Abstract
The gut microbiota‐brain axis has been recognized as a network of connections that provides communication between the gut microflora and both central and autonomic nervous system. The gut microbiota alteration has been targeted for therapy in various neurodegenerative and psychiatric disbalances. Psychobiotics are probiotics that contribute beneficially to the brain function and the host mental health as a result of an interaction with the commensal gut bacteria, although their mechanism of action has not been completely revealed. In this state‐of‐art review, the findings about the potential therapeutic effects of the psychobiotics alone or in combination with conventional medicine in the treatment of neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease, as well as in some psychiatric diseases like depression, schizophrenia, and bipolar disorder, have been summarized. The evidence of the psychobiotics therapeutic outcomes obtained in preclinical and clinical trials have been given respectively for the observed neurodegenerative and psychiatric disorders.
Keywords: alzheimer disease, gut microbiota, parkinson disease, psychiatric disease, psychobiotics
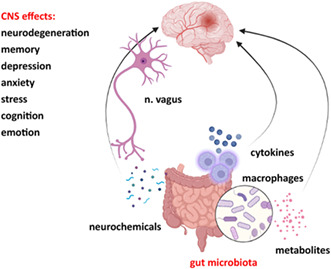
Gut microbiota communicates with the brain in a bidirectionnal manner via n.vagus, microbial metabolites such as short chain fatty acids, cytokines, and neurochemicals.Gut microbiota dysbiosis has been implicated in pathophysiology of neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases, as well as of the psychiatric disorders like depression, schizophrenia and bipolar disorder.
1. INTRODUCTION
The probiotics usage has been known much earlier than microbes were discovered. Fermented dairy products were painted on Egyptian hieroglyphs, and Tibetan peasants traditionally used fermented yak milk to preserve it during long journeys. In the early 19th century, scientists noticed the apparent health effects of fermented dairy products. Although Pasteur identified the responsible bacteria and yeasts for the fermentation process, no health effects had been attributed to the microbes. 1 In 1908, Metchnikoff associated the Bulgarians' long life span with the Lactobacillus species from regularly consuming fermented milk and their presence in the gut. 2 Tissier isolated Bifidobacterium species in infants and claimed that they could displace gut pathogens. 3 These findings have catalyzed research into health‐promoting microbes and their role in disease prevention. In one of the earliest human studies, in 1922, Lactobacillus acidophilus was used in 30 patients with chronic constipation, diarrhea, and eczema, showing improvement in all three conditions. 4 Soon after, the beneficial effects of Lactobacillus acidophilus were confirmed in patients with constipation and mental illness. 5 Again, scientists brought to the light bidirectional communication between brain and gut, claiming that emotional state could modify gut function. Moreover, Canon 6 showed that certain parts of the gastrointestinal tract, as well as the urinary bladder, were susceptible to mental state.
In the 21st century, the growing scientific research highlighted the bond between the brain and the gut. 7 , 8 Alterations in the gastrointestinal microbiome, known as dysbiosis, have been observed during the onset and development of mental disorders. Numerous adverse psychiatric reactions to antibiotics have been reported, even in patients with no history of psychiatric illness. 9 To manage depression, Logan and Katzman (2005) 10 proposed the probiotics as concomitant therapy with antidepressants. Many studies were done to shed light on the interplay between the gut microbiome and the brain, and thus, the digestive system‐brain axis concept was created. 11 Preclinical and clinical studies suggested that administration of beneficial microbes may reduce depression, anxiety, stress, neuroinflammation, neurodegeneration, eliminate panic attacks, hypochondriac behavior and somatization, and improve cognition. 12 Furthermore, early‐life perturbations in the gastrointestinal microbiome influenced neurodevelopment, with emerging mental health issues years later. 13
Dinan et al. (2013) 14 coined the term psychobiotics, as probiotics, which, if taken in appropriate amounts, have positive mental health effects. Magnetic resonance spectroscopy revealed that the oral administration of Lactobacillus leads to increased levels of γ‐aminobutyric acid (GABA), N‐acetylaspartate, and glutamate in the brain, assuming that these neuroactive molecules were enrolled in the mechanism of Lactobacillus strain action. Sarkar et al. (2016) 15 suggested that the definition of psychobiotics could be expanded to any substance that confer positive alterations in the microbiome. The prebiotics could also be part of the psychobiotics, supporting the psychobiotic bacteria growth and thus contributing mental health. Afterward, similar terminology has emerged: paraprobiotics, also known as inactivated probiotics, and postbiotics, which are non‐viable bacterial cells, their products, or metabolites of live probiotic microorganisms. Both of them showed physiological benefits to the host. 16 Recently, it has been suggested that the concept of postbiotics should be expanded to comprise paraprobiotics in the definition. 17 Although many details in the mechanism of action remain unraveled, today, it is believed that the intestinal microbiome affects the brain and human behavior.
