Abstract
The ‘MHC-I (major histocompatibility complex class I)-opathy’ concept describes a family of inflammatory conditions with overlapping clinical manifestations and a strong genetic link to the MHC-I antigen presentation pathway. Classical MHC-I-opathies such as spondyloarthritis, Behçet’s disease, psoriasis and birdshot uveitis are widely recognised for their strong association with certain MHC-I alleles and gene variants of the antigen processing aminopeptidases ERAP1 and ERAP2 that implicates altered MHC-I peptide presentation to CD8+T cells in the pathogenesis. Progress in understanding the cause and treatment of these disorders is hampered by patient phenotypic heterogeneity and lack of systematic investigation of the MHC-I pathway.
Here, we discuss new insights into the biology of MHC-I-opathies that strongly advocate for disease-overarching and integrated molecular and clinical investigation to decipher underlying disease mechanisms. Because this requires transformative multidisciplinary collaboration, we introduce the EULAR study group on MHC-I-opathies to unite clinical expertise in rheumatology, dermatology and ophthalmology, with fundamental and translational researchers from multiple disciplines such as immunology, genomics and proteomics, alongside patient partners. We prioritise standardisation of disease phenotypes and scientific nomenclature and propose interdisciplinary genetic and translational studies to exploit emerging therapeutic strategies to understand MHC-I-mediated disease mechanisms. These collaborative efforts are required to address outstanding questions in the etiopathogenesis of MHC-I-opathies towards improving patient treatment and prognostication.
Keywords: Arthritis, Psoriatic; Behcet Syndrome; Immune System Diseases; Spondylitis, Ankylosing
The inception of the MHC-I-opathy family
Inflammation against self is orchestrated by a continuum of incompletely understood innate and adaptive immune mechanisms. The term ‘autoinflammatory’ refers to inflammation against self, caused by abnormal innate immunity, whereas ‘autoimmunity’ is caused by aberrant adaptive immunity.1 2 Since this dichotomous definition overlooked conditions such as psoriasis (PsO) and Behçet’s disease (BD), the concept of ‘mixed-pattern’ or ‘intermediate’ diseases was proposed.3
Genome-wide association studies (GWAS) of MHC-I-associated diseases, such as BD (associated with HLA-B*51),4 5 PsO (associated with HLA-C*06:02),6–8 HLA-B*27-associated spondyloarthritis (SpA)9–11 HLA-B*27-associated anterior uveitis (AU)12 and HLA-A*29-associated birdshot uveitis (BU),13 14 revealed that these ‘intermediate diseases’ share a distinguishable genetic background defined by MHC-I genes, the antigen processing genes ERAP1 and ERAP2, and the IL-17 pathway gene IL23R. Such genetic overlap implicates MHC-I peptide presentation as the key mechanistic commonality. Furthermore, it substantiates the idea that BD, PsO, SpA and BU belong to a distinct disease cluster known as ‘MHC-I-opathies’.15
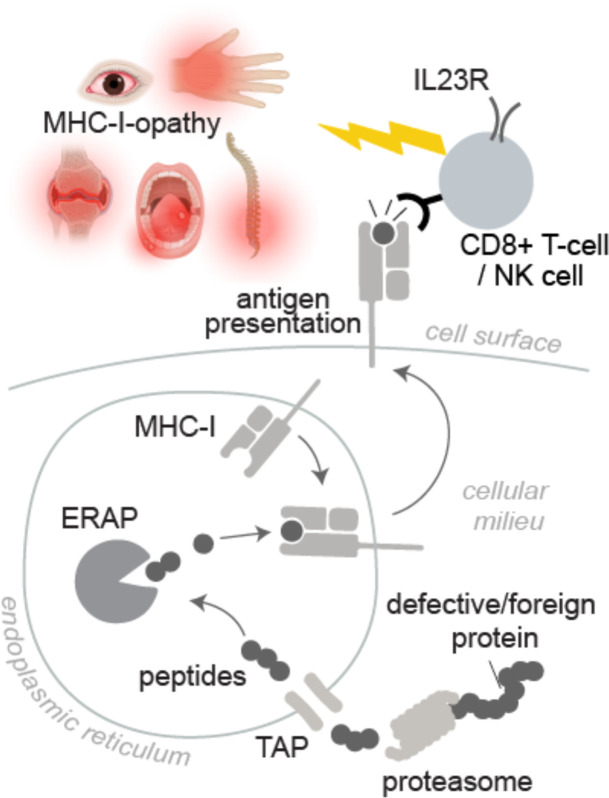
There is ongoing debate and incomplete evidence regarding underlying mechanisms of MHC-I-opathies.16–18 MHC-I proteins (also called HLA-A, HLA-B and HLA-C) bind short peptides from degraded or pathogenic proteins, which have been proteolysed inside the cell by the proteasome.19–21 Most MHC-I peptides are derived from proteins from the host. ERAP1 and ERAP2 are endoplasmic reticulum aminopeptidases associated with antigen processing that trim a certain fraction of these peptides if they are not short enough before loading them onto MHC-I molecules.22 This process enables MHC-I to present tens of thousands of peptides on the cell surface, collectively referred to as the ‘immunopeptidome’.23 CD8+T cells read out the immunopeptidome by binding to the peptide-MHC-I complexes with their T cell receptors (TCR) (figure 1). MHC-I molecules can also bind to killer-cell immunoglobulin-like receptors (KIRs) and other receptors on natural killer (NK) cells.24
Figure 1.
An overview of the role of the MHC-I pathway in MHC-I-opathies. The proteasome produces peptide fragments that are transported into the endoplasmic reticulum by the transporter associated with antigen processing (TAP) and trimmed by ERAP1 and ERAP2 (ERAP) to a length of 8-11 amino acids before binding to MHC-I molecules. After trafficking to the cell surface the MHC-I-peptide complex is “read out” by surveying immune cells, triggering antigen-specific CD8+ T cell responses or natural killer (NK) cell activation. MHC-I-opathies are genetically associated with functionally distinct variants of MHC-I and ERAP which alter the peptide repertoire presented by MHC-I. Autoreactive T cells in the periphery that escape tolerance mechanisms and promote inflammation against self-peptide epitopes. Biorender software was used to create elements from this figure under an academic license.
There is much conjecture about the cause(s) of MHC-I-opathies15 25 26 and several popular hypotheses have been proposed which are not necessarily mutually exclusive. The primary hypothesis for the cause of MHC-I-opathies is that disease-associated MHC-I alleles present specific immunogenic peptides that trigger ‘autoimmune’ reactions (ie, the arthritogenic peptide theory).27 The genetic association with ERAP genes also supports this hypothesis since the activity of these enzymes can modify the immunopeptidome.28 Proof of concept has been shown in PsO and recently in SpA and HLA-B*27+AU.29 The identification of CD8+T cells in PsO react against melanocytes in the context of HLA-C06:02 as skin-specific target cells of the psoriatic autoimmune response,30–32 while CD8+T cells from synovial and eye fluid of SpA and AU patients recognise both self and microbial peptides presented by HLA-B*27.29
There is still no conclusive evidence that this mechanism underlies other MHC-I-opathies since mechanistic studies are technically challenging to conduct, owing to multiorgan involvement having complex tissues, which requires labour-intensive technology to screen for many epitopes. Consequently, several alternative theories for MHC-I-opathies have been proposed, one of which suggests that MHC-I protein misfolding directly leads to inflammation. According to this theory, predisposing MHC-I molecules may exhibit properties which could cause excessive misfolding and accumulation in the ER, promoting the ‘unfolded protein response’.33–38 Studies of (HLA-B*27) transgenic animals and cellular models support this hypothesis, but there is a paucity of translational evidence.39–43 A third popular hypothesis suggests that the predisposing MHC-I alleles are recognised by KIRs or leucocyte immunoglobulin-like receptors (LILRs) on the cell surface of NK cells.44 45
In our opinion, the first hypothesis applies to the majority of MHC-I-opathies (with the most robust evidence for PsO and SpA), but definitive proof for CD8+T cell-mediated pathologies is lacking for several other conditions. Hypotheses 2 and 3 may also apply to certain conditions. For example, in BD, ERAP1 may mediate HLA-B51 recognition via NK cells,17 46 47 and pathogens that can cause reactive arthritis induce unfolded protein responses in HLA-B*27-positive individuals.48 While these other pathways and mechanisms are implicated, including the very interesting interactions of altered microbiomes in patients,49 we focus our discussion on the MHC-I pathway as the key determinant for this family of complex conditions.
The many faces and challenges of MHC-I-opathies
Several conditions are considered to be ‘classical’ MHC-I-opathies (PsO, psoriatic arthritis (PsA), SpA, B*27-AU, BD and BU) and share strikingly similar clinical symptoms (table 1).
Table 1.
Summary of tissue involvement per MHC-I-opathy, organised per clinical specialty (references underlying the summarised data and scores can be found in online supplemental table 1
| Disease | PsO* | PsA† | SpA | B*27 AU | BD | BU | |
| Medical specialty | Primary risk MHC-I-allele(s) | C*06 | C*06/B*27 | B*27 | B*27 | B*51 | A*29 |
| Prognosis worse when primary MHC-I allele present | 3 | 0 | 3 | n.a | 3 | 0 | |
| Ophthalmology | Uveitis‡ | 1 | 1 | 3 | 3 | 3 | 3 |
| Dermatology | Oral ulcerations | 0 | 1 | 1 | 0 | 3 | 0 |
| Dermatology | Genital ulcerations | 0 | 0 | 0 | 0 | 3 | 0 |
| Dermatology | Psoriasiform dermatitis§ | 3 | 3 | 2 | 2 | 1 | 1 |
| Dermatology | Pustular lesions¶ | 2 | 2 | 1 | 0 | 3 | 0 |
| Dermatology | Erythema nodosum-like lesions | 0 | 0 | 0 | 0 | 3 | 0 |
| Rheumatology | Spondylitis | 1 | 3 | 3 | 3 | 1 | 0 |
| Rheumatology | Arthritis | 2 | 3 | 3 | 2 | 3 | 0 |
| Rheumatology | Enthesitis | 2 | 3 | 3 | 3 | 1 | 0 |
| Rheumatol/immunol | Vasculitis** | 1 | 1 | 1 | 0 | 3 | 0 |
| Gastroenterology | Inflammatory bowel disease | 1 | 1 | 2 | 1 | 2 | 0 |
| Internal medicine | Comorbid hypertension | 1 | 2 | 2 | 0 | 0 | 2 |
| Neurol/Int Med/cardiol | Comorbid cardiovasc disease | 2 | 2 | 2 | 0 | 1 | 0 |
| Legend: | 3 | part of the disease ectrum | |||||
| 2 | regularly reported | ||||||
| 1 | infrequently reported | ||||||
| 0 | |||||||
3 part of the disease spectrum.
