Abstract
Aims
We conducted a meta-analysis of randomised controlled trials (RCTs) of implantable haemodynamic monitoring (IHM)-guided care.
Methods
PubMed and Ovid MEDLINE were searched for RCTs of IHM in patients with heart failure (HF). Outcomes were examined in total (first and recurrent) event analyses.
Results
Five trials comparing IHM-guided care with standard care alone were identified and included 2710 patients across ejection fraction (EF) ranges. Data were available for 628 patients (23.2%) with heart failure with preserved ejection fraction (HFpEF) (EF ≥50%) and 2023 patients (74.6%) with heart failure with a reduced ejection fraction (HFrEF) (EF <50%). Chronicle, CardioMEMS and HeartPOD IHMs were used. In all patients, regardless of EF, IHM-guided care reduced total HF hospitalisations (HR 0.74, 95% CI 0.66 to 0.82) and total worsening HF events (HR 0.74, 95% CI 0.66 to 0.84). In patients with HFrEF, IHM-guided care reduced total worsening HF events (HR 0.75, 95% CI 0.66 to 0.86). The effect of IHM-guided care on total worsening HF events in patients with HFpEF was uncertain (fixed-effect model: HR 0.72, 95% CI 0.59 to 0.88; random-effects model: HR 0.60, 95% CI 0.32 to 1.14). IHM-guided care did not reduce mortality (HR 0.92, 95% CI 0.71 to 1.20). IHM-guided care reduced all-cause mortality and total worsening HF events (HR 0.80, 95% CI 0.72 to 0.88).
Conclusions
In patients with HF across all EFs, IHM-guided care reduced total HF hospitalisations and worsening HF events. This benefit was consistent in patients with HFrEF but not consistent in HFpEF. Further trials with pre-specified analyses of patients with an EF of ≥50% are required.
PROSPERO registration number
CRD42021253905.
Keywords: Heart Failure, Meta-Analysis
WHAT IS ALREADY KNOWN ON THIS TOPIC
Implantable haemodynamic monitoring (IHM) to guide treatment has a class IIb recommendation in the 2021 European Society of Cardiology Heart Failure Guidelines.
WHAT THIS STUDY ADDS
This is the first meta-analysis to examine the effectiveness of IHM-guided care across a range of ejection fractions (EFs), combining data from the five randomised trials that investigated IHM-guided treatment, including a pre-COVID-19 sensitivity analysis from the recent Hemodynamic-Guided Management of Heart Failure (GUIDE-HF) trial. This study demonstrates that IHM-guided treatment was effective at reducing worsening heart failure (HF) events in patients with an EF of <50%; it is uncertain if patients with HF with preserved EF receive the same benefit.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study indicates that future trials should focus on people with an EF of ≥50% with pre-specified analyses to confirm the effectiveness of IHM-guided care in this population. IHM-guided treatment can be considered as a strategy in patients with an EF of <50%.
Introduction
Remote monitoring using implanted devices may provide useful information about the natural history of congestion leading to decompensation in people with heart failure (HF). Early identification of increasing cardiopulmonary pressures and intervention to reduce these might decrease the risk of subsequent HF hospitalisation. Based on the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients (CHAMPION) trial, one implantable pulmonary artery pressure monitor (CardioMEMS, Abbott, Illinois, USA) received a class IIb recommendation to reduce HF hospitalisations in the 2021 European Society of Cardiology Heart Failure guidelines.1 In the Hemodynamic-Guided Management of Heart Failure (GUIDE-HF) trial, the largest randomised controlled trial (RCT) to compare implantable haemodynamic monitoring (IHM) with standard care, IHM-guided care did not reduce HF hospitalisations overall, but sensitivity analyses suggested a modest benefit before the COVID-19 pandemic had an impact on patient management.2
No previous meta-analysis has included data from both the GUIDE-HF and the Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy (LAPTOP-HF) study, the latter of which was an international RCT that reported its findings in 2016 (table 1). Additionally, despite IHM randomised trials recruiting patients across a range of ejection fractions (EFs), no meta-analysis reported the effect of IHM on the reduction of HF hospitalisations and related events in subgroups of patients with HF with preserved ejection fraction (HFpEF) or heart failure with a reduced ejection fraction (HFrEF). This type of monitoring is of particular interest in patients with HFpEF, in whom evidence-based treatment options are limited. Therefore, in this meta-analysis of all randomised trials using IHM, we investigated whether treatment guided by such monitoring reduced the risk of total (first and recurrent) HF hospitalisations, total worsening HF events (HF hospitalisation and emergency department (ED) and urgent clinic visit for intravenous HF therapy) and all-cause mortality, when compared with standard care, in patients with HF across a range of EFs.
Table 1.
