Abstract
Objectives
Based on primary results from ORAL Surveillance, an event-driven clinical trial of risk-enriched patients, identify subpopulations with different relative risk (ie, ‘high-risk’ and ‘low-risk’) with tofacitinib versus tumour necrosis factor inhibitors (TNFi).
Methods
Patients with rheumatoid arthritis aged ≥50 years with ≥1 additional cardiovascular risk factor received tofacitinib 5 or 10 mg two times a day or TNFi. Prior analyses had identified age and smoking as risk factors of particular interest across safety outcomes. Hazard ratios (HRs) and incidence rates were evaluated by age and smoking individually and in combination. Results were validated across tofacitinib development programmes.
Results
‘Age ≥65 years or ever smoker’ defined a group (‘high-risk’) with increased risk of malignancies (excluding non-melanoma skin cancer), major adverse cardiovascular events, myocardial infarction, venous thromboembolism and all-cause death with tofacitinib (combined doses) versus TNFi (HRs 1.41–5.19). In patients ‘aged <65 years and never smokers’ (’low-risk’), there was no detectable risk increase with tofacitinib versus TNFi (HRs ≈1.0) up to 6 years of follow-up, and absolute risk remained low and was corroborated across tofacitinib rheumatoid arthritis, psoriatic arthritis and ulcerative colitis programmes with up to 10 years of observation.
Conclusions
This posthoc analysis of ORAL Surveillance identified two tofacitinib subpopulations with different relative risk versus TNFi. High risk was confined to patients defined by distinct risk factors age ≥65 years or smoking, and these differentiating risk factors accounted for the excess risk observed with tofacitinib versus TNFi. These findings can guide individualised benefit/risk assessment and clinical decision-making on treatment with tofacitinib.
Trial registration numbers
NCT02092467, NCT01262118, NCT01484561, NCT00147498, NCT00413660, NCT00550446, NCT00603512, NCT00687193, NCT01164579, NCT00976599, NCT01059864, NCT01359150, NCT02147587, NCT00960440, NCT00847613, NCT00814307, NCT00856544, NCT00853385, NCT01039688, NCT02281552, NCT02187055, NCT02831855, NCT00413699, NCT00661661, NCT00787202, NCT01465763, NCT01458951, NCT01458574, NCT01470612, NCT01877668, NCT01882439, NCT01976364.
Keywords: Antirheumatic Agents, Arthritis, Therapeutics, Tumor Necrosis Factor Inhibitors
WHAT IS ALREADY KNOWN ON THIS TOPIC
Primary findings from ORAL Surveillance indicated that patients with rheumatoid arthritis aged ≥50 years with ≥1 additional cardiovascular risk factor have an increased risk of major adverse cardiovascular events (MACE) and malignancies (excluding non-melanoma skin cancer) with tofacitinib compared with tumour necrosis factor inhibitors (TNFi).
Increased risk of MACE with tofacitinib versus TNFi was primarily observed in patients with a history of atherosclerotic cardiovascular disease.
Previous analyses from ORAL Surveillance identified age and smoking as independent (ie, across treatment groups) risk factors of interest across safety outcomes.
WHAT THIS STUDY ADDS
This posthoc analysis of ORAL Surveillance identified high-risk and low-risk populations with different relative risk with tofacitinib versus TNFi.
Higher risk versus TNFi was confined to a subgroup of patients defined by distinct, readily identifiable risk factors, age 65 years or older and long-time smoking (current or past).
In ‘low-risk’ patients who were younger than 65 years and had never smoked, there was no detectable risk increase versus TNFi with up to 6 years of follow-up in ORAL Surveillance, and absolute risk remained low and was corroborated across tofacitinib rheumatoid arthritis, psoriatic arthritis and ulcerative colitis development programmes with up to 10 years of observations.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These easily identifiable and clinically practical subpopulations with different relative risk versus TNFi (ie, ‘high-risk’ and ‘low-risk’) can better guide individualised benefit/risk assessment and clinical decision-making on treatment with tofacitinib.
Introduction
ORAL Surveillance was a large, randomised, open-label, event-driven clinical trial in patients with rheumatoid arthritis (RA) designed to demonstrate non-inferiority of tofacitinib versus TNF inhibitors (TNFi) for the coprimary endpoints of adjudicated major adverse cardiovascular events (MACE) and adjudicated malignancies (excluding non-melanoma skin cancer (NMSC)).1 The U.S. FDA required the study to be of sufficient size and duration to evaluate long-latency and rare events.2 For the study to be declared complete, ≥1500 patients had to be followed for ≥3 years, and ≥103 MACE, and ≥138 malignancies (excluding NMSC), had to accrue.1 The study started in 2014, enrolled 4362 patients and completed 6 years later.
To ensure enough cardiovascular (CV) and malignancy events accrued in a reasonable timeframe, ORAL Surveillance enrolled patients with RA with higher-than-average risk; patients had to be at least 50 years old and have at least one additional CV risk factor.1 Previous studies have shown a relationship and shared risk factors between CV disease and malignancy, for example, data from Dutch and US cohorts found an association between the 10-year atherosclerotic cardiovascular disease (ASCVD) risk scores and risk of future cancer.3 4 Importantly, a wide range of CV risk factors were applied as eligibility criteria, therefore, a 52-year-old with mild hypertension and a 71-year-old with prior myocardial infarction (MI) would both have been eligible despite very different risk levels. Indeed, enrolled patients were found to be distributed across a continuum of risk.5 For example, close to one-third of the overall ORAL Surveillance study population had only low-to-borderline predicted 10-year risk of ASCVD.5
Guided by initial thematic analyses of clinically meaningful factors in ORAL Surveillance, in which age and smoking were consistently identified as independent (ie, across treatment groups) risk factors of particular interest across safety outcomes, we aimed to find easily identifiable and clinically practical subpopulations with different relative risk vs TNFi (ie, ‘high-risk’ and ‘low-risk’) to better guide individualised benefit/risk assessment and clinical decision-making. For risk minimisation and product labelling purposes, it was important to identify the risk factors (individual or combinations thereof) accounting for the increased risk observed with tofacitinib versus TNFi (ie, differentiating risk factors) and, equally important, where the reverse was true, that is, the absence of these risk factors produced risk estimates with no apparent risk difference between treatments (ie, HR at or below 1). Analyses were repeated in the larger and longer tofacitinib RA development programme, and tofacitinib psoriatic arthritis (PsA) and ulcerative colitis (UC) development programmes to assess consistency and validate results.
