Abstract
Objectives
To evaluate incident cardiovascular outcomes and imaging phenotypes in UK Biobank participants with previous cancer.
Methods
Cancer and cardiovascular disease (CVD) diagnoses were ascertained using health record linkage. Participants with cancer history (breast, lung, prostate, colorectal, uterus, haematological) were propensity matched on vascular risk factors to non-cancer controls. Competing risk regression was used to calculate subdistribution HRs (SHRs) for associations of cancer history with incident CVD (ischaemic heart disease (IHD), non-ischaemic cardiomyopathy (NICM), heart failure (HF), atrial fibrillation/flutter, stroke, pericarditis, venous thromboembolism (VTE)) and mortality outcomes (any CVD, IHD, HF/NICM, stroke, hypertensive disease) over 11.8±1.7 years of prospective follow-up. Linear regression was used to assess associations of cancer history with left ventricular (LV) and left atrial metrics.
Results
We studied 18 714 participants (67% women, age: 62 (IQR: 57–66) years, 97% white ethnicities) with cancer history, including 1354 individuals with cardiovascular magnetic resonance. Participants with cancer had high burden of vascular risk factors and prevalent CVDs. Haematological cancer was associated with increased risk of all incident CVDs considered (SHRs: 1.92–3.56), larger chamber volumes, lower ejection fractions, and poorer LV strain. Breast cancer was associated with increased risk of selected CVDs (NICM, HF, pericarditis and VTE; SHRs: 1.34–2.03), HF/NICM death, hypertensive disease death, lower LV ejection fraction, and lower LV global function index. Lung cancer was associated with increased risk of pericarditis, HF, and CVD death. Prostate cancer was linked to increased VTE risk.
Conclusions
Cancer history is linked to increased risk of incident CVDs and adverse cardiac remodelling independent of shared vascular risk factors.
Keywords: epidemiology, magnetic resonance imaging, epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Few studies have reported associations of past cancer with incident cardiovascular outcomes in large population-based cohorts, and none have included cardiovascular imaging.
WHAT THIS STUDY ADDS
We studied 18 714 UK Biobank participants with history of six common cancers and an equal number of non-cancer comparators propensity matched on vascular risk factors. Our results demonstrate association of cancer history with increased risk of a wide range of incident cardiovascular disease and mortality outcomes over 12 years of prospective follow-up. In participants with cardiovascular magnetic resonance (n=1354), cancer history was linked to adverse cardiac remodelling. The greatest range and magnitude of risk was observed in those with past breast and haematological cancers.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
People with past cancer have heightened cardiovascular risk, which appears independent of vascular risk factors and persists several years after initial cancer diagnosis. This study highlights the specific cardiovascular care needs of patients with cancer and supports consideration of cancer-specific exposures in cardiovascular risk stratification.
Introduction
Patients with cancer history represent a growing cohort at heightened cardiovascular risk, attributed to shared vascular risk factors, cardiotoxicities of cancer therapies, and biological processes related to the cancer itself.1 2 There is differential propensity to cardiovascular disease (CVD) across cancer sites, reflecting variation in these risk exposures.3 4
Existing work indicates highest risk of cardiovascular complications to be in the first year after cancer diagnosis.5 Few researchers have examined longer term cancer-specific cardiovascular risk in population samples. Such analyses are important for informing cardiovascular risk stratification, surveillance, and treatment of patients with past cancer.
Cardiovascular imaging has a key role in detecting subclinical cardiotoxicity. However, associations of cancer with cardiovascular remodelling in population cohorts have not been previously reported.
We evaluated cardiovascular health in 18 714 UK Biobank participants with previous cancer, characterising disease and risk factor burden, incident disease and mortality outcomes, and cardiovascular remodelling patterns.
Methods
Setting and study population
The UK Biobank includes over 500 000 participants aged 40–69 years, characterised in detail at baseline recruitment (2006–2010).6 Incident health events are prospectively tracked through extensive health record linkages (Hospital Episode Statistics (HES), cancer register, death register). The UK Biobank Imaging Study, which includes cardiovascular magnetic resonance (CMR), is underway and aims to scan 100 000 of the original participants.
Ascertainment of cancer history
Cancer history was ascertained from cancer registry and HES records (online supplemental table 1). We created six categories (lung, breast, prostate, haematological, uterus, colorectal) to capture the most common cancer sites.7 The primary cancer site was defined from the first code for cancer in any of the linked databases.
heartjnl-2022-321888supp001.pdf (9.6MB, pdf)
Ascertainment of incident cardiovascular outcomes
We defined incident CVD (ischaemic heart disease (IHD), stroke, atrial fibrillation (AF)/flutter, heart failure (HF), non-ischaemic cardiomyopathies (NICM), venous thromboembolism (VTE; deep vein thrombosis (DVT), pulmonary embolus (PE)), pericarditis) and mortality outcomes (IHD, stroke, hypertensive diseases, HF or NICMs) using HES and death registration records (online supplemental table 2).
CMR acquisition and analysis
CMR scans were performed according to predefined protocols and analysed using automated pipelines.8–10 These are research scans without any clinical indication. The following metrics were included: left ventricular (LV) end-diastolic volume (LVEDV), LV ejection fraction (LVEF), LV global function index (LVGFI), LV global longitudinal strain (GLS), left atrial (LA) maximum volume (LAV) and LA ejection fraction (LAEF).
Statistical analysis
Statistical analysis was performed using R studio V.4.1.0 (https://www.R-project.org/) and Stata V.17.11 Baseline characteristics are presented as number (percentage) for categorical variables, mean (SD) for normally distributed continuous variables and median (IQR) for non-normally distributed continuous variables. A propensity matched non-cancer comparator cohort was created with a priori selection of covariates (online supplemental figure 1, tables 3 and 4). Comparators were participants without record of cancer at baseline. Each cancer exposed participant was matched to one non-exposed participant using nearest neighbour propensity score matching on 20 predefined baseline covariates. Pairs were discarded if no matching participant had logit propensity score within 0.2 SDs of the case.12 Balance of covariates was assessed in the unmatched and matched samples using the standardised mean difference between exposed and non-exposed groups (online supplemental figure 2). Missing data values were imputed using single centre imputation from the multiple chained equation algorithm.
Competing risks regression was used to calculate subdistribution HRs (SHR) and 95% CIs for the association of cancer history at baseline with incident disease and mortality outcomes. Participants with the outcome of interest at baseline were excluded from analyses for that outcome (but included in analyses of other outcomes). Incident events were first occurrence of the outcome after baseline. Prevalent events were conditions present at baseline. The censor date was 26 March 2021, providing mean prospective follow-up of 11.8±1.7 years. We performed sensitivity analyses using cause-specific Cox regression, limiting to cases with complete data (no imputation), and to cancers diagnosed within 5 years prior to baseline. Given possible heterogeneities within the haematological cancer category, we examined associations with incident outcomes within its subcategories (lymphoma, leukaemia, myeloma). We tested for interaction of cancer exposure with time by defining time from cancer diagnosis to baseline for cases and assigning the same time to their matched controls.