Randomised controlled trials of IHM-guided HF management compared with standard care
| Trial | First author and year | Design, country | Primary efficacy endpoint | Numbers enrolled | EF | NYHA class | Previous HF event | Follow-up |
| COMPASS-HF | Bourge et al,16 2008 | Single-blind,* multicentre RCT; USA | HF hospitalisation and ED and urgent clinic visit for intravenous therapy (included hypovolaemic events) | 274 | No EF inclusion criterion | III-IV | ≤6 months (or ED visit) | 6 months |
| CHAMPION | Abraham et al,18 2011 | Single-blind,* multicentre RCT; USA | HF hospitalisation | 550 | No EF inclusion criterion | III | ≤12 months | 6 months |
| REDUCE-HF | Adamson et al,10 2011 | Single-blind,* multicentre RCT; USA | HF hospitalisation and ED and urgent clinic visit for intravenous therapy | 400 | No EF inclusion criterion | II-III | ≤12 months | 12 months |
| LAPTOP-HF | Abraham et al,17 2016 | Multicentre RCT (no blinding); USA and New Zealand |
HF hospitalisation and complications from HF therapy | 486 | No EF inclusion criterion | III | ≤12 months (or BNP ≥400 pg/mL or NT-proBNP ≥1500 pg/mL) |
12 months |
| GUIDE-HF | Lindenfeld et al,2 2021 | Single-blind,* multicentre RCT; USA and Canada | All-cause mortality and HF hospitalisation and ED and urgent clinic visit for intravenous therapy | 1000 | No EF inclusion criterion | II – IV | ≤12 months (or BNP ≥250 pg/mL or NT-proBNP ≥1000 pg/mL) |
12 months |
*Patients but not investigators were blinded to haemodynamic data.
BNP, brain natriuretic peptide; CHAMPION, CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients; COMPASS-HF, Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure; ED, emergency department; EF, ejection fraction; GUIDE-HF, Hemodynamic-Guided Management of Heart Failure; HF, heart failure; IHM, implantable haemodynamic monitor; LAPTOP-HF, Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy; NT-proBNP, N- terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; RCT, randomised controlled trial; REDUCE-HF, Reducing Events in Patients with Chronic Heart Failure.
Methods
Search strategy and data extraction
A systematic review of RCTs in patients with HF was performed, comparing IHM-guided care versus standard therapy. This meta-analysis was registered on PROSPERO (CRD42021253905). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to conduct the literature search, data extraction and reporting. A PRISMA checklist is included in online supplemental appendix, table S1). Bias was assessed using the Revised Cochrane Risk-of-Bias Tool for Randomised Trials 3 (online supplemental appendix, table S2). Searches were performed on public databases (PubMed and Ovid MEDLINE) between 1 May 2020 and 5 September 2022 using the terms “heart failure AND implantable AND haemodynamic monitoring” and “left atrial pressure AND heart failure AND monitoring” and “pulmonary artery pressure monitoring AND heart failure”. All studies published up to 5 September 2022 were eligible. No restriction was placed on study size, language or country of publication. Titles and abstracts were screened based on pre-specified inclusion criteria using the population, intervention, comparator, outcomes and study framework:
heartjnl-2022-321885supp001.pdf (549.7KB, pdf)
Population: patients with HF.
Intervention: IHM-guided care.
Comparator: standard care.
-
Outcomes of interest.
HF hospitalisation.
Worsening HF events (HF hospitalisation and ED and urgent clinic visits for intravenous HF therapy).
All-cause mortality.
All-cause mortality and HF hospitalisation.
All-cause mortality and worsening HF events.
Study design: RCTs.
Full-text articles of original trial reports and published articles with retrospective analyses of the RCTs were included. Data presented at conferences were included if the presentation was available and verifiable by the researchers. Hazard ratios (HRs) and incidence rate ratios (IRRs) for endpoints were recorded. IRRs are approximations of HRs and were included as the effect estimate if HRs were not available as has previously been reported.4–6 If either HR or IRR was not reported in the literature, the IRR was calculated using event numbers and study cohort time at risk. Ninety-five per cent CIs were calculated if only a p value and effect estimate were reported.7 Numbers of patients in EF subgroups and their numbers of events were calculated from available data where necessary. Two researchers (JPC and MMYL) independently extracted and analysed the data. Results were compared and differences resolved by consensus with opinion from a third author. All authors reviewed the analysis results and contributed to drafting the report. If data were not available, the original study authors were contacted and data were requested.
Statistical analysis
Statistical analyses were performed using Stata17. As the trials investigated three devices across different decades we used a random effects (DerSimonian and Laird (D+L)8) model so that differences in the studies’ designs and cohorts would be accounted for within the analysis. We performed sensitivity analyses of each meta-analysis using fixed-effect models. Only the result of the fixed-effect model in patients with HFpEF is reported, as the other fixed-effect analyses were consistent with the reported random effect models. I2 statistic for percentage heterogeneity was computed with corresponding p values. Forest plots graphically report the pooled effect size estimates, the degree of heterogeneity and the weighted contribution each study made to the analyses. All outcomes were examined in total events (first and recurrent) analyses.
Definitions of HFpEF and HFrEF
HFpEF was defined as HF with an EF of ≥50% in keeping with the 2021 European Society of Cardiology Heart Failure guidelines1 and the recently proposed universal definition of HF.9 HFrEF was defined as HF with an EF of <50%, with the inclusion of patients with heart failure with mildly reduced ejection fraction (HFmrEF, EF 41%–49%) as well as patients with an EF of ≤40%.1 9 There were insufficient data available to further subclassify the trial cohorts into a HFmrEF subgroup. Only Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure (COMPASS-HF) pre-specified an analysis of patients with an EF of ≥50%. While EF was not an inclusion criterion for the Reducing Events in Patients with Chronic Heart Failure (REDUCE-HF) trial, patients included in that trial had severely impaired systolic function with a mean (±SD) EF of 23%±7%.10 Patients from the REDUCE-HF trial were therefore included in the HFrEF (EF <50%) analysis. Similarly, patients from LAPTOP-HF, in whom the mean EF was 30%±15%11 were included in the HFrEF (EF <50%) analysis.
Efficacy endpoints
The primary efficacy endpoints for each of the included trials were examined in total (first and recurrent) event analyses comparing the effect of IHM-guided care with standard care alone. These endpoints were as follows:
COMPASS-HF and REDUCE-HF: total worsening HF events. HF hospitalisations for less than 24 hours were included in the composite endpoint in REDUCE-HF.