Methods
Study design and patients
ORAL Surveillance
ORAL Surveillance (NCT02092467) was a phase IIIb/IV randomised, open-label, non-inferiority, safety endpoint study conducted from March 2014 to July 2020 in patients with active moderate-to-severe RA despite methotrexate treatment who were aged ≥50 years with ≥1 additional CV risk factor (current smoking, hypertension, high-density lipoprotein cholesterol <40 mg/dL, diabetes mellitus, family history of premature coronary heart disease, RA-associated extra-articular disease and/or history of coronary artery disease).1
Patients were randomised 1:1:1 to receive oral tofacitinib 5 or 10 mg two times a day or subcutaneous TNFi (adalimumab 40 mg every 2 weeks (North America) or etanercept 50 mg once a week (rest of the world)). All patients continued their pre-study stable dose of methotrexate unless modification was clinically indicated. In February 2019, the tofacitinib 10 mg two times a day dose was reduced to 5 mg two times a day after the Data Safety Monitoring Board noted an increased frequency of pulmonary embolism in patients receiving tofacitinib 10 mg two times a day versus TNFi and an increase in overall mortality with tofacitinib 10 vs 5 mg two times a day and TNFi.
RA, PsA and UC development programmes
This exploratory analysis includes data for patients who received ≥1 dose of tofacitinib in clinical trials and open-label LTE studies across RA (excluding ORAL Surveillance), PsA and UC. Full details of the individual studies can be found in online supplemental table S3.
ard-2022-223715supp001.pdf (2MB, pdf)
All cohorts included patients aged ≥18 years who received tofacitinib as monotherapy (RA and UC) or with background conventional synthetic disease-modifying antirheumatic drugs (RA, PsA). All studies have been completed. Final data for the RA, PsA and UC cohorts are from 18 January 2019, 31 July 2019 and 24 August 2020, respectively.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Outcomes
This analysis focused on events of malignancies (excluding NMSC), MACE, MI (fatal and non-fatal), venous thromboembolism (VTE) and all-cause death. In ORAL Surveillance, these events were adjudicated by an external, independent adjudication committee. MACE was defined as the composite of CV death, non-fatal MI and non-fatal stroke. Similarly, events of malignancies (excluding NMSC), MACE and MI were adjudicated in the tofacitinib development programmes. See online supplemental table S1) for definition of outcomes.
Statistical analyses
ORAL Surveillance was powered to assess non-inferiority for risk of MACE and malignancies (excluding NMSC) with combined tofacitinib doses versus TNFi. To maximise statistical precision and power in these posthoc analyses, data for the combined doses of tofacitinib were prioritised. HRs and two-sided 95% CIs comparing tofacitinib and TNFi were estimated using Cox proportional hazard regression models. Incidence rates (IRs) and two-sided 95% CIs for malignancies (excluding NMSC), MACE, MI, VTE and all-cause death were reported as the number of unique patients with events per 100 patient-years. See online supplemental table S1 for definition of censoring times for the different analyses. Exact Poisson, adjusted for exposure, was used to calculate 95% CIs for the crude IR.
Prior analyses had consistently identified age and smoking as independent risk factors of particular interest across safety outcomes and were therefore assessed here, individually and in combination.1 6 7 Informed by the definition of the geriatric population from the ICH Topic E7 (Studies in Support of Special Populations: Geriatrics), a cut-off of 65 years of age was a pre-specified analysis in ORAL Surveillance and also applied in these analyses.1 8 IRs and HRs with tofacitinib versus TNFi were also determined by 5-year intervals of age.
Further analyses were conducted to identify risk factors accounting for the increased risk observed with tofacitinib versus TNFi (ie, differentiating risk factors) while when absent produced tofacitinib risk estimates with no difference versus TNFi (ie, HR≈1.0). HRs (95% CIs) comparing tofacitinib and TNFi (ORAL Surveillance) and IRs (95% CIs) for each treatment group were calculated for subgroups of patients defined by age (≥ or <65 years of age), or by smoking status (current, past or never smoking), or by composites thereof.
For the subgroups defined by ‘age ≥65 years or ever smoker’ (‘high-risk’) and ‘age <65 years and never smoker’ (‘low-risk’), number needed to harm (NNH) was calculated as the reciprocal of the difference in IRs between tofacitinib and TNFi. Positive NNH was defined as patient-years of tofacitinib exposure needed for one more patient to report an additional event versus TNFi. Negative NNH was defined as the reverse. The differential or interaction effect of treatment group (tofacitinib vs TNFi) and high-risk versus low-risk was assessed using the difference of the incidence rate difference (IRD, comparing tofacitinib and TNFi) between high-risk and low-risk and its SE. The two-sided interaction p value was calculated assuming normal approximation to the difference of IRD. In published criteria for how to evaluate the credibility of subgroup analyses, it is advised to not use a specified p value threshold, but p<0.1 are generally supportive of the hypothesis.9 Cumulative probability plots using Kaplan-Meier estimates were generated for analysis of time to events. IRs (95% CI) of all outcomes were determined by high-risk and low-risk in the tofacitinib RA, PsA and UC development programmes.
All analyses were posthoc. Across these exploratory analyses, no multiplicity adjustments were applied.
Results
Patients
In ORAL Surveillance, 4362 patients were randomised and treated (tofacitinib 5 mg two times a day, n=1455; tofacitinib 10 mg two times a day, n=1456; TNFi, n=1451).1 In addition, this analysis includes 9904 tofacitinib-exposed patients from completed studies in the development programmes: 7964 with RA (excluding ORAL Surveillance), 783 with PsA and 1157 with UC.10 Table 1 (online supplemental table S2 by low-risk/high-risk) summarises demographics and baseline characteristics for patients in ORAL Surveillance and in all patients who received ≥1 dose of tofacitinib in the RA, PsA and UC development programmes. Compared with the tofacitinib development programmes, patients included in ORAL Surveillance represented a risk-enriched population as reflected in a higher proportion of patients aged ≥65 years, current smokers and patients with a history of diabetes, hypertension and ASCVD (table 1).
Table 1.