2. GUT MICROBIOTA‐BRAIN AXIS
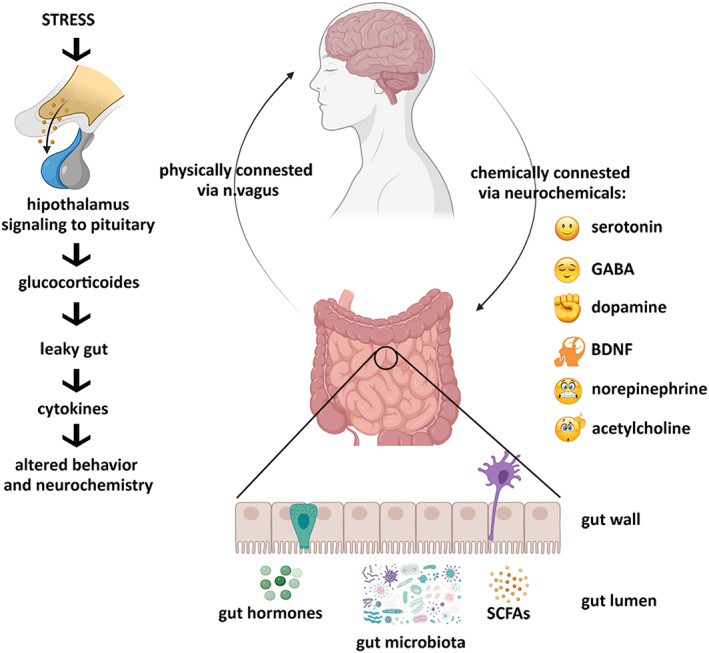
A key step in understanding the psychobiotics mechanism lies in studying the ongoing communication between the gut microbiota and the brain. 15 In humans, substantial evidence of this interaction happened more than 25 years ago. Patients suffering from hepatic encephalopathy showed the dramatic improvements after oral antibiotics. 18 The intestinal flora interacts closely in a bidirectional manner, linking to the intestine permeability, immune system, and entero‐endocrine signaling with the cognitive and emotional brain centers. 19 The gut microbiota‐brain axis binds the neuroendocrine and neuroimmune systems, as well as the autonomic nervous system with the intestinal microbiota. 19 , 20 , 21
The hypothalamic–pituitary–adrenal axis (HPA) is considered to be the main part of the neuroendocrine system that provides an adequate hormonal response to the stress. 22 The stress hormones, glucocorticoids, disrupt gut barrier integrity through alterations in tight junctions, leading to a leaky gut and triggering inflammatory immune response (Figure 1). 23
FIGURE 1.
Gut microbiota–brain axis. Brain acts on the gut microbiome through the HPA axis and vagus nerve. In turn, gut dysbiosis acts on the brain through the vagus mechanism and immune endocrine pathway to cause neuronal degeneration and behavioral abnormalities.
It is recognized that early colonization of the intestinal microbiota affects certain aspects of brain function and behavior, including neuroendocrine responses to stress. Sudo et al. (2004) 24 showed that germ‐free mice exerted enhanced physiological reactions to stress in comparison to control. However, following the gut microbiota recolonization with probiotics, these abnormal reactions were reversible. The manifestation of homeostatic effects of probiotics on neuroendocrine physiology is highly significant, and it indicates new therapeutic possibilities. 25
The development of the intestinal immune system largely depends on exposure to microorganisms. 26 Gut microbiome alteration is associated with aberrant immune response due to the overproduction of cytokines through the HPA axis modulation. 27 The elevation of circulating pro‐inflammatory cytokines IL‐6 and TNF‐α, with concomitant activation of microglia, brain resident macrophages, is involved in induced depressive and anxiety states, and other affective disorders. 28 , 29 Nonetheless, certain probiotics can reverse the microglia activation and decrease the levels of pro‐inflammatory cytokines, mediating anti‐inflammatory response. 15
The vagus nerve is pivotal in coordinating parasympathetic activity with afferent terminals under the intestinal epithelium. Gut microbiota signals, transported to the brain, alter host behavior and possibly cause lethargy, loss of appetite, depression, or anxiety state. 30 In several animal studies, it has been found that n. vagus mediated the interplay between psychobiotics and their psychophysiological effects, since vagotomy has eliminated the response to psychobiotic administration. 31 , 32 , 33
The enteric nervous system (ENS) is responsible for coordinating the various digestive functions by forward and backward brain signal propagation, all via the vagus nerve. 34 Myenteric neurons are close to the gut lumen, facilitating their contact with the microbiota. 8 In addition, plenty of evidence suggested that gut bacteria modulated the ENS, regulating electrophysiological thresholds of myenteric neurons. 32 , 35 , 36 , 37
According to both preclinical and clinical evidences, changes in beneficial bacteria may have significant health consequences, while certain factors such as infection, drug use, diet, exercise, environment, social interactions, and stress can alter the microbiome. 12 , 38 , 39 This determined changes in motility and intestinal secretion, caused visceral hypersensitivity, and lead to modification of the enteroendocrine and immune system. 19
3. PSYCHOBIOTICS AND NEUROACTIVE MOLECULES
Gut microbiota produces a broad range of neuroactive molecules, signaling in the crosstalk between the gut microbiome and host metabolism (Figure 1). Due to the chemical and functional resemblance, these metabolites act as human neurotransmitters or neuromodulators. Serotonin (5‐HT), dopamine (DA), noradrenaline (NA), GABA, acetylcholine (Ach), as well as short‐chain fatty acids (SCFAs), produced by the gut microbiota via the metabolism of indigestible fibers, are of special interest. 40 , 41 , 42 , 43
Altered levels of 5‐HT and DA are implicated in several mental health and neurological diseases. 8 , 44 NA is able to modulate cognitive functions, learning, memory processes, and mood disturbances. 45 GABA and Ach are the main inhibitory/excitatory neurotransmitters. Muller et al. (2021) 46 demonstrated that SCFAs composition is associated with psychiatric and gastrointestinal symptoms in adults with affective or anxiety disorders. Therefore, psychobiotics control the neural excitatory‐inhibitory balance and modulation of the host's response to anxiety and depression. Due to these effects of microbially produced neuroactive molecules, psychobiotics have been suggested as a promising alternative or supportive therapy in treating neuropsychiatric disorders. In Table 1 results of different psychobiotic treatments associated with neurodegenerative and psychiatric diseases are summarized.
TABLE 1.
