Abstract
Background
Preterm infants commonly receive red blood cell (RBC), platelet and fresh frozen plasma (FFP) transfusions. The aim of this Neonatal Transfusion Network survey was to describe current transfusion practices in Europe and to compare our findings to three recent randomised controlled trials to understand how clinical practice relates to the trial data.
Methods
From October to December 2020, we performed an online survey among 597 neonatal intensive care units (NICUs) caring for infants with a gestational age (GA) of <32 weeks in 18 European countries.
Results
Responses from 343 NICUs (response rate: 57%) are presented and showed substantial variation in clinical practice. For RBC transfusions, 70% of NICUs transfused at thresholds above the restrictive thresholds tested in the recent trials and 22% below the restrictive thresholds. For platelet transfusions, 57% of NICUs transfused at platelet count thresholds above 25×109/L in non-bleeding infants of GA of <28 weeks, while the 25×109/L threshold was associated with a lower risk of harm in a recent trial. FFP transfusions were administered for coagulopathy without active bleeding in 39% and for hypotension in 25% of NICUs. Transfusion volume, duration and rate varied by factors up to several folds between NICUs.
Conclusions
Transfusion thresholds and aspects of administration vary widely across European NICUs. In general, transfusion thresholds used tend to be more liberal compared with data from recent trials supporting the use of more restrictive thresholds. Further research is needed to identify the barriers and enablers to incorporation of recent trial findings into neonatal transfusion practice.
Keywords: neonatology, child health, data collection, epidemiology, healthcare disparities
WHAT IS ALREADY KNOWN ON THIS TOPIC
Neonates frequently receive red blood cell (RBC), platelet or fresh frozen plasma (FFP) transfusions.
Two recent trials showed no difference in death or neurodevelopmental delay at 2 years’ corrected age between liberal and restrictive RBC transfusion thresholds.
One recent trial showed a reduction in the combined risk of mortality and major bleeding in the restrictive versus the liberal platelet transfusion threshold.
WHAT THIS STUDY ADDS
RBC transfusion practices across Europe vary widely.
Over 50% of European neonatal intensive care units use platelet count thresholds above 25×109/L for non-bleeding neonates, potentially exposing neonates to increased risk of mortality and bleeding.
There is substantial variation in transfusion volume and duration, particularly for platelets and FFP, reflecting lack of evidence to support practice.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Researchers might use these data to investigate the impact of different transfusion practices on regional differences in short-term and long-term clinical outcomes.
Our survey data will help align regional, national and international practice guidelines with the currently best available evidence.
Policy makers might use our data to better understand regional differences in healthcare uses and costs and to assist in planning future healthcare strategies.
Introduction
Blood component transfusions of red blood cells (RBCs), platelets and fresh frozen plasma (FFP) are commonly administered to preterm infants, but the evidence base for these transfusions, particularly for platelets, has thus far been limited.1 Since 2019, three large randomised controlled trials (RCTs) were published. The Effects of Transfusion Thresholds on Neurocognitive Outcomes of Extremely Low-Birth-Weight Infants (ETTNO) and Transfusion of Prematures (TOP) trials reported no difference between the effects of liberal versus restrictive RBC thresholds on death or neurocognitive deficit at 2 years’ corrected age.2–4 The Platelets for Neonatal Transfusion 2/Management of Thrombocytopenia in Special Subgroup: Neonates (PlaNeT-2/MATISSE) trial compared liberal (50×109/L) versus restrictive (25×109/L) platelet count thresholds, reporting a higher rate of death and major bleeding in the liberal platelet transfusion threshold group (26% vs 19%).5 This effect was shown to be present irrespective of varying baseline risk of outcome.6 The extent to which findings of the aforementioned trials correspond with clinical practice in Europe is unknown. The aims of the study were to describe current transfusion practices and to compare these to the recently generated evidence from clinical trials.
Methods
This survey was performed by the Neonatal Transfusion Network (NTN) (www.neonataltransfusionnetwork.com), an international research group which aims to generate evidence to improve clinical practice in neonatal transfusion medicine. An NTN panel of four neonatologists (EL, ED, CD and CCR), one trainee neonatologist (AS), three haematologists (SJS, HN and KF) with paediatric transfusion expertise, and one clinical epidemiologist (SFFG) developed a preliminary list of questions. Topics included RBC, platelet and FFP transfusion practices in premature neonates of less than 32 weeks’ gestational age (GA) at birth, addressing transfusion thresholds or indications, durations and volumes, concomitant use of diuretics, withholding enteral feeding and parental consent. We used a ranking procedure to obtain a final set of 31 questions, which we entered into LimeSurvey (LimeSurvey GmbH, Hamburg, Germany) (online supplemental materials) Neonatologists from 18 European countries volunteered to disseminate the survey. These national coordinators received a password protected link to the questionnaire, which they disseminated between October and December 2020 to neonatal intensive care unit (NICUs) providing care for infants born at <32 weeks of GA. In the United Kingdom only larger regional NICUs were approached for participation, as these are known to dictate local transfusion practices. We limited responses to one per NICU. National coordinators were free to use their own contacts or use an existing neonatal network (online supplemental materials).
fetalneonatal-2022-324619supp001.pdf (370.1KB, pdf)
We extracted the LimeSurvey data to SPSS V.27, for data cleaning and analysis, by two authors working independently. We used GraphPad Prism V.9.0.1 for Windows (GraphPad Software, San Diego, California USA) for graphs. We excluded confirmed double entries, ineligible responses and responses that were>75% blank, and converted haematocrit to haemoglobin using this formula: haemoglobin (g/L)=haematocrit(%)×300.