Demographic and baseline disease characteristics of patients in ORAL Surveillance and the tofacitinib RA, PsA and UC development programmes
| ORAL Surveillance | Tofacitinib development programme | |||
| N=4362 | RA* N=7964 |
PsA N=783 |
UC N=1157 |
|
| Female, % (n) | 78.2% (3410) | 81.9% (6522) | 54.7% (428) | 41.3% (478) |
| Duration of disease (years), mean/median | 10.4/8.0 | 8.1/5.6 | 7.7/5.5 | 8.2/6.3 |
| Age, mean (SD) | 61.2 (7.1) | 52.6 (12.1) | 48.7 (12.0) | 41.3 (13.9) |
| ≥65 years of age, % (n) | 31.0% (1353) | 15.9% (1270) | 9.2% (72) | 6.7% (77) |
| Smoking status†, % (n) | ||||
| Current | 26.7% (1166) | 17.2% (1366) | 17.9% (140) | 5.1% (59) |
| Past | 21.5% (937) | 17.4% (1388) | 20.2% (158) | 30.9% (357) |
| Never | 51.8% (2259) | 62.7% (4996) | 61.9% (485) | 64.0% (740) |
| History of other CV risk factors, % (n) | ||||
| Diabetes mellitus | 17.4% (759) | 8.2% (651) | 13.7% (107) | 4.1% (48)‡ |
| Hyperlipidaemia | 35.2% (1534) | 19.3% (1534) | 21.3% (107) | NA |
| Hypertension | 66.0% (2878) | 35.4% (2818) | 39.1% (306) | 13.9% (161)‡ |
| Coronary artery disease | 11.4% (497) | 1.6% (126) | 5.6% (44) | 1.6% (18) |
| ASCVD | 14.7% (640) | 3.4% (274) | 6.5% (51) | 3.9% (45) |
| Treatment history, % (n) | ||||
| Prior TNFi | 7.6% (330) | 15.6% (1245) | 48.1% (377) | 54.4% (1124) |
| Statin at baseline‡ | 23.4% (1020) | 7.8% (620) | 12.8% (100) | 6.4% (74) |
| Aspirin at baseline‡ | 15.3% (667) | 6.9% (551) | 6.4% (50) | NA |
*Excluding ORAL Surveillance.
†In the tofacitinib RA development programme, 2.7% (N=214) of patients had unknown smoking status. Patients <65 years old with unknown smoking status were not included in the low-risk group. 25 patients in the high-risk group had unknown smoking status.
‡Based on day 1 of treatment.
ASCVD, atherosclerotic cardiovascular disease; CV, cardiovascular; n, number of patients with characteristic; NA, not available; RA, rheumatoid arthritis; TNFi, tumour necrosis factor inhibitor; UC, ulcerative colitis.
Tofacitinib relative risk versus TNFi and role of age and smoking as individual risk factors
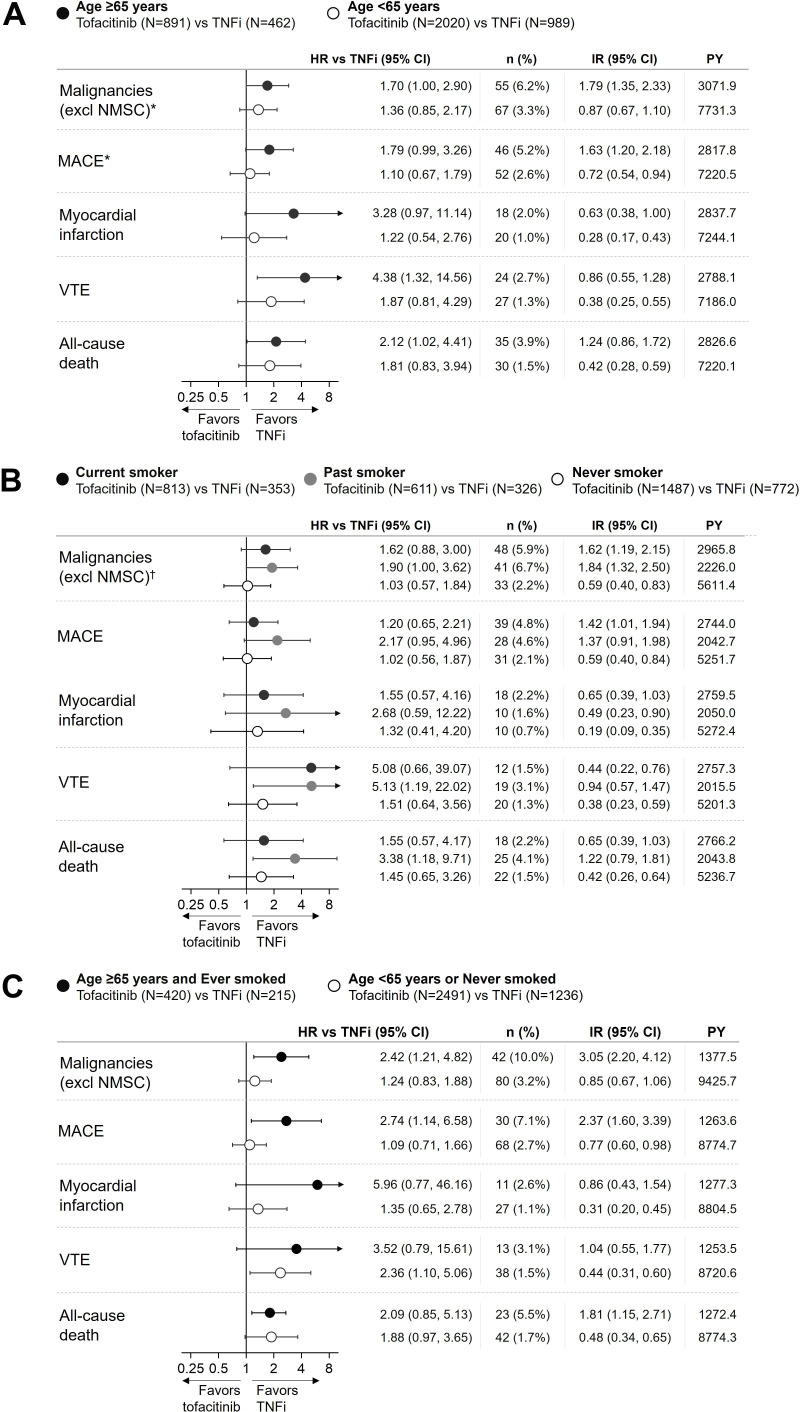
Age ≥65 years (figure 1A) and ever (ie, current or past) smoking (figure 1B) were both independent overall risk factors with associations with absolute (IRs) and relative (HRs) risk with tofacitinib versus TNFi of malignancies (excluding NMSC), MACE, MI, VTE and all-cause death in ORAL Surveillance.1 6 7 Even though less pronounced, an increased risk versus TNFi (HRs >1) was observed in patients who were <65 years as well as in never smokers for certain outcomes.