LAPTOP-HF: total HF hospitalisation and complications of HF treatment such as hypotension and acute renal failure.
CHAMPION: total HF hospitalisation.
GUIDE-HF: all-cause mortality and total worsening HF events.
Recurrent events were analysed in a negative binomial regression model in COMPASS-HF and an Andresen-Gill model in CHAMPION, REDUCE-HF, LAPTOP-HF and GUIDE-HF. As these methods yield very similar results in simulations12 and trial datasets,13 they were used in the meta-analysis. Meta-analysis was performed for (1) total HF hospitalisations, (2) total worsening HF events (HF hospitalisation and ED visit and urgent clinic visit for intravenous HF therapy), (3) all-cause mortality, (4) all-cause mortality and total HF hospitalisations, and (5) all-cause mortality and total worsening HF events. Only hospitalisations for greater than 24 hours in REDUCE-HF were included in the total HF hospitalisation analysis (aforementioned item 1). All-cause mortality data from COMPASS-HF, CHAMPION, REDUCE-HF and GUIDE-HF were pooled. The GUIDE-HF main trial results were published in 2021, followed by an analysis examining the impact of the COVID-19 pandemic on that trial’s event rates.14 We performed a sensitivity analysis examining the rate of HF events including the pre-COVID event rates and the results from the other four included trials.
Results
Literature review and search strategy
A total of 1373 articles were identified by searching electronic databases. Two further articles11 15 were found by hand searching references and internet searches. A PRISMA flowchart outlines the search process and identification of relevant articles (online supplemental appendix, figure S1). Five RCTs were identified (table 1): COMPASS-HF,16 CHAMPION,17 18 REDUCE-HF,10 LAPTOP-HF11 15 and GUIDE-HF.2 The 18-month results for the CHAMPION trial were used in this meta-analysis. The HR for HF hospitalisation at 1 year was reported for 455 of the 486 randomised in LAPTOP-HF by the lead investigator at the annual meeting of the Heart Failure Association of the European Society of Cardiology in 2017.15
Trial characteristics
COMPASS-HF, CHAMPION and REDUCE-HF were conducted in the USA. LAPTOP-HF was conducted in the USA and New Zealand. GUIDE-HF was conducted in the USA and Canada. The COMPASS-HF and REDUCE-HF studies evaluated the Chronicle pressure sensor (Medtronic, Minnesota, USA). The CardioMEMS device (Abbott, Illinois, USA) was investigated in CHAMPION and GUIDE-HF. The HeartPOD device (St. Jude, Minnesota, USA) was investigated in LAPTOP-HF (table 1). The main trial characteristics are summarised in table 2.
Table 2.
Key baseline characteristics of patients enrolled in randomised controlled trials of IHM-guided HF management compared with standard care
| COMPASS-HF | CHAMPION | REDUCE-HF | LAPTOP-HF | GUIDE-HF | |
| Age (years) | IHM arm: 58±14 Control arm: 58±13 |
IHM arm: 61±13 Control arm: 62±13 |
IHM arm: 55±15 Control arm: 55±15 |
IHM and control arms: 62±12 | IHM arm: 71 (64-76) Control arm: 70 (64-77) |
| Sex (male, %) | IHM arm: 66 Control arm: 64 |
IHM arm: 72 Control arm: 73 |
IHM arm: 70 Control arm: 67 |
IHM and control arms: 75 | IHM arm: 62 Control arm: 63 |
| Race (%) | IHM arm
Control arm
|
IHM arm
Control arm
|
IHM arm
Control arm
|
n/r | IHM arm
Control arm
|
| EF (n=participants) | ≥50%: 70 <50%: 204 |
≥50%: 66 ≥40–49%: 53 ≤40%: 456 <40%: 430 |
IHM and control arms: mean EF 23%±7 | IHM and control arms, EF >35%: 121 IHM and control arms, EF ≤35%: 365 Mean EF 30%±15 |
>50%: 321 ≤50%: 679 |
| NYHA class (%) | IHM arm:
Control arm:
|
IHM arm: III only Control arm: III only |
IHM arm:
Control arm:
|
IHM arm: III only Control arm: III only |
IHM arm:
Control arm:
|
| Ischaemic aetiology (%) | IHM arm: 47 Control arm: 44 |
IHM arm: 59 Control arm: 62 |
IHM arm: 45 Control arm: 44 |
IHM and control arms: 46 | IHM arm: 42 Control arm: 38 |
| Diuretics (%) | IHM arm: 93 Control arm: 99 |
IHM arm: 92 Control arm: 92 |
IHM arm: 93 Control arm: 97 |
n/r | IHM arm: 95 Control arm: 95 |
| ACEi/ARB (%) | IHM arm: 85 Control arm: 81 |
IHM arm: 76 Control arm: 79 |
IHM arm: 92 Control arm: 92 |
n/r | IHM arm*: 64 Control arm*: 64 |
| Beta blockers (%) | IHM arm: 83 Control arm: 81 |
IHM arm: 90 Control arm: 91 |
IHM arm: 96 Control arm: 96 |
n/r | IHM arm: 89 Control arm: 88 |
*ARNI/ACEi/ARB.
ACEi, ACE inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor blocker-neprilysin inhibitor; CHAMPION, CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients; COMPASS-HF, Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure; EF, ejection fraction; GUIDE-HF, Hemodynamic-Guided Management of Heart Failure; HF, heart failure; IHM, implantable haemodynamic monitoring; LAPTOP-HF, Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy; n/r, not reported; NYHA, New York Heart Association; REDUCE-HF, Reducing Events in Patients with Chronic Heart Failure.