2 regularly report.
1 infrequently reported.
0 either unknown / no reports / not present.
*Psoriasis: besides plaque psoriasis. This encompasses other forms of psoriatic disease like psoriasis guttate and (several types of) pustular psoriasis.
†PsA: both axial and peripheral disease.
‡Uveitis anterior is the main subtype reported in PsO, PsA, SpA, whereas in Behçet’s multiple anatomical subtypes of uveitis are reported. BU manifests as posterior uveitis.
§Psoriasiform lesions: refers to the several types of psoriasis; classical plaque psoriasis, guttate, nail lesions and erythematous as well as pustular lesions.
¶Pustular lesions: covers acneiform, papulopustular and non-follicular pustules.
**Vasculitis in PsO as well as in PsA and SpA vasculitis is in the large vessels (aortitis); in B27-AU and BU not reported outside the eye; in Behçet’s vasculitis is in all types of vessels, arteries and veins.
BU, birdshot uveitis; PsA, psoriatic arthritis; PsO, psoriasis; SpA, spondyloarthritis.
ard-2022-222852supp001.pdf (124.4KB, pdf)
BU is a rare and severe type of uveitis, leading to retinal damage and vision loss that exclusively affects HLA-A*29-positive individuals.50 51 Although it is unclear which other clinical features are shared between BU and other MHC-I-opathies, 1 study of 118 cases revealed that many patients also suffer from arthralgia and PsO.52 We also discuss PsA because it shares many characteristics with PsO, including strong association with MHC-I alleles and IL23R.7 8 53–55 While some patients with inflammatory bowel disease may have similar symptoms,56 we will only discuss classical MHC-I-opathies here.
MHC-I-opathies overlap in their pattern of organ involvement (table 1). Uveitis, for instance, is a disease feature reported in every classical MHC-I-opathy, although with different prevalence and anatomical location (anterior/posterior).57 58 Sacroiliitis is present in patients with SpA, PsA as well as BD.59–61 Cutaneous involvement is also a shared feature of MHC-I-opathies (table 1).
However, not every patient exhibits the symptomatic hallmarks of every clinical entity. For example, arterial, venous and neurological complications are common in BD, but infrequent in other MHC-I-opathies.18 62 For several MHC-I-opathies, patients with the associated risk MHC-I alleles are more likely to manifest early-onset disease and a worse prognosis.63–66 Furthermore, substantial clinical and geographical variation in disease phenotypes exists, for example, the prevalence of gastrointestinal involvement in BD in Asian versus European populations.67 68
What you (do not) see is what you (do not) get!
The clinical management of MHC-I-opathy patients is complicated by heterogeneity in age of onset, symptoms and disease course. Unlike cases with commonly recognised symptoms (e.g., uveitis in SpA patients), asymptomatic or atypical involvement of the skin, bowel or other comorbidities in patients may be overlooked (table 1). For example, reexamination of SpA patients revealed that up to one-third may have comorbid PsO.69 In the DUET study, over 40% of patients with B*27-AU were diagnosed with SpA or PsA on re-evaluation by a rheumatologist,70 which was confirmed by other studies.71 Large population-level data also correlate disease manifestations of MHC-I-opathies such as uveitis, PsO, PsA and BD.58 72 73 Observations from well-powered cohort studies substantiate that oral disease, which is a hallmark of BD, is also linked to SpA.74–76 Despite considerable phenotypic heterogeneity, these studies support that MHC-I-opathies are interconnected conditions that cannot be understood in isolation and require a multidisciplinary approach.
The human phenotype ontology (HPO) provides a framework for standardised nomenclature of disease symptoms, which can facilitate improved classification of disease phenotypes.77 Although originally designed to systematically capture the clinical manifestations of rare, monogenic conditions, HPO has more recently been used to successfully infer several rare phenotypes of the UK Biobank.78 In its current form, the HPO may not be optimal for the annotation of the clinical spectrum of patients with MHC-I-opathies. As a result, the EULAR study group aims to evaluate the HPO and adapt it to fit the symptoms of MHC-I-opathies. The spectrum of MHC-I-opathies will benefit from standardisation of disease manifestations, allowing existing cohorts to be merged into a well-powered study. The precise delineation of clinical phenotypes will allow us to relate them to molecular endotypes. We expect that this process will facilitate the discovery (and validation) of better diagnostic, prognostic and therapeutic biomarkers.
A common genetic architecture
MHC-I, the tip of the iceberg
Strong genetic association with certain MHC-I alleles is the hallmark of the MHC-I-opathy cluster: MHC-I association studies date back to 1973 with the discovery of the association of HLA-B*27 and SpA as well as HLA-B*51 and BD (formerly ‘HL-A5’),79–81 followed by reports on HLA-C*06:02 (previously known as ‘HLA-Cw6’) and PsO in 1977,82 83 and the association between HLA-A*29 and BU in 198284 (table 2). In comparison to genes associated with complex inflammatory conditions, the effect size of MHC-I alleles accounts for a disproportionate amount of genetic risk. For almost 50 years, researchers have struggled to understand the role these class I alleles play in their disease biology.
Table 2.
Reported HLA class I associations in four MHC-I-opathies
| MHC-I-opathy | Prevalence | Primary HLA class I association | % cases negative for primary HLA class I allele | Independent* HLA class I associations |
| Birdshot uveitis | 1-5/500 000 | HLA-A*29:02 | 0 |
HLA-A*30
13 14
HLA-A*33 14 |
| Spondyloarthritis† | 0.5% | HLA-B*27 | ~ <30 |
HLA-B*40
85 86
HLA-A*02 85 HLA-B*07 85 HLA-B*57 85 HLA-C*15 86 |
| Behçet’s Disease | 0.19-120/100 0009 | HLA-B*51 | ~30–70 |
HLA-A*02
87
HLA-B*27 87 HLA-B*57 87 HLA-A*03 87 HLA-B*15 87 HLA-B*49 87 HLA-A*26 87 89 HLA-C*07 89 |
| Psoriasis | 2–4% | HLA-C*06:02 | ~30–70 |
HLA-A*02
122
HLA-B*27 122 HLA-B*07 122 HLA-C*07 176 |
*Identified by statistical adjusting for primary associated HLA class I allele.
†Majority of data are from genetic studies in ankylosing spondylitis. Includes both risk and protective alleles.
Interestingly, recent fine-mapping studies showed that statistical adjustment for HLA-B*27 in SpA revealed independent associations for other MHC-I alleles, including HLA-A*02:01, HLA-B*07, HLA-B*57 and HLA-B*40 85 86 (table 2). This is significant because it also implicates the MHC-I pathway for cases lacking the primary MHC-I risk allele and strongly incriminates peptide presentation rather than alternative mechanisms.
Association with several of these alleles was also found after correcting for the primary risk MHC-I allele in PsO (HLA-A*02:01, HLA-B*27 and HLA-B*07), BD (eg, HLA-B*27 and HLA-B*57), PsA (eg, HLA-B*07 and HLA-A*02) and AU.7 12 87–90 To date, small GWAS in the rare BU had limited power to detect HLA-A*29-independent loci in detail, but also reported independent risk MHC-I alleles.13 14 These findings raise the possibility that an ensemble of disease-overarching MHC-I alleles contribute to MHC-I-opathy susceptibility.
Therefore, functional studies that consider only one MHC-I allele may not capture the complexity of the MHC-I pathway in patients. This emphasises the need to use primary patient tissues to investigate disease mechanisms. It remains to be determined whether the full MHC haplotype (including ‘secondary’ risk MHC-I alleles) improves patient stratification. Large population-based studies (ie, UK Biobank) support that MHC-I alleles are associated with a variety of health biomarkers.91 A first step into this direction could be the conduction of a multiancestral MHC-I-opathy GWAS analysis by combining several available large-scale genome-wide datasets and interrogating the MHC for different phenotypic states.
The devil is in the ERAP1 and ERAP2 details
Perhaps one of the major accomplishments for the progress in the understanding of MHC-I-opathies was the discovery of the association with the ERAP1 and/or ERAP2 genes.4 6 7 9 12–14 92–95 These genes encode two ER-resident enzymes specialised in trimming peptides to facilitate or prevent their binding in the groove of MHC-I.96 97 By generating and destroying peptide epitopes, ERAPs can affect CD8+T cell and NK cell responses.98–101 Genetic variants in ERAP1 and ERAP2 affect the enzymatic activity and expression levels of these enzymes.93 102 Consequently, a change in ERAP activity may expose CD8+T cells to altered peptide repertoires (self or non-self) via MHC-I risk alleles, which can be harmful.28
Genetic association between ERAP1 and MHC-I-opathies is typically observed in individuals carrying the primary risk MHC-I.4 6 11 13 85 93 Coding variants in ERAP1 organise into several common haplotypes often referred to as ERAP1 ‘allotypes’103 104 that exhibit a wide range of enzymatic activities towards peptide substrates and differentially shape the immunopeptidome of MHC-I.28 105 Risk polymorphisms in ERAP1 (and ERAP2) are also strongly associated with mRNA and protein expression levels of these aminopeptidases.50 102 106 Haplotype-based analyses have singled out specific ERAP1 allotypes as risk factors for MHC-I-opathies. While several terms have been proposed for ERAP1 allotypes, standardised nomenclature has yet to be widely adopted. One functionally distinct ERAP1 allotype (often referred to as Haplotype 10 (hap10)) is a risk factor for BD and BU,93 107 but protective for SpA, AU and PsO.28 108 Interestingly, in PsO, the protective hap10 was less effective in generating the autoantigenic epitope than the risk haplotypes of ERAP1, leading to lower HLA-C expression and immunogenicity of melanocytes.31
ERAP1 may also influence NK cell responses via inhibitory receptors NKG2A/CD94 (also expressed by CD8+T cells109) to non-classical MHC-I molecule HLA-E.46 The inhibitory activity of HLA-E requires the presentation of a signal sequence from MHC-I molecules, which are also present in HLA-A29, HLA-B27 and HLA-B51.110–112 Therefore, ERAPs may also affect NK cells and CD8+T cells via MHC-I-related molecules, as was previously shown in cancer models.46 113 Although KIR receptors can recognise immunopeptidome changes caused by ERAP1, KIR genes do not influence HLA-B*27 and ERAP1-mediated ankylosing spondylitis risk.114 115 This suggests that the disease mechanisms mediated by ERAP1 and MHC-I are less dependent on KIRs.