The TOP and ETTNO trial had only recently been published at the moment of survey dissemination.2 3 Therefore, we did not aim to assess implementation of their results but instead to assess how current clinical practice compared with the thresholds tested in these trials. To make the comparison, we combined the two trials to select one liberal and one restrictive ‘ETTNO/TOP threshold’ for each of our 15 survey clinical scenarios: ‘air’, ‘low flow’, ‘high flow </>30% FiO2’ and ‘intubated’ for three postnatal age groups. Where ETTNO and TOP thresholds differed, we selected the higher liberal value and the lower restrictive. The low flow and high flow’ clinical scenarios could not be assigned to either the ‘critical’ or the ‘non-critical’ strategies in the ETTNO and TOP trials because of overlapping definitions. Therefore, some clinical scenarios were classified as both critical and non-critical in one or both trials, leading to relatively wide ETTNO/TOP threshold ranges (online supplemental tables S1 and S2). We calculated the proportion of reported thresholds in our survey that were at or above the liberal ETTNO/TOP threshold, between the liberal and restrictive ETTNO/TOP threshold or at or below the restrictive ETTNO/TOP threshold, for all clinical scenarios for both RBC and platelet transfusion thresholds.
We performed two sensitivity analyses. To estimate the effect of non-responder bias, we compared early versus late responders. This is an established method to estimate if responders answered survey questions differently from non-responders, where late responders are considered a proxy for non-responders. We combined the first and last 20% of entries in each country to form the early-responder and late-responder groups. We also assessed the effect of varying response rates between countries in a weighted sensitivity analysis, where entries received a weight equal to one divided by the response rate in their respective country.
Results
Response rate
After removal of seven duplicate responses, 10 ineligible responses and 168 responses which were >75% blank, responses from 343 NICUs were included, yielding an overall response rate of 57% (343/597). The response rate per country varied between 21% and 100% (median 81%). We included NICUs in Austria (7 of a total of 7 units), Belgium (16/19), Finland (5/5), Germany (112/160), Hungary (21/21), Italy (49/105), Malta (1/1), the Netherlands (9/9), Norway (6/6), Poland (18/36), Portugal (10/11), Romania (4/19), Slovakia (10/13), Slovenia (3/4), Spain (41/111), Sweden (8/8), Switzerland (6/8) and the United Kingdom (17/55).UK
RBC transfusion thresholds
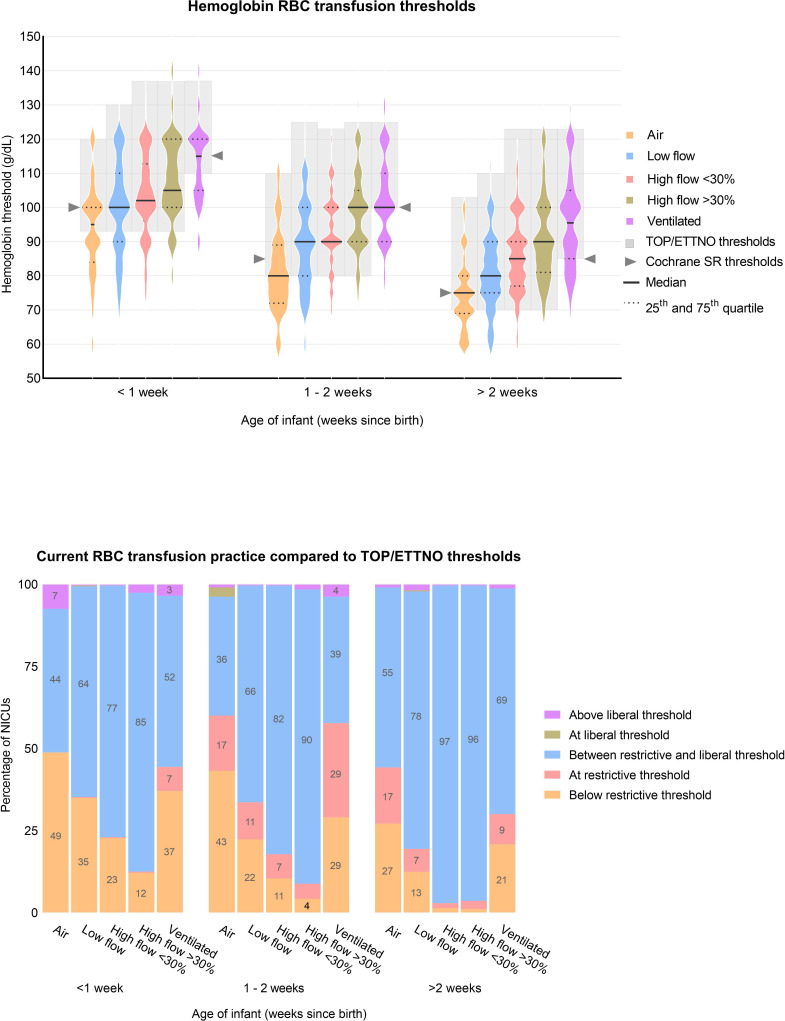
A violin plot of haemoglobin thresholds for RBC transfusions is shown in figure 1A. A total of 104 NICUs used haematocrit thresholds, which were converted into haemoglobin. Higher haemoglobin thresholds were adopted as the degree of respiratory support intensified. On average, over all 15 clinical scenarios, 22% of reported thresholds were below, and 8% at the restrictive ETTNO/TOP threshold, 68% were in between the restrictive and liberal ETTNO/TOP threshold, <1% at the liberal threshold and 2% above the liberal ETTNO/TOP threshold (figure 1B).
