Abstract
Aims
To report the baseline intraocular pressure (IOP) characteristics and its diurnal fluctuation in the Laser in Glaucoma and Ocular Hypertension China cohort.
Methods
622 primary open-angle glaucoma (POAG) patients and 149 ocular hypertension (OHT) patients were recruited at Zhongshan Ophthalmic Center from 2015 to 2019. Standardised ocular examinations were performed including IOP measurement using the Goldmann applanation tonometer. Daytime phasing IOP was recorded at 8:00, 10:00, 11:30, 14:30, 17:00 hour.
Results
The mean baseline IOP was 20.2 mm Hg for POAG patients and 24.4 mm Hg for OHT. Multiple regression analysis revealed that thicker central corneal thickness (CCT) was correlated with higher IOP in both POAG and OHT. Male gender and younger age were correlated with higher IOP only for POAG. As for diurnal IOP fluctuation, mean IOP fluctuation was 3.4 mm Hg in POAG eyes and 4.4 mm Hg in OHT. The peak and trough IOP occurred at 8:00 and 14:30 hour in both POAG and OHT eyes.
Conclusions
Younger age, male gender and thicker CCT are correlated to higher IOP in POAG patients while only thicker CCT is related to higher IOP in OHT patients. Peak IOP appears mostly at early morning or late afternoon and trough value occurs mostly at early afternoon.
Keywords: glaucoma, intraocular pressure
Introduction
Glaucoma, a group of disease characterised by optic nerve injury and progressive visual impairment, is regarded as one of the world’s leading causes of irreversible blindness. Although it is a multifactorial disease, intraocular pressure (IOP) remains the only verified modifiable risk factor for the condition. Evidence suggests that high IOP1–3 and IOP fluctuation4 are both potential risk factors for the onset and progression of glaucomatous optic neuropathy. Thus, it is of great clinical interest to fully understand characteristics of IOP in ocular hypertension (OHT) and primary open angle glaucoma (POAG) patients for glaucoma prevention and prognosis.
Many factors, including age,5–8 central cornea thickness (CCT)9 10 and spherical equivalent11 12 have been reported to be associated with IOP. However, all these results are inconsistent even in similar ethnicity and most of the studies are normal population based. Relatively little information on IOP and its related factors have been available in OHT or POAG patients.13 14 Since the ocular biometry can be different between normal and glaucoma eyes,15 the risk factors for normal subjects may or may not influence the IOP of POAG or OHT patients.14 On the other hand, it is acknowledged that IOP is not fixed, but varies during the 24-hour cycle.16 Therefore, monitoring a patient’s IOP during the daytime or over a 24-hour period known as phasing has obvious pragmatic benefits in the management of glaucoma.
The Laser in Glaucoma and Ocular Hypertension (LiGHT) China Trial is a single centre, prospective, randomised controlled trial, aiming to compare eye drops vs selective laser trabeculoplasty (SLT) as the first-line treatment for newly diagnosed patients with POAG or OHT. The purpose of this paper is to report the baseline IOP characteristics and its diurnal fluctuation in the LiGHT China cohort.
Methods
Subjects
Eligible patients were recruited at the Zhongshan Ophthalmic Centre from March 2015 to January 2019. A total of 771 patients aged 18 years and above who met the eligibility criteria were enrolled in the LiGHT China. The details about the LiGHT China design have been published previously.17 Briefly, patients with newly diagnosed, untreated POAG in one or both eyes (including normal tension glaucoma (NTG)) or OHT qualifying for treatment according to National Institute for Health and Care Excellence guidelines18 were enrolled. Exclusion criteria included contraindications to SLT, unable to accept randomisation, having visually significant cataract or were having treatment for another ophthalmic condition, having any history of treatment for POAG or OHT or previous intraocular surgery. Written informed consent was also obtained from all study participants.