Figure 1.
Risk of malignancies (excluding NMSC), MACE, MI, VTE and all-cause death with tofacitinib versus TNFi in ORAL Surveillance by (A) age (≥ or <65 years) or (B) history of smoking (current, past or never) or (C) composite of age and smoking (‘Age ≥65 years and Ever smoked’ or ‘Age <65 years or Never smoked’). HRs (95% CIs), shown on a logarithmic scale, are based on a simple Cox proportional hazard model comparing combined tofacitinib doses versus TNFi. Arrow heads indicate that CI extends beyond the graph axis. IRs express the number of patients with first events per 100 PY. IRs, n and PY are for combined tofacitinib doses. *Results previously reported in Ytterberg et al.1 †Results reported in Curtis et al.7 IR, incidence rate; MACE, major adverse cardiovascular events; n, number of evaluable patients; N, number of patients with events; NMSC, non-melanoma skin cancer; PY, patient-years; TNFi, tumour necrosis factor inhibitor; VTE, venous thromboembolism.
Analyses of risk with tofacitinib by 5-year age intervals indicated a risk continuum with increasing age where the inflection point seemed to appear at or around ≥65 years of age (online supplemental figure S1).
An analysis of smoking duration showed that the majority of current and past smokers in ORAL Surveillance were long-time smokers. More than 90% of patients treated with tofacitinib in ORAL Surveillance that had ever smoked (ie, current or past smokers) had a smoking duration of more than 10 years. Median duration of smoking in current and past smokers was 35.0 and 39.0 years, respectively. Majority of past smokers had been smoke-free for 10 years or longer (table 2).
Table 2.
Smoking duration in current and past smokers and time since smoking cessation in past smokers in ORAL Surveillance
| Current smokers | Past smokers | |||
| Tofacitinib N=811 |
TNFi N=352 |
Tofacitinib N=605 |
TNFi N=322 |
|
| Smoking Duration (years) | ||||
| Mean (SD) | 32.7 (13.1) | 31.9 (13.2) | 37.2 (13.0) | 39.9 (12.2) |
| Median (Range) | 35.0 (0.02, 67.00) | 34.2 (0.02, 69.00) | 39.0 (0.00, 69.00) | 41.0 (1.00, 70.00) |
| Smoking duration levels*, % (n) | ||||
| >10 years | 91.4% (741) | 91.2% (321) | 96.2% (582) | 98.4% (317) |
| >5–10 years | 4.4% (36) | 3.7% (13) | 2.3% (14) | 0.9% (3) |
| >0–5 years | 4.2% (34) | 5.1% (18) | 1.5% (9) | 0.6% (2) |
| Time since smoking cessation levels, % (n) | ||||
| ≥10 years | – | – | 61.5% (372) | 71.7% (231) |
| <10 years | – | – | 38.5% (233) | 28.3% (91) |
| <5 years | – | – | 21.8% (132) | 15.2% (49) |
| <1 year | – | – | 5.6% (34) | 3.1% (10) |
*Information on smoking duration was missing on eight patients (two current and six past smokers) treated with tofacitinib and five patients (one current and four past smokers) treated with TNFi.
n, number of patients with characteristic; TNFi, tumour necrosis factor inhibitor.
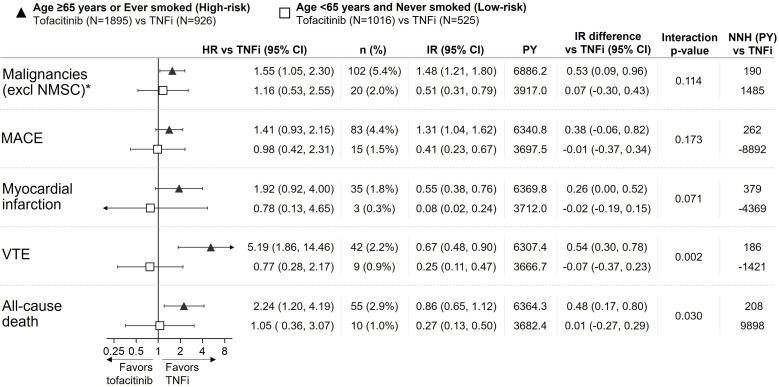
Tofacitinib relative risk versus TNFi and composites of age and smoking as differentiating risk factors
Figures 1C and 2 show the relative risk (HRs) for malignancies (excluding NMSC), MACE, MI, VTE and all-cause death with tofacitinib versus TNFi in subgroups defined by composites of age and smoking. In patients with both risk factors, that is, ‘age≥65 years and ever smoker’ (figure 1C), or one risk factor, that is, ‘age ≥65 years or ever smoker’ (figure 2; online supplemental figure S2 by tofacitinib dose), the risk was higher with tofacitinib versus TNFi, and for some endpoints, the 95% CI for the HR excluded 1. It was only in patients with neither risk factor, that is, ‘age <65 years and never smoker’ group that we could not detect a higher risk with tofacitinib vs TNFi (ie, HRs≈1.0) for any of these events (figure 2). Based on IRD, the p values for the treatment-by-risk high/low interaction for these events with combined doses of tofacitinib versus TNFi ranged from 0.002 to 0.173 (figure 2), supporting that this composite of age ≥65 years or ever smoker represents a differentiating risk factor for these events.9
Figure 2.
Risk of malignancies (excluding NMSC), MACE, MI, VTE and all-cause death with tofacitinib versus TNFi in ORAL Surveillance by subgroups of high-risk and low-risk patients. HRs (95% CIs), shown on a logarithmic scale, are based on a simple Cox proportional hazard model comparing combined tofacitinib doses versus TNFi. Arrow heads indicate that CI extends beyond the graph axis. IRs express the number of patients with first events per 100 PY. Treatment-by-risk interaction p values were calculated based on IR differences (two-sided, normal approximation of difference in IR). NNH (PY) should be interpreted as the number of patient-years of exposure to tofacitinib required to have one additional event versus TNFi. All data are for combined tofacitinib doses. *Results reported in Curtis et al.7 IR, incidence rate; MACE, major adverse cardiovascular events; N, number of evaluable patients; n, number of patients with events; NMSC, non-melanoma skin cancer; NNH, numbers needed to harm; PY, patient-years; TNFi, tumour necrosis factor inhibitor; VTE, venous thromboembolism.