In contrast to ERAP1, ERAP2 genetic variants are not associated with all MHC-I-opathies (eg, BD). Also, ERAP2 is associated with SpA regardless of HLA-B*27 status. Because there is also epistasis between ERAP1 and HLA-B*40 in SpA (independent of HLA-B*27),85 it is possible that ERAP2 modifies disease in SpA via alternative risk MHC-I alleles. Functional studies support that ERAP2 significantly affects the immunopeptidome of many MHC-I alleles, including HLA-B*40 115 116
Note that ERAP2 allotypes co-occur non-randomly with ERAP1 allotypes.93 105 Furthermore, although HLA-A*29 is common in many regions, HLA-A*29-positive individuals who carry both ERAP1 and ERAP2 risk alleles are only observed in countries where BU is prevalent.93 Therefore, an individual’s ERAP1 and ERAP2 allotypes along with their MHC-I profile (and T cell repertoire) are most likely to determine their susceptibility to MHC-I-opathies.117
Studies linking ERAP genotypes with clinical end points may have potential,118 119 but we would like to emphasise that these studies should be carefully controlled and well powered. Both ERAP1 and ERAP2 are common denominators of MHC-I-opathies, which place antigenic peptide presentation at the heart of their pathogenesis.
IL23R and T cells
There are many other genes associated with conditions within the MHC-I-opathy spectrum that have been discovered through GWAS. While they are important to disease biology, we only briefly discuss IL23R, a receptor for IL-23 expressed by T cells (and innate lymphocytes), because it is common to MHC-I-opathies and is associated with disease severity and phenotypes.8 118 120–122 Fascinatingly, despite IL23R expression by CD4+T cells, epigenetic analyses implicate CD8+T cells as major perpetrators of MHC-I-opathies.88 Interleukin-17-producing CD8+T cells (termed ‘Tc17’) infiltrating skin and synovial lesions in PsO, BD, SpA and PsA patients express IL23R.123–125 Tc17 cells are also more abundant in patients with BU.126 127 IL23R’s role in the pathophysiology of MHC-I-opathies is incompletely understood, but likely to be tissue-dependent.128 This may explain why patients with PsO129 and PsA130 exhibit clinical response to therapy that disrupts T cell IL-23 signalling, while initial trials were less successful in SpA.131 132 A better understanding of clinical and molecular features will help overcome challenges posed by patient heterogeneity as well as identify therapeutic biomarkers which will guide the selection of candidates eligible for treatment with IL-23 inhibitors.21 128 131–134
Unmet needs in MHC-I-opathy pathophysiology understanding
Evidence for autoreactive CD8+ T cell involvement
A number of immunopeptidome studies in cell models have shown that polymorphisms in ERAP cause change in the peptides presented by HLA-B27, HLA-B51, HLA-A29 and other MHC-I alleles.28 115 116 Circumstantial evidence suggests that these enzymes introduce or remove peptides that bind to risk MHC-I alleles and signal CD8+T cells to attack healthy tissues. The fact that CD8+T cells are clonotypically expanded in patients with SpA, PsO and PsA supports this concept.135–138 In BD, carriers of the disease-associated ERAP1 allotype107 show enrichment for circulating antigen-experienced effector CD8+T cells and ERAP1 modulation influenced CD8+T cell responses.107 The lack of identification of causative autoantigens or indeed alloantigens has resulted in discussion about whether CD8+T cells drive pathology in MHC-I-opathies.17 Regardless, autoantigen-derived peptide recognition by CD8+T cells in patients has previously been reported, including an HLA-B51-presented peptide derived from a stress-inducible autoantigen in BD,139 HLA-C06:02 presented peptide from innate host defence protein LL-37 in PsO,140 and HLA-B27-restricted epitope from a peptide hormone receptor and cartilage-derived peptides in SpA.141 142
To date, the most compelling conceptual proof that CD8+T cells mediate autoimmune inflammation is based on studies of PsO, and very recently in HLA-B*27-positive SpA and AU patients.29 30 Skin lesional CD8+T cells in PsO can recognise an HLA-C06:02-restricted autoantigen epitope from ADAMTSL5 highly expressed in skin melanocytes.30 31 ADAMTSL5-specific CD8+T cells secrete PsO-promoting cytokines (eg, IL-17) specifically after recognising melanocyte-peptide processed by ERAP1 and presented by the disease-associated MHC-I HLA-C06:02.30–32 Here, the immunogenicity of melanocytes for self-reactive CD8+T cell responses was increased by disease-associated ERAP1 haplotypes through greater supply of the peptide autoantigen.30 It has, therefore, been suggested that pharmacological modulation of ERAP activity towards precursor peptides specifically presented by MHC-I alleles could reverse inflammation in MHC-I-associated diseases.143 144
Researchers recently found that tissue-infiltrating CD8+T cells shared TCRs in eye liquid as well as synovial fluid of HLA-B*27-positive patients with AS and AU.29 These CD8+T cells specifically recognise microbial (eg, YEIH protein from reactive arthritis-triggering pathogens) and self-antigens (eg, peptides from GPER1 or PRPF3 proteins) specifically within the context of HLA-B27. According to these findings, environmental pathogens may trigger autoimmunity via CD8+T cell activation in MHC-I-opathies, thus supporting the primary hypothesis of the MHC-I-opathy pathogenesis. Future research might explore whether HLA-B27 presentations of these peptides are affected by risk allotypes of ERAP1 and whether pharmacological targeting of ERAPs interferes with these responses.
It remains unclear why of the thousands of self-peptides in the immunopeptidome only a minority become immunogenic, while the majority remain tolerable. However, T cell autoantigens often have post-translational modifications or show altered binding conformation.145–147
What triggers CD8+T cell self-reactivity in MHC-I-opathies remains unknown. The classical view is that negative selection in the thymus eliminates autoreactive T cells. Some self-reactive CD8+T cells manage to escape this filtering process and are reintroduced into the circulation (sometimes at high frequencies) but kept in check by tolerance mechanisms.148–150
Interestingly, recent work suggests that thymic regulatory T-cells, rather than negative selection of autoreactive T cells, enforce protection against autoimmunity.148 151 Here, the cytokine IL-23 eliminates thymic regulatory T cells in an IL23R-dependent manner,152 while selectively enriching IL23R-expressing CD8+T cells.153 Moreover, there is no sharp affinity threshold for the recognition of MHC-peptide complex by TCRs, and CD8+T cells with otherwise low affinity TCRs can be activated by a large increase in presented autoantigen.154 155 This also fits with the recently proposed ‘autoimmune surveillance of hypersecreting mutants’ theory that links high autoantigen levels to T-cell autoimmunity.156 Cross-presentation of extracellular antigens in dendritic cells can also lead to the entry of extracellular antigens into the MHC class I pathway, thereby greatly expanding the potential pool of immunogenic peptides. Conceptually, this integrates the possibility of microbial agents causing disease, as demonstrated for SpA, and AU.29 Virus-triggered clonal CD8+T cell responses are processed through MHC class I, and some of these responses are controlled by ERAP1.157
Recent technological advancements which have increased the sensitivity and scale of analysing immunopeptidomes of primary patient tissues (ideally sampled at the affected organs) as-well as high-throughput profiling of (auto)antigen-specific T-cell repertoires (ie, single-cell TCR sequencing) may help identify CD8+T cell-mediated disease mechanisms in MHC-I-opathies in greater detail.158–161
Towards MHC-I pathway therapy
This study group’s ultimate goal is to improve disease outcome of MHC-I-opathies. Although definite disease mechanisms need to be established, available clinical and molecular evidence allow us to outline several potential strategies. Given that MHC-I is considered a root cause for MHC-I-opathies, therapeutic targeting of antigen processing and presentation seems self-evident. This may be achieved by interventions aimed at disrupting cytokine signalling (see section IL23R and T cells) or strategies that facilitate restoration of the microbiome.48 Patients with MHC-I-opathies may have an altered microbiota,162–164 but healthy individuals may also show microbiota compositions that cluster according to their HLA alleles (eg, HLA-B*27, HLA-A*29).165 Emerging T cell-antigen discovery approaches within the microbiome may provide an exciting field for upcoming studies.166 In case of autoantigen-mediated pathology, it may be possible to specifically negate T cell interaction by antibodies or small compounds that specifically block access to MHC-I-peptide complexes. T-cell engagement may also be blocked by preventing or changing the abundance of target peptide presentation by manipulation upstream of MHC-I, including the cellular proteome (eg, chemotherapy), or pharmacological inhibition or modulation of the proteasome, TAP or the antigen loading complex,167–171 although with limitations in specificity at the cost of potential adverse effects.