Figure 1.
(A) Violin plots of haemoglobin-based RBC transfusion thresholds for different postnatal age and respiratory support levels in infants born at 30%=infants on 30% oxygen by non-invasive respiratory support (including continuous positive airway pressure, biphasic intermittent positive airway pressure (synchronised or unsynchronised) and nasal high flow). Ventilated=infants who are intubated and ventilated. The grey boxes highlight the values between the restrictive and liberal ETTNO/TOP thresholds (online supplemental table S1). Cochrane SR refers to the restrictive thresholds of previous trials, summarised in a Cochrane systematic review by Whyte et al. Number of datapoints per violin plot: 325, 323, 324, 324, 326, 327, 326, 325, 324, 327, 327, 327, 324, 325 and 326 (from left to right). Violin plots are a combination of a boxplot (showing median and IQRs) with a kernel density plot (showing the distribution of the data). The wider the plot, the more NICUs selected this threshold. (B) 100% stacked bar chart showing for each clinical scenario the proportion of the reported transfusion thresholds (from top to bottom) that were above the liberal ETTNO/TOP threshold, at the liberal ETTNO/TOP threshold, between the liberal and restrictive ETTNO/TOP threshold, at the restrictive ETTNO/TOP threshold or below the restrictive ETTNO/TOP threshold. ETTNO, Effects of Transfusion Thresholds on Neurocognitive Outcomes of Extremely Low-Birth-Weight Infants; NICU, neonatal intensive care unit; RBC, red blood cell; TOP, Transfusion of Prematures.
Platelet transfusion thresholds
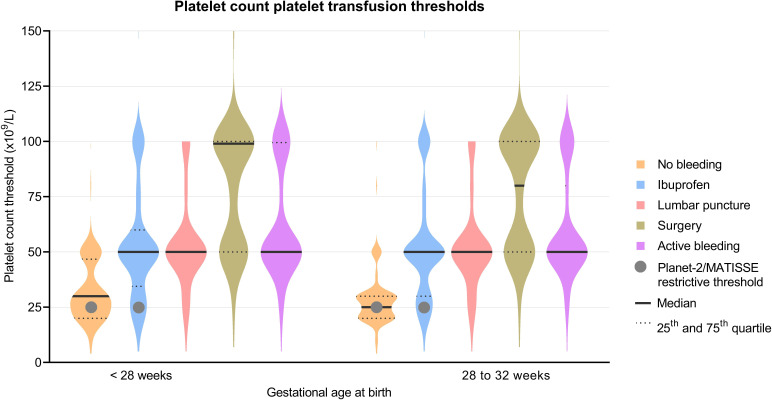
A violin plot of platelet count thresholds for platelet transfusions is shown in figure 2. Platelet transfusion thresholds above 25×109/L were used in 57% (188/332) and 47% (158/333) of NICUs for a non-bleeding infant of <28 weeks’ GA or 28–32 weeks’ GA, respectively. For infants treated with ibuprofen, platelet transfusion thresholds above 25×109/L were used in 84% (249/297 and 248/297) of NICUs for infants of <28 weeks’ GA and infants of 28–32 weeks’ GA. Thresholds of 20×109/L or less were used in 27% (91/332) for infants with GA of <28 weeks without bleeding, 34% (114/333) for infants with GA of 28–32 weeks without bleeding, 8% (25/297) for infants with GA of <28 weeks and ibuprofen and 9% (26/297) of infants with GA of 28–32 weeks with ibuprofen. Infants with lumbar puncture, surgery or active bleeding could not be compared with the trial results as they were allowed additional transfusions at the discretion of the treating neonatologist according to the RCT protocol in the PlaNeT-2/MATISSE trial.
Figure 2.
Violin plots of platelet count transfusion thresholds for different clinical scenarios. Datapoints per violin plot: 332, 297, 316, 311, 329, 333, 297, 317, 311 and 331, from left to right. Violin plots are a combination of a boxplot (showing median and IQRs) with a kernel density plot (showing the distribution of the data). The wider the plot, the more NICUs selected this threshold. MATISSE, Management of Thrombocytopenia in Special Subgroup.