Summary of different psychobiotic treatment results in neurodegenerative and psychiatric diseases
| Subjects | Treatment | Outcome | Authors, year |
|---|---|---|---|
| Alzheimer's disease | |||
|
3 × Tg‐AD† mice |
Lactobacillus plantarum PS128 |
|
Huang et al., 2021 47 |
| 15–17 months old Lister Hooded rats | Lactobacillus acidophilus CUL60, Lactobacillus acidophilus CUL21, Bifidobacterium bifidum CUL20, and Bifidobacterium lactis CUL34 |
|
O'Hagan et al., 2017 48 |
| Aβ†‐injected ddY mice | Bifidobacterium breve A1 |
|
Kobayashi et al., 2017 49 |
|
D‐galactose‐injected Wistar rats |
Lactobacillus plantarum MTCC1325 |
|
Nimgapalle and Kuna, 2017 50 |
| 3 × Tg‐AD† mice |
SLAB51 formulation: Streptococcus thermophilus, Bifidobacterium. longum, Bifidobacterium breve, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus. paracasei, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus brevis |
|
Bonfilli et al., 2017 51 |
| Aβ†‐injected Wistar rats | Lactobacillus acidophilus, Lactobacillus fermentum, Bifidobacterium lactis, and Bifidobacterium longum |
|
Athari et al., 2018 52 |
| 5XFAD Tg mice | Lactobacillus plantarum C29 |
|
Lee et al., 2021 53 |
| AD† patients | Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum |
|
Akbari et al., 2016 54 |
| AD† patients | Lactobacillus fermentum, Lactobacillus plantarum, Bifidobacterium lactis, Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum |
|
Agahi et al., 2018 55 |
| AD† patients | Lactobacillus casei W56, Lactococcus lactis W19, Lactobacillus acidophilus W22, Bifidobacterium lactis W52, Lactobacillus paracasei W20, Lactobacillus plantarum W62, Bifidobacterium lactis W51, Bifidobacterium bifidum W23 and Lactobacillus salivarius W24 |
|
Leblhuber et al., 2018 56 |
| AD† patients | Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium longum, and selenium |
|
Tamtaji et al., 2019 57 |
| Parkinson's disease | |||
| MitoPark PD† mice | Bifidobacterium bifidum, Bifidobacterium longum, Lactobacillus rhamnosus, Lactobacillus rhamnosus GG, Lactobacillus plantarum LP28, and Lactococcus lactis subsp. lactis |
|
Hsieh et al., 2020 58 |
| MPTP†‐induced PD† in C57BL/6 mice | Lactobacillus rhamnosus GG, Bifidobacterium animalis lactis, and Lactobacillus acidophilus |
|
Srivastav et al., 2019 59 |
| 6‐OHDA†‐induced PD† in C57BL/6 mice | SLAB51 formulation |
|
Castelli et al., 2020 60 |
| MPTP†‐induced PD† in C57BL/6 mice | Lactobacillus plantarum CRL2130, Streptococcus thermophilus CRL807, and Streptococcus thermophilus CRL808 |
|
Perez Visnuk et al., 2020 61 |
| PD† patients | Lactobacillus acidophilus and Bifidobacterium infantis |
|
Georgescu et al., 2016 62 |
| PD† patients | Enterococcus faecium, Lactobacillus acidophilus, Lactobacillus paracasei, Lactobacillus rhamnosus, Bifidobacterium longum, Bifidobacterium bifidum, and Lactobacillus reuteri |
|
Tan et al., 2021 63 |
| PD† patients | Lactobacillus casei Shirota |
|
Cassani et al., 2011 64 |
| PD† patients | Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri, and Lactobacillus fermentum |
|
Borzabadi et al., 2018 65 |
| PD† patients | Streptococcus salivarius subsp. thermophilus, Enterococcus faecium, Lactobacillus rhamnosus GG, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus delbrueckii subsp bulgaricus, and Bifidobacterium (breve and animalis subsp. lactis |
|
Barichella et al., 2016 66 |
| Depression | |||
| Mice exposed to chronic restraint stress | Bifidobacterium adolescentis |
|
Guo et al., 2019 67 |
| Corticosterone‐induced depression in mice | Lactobacillus paracasei PS23, live or heat‐killed | Live PS23:
|
Wei et al., 2019 68 |
| Healthy Swiss mice | Lactobacillus plantarum 286 |
|
Barros‐ Santos et al., 2020 69 |
| Wistar rats exposed to chronic unpredictable mild stress | Lactobacillus rhamnosus JB‐1 |
|
Kochalska et al., 2020 70 |
| C57BL/6J mice exposed to chronic stress | Bifidobacterium breve CCFM1025 |
|
Tian et al., 2020 71 |
| Corticosterone‐induced depression in Sprague–Dawley rats | Lactobacillus plantarum DP189 |
|
Zhao et al., 2020 72 |
| Lipopolysaccharide‐induced anxiety in adolescent CD1 mice | BGOS† |
|
Savignac et al., 2016 73 |
| C57BL/6J mice exposed to chronic psychosocial stress | FOS† + GOS† |
|
Burokas et al., 2017 74 |
| Sprague–Dawley rats exposed to chronic unpredictable mild stress | FOS† |
|
Chi et al., 2020 75 |
| C57BL/6J mice exposed to subchronic and mild social defeat stress | Heat‐killed Lactobacillus helveticus strain MCC1848 |
|
Maehata et al., 2019 76 |
| Healthy C57BL/6 mice | Heat‐killed Lactobacillus fermentum and Lactobacillus delbrueckii |
|
Warda et al., 2019 77 |
| Healthy C57BL/6J mice | Heat‐killed Enterococcus faecalis strain EC‐12 |
|
Kambe et al., 2020 78 |
| Adults with moderate mood swings | Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 |
|
Romijn et al., 2017 79 |
| Moderately stressed adults | Lactobacillus plantarum DR7 |
|