Inhibiting or, depending on the disease, enhancing the action of ERAP1 and ERAP2 may be a promising approach, since these enzymes are highly specialised for antigen presentation, and much is known about their structure and function to allow the development of inhibitors or enhancers.143 144 172 The fact that their impact on antigen presentation may be limited to a part of the immunopeptidome,173 may constitute a middle ground between single antigen strategies (antibodies for MHC/peptide complex) and general suppression of the MHC-I pathway. Most of these therapeutic ‘options’ are still in their infancy and require translational studies in suitable preclinical models. Although the HLA-B*27-transgenic rodent models,174 have provided valuable insights into the disease mechanisms of MHC-I-opathies, there remains an unmet need for additional transgenic MHC-I models. To determine if it is possible to target the MHC-I pathway therapeutically in patients, these models should be ‘fully’ humanised and capture a broader spectrum of clinical and molecular characteristics.
Mission of the EULAR study group on MHC-I-opathies
As a result of the complexity of the clinical phenotypes and the lack of knowledge about the underlying mechanisms of MHC-I-opathies, international cross-disciplinary collaborations and complementary scientific expertise are urgently needed. The EULAR study group on MHC-I-opathies provides an international network that brings medical specialists, translational and fundamental scientists under one umbrella with the aim of cooperatively overcoming long-standing unmet needs in the disease management and understanding of the biology of MHC-I-opathies.
The study group (currently >50 participants: dermatologists, ophthalmologists, rheumatologist, scientists and patient representatives from >15 countries) was founded in 2020 amidst the COVID-19 pandemic. The global pandemic restricted initial discussion to online meetings. An inaugural meeting took place in May 2022, in Amsterdam, followed by a meeting during EULAR in June 2022 in Copenhagen. Study group research and collaborations will focus on the pathophysiology of MHC-I pathway in these conditions. Briefly, the study group aims are summarised in box 1 and the objective is to harmonise, facilitate and improve research methodology and terminology, study disease mechanisms more collectively; foster basic and translational knowledge exchange in an interdisciplinary fashion through meetings via symposia during EULAR meetings(https://www.eular.ch/myUploadData/files/study_group_aims_mhc_i_opathy_for_web.pdf) and disseminate progress via social media (eg, an open Linked-in page for interested colleagues, https://www.linkedin.com/groups/12722534/). To accomplish these objectives, the Study Group formed several multidisciplinary task forces composed of clinicians, biologists and patient representatives to prioritise unmet research needs that would require cross-European collaboration. For example, one of the task forces aims to conduct meta-analysis of GWAS data of the MHC-I-opathies to fine map the MHC and identify novel risk loci in relation to clinical features. Another task force currently works on evaluation of a patient-reported symptom infrastructure, which has already been successfully employed in COVID-19 studies.175 Although currently all work within the study group is contributed in kind by its members, the rapidly growing study group aims to apply for external funding for research. This will also be required to achieve more ambitious goals, such as the collection of biomaterials to foster innovative research by deep immunoprofiling (eg, T-cell repertoires, MHC-I immunopeptidomes) and translational studies (eg, ERAP modulation in patient tissues). The EULAR study group will complement their scientific objectives with the organisation of interactive workshops and symposia connected to EULAR to exchange basic, translational and clinical knowledge in an interdisciplinary fashion and further facilitate the growth of the study group by inclusion of physicians and scientists active in this field.
Box 1. The aims of the EULAR study group on "MHC-I-opathy".
Multidisciplinary collaboration between rheumatologists, dermatologists and ophthalmologists for consensus and standardised annotation of disease symptoms.
Detailed phenotypic evaluation by patient-reported symptoms/outcomes.
Integration of GWAS data of MHC-I-opathy-related diseases, across a larger number of existing cohorts, to facilitate fine mapping of the genetic basis.
Harmonisation of the nomenclature (eg, ERAP allotypes) and provide expert synthesis of current best practice for the study of key aspects of the biology in MHC-I-opathies.
Establishment of a pan-European consortium with standardised clinicopathological disease phenotypes from aim 1 and 2, (complemented by molecular data on ERAP and MHC-I haplotypes and possibly other biological data such as metagenomics to assess microbiome involvement and TCR-repertoire data) for improved disease classification, diagnostic criteria and prognostic biomarkers for prediction of disease progression and efficacy of (type of) therapy.
Evaluation of MHC-I-opathies in different ethnic backgrounds, given the massive heterogeneity within class-1 antigens.
Patient participation: involvement of patient research partners.
In conclusion, the EULAR study group on MHC-I-opathies bridges a variety of medical scientific disciplines with the ambitious joint objective to conduct an integrated investigation of MHC-I-opathies to discover the cause and cure for a variety of complex inflammatory conditions.
Footnotes
Handling editor: Josef S Smolen
Twitter: @jonas_kuiper, @tonykenna3
Contributors: The final version was approved by all authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JCP: Grants or contracts from any entity German Research Foundation grants PR 241/5-2Consulting fees Boehringer IngelheimPayment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events paid activities as a speaker for Almirall, Boehringer Ingelheim, Janssen-Cilag, Novartis and Pfizer PK Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid All unpaid: Human Immunology (Elsevier) Editorial Board, Frontiers in Immunology Guest associate, Editor and review editor. Editorial board of International Journal of Immunogenetics SS: all support for the present manuscript (eg, funding, provision of study materials, medical writing, article processing charges, etc. Deutsche Forschungsgemeinschaft (DFG) to Jacobs University Bremen DM Grants or contracts from any entity Government of MaltaPayment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events Sanofi, UriageSupport for attending meetings and/or travel Avene, Bioderma, UriageLeadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid Maltese Association of Dermatologists and VenereologistsIvan IP Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events Novartis, Eli Lilly, Pfizer, Abbvie, Honoraria for lectures, payments directly to me RS Support for attending meetings and/or travel Abbvie and PfizerIna IK Consulting fees Amgen, Boehringer, GSK, SobiPayment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events Abbvie, Amgen. Boehringer, GSK, Janssen, Lilly, MSD, Novartis, Pfizer, Sobi MvdS Grants or contracts from any entity Novartis, UCB, EliLillyPayment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events UCBSupport for attending meetings and/or travel UCBParticipation on a Data Safety Monitoring Board or Advisory Board Novartis, UCB, Abbvie GB Grants or contracts from any entity GSK, PfizerPayment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events GSK, AstraZeneca, Pfizer, Abbvie, Aenorasis, Novartis, Lilly IL Consulting fees Novartis, Alimera lT-T Consulting fees AbbVie, Turkey, NovartisPayment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events AbbVie, Turkey FC Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events LillySupport for attending meetings and/or travel UCB, NovartisParticipation on a data safety monitoring board or Advisory Board UCB, Novartis DMZ Grants or contracts from any entity European Society of OphthalmologyPayment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events Zentiva, Alkaloid d.o.o., Inspharma d.o.o. MB Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events Pfizer, Viatris, Lilly, MSD TJK All support for the present manuscript (eg, funding, provision of study materials, medical writing, article processing charges, etc). National Health & Medical Research Council GNT2011115Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid President, Australian Society for Medical Research MB Grants or contracts from any entity PFIZERConsulting fees PFIZERPayment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events FRENESIUS KABI, LILLYSupport for attending meetings and/or travel BIOGEN, PFIZER, JANSSEN RJUL Consulting fees UCB, Novartis, Abbvie, Eli-LillyPayment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events UCB, Novartis, Abbvie, Eli-Lilly, Amgen JN All research funding support for the present manuscript: NEI-NIH R01EY033495—research funds and R01EY031383—research fundsHonoraria for lectures: Harvard University, Northwestern University, Massachusetts General Hospital.Support for attending meetings and/or travel: NYU Department of Medicine, NIH-NEIParticipation Medical Advisory Board: ABDA (American Behçet’s Disease Association) DGMcG Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events Janssen, Abbvie, Novartis, UCB, BMS, Lilly.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Medzhitov R. The spectrum of inflammatory responses. Science 2021;374:1070–5. 10.1126/science.abi5200 [DOI] [PubMed] [Google Scholar]
- 2. Szekanecz Z, McInnes IB, Schett G, et al. Autoinflammation and autoimmunity across rheumatic and musculoskeletal diseases. Nat Rev Rheumatol 2021;17:585–95. 10.1038/s41584-021-00652-9 [DOI] [PubMed] [Google Scholar]