Fresh frozen plasma
Eleven percent of NICUs (38/332) performed routine coagulation tests on infants born at <32 weeks’ GA. FFP was used for the following indications: coagulopathy with active bleeding, 93% (320/343); active bleeding without coagulopathy, 46% (158/343); coagulopathy without active bleeding, 39% (133/343); sepsis, 26.5% (91/343); and hypotension, 25% (85/343).
Duration, volume and rates of transfusion
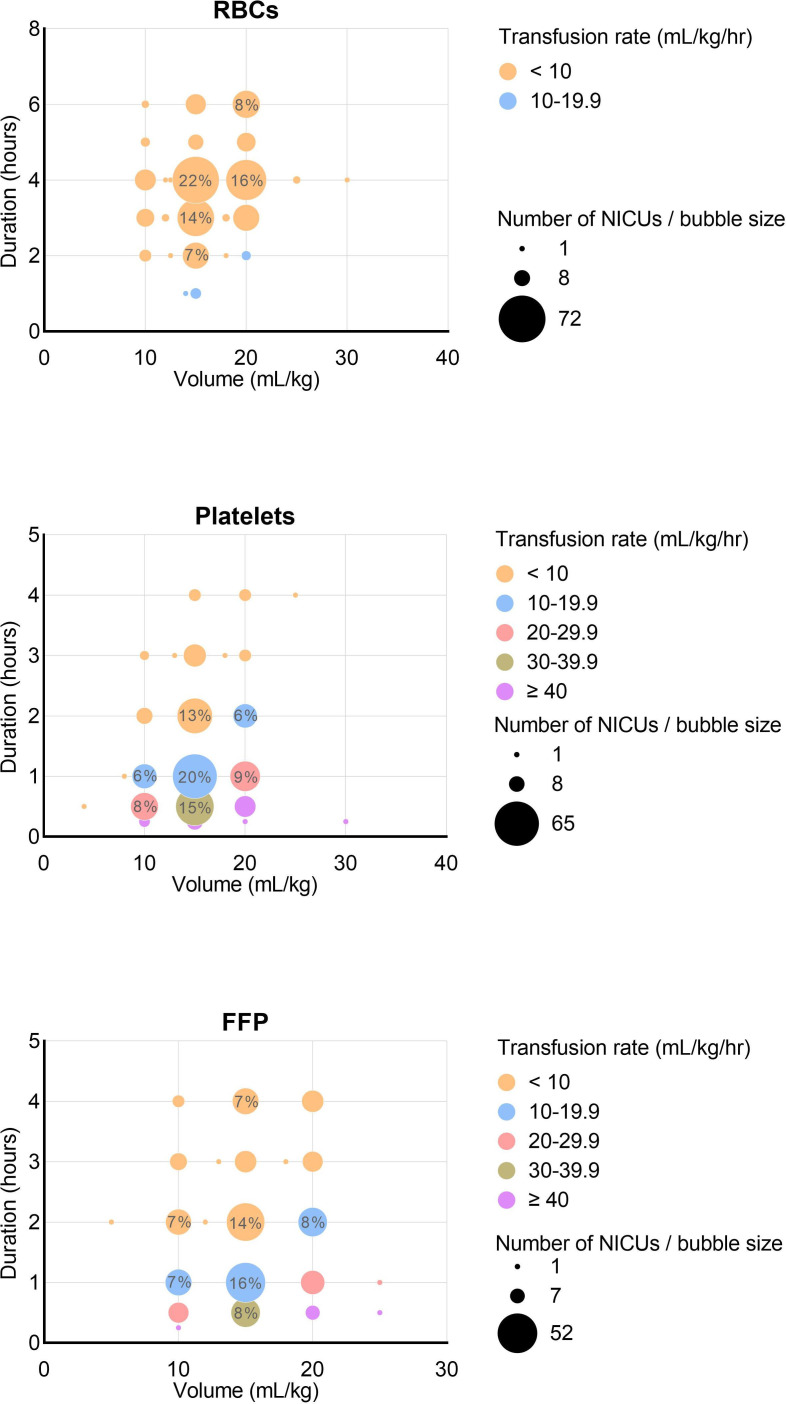
Transfusion volume and duration are depicted in figure 3. We calculated transfusion rates in millilitre per kilogram per hour by dividing volume (mL/kg) by duration (hours). Volumes ranged between 10 mL/kg and 20 mL/kg in 99% of transfusions. The median volume was 15 mL/kg for RBC (IQR 15–20), platelet transfusions (IQR 15–15) and FFP (IQR 15–20). Duration ranged between 1 hour and 7 hours for RBC transfusions (median 4, IQR 3–4), 15 min and 4 hours for platelets (median 1, IQR 0.5–2.0), and 30 min and 4 hours for FFP (median 2, IQR 1–3). Transfusion rates varied between 3.3 mL/kg/hour and 15.0 mL/kg/hour for RBCs (median 4.0, IQR 3.8–5.0), 3.3 mL/kg/hour and 60.0 mL/kg/hour for platelets (excluding two outliers at 120 and 80 mL/kg/hour) (median 15.0, IQR 7.5–20.0) and 2.5 mL/kg/hour and 50.0 mL/kg/hour for FFP (median 10.0, IQR 5–15).