Linear regression was used to estimate association of cancer exposure with each CMR metric, reporting standardised beta coefficients, 95% CIs, and p values. For this analysis, cancer status was ascertained at imaging (any cancer diagnosis had been established prior to imaging). The samples all matched well on overall propensity score; individual covariates that were less well matched were included as covariates in final models, as per Nguyen et al (online supplemental figure 3).13 We repeated the analysis excluding individuals with CVD at time of imaging. A two-sided significance level of 0.05 was used for all comparisons.
Results
Baseline characteristics
We analysed 18 714 participants with past cancer (online supplemental figure 4). Smoking was most common in those with lung (82.9%), colorectal (54.4%) and prostate (53.0%) cancer (table 1). Diabetes was most common in lung (9.9%), uterine (9.5%), and colorectal (8.8%) cancer. The highest rates of hypertension were in prostate (45.6%), colorectal (39.5%), and uterine (38.4%) cancer. Individuals with uterine cancer had the highest average body mass index. Among those with cancer, 17.6% had pre-existing CVD (table 2).
Table 1.
Baseline participant characteristics
| Cases | Controls | Breast | Lung | Prostate | Colorectal | Uterus | Haem | |
| N | 18 714 | 18 714 | 9531* | 313 | 3291 | 2412 | 937 | 2230 |
| Age | 62 (57–66) | 62 (57–66) | 61 (56–65) | 62 (58–66) | 65 (62–67) | 63 (59–66) | 63 (59–66) | 60 (53–65) |
| Men | 6095 (32.6) | 6095 (32.6) | 0 (0) | 170 (54.3) | 3291 (100) | 1383 (57.3) | 0 (0) | 1251 (56.1) |
| Women | 12 619 (67.4) | 12 619 (67.4) | 9531 (100) | 143 (45.7) | 0 (0) | 1029 (42.7) | 937 (100) | 979 (43.9) |
| White ethnicity | 18 002 (96.7) | 18 025 (96.7) | 9201 (96.9) | 301 (96.2) | 3143 (96.1) | 2324 (96.6) | 910 (97.5) | 2146 (96.7) |
| BAME | 617 (3.3) | 611 (3.3) | 299 (3.2) | 12 (3.8) | 129 (3.9) | 81 (3.4) | 23 (2.5) | 73 (3.3) |
| Townsend score | −2.3 (−3.7 to 0.3) | −2.3 (−3.7 to 0.3) | −2.3 (−3.7 to 0.2) | −0.7 (−3.3 to 2.5) | −2.4 (−3.8 to −0.1) | −2.2 (−3.7 to 0.4) | −2.2 (−3.6 to 0.0) | −2.2 (−3.6 to 0.5) |
| Degree or professional qualification | 8329 (45.5) | 8300 (45.4) | 4259 (45.5) | 96 (32.1) | 1513 (47.1) | 1022 (43.3) | 382 (42.0) | 1057 (48.5) |
| SBP (mm Hg) | 140.2±19.2 | 140.1±19.1 | 138.5±19.4 | 137.7±19.3 | 145.0±17.8 | 142.6±19.2 | 141.2±18.7 | 137.5±18.9 |
| DBP (mm Hg) | 82.0±10.1 | 82.0±10.0 | 81.4±9.9 | 81.5±11.2 | 84.0±9.9 | 82.6±10.1 | 82.1±9.6 | 81.1±10.6 |
| HR (bpm) | 70.5 (63.5–78.5) | 70(63-78) | 71.5(65-79) | 75 (67–83.5) | 67.5 (60.5–75.5) | 69.5 (62.5–77.5) | 71(64-78) | 70.5(63-80) |
| BMI (kg/m2) | 26.8 (24.2–30.0) | 26.7 (24.1–29.9) | 26.4 (23.7–29.7) | 26.7 (24.3–30.1) | 27.4 (25.1–30.0) | 27.2 (24.7–30.2) | 28.4 (24.7–33.7) | 26.8 (24.2–30.0) |
| Physical activity (METS/week) | 1695 (754–3426) | 1742 (782–3471) | 1695 (777–3336) | 1175 (375–2799) | 1874 (817–3848) | 1626 (704–3412) | 1624 (710–3506) | 1578 (693–3279) |
| Ever smoked | 8909 (48.0) | 9141 (49.2) | 4225 (44.6) | 257 (82.9) | 1725 (53.0) | 1304 (54.4) | 342 (36.8) | 1056 (47.6) |
| HbA1c (mmol/mol) | 36 (33.5–38.7) | 35.9 (33.4–38.5) | 36 (33.7–38.5) | 37 (34.1–39.7) | 36 (33.4–38.6) | 36 (33.4–39.1) | 36.4 (34.1–39.2) | 35.5 (32.8–38.4) |
| Random glucose (mmol/L) | 5.0 (4.7–5.4) | 5.0 (4.6–5.4) | 5.0 (4.7–5.4) | 4.9 (4.6–5.4) | 5.0 (4.7–5.5) | 5.1 (4.7–5.5) | 5.0 (4.7–5.5) | 5.0 (4.6–5.4) |
| Total cholesterol (mmol/L) | 5.8±1.2 | 5.8±1.2 | 6.0±1.2 | 5.6±1.3 | 5.4±1.1 | 5.6±1.2 | 5.9±1.2 | 5.6±1.2 |
| HDL (mmol/L) | 1.4 (1.2–1.7) | 1.4 (1.2–1.7) | 1.6 (1.3–1.8) | 1.3 (1.1–1.6) | 1.3 (1.1–1.5) | 1.4 (1.1–1.7) | 1.5 (1.3–1.7) | 1.3 (1.1–1.6) |
| LDL (mmol/L) | 3.5 (2.9–4.2) | 3.6 (2.9–4.2) | 3.6 (3.0–4.3) | 3.4 (2.8–4.1) | 3.4 (2.8–4.0) | 3.4 (2.8–4.1) | 3.6 (3.0–4.3) | 3.5 (2.9–4.1) |
| Triglyceride level (mmol/L) | 1.6 (1.1–2.2) | 1.5 (1.1–2.2) | 1.5 (1.1–2.1) | 1.7 (1.2–2.3) | 1.7 (1.2–2.4 | 1.7 (1.2–2.4) | 1.6 (1.2–2.2) | 1.6 (1.1–2.4) |
| Diabetes | 1222 (6.5) | 1238 (6.6) | 463 (4.9) | 31 (9.9) | 264 (8.0) | 211 (8.8) | 89 (9.5) | 164 (7.4) |
| Hypertension | 6421 (34.3) | 6443 (34.4) | 2761 (29.0) | 108 (34.5) | 1499 (45.6) | 953 (39.5) | 360 (38.4) | 740 (33.2) |
| High cholesterol | 5659 (30.2) | 5627 (30.1) | 2272 (23.8) | 115 (36.7) | 1431 (43.5) | 882 (36.6) | 304 (32.4) | 655 (29.4) |
Count variables are shown as N (%). Continuous variables are shown as mean±SD or median (IQR) if skewed.