All participants underwent implantation of haemodynamic monitors and were randomised to receive HF care guided by haemodynamic data or receive standard care. COMPASS-HF, CHAMPION, REDUCE-HF and GUIDE-HF were single-blind studies where investigators, but not patients, had access to the treatment group haemodynamic data. Patients were unaware of their randomised assignment groups in these four trials. LAPTOP-HF had no blinding (ie, patients and investigators were aware of the intervention assignment groups).
Patients with HF regardless of EF
Total HF hospitalisations
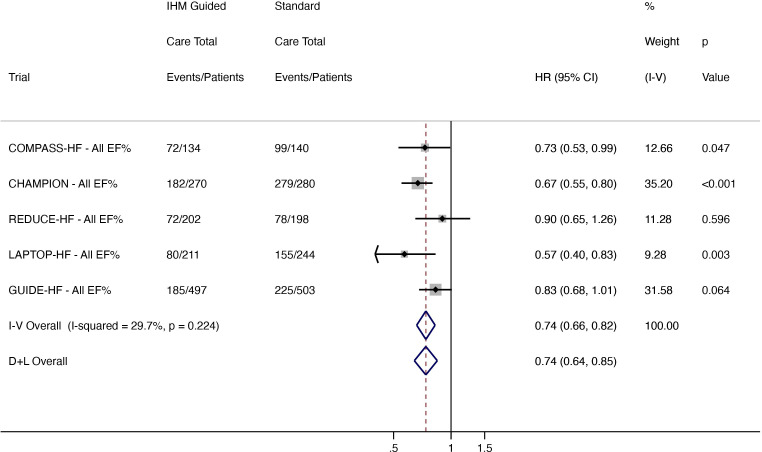
There were 591 hospitalisations in 1314 patients receiving IHM-guided care compared with 836 events in 1365 standard care patients. HF hospitalisations were reduced in the IHM-guided care arm by 26% (HR 0.74, 95% CI 0.64 to 0.85; low heterogeneity (I2 29.7%)) (figure 1).
Figure 1.
Total (first and recurrent) HF hospitalisations in all patients regardless of EF. CHAMPION, CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients; COMPASS-HF, Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure; D+L, DerSimonian and Laird; EF, ejection fraction; GUIDE-HF, Hemodynamic-Guided Management of Heart Failure; HF, heart failure; IHM, implantable haemodynamic monitor; I-V, inverse variance; LAPTOP-HF, Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy; REDUCE-HF, Reducing Events in Patients with Chronic Heart Failure.
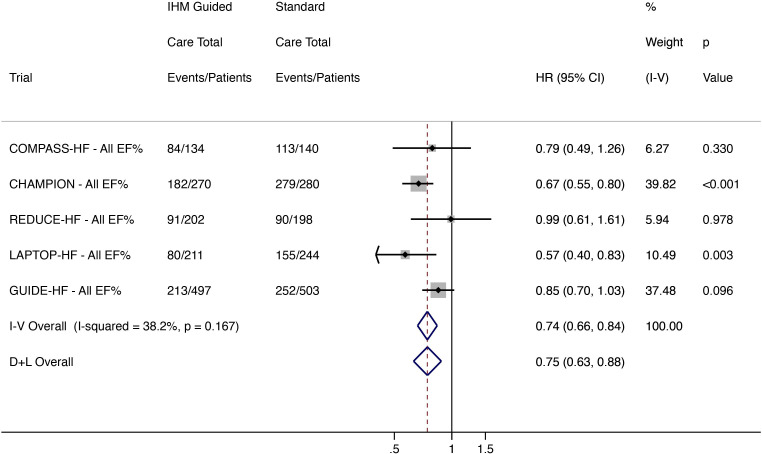
Total worsening HF events
There were 650 composite outcome events in 1314 patients receiving IHM-guided care and 889 events in 1365 standard care patients. IHM-guided care reduced total worsening HF events by 26% (HR 0.75, 95% CI 0.63 to 0.88; low heterogeneity (I2 38.2%)) (figure 2). In a sensitivity analysis of pre-COVID-19 event rates in the GUIDE-HF trial, IHM-guided care reduced HF events by 29% (HR 0.71, 95% CI 0.63 to 0.81; low heterogeneity (I2 2.9%)).
Figure 2.
Total (first and recurrent) worsening HF events (HF hospitalisation and ED and urgent clinic visit for intravenous HF therapy) in all patients regardless of EF. CHAMPION, CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients; COMPASS-HF, Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure; D+L, DerSimonian and Laird; ED, emergency department; EF, ejection fraction; GUIDE-HF, Hemodynamic-Guided Management of Heart Failure; HF, heart failure; IHM, implantable haemodynamic monitor; I-V, inverse variance; LAPTOP-HF, Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy; REDUCE-HF, Reducing Events in Patients with Chronic Heart Failure.