- 3. McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med 2006;3:e297. 10.1371/journal.pmed.0030297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirino Y, Bertsias G, Ishigatsubo Y, et al. Genome-wide association analysis identifies new susceptibility loci for Behçet’s disease and epistasis between HLA-B*51 and ERAP1. Nat Genet 2013;45:202–7. 10.1038/ng.2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takeuchi M, Mizuki N, Meguro A, et al. Dense genotyping of immune-related loci implicates host responses to microbial exposure in Behçet’s disease susceptibility. Nat Genet 2017;49:438–43. 10.1038/ng.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strange A, Capon F, Spencer CCA, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet 2010;42:985–90. 10.1038/ng.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bowes J, Ashcroft J, Dand N, et al. Cross-phenotype association mapping of the MHC identifies genetic variants that differentiate psoriatic arthritis from psoriasis. Ann Rheum Dis 2017;76:1774–9. 10.1136/annrheumdis-2017-211414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stuart PE, Nair RP, Tsoi LC, et al. Genome-wide association analysis of psoriatic arthritis and cutaneous psoriasis reveals differences in their genetic architecture. Am J Hum Genet 2015;97:816–36. 10.1016/j.ajhg.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burton PR, Clayton DG, Cardon LR, et al. Association scan of 14,500 nonsynonymous snps in four diseases identifies autoimmunity variants. Nat Genet 2007;39:1329–37. 10.1038/ng.2007.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reveille JD, Sims A-M, Danoy P, et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet 2010;42:123–7. 10.1038/ng.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Evans DM, Spencer CCA, Pointon JJ, et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet 2011;43:761–7. 10.1038/ng.873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang X-F, Li Z, De Guzman E, et al. Genomewide association study of acute anterior uveitis identifies new susceptibility loci. Invest Ophthalmol Vis Sci 2020;61:3. 10.1167/iovs.61.6.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuiper JJW, Van Setten J, Ripke S, et al. A genome-wide association study identifies a functional ERAP2 haplotype associated with birdshot chorioretinopathy. Hum Mol Genet 2014;23:6081–7. 10.1093/hmg/ddu307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gelfman S, Monnet D, Ligocki AJ, et al. ERAP1, ERAP2, and two copies of HLA-aw19 alleles increase the risk for birdshot chorioretinopathy in HLA-A29 carriers. Invest Ophthalmol Vis Sci 2021;62:3. 10.1167/iovs.62.14.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGonagle D, Aydin SZ, Gül A, et al. MHC-I-opathy’-unified concept for spondyloarthritis and behçet disease. Nat Rev Rheumatol 2015;11:731–40. 10.1038/nrrheum.2015.147 [DOI] [PubMed] [Google Scholar]
- 16. McGonagle D, Aydın S, Gül A, et al. Reply to: behçet’s disease: an MHC-I-opathy? undefined. 2017. Available: https://www.semanticscholar.org/paper/Reply-to%3A-Beh%C3%A7et%27s-disease%3A-an-MHC-I-opathy-McGonagle-Ayd%C4%B1n/5840d4ead0babea3fdb727ffb06b271c7c9f7993 [Accessed 14 Oct 2022]. [PubMed]
- 17. Giza M, Koftori D, Chen L, et al. Is behçet’s disease a “class 1-opathy”? The role of HLA-B*51 in the pathogenesis of behçet’s disease. Clin Exp Immunol 2018;191:11–8. 10.1111/cei.13049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hatemi G, Karatemiz G, Yazici H. Behçet’s disease: an MHC-I-opathy? Clin Exp Rheumatol 2017;35 Suppl 104:5. [PubMed] [Google Scholar]
- 19. Rock KL, Reits E, Neefjes J. Present yourself! by MHC class I and MHC class II molecules. Trends Immunol 2016;37:724–37. 10.1016/j.it.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yewdell JW, Hollý J. DRiPs get molecular. Curr Opin Immunol 2020;64:130–6. 10.1016/j.coi.2020.05.009 [DOI] [PubMed] [Google Scholar]
- 21. Rock KL, York IA, Goldberg AL. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat Immunol 2004;5:670–7. 10.1038/ni1089 [DOI] [PubMed] [Google Scholar]
- 22. Blees A, Januliene D, Hofmann T, et al. Structure of the human MHC-I peptide-loading complex. Nature 2017;551:525–8. 10.1038/nature24627 [DOI] [PubMed] [Google Scholar]
- 23. Admon A, Bassani-Sternberg M. The human immunopeptidome project, a suggestion for yet another postgenome next big thing. Mol Cell Proteomics 2011;10:111. 10.1074/mcp.O111.011833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mbiribindi B, Mukherjee S, Wellington D, et al. Spatial clustering of receptors and signaling molecules regulates NK cell response to peptide repertoire changes. Front Immunol 2019;10:605. 10.3389/fimmu.2019.00605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gill T, Rosenbaum JT. Putative pathobionts in HLA-B27-associated spondyloarthropathy. Front Immunol 2020;11:586494. 10.3389/fimmu.2020.586494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garrido-Mesa J, Brown MA. T cell repertoire profiling and the mechanism by which HLA-B27 causes ankylosing spondylitis. Curr Rheumatol Rep 2022;24:398–410. 10.1007/s11926-022-01090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mauro D, Thomas R, Guggino G, et al. Ankylosing spondylitis: an autoimmune or autoinflammatory disease? Nat Rev Rheumatol 2021;17:387–404. 10.1038/s41584-021-00625-y [DOI] [PubMed] [Google Scholar]
- 28. López de Castro JA. How ERAP1 and ERAP2 shape the peptidomes of disease-associated MHC-I proteins. Front Immunol 2018;9:2463. 10.3389/fimmu.2018.02463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang X, Garner LI, Zvyagin IV, et al. Autoimmunity-associated T cell receptors recognize HLA-B*27-bound peptides. Nature 2022;612:771–7. 10.1038/s41586-022-05501-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arakawa A, Reeves E, Vollmer S, et al. Erap1 controls the autoimmune response against melanocytes in psoriasis by generating the melanocyte autoantigen and regulating its amount for HLA-C*06:02 presentation. J Immunol 2021;207:2235–44. 10.4049/jimmunol.2100686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arakawa A, Siewert K, Stöhr J, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med 2015;212:2203–12. 10.1084/jem.20151093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonifacio KM, Kunjravia N, Krueger JG, et al. Cutaneous expression of a disintegrin-like and metalloprotease domain containing thrombospondin type 1 motif-like 5 (ADAMTSL5) in psoriasis goes beyond melanocytes. J Pigment Disord 2016;3:244. 10.4172/2376-0427.1000244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Loll B, Rückert C, Uchanska-Ziegler B, et al. Conformational plasticity of HLA-B27 molecules correlates inversely with efficiency of negative T cell selection. Front Immunol 2020;11:179. 10.3389/fimmu.2020.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blanco-Gelaz MA, Suárez-Alvarez B, Díaz-Peña R, et al. Hla-B27 polymorphism at position 116 critically influences the association with TAP/tapasin, intracellular trafficking and conformational homodimers formation. Mol Immunol 2009;46:1304–11. 10.1016/j.molimm.2008.11.023 [DOI] [PubMed] [Google Scholar]
- 35. Abualrous ET, Fritzsche S, Hein Z, et al. F pocket flexibility influences the tapasin dependence of two differentially disease-associated MHC class I proteins. Eur J Immunol 2015;45:1248–57. 10.1002/eji.201445307 [DOI] [PubMed] [Google Scholar]
- 36. Hein Z, Uchtenhagen H, Abualrous ET, et al. Peptide-independent stabilization of MHC class I molecules breaches cellular quality control. J Cell Sci 2014;127:2885–97. 10.1242/jcs.145334 [DOI] [PubMed] [Google Scholar]
- 37. Granados DP, Tanguay P-L, Hardy M-P, et al. Er stress affects processing of MHC class I-associated peptides. BMC Immunol 2009;10:10. 10.1186/1471-2172-10-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Almeida SF, Fleming JV, Azevedo JE, et al. Stimulation of an unfolded protein response impairs MHC class I expression. J Immunol 2007;178:3612–9. 10.4049/jimmunol.178.6.3612 [DOI] [PubMed] [Google Scholar]
- 39. Turner MJ, Sowders DP, DeLay ML, et al. HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response. J Immunol 2005;175:2438–48. 10.4049/jimmunol.175.4.2438 [DOI] [PubMed] [Google Scholar]
- 40. Robinson PC, Lau E, Keith P, et al. ERAP2 functional knockout in humans does not alter surface heavy chains or HLA-B27, inflammatory cytokines or endoplasmic reticulum stress markers. Ann Rheum Dis 2015;74:2092–5. 10.1136/annrheumdis-2015-207467 [DOI] [PubMed] [Google Scholar]
- 41. Kenna TJ, Lau MC, Keith P, et al. Disease-associated polymorphisms in ERAP1 do not alter endoplasmic reticulum stress in patients with ankylosing spondylitis. Genes Immun 2015;16:35–42. 10.1038/gene.2014.62 [DOI] [PubMed] [Google Scholar]
- 42. Ambarus CA, Yeremenko N, Baeten DL. Altered cytokine expression by macrophages from HLA-B27-positive spondyloarthritis patients without evidence of endoplasmic reticulum stress. Rheumatol Adv Pract 2018;2:rky014. 10.1093/rap/rky014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Campbell EC, Fettke F, Bhat S, et al. Expression of MHC class I dimers and ERAP1 in an ankylosing spondylitis patient cohort. Immunology 2011;133:379–85. 10.1111/j.1365-2567.2011.03453.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Díaz-Peña R, Vidal-Castiñeira JR, Mulero J, et al. Activating killer immunoglobulin-like receptors genes are associated with increased susceptibility to ankylosing spondylitis. Clin Exp Immunol 2015;180:201–6. 10.1111/cei.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cauli A, Shaw J, Giles J, et al. The arthritis-associated HLA-B*27:05 allele forms more cell surface B27 dimer and free heavy chain ligands for KIR3DL2 than HLA-B*27:09. Rheumatology (Oxford) 2013;52:1952–62. 10.1093/rheumatology/ket219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cifaldi L, Romania P, Falco M, et al. ERAP1 regulates natural killer cell function by controlling the engagement of inhibitory receptors. Cancer Res 2015;75:824–34. 10.1158/0008-5472.CAN-14-1643 [DOI] [PubMed] [Google Scholar]