*39 males excluded
BAME, black, Asian and minority ethnic; BMI, body mass index; DBP, diastolic blood pressure; Haem, haematological; HbA1c, glycated haemoglobin; HDL, high density lipoprotein; LDL, low density lipoprotein; MET, metabolic equivalent; SBP, systolic blood pressure.
Table 2.
Prevalent and incident cardiovascular diseases and mortality
| Cases | Controls | Breast | Lung | Prostate | Colorectal | Uterus | Haem | |
| N (total) | 18 714 | 18 714 | 9531 | 313 | 3291 | 2412 | 937 | 2230 |
| Prevalent CVDs (N, %) | 3289 (17.6) | 2856 (15.3) | 1119 (11.7) | 116 (37.1) | 805 (24.5) | 554 (23.0) | 121 (12.9) | 574 (25.7) |
| IHD | 1238 (6.6) | 1286 (6.9) | 348 (3.7) | 45 (14.4) | 375 (11.4) | 222 (9.2) | 45 (4.8) | 203 (9.1) |
| NICM | 52 (0.3) | 33 (0.2) | 21 (0.2) | 1 (0.3) | 11 (0.3) | 7 (0.3) | 2 (0.2) | 10 (0.4) |
| HF | 152 (0.8) | 97 (0.5) | 44 (0.5) | 7 (2.2) | 38 (1.2) | 21 (0.9) | 4 (0.4) | 38 (1.7) |
| AF/flutter | 431 (2.3) | 394 (2.1) | 111 (1.2) | 23 (7.3) | 138 (4.2) | 71 (2.9) | 14 (1.5) | 74 (3.3) |
| Stroke | 426 (2.3) | 448 (2.4) | 160 (1.7) | 18 (5.8) | 100 (3.0) | 63 (2.6) | 15 (1.6) | 70 (3.1) |
| Pericarditis | 35 (0.2) | 22 (0.1) | 17 (0.2) | 1 (0.3) | 7 (0.2) | 4 (0.2) | 0 | 6 (0.3) |
| VTE (DVT/PE) | 955 (5.1) | 576 (3.1) | 418 (4.4) | 21 (6.7) | 136 (4.1) | 166 (6.9) | 41 (4.4) | 173 (7.8) |
| Incident CVDs (N, %) (rate per 1000 person-years) |
5753 (30.7) (21.5) |
4594 (24.5) (16.3) |
2130 (22.3) (14.7) |
155 (49.5) (32.3) |
1335 (40.6) (27.6) |
803 (33.3) (22.8) |
250 (26.7) (15.9) |
1080 (48.4) (30.7) |
| IHD | 1584 (8.5) (7.8) |
1425 (7.6) (7.0) |
560 (5.9) (5.5) |
40 (12.8) (19.4) |
385 (11.7) (12.3) |
245 (10.2) (20.8) |
68 (7.3) (6.9) |
286 (12.8) (14.1) |
| NICM | 225 (1.2) (1.0) |
134 (0.7) (0.6) |
90 (0.9) (0.8) |
2 (0.6) (0.7) |
38 (1.2) (1.1) |
31 (1.3) (1.2) |
7 (0.7) (0.6) |
57 (2.6) (2.5) |
| HF | 950 (5.1) (4.3) |
705 (3.8) (3.2) |
337 (3.5) (3.2) |
32 (10.2) (12.5) |
205 (6.2) (5.8) |
107 (4.4) (4.2) |
42 (4.5) (3.9) |
227 (10.2) (10.0) |
| AF/flutter | 1539 (8.2) (7.2) |
1317 (7.0) (6.1) |
555 (5.8) (5.4) |
38 (12.1) (15.4) |
382 (11.6) (11.6) |
236 (9.8) (9.7) |
69 (7.4) (6.3) |
259 (11.6) (11.8) |
| Stroke | 590 (3.2) (2.7) |
477 (2.5) (2.2) |
211 (2.2) (2.0) |
16 (5.1) (6.6) |
148 (4.5) (4.4) |
83 (3.4) (3.3) |
30 (3.2) (2.8) |
102 (4.6) (4.6) |
| Pericarditis | 188 (1.0) (0.8) |
94 (0.5) (0.4) |
75 (0.8) (0.7) |
12 (3.8) (4.8) |
28 (0.9) (0.8) |
19 (0.8) (0.7) |
7 (0.7) (0.6) |
47 (2.1) (2.0) |
| VTE (DVT/PE) | 677 (3.6) (3.4) |
442 (2.4) (2.1) |
302 (3.2) (2.9) |
15 (4.8) (5.8) |
149 (4.5) (4.3) |
82 (3.4) (3.4) |
27 (2.9) (2.7) |
102 (4.6) (4.7) |
| Mortality outcomes (N, %) (rate per 1000 person-years) |
3514 (18.8) (17.0) |
1582 (8.5) (7.2) |
1454 (15.3) (13.5) |
160 (51.1) (59.0) |
683 (20.8) (18.9) |
499 (20.7) (19.1) |
113 (12.1) (10.4) |
605 (27.1) (25.7) |
| Any CVD | 287 (1.5) (1.4) |
265 (1.4) (1.2) |
74 (0.8) (0.7) |
17 (5.4) (6.3) |
83 (2.5) (2.3) |
54 (2.2) (2.1) |
12 (1.3) (1.1) |
47 (2.1) (2.0) |
| IHD | 154 (0.8) (0.7) |
160 (0.9) (0.7) |
24 (0.3) (0.2) |
14 (4.5) (5.2) |
53 (1.6) (1.5) |
34 (1.4) (1.3) |
3 (0.3) (0.3) |
26 (1.2) (1.1) |
| HF/NICM | 37 (0.2) (0.2) |
17 (0.1) (0.1) |
17 (0.2) (0.2) |
0 | 7 (0.2) (0.2) |
5 (0.2) (0.2) |
3 (0.3) (0.3) |
5 (0.2) (0.2) |
| Stroke | 65 (0.3) (0.3) |
60 (0.3) (0.3) |
21 (0.2) (0.2) |
2 (0.6) (0.7) |
16 (0.5) (0.4) |
11 (0.5) (0.4) |
5 (0.5) (0.5) |
10 (0.4) (0.4) |
| Hypertensive diseases | 21 (0.1) (0.1) |
9 (0.1) (0.04) |
8 (0.1) (0.1) |
0 | 5 (0.2) (0.1) |
3 (0.1) (0.1) |
2 (0.2) (0.2) |
3 (0.1) (0.1) |
Figures are numbers of participant with each condition/outcome. Percentages are shown in brackets with denominator taken as the total number of participants in each category (‘total’ row). Prevalent CVDs were present at baseline recruitment. Incident CVDs represent first occurrence of the condition after baseline.