Chong et al., 2019 80 |
| Moderately stressed adults | Lactobacillus plantarum P8 |
|
Lew et al., 2019 81 |
| Healthy adults | Heat‐killed Lactobacillus paracasei MCC1849 |
|
Murata et al., 2018 82 |
| Young adult students preparing for the national examination | Heat‐inactivated Lactobacillus gasseri CP2305 |
|
Nishida et al., 2019 83 |
| Patients with major depressive disorder | Lactobacillus plantarum 299v + SSRI |
|
Rudzki et al., 2019 84 |
| Adult patients with moderate depression | Lactobacillus casaei + Lactobacillus acidofilus + Lactobacillus bulgarigus + Lactobacillus rhamnosus + Bifidobacterium breve + Bifidobacterium longum + Streptococcus thermophilus + FOS + fluoxetine |
|
Ghorbani et al., 2018 85 |
| Adult patients with mild‐to‐moderate depression |
Lactobacillus helveticus Rosell® − 52 + Bifidobacterium longum Rosell® − 175 + SAMe† + magnesium oxide + vitamin B6 |
|
Ullah et al., 2022 86 |
| Adult patients with major depressive disorder |
Freeze‐dried Bifidobacterium breve CCFM1025 |
|
Tian et al., 2022 87 |
| Schizophrenia | |||
| Sprague Dawley rats | BGOS† + olanzapine |
|
Kao et al., 2018 88 |
| C57BL/6 mice on a high‐fat diet | Akkermansia muciniphilasub (Akk I subtype, GP01 strain) + olanzapine |
|
Huang et al., 2021 89 |
| Sprague Dawley rats | FOS† + GOS† |
|
Savignac et al., 2013 90 |
| BALB/c mice | Lactobacillus rhamnosus JB‐1 |
|
Janik et al., 2016 91 |
| C57BL/6J mice | FOS† + GOS† |
|
Burokas et al., 2017 92 |
| Sprague Dawley rats | BGOS† |
|
Gronier et al., 2018 93 |
| SCZ† patients | Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis Bb12 |
|
Dickerson et al., 2014 94 |
| Patients with SCZ† and schizoaffective disorder | Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis Bb12 |
|
Tomasik et al., 2015 95 |
| SCZ† patients | Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis Bb12 |
|
Severance et al., 2017 96 |
| Bipolar disorder | |||
| BD† patients | Bifidobacterium bifidum, Bifidobacterrium lactis, Bifidobacterium longum, and Lactobacillus acidophilus |
|
Shahrbabaki et al., 2020 97 |
| BD† patients |
Lactobacillus casei W56, Lactobacillus acidophilus W22, Lactobacillus paracasei W20, Bifidobacterium lactis W51, Lactobacillus salivarius W24, Lactococcus lactis W19, Bifidobacterium lactis W52, Lactobacillus plantarum W62, Bifidobacterium bifidum W23 |
|
Reininghaus et al., 2020 98 |
Abbreviations: AD, Alzheimer's disease; AchE, acetylcholine esterase; Ach, acetylcholine; ALT, alanine‐transaminase; AST, aspartate‐transaminase; Aβ, amyloid beta; BD, bipolar disorder; BDNF, brain‐derived neurotrophic factor; B‐GOS, Bimuno™ galacto‐oligosaccharide; CBM, complete bowel movements; CRP, C‐reactive peptide; FOS, fructo‐oligosaccharides; GIP, gastric inhibitory polypeptide; GOS, galacto‐oligosaccharides; GSH, glutathione; HAM‐D, Hamilton Depression Rating Scale; HDRS‐24, Hamilton Depression Rating scale‐24 Items; MADRS, Montgomery‐Asberg Depression Rating Scale; MAO‐B, monoamine oxidase B; MDA‐ malondialdehyde; MMSE, Mini‐Mental State Examination; MPTP, 1‐methyl‐4‐phenyl‐1,2,3,6,‐tetrahydropyridine; NMDA, N‐methyl–D‐aspartate; PANSS, Positive and Negative Syndrome Scale; PD, Parkinson's disease; PHQ‐9, Patient Health Questionnaire‐9; PPARγ, peroxisome proliferator‐activated receptor gamma; SAMe, S‐adenosyl‐L‐methionine disulfate p‐toluenesulfonate; SBM, spontaneous bowel movements; SCFAs, short‐chain fatty acids; †SCZ, schizophrenia; SOD, superoxide‐dismutase; TAC, total antioxidant capacity; TH, tyrosine hydroxylase; TYM, Test Your Memory; 6‐OHDA, 6‐hydroxydopamine.
4. NEURODEGENERATIVE DISEASES TREATMENT WITH PSYCHOBIOTICS
4.1. Alzheimer's disease
AD is a chronic neurodegenerative disease with a progressive decline in cognitive and memory function. Recently, poor gut microbiota diversity in AD patients has gained an ongoing interest followed by finding an additional molecular pathogenesis for AD. Novel findings suggest that autoimmune and autoinflammatory mechanisms are engaged in AD. 99 The data suggested that SCFAs, such as butyric, propionic, acetic acids, and microbial metabolites in colon reduced the AD neuropathological features and other neurodegenerative diseases by providing alternative energy sources to the brain. 100 Moreover, selected SCFAs may modulate neuroinflammation, a significant pathomechanism of the early and preclinical course of AD. Amyloid β abnormality, tau phosphorylation, neurotransmitter dysregulation, and oxidative stress in AD followed the derangement in the gut microbiota composition. 47 , 101 In addition, several probiotic strains, such as Lactobacillus plantarum and Bifidobacterium infantis, enhance gut barrier function via upregulation of tight junction expression and production of SCFAs. 102 Bearing in mind the richness of the gut milieu with endotoxins and amyloid β, the maintenance of secure gut barrier is requisite to avoid inflammation.