- 47. D’Amico S, D’Alicandro V, Compagnone M, et al. ERAP1 controls the interaction of the inhibitory receptor KIR3DL1 with HLA-B51:01 by affecting natural killer cell function. Front Immunol 2021;12:778103. 10.3389/fimmu.2021.778103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barton A, Hill J, Bibi S, et al. Genetic susceptibility to enteric fever in experimentally challenged human volunteers. Infect Immun 2022;90:e0038921. 10.1128/iai.00389-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Manasson J, Blank RB, Scher JU. The microbiome in rheumatology: where are we and where should we go? Ann Rheum Dis 2020;79:727–33. 10.1136/annrheumdis-2019-216631 [DOI] [PubMed] [Google Scholar]
- 50. Kuiper JJW, Venema WJ. HLA-A29 and birdshot uveitis: further down the rabbit hole. Front Immunol 2020;11:599558. 10.3389/fimmu.2020.599558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pulido JS, Canal I, Salomão D, et al. Histological findings of birdshot chorioretinopathy in an eye with ciliochoroidal melanoma. Eye (Lond) 2012;26:862–5. 10.1038/eye.2012.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pagnoux C, Mahr A, Aouba A, et al. Extraocular manifestations of birdshot chorioretinopathy in 118 French patients. Presse Med 2010;39:e97–102. 10.1016/j.lpm.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 53. Okada Y, Han B, Tsoi LC, et al. Fine mapping major histocompatibility complex associations in psoriasis and its clinical subtypes. Am J Hum Genet 2014;95:162–72. 10.1016/j.ajhg.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Winchester R, Giles J, Jadon D, et al. Implications of the diversity of class I HLA associations in psoriatic arthritis. Clin Immunol 2016;172:29–33. 10.1016/j.clim.2016.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. FitzGerald O, Haroon M, Giles JT, et al. Concepts of pathogenesis in psoriatic arthritis: genotype determines clinical phenotype. Arthritis Res Ther 2015;17:115. 10.1186/s13075-015-0640-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schwartzman M, Ermann J, Kuhn KA, et al. Spondyloarthritis in inflammatory bowel disease cohorts: systematic literature review and critical appraisal of study designs. RMD Open 2022;8:e001777. 10.1136/rmdopen-2021-001777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cantini F, Nannini C, Cassarà E, et al. Uveitis in spondyloarthritis: an overview. J Rheumatol Suppl 2015;93:27–9. 10.3899/jrheum.150630 [DOI] [PubMed] [Google Scholar]
- 58. Wang K, Gaitsch H, Poon H, et al. Classification of common human diseases derived from shared genetic and environmental determinants. Nat Genet 2017;49:1319–25. 10.1038/ng.3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gur A, Sarac AJ, Burkan YK, et al. Arthropathy, quality of life, depression, and anxiety in behcet’s disease: relationship between arthritis and these factors. Clin Rheumatol 2006;25:524–31. 10.1007/s10067-005-0100-6 [DOI] [PubMed] [Google Scholar]
- 60. Poddubnyy D, Jadon DR, Van den Bosch F, et al. Axial involvement in psoriatic arthritis: an update for rheumatologists. Semin Arthritis Rheum 2021;51:880–7. 10.1016/j.semarthrit.2021.06.006 [DOI] [PubMed] [Google Scholar]
- 61. Benavent D, Plasencia C, Poddubnyy D, et al. Unveiling axial involvement in psoriatic arthritis: an ancillary analysis of the ASAS-perspa study. Semin Arthritis Rheum 2021;51:766–74. 10.1016/j.semarthrit.2021.04.018 [DOI] [PubMed] [Google Scholar]
- 62. Kötter I, Lötscher F. Behçet’s syndrome apart from the triple symptom complex: vascular, neurologic, gastrointestinal, and musculoskeletal manifestations. A mini review. Front Med (Lausanne) 2021;8:639758. 10.3389/fmed.2021.639758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Prinz JC. Human leukocyte antigen-class I alleles and the autoreactive T cell response in psoriasis pathogenesis. Front Immunol 2018;9:954. 10.3389/fimmu.2018.00954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maldini C, Lavalley MP, Cheminant M, et al. Relationships of HLA-B51 or b5 genotype with Behcet’s disease clinical characteristics: systematic review and meta-analyses of observational studies. Rheumatology (Oxford) 2012;51:887–900. 10.1093/rheumatology/ker428 [DOI] [PubMed] [Google Scholar]
- 65. Arévalo M, Gratacós Masmitjà J, Moreno M, et al. Influence of HLA-B27 on the ankylosing spondylitis phenotype: results from the REGISPONSER database. Arthritis Res Ther 2018;20:221. 10.1186/s13075-018-1724-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang S, Wang Y, Peng L, et al. Comparison of clinical features in HLA-B27 positive and negative patients with axial spondyloarthritis: results from a cohort of 4,131 patients. Front Med (Lausanne) 2020;7:609562. 10.3389/fmed.2020.609562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Leccese P, Yazici Y, Olivieri I. Behcet’s syndrome in nonendemic regions. Curr Opin Rheumatol 2017;29:12–6. 10.1097/BOR.0000000000000349 [DOI] [PubMed] [Google Scholar]
- 68. Skef W, Hamilton MJ, Arayssi T. Gastrointestinal Behçet’s disease: a review. World J Gastroenterol 2015;21:3801–12. 10.3748/wjg.v21.i13.3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Meier K, Schloegl A, Poddubnyy D, et al. Skin manifestations in spondyloarthritis. Ther Adv Musculoskelet Dis 2020;12. 10.1177/1759720X20975915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Haroon M, O’Rourke M, Ramasamy P, et al. A novel evidence-based detection of undiagnosed spondyloarthritis in patients presenting with acute anterior uveitis: the duet (Dublin uveitis evaluation tool). Ann Rheum Dis 2015;74:1990–5. 10.1136/annrheumdis-2014-205358 [DOI] [PubMed] [Google Scholar]
- 71. Monnet D, Breban M, Hudry C, et al. Ophthalmic findings and frequency of extraocular manifestations in patients with HLA-B27 uveitis: a study of 175 cases. Ophthalmology 2004;111:802–9. 10.1016/j.ophtha.2003.07.011 [DOI] [PubMed] [Google Scholar]
- 72. Chaiyabutr C, Ungprasert P, Silpa-Archa N, et al. Psoriasis and risk of uveitis: a systematic review and meta-analysis. Biomed Res Int 2020;2020:9308341. 10.1155/2020/9308341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hahn HJ, Kwak SG, Kim D-K, et al. Association of behçet disease with psoriasis and psoriatic arthritis. Sci Rep 2021;11:2531. 10.1038/s41598-021-81972-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lee YC, Jeong SJ, Eun Y-G, et al. Risk of autoimmune diseases in recurrent aphthous ulcer patients: a nationwide population study. Oral Dis 2021;27:1443–50. 10.1111/odi.13659 [DOI] [PubMed] [Google Scholar]
- 75. Abbood HM, Pathan E, Cherukara GP. The link between ankylosing spondylitis and oral health conditions: two nested case-control studies using data of the UK biobank. J Appl Oral Sci 2018;27:e20180207. 10.1590/1678-7757-2018-0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lin K-C, Tsai LL, Ko EC, et al. Comorbidity profiles among patients with recurrent aphthous stomatitis: a case-control study. J Formos Med Assoc 2019;118:664–70. 10.1016/j.jfma.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 77. Robinson PN. Deep phenotyping for precision medicine. Hum Mutat 2012;33:777–80. 10.1002/humu.22080 [DOI] [PubMed] [Google Scholar]
- 78. Tcheandjieu C, Aguirre M, Gustafsson S, et al. A phenome-wide association study of 26 Mendelian genes reveals phenotypic expressivity of common and rare variants within the general population. PLoS Genet 2020;16:e1008802. 10.1371/journal.pgen.1008802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schlosstein L, Terasaki PI, Bluestone R, et al. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med 1973;288:704–6. 10.1056/NEJM197304052881403 [DOI] [PubMed] [Google Scholar]
- 80. Brewerton DA, Hart FD, Nicholls A, et al. Ankylosing spondylitis and HL-A 27. Lancet 1973;1:904–7. 10.1016/s0140-6736(73)91360-3 [DOI] [PubMed] [Google Scholar]
- 81. Ohno S, Aoki K, Sugiura S, et al. Letter: HL-A5 and Behçet’s disease. Lancet 1973;2:1383–4. 10.1016/s0140-6736(73)93343-6 [DOI] [PubMed] [Google Scholar]
- 82. Tiilikainen A, Lassus A, Pirskanen R, et al. An attempt to evaluate ia type antigens in patients with psoriasis or myasthenia gravis. Tissue Antigens, 1977: 10. [Google Scholar]
- 83. Tsuji K, Nose Y, Hoshino K. Further study on HLA-C, -D and ia antigens in psoriasis vulgaris. Tissue Antigens, 1977: 10. [Google Scholar]
- 84. Nussenblatt RB, Mittal KK, Ryan S, et al. Birdshot retinochoroidopathy associated with HLA-A29 antigen and immune responsiveness to retinal S-antigen. Am J Ophthalmol 1982;94:147–58. 10.1016/0002-9394(82)90069-1 [DOI] [PubMed] [Google Scholar]
- 85. Cortes A, Pulit SL, Leo PJ, et al. Major histocompatibility complex associations of ankylosing spondylitis are complex and involve further epistasis with ERAP1. Nat Commun 2015;6:7146. 10.1038/ncomms8146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang G, Kim T-H, Li Z, et al. MHC associations of ankylosing spondylitis in east asians are complex and involve non-HLA-B27 HLA contributions. Arthritis Res Ther 2020;22:74. 10.1186/s13075-020-02148-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ombrello MJ, Kirino Y, de Bakker PIW, et al. Behçet disease-associated MHC class I residues implicate antigen binding and regulation of cell-mediated cytotoxicity. Proc Natl Acad Sci U S A 2014;111:8867–72. 10.1073/pnas.1406575111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bowes J, Budu-Aggrey A, Huffmeier U, et al. Dense genotyping of immune-related susceptibility loci reveals new insights into the genetics of psoriatic arthritis. Nat Commun 2015;6:6046. 10.1038/ncomms7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Su G, Zhong Z, Zhou Q, et al. Identification of novel risk loci for behçet’s disease-related uveitis in a chinese population in a genome-wide association study. Arthritis Rheumatol 2022;74:671–81. 10.1002/art.41998 [DOI] [PubMed] [Google Scholar]