AF, atrial fibrillation; CVD, cardiovascular disease; DVT, deep vein thrombosis; Haem, haematological; HF, heart failure; IHD, ischaemic heart disease; NICM, non-ischaemic cardiomyopathies; PE, pulmonary embolism; VTE, venous thromboembolism.
Incident events
Almost one-third of participants with cancer developed one of the incident CVDs (table 2). The highest rates of incident CVD were in participants with lung (49.5%), haematological (48.4%), and prostate (40.6%) cancer. Incident IHD, AF/flutter and HF were the top three incident CVDs across all cancers. Over the study period, 18.8% of participants with cancer died compared with 8.5% of controls. In those with cancer, 8.2% (287/3514) of deaths were primary cardiovascular deaths.
Breast cancer
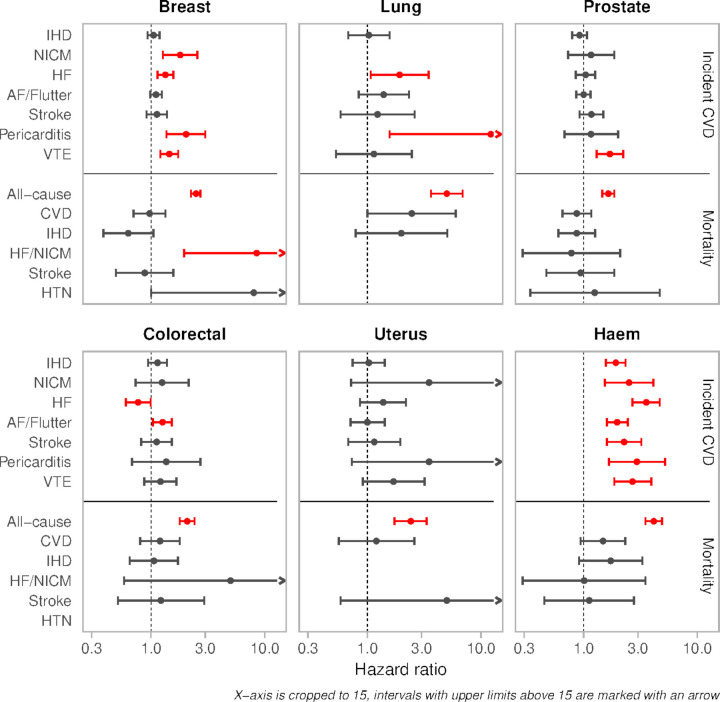
Among participants with breast cancer, 22.3% (2130/9531) developed one of the incident CVDs considered and 15.3% (1454/9531) died. The most common incident CVDs were IHD (5.9%), AF/flutter (5.8%), HF (3.5%), VTE (3.2%) and stroke (2.2%). NICMs occurred in 0.9% and pericarditis in 0.8% of participants with breast cancer. A total of 5.1% (74/1454) of all deaths were primary cardiovascular deaths. The most common causes of CVD death were stroke and IHD.
Compared with matched non-cancer controls, those with past breast cancer had over twofold greater risk of incident pericarditis (SHR 2.03 (1.36, 3.00); p=0.0004), 80% greater risk of incident NICM (SHR 1.80 (1.27, 2.56), p=0.0008), and 45% greater risk of incident VTE (SHR 1.45 (1.21, 1.73); p=6.61×10−5) (table 3, figure 1). Breast cancer history was associated with 8.5-fold greater risk of death from HF or NICM (SHR 8.50 (1.95, 36.97); p=0.004) and eightfold greater risk of death from hypertensive diseases (SHR 8.00 (1.00, 64.07); p=0.05).
Table 3.
Associations of cancer patients with incident cardiovascular events compared with propensity matched non-cancer controls
| Breast | Lung | Prostate | Colorectal | Uterus | Haematological | |
| Incident disease | ||||||
| IHD | 1.05 (0.93, 1.19) | 1.03 (0.68, 1.57) | 0.92 (0.79, 1.07) | 1.14 (0.94, 1.38) | 1.03 (0.74, 1.42) | 1.92 (1.57, 2.34) |
| 0.428 | 0.899 | 0.297 | 0.181 | 0.868 | 2.02×10−10 | |
| NICM | 1.80 (1.27, 2.56) | – | 1.16 (0.73, 1.86) | 1.25 (0.73, 2.14) | 3.49 (0.72, 16.78) | 2.51 (1.54, 4.10) |
| 0.0008 | – | 0.543 | 0.416 | 0.121 | 0.002 | |
| Heart failure | 1.34 (1.14, 1.57) | 1.92 (1.07, 3.46) | 1.04 (0.85, 1.26) | 0.77 (0.60, 0.99) | 1.38 (0.86, 2.18) | 3.56 (2.69, 4.66) |
| 0.0004 | 0.029 | 0.72 | 0.044 | 0.181 | 1.19×10−19 | |
| AF/flutter | 1.11 (0.98, 1.25) | 1.39 (0.84, 2.32) | 1.00 (0.86, 1.15) | 1.26 (1.04, 1.52) | 1.00 (0.71, 1.42) | 1.97 (1.60, 3.22) |
| 0.114 | 0.206 | 0.969 | 0.02 | 0.996 | 4.43×10−6 | |
| Stroke | 1.13 (0.91, 1.38) | 1.23 (0.58, 2.61) | 1.17 (0.92. 1.49) | 1.12 (0.82, 1.52) | 1.15 (0.68, 1.95) | 2.27 (1.60, 2.44) |
| 0.259 | 0.575 | 0.194 | 0.48 | 0.59 | 2.62×10−10 | |
| Pericarditis | 2.03 (1.36, 3.00) | 12.18 (1.57, 94.63) | 1.16 (0.68, 2.01) | 1.36 (0.68, 2.72) | 3.49 (0.73, 16.95) | 2.94 (1.67, 5.21) |
| 0.0004 | 0.017 | 0.585 | 0.385 | 0.119 | 0.0002 | |
| VTE | 1.45 (1.21, 1.73) | 1.14 (0.53, 2.46) | 1.70 (1.30, 2.23) | 1.21 (0.87, 1.67) | 1.70 (0.91, 3.19) | 2.69 (1.86, 3.94) |
| 6.61×10-5 | 0.736 | 0.0001 | 0.2639 | 0.095 | 2.47×10−7 | |
| Mortality outcomes | ||||||
| All-cause | 2.48 (2.25, 2.72) | 5.00 (3.63, 6.89) | 1.65 (1.46, 1.86) | 2.08 (1.79, 2.41) | 2.41 (1.73, 3.32) | 4.14 (3.49, 4.90) |
| 3.65×10–80 | 7.25×10-21 | 2.40×10–16 | 1.30×10–21 | 3.06×10-7 | 3.10×10−59 | |
| Any CVD | 0.97 (0.70, 1.34) | 2.46 (1.00, 5.99) | 0.87 (0.65, 1.17) | 1.20 (0.80, 1.79) | 1.20 (0.56, 2.59) | 1.48 (0.94, 2.32) |
| 0.871 | 0.05 | 0.371 | 0.374 | 0.64 | 0.087 | |
| IHD | 0.63 (0.38, 1.05) | 1.99 (0.79, 5.05) | 0.87 (0.60, 1.26) | 1.06 (0.65, 1.72) | – | 1.73 (0.91, 3.29) |
| 0.079 | 0.14 | 0.461 | 0.820 | – | 0.090 | |
| Heart failure or NICM | 8.50 (1.95, 36.97) | – | 0.78 (0.29, 2.10) | 5.00 (0.58, 42.95) | – | 1.01 (0.29, 3.49) |
| 0.004 | – | 0.615 | 0.142 | – | 0.991 | |
| Stroke | 0.88 (0.49, 1.57) | – | 0.94 (0.47, 1.86) | 1.22 (0.51, 2.94) | 5.00 (0.58, 42.95) | 1.12 (0.45, 2.77) |
| 0.656 | – | 0.853 | 0.652 | 0.142 | 0.806 | |
| Hypertensive diseases | 8.00 (1.00, 64.07) | – | 1.25 (0.34, 4.66) | – | – | – |
| 0.050 | – | 0.741 | – | – | – |
Results are subdistribution HR (95% CI) and p value associated with cancer exposure (vs no cancer). Blank cells indicate that no analysis was performed due to small number of outcomes (<5) in that category. Comparators are matched on age, sex, ethnicity, deprivation, education, blood pressure, heart rate, body mass index, glycated haemoglobin, random glucose, total cholesterol, high density lipoprotein, low density lipoprotein, triglyceride level, physical activity, smoking, diabetes, hypertension and high cholesterol. The bold cells represent statistically significant associations.