4.1.1. Preclinical probiotic supplementation in AD
Research on rodents showed that memory storage and cognition began to decline with age and were severely damaged in AD. 103 Germ‐free mice displayed a decreased level of tight junction protein and decreased level of brain‐derived neurotrophic factor (BDNF) and N‐methyl‐D‐aspartate (NMDA) receptor expression in the cortex and hippocampus. 104 BDNF and NMDA are proven to play a pivotal role in neuroplasticity, which loss is a significant indicator of the AD etiology. 105 GABA and glutamine levels, brain metabolites, are enhanced by long‐term administration of Lactobacillus spp. and Bifidobacterium spp. to aging rats, improving task‐specific memory. 48 Moreover, Li et al. (2020) 106 noticed a correlation between the gut microbiota changes and increased amyloid β deposition in mice through stimulation of the MAPK signaling pathway.
Attempts have been made to untangle the effects of different probiotic strains on AD. Kobayashi et al. (2017) 49 observed the anti‐inflammatory effects of Bifidobacterium breve A1 strain and amelioration of cognitive dysfunction in AD mice. Nimgampalle and Kuna (2017) 50 showed that Lactobacillus plantarum reduced amyloid β load and increased the acetylcholine level in the cortex and hippocampus, with improved spatial memory in AD mice. In addition, Bonfilli et al (2017) 51 reported that SLAB51 probiotic formulation (Bifidobacteria and Lactobacilli mixture) decreased amyloid β aggregations and positively influenced on inflammatory cytokines levels, preventing the onset and delaying the AD progression in early‐stage AD. These probiotic strains were found to restore synaptic plasticity, followed by a decrease in microglial activation and increased BDNF, improving cognitive function and spatial learning. 48 Another study found that the L.acidophilus, L.fermentum, B.lactis, and B. longum mixture attenuated the learning deficits and oxidative stress and improved spatial memory. 52 L. plantarum was also shown to regulate microglia activation, reduced amyloid β load via suppressing NF‐κB activation in AD mice. 53 These findings indicated a possible role of specific probiotic strains in enhancing memory and cognitive function.
4.1.2. Clinical probiotic supplementation in AD
The Firmicutes/Bacteroidetes ratio has emerged as an indicator of intestinal microbiota health. It is shown that AD patients have decreased Firmicutes level along with increased Bacteroidetes level. 107 Eskelinen et al. (2009) 108 reported (21‐year follow‐up study of 1409 volunteers, aged 65–79) that the 3–5 cups of coffee per day in the middle ages reduced the risk of developing AD. The neuroprotective effect of coffee could be attributed to the antioxidant activity of polyphenols and the coffee seed fibers effected increased Firmicutes/Bacteroidetes ratio, bringing to reduced inflammation. 109 However, the beneficial outcome of probiotics in AD patients is still lack of evidence because only 4 clinical trials based on Lactobacillus and Bifidobacterium lasting for maximum 12 weeks have been conducted. The results indicated that probiotics played a significant role in mitigating AD‐like symptoms in a multi‐targeted approach enhancing cognitive function. 54 , 55 , 56 , 57 In spite of current supportive evidence on health benefits, large‐scale, long‐term, randomized clinical trials are needed to posit a curative role of probiotics in AD patients.
4.2. Parkinson's disease
Parkinson's disease (PD) is a neurodegenerative disorder associated with the progressive loss of dopaminergic neurons in substantia nigra and abnormal intracellular aggregation of α‐synuclein. 110 Motor impairment and characteristic brain pathology do not appear as symptoms until a fairly advanced stage of the disease. On the other hand, intestinal dysfunction, such as bloating, delayed gastric emptying, constipation, prolonged intestinal transit time with incomplete defecation, occurs years before the initiation of motor impairment. 111 , 112
Dysbiosis in gut microbiota has also been reported in PD. Decrease of Prevotellaceae in stool samples of PD patients, 113 along with increased Lactobacilliceae, are found to be associated with lower ghrelin level, a gut hormone that maintains normal dopamine function. 114 A study observed an antiinflammatory bacteria depletion, such as genus Blautia, Roseburia, and Coprococcus in stool samples of PD patients, along with a reduction in Lactococcus bacteria. 115 It is assumed that this shift from predominantly antiinflammatory phenotype toward the proinflammatory phenotype of the gut microbiota contributes the increased gut permeability and decreased dopamine function. 116 Furthermore, a significantly reduced abundance of SCFA (butyrate, acetate, propionate)‐producing bacteria was found in PD patients. 117
4.2.1. Preclinical probiotic supplementation in PD
PD mice supplemented daily with a six probiotic strains mixture (B. bifidum, B. longum, L. rhamnosus, L. rhamnosus GG, L. plantarum, and L. lactis) for 16 weeks showed better balance, gait, and coordination which persisted for 8 weeks. Moreover, a reduced dopaminergic neuronal degeneration was observed. 58
Neuroprotective probiotics effect was also reported in rotenone‐ and 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP)‐induced PD mice model. L. rhamnosus GG, B. animalis lactis, and L. acidophilus mixture enhanced the butyrate production and elevated the BDNF level, which are associated with rescuing dopaminergic neurons from toxicity and promoting cell survival and proliferation. Furthermore, an inhibition of monoamine oxidase B contributed to the dopamine synthesis and survival of dopaminergic neurons. 59 In accordance, the study conducted on a 6‐hydroxydopamine‐induced PD mouse model with a nine probiotic strains cocktail named SLAB51 showed that the reduced neuronal loss was mediated through the BDNF upregulation. Also, the peroxisome proliferator‐activated receptor gamma (PPAR‐γ) signaling pathway was activated, with ameliorating antiinflammatory and antioxidative effects., 60 Perez Visnuk et al. (2020) 61 reported proinflammatory cytokines IL‐6 and TNF‐α reduction, with an antiinflammatory cytokine IL‐10 increment in PD mice treated with probiotics.