Baseline IOP measurement
The key points of the protocol have been attached as an online supplemental appendix.17 The series of examinations started with a standardised questionnaire that consisted of questions on the participants’ personal information, general health condition, past history, family history, lifestyle and so forth. Complete ophthalmological examinations including Goldmann applanation tonometry (GAT), Schiotz tonometer, slit-lamp examination, gonioscopy, automated visual field test and Heidelberg Retinal Tomograph disc imaging were performed. The refractive error was calculated as the spherical equivalent measured with an autorefractor (SE=spherical +1/2 cylindrical power). The CCT was measured with type A ultrasound. GAT was performed by technicians who had been trained followed the protocol and passed the consistency assessment before recruitment. The average of two readings was recorded and more readings were required if the difference between the first two readings is >1 mm Hg. Calibration of tonometry was checked on a weekly basis. IOP phasing was not included in the protocol of the Trial but was an alternative diagnostic item. Daytime phasing IOP was recorded using GAT at five time-intervals during the day (8:00, 10:00, 11:30, 14:30, 17:00 hour). All examinations were based on standard operating procedures and performed by examiners blinded to trial.
bjophthalmol-2021-320128supp001.pdf (614.6KB, pdf)
Statistical analysis
Definitions of the terms used to describe fluctuation are shown below: (1) Peak IOP: highest IOP recorded in the stated time period; (2) Trough IOP: lowest IOP recorded in the stated time period; (3) IOP fluctuation: Peak IOP minus trough measured in the stated time period and (4) Mean amplitude of IOP excursions (MAPE): MAPE was calculated as the arithmetic mean value of the relevant IOP fluctuations meeting this criterion.19 All categorical data were represented by frequency with percentage and it was analysed by χ2, Fisher’s exact test. Continuous data were presented by mean with SD and tested by Student’s t-test. Pearson correlation analysis and multivariate regression analysis were used to analyse the association with IOP. All p values were two sided and were considered statistically significant when p<0.05. Statistical analysis was carried out using a commercially available statistical software package (SPSS for Windows, V.26.0).
Results
A total of 1105 POAG eyes and 271 OHT eyes of 771 participants (both eyes were eligible in 605 subjects, only the right eye was eligible in 73 subjects, and only the left eye in 93 subjects) were enrolled in the LiGHT China. Of the 1376 eyes identified, 945 POAG eyes and 264 OHT eyes accepted daytime phasing IOP measurements.
The mean age of the POAG patients was 49.76±17.19 years, and 364 (58.5%) were male. For OHT patients, the mean age was 38.81±14.69 years, and 72 (48.3%) were male. Mean IOP was 20.4±5.4 mm Hg for eyes diagnosed with POAG and 24.4±3.2 mm Hg for OHT eyes. OHT eyes had higher IOP and thicker CCT than POAG eyes with a statistical significance (p<0.001) (table 1). Notably, in patients with both eyes eligible, right eyes were more myopic than left eyes in both diagnostic group (both p<0.05). Also, in POAG group, IOP of left eyes was higher than that of right eyes with a statistical significance (p=0.003) (table 2).
Table 1.
Baseline characteristics of 771 subjects in the LiGHT China
| Parameters | OHT (n=149) | POAG (n=622) |
| Age, years, mean (SD) | 38.81±14.69 | 49.76±17.19 |
| Gender, male (%) | 72 (48.3) | 364 (58.5) |
| IOP, mm Hg, mean (SD)* | 24.4±3.2 | 20.4±5.4 |
| SE, dioptres, mean (SD)* | −3.10±3.52 | −2.72±3.84 |
| CCT, um, mean (SD)* | 544.64±29.13 | 536.08±33.54 |
*Data from 1376 eligible eye.
CCT, central cornea thickness; IOP, intraocular pressure; LiGHT, Laser in Glaucoma and Ocular Hypertension; OHT, ocular hypertension; POAG, primary open angle glaucoma; SE, spherical equivalent.
Table 2.
Baseline eye characteristics of patients eligible for both eyes
| Mean±SD | Right eyes | Left eyes | P value | |
| POAG (n=483) | IOP, mm Hg | 20.1±5.3 | 20.5±5.4 | 0.003* |
| SE, dioptes | −2.82±3.93 | −2.67±3.82 | 0.019* | |
| CCT, µm | 536.13±32.49 | 536.87±33.43 | 0.063 | |
| OHT (n=122) | IOP, mm Hg | 24.4±3.4 | 24.4±3.1 | 0.807 |
| SE, dioptres | −3.33±3.51 | −3.10±3.47 | 0.018* | |
| CCT, µm | 545.33±29.46 | 545.89±29.76 | 0.330 |
*P<0.05 level.