Figure 3.
Transfusion volume, duration and rates for RBC, platelet and plasma transfusions. Bubble size and values within larger bubbles represent the number and percentage of NICUs. Platelet transfusion volume outlier at 4 mL/kg represents one NICU providing hyperconcentrated platelet transfusions. Datapoints for RBC, platelet and FFP volumes: 335, 335 and 336. Datapoints for RBC, platelet and FFP durations: 333, 330 and 335. FFP, fresh frozen plasma; NICU, neonatal intensive care unit; RBC, red blood cell.
Diuretics, enteral feeding and parental consent
Diuretics were ‘always’ or ‘sometimes’ prescribed in conjunction with RBC, platelet and FFP transfusions in 47% (154/329), 18% (57/322) and 28% (92/323) of NICUs, respectively. Enteral feeding was always or sometimes withheld during RBC transfusion in 28% (94/337) and 9% (31/337) of NICUs, respectively. Parental consenting for non-emergency transfusion practices varied, with 8% of NICUs (28/335) requiring no consent, 9% (31/335) requiring verbal consent only, 6% (20/335) requiring verbal consent documented by a clinician, 70% (241/335) requiring verbal and written consent and 4% (15/335) used other forms of consent, not otherwise specified.
Sensitivity analyses
Our weighted analysis showed no substantial changes compared with our primary analysis, suggesting that bias because of varying response rates between countries was likely limited (online supplemental tables S3–S6). The results of our non-responder analysis indicated that non-response bias may have resulted in an underestimation of platelet transfusion thresholds and the proportion of NICUs giving FFP for indications other than active bleeding, as these were higher in the late responders (online supplemental table S7)
Discussion
Despite recent evidence from clinical trials, we demonstrated substantial variation in transfusion practices for RBC, platelets and FFP, regarding thresholds, volume and rate of transfusion across European NICUs. To our knowledge, this is the first survey to assess neonatal transfusion practices across Europe. Other surveys included only selected countries or included mostly non-European NICUs.7 8 A retrospective cohort study of North American NICU blood component transfusion thresholds for all infants between 2013 and 2016 by Patel et al also found wide variation in practice with regard to transfusion thresholds.9
Comparison with RBC trials
The large variation in RBC transfusion thresholds probably reflects the lack of international and European consensus in transfusion criteria, and the lack of evidence until recently. Prior to 2020, clinical practice was partly based on two RCTs (the Premature Infants in Need of Transfusion and Iowa trials), which have been summarised in systematic reviews and national guidelines supporting the use of restrictive thresholds.2–4 10–15 These thresholds were roughly similar to those tested in the TOP and ETTNO trials (online supplemental table S2). In our survey, reported use of thresholds similar to or above the previously tested liberal thresholds was rather rare. However the majority (70%) of thresholds reported were above the TOP/ETTNO restrictive thresholds, while surprisingly, 22% reported thresholds below the restrictive thresholds. These results could be due to implementation of evidence from earlier trials but could also indicate that neonatologists are uncertain regarding restrictive thresholds. Arguments against restrictive thresholds include the possibility of minor neurodevelopmental abnormalities or impairments that become apparent at a later age, which have not been assessed in existing trials. Arguments against a liberal threshold include the lack of clinical benefit of liberal thresholds in clinical trials, and observational studies suggesting potential transfusion-related harm.16–20 The reasons for the use of such low haemoglobin thresholds in 22% of units are unclear but may represent an underestimation of transfusion threshold, as we had to use wide ranges for several clinical scenarios as described previously (online supplemental table S1). Without further clinical trials, it is not known whether these low haemoglobin thresholds are safe for neonates, as various preclinical studies have suggested adverse outcomes following prolonged severe anaemia.21–23
Comparison with platelet transfusion trials
In our survey, 47%–57% of NICUs indicated using platelet count thresholds above 25×109/L for stable non-bleeding infants. Given the strong and concerning evidence for platelet transfusion-mediated harm, the use of platelet transfusion thresholds above 25×109/L for non-bleeding neonates should be discouraged while we await results from long-term neurodevelopmental analyses. Our study also showed that 27%–34% of NICUs use thresholds equal to or lower than 20×109/L for non-bleeding infants. The use of thresholds lower than those tested in trials may be an acceptable practice for platelet transfusions, given the evidence in favour of the restrictive versus liberal threshold, but again this needs to be tested in a clinical trial. Possible explanations for platelet transfusion-mediated adverse effects include the role of platelets in inflammatory and immunoregulatory responses,24 a developmental mismatch between adult donor platelets and neonatal platelets25 26 and volume-mediated effects, since platelet transfusions are given at relatively high volumes and rates compared with adult transfusions.27
Fresh frozen plasma
There are no recent trials investigating FFP transfusion indications, but based on observational and adult data, most guidelines recommend that FFP should not be administered to non-bleeding infants to correct abnormalities of the coagulation screen alone.15 28–30 Minor coagulation abnormalities are poor predictors of bleeding risk and FFP administration often will not correct these abnormalities.30 31 In addition, they can be difficult to interpret, particularly for very preterm babies. We found that 39% of NICUs in our survey administered FFP in case of coagulopathy without bleeding. Furthermore, 25% of NICUs transfused FFP to treat hypotension, for which there is no robust evidence, yet there are potential risks such as transfusion-related acute long injury and severe allergic reactions.32 These findings indicate an urgent need for clinical trials in this area.
