765 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnis-2022-019849supp001.pdf (2.6MB, pdf)
jnis-2022-019849supp002.pdf (511.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author(s) upon reasonable request.