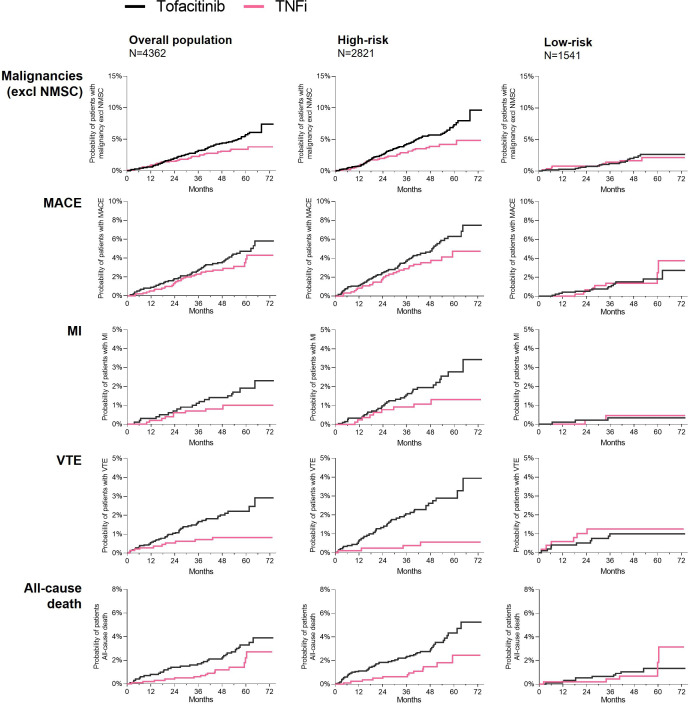
Cumulative probability of events with tofacitinib and TNFi in high-risk and low-risk subpopulations
‘Age ≥65 years or ever smoker’ and ‘age <65 years and never smoker’ are hereafter referred to as ‘high-risk’ and ‘low-risk’, respectively. To further assess absolute and relative risk of malignancies (excluding NMSC), MACE, MI, VTE and all-cause death with tofacitinib versus TNFi over time in high-risk and low-risk patients, cumulative probability curves were generated (figure 3). These curves confirmed that there were two tofacitinib subpopulations with different relative risk versus TNFi: one subpopulation (‘high-risk’) of patients which had higher risk with tofacitinib versus TNFi for all these outcomes and one subpopulation (‘low-risk’) for which the curves representing treatment with tofacitinib and TNFi overlapped and/or crossed, and we could not detect a difference between tofacitinib and TNFi up to 6 years of follow-up in ORAL Surveillance.
Figure 3.
Cumulative probability of patients with malignancies (excluding NMSC), MACE, MI, VTE and all-cause death in ORAL Surveillance overall population and by subgroups of high-risk and low-risk patients. Overall population received tofacitinib 5 mg or 10 mg two times a day (N=2911) or TNFi (N=1451). High-risk patients were ≥65 years of age or ever smoker (tofacitinib, N=1895; TNFi, N=926). Low-risk patients were <65 years of age and never smoker (tofacitinib, N=1016; TNFi, N=525). Cumulative probabilities of events were calculated based on Kaplan-Meier estimates. Cumulative probability plots for malignancies (excluding NMSC) and MACE in overall population have been reported in Ytterberg et al 1 and are included for reference. MACE, major adverse cardiovascular events; MI, myocardial infarction; NMSC, non-melanoma skin cancer; TNFi, tumour necrosis factor inhibitor; VTE, venous thromboembolism.
Absolute risk in low-risk patients in ORAL Surveillance
Absolute risk of malignancies (excluding NMSC), MACE, MI, VTE and all-cause death in low-risk patients (figure 2) was low as reflected in low event rates and IRs. NNH based on the IR differences with tofacitinib versus TNFi indicated that 1485 and 9898 low-risk patients would need to be treated with tofacitinib for 1 year to have one additional event of, respectively, malignancies (excluding NMSC) and all-cause mortality versus TNFi. For MACE, MI and VTE in low-risk patients, IRD and NNH were negative and in favour of tofacitinib versus TNFi. The relatively large NNH (positive or negative numbers) principally reflect similar absolute risk with tofacitinib versus TNFi in low-risk patients.
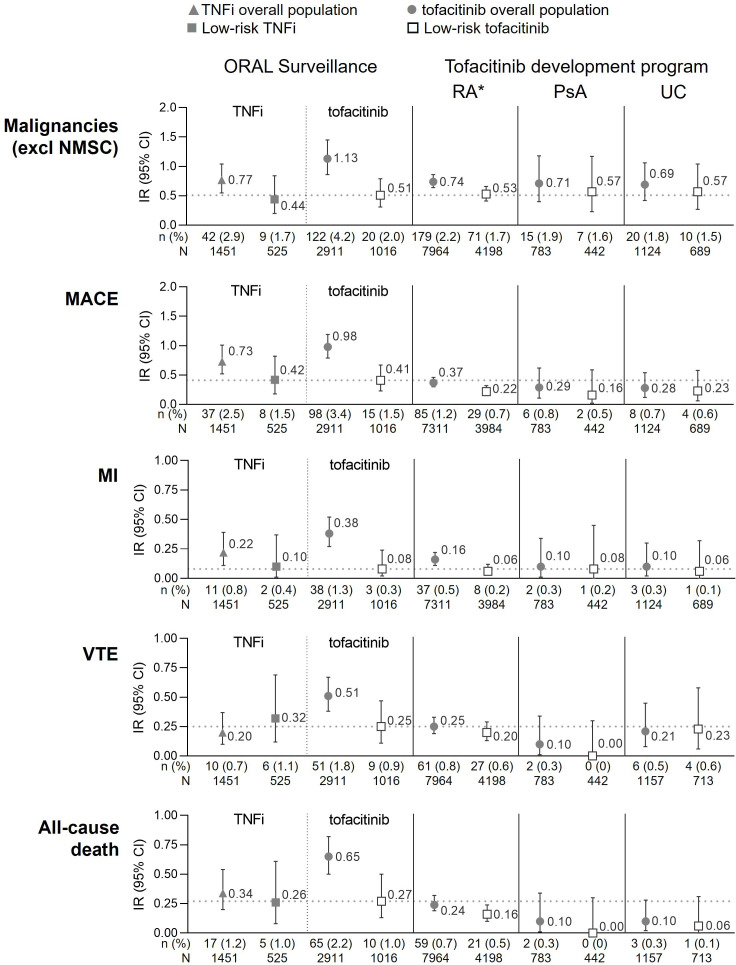
Absolute risk in low-risk patients in tofacitinib RA, PsA and UC clinical development programmes
ORAL Surveillance was a substantial RA study, in terms of number of patients and exposure time. However, it makes up only a third of the overall tofacitinib experience in RA. Specifically, 34.1% (1016/2911) of patients treated with tofacitinib in ORAL Surveillance were <65 years of age and never smokers, and this population had low absolute risk and no detectable excessive risk of malignancies (excluding NMSC), MACE, MI, VTE and all-cause death versus TNFi. To increase the precision of absolute low-risk estimates, we conducted additional analyses in the tofacitinib development programmes of RA, PsA and UC, collectively representing 25 437 patient years of tofacitinib exposure and safety observations extending up to 10.5 years.10 52.7% (4198/7964) of patients in the tofacitinib RA development programme were <65 years of age and never smokers, representing more than 4000 patients and 13 497 patient years of tofacitinib exposure in the low-risk group of interest (table 3). In the tofacitinib PsA and UC development programmes, 56.4% and 59.6% of patients were <65 years of age and never smokers.