CCT, central cornea thickness; IOP, intraocular pressure; OHT, ocular hypertension; POAG, primary open-angle glaucoma; SE, spherical equivalent.
Pearson correlation analysis demonstrated that higher IOP was significantly correlated to thicker CCT and younger age in OHT group (all p<0.05). For POAG patients, younger age, male gender, thicker CCT, lower SE were all correlated with increasing IOP with a statistical significance (all p<0.01) (table 3). With regard to the results of multiple regression analysis, in both groups, higher IOP was still significantly correlated to thicker CCT (all p<0.05), but not with spherical equivalence. Additionally, a statistic significant correlation was noted between increasing IOP and male gender, younger age in POAG group (all p<0.01) while this correlation was not significant in OHT group (table 3).
Table 3.
Correlation coefficients and multivariate regression analysis between IOP and other variables
| Variables | Correlation coefficients | Multivariate regression analysis | ||||||
| OHT | POAG | OHT | POAG | |||||
| Pearson correlation | P value | Pearson correlation | P value | β | P value | β | P value | |
| Age (years) | −0.16 | 0.045* | −0.23 | <0.001* | −0.033 | 0.063 | −0.066 | <0.001* |
| Central cornea thickness (μm) | 0.20 | 0.012* | 0.16 | <0.001* | 0.019 | 0.022* | 0.019 | 0.002* |
| Spherical equivalent (dioptres) | −0.013 | 0.873 | −0.012 | 0.003* | 0.049 | 0.501 | 0.047 | 0.472 |
| Gender, female/male†‡ | — | 0.052 | — | <0.001* | −0.707 | 0.137 | −1.449 | 0.001* |
*P<0.05 level.
†Student’s t-test was used to compare the IOP of different gender.
‡Coding for dummy variables: male=0, female=1.
IOP, intraocular pressure; OHT, ocular hypertension; POAG, primary open-angle glaucoma.
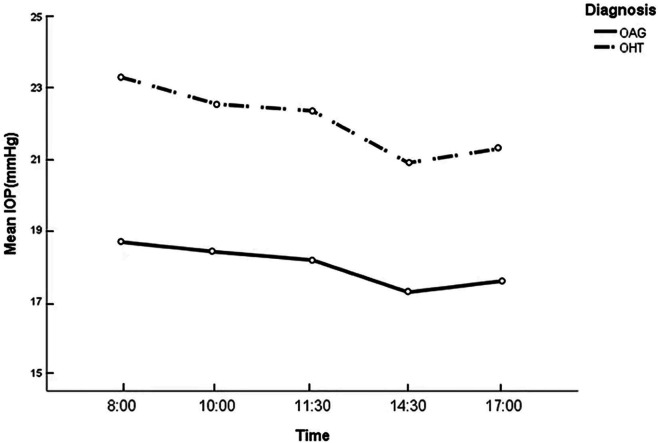
As for the diurnal variation in IOP, the highest IOP (POAG: 18.7±5.1 mm Hg, OHT: 23.3±3.3 mm Hg) occurred at 8:00 hour and gradually decreased during the day to reach its lowest value (POAG: 17.3±4.5 mm Hg, OHT: 20.9±2.9 mm Hg) at 14:30 hour (figure 1). The vast majority of eyes had their peak IOP recorded at 8:00 hour (POAG: 51.3%, OHT: 56.4%) and the lowest IOP values mostly occurred at 14:30 hour in both diagnostic groups (POAG: 31.7%, OHT: 42.8%). Additionally, on average, OHT eyes showed larger IOP fluctuation than POAG eyes in the daytime phasing curve (p<0.001). OHT eyes had higher level of MAPE than POAG eyes in the daytime phasing (p<0.001). In POAG group, there was no significant difference among different severities sub-groups with mean IOP (p=0.631), daytime peak IOP (p=0.476), daytime trough IOP (p=0.769), IOP fluctuation (p=0.425) or MAPE (p=0.159) (table 4).
Figure 1.