AF, atrial fibrillation; CVD, cardiovascular disease; IHD, ischaemic heart disease; NICM, non-ischaemic cardiomyopathies; VTE, venous thromboembolism.
Figure 1.
Associations of cancer exposure with incident cardiovascular disease and mortality outcomes. Results are association of cancer exposure with incident outcomes presented as subdistribution HRs and 95% CIs from competing risk regression, except for all-cause death where we report HR from Cox hazard proportional regression. HRs and 95% CIs are presented on a log10 scale. The comparators are propensity matched non-cancer controls. The dots represent the point estimate, and the intervals are the CIs. The greyed-out intervals indicate statistically non-significant associations. AF, atrial fibrillation; CVD, cardiovascular disease; NICM, non-ischaemic cardiomyopathies; Haem, haematological; HF, heart failure; HTN, hypertension; IHD, ischaemic heart disease.
Lung cancer
Among the cancer sites considered, participants with a history of lung cancer (n=313) had the highest rates of incident CVD (49.4%), all-cause death (51.1%), and CVD death (5.4%). The most common incident CVDs were IHD (12.8%), AF/flutter (12.1%) and HF (10.2%). Among participants with lung cancer who died, 10.1% (17/160) died of a primary cardiovascular cause.
Lung cancer was associated with over 12-fold greater risk of incident pericarditis (SHR 12.18 (1.57, 94.63); p=0.017), 88% greater risk of incident HF (SHR 1.88 (1.07, 3.29); p=0.029), and almost 2.5-fold greater risk of CVD death (SHR 2.46 (1.00, 5.99); p=0.05). The risk of IHD death was increased in lung cancer patients, although with wide CIs (SHR 1.99 (0.79, 5.05); p=0.14).
Prostate cancer
Among 3291 participants with prostate cancer, 40.6% developed incident CVD and 20.8% died. Primary cardiovascular deaths contributed 12.2% (83/683) of all deaths. The most common incident CVDs were IHD (11.7%), AF/flutter (11.6%), and HF (6.2%). Incident stroke and VTE each occurred in 4.5%, NICMs in 1.2% and pericarditis in 0.9%.
Compared with matched non-cancer controls, participants with prostate cancer had increased risk of incident VTE (SHR 1.70 (1.30, 2.23); p=0.0001) and all-cause death (HR 1.65 (1.46, 1.86); p=2.40×10−16). Associations with all other outcomes were statistically non-significant.
Colorectal cancer
One-third (803/2412) of participants with colorectal cancer developed incident CVD, 20.7% died and 2.2% died of primary cardiovascular causes (10.8% of all deaths: 54/499). The most common incident CVDs were IHD (10.2%), AF/flutter (9.8%), and HF (4.4%).
Participants with colorectal cancer had 26% greater risk of incident AF/flutter (SHR 1.26 (1.04, 1.52); p=0.02) compared with matched non-cancer controls. Colorectal cancer was associated with higher risk of HF/NICM death, but with wide CIs (SHR 5.00 (0.58, 42.95); p=0.14). Aside from all-cause death, there was no statistically significant difference in risk of any other outcome.
Uterine cancer
Among the 937 participants with uterine cancer, 26.7% developed incident CVD and 12.1% died. Primary cardiovascular deaths contributed 10.6% (12/113) of all deaths. The most common incident CVDs were AF/flutter (7.4%), IHD (7.3%) and HF (4.5%). Incident stroke occurred in 3.2%, VTE in 2.9% and NICMs and pericarditis were each observed in 0.7% of individuals.
Compared with matched non-cancer controls, uterine cancer patients had increased (statistically non-significant) risk of incident NICM (SHR 3.49 (0.72, 16.78); p=0.12), pericarditis (SHR 3.49 (0.73, 16.95); p=0.12) and stroke death (SHR 5.00 (0.58, 42.95); p=0.14).
Haematological cancer
Among 2230 participants with past haematological cancer, 48.4% (n=1080) developed incident CVD and 27.1% died. A total of 7.8% (47/605) of all deaths were attributed to a primary cardiovascular cause. The most common CVDs were IHD (12.8%), AF/flutter (11.6%), and HF (10.2%). Incident stroke and VTE each occurred in 4.6%, NICMs in 2.6% and pericarditis in 2.1% of haematological cancer patients.
Participants with past haematological cancer had significantly greater risk of all incident CVDs (table 3, figure 1). The risk of incident HF was increased by over 3.5-fold (SHR 3.56 (2.69, 4.66); p=1.19×10−19), pericarditis by almost threefold (SHR 2.94 (1.67, 5.21); p=0.0002)], and there was over 2.5-fold greater risk of both incident VTE (SHR 2.69 (1.86, 3.94); p=2.47×10−7] and NICM (SHR 2.51 (1.54, 4.10); p=0.002). There was almost twofold increased risk of incident AF/flutter (SHR 1.97 (1.60, 2.44); p=2.62×10−10) and IHD (SHR 1.92 (1.57, 2.34); p=2.02×10−10). Associations with CVD mortality outcomes were statistically non-significant; however, participants with a history of haematological cancer appeared at higher risk of CVD (SHR 1.48 (0.94, 2.32); p=0.087) and IHD (SHR 1.73 (0.91, 3.29); p=0.090) death.