Table 3.
Tofacitinib treatment exposure in high-risk and low-risk populations in ORAL Surveillance and RA, PsA, UC development programmes
| ORAL Surveillance (RA) | Tofacitinib development programme | |||
| RA* | PsA | UC | ||
| Overall population | ||||
| N | 2911 | 7964 | 783 | 1157 |
| Exposure (PY) | 10 922 | 23 497 | 2038 | 2814 |
| Follow-up; mean, max (years) | 3.8, 6.1 | 3.0, 10.5 | 2.6, 4.8 | 2.4, 7.8 |
| High-risk: ≥65 years or ever smoked | ||||
| N | 1895 | 3577 | 341 | 444 |
| % | 65.1% | 44.9% | 43.6% | 38.4% |
| Exposure (PY) | 6986 | 9961 | 837 | 1113 |
| Low-risk: <65 years and never smoked | ||||
| N | 1016 | 4198 | 442 | 713 |
| % | 34.9% | 52.7% | 56.4% | 61.6% |
| Exposure (PY) | 3937 | 13 168 | 1201 | 1702 |
PY was calculated from the first dose of tofacitinib to the last contact date in ORAL Surveillance, and from the first dose of tofacitinib to the last dose of tofacitinib for all other development programmes.
*Excluding ORAL Surveillance.
N, number of patients treated with tofacitinib; PsA, psoriatic arthritis; PY, patient-years; RA, rheumatoid arthritis; UC, ulcerative colitis.
Figure 4 shows absolute risk (ie, IRs (95% CIs)) of malignancies (excluding NMSC), MACE, MI, VTE and all-cause death in the overall population and in low-risk patients treated with tofacitinib in the overall RA, PsA and UC development programmes compared with absolute risk with TNFi and tofacitinib in ORAL Surveillance (RA controlled phase and high-risk patient data in online supplemental figure S3). Consistently, IRs for malignancies (excluding NMSC), MACE, MI, VTE and all-cause death in low-risk patients treated with tofacitinib in the RA development programme were similar to those observed in low-risk patients in ORAL Surveillance; however, the precision of the estimate was higher, that is, the 95% CIs were narrower. Similarly, IRs of malignancies (excluding NMSC), MACE, MI, VTE and all-cause death in the PsA and UC tofacitinib development programmes in overall populations and low-risk patients were similar to those observed in tofacitinib-treated RA low-risk patients in ORAL Surveillance and the RA development programme (figure 4).
Figure 4.
Risk of malignancies (excluding NMSC), MACE, MI, VTE and all-cause death in low-risk populations in ORAL Surveillance and tofacitinib clinical development programmes. Low-risk patients were <65 years of age and never smoker. Horizontal dotted line represents IR in low-risk patients treated with tofacitinib in ORAL Surveillance. IRs express the number of patients with first events per 100 PY. All data are for combined tofacitinib doses. *Excluding ORAL Surveillance. Data from ORAL Surveillance overall populations have previously been published and are included for reference; malignancies (excluding NMSC) and MACE (Ytterberg et al 1), MI (Charles-Schoeman et al 5). Also, previously published are data from the tofacitinib RA and PsA development programmes on MACE (Burmester et al 25) and VTE (Mease et al 26). IR, incidence rate; MACE, major adverse cardiovascular events; MI, myocardial infarction; N, number of evaluable patients; n, number of patients with events; NMSC, non-melanoma skin cancer; PsA, psoriatic arthritis; PY, patient-years; RA, rheumatoid arthritis; TNFi, tumour necrosis factor inhibitor; UC, ulcerative colitis; VTE, venous thromboembolism.
Discussion
Primary findings in ORAL Surveillance demonstrated a higher incidence of MACE and malignancies with tofacitinib versus TNFi.1 5 In this posthoc analysis, two clinically practical and easily identifiable subpopulations were found that demarcated tofacitinib-treated patients with increased risk of these events from patients with similar risk relative to TNFi-treated patients.
Given the design of ORAL Surveillance, enough events accrued to allow for posthoc identification of patients at higher risk with tofacitinib versus TNFi. Initial analyses identified age and smoking as potential independent risk factors, and more than 80% of tofacitinib-treated patients with MACE, MI, malignancies (excluding NMSC), VTE or all-cause death were accounted for by the combination of these two risk factors. Of note, ‘having ever smoked’ was found to largely correspond to substantial smoking history. Specifically, more than 90% of current or past smokers in ORAL Surveillance were long-time smokers with more than 10 years of smoking and a median smoking history over 30 years on study entry.
We found an exacerbation in risk with the combination of risk factors age 65 years or older and having ever smoked, and patients with at least one of these risk factors had a disproportionately larger risk increase with tofacitinib versus TNFi, while in patients without these two differentiating risk factors, we could not detect a risk difference for malignancies (excluding NMSC), MACE, MI, VTE or all-cause death between tofacitinib and TNFi.