Volume, duration and rate of transfusion
Transfusion duration and rate were marked in their variation. The cause for this variation is unclear, but evidence to guide practice is lacking. On average, volumes were comparable to those used in the recent trials (15 mL/kg for PlaNeT-2/MATISSE and TOP, 20 mL/kg for ETTNO). Only a few small studies have assessed neonatal transfusion volume and suggested that these volumes are tolerated, though there is also evidence for harm.14 33–38 Duration or rates of transfusions were not reported in the ETTNO and TOP trials. High-volume or high-rate transfusions may increase the risk of transfusion associated circulatory overload (TACO), a leading cause of transfusion-associated morbidity and mortality in adults.39 Neonatal TACO is poorly defined and the true incidence of this and other transfusion-related lung injuries in neonates is not known; observational studies have shown variable outcomes.37 40–42 However, it should be noted that weight-related volumes transfused to non-bleeding neonates (usually 10–20 mL/kg) are commonly higher than those for adults (typically, 350 mL for packed red cells and 200–300 mL for platelets, which equates to <5 mL/kg for an 80 kg adult).27 43 44 Moreover, the vulnerability of the cerebral vasculature and increased risk of intraventricular haemorrhage in preterm infants should also be considered. Further high-quality research is urgently needed to optimise transfusion rates and volumes.
Limitations and strengths
There are some limitations to our survey. First, we requested one response per neonatal unit, which may mask intraunit variation. Second, we did not ask for detailed transfusion product information because we anticipated that these data were not always available to neonatologists. Third, our eligibility criteria were broad; we only defined that NICUs should care for neonates born at <32 weeks’ GA. We chose this pragmatic approach to be able to paint a picture of current transfusion practices across Europe and because NICU level definitions differ per country. Lastly, combining the ETTNO and TOP trials was not always possible due to varying clinical definitions, highlighting the need for uniform transfusion indications in future trials. Strengths of our survey include the relatively high response rate despite the ongoing pandemic, use of sensitivity analyses and the wide range of countries included. This is, to our knowledge, the first study to map European neonatal transfusion practices, and given the recently published trials, our data are timely and provide a valuable starting point for further studies and highlight the need for guideline development.
Conclusions
In Europe, transfusion practices for preterm infants vary widely. Transfusion thresholds tend to be more liberal compared with data from recent trials supporting the use of more restrictive thresholds. Transfusion indications, volume, duration, concomitant use of diuretics, withholding enteral feeds and parental consent requirements differ considerably. These areas, including factors affecting the implementation of research findings, deserve further attention and clinical research.
Acknowledgments
The authors thank Laura Moschino, Camila Caram-Deelder, the neonatal networks that were involved, Ann Kennedy, the National Perinatal Epidemiology Unit, University of Oxford, and all neonatologists who took the time to fill in the survey.
Footnotes
Twitter: @dralexscrivens, @fracardo
Collaborators: The study was conducted by the executive council members and other members of the Neonatal Transfusion Network (NTN). The NTN is an interdisciplinary, international research network focused on optimising neonatal transfusion practices and research worldwide. It is governed by a steering board and includes over 130 members from over 35 countries. The network is endorsed by the European Society for Pediatric Research, the European Blood Alliance, the European Foundation for the Care of Newborn Infants and the International Hemovigilance Network (www.neonataltransfusionnetwork.com).
Contributors: The study was planned and executed by the members of the Neonatal Transfusion Network steering committee (CCR, CD, ED, EL, HVN, JvbB, KF, SS, SFG, AS, HS, LH, NH and NJR) with significant input from MAC, KB, FSC, FC, RF, SGh, JL, KM, TM, US, HS, MS, TS and GZ, who also facilitated region-specific data collection. S F-G is guarantor.
Funding: The study was in part funded by the generous support and grant (PPOC21-08/L2588 and RES/00264) from Sanquin Blood Supply Foundation, Amsterdam, the Netherlands, and a postdoctoral research grant (RGP2020-09/PDRG-02/04) from The European Society for Pediatric Research, Geneva, Switzerland.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request by email to the corresponding and the senior author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethical approval was not required as no patient-specific data were collected.