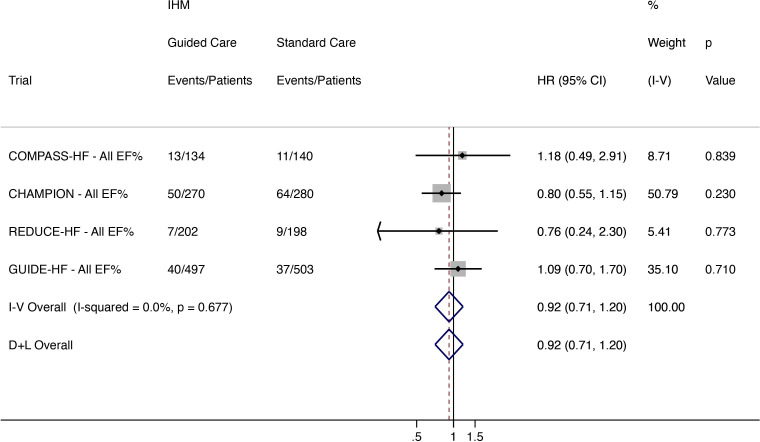
All-cause mortality
Mortality was reported in the COMPASS-HF, CHAMPION, REDUCE-HF and GUIDE-HF trials. Of 1103 patients, 110 (10·0%) in the IHM-guided care arm died compared with 121/1121 (10·8%) receiving standard care. IHM-guided care did not reduce all-cause mortality (HR 0.92, 95% CI 0.71 to 1.20; low heterogeneity (I2 0%)) (figure 3).
Figure 3.
All-cause mortality in all patients regardless of EF for (COMPASS-HF, CHAMPION, REDUCE-HF and GUIDE-HF). CHAMPION, CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients; COMPASS-HF, Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure; D+L, DerSimonian and Laird; EF, ejection fraction; GUIDE-HF, Hemodynamic-Guided Management of Heart Failure; HF, heart failure; IHM, implantable haemodynamic monitor; I-V, inverse variance; LAPTOP-HF, Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy; REDUCE-HF, Reducing Events in Patients with Chronic Heart Failure.
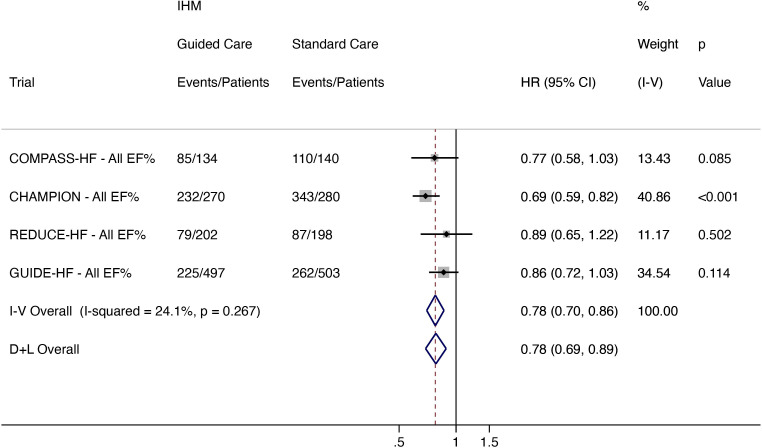
All-cause mortality and total HF hospitalisation
Data were available from COMPASS-HF, CHAMPION, REDUCE-HF and GUIDE-HF. A total of 621 events in 1103 patients occurred in the IHM arm and 802 events occurred in 1121 standard care patients. IHM-guided care, compared with standard care, reduced all-cause mortality and total HF hospitalisation by 22% (HR 0.78, 95% CI 0.69 to 0.89; low heterogeneity (I2 24.1%)) (figure 4).
Figure 4.
Total (first and recurrent) HF hospitalisation and all-cause mortality in all patients regardless of EF (COMPASS-HF, CHAMPION, REDUCE-HF and GUIDE-HF). CHAMPION, CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients; COMPASS-HF, Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure; D+L, DerSimonian and Laird; EF, ejection fraction; GUIDE-HF, Hemodynamic-Guided Management of Heart Failure; HF, heart failure; IHM, implantable haemodynamic monitor; I-V, inverse variance; LAPTOP-HF, Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy; REDUCE-HF, Reducing Events in Patients with Chronic Heart Failure.
All-cause mortality and total worsening HF events
A total of 680 events occurred in 1103 patients in the IHM arm and 855 events in 1121 standard care patients. IHM-guided care reduced all-cause mortality and worsening HF events by 20% (HR 0.81, 95% CI 0.69 to 0.94; moderate heterogeneity (I2 49.9%)). (online supplemental appendix, figure S2).
Patients with HFpEF (EF ≥50%)
Data were available from COMPASS-HF and CHAMPION for 136 patients with an EF of ≥50%.19 20 In the GUIDE-HF trial, a HR and event numbers were reported, but patient numbers for each randomised treatment group were not available.2
Total worsening HF events
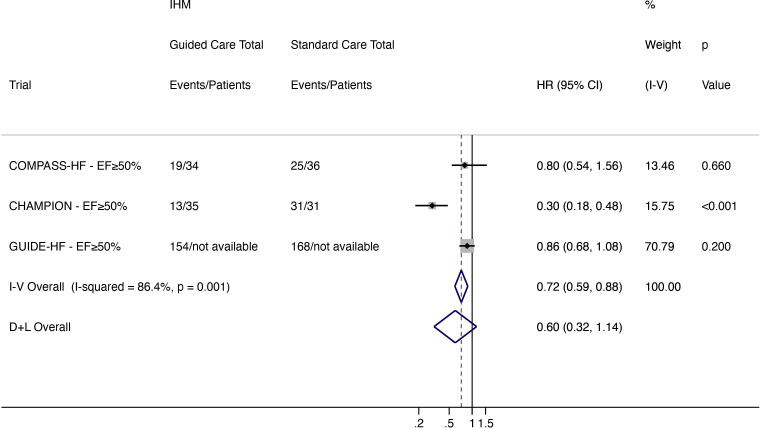
A total of 186 events occurred in patients receiving IHM-guided treatment compared with 224 events in the standard care arm. IHM-guided care reduced worsening HF when analysed using a fixed effect model (HR 0.72, 95% CI 0.59 to 0.88) but not a random-effects model (HR 0.60, 95% CI 0.32 to 1.14; high heterogeneity (I2 86.4%)) (figure 5).