The risk difference for long-latency events between tofacitinib and TNFi observed at the overall study population level emerged after approximately two years of follow-up in ORAL Surveillance. Since JAK inhibitors are used chronically, patients without risk factors could potentially be exposed for prolonged periods of time during which a small risk increase could become clinically relevant. Analyses of absolute and relative risk over time found that the excess risk with tofacitinib versus TNFi was confined to patients defined by the composite of age ≥65 years or ever smoking (‘high-risk’), and these differentiating risk factors accounted for the excess risk of malignancies (excluding NMSC), MACE, MI, VTE or all-cause death observed with tofacitinib versus TNFi. On the other hand, in patients who were younger than 65 and had never smoked (‘low-risk’), but who all had prevalent other CV risk factors per ORAL Surveillance eligibility criteria, we could not detect a difference between tofacitinib and TNFi even up to 6 years of follow-up which is longer than the median drug survival in RA.11 This observation was consistent across outcomes including malignancies (excluding NMSC), MACE, MI, VTE and all-cause death, and the magnitude of absolute risk in the low-risk group remained low over time and was similar to TNFi with large NNH.
The composite of age ≥65 years or ever smoker was strongly associated with absolute risk of malignancies (excluding NMSC), MACE, MI, VTE and all-cause death in ORAL Surveillance, and, as mentioned above, with treatment with tofacitinib, most events (>82%) occurred in the 65% of patients in this high-risk subgroup. There were accordingly few events in the low-risk group, patients <65 years of age that had never smoked, which made up 35% of the overall study population. Consequently, it can be argued that there is not sufficient precision in the absolute and relative risk estimates in this low-risk population in ORAL Surveillance. Even though ORAL Surveillance was a large and long study, it makes up only a third of the overall tofacitinib experience (excluding ORAL Surveillance) in RA and therefore, it was important to validate the absolute risk estimate in ORAL Surveillance within the larger tofacitinib RA clinical programme which extended up to 10 years and included more than 4000 patients in the low-risk group of interest. Importantly, we corroborated the absolute risk estimates from ORAL Surveillance and with higher precision, with data from the overall population and the low-risk population of the more extensive tofacitinib RA development programme. Moreover, the magnitude of the absolute risk in the low-risk group was low also in relation to published rates in randomised controlled trials and their LTEs in RA populations treated with TNFi and other biologics,12–18 and this finding was confirmed also in the other tofacitinib development programmes of PsA and UC. A limitation of these analyses is that the average follow-up time in the tofacitinib clinical development programmes (3.0, 2.6 and 2.4 years in the RA, PsA and UC programmes, respectively) was shorter than in ORAL Surveillance (3.8 years).
Recognising limitations associated with the posthoc nature of these results, multiple aspects were considered to improve robustness and confirm validity of findings. First, only data for the combined doses of tofacitinib (with larger number of patients and events vs per individual dose) were used for increased precision. In ORAL Surveillance, p values (0.002–0.173 for the outcomes) for the interactions between treatment groups and subgroups of high-risk/low-risk patients lent support to the presence of a differential treatment effect for the two subgroups of patients. Second, the analyses are based on risk factors (ie, older age and smoking) already identified as major risk factors of malignancies, MACE and VTE in the general and RA populations.3 19–24 Finally, we cannot, based on these posthoc analyses, rule out that there is an increased relative risk of safety outcomes with tofacitinib versus TNFi in the low-risk group. However, if such an increased risk is present, we show that the absolute risk is low, as indicated by low or no difference in IRs over time and high NNH. Moreover, we have recently reported on history of ASCVD as another differentiating risk factor.5 However, whereas the combination of age and smoking is capable of differentiating risk across major outcomes, history of ASCVD is specific for MACE. These are all factors that need to be considered in an individualised benefit/risk assessment.
The analyses presented herein had a particular focus on the identification of a high-risk and a low-risk population. Future investigations should aim at assessing whether the high-risk population can be further segmented into different relative risk levels.
In summary, ORAL Surveillance identified a high-risk and low-risk tofacitinib population with different relative risk vs TNFi. Higher risk versus TNFi was confined to a subgroup of patients defined by distinct, readily identifiable risk factors, age 65 years or older and long-time smoking (current or past), and these differentiating risk factors accounted for the excess risk observed with tofacitinib versus TNFi. In ‘low-risk’ patients who were younger than 65 and had never smoked, but who all had prevalent other CV risk factors per ORAL Surveillance inclusion criteria, there was no detectable risk increase vs TNFi with up to 6 years of follow-up in ORAL Surveillance and the magnitude of absolute risk remained low and was corroborated across tofacitinib programmes with up to 10 years of observations. It is acknowledged that the findings are posthoc, nevertheless, the results are clinically important and appear generalizable. These findings can guide individualised benefit/risk assessment and clinical decision-making on treatment with tofacitinib.
Acknowledgments
Select data in this manuscript were previously presented at ACR Convergence 2021.6 The authors would like to thank the patients, investigators and study teams involved in the study. The authors would like to thank Hernan Valdez for invaluable scientific discussions and guidance and Joseph Wu and Kenneth Kwok, employees and shareholders of Pfizer Inc, for their contribution to the statistical analyses.
Footnotes
Handling editor: Josef S Smolen
Contributors: LEK, SD, AY, CW, EN, IM, JR and BB conceived or designed the study and data analyses. CW analysed the data. All authors had access to the data, were involved in interpretation of data and reviewed and approved the manuscript’s content before submission. LEK accepts final responsibility for this work and controlled the decision to publish.
Funding: This study was sponsored by Pfizer Inc.
Competing interests: LEK has received fees for speaking and consultancy from Pfizer, AbbVie, Amgen, Galapagos, UCB, Gilead, Biogen, BMS, MSD, Novartis, Eli Lilly and Janssen pharmaceuticals. LEK has received IIT research grants from Novo, UCB, Eli Lilly, Novartis and AbbVie. SD reports consultancy fees from AbbVie, Alimentiv, Allergan, Amgen, Applied Molecular Transport, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Dr Falk Pharma, Eli Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, Morphic, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, Teladoc Health, TiGenix, UCB Inc., Vial, Vifor. SD reports lecture fees from AbbVie, Amgen, Ferring Pharmaceuticals Inc., Gilead, Janssen, Mylan, Pfizer, Takeda. AY, CW, EN, IM, JR and BB are employees and stockholders of Pfizer Inc.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. On request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-resultsformoreinformation.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All studies included in this manuscript were conducted in accordance with the Declaration of Helsinki, International Council on Harmonisation Guidelines for Good Clinical Practice and local country regulations and were approved by each centre’s Institutional Review Board or Independent Ethics Committee. Participants gave informed consent to participate in the study before taking part.