References
- 1. Fustolo-Gunnink SF, Roehr CC, Lieberman L, et al. Platelet and red cell transfusions for neonates: lifesavers or Trojan horses? Expert Rev Hematol 2019;12:797–800. 10.1080/17474086.2019.1657824 [DOI] [PubMed] [Google Scholar]
- 2. Kirpalani H, Bell EF, Hintz SR, et al. Higher or lower hemoglobin transfusion thresholds for preterm infants. N Engl J Med Overseas Ed 2020;383:2639–51. 10.1056/NEJMoa2020248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Franz AR, Engel C, Bassler D, et al. Effects of liberal vs restrictive transfusion thresholds on survival and neurocognitive outcomes in extremely low-birth-weight infants: the ETTNO randomized clinical trial. JAMA 2020;324:560–70. 10.1001/jama.2020.10690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang P, Wang X, Deng H, et al. Restrictive versus liberal transfusion thresholds in very low birth weight infants: a systematic review with meta-analysis. PLoS One 2021;16:e0256810–4. 10.1371/journal.pone.0256810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curley A, Stanworth SJ, Willoughby K, et al. Randomized trial of platelet-transfusion thresholds in neonates. N Engl J Med 2019;380:242–51. 10.1056/NEJMoa1807320 [DOI] [PubMed] [Google Scholar]
- 6. Fustolo-Gunnink SF, Fijnvandraat K, van Klaveren D, et al. Preterm neonates benefit from low prophylactic platelet transfusion threshold despite varying risk of bleeding or death. Blood 2019;134:2354–60. 10.1182/blood.2019000899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guillén U, Cummings JJ, Bell EF, et al. International survey of transfusion practices for extremely premature infants. Semin Perinatol 2012;36:244–7. 10.1053/j.semperi.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cremer M, Sola-Visner M, Roll S, et al. Platelet transfusions in neonates: practices in the United States vary significantly from those in Austria, Germany, and Switzerland. Transfusion 2011;51:2634–41. 10.1111/j.1537-2995.2011.03208.x [DOI] [PubMed] [Google Scholar]
- 9. Patel RM, Hendrickson JE, Nellis ME, et al. Variation in neonatal transfusion practice. J Pediatr 2021;235:92–9. 10.1016/j.jpeds.2021.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics 2005;115:1685–91. 10.1542/peds.2004-1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whyte RK, Kirpalani H, Asztalos EV, et al. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics 2009;123:207–13. 10.1542/peds.2008-0338 [DOI] [PubMed] [Google Scholar]
- 12. Kirpalani H, Whyte RK, Andersen C, et al. The premature infants in need of transfusion (pint) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr 2006;149:301–7. 10.1016/j.jpeds.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 13. Whyte R, Kirpalani H, Cochrane Neonatal Group . Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants. Cochrane Database Syst Rev 2011;115. 10.1002/14651858.CD000512.pub2 [DOI] [PubMed] [Google Scholar]
- 14. Venkatesh V, Khan R, Curley A, et al. The safety and efficacy of red cell transfusions in neonates: a systematic review of randomized controlled trials. Br J Haematol 2012;158:370–85. 10.1111/j.1365-2141.2012.09180.x [DOI] [PubMed] [Google Scholar]
- 15. New HV, Berryman J, Bolton-Maggs PHB, et al. Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol 2016;175:784–828. 10.1111/bjh.14233 [DOI] [PubMed] [Google Scholar]
- 16. Juul SE, Vu PT, Comstock BA, et al. Effect of high-dose erythropoietin on blood transfusions in extremely low gestational age neonates: post hoc analysis of a randomized clinical trial. JAMA Pediatr 2020;174:933–43. 10.1001/jamapediatrics.2020.2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patel RM, Lukemire J, Shenvi N, et al. Association of blood donor sex and age with outcomes in very low-birth-weight infants receiving blood transfusion. JAMA Netw Open 2021;4:e2123942–11. 10.1001/jamanetworkopen.2021.23942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rose AT, Saroha V, Patel RM. Transfusion-related gut injury and necrotizing enterocolitis. Clin Perinatol 2020;47:399–412. 10.1016/j.clp.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fontana C, Raffaeli G, Pesenti N, et al. Red blood cell transfusions in preterm newborns and neurodevelopmental outcomes at 2 and 5 years of age. Blood Transfus 2022;20:40–9. 10.2450/2020.0207-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Benavides A, Conrad AL, Brumbaugh JE, et al. Long-Term outcome of brain structure in female preterm infants: possible associations of liberal versus restrictive red blood cell transfusions. J Matern Fetal Neonatal Med 2021;34:3292–9. 10.1080/14767058.2019.1683157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MohanKumar K, Namachivayam K, Song T, et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat Commun 2019;10. 10.1038/s41467-019-11199-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh G, Wallin DJ, Abrahante Lloréns JE, et al. Dose- and sex-dependent effects of phlebotomy-induced anemia on the neonatal mouse hippocampal transcriptome. Pediatr Res 2022;92:1–9. 10.1038/s41390-021-01832-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arthur CM, Nalbant D, Feldman HA, et al. Anemia induces gut inflammation and injury in an animal model of preterm infants. Transfusion 2019;9:1233–45. 