- 90. Robinson PC, Claushuis TAM, Cortes A, et al. Genetic dissection of acute anterior uveitis reveals similarities and differences in associations observed with ankylosing spondylitis. Arthritis Rheumatol 2015;67:140–51. 10.1002/art.38873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sakaue S, Kanai M, Tanigawa Y, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet 2021;53:1415–24. 10.1038/s41588-021-00931-x [DOI] [PubMed] [Google Scholar]
- 92. Sun L-D, Cheng H, Wang Z-X, et al. Association analyses identify six new psoriasis susceptibility loci in the Chinese population. Nat Genet 2010;42:1005–9. 10.1038/ng.690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kuiper JJW, Setten J van, Devall M, et al. Functionally distinct ERAP1 and ERAP2 are a hallmark of HLA-A29- (birdshot) uveitis. Hum Mol Genet 2018;27:4333–43. 10.1093/hmg/ddy319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Robinson PC, Costello M-E, Leo P, et al. ERAP2 is associated with ankylosing spondylitis in HLA-B27-positive and HLA-B27-negative patients. Ann Rheum Dis 2015;74:1627–9. 10.1136/annrheumdis-2015-207416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tang H, Jin X, Li Y, et al. A large-scale screen for coding variants predisposing to psoriasis. Nat Genet 2014;46:45–50. 10.1038/ng.2827 [DOI] [PubMed] [Google Scholar]
- 96. Haroon N, Inman RD. Endoplasmic reticulum aminopeptidases: biology and pathogenic potential. Nat Rev Rheumatol 2010;6:461–7. 10.1038/nrrheum.2010.85 [DOI] [PubMed] [Google Scholar]
- 97. Weimershaus M, Evnouchidou I, Saveanu L, et al. Peptidases trimming MHC class I ligands. Curr Opin Immunol 2013;25:90–6. 10.1016/j.coi.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 98. Mpakali A, Maben Z, Stern LJ, et al. Molecular pathways for antigenic peptide generation by ER aminopeptidase 1. Mol Immunol 2019;113:50–7. 10.1016/j.molimm.2018.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tedeschi V, Paldino G, Paladini F. The impact of the “mis-peptidome” on HLA class I-mediated diseases: contribution of ERAP1 and ERAP2 and effects on the immune response. Int J Mol Sci 2020;21:9608. 10.3390/ijms21249608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Babaie F, Hosseinzadeh R, Ebrazeh M, et al. The roles of ERAP1 and ERAP2 in autoimmunity and cancer immunity: new insights and perspective. Mol Immunol 2020;121:7–19. 10.1016/j.molimm.2020.02.020 [DOI] [PubMed] [Google Scholar]
- 101. de Castro JAL, Stratikos E. Intracellular antigen processing by ERAP2: molecular mechanism and roles in health and disease. Hum Immunol 2019;80:310–7. 10.1016/j.humimm.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 102. Hanson AL, Cuddihy T, Haynes K, et al. Genetic variants in ERAP1 and ERAP2 associated with immune-mediated diseases influence protein expression and the isoform profile. Arthritis Rheumatol 2018;70:255–65. 10.1002/art.40369 [DOI] [PubMed] [Google Scholar]
- 103. Ombrello MJ, Kastner DL, Remmers EF. Endoplasmic reticulum-associated amino-peptidase 1 and rheumatic disease: genetics. Curr Opin Rheumatol 2015;27:349–56. 10.1097/BOR.0000000000000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Reeves E, Colebatch-Bourn A, Elliott T, et al. Functionally distinct ERAP1 allotype combinations distinguish individuals with ankylosing spondylitis. Proc Natl Acad Sci U S A 2014;111:17594–9. 10.1073/pnas.1408882111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hutchinson JP, Temponeras I, Kuiper J, et al. Common allotypes of ER aminopeptidase 1 have substrate-dependent and highly variable enzymatic properties. J Biol Chem 2021;296:100443. 10.1016/j.jbc.2021.100443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Costantino F, Talpin A, Evnouchidou I, et al. ERAP1 gene expression is influenced by nonsynonymous polymorphisms associated with predisposition to spondyloarthritis. Arthritis Rheumatol 2015;67:1525–34. 10.1002/art.39072 [DOI] [PubMed] [Google Scholar]
- 107. Cavers A, Kugler MC, Ozguler Y, et al. Behçet’s disease risk-variant HLA-B51/ERAP1-hap10 alters human CD8 T cell immunity. Ann Rheum Dis 2022;81:1603–11. 10.1136/ard-2022-222277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Roberts AR, Appleton LH, Cortes A, et al. ERAP1 association with ankylosing spondylitis is attributable to common genotypes rather than rare haplotype combinations. Proc Natl Acad Sci U S A 2017;114:558–61. 10.1073/pnas.1618856114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Colonna M, Moretta A, Vély F, et al. A high-resolution view of NK-cell receptors: structure and function. Immunol Today 2000;21:428–31. 10.1016/s0167-5699(00)01697-2 [DOI] [PubMed] [Google Scholar]
- 110. Lee N, Llano M, Carretero M, et al. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci U S A 1998;95:5199–204. 10.1073/pnas.95.9.5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lampen MH, Hassan C, Sluijter M, et al. Alternative peptide repertoire of HLA-E reveals a binding motif that is strikingly similar to HLA-A2. Mol Immunol 2013;53:126–31. 10.1016/j.molimm.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 112. Braud V, Jones EY, McMichael A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur J Immunol 1997;27:1164–9. 10.1002/eji.1830270517 [DOI] [PubMed] [Google Scholar]
- 113. Cifaldi L, Lo Monaco E, Forloni M, et al. Natural killer cells efficiently reject lymphoma silenced for the endoplasmic reticulum aminopeptidase associated with antigen processing. Cancer Res 2011;71:1597–606. 10.1158/0008-5472.CAN-10-3326 [DOI] [PubMed] [Google Scholar]
- 114. Cauli A, Dessole G, Piga M, et al. Expression analysis of HLA-E and NKG2A and NKG2C receptors points at a role for natural killer function in ankylosing spondylitis. RMD Open 2018;4:e000597. 10.1136/rmdopen-2017-000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hanson AL, Vukcevic D, Leslie S, et al. Epistatic interactions between killer immunoglobulin-like receptors and human leukocyte antigen ligands are associated with ankylosing spondylitis. PLoS Genet 2020;16:e1008906. 10.1371/journal.pgen.1008906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Venema WJ, Hiddingh S, de Boer JH, et al. ERAP2 increases the abundance of a peptide submotif highly selective for the birdshot uveitis-associated HLA-A29. Front Immunol 2021;12:634441. 10.3389/fimmu.2021.634441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. López de Castro JA, Alvarez-Navarro C, Brito A, et al. Molecular and pathogenic effects of endoplasmic reticulum aminopeptidases ERAP1 and ERAP2 in MHC-I-associated inflammatory disorders: towards a unifying view. Mol Immunol 2016;77:193–204. 10.1016/j.molimm.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 118. Ruyssen-Witrand A, Luxembourger C, Cantagrel A, et al. Association between IL23R and ERAP1 polymorphisms and sacroiliac or spinal MRI inflammation in spondyloarthritis: DESIR cohort data. Arthritis Res Ther 2019;21:22. 10.1186/s13075-018-1807-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nossent JC, Johnsen S, Bakland G. The influence of ERAP1 gene variants on clinical phenotype in ankylosing spondylitis. Scand J Rheumatol 2016;45:474–9. 10.3109/03009742.2016.1150507 [DOI] [PubMed] [Google Scholar]
- 120. Ortiz-Fernández L, Carmona F-D, Montes-Cano M-A, et al. Genetic analysis with the immunochip platform in Behçet disease. Identification of residues associated in the HLA class I region and new susceptibility loci. PLoS One 2016;11:e0161305. 10.1371/journal.pone.0161305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Nikamo P, Lysell J, Ståhle M. Association with genetic variants in the IL-23 and NF-κB pathways discriminates between mild and severe psoriasis skin disease. J Invest Dermatol 2015;135:1969–76. 10.1038/jid.2015.103 [DOI] [PubMed] [Google Scholar]
- 122. Yin X, Low HQ, Wang L, et al. Genome-wide meta-analysis identifies multiple novel associations and ethnic heterogeneity of psoriasis susceptibility. Nat Commun 2015;6:6916. 10.1038/ncomms7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Vural S, Kerl K, Ertop Doğan P, et al. Lesional activation of Tc 17 cells in Behçet disease and psoriasis supports HLA class I-mediated autoimmune responses. Br J Dermatol 2021;185:1209–20. 10.1111/bjd.20643 [DOI] [PubMed] [Google Scholar]
- 124. Steel KJA, Srenathan U, Ridley M, et al. Polyfunctional, proinflammatory, tissue-resident memory phenotype and function of synovial interleukin-17A+CD8+ T cells in psoriatic arthritis. Arthritis Rheumatol 2020;72:435–47. 10.1002/art.41156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Phadungsaksawasdi P, Fujiyama T, Kurihara K, et al. PD-1 expression defines epidermal CD8+CD103+ T cells preferentially producing IL-17A and using skewed TCR repertoire in psoriasis. J Invest Dermatol 2021;141:2426–35. 10.1016/j.jid.2021.03.011 [DOI] [PubMed] [Google Scholar]
- 126. Kuiper J, Rothova A, de Boer J, et al. The immunopathogenesis of birdshot chorioretinopathy; a bird of many feathers. Prog Retin Eye Res 2015;44:99–110. 10.1016/j.preteyeres.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 127. Dagur PK, Biancotto A, Stansky E, et al. Secretion of interleukin-17 by CD8+ T cells expressing CD146 (MCAM). Clin Immunol 2014;152:36–47. 10.1016/j.clim.2014.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. McGonagle D, Watad A, Sharif K, et al. Why inhibition of IL-23 lacked efficacy in ankylosing spondylitis. Front Immunol 2021;12:614255. 10.3389/fimmu.2021.614255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol 2020;156:649–58. 10.1001/jamadermatol.2020.0723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kristensen LE, Keiserman M, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 keepsake 1 trial. Ann Rheum Dis 2022;81:225–31. 10.1136/annrheumdis-2021-221019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Deodhar A, Gensler LS, Sieper J, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol 2019;71:258–70. 10.1002/art.40728 [DOI] [PubMed] [Google Scholar]
- 132. Baeten D, Østergaard M, Wei JC-C, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis 2018;77:1295–302. 10.1136/annrheumdis-2018-213328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Coates LC, Corp N, van der Windt DA, et al. Grappa treatment recommendations: an update from the 2020 grappa annual meeting. J Rheumatol 2021:jrheum.201681. 10.3899/jrheum.201681 [DOI] [PubMed] [Google Scholar]