References
- 1. Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386:316–26. 10.1056/NEJMoa2109927 [DOI] [PubMed] [Google Scholar]
- 2. FDA . Approval letter - xeljanz (tofacitinib); 2012. Available: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203214Orig1s000Approv.pdf
- 3. Koene RJ, Prizment AE, Blaes A, et al. Shared risk factors in cardiovascular disease and cancer. Circulation 2016;133:1104–14. 10.1161/CIRCULATIONAHA.115.020406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lau ES, Paniagua SM, Liu E, et al. Cardiovascular risk factors are associated with future cancer. JACC CardioOncol 2021;3:48–58. 10.1016/j.jaccao.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charles-Schoeman C, Buch MH, Dougados M, et al. Risk of major adverse cardiovascular events with tofacitinib versus tumour necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: a post hoc analysis from oral surveillance. Ann Rheum Dis 2023;82:119–29. 10.1136/ard-2022-222259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charles-Schoeman C, Buch M, Dougados M, et al. Risk factors for major adverse cardiovascular events in patients aged ≥ 50 years with RA and ≥ 1 additional cardiovascular risk factor: results from a phase 3b/4 randomized safety study of tofacitinib vs TNF inhibitors [abstract]. Arthritis Rheumatol 2021;73 (suppl 9). [Google Scholar]
- 7. Curtis JR, Yamaoka K, Chen Y-H, et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: results from the open-label, randomised controlled oral surveillance trial. Ann Rheum Dis 2023;82:331–43. 10.1136/ard-2022-222543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. European medicines agency . ICH topic E 7. studies in support of special populations: geriatrics; Available: https://www.ema.europa.eu/en/ich-e7-studies-support-special-populations-geriatrics-scientific-guideline#current-effective-version-section1994 [Accessed 27 Mar 2021].
- 9. Sun X, Briel M, Walter SD, et al. Is a subgroup effect believable? updating criteria to evaluate the credibility of subgroup analyses. BMJ 2010;340:bmj.c117. 10.1136/bmj.c117 [DOI] [PubMed] [Google Scholar]
- 10. Burmester GR, Nash P, Sands BE, et al. Adverse events of special interest in clinical trials of rheumatoid arthritis, psoriatic arthritis, ulcerative colitis and psoriasis with 37 066 patient-years of tofacitinib exposure. RMD Open 2021;7:e001595. 10.1136/rmdopen-2021-001595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pope JE, Keystone E, Jamal S, et al. Persistence of tofacitinib in the treatment of rheumatoid arthritis in open-label, long-term extension studies up to 9.5 years. ACR Open Rheumatol 2019;1:73–82. 10.1002/acr2.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burmester GR, Gordon KB, Rosenbaum JT, et al. Long-term safety of adalimumab in 29,967 adult patients from global clinical trials across multiple indications: an updated analysis. Adv Ther 2020;37:364–80. 10.1007/s12325-019-01145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curtis JR, Mariette X, Gaujoux-Viala C, et al. Long-term safety of certolizumab pegol in rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis and Crohn’s disease: a pooled analysis of 11 317 patients across clinical trials. RMD Open 2019;5:e000942. 10.1136/rmdopen-2019-000942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schiff MH, Kremer JM, Jahreis A, et al. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther 2011;13:R141. 10.1186/ar3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rubbert-Roth A, Sebba A, Brockwell L, et al. Malignancy rates in patients with rheumatoid arthritis treated with tocilizumab. RMD Open 2016;2:e000213. 10.1136/rmdopen-2015-000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinblatt ME, Moreland LW, Westhovens R, et al. Safety of abatacept administered intravenously in treatment of rheumatoid arthritis: integrated analyses of up to 8 years of treatment from the abatacept clinical trial program. J Rheumatol 2013;40:787–97. 10.3899/jrheum.120906 [DOI] [PubMed] [Google Scholar]
- 17. Emery P, Furst DE, Kirchner P, et al. Risk of malignancies in patients with rheumatoid arthritis treated with rituximab: analyses of global postmarketing safety data and long-term clinical trial data. Rheumatol Ther 2020;7:121–31. 10.1007/s40744-019-00183-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Vollenhoven RF, Emery P, Bingham CO, et al. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann Rheum Dis 2013;72:1496–502. 10.1136/annrheumdis-2012-201956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Cock D, Hyrich K. Malignancy and rheumatoid arthritis: epidemiology, risk factors and management. Best Pract Res Clin Rheumatol 2018;32:869–86. 10.1016/j.berh.2019.03.011 [DOI] [PubMed] [Google Scholar]
- 20. Gregson J, Kaptoge S, Bolton T, et al. Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol 2019;4:163–73. 10.1001/jamacardio.2018.4537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Molander V, Bower H, Frisell T, et al. Risk of venous thromboembolism in rheumatoid arthritis, and its association with disease activity: a nationwide cohort study from Sweden. Ann Rheum Dis 2021;80:169–75. 10.1136/annrheumdis-2020-218419 [DOI] [PubMed] [Google Scholar]
- 22. Omair MA, Alkhelb SA, Ezzat SE, et al. Venous thromboembolism in rheumatoid arthritis: the added effect of disease activity to traditional risk factors. Open Access Rheumatol 2022;14:231–42. 10.2147/OARRR.S284757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sepriano A, Kerschbaumer A, Bergstra SA, et al. Safety of synthetic and biological dmards: a systematic literature review Informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2023;82:107–18. 10.1136/ard-2022-223357 [DOI] [PubMed] [Google Scholar]
- 24. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17–28. 10.1136/annrheumdis-2016-209775 [DOI] [PubMed] [Google Scholar]
- 25. Burmester GR, Curtis JR, Yun H, et al. An integrated analysis of the safety of tofacitinib in psoriatic arthritis across phase III and long-term extension studies with comparison to real-world observational data. Drug Saf 2020;43:379–92. 10.1007/s40264-020-00904-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mease P, Charles-Schoeman C, Cohen S, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis 2020;79:1400–13. 10.1136/annrheumdis-2019-216761 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2022-223715supp001.pdf (2MB, pdf)
Data Availability Statement
Data are available on reasonable request. On request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-resultsformoreinformation.