4.2.2. Clinical probiotic supplementation in PD
Several clinical studies reported beneficial probiotics effects in PD patients, especially in combating constipation, a common symptom in PD with a prevalence of up to 70%. 62 , 63 , 118 The first clinical trial conducted in 2011 highlighted that fermented milk with Lactobacillus casei administration improved stool consistency, defecation, decreased bloating, and abdominal pain in PD patients suffering from constipation. 64 Delayed gastric emptying, also common in PD patients, was found to be accelerated after the Lactobacillus reuteri administration. 119 In the randomized, double‐blind, placebo‐controlled clinical study, L. acidophilus, L. fermentum, and B. bifidum supplementation for 12 weeks reduced the expression of proinflammatory cytokines (IL‐1, TNF‐α) and oxidative markers, and increased the antiinflammatory factors expression (TGF‐β, PPAR‐γ). 65 According to Barichella et al. (2016), 66 the number of spontaneous and total bowel movements were improved in PD patients treated with probiotics for 4 weeks.
5. POSSIBLE PSYCHOBIOTIC TREATMENT IN THE MOST COMMON PSYCHIATRIC DISEASES
5.1. Depression
Although the depression is a complex chronic mood disorder associated with various etiology factors, there is a growing scientific evidence that the intestinal microbiota derangement is implicated in its pathophysiology. 120 Luo et al. (2018) 121 showed that changes in the gut microbiota composition altered mice behavior toward anxiety, depression, and even autism. The fecal microbiota transplantation performed from depression suffering patients into rats with entire microbiota previously removed, resulted in depression‐like behavior of the animals, suggesting that the transplantation of the abnormal microbiome could lead to the depression “transmission.” 122 Clinical studies indicated that the gut microbiota of depressed patients differs significantly from healthy subjects. 8 Antibiotics can also destroy important intestinal microorganisms and thus induce digestive‐brain dysfunction, which may cause an increased incidence of various diseases, including mental disorders like depression. 123
The signal dysfunction of GABA, a major inhibitory neurotransmitter in the CNS, is associated with anxiety and depression. Lactobacillus and Bifidobacterium are capable of metabolizing glutamate to GABA. An in vivo experiment with L. rhamnosus reported alterations in GABA receptor expression in brain regions related to stress. 8 In addition, pathogens and their metabolites may induce brain inflammation through circulation and cytokine cascade reactions, which further affect the various brain processes involved in mood and behavior. 124 Pro/prebiotics exerted the beneficial effects as adjuvant therapy in mood disorders through the regulation of inflammatory markers, neurotransmission of serotonin, GABA, and BDNF and reducing HPA activity. 14 , 125
5.1.1. Preclinical probiotic supplementation in depression
The psychobiotics positive effects in depressive disorders are studied mostly on three rodent models, such as the corticosterone‐induced depression model, chronic unpredictable mild stress, and chronic restraint stress model. The most tested probiotics were L. plantarum, L. casei, L. rhamnosus, B. breve, B. infantis, and B. adolescentis. 67 , 68 , 69 , 70 , 71 , 72 These studies showed attenuation of proinflammatory cytokines (IL‐1β and TNF‐α), apoptosis (downregulation of BAX with upregulation of Bcl‐2), and the tryptophan increase (a serotonergic precursor), BDNF, and GABA, along with mitigation of anxiety‐ and depression‐like behavior.
The prebiotics effects on mood disorders have been examined to a much less extent than probiotics. Nevertheless, studies done on fructooligosaccharides (FOS) and galactooligosaccharides (GOS) corroborated the psychobiotics effects regarding the alteration of behavior and neurochemistry. FOS and GOS supplementation, alone or in combination, yielded antidepressant outcomes. 73 , 74 , 75 Supplementation with postbiotics, heat‐killed L. helveticus, 76 L. fermentum, and L. delbrueckii, 77 or heat‐killed Enteroccocus fecalis 78 subtly but distinctly changed the gut microbiota composition, reduced the baseline corticosterone level, and decreased anxiety‐ and depression‐like behavior.
5.1.2. Clinical probiotic supplementation in depression
The clinical trials’ results regarding the effects of different probiotic strains in mitigating depressive‐like behavior are inconsistent. Three clinical trials demonstrated that the probiotic supplementation did not reverse behavioral deficits in patients with depression. 79 , 80 , 81 In contrast, several studies showed beneficial neurobehavioral effects of probiotics and prebiotics in combination. 126 , 127 Moreover, heat‐killed Lactobacillus paracasei were efficient in maintaining the desirable mood state in healthy adults. 82 Heat‐killed Lactobacillus gasseri reduced anxiety and altered the gut microbiota composition in young adult students preparing for the national examination. 83
In concomitant therapy with antidepressants, probiotics exerted improvement in overall mood. 84 Ghorbani et al. (2018) 85 reported the improved depression clinical symptoms when specific probiotics and FOS were applied as an adjuvant therapy to the antidepressant drug fluoxetine.