Figure 5.
Total (first and recurrent) worsening HF events (HF hospitalisation and ED and urgent clinic visit for intravenous HF therapy) in patients with HFpEF (EF ≥50%). CHAMPION, CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients; COMPASS-HF, Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure; D+L, DerSimonian and Laird; ED, emergency department; EF, ejection fraction; GUIDE-HF, Hemodynamic-Guided Management of Heart Failure; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; IHM, implantable haemodynamic monitor; I-V, inverse variance; LAPTOP-HF, Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy; REDUCE-HF, Reducing Events in Patients with Chronic Heart Failure.
Patients with HFrEF (EF<50%)
Data were available for 659 patients with an EF<50% from COMPASS-HF and LAPTOP-HF and for 856 patients with an EF≤40% from CHAMPION and REDUCE-HF. HRs and event numbers were reported for patients with an EF 40–50% and an EF<40% in GUIDE-HF but patient numbers were not available for each EF category in this trial.2
Total worsening HF events
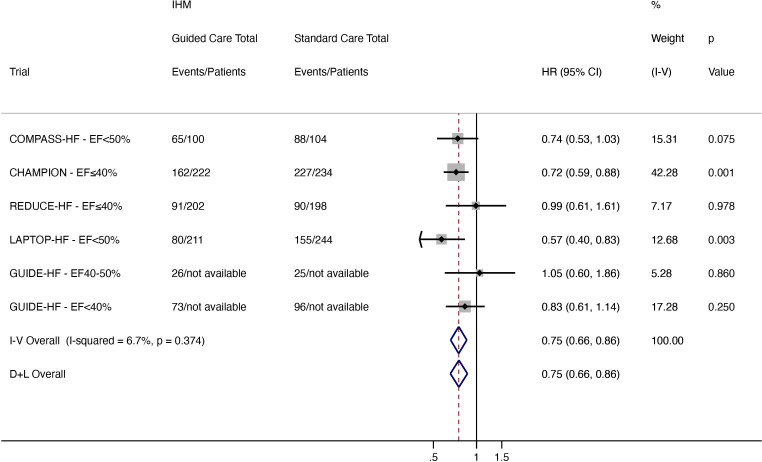
A total of 497 events occurred in the IHM-guided care arm compared with 681 events in the standard care arm. IHM-guided care reduced worsening HF by 25% when compared with standard care (HR 0.75, 95% CI 0.66 to 0.86; low heterogeneity (I2 6.7%)) (figure 6)
Figure 6.
Total (first and recurrent) worsening HF events (HF hospitalisation and ED and urgent clinic visit for intravenous HF therapy) in patients with HFrEF (EF<50%). CHAMPION, CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients; COMPASS-HF, Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure; D+L, DerSimonian and Laird; ED, emergency department; EF, ejection fraction; GUIDE-HF, Hemodynamic-Guided Management of Heart Failure; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; IHM, implantable haemodynamic monitor; I-V, inverse variance; LAPTOP-HF, Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy; REDUCE-HF, Reducing Events in Patients with Chronic Heart Failure.
Discussion
The main results of this meta-analysis support the use of IHM in patients with symptomatic HF (irrespective of EF), demonstrating a 26% reduction in the risk of total worsening HF events, including hospital admission. This is the first meta-analysis to include total HF events from all IHM randomised trials, including LAPTOP-HF and the recently reported GUIDE-HF. We also report for the first time meta-analyses of the effectiveness of IHM-guided care in patients with HFrEF and HFpEF. The finding of a reduction in total worsening HF events in all patients regardless of EF was also present in patients with HFrEF, who comprised the majority of patients. The same benefit was not consistent in patients with HFpEF.
Patients with an EF of <50% have been shown to have higher resting intracardiac and pulmonary pressures than those with HFpEF, and in turn, patients with higher pressures are at greater risk of decompensation from even small rises in pressure.21–23 The relative reduction of total worsening HF events with IHM monitoring observed in this meta-analysis was comparable with the magnitude of benefit found in patients with HFrEF treated with an angiotensin receptor blocker-neprilysin inhibitor in the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial (sacubitril/valsartan reduced HF hospitalisations by 21% compared with enalapril) and the sodium glucose cotransporter 2 inhibitor dapagliflozin in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial (dapagliflozin reduced HF hospitalisations by 30% compared with placebo).24 25
Mortality data were not reported from LAPTOP-HF, and only 231 deaths were reported during the overall short average follow-up in the other four trials. With low numbers of deaths and limited follow-up periods, no IHM trial demonstrated a reduction in mortality with IHM-guided care. Accordingly, the 22% reduction in the composite endpoint all-cause mortality and HF hospitalisation with IHM-guided care observed in this analysis was driven by the favourable effect on hospitalisations. The rate of HF hospitalisations calculated for the standard care arms in individual trials in this analysis ranged from 42 per 100 patient-years in REDUCE-HF to an estimated 147 per 100 patient-years in COMPASS-HF. The reported rate of total HF hospitalisations in the standard care arm of GUIDE-HF, which investigated the only currently available IHM (CardioMEMS) in the most contemporary HF population, was 49.7 per 100 patient-years.2 This was markedly higher than the composite event rate for total HF hospitalisations and cardiovascular deaths in the placebo group of DAPA-HF (21.6 per 100 patient-years).26 The substantially higher hospitalisation rates in the IHM trials highlight two considerations. First, that patients in these trials were highly selected and prognostically vulnerable. Second, rates of hospitalisation will differ between the healthcare setting in which the IHM trials were conducted (USA predominantly) and that of other diverse settings of care as indicated by event rates in more international contemporary HF trials. The effectiveness, including cost-effectiveness, of such a targeted intervention as IHM and the system of care required to deliver it may accordingly differ, depending on the setting and is an important consideration for future IHM studies (online supplemental appendix, table S3).