Diurnal variation in intraocular pressure in POAG and OHT eyes. IOP intraocular pressure; OHT, ocular hypertension; POAG, primary open-angle glaucoma.
Table 4.
Daytime phasing IOP measurements of included eyes
| Mean IOP, mm Hg (mean±SD) | Peak IOP, mm Hg (mean±SD) | Trough IOP, mm Hg (mean±SD) | IOP fluctuation, mm Hg (mean±SD) | MAPE, mm Hg (mean±SD) | |
| POAG (n=945 eyes) | 18.0±4.7 | 19.8±5.3 | 16.4±4.2 | 3.4±2.2 | 2.4±1.7 |
| Mild POAG (n=598 eyes) | 18.1±4.6 | 20.0±6.5 | 16.4±4.1 | 3.6±4.5 | 2.4±1.7 |
| Moderate POAG (n=251 eyes) | 18.2±5.0 | 20.0±5.6 | 16.5±4.5 | 3.5±2.3 | 2.4±1.7 |
| Severe POAG (n=96 eyes) | 17.7±4.6 | 19.2±5.0 | 16.1±4.2 | 3.1±1.9 | 2.1±1.3 |
| NTG (n=540 eyes) | 15.1±2.5 | 16.7±4.9 | 13.8±2.3 | 2.9±4.4 | 1.9±1.2 |
| OHT (n=264 eyes) | 22.1±2.7 | 24.4±3.2 | 20.0±2.4 | 4.4±2.4 | 2.8±1.6 |
The severity of POAG is classified according to MD value of baseline visual field (mild POAG: MD value ≥6; moderate POAG: −6>MD value ≥12; severe POAG: MD value≤12).
IOP, intraocular pressure; MAPE, mean amplitude of IOP excursion; NTG, normal tension glaucoma; OHT, ocular hypertension; POAG, primary open-angle glaucoma.
A total of 1105 eyes of 622 OAG patients were enrolled in LiGHT China Trial, among which 620 eyes in 317 patients were recorded with baseline IOP lower than 21 mm Hg (51.0% in OAG). There is no detailed description or inclusion criteria of NTG in the protocol of the trial. We named them here NTG patients. The mean age of the enrolled NTG patients was 52.69±17.16 years old, including 155 males and 162 females. The mean baseline IOP of NTG eyes was 16.7±2.5 mm Hg (Mild OAG:16.8±2.4 mm Hg, Mod OAG:16.8±2.7 mm Hg, Severe OAG: 16.2±2.7 mm Hg). A total of 540 NTG eyes accepted daytime phasing IOP measurements. Similarly, the majority of eyes had their peak IOP recorded at 8:00 hour (52.6%) and the trough IOP values mostly occurred at 14:30 hour (29.6%) (table 4).
Discussion
IOP is an important indicator in the development and progression of glaucoma, thus fully understanding risk factors of elevated IOP and IOP fluctuation is of great significance. To our knowledge, this study is the first to report the baseline IOP, its associated factors and diurnal fluctuation of the POAG and OHT patients from the LiGHT China Trial.
The mean baseline IOP in OHT patients was 24.4±3.2 mm Hg, similar to 24.9±2.7 mm Hg reported in the Ocular Hypertension Treatment Study3 and other studies that included OHT patients.12 Average baseline IOP of POAG patients was 20.2±5.4 mm Hg, which was also similar to 20.7±4.1 mm Hg in the Early Manifest Glaucoma Trial20 and other studies.21 OHT eyes had significant higher baseline IOP and thicker CCT than POAG eyes in our cohort. As evidence, the results in our study have confirmed a positive relationship between IOP and CCT, which has been documented consistently in the literature.6 9 10 Our data revealed that an increase of 10 µm in CCT was associated with an increase of 0.21 mm Hg in OHT and 0.25 mm Hg in POAG, which close to a 0.23 mm Hg elevation reported in the Liwan Eye Study in China.10
The role of age and its relationship with IOP still remains controversial. Numerous studies have discovered a positive association between older age and higher IOP level.5 22 However, in our study, multivariate analysis showed a significant negative correlation between age and IOP in POAG patients, consistent with the results of studies conducted in Asia populations.6 7 9 Ageing is relevant to reduced production of aqueous humor,23 which may be the reason for the reduction of IOP. But conversely, age-related structural changes in the trabecular meshwork can also increase the resistance to aqueous humour outflow and lead to elevated IOP.24 Briefly, different changes in aqueous humour circulation may have caused those two opposite results. As for the observed gender difference in IOP of POAG patients, it was hard to explain, probably because of hormonal difference.25
Refractive error is postulated to influence IOP by altering the shape of the eye (axial elongation and scleral thinning) and subjecting it to greater stress as the spherical equivalent decreases.11 12 26 Accordingly, some studies have found a negative association between IOP and SE,6 11 however, this association was not significant after controlling for age.