5.2. Schizophrenia
Schizophrenia is a debilitating psychiatric disorder, with symptoms characterized as positive (aberrant flow of thoughts, delusions, hallucinations), and negative (social withdrawal, lack of motivation, apathy). 128 Recently, dysbiosis has also been noticed in schizophrenic patients. Shen et al. (2018) 129 and Zhang et al. (2020) 130 found reduced Roseburia and Faecalibacterium levels in stool samples in schizophrenia patients. Noteworthy, both genera produce butyrate, maintaining the intestinal barrier secure. 131 , 132 Schizophrenia incidence has been found to correlate with the Clostridium difficile gut increase due to the phenylalanine derivatives production which controls catecholamine levels. Catecholamines, especially dopamine, are notably elevated in schizophrenia. 133 , 134 Interestingly, a significant Lactobacilli elevation in schizophrenia patients was noticed, which even correlated with the severity of symptoms. 135
5.2.1. Preclinical probiotic supplementation in schizophrenia
In female rats with olanzapine therapy, an increase in Firmicutes and a decrease in Bacteroidetes, with elevated systemic inflammatory markers IL‐6, IL‐8, TNF‐α, and IL‐1β were reported. 136 In addition, several studies observed that prebiotics and probiotics could alleviate the side effects caused by antipsychotic therapy. Kao et al. (2018) 88 and Huang et al. (2021) 89 showed that the use of prebiotics and probiotics, respectively, as adjuvant therapy to olanzapine attenuated weight and metabolic disturbances in female animals.
Prebiotics (FOS and GOS) or certain probiotic strains (Lactobacillus rhamnosus and Bifidobacterium infantis) administration in animal models was found to elevate hippocampal levels of BDNF, GABA, and NMDA receptor gene expression. 90 , 91 , 92 , 93 These changes are relevant to schizophrenia, given that NMDA and GABA hypofunction in signaling, and decreased level of BDNF are thought to contribute to cognitive decline and psychotic symptoms. 137
5.2.2. Clinical probiotic supplementation in schizophrenia
Dickerson et al. (2014) 94 investigated the effects of a Bifidobacterium lactis and Lactobacillus rhamnosus formulation, as adjuvant antipsychotic treatment. The probiotics failed to impact positive and negative manifestations, however, they elevated the BDNF level and improved gastrointestinal symptoms. 95 The beneficial probiotics usage was observed in seronegative male schizophrenia patients, decreasing Candida albicans IgG serum levels. 96 Flowers et al. (2019) 138 found that the prebiotic raw unmodified potato starch supplementation in patients with antipsychotic therapy increased the Actinobacteria abundance. As patients with schizophrenia often suffer from enhanced stress response, compromised nutritional status, enhanced inflammatory status, and constipation, probiotics have promising therapeutic potential, alone or in combination with antipsychotics. 139
5.3. Bipolar disorder
Bipolar disorder is severe neuropsychiatric disease, characterized by extreme mood swings that include emotional highs (mania) and lows (depression) and may also have gut dysbiosis etiology. 140 Bipolar mania is twice as likely in patients who have been recently treated with antibiotics than in other patients. 141 Actinobacteria and Corinobacteria increased levels, and Faecalibacterium and Ruminococcaceae decreased levels were reported in bipolar patients' stool samples, which correlated with the severity of symptoms. 142 , 143 Although Aizawa et al. (2019) 144 reported no difference in the amount of Lactobacillus and Bifidobacterium stool samples of bipolar disorder patients comparing to healthy controls, a negative correlation was found in the Lactobacillus level and sleep, and the Bifidobacterium level and cortisol.
The HPA axis dysregulation, chronic inflammation, and abnormal monoamine function could cause bipolar disorder. 145 , 146 , 147 , 148 Hence, psychobiotic supplementation could be useful through the modulation of the aforementioned bipolar disorder causes and may help in treating microbiome dysbiosis and increased intestinal permeability. Also, given the high prevalence of gastrointestinal symptoms (diarrhea, satiety) in this patients, psychobiotic supplementation could form a potential add‐on therapy.
5.3.1. Clinical probiotic supplementation in bipolar disorder
Akkasheh et al. (2016) 149 reported that probiotics containing Bifidobacterium bifidum, Lactobacillus casei, and Lactobacillus acidophilus significantly reduced depressive symptoms. In line with this, Shahrbabaki et al. (2020) 97 indicated that probiotic consumption alleviated the severity of mania and depression over time. Significant improvements to attention and psychomotor processing speed were achieved after 1 and 3 months of probiotics treatment, respectively, indicating the potential beneficial effects of probiotics in improving cognitive function. 98 The lower rehospitalization rate was reported after probiotic supplementation as add‐on therapy in bipolar disorder patients . 150
6. CONCLUSION
“Let food be thy medicine and medicine be thy food” is one of the antient quote that is so important nowadays. 151 Recent studies have justified it considering a modified version: “Let food for your microbes be medicine for your brain.” 11 Abnormal microbiota is undoubtedly involved in the etiology and pathophysiology of neurodegenerative disorders, behavioral and mood alterations, and it is likely to be the target of future therapies. However, in line with differences in food preferences, environment, and lifestyle, an outstanding issue is to define the normal gut microbiota, due to interindividual and geographic differences in gut microbiota composition.
Studying the whole ecosystem, gut microbiota and probiotics, is requisite in understanding the interplay between the gut and host health. The novel class of probiotics – psychobiotics – has shown to be helpful in improving CNS functions, such as neuronal degeneration, memory, depression, anxiety, and mood, modulating the HPA, inflammation, and neurochemical production. The usage of psychobiotics alone or in combination with conventional medicine could contribute to the development of new therapeutic strategies; however, more research is needed due to the scarcity of the clinical data.