To date, the main source of information on the effect of IHM in patients with HFpEF has been the CHAMPION trial. In that trial, the rate of HF hospitalisation was 41 events per 100 patient-years in the IHM arm compared with 139 events per 100 patient-years in the standard care arm, giving a 70% relative risk reduction in HF hospitalisation among patients with an EF of ≥50% when treatment was guided by IHM.19 Again, the rate of HF hospitalisation was substantially higher in CHAMPION than observed in other contemporary trials of patients with HFpEF. In PARAGON-HF, the rate of HF hospitalisation and cardiovascular death in the valsartan group was 14.6 per 100 patient-years.27 The reliability of the relative reduction for HF hospitalisation in patients with HFpEF reported in the CHAMPION trial is limited by the small number of patients (n=66) included in that analysis. Our new meta-analysis adds data on patients with HFpEF from COMPASS-HF and GUIDE-HF. With an additional 562 patients and 366 events, the fixed effect model demonstrated patients with HFpEF receiving IHM-guided treatment had a 28% relative reduction in total worsening HF events. In the random-effects model, the average reduction was similar, but the CIs were wide, encompassing a potential 68% reduction to a 14% increase in such events with IHM-guided care. The difference in significance levels between models is in keeping with the high heterogeneity in the pooled sample. The effectiveness of IHM-guided treatment in patients with HFpEF remains uncertain and further trials are required with analyses in this population pre-specified in the study designs. On the available evidence, the patients who might benefit the most from IHM would have several characteristics, including a history of volume overload (as indicated by a recent HF hospitalisation), persisting symptoms and an EF of <50%.
This analysis has limitations. First, only two trials examined an IHM that is currently available (CardioMEMS), and three IHMs were examined over 18 years of investigation during which time the background management of patients with HF evolved with advancements in drug and device therapies.24 25 28–30 Each IHM measured a different haemodynamic parameter. However, the IHM’s haemodynamic measures were physiologically related (eg, ePAD (COMPASS-HF) provided a surrogate estimate for left atrial pressure (LAPTOP-HF)). Potential sources of bias exist. REDUCE-HF was terminated following concerns regarding 4-year pressure sensor failure in patients from other Chronicle device studies. A total of 400 patients from a recruitment target of 1300 patients had enrolled at the point of study termination. Consequently, REDUCE-HF was underpowered, with only 181 events reported compared with the 648 events expected. LAPTOP-HF was also terminated early after 1 year due to periprocedural safety concerns,11 and mortality data from this trial were not available. The meta-analysis effect estimates may have changed had both the REDUCE-HF and LAPTOP-HF trials achieved target recruitment. However, the inclusion of these studies in the meta-analysis reduced selection bias by including at least 1 year of follow-up data on clinically relevant outcomes from these RCTs. Based on patients in REDUCE-HF and LAPTOP-HF having a mean EF of 23%±7% and 30%±15%, respectively, both trials were included in the HFrEF analysis. The initial REDUCE-HF inclusion criteria also required participants to have an implantable cardioverter–defibrillator (ICD), favouring recruitment from a population with more severe HFrEF, the patient group in whom ICD implantation predominates. We cannot, however, completely exclude the possibility that some patients had EFs above these ranges. Individual cohort numbers were not available from all studies for all EF groups. We did not have individual participant level data to test the interaction between EF and IHM-guided care.
Conclusions
IHM-guided treatment reduced total HF hospitalisation and worsening HF events. In subgroup analyses, patients with HFrEF appear to benefit from IHM-guided care, but whether the same benefit is present in patients with HFpEF remains uncertain. Further trials with pre-specified analyses of patients with an EF of ≥50% are required.
Footnotes
Contributors: JPC conceived and designed the study, extracted the data, performed the analyses and contributed to the writing of the manuscript. MMYL extracted the data, performed the analyses and contributed to the writing of the manuscript. JJVM, RSG and MCP contributed to the writing of the manuscript. PSJ conceived and designed the study, contributed to the writing of the manuscript and is the guarantor of this study.
Funding: JJVM, MCP and PSJ are supported by a British Heart Foundation Centre of Research Excellence Grant (RE/18/6/34217).
Competing interests: JPC and MMYL have no declarations. RSG received research support from Abbott and Boston Scientific and speaker fees from Abbott and Boston Scientific. JJVM received payments through Glasgow University from work on clinical trials, consulting and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardurion, Cytokinetics, DalCor, GSK, KBP Biosciences, Novartis, Pfizer and Theracos, and personal payments from Abbott, Hikma, Ionis, Sun Pharmaceuticals and Servier. MCP received lecture fees from AstraZeneca and Eli Lilly; personal fees from Novo Nordisk, AstraZeneca, NAPP Pharmaceuticals, Takeda Pharmaceutical, Alnylam, Bayer, Resverlogix and Cardiorentis; and grants and personal fees from Boehringer Ingelheim and Novartis. PSJ received consulting fees, advisory board fees and lecture fees from Novartis; advisory board fees from Cytokinetics; and grant support from Boehringer Ingelheim.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. All five trials included in this meta-analysis were approved by a local ethics committee at each of the participating trials sites and complied with the Declaration of Helsinki. The participants gave informed consent to participate in the study before taking part.