As a physical phenomenon, IOP is known to be dynamic with short-term and long-term fluctuations. In our study, multiple IOP measurements during office time demonstrated that IOP reached a peak early in the morning and decreased steadily during the day, which was similar with other studies.27 28 An average of 3.4 mm Hg IOP fluctuation in POAG eyes and 4.4 mm Hg in OHT eyes were reported in daytime phasing, and IOP fluctuation in OHT eyes was larger than that in OHT eyes, which were both within ‘normal’ range.16 The MAPE of OHT eyes was also higher than that of POAG eyes. Besides, the mean MAPE of POAG eyes in our study was 4.18 mm Hg and similar to the mean MAPE reported in another study (4.16 mm Hg).19 The daytime phasing demonstrated significant larger variation in IOP of OHT eyes than POAG eyes, possibly supporting the findings that IOP fluctuation might not be an independent risk factor for conversion from OHT to glaucoma.29 30
It is also interesting to mention that in both POAG and OHT patients the right eyes were more myopic than left eyes, which confirmed the findings that right eyes have longer axial length than left eyes.31 However, the interocular IOP difference noted only in POAG patients was difficult for us to explain.
Potential limitations of our study should also be discussed. First, 24 hours IOP phasing with large sample size should be needed to get more accurate results, which will be shown in our other studies. Besides, risk factors in our study are still limited. Some parameters, such as ambulatory blood pressure9 11 and axial length,32 proved to be potential predictors of IOP were not included in our study.
In conclusion, for POAG patients, higher IOP is correlated to younger age, male gender, thicker CCT, whereas in patients with OHT, only thicker CCT seems to be risk factors of higher IOP. IOP of POAG or OHT eyes varies and reaches the peak value mostly at early morning or late afternoon and the trough value mostly at early afternoon.
Acknowledgments
We are grateful to Xiaoyu Cai, Liming Chen, Xin Yi, Yuning Zhang, Mingjie Deng, Zhikun Ouyang, Xiaoxiao Cai, Zongyi Zhan, Shitong Huang, Yunzhi Xu for their contribution to the collection of baseline data. Thanks to Amanda Davis for her valuable help during preparation and recruitment, and Emily Dowse for her efforts on data quality.
Footnotes
Twitter: @gusgazzard
YY and XZ contributed equally.
Contributors: YF and XZ performed the analysis of the data, and contributed equally in writing the manuscript. ZC and YW were contributors in data collection and quality control. QY and YF contributed to the revision. YF and NN contributed to data quality control. GG and MY were the leader of the study group, designed and in charge of the study, and were also responsible for quality control of the trial. MY was responsible for the overall content as guarantor. All authors read and approved the final manuscript.
Funding: This work was supported by the Moorfields Eye Charity (grant number 166186); the British Council for Prevention Of Blindness (grant number 165223) and the Natural Science Foundation of Guangdong Province (grant number 2021A1515010604).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
LiGHT China Trial Study Group:
Mingkai Lin, Xing Liu, Xiulan Zhang, Jian Ge, Jingjing Huang, Yunlan Ling, Yimin Zhong, Paul Foster, Yuzhen Jiang, Yangfan Yang, Jiangang Xu, Chengguo Zuo, Hui Xiao, Yixiang Huang, Yuantao Hao, Yanmei Fan, Pingping Liu, Mingjie Deng, Yiming Ye, Zidong Chen, Zhikun Ouyang, Xiaoxiao Cai, Qingshu Ge, Zongyi Zhan, Shitong Huang, Yunzhen Wang, and Yunzhi Xu
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study design followed the tenets of the Declaration of Helsinki and had been approved by ethical committee of Zhongshan Ophthalmic Center, Sun Yat-sen University (reference number 2014MEKY054).