Associations with incident events were broadly similar across myeloma, leukaemia, and lymphomas (online supplemental tables 5 and 6).
Sensitivity analyses
In analyses limiting to cases with complete data, associations remained similar across all outcomes (online supplemental tables 7 and 8). The results were consistent in cause-specific Cox regression models (online supplemental table 9) and when restricting to participants diagnosed with cancer within 5 years of baseline (online supplemental tables 10 and 11). The interaction of cancer exposure with time from diagnosis was non-significant for all models, except for the association of lung cancer with incident stroke, where risk was higher in the earlier years after cancer incidence.
Associations with CMR metrics
We investigated associations of past cancer with cardiovascular phenotypes in 1354 participants who had CMR data available (online supplemental table 12). Compared with matched non-cancer controls, participants with past haematological cancer had larger LVEDV, poorer LV function by both LVEF and LV GLS, larger LAV, and lower LAEF (table 4, figure 2). Breast cancer was associated with significantly poorer LV function by LVEF and LVGFI. These relationships were similar in individuals without CVD at imaging (online supplemental table 13).
Table 4.
Association of cancer with CMR metrics
| Breast | Lung | Prostate | Colorectal* | Uterus* | Haem* | |
| LVM (g) | 0.07 (−0.05, 0.18) | −0.41 (−1.27, 0.46) | −0.01 (−0.14, 0.12) | −0.23 (−0.78, 0.32) | 0.14 (−0.23, 0.51) | 0.11 (−0.11, 0.33) |
| 0.27 | 0.33 | 0.84 | 0.40 | 0.45 | 0.33 | |
| LVEDV (mL) | 0.10 (−0.01, 0.22) | −0.56 (−1.48, 0.35) | 0.05 (−0.08, 0.18) | −0.32 (−0.85, 0.22) | −0.01 (−0.37, 0.35) | 0.22 (−0.00, 0.44) |
| 0.07 | 0.21 | 0.44 | 0.24 | 0.94 | 0.05 | |
| LVEF (%) | −0.18 (−0.30, −0.06) | 0.62 (−0.26, 1.50) | 0.02 (−0.10, 0.15) | −0.12 (−0.60, 0.36) | 0.03 (−0.34, 0.41) | −0.28 (−0.49, −0.06) |
| 0.003 | 0.15 | 0.73 | 0.61 | 0.87 | 0.01 | |
| LVGFI (%) | −0.14 (−0.26, −0.02) | 0.25 (−0.68, 1.18) | 0.05 (−0.07, 0.18) | −0.13 (−0.59, 0.34) | −0.06 (−0.45, 0.33) | −0.18 (−0.39, 0.04) |
| 0.02 | 0.56 | 0.41 | 0.58 | 0.76 | 0.10 | |
| LV GLS (%) | −0.02 (−0.13, 0.10) | −0.87 (−1.71, −0.04) | −0.03 (−0.17, 0.11) | 0.38 (−0.26, 1.02) | 0.33 (−0.14, 0.80) | 0.25 (0.03, 0.47) |
| 0.78 | 0.05 | 0.65 | 0.24 | 0.17 | 0.02 | |
| LAV max (mL) | 0.08 (−0.04, 0.20) | −0.82 (−1.69, 0.05) | 0.02 (−0.11, 0.16) | −0.35 (−0.76, 0.05) | −0.01 (−0.39, 0.37) | 0.30 (0.06, 0.53) |
| 0.18 | 0.06 | 0.75 | 0.09 | 0.96 | 0.01 | |
| LAEF (%) | −0.12 (−0.24, 0.00) | 0.42 (−0.11, 0.94) | −0.02 (−0.15, 0.11) | 0.15 (−0.24, 0.54) | −0.07 (−0.41, 0.27) | −0.33 (−0.56, −0.11) |
| 0.06 | 0.11 | 0.74 | 0.45 | 0.68 | 0.004 |
The results are standardised beta-coefficients and 95% CIs, thus representing SD change in CMR metrics with change in cancer exposure status from non-cancer to cancer; for SD of each metric, please refer to online supplemental table 5. The bold and yellow shaded cells represent statistically significant associations.
*Doubly robust model.
GLS, LV global longitudinal strain; LA, left atrium; LAEF, LA ejection fraction; LAV, LA maximum volume; LV, left ventricle; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; LVGFI, LV global function index; LVM, LV mass.
Figure 2.
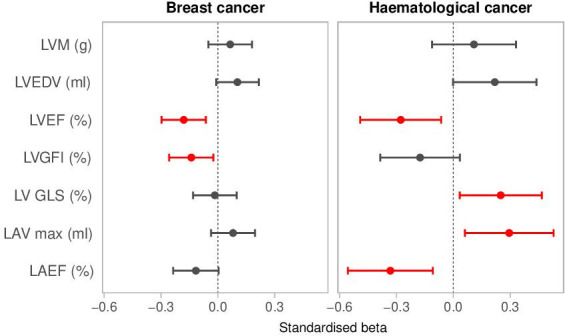
Association of breast and haematological cancer exposure with CMR metrics. Results are standardised beta-coefficients and 95% CIs, thus representing SD change in CMR metrics with change in cancer exposure status from non-cancer to cancer. CMR, cardiovascular magnetic resonance; GLS, LV global longitudinal strain; LA, left atrium; LAEF, LA ejection fraction; LAV, LA maximum volume; LV, left ventricle; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; LVGFI, LV global function index; LVM, LV mass.
Discussion
Summary of findings
In this large population-based study, covering an average of 12 years prospective follow-up, past cancer was linked to increased risk of a wide range of incident cardiovascular outcomes and adverse remodelling, independent of shared vascular risk factors. Previous haematological cancer was linked to increased incidence of all CVDs considered, poorer LV function (by LVEF and GLS), larger LV and LA size, and poorer LA function (lower LAEF). Past breast cancer was linked to increased incidence of NICM, HF, pericarditis, VTE, HF/NICM mortality, hypertensive disease death, and poorer LV function (by LVEF and LVGFI). Lung cancer was associated with increased risk of incident HF, pericarditis and CVD death. Colorectal cancer was associated with increased risk of incident AF/flutter. Prostate cancer was linked to increased VTE risk.
Comparison with previous work
The most common incident CVDs in our cancer-exposed cohort were IHD, AF/flutter, and HF. This distribution reflects both the risk factor profile of individuals with cancer and general population trends.14 Consistent with previous reports, we found high burden of vascular risk factors in participants with cancer.15 16 The observed CVD patterns are similar to studies from China and the USA.15 17 In our cancer cohort, 8.2% of deaths were attributed to primary cardiovascular causes. Similarly, an analysis of the UK Clinical Primary Records Datalink identified CVD as the primary cause of death in 9.7% of men and 7.7% of women with cancer.18
Our work extends previous reports by isolating cardiovascular risk associated with cancer independent of shared risk factors. A recent study from the UK used linked primary care and hospitalisation records to examine risk of incident disease-specific CVDs in patients with cancer independent of vascular risk factors.3 Our findings validate these observations in an independent cohort and provide new insights by considering disease associations alongside CMR remodelling.