References
- 1. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet 2021;398:991–1001. 10.1016/S0140-6736(21)01754-2 [DOI] [PubMed] [Google Scholar]
- 3. Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 4. Ost D, Tepper J, Mihara H, et al. Duration of anticoagulation following venous thromboembolism: a meta-analysis. JAMA 2005;294:706–15. 10.1001/jama.294.6.706 [DOI] [PubMed] [Google Scholar]
- 5. Hernán MA. The hazards of hazard ratios. Epidemiology 2010;21:13–15. 10.1097/EDE.0b013e3181c1ea43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gillies CL, Abrams KR, Lambert PC, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007;334:299. 10.1136/bmj.39063.689375.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ 2011;343:d2090. 10.1136/bmj.d2090 [DOI] [PubMed] [Google Scholar]
- 8. DerSimonian R, Laird N. Meta-Analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 9. Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the heart failure Society of America, heart failure association of the European Society of cardiology, Japanese heart failure Society and writing Committee of the universal definition of heart failure. Eur J Heart Fail 2021;23:352–80. 10.1002/ejhf.2115 [DOI] [PubMed] [Google Scholar]
- 10. Adamson PB, Gold MR, Bennett T, et al. Continuous hemodynamic monitoring in patients with mild to moderate heart failure: results of the reducing decompensation events utilizing intracardiac pressures in patients with chronic heart failure (REDUCEhf) trial. Congest Heart Fail 2011;17:248–54. 10.1111/j.1751-7133.2011.00247.x [DOI] [PubMed] [Google Scholar]
- 11. Abraham WT, Adamson PB, Costanzo MR, et al. Hemodynamic monitoring in advanced heart failure: results from the LAPTOP-HF trial. J Card Fail 2016;22:940. 10.1016/j.cardfail.2016.09.012 [DOI] [Google Scholar]
- 12. Jahn-Eimermacher A. Comparison of the Andersen–Gill model with Poisson and negative binomial regression on recurrent event data. Comput Stat Data Anal 2008;52:4989–97. 10.1016/j.csda.2008.04.009 [DOI] [Google Scholar]
- 13. Rogers JK, Jhund PS, Perez A-C, et al. Effect of rosuvastatin on repeat heart failure hospitalizations: the corona trial (controlled rosuvastatin multinational trial in heart failure). JACC Heart Fail 2014;2:289–97. 10.1016/j.jchf.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 14. Zile MR, Desai AS, Costanzo MR, et al. The GUIDE-HF trial of pulmonary artery pressure monitoring in heart failure: impact of the COVID-19 pandemic. Eur Heart J 2022;43:2603–18. 10.1093/eurheartj/ehac114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abraham WT. Cumulative hazard rate for heart failure hospitalization over 12 months in LAPTOP-HF. ESC/HFA heart failure Congress 2017; Paris: European Society of cardiology / heart failure association 2017.
- 16. Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol 2008;51:1073–9. 10.1016/j.jacc.2007.10.061 [DOI] [PubMed] [Google Scholar]
- 17. Abraham WT, Stevenson LW, Bourge RC, et al. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the champion randomised trial. Lancet 2016;387:453–61. 10.1016/S0140-6736(15)00723-0 [DOI] [PubMed] [Google Scholar]
- 18. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–66. 10.1016/S0140-6736(11)60101-3 [DOI] [PubMed] [Google Scholar]
- 19. Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:935–44. 10.1161/CIRCHEARTFAILURE.113.001229 [DOI] [PubMed] [Google Scholar]
- 20. Zile MR, Bourge RC, Bennett TD, et al. Application of implantable hemodynamic monitoring in the management of patients with diastolic heart failure: a subgroup analysis of the COMPASS-HF trial. J Card Fail 2008;14:816–23. 10.1016/j.cardfail.2008.07.235 [DOI] [PubMed] [Google Scholar]
- 21. Zile MR, Bennett TD, St John Sutton M, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008;118:1433–41. 10.1161/CIRCULATIONAHA.108.783910 [DOI] [PubMed] [Google Scholar]
- 22. Zile MR, Adamson PB, Cho YK, et al. Hemodynamic factors associated with acute decompensated heart failure: part 1--insights into pathophysiology. J Card Fail 2011;17:282–91. 10.1016/j.cardfail.2011.01.010 [DOI] [PubMed] [Google Scholar]
- 23. Stevenson LW, Zile M, Bennett TD, et al. Chronic ambulatory intracardiac pressures and future heart failure events. Circ Heart Fail 2010;3:580–7. 10.1161/CIRCHEARTFAILURE.109.923300 [DOI] [PubMed] [Google Scholar]
- 24. McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 25. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 26. Jhund PS, Ponikowski P, Docherty KF, et al. Dapagliflozin and recurrent heart failure hospitalizations in heart failure with reduced ejection fraction: an analysis of DAPA-HF. Circulation 2021;143:1962–72. 10.1161/CIRCULATIONAHA.121.053659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-Neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–20. 10.1056/NEJMoa1908655 [DOI] [PubMed] [Google Scholar]
- 28. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med Overseas Ed 2021;385:1451–61. 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 29. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with Empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 30. Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-Resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329–38. 10.1056/NEJMoa0906431 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2022-321885supp001.pdf (549.7KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.