References
- 1. Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 2003;121:48–56. 10.1001/archopht.121.1.48 [DOI] [PubMed] [Google Scholar]
- 2. Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology 2004;111:1627–35. 10.1016/j.ophtha.2004.02.017 [DOI] [PubMed] [Google Scholar]
- 3. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:701–13. 10.1001/archopht.120.6.701 [DOI] [PubMed] [Google Scholar]
- 4. Asrani S, Zeimer R, Wilensky J, et al. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma 2000;9:134–42. 10.1097/00061198-200004000-00002 [DOI] [PubMed] [Google Scholar]
- 5. Bonomi L, Marchini G, Marraffa M, et al. Prevalence of glaucoma and intraocular pressure distribution in a defined population. The Egna-Neumarkt Study. Ophthalmology 1998;105:209–15. 10.1016/S0161-6420(98)92665-3 [DOI] [PubMed] [Google Scholar]
- 6. Kawase K, Tomidokoro A, Araie M, et al. Ocular and systemic factors related to intraocular pressure in Japanese adults: the Tajimi study. Br J Ophthalmol 2008;92:1175–9. 10.1136/bjo.2007.128819 [DOI] [PubMed] [Google Scholar]
- 7. Chua J, Chee ML, Chin CWL, et al. Inter-Relationship between ageing, body mass index, diabetes, systemic blood pressure and intraocular pressure in Asians: 6-year longitudinal study. Br J Ophthalmol 2019;103:196–202. 10.1136/bjophthalmol-2018-311897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Han X, Niu Y, Guo X, et al. Age-Related changes of intraocular pressure in elderly people in southern China: Lingtou eye cohort study. PLoS One 2016;11:e0151766. 10.1371/journal.pone.0151766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tomoyose E, Higa A, Sakai H, et al. Intraocular pressure and related systemic and ocular biometric factors in a population-based study in Japan: the Kumejima study. Am J Ophthalmol 2010;150:279–86. 10.1016/j.ajo.2010.03.009 [DOI] [PubMed] [Google Scholar]
- 10. Wang D, Huang W, Li Y, et al. Intraocular pressure, central corneal thickness, and glaucoma in chinese adults: the liwan eye study. Am J Ophthalmol 2011;152:454–62. 10.1016/j.ajo.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 11. Han X, Yang T, Zhang J, et al. Longitudinal changes in intraocular pressure and association with systemic factors and refractive error: Lingtou Eye Cohort Study. BMJ Open 2018;8:e019416. 10.1136/bmjopen-2017-019416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma D, Wei S, Sun Y, et al. Distribution of IOP and its relationship with refractive error and other factors: the Anyang University Students Eye Study. Int J Ophthalmol 2021;14:554–9. 10.18240/ijo.2021.04.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahn MW, Lee JW, Shin JH, et al. Relationship between intraocular pressure and parameters of obesity in ocular hypertension. Int J Ophthalmol 2020;13:794–800. 10.18240/ijo.2020.05.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weih LM, Mukesh BN, McCarty CA, et al. Association of demographic, familial, medical, and ocular factors with intraocular pressure. Arch Ophthalmol 2001;119:875–80. 10.1001/archopht.119.6.875 [DOI] [PubMed] [Google Scholar]
- 15. Vasile P, Valeria C, Speranţa S, et al. Sympathetic context of the disease - a new era in glaucoma management. Rom J Ophthalmol 2021;65:15–19. 10.22336/rjo.2021.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quaranta L, Katsanos A, Russo A, et al. 24-hour intraocular pressure and ocular perfusion pressure in glaucoma. Surv Ophthalmol 2013;58:26–41. 10.1016/j.survophthal.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 17. Yang Y, Jiang Y, Huang S, et al. Laser in Glaucoma and Ocular Hypertension Trial (LIGHT) in China A Randomized Controlled Trial: Design and Baseline Characteristics. Am J Ophthalmol 2021;S0002-9394:00219–1. [DOI] [PubMed] [Google Scholar]