Participants with previous haematological cancer had significantly increased risk of all incident CVDs. They also had increased size and poorer function of both the LA and LV. Haematological cancer patients are exposed to many cardiotoxic cancer therapies such as tyrosine kinase inhibitors,19 cyclophosphamide,20 anthracyclines,21 and mediastinal radiotherapy.22 The observed pattern of LV remodelling associated with haematological cancer may reflect subclinical cardiotoxicity, indicating a dilated LV with lower ejection fraction and poorer longitudinal function, and is consistent with our finding of increased risk of incident NICM and HF. The atrial remodelling patterns of a dilated and poorly functioning LA may reflect haemodynamic consequences of increased LV filling pressures that accompanies HF. There may also be direct effects on the atria via radiotherapy or other treatments. Regardless of underlying mechanism, atrial remodelling is both precipitated by and predisposes to AF, which we found to be significantly associated with haematological cancer history. We also found increased risk of stroke associated with past haematological cancer, which is likely driven by both ischaemic and haemorrhagic mechanisms, with the latter precipitated by coagulopathies related to the primary cancer and greater use of anticoagulants in these patients.
Increased risk of VTE was observed in participants with haematological, breast, and prostate cancer. Many factors promote a prothrombotic state in the setting of cancer, such as the systemic biological processes of the cancer itself, tumour compression effects, chemotherapy, and long-term indwelling venous catheters. Previous studies have documented augmented risk of VTE in patients with cancer.23 In our study, the magnitude of increased VTE risk was highest among participants with past haematological cancer.
Radiation-induced heart disease has a range of possible manifestations.24 Mediastinal radiotherapy has been linked to initiation and progression of atherosclerosis. Patients with lymphomas are often exposed to mediastinal radiotherapy, which may be a driver of the increased risk of IHD in participants with previous haematological cancer in our cohort. Our findings are consistent with a previous study by van Nimwegen et al,25 who also report increased risk of IHD in Hodgkin lymphoma survivors and attribute this, in part, to radiotherapy exposure.
Participants with previous lung, breast or haematological cancer had increased risk of pericardial disease, with lung cancer patients having a markedly increased risk (over 12-fold). This may reflect metastatic disease presentations. Pericardial disease may also be an adverse consequence of mediastinal radiotherapy,24 which is common in all three cancers.
Participants with breast cancer had increased risk of incident HF, incident NICMs and death from HF or NICM. Furthermore, breast cancer history was associated with poorer LV function by LVGFI and LVEF. These observations likely reflect cardiotoxicity linked to breast cancer therapies.21 26 An interesting observation in our results was a markedly increased risk of death due to hypertensive disease (eightfold increase) in participants with previous breast cancer, which may reflect suboptimal control of hypertension in this cohort.
Participants with uterine cancer had the highest average body mass index of all cancers, high rates of hypertension and diabetes and increased risk of stroke death. The clustering of cardiometabolic factors has been previously reported in uterine cancer.27 28 In our analysis, uterine cancer was linked to increased stroke mortality but with very wide CIs.
Clinical implications
Patients with cancer have a constellation of demographic and clinical risk factors that place them at higher cardiovascular risk. Our findings underscore the importance of controlling modifiable risk factors for all patients during and after their cancer treatment, as well as specific areas of risk where surveillance and/or preventive strategies should be focused. Importantly, we demonstrate that past cancer confers an increased risk of cardiovascular events, independent of traditional vascular risk factors and that this risk may extend several years beyond the initial cancer diagnosis. Thus, our results support consideration of cancer-specific exposures in cardiovascular risk stratification and lower thresholds for treatment of modifiable risk factors in this patient group. We demonstrate particular vulnerability of individuals with past breast and haematological cancer, who appeared at greatest risk, both with regards risk of incident clinical disease and adverse cardiac remodelling.
We found significant associations between breast and haematological cancer history and selected CMR metrics, even in the absence of prevalent CVD. The most consistent associations were observed with LVEF. We also demonstrate potential value of LVGFI, GLS, and LAEF as emerging novel imaging biomarkers of subclinical disease.
Limitations
Ascertainment of incident outcomes from health records may be subject to miscoding. We may be underpowered to detect associations in cancers with small sample sizes (eg, lung and uterine). Our dataset does not permit characterisation by cancer histology or stage. Information about specific cancer therapies was not available, and we cannot make inferences about treatment-specific effects. We are unable to consider ethnic disparities as our sample comprises a predominantly white cohort; future studies in more diverse cohorts are needed.
Conclusions
Individuals with past cancer have heightened cardiovascular risk, which appears independent of vascular risk factors and persists several years after initial cancer diagnosis. The pattern of CVDs varies by cancer site, likely reflecting specific characteristics of the cancer and its therapies. CMR measures of LV and LA structure and function provide preclinical indicators of cardiovascular health in this context.
Acknowledgments
This study was conducted using the UK Biobank resource under access application 2964. We would like to thank all the UK Biobank participants, staff involved with planning, collection and analysis, including core lab analysis of the CMR imaging data.
Footnotes
Twitter: @zahra_raisi, @ProfEmmaCrosbie, @fmw22, @MMamas1973, @s_e_petersen
Contributors: ZR-E and SEP conceptualised the idea and design the statistical analysis plan. JC advised on statistical analysis and performed the analysis. CM provided support for the data analysis. ZR-E wrote the manuscript. SEP, SN and NCH provided overall supervision for the work. SEP is the guarantor of the work. All coauthors reviewed the manuscript provided critical review of the work.