10.1111/trf.15254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Reilly D, Murphy CA, Drew R, et al. Platelets in pediatric and neonatal sepsis: novel mediators of the inflammatory cascade. Pediatr Res 2022;91:359–67. 10.1038/s41390-021-01715-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferrer-Marín F, Sola-Visner M. Neonatal platelet physiology and implications for transfusion. Platelets 2022;33:1–9. 10.1080/09537104.2021.1962837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waubert de Puiseau M, Sciesielski LK, Meyer O, et al. Pooling, room temperature, and extended storage time increase the release of adult‐specific biologic response modifiers in platelet concentrates: a hidden transfusion risk for neonates? Transfusion 2020;60:1828–36. 10.1111/trf.15827 [DOI] [PubMed] [Google Scholar]
- 27. Curley A, Stanworth SJ, New H. A randomized trial of neonatal platelet transfusion thresholds. Reply. N Engl J Med 2019;380:1584–5. 10.1056/NEJMc1902638 [DOI] [PubMed] [Google Scholar]
- 28. Motta M, Del Vecchio A, Chirico G. Fresh frozen plasma administration in the neonatal intensive care unit: evidence-based guidelines. Clin Perinatol 2015;42:639–50. 10.1016/j.clp.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 29. Houben NAM, Heeger LE, Stanworth SJ, et al. Changes in the use of Fresh-Frozen plasma transfusions in preterm neonates: a single center experience. J Clin Med 2020;9:3789. 10.3390/jcm9113789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keir AK, Stanworth SJ. Neonatal plasma transfusion: an evidence-based review. Transfus Med Rev 2016;30:174–82. 10.1016/j.tmrv.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 31. Segal JB, Dzik WH, Transfusion Medicine/Hemostasis Clinical Trials Network . Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion 2005;45:1413–25. 10.1111/j.1537-2995.2005.00546.x [DOI] [PubMed] [Google Scholar]
- 32. Osborn DA, Evans N. Early volume expansion versus inotrope for prevention of morbidity and mortality in very preterm infants. Cochrane Database Syst Rev 2001;2001:CD002056. 10.1002/14651858.CD002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paul DA, Leef KH, Locke RG, et al. Transfusion volume in infants with very low birth weight: a randomized trial of 10 versus 20 mL/kg. J Pediatr Hematol Oncol 2002;24:43–6. 10.1097/00043426-200201000-00012 [DOI] [PubMed] [Google Scholar]
- 34. Wong H, Connelly R, Day A, et al. A comparison of high and standard blood transfusion volumes in premature infants. Acta Paediatr 2005;94:624–5. 10.1111/j.1651-2227.2005.tb01949.x [DOI] [PubMed] [Google Scholar]
- 35. Khodabux CM, Hack KEA, von Lindern JS, et al. A comparative cohort study on transfusion practice and outcome in two Dutch tertiary neonatal centres. Transfus Med 2009;19:195–201. 10.1111/j.1365-3148.2009.00934.x [DOI] [PubMed] [Google Scholar]
- 36. Mallett LH, Govande VP, Shetty A, et al. Safety and efficacy of packed red blood cell transfusions at different doses in very low birth weight infants. Baylor Univ Med Cent Proc 2016;29:128–30. 10.1080/08998280.2016.11929387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rashid N, Al-Sufayan F, Seshia MMK, et al. Post transfusion lung injury in the neonatal population. J Perinatol 2013;33:292–6. 10.1038/jp.2012.114 [DOI] [PubMed] [Google Scholar]
- 38. Choi EK, Shin J, Kim G-H, et al. Hemodynamics of different volumes of red blood cell transfusion in preterm infants. Pediatr Int 2021;63:410–4. 10.1111/ped.14380 [DOI] [PubMed] [Google Scholar]
- 39. Grey S, Bolton‐Maggs P. Pulmonary complications of transfusion: changes, challenges, and future directions. Transfus Med 2020;30:442–9. 10.1111/tme.12709 [DOI] [PubMed] [Google Scholar]
- 40. Grev J, Stanclova M, Ellsworth M, et al. Does red blood cell Transfusion-Related acute lung injury occur in premature infants? A retrospective cohort analysis. Am J Perinatol 2017;34:14–18. 10.1055/s-0036-1584142 [DOI] [PubMed] [Google Scholar]
- 41. Kovatis KZ, Di Fiore JM, Martin RJ, et al. Effect of blood transfusions on intermittent hypoxic episodes in a prospective study of very low birth weight infants. J Pediatr 2020;222:65–70. 10.1016/j.jpeds.2020.03.015 [DOI] [PubMed] [Google Scholar]
- 42. Poppe JA, van Essen T, van Weteringen W, et al. Cardiorespiratory monitoring of red blood cell transfusions in preterm infants. Eur J Pediatr 2022;181:489–500. 10.1007/s00431-021-04218-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keir AK, New H, Robitaille N, et al. Approaches to understanding and interpreting the risks of red blood cell transfusion in neonates. Transfus Med 2019;29:231–8. 10.1111/tme.12575 [DOI] [PubMed] [Google Scholar]
- 44. UpToDate . Blood components: indications and dosing in adults. Available: https://www.uptodate.com/contents/image?imageKey=HEME%2F53854 [Accessed 10 Oct 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
fetalneonatal-2022-324619supp001.pdf (370.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data are available upon reasonable request by email to the corresponding and the senior author.