- 18. National Institute for Health and Care Excellence, . NICE L. NICE: Guidance on Glaucoma: Diagnosis and management of chronic open angle glaucoma and other ocular hypertension [Internet]. DoH, 2010. Available: www.nice.org.uk/guidance/CG85fullguideline [PubMed]
- 19. Zhai R, Cheng J, Xu H, et al. Mean amplitude of intraocular pressure excursions: a new assessment parameter for 24-h pressure fluctuations in glaucoma patients. Eye 2021;35:326–33. 10.1038/s41433-020-0845-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leske MC, Heijl A, Hyman L, et al. Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology 1999;106:2144–53. 10.1016/s0161-6420(99)90497-9 [DOI] [PubMed] [Google Scholar]
- 21. Lascaratos G, Garway-Heath DF, Burton R, et al. The United Kingdom Glaucoma Treatment Study: a multicenter, randomized, double-masked, placebo-controlled trial: baseline characteristics. Ophthalmology 2013;120:2540–5. 10.1016/j.ophtha.2013.07.054 [DOI] [PubMed] [Google Scholar]
- 22. Rochtchina E, Mitchell P, Wang JJ. Relationship between age and intraocular pressure: the Blue Mountains Eye Study. Clin Exp Ophthalmol 2002;30:173–5. 10.1046/j.1442-9071.2002.00519.x [DOI] [PubMed] [Google Scholar]
- 23. Brubaker RF, Nagataki S, Townsend DJ, et al. The effect of age on aqueous humor formation in man. Ophthalmology 1981;88:283–8. 10.1016/S0161-6420(81)35037-4 [DOI] [PubMed] [Google Scholar]
- 24. Liu B, McNally S, Kilpatrick JI, et al. Aging and ocular tissue stiffness in glaucoma. Surv Ophthalmol 2018;63:56–74. 10.1016/j.survophthal.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 25. Feola AJ, Sherwood JM, Pardue MT, et al. Age and Menopause Effects on Ocular Compliance and Aqueous Outflow. Invest Ophthalmol Vis Sci 2020;61:16. 10.1167/iovs.61.5.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmid KL, Li RWH, Edwards MH, et al. The expandability of the eye in childhood myopia. Curr Eye Res 2003;26:65–71. 10.1076/ceyr.26.2.65.14513 [DOI] [PubMed] [Google Scholar]
- 27. David R, Zangwill L, Briscoe D, et al. Diurnal intraocular pressure variations: an analysis of 690 diurnal curves. Br J Ophthalmol 1992;76:280–3. 10.1136/bjo.76.5.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gautam N, Kaur S, Kaushik S, et al. Postural and diurnal fluctuations in intraocular pressure across the spectrum of glaucoma. Br J Ophthalmol 2016;100:537–41. 10.1136/bjophthalmol-2015-306861 [DOI] [PubMed] [Google Scholar]
- 29. Medeiros FA, Weinreb RN, Zangwill LM, et al. Long-term intraocular pressure fluctuations and risk of conversion from ocular hypertension to glaucoma. Ophthalmology 2008;115:934–40. 10.1016/j.ophtha.2007.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hopf S, Schwantuschke D, Pfeiffer N, et al. The impact of intraocular pressure fluctuations and other factors on conversion of ocular hypertension to primary open-angle glaucoma. Int Ophthalmol 2020;40:1403–10. 10.1007/s10792-020-01306-7 [DOI] [PubMed] [Google Scholar]
- 31. Mahroo OA, Williams C, Hysi PG, et al. Interocular asymmetries in axial length and refractive error in 4 cohorts. Ophthalmology 2015;122:648–9. 10.1016/j.ophtha.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 32. Bikbov MM, Kazakbaeva GM, Zainullin RM, et al. Intraocular Pressure and Its Associations in a Russian Population: The Ural Eye and Medical Study. Am J Ophthalmol 2019;204:130–9. 10.1016/j.ajo.2019.02.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjophthalmol-2021-320128supp001.pdf (614.6KB, pdf)
Data Availability Statement
Data are available on reasonable request.