Not all psychobiotics are probiotics, and vice versa, not all probiotics have psychobiotic potential. An increasing number of preclinical and clinical research reported these promising psychobiotics effects, with no side effects. At present, the discrepancies between the results from laboratories occur due to the rapid microbiome testing commercialization leading to the misinterpretation of the results and the lack of consensus in general. Therefore, multi‐omics approach would enable comprehensive insight into the linkage between the gut microbiota composition and related diseases, as a promising tool for further research.
CONFLICT OF INTEREST
The authors declare they have no competing interests.
ACKNOWLEDGMENT
This work was supported by the Autonomous Province of Vojvodina, Republic of Serbia, Grants No: 142‐451‐3120/2022‐01.
Drljača J, Milošević N, Milanović M, Abenavoli L, Milić N. When the microbiome helps the brain‐current evidence. CNS Neurosci Ther. 2023;29(Suppl. 1):43‐58. doi: 10.1111/cns.14076
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Barnett JA. A history of research on yeasts 2: Louis Pasteur and his contemporaries, 1850‐1880. Yeast. 2000;16(8):755‐771. doi: [DOI] [PubMed] [Google Scholar]
- 2. Podolsky SH. Metchnikoff and the microbiome. Lancet. 2012;380(9856):1810‐1811. doi: 10.1016/s0140-6736(12)62018-2 [DOI] [PubMed] [Google Scholar]
- 3. Tissier H. Treatment of intestinal infections using bacterial flora of the intestine. Crit Rev Soc Biol. 1906;60:359‐361. [Google Scholar]
- 4. Rettger LF, Cheplin HA. Bacillus acidophilus and its therapeutic application. Arch Intern Med. 1922;29(3):357‐367. [Google Scholar]
- 5. Kopeloff N, Blackman N, McGinn B. The incidence of Lactobacillus acidophilus in adults. J Infect Dis. 1932;1:426‐429. [Google Scholar]
- 6. Canon WB. The influence of emotional states on the functions of the alimentary canal. Am J Med Sci. 1909;137:480‐487. [Google Scholar]
- 7. Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin‐releasing factor (CRF) mRNA, median eminence CRF content and stress‐induced release in adult rats. Brain Res Mol Brain Res. 1993;18(3):195‐200. doi: 10.1016/0169-328x(93)90189-v [DOI] [PubMed] [Google Scholar]
- 8. Foster JA, McVey Neufeld KA. Gut‐brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305‐312. doi: 10.1016/j.tins.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 9. Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21(6):738‐748. doi: 10.1038/mp.2016.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Logan AC, Katzman M. Major depressive disorder: probiotics may be an adjuvant therapy. Med Hypotheses. 2005;64(3):533‐538. doi: 10.1016/j.mehy.2004.08.019 [DOI] [PubMed] [Google Scholar]
- 11. Cryan JF, O'Riordan KJ, Cowan CSM, et al. The Microbiota‐Gut‐Brain Axis. Physiol Rev. 2019;99(4):1877‐2013. doi: 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- 12. Karakan T, Ozkul C, Küpeli Akkol E, Bilici S, Sobarzo‐Sánchez E, Capasso R. Gut‐Brain‐Microbiota Axis: Antibiotics and Functional Gastrointestinal Disorders. Nutrients. 2021;13(2):389. doi: 10.3390/nu13020389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tang F, Reddy BL, Saier MH Jr. Psychobiotics and their involvement in mental health. J Mol Microbiol Biotechnol. 2014;24(4):211‐214. doi: 10.1159/000366281 [DOI] [PubMed] [Google Scholar]
- 14. Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720‐726. doi: 10.1016/j.biopsych.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 15. Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the Manipulation of Bacteria‐Gut‐Brain Signals. Trends Neurosci. 2016;39(11):763‐781. doi: 10.1016/j.tins.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barros CP, Guimaraes JT, Esmerino EA, et al. Paraprobiotics and postbiotics: concepts and potential applications in dairy products. Curr Opin Food Sci. 2020;32:1‐8. [Google Scholar]
- 17. Collado MC, Vinderola G, Salminen S. Postbiotics: facts and open questions. A position paper on the need for a consensus definition. Benef Microbes. 2019;10(7):711‐719. doi: 10.3920/BM2019.0015 [DOI] [PubMed] [Google Scholar]
- 18. Morgan MY. The treatment of chronic hepatic encephalopathy. Hepatogastroenterology. 1991;38(5):377‐387. [PubMed] [Google Scholar]
- 19. Carabotti M, Scirocco A, Maselli MA, Severi C. The gut‐brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203‐209. [PMC free article] [PubMed] [Google Scholar]
- 20. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37(9):1369‐1378. doi: 10.1016/j.psyneuen.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 21. Tilocca B, Pieroni L, Soggiu A, et al. Gut‐Brain Axis and Neurodegeneration: State‐of‐the‐Art of Meta‐Omics Sciences for Microbiota Characterization. Int J Mol Sci. 2020;21(11):4045. doi: 10.3390/ijms21114045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramírez‐Rentería C, Ferreira‐Hermosillo A, Marrero‐Rodríguez D, Taniguchi‐Ponciano K, Melgar‐Manzanilla V, Mercado M. An Update on Gastroenteropancreatic Neuroendocrine Neoplasms: From Mysteries to Paradigm Shifts. Arch Med Res. 2020;51(8):765‐776. doi: 10.1016/j.arcmed.2020.06.018 [DOI] [PubMed] [Google Scholar]
- 23. Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J, Forsythe P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology. 2016;63:217‐227. doi: 10.1016/j.psyneuen.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 24. Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic‐pituitary‐adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263‐275. doi: 10.1113/jphysiol.2004.063388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farzi A, Fröhlich EE