- 134. Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Liu J, Chang H-W, Huang Z-M, et al. Single-cell RNA sequencing of psoriatic skin identifies pathogenic tc17 cell subsets and reveals distinctions between CD8+ T cells in autoimmunity and cancer. J Allergy Clin Immunol 2021;147:2370–80. 10.1016/j.jaci.2020.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hanson AL, Nel HJ, Bradbury L, et al. Altered repertoire diversity and disease-associated clonal expansions revealed by T cell receptor immunosequencing in ankylosing spondylitis patients. Arthritis Rheumatol 2020;72:1289–302. 10.1002/art.41252 [DOI] [PubMed] [Google Scholar]
- 137. Kim S-M, Bhonsle L, Besgen P, et al. Analysis of the paired TCR α- and β-chains of single human T cells. PLoS One 2012;7:e37338. 10.1371/journal.pone.0037338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Penkava F, Velasco-Herrera MDC, Young MD, et al. Single-cell sequencing reveals clonal expansions of pro-inflammatory synovial CD8 T cells expressing tissue-homing receptors in psoriatic arthritis. Nat Commun 2020;11:4767. 10.1038/s41467-020-18513-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yasuoka H, Okazaki Y, Kawakami Y, et al. Autoreactive CD8+ cytotoxic T lymphocytes to major histocompatibility complex class I chain-related gene A in patients with Behçet’s disease. Arthritis Rheum 2004;50:3658–62. 10.1002/art.20597 [DOI] [PubMed] [Google Scholar]
- 140. Lande R, Botti E, Jandus C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun 2014;5:5621. 10.1038/ncomms6621 [DOI] [PubMed] [Google Scholar]
- 141. Fiorillo MT, Maragno M, Butler R, et al. CD8 (+) T-cell autoreactivity to an HLA-B27-restricted self-epitope correlates with ankylosing spondylitis. J Clin Invest 2000;106:47–53. 10.1172/JCI9295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Atagunduz P, Appel H, Kuon W, et al. HLA-B27-Restricted CD8+ T cell response to cartilage-derived self peptides in ankylosing spondylitis. Arthritis Rheum 2005;52:892–901. 10.1002/art.20948 [DOI] [PubMed] [Google Scholar]
- 143. Stratikos E. Regulating adaptive immune responses using small molecule modulators of aminopeptidases that process antigenic peptides. Curr Opin Chem Biol 2014;23:1–7. 10.1016/j.cbpa.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 144. Laura M, Ronan G, Vy LB, et al. Modulators of hERAP2 discovered by high-throughput screening. Eur J Med Chem 2021;211:113053. 10.1016/j.ejmech.2020.113053 [DOI] [PubMed] [Google Scholar]
- 145. Ge C, Weisse S, Xu B, et al. Key interactions in the trimolecular complex consisting of the rheumatoid arthritis-associated DRB1*04:01 molecule, the major glycosylated collagen II peptide and the T-cell receptor. Ann Rheum Dis 2022;81:480–9. 10.1136/annrheumdis-2021-220500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bankovich AJ, Girvin AT, Moesta AK, et al. Peptide register shifting within the MHC groove: theory becomes reality. Mol Immunol 2004;40:1033–9. 10.1016/j.molimm.2003.11.016 [DOI] [PubMed] [Google Scholar]
- 147. Wei P, Yang Y, Liu Z, et al. Characterization of autoantigen presentation by HLA-C*06:02 in psoriasis. J Invest Dermatol 2017;137:2238–41. 10.1016/j.jid.2017.05.036 [DOI] [PubMed] [Google Scholar]
- 148. Yu W, Jiang N, Ebert PJR, et al. Clonal deletion prunes but does not eliminate self-specific αβ CD8 (+) T lymphocytes. Immunity 2015;42:929–41. 10.1016/j.immuni.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Richards DM, Ruggiero E, Hofer A-C, et al. The contained self-reactive peripheral T cell repertoire: size, diversity, and cellular composition. J Immunol 2015;195:2067–79. 10.4049/jimmunol.1500880 [DOI] [PubMed] [Google Scholar]
- 150. Boehncke W-H, Brembilla NC. Autoreactive T-lymphocytes in inflammatory skin diseases. Front Immunol 2019;10:1198. 10.3389/fimmu.2019.01198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Haftmann C, Zwicky P, Ingelfinger F, et al. Protection against autoimmunity is driven by thymic epithelial cell-mediated regulation of Treg development. Sci Immunol 2021;6:eabf3111. 10.1126/sciimmunol.abf3111 [DOI] [PubMed] [Google Scholar]
- 152. Li H, Hsu H-C, Wu Q, et al. Il-23 promotes TCR-mediated negative selection of thymocytes through the upregulation of IL-23 receptor and RORγt. Nat Commun 2014;5:4259. 10.1038/ncomms5259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ball JA, Clear A, Aries J, et al. Retinoic acid-responsive CD8 effector T cells are selectively increased in IL-23-rich tissue in gastrointestinal GVHD. Blood 2021;137:702–17. 10.1182/blood.2020005170 [DOI] [PubMed] [Google Scholar]
- 154. Pettmann J, Huhn A, Abu Shah E, et al. The discriminatory power of the T cell receptor. Elife 2021;10:e67092. 10.7554/eLife.67092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wang J, Jelcic I, Mühlenbruch L, et al. HLA-dr15 molecules jointly shape an autoreactive T cell repertoire in multiple sclerosis. Cell 2020;183:1264–81. 10.1016/j.cell.2020.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Korem Kohanim Y, Tendler A, Mayo A, et al. Endocrine autoimmune disease as a fragility of immune surveillance against hypersecreting mutants. Immunity 2020;52:872–84. 10.1016/j.immuni.2020.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kemming J, Reeves E, Nitschke K, et al. ERAP1 allotypes shape the epitope repertoire of virus-specific CD8+ T cell responses in acute hepatitis C virus infection. J Hepatol 2019;70:1072–81. 10.1016/j.jhep.2019.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Prinz JC. Antigen processing, presentation, and tolerance: role in autoimmune skin diseases. J Invest Dermatol 2022;142:750–9. 10.1016/j.jid.2021.05.009 [DOI] [PubMed] [Google Scholar]
- 159. Klaeger S, Apffel A, Clauser KR, et al. Optimized liquid and gas phase fractionation increases HLA-peptidome coverage for primary cell and tissue samples. Mol Cell Proteomics 2021;20:100133. 10.1016/j.mcpro.2021.100133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Stopfer LE, Gajadhar AS, Patel B, et al. Absolute quantification of tumor antigens using embedded MHC-I isotopologue calibrants. Proc Natl Acad Sci U S A 2021;118:e2111173118. 10.1073/pnas.2111173118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ma K-Y, Schonnesen AA, He C, et al. High-throughput and high-dimensional single-cell analysis of antigen-specific CD8+ T cells. Nat Immunol 2021;22:1590–8. 10.1038/s41590-021-01073-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Stoll ML, DeQuattro K, Li Z, et al. Impact of HLA-B27 and disease status on the gut microbiome of the offspring of ankylosing spondylitis patients. Children (Basel) 2022;9:569. 10.3390/children9040569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Asquith M, Sternes PR, Costello M-E, et al. HLA alleles associated with risk of ankylosing spondylitis and rheumatoid arthritis influence the gut microbiome. Arthritis Rheumatol 2019;71:1642–50. 10.1002/art.40917 [DOI] [PubMed] [Google Scholar]
- 164. Berland M, Meslier V, Berreira Ibraim S, et al. Both disease activity and HLA-B27 determine gut microbiome dysbiosis in spondyloarthritis. Arthritis Rheumatol 2022. 10.1002/art.42289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Andeweg SP, Keşmir C, Dutilh BE. Quantifying the impact of human leukocyte antigen on the human gut microbiota. MSphere 2021;6:e0047621. 10.1128/mSphere.00476-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Pedersen TK, Brown EM, Plichta DR, et al. The CD4+ T cell response to a commensal-derived epitope transitions from a tolerant to an inflammatory state in crohn’s disease. Immunity 2022;55:1909–23. 10.1016/j.immuni.2022.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259–71. 10.1084/jem.20052494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Shi G-N, Hu M, Chen C, et al. Methotrexate enhances antigen presentation and maturation of tumour antigen-loaded dendritic cells through NLRP3 inflammasome activation: a strategy for dendritic cell-based cancer vaccine. Ther Adv Med Oncol 2021;13:1758835920987056. 10.1177/1758835920987056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Verbrugge SE, Scheper RJ, Lems WF, et al. Proteasome inhibitors as experimental therapeutics of autoimmune diseases. Arthritis Res Ther 2015;17:17. 10.1186/s13075-015-0529-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Momburg F, Roelse J, Howard JC, et al. Selectivity of MHC-encoded peptide transporters from human, mouse and rat. Nature 1994;367:648–51. 10.1038/367648a0 [DOI] [PubMed] [Google Scholar]
- 171. Ilca FT, Neerincx A, Wills MR, et al. Utilizing TAPBPR to promote exogenous peptide loading onto cell surface MHC I molecules. Proc Natl Acad Sci U S A 2018;115:E9353–61. 10.1073/pnas.1809465115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Maben Z, Arya R, Rane D, et al. Discovery of selective inhibitors of endoplasmic reticulum aminopeptidase 1. J Med Chem 2020;63:103–21. 10.1021/acs.jmedchem.9b00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Admon A. ERAP1 shapes just part of the immunopeptidome. Hum Immunol 2019;80:296–301. 10.1016/j.humimm.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 174. Breban M, Glatigny S, Cherqaoui B, et al. Lessons on spa pathogenesis from animal models. Semin Immunopathol 2021;43:207–19. 10.1007/s00281-020-00832-x [DOI] [PubMed] [Google Scholar]
- 175. Boekel L, Hooijberg F, Vogelzang EH, et al. Spinning straw into gold: description of a disruptive rheumatology research platform inspired by the COVID-19 pandemic. Arthritis Res Ther 2021;23:207. 10.1186/s13075-021-02574-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Li J, Li X, He F, et al. Cross-sectional study reveals that HLA-C*07:02 is a potential biomarker of early onset/lesion severity of psoriasis. Exp Dermatol 2020;29:639–46. 10.1111/exd.14127 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2022-222852supp001.pdf (124.4KB, pdf)