Funding: ZR-E recognizes the National Institute for Health Research (NIHR) Integrated Academic Training programme which supports her Academic Clinical Lectureship post and was also supported by British Heart Foundation Clinical Research Training Fellowship No. FS/17/81/33318. EJC is supported by an NIHR Advanced Fellowship (NIHR300650) and the NIHR Manchester Biomedical Research Centre (IS-BRC-1215-20007). FMW is co-Director of the CanTest Collaborative, which is funded by Cancer Research UK (CC8640/A23385). SN and CM were supported by the Oxford NIHR Biomedical Research Centre and SN by Oxford NIHR Biomedical Research Centre and the Oxford British Heart Foundation Centre of Research Excellence. SEP acknowledges support from the 'SmartHeart' EPSRC programme grant (www. nihr.ac.uk; EP/P001009/1) and the European Union's Horizon 2020 research and innovation programme under grant agreement No 825903 (euCanSHare project). SEP and SN acknowledge the British Heart Foundation for funding the manual analysis to create a cardiovascular magnetic resonance imaging reference standard for the UK Biobank imaging-resource in 5000 CMR scans (www.bhf.org.uk; PG/14/89/31194). NCH acknowledges support from MRC (MC_PC_21003; MC_PC_21001) and NIHR Southampton Biomedical Research Centre. CHM is supported directly and indirectly from the NIHR Biomedical Research Centres at University College London Hospitals and Barts Health NHS Trusts. Barts Charity (G-002346) contributed to fees required to access UK Biobank data [access application #2964]. This article is supported by the London Medical Imaging and Artificial Intelligence Centre for Value Based Healthcare (AI4VBH), which is funded from the Data to Early Diagnosis and Precision Medicine strand of the government’s Industrial Strategy Challenge Fund, managed and delivered by Innovate UK on behalf of UK Research and Innovation (UKRI). Views expressed are those of the authors and not necessarily those of the AI4VBH Consortium members, the NHS, Innovate UK, or UKRI. This project was enabled through access to the MRC eMedLab Medical Bioinformatics infrastructure, supported by the Medical Research Council (www.mrc.ac.uk; MR/L016311/1). The funders did not have any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: SEP provides consultancy to Cardiovascular Imaging Inc, Calgary, Alberta, Canada. The remaining authors have nothing to disclose.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. This research was conducted using the UK Biobank resource under access application 2964. UK Biobank will make the data available to all bona fide researchers for all types of health-related research that is in the public interest, without preferential or exclusive access for any persons. All researchers will be subject to the same application process and approval criteria as specified by UK Biobank. For more details on the access procedure, see the UK Biobank website: http://www.ukbiobank.ac.uk/register-apply.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study complies with the Declaration of Helsinki; the work was covered by the ethical approval for UK Biobank studies from the NHS National Research Ethics Service on 17 June 2011 (Ref 11/NW/0382) and extended on 18 June 2021 (Ref 21/NW/0157) with written informed consent obtained from all participants. Participants gave informed consent to participate in the study before taking part.
References
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018;103:356–87. 10.1016/j.ejca.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 2. Koene RJ, Prizment AE, Blaes A, et al. Shared risk factors in cardiovascular disease and cancer. Circulation 2016;133:1104–14. 10.1161/CIRCULATIONAHA.115.020406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strongman H, Gadd S, Matthews A, et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet 2019;394:1041–54. 10.1016/S0140-6736(19)31674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raisi-Estabragh Z, Kobo O, Freeman P, et al. Temporal trends in disease-specific causes of cardiovascular mortality amongst patients with cancer in the USA between 1999 and 2019. Eur Heart J Qual Care Clin Outcomes 2022;9:qcac016:54–63. 10.1093/ehjqcco/qcac016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European Society for therapeutic radiology and oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022;43:4229–361. 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
- 6. UK Biobank Coordinating Centre . UK Biobank: protocol for a large-scale prospective epidemiological resource. UKBB-PROT-09-06 (main phase); 2007: 1–112. https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf
- 7. Office for National Statistics . Cancer registration statistics, England. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/final2016 [Accessed 24 Nov 2020].
- 8. Hann E, Popescu IA, Zhang Q, et al. Deep neural network ensemble for on-the-fly quality control-driven segmentation of cardiac MRI T1 mapping. Med Image Anal 2021;71:102029. 10.1016/j.media.2021.102029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bai W, Sinclair M, Tarroni G, et al. Automated cardiovascular magnetic resonance image analysis with fully convolutional networks. Journal of Cardiovascular Magnetic Resonance 2018;20:1–12. 10.1186/s12968-018-0471-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petersen SE, Matthews PM, Francis JM, et al. UK Biobank’s cardiovascular magnetic resonance protocol. Journal of Cardiovascular Magnetic Resonance 2015;18:8. 10.1186/s12968-016-0227-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. StataCorp . Stata statistical software: release 14. 2015. College Station, TX: StataCorp LP, 2015. [Google Scholar]
- 12. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150–61. 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nguyen T-L, Collins GS, Spence J, et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol 2017;17:1–8. 10.1186/s12874-017-0338-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Timmis A, Vardas P, Townsend N, et al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J 2022;43:716–99. 10.1093/eurheartj/ehab892 [DOI] [PubMed] [Google Scholar]
- 15. Liu D, Ma Z, Yang J, et al. Prevalence and prognosis significance of cardiovascular disease in cancer patients: a population-based study. Aging 2019;11:7948–60. 10.18632/aging.102301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X, Pawlikowski M, Olivo-Marston S, et al. Ten-year cardiovascular risk among cancer survivors: the National health and nutrition examination survey. PLoS One 2021;16:e0247919. 10.1371/journal.pone.0247919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J 2019;40:3889–97. 10.1093/eurheartj/ehz766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alam N, Wright AK, Ashcroft DM, et al. Cancer and cardiovascular disease. The Lancet 2020;395:1903–4. 10.1016/S0140-6736(20)30222-1 [DOI] [PubMed] [Google Scholar]
- 19. Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer 2007;7:332–44. 10.1038/nrc2106 [DOI] [PubMed] [Google Scholar]
- 20. Duléry R, Mohty R, Labopin M, et al. Early cardiac toxicity associated with post-transplant cyclophosphamide in allogeneic stem cell transplantation. JACC CardioOncol 2021;3:250–9. 10.1016/j.jaccao.2021.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Volkova M, Russell R. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev 2012;7:214–20. 10.2174/157340311799960645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ratosa I, Ivanetic Pantar M. Cardiotoxicity of mediastinal radiotherapy. Rep Pract Oncol Radiother 2019;24:629–43. 10.1016/j.rpor.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cohen AT, Katholing A, Rietbrock S, et al. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb Haemost 2017;117:57–65. 10.1160/TH15-08-0686 [DOI] [PubMed] [Google Scholar]
- 24. Belzile-Dugas E, Eisenberg MJ. Radiation-induced cardiovascular disease: review of an underrecognized pathology. J Am Heart Assoc 2021;10:21686. 10.1161/JAHA.121.021686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Nimwegen FA, Schaapveld M, Janus CPM, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med 2015;175:1007–17. 10.1001/jamainternmed.2015.1180 [DOI] [PubMed] [Google Scholar]
- 26. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92. 10.1056/NEJM200103153441101 [DOI] [PubMed] [Google Scholar]
- 27. Kitson SJ, Lindsay J, Sivalingam VN, et al. The unrecognized burden of cardiovascular risk factors in women newly diagnosed with endometrial cancer: a prospective case control study. Gynecol Oncol 2018;148:154–60. 10.1016/j.ygyno.2017.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crosbie EJ, Kitson SJ, McAlpine JN, et al. Endometrial cancer. The Lancet 2022;399:1412–28. 10.1016/S0140-6736(22)00323-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2022-321888supp001.pdf (9.6MB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. This research was conducted using the UK Biobank resource under access application 2964. UK Biobank will make the data available to all bona fide researchers for all types of health-related research that is in the public interest, without preferential or exclusive access for any persons. All researchers will be subject to the same application process and approval criteria as specified by UK Biobank. For more details on the access procedure, see the UK Biobank website: http://www.ukbiobank.ac.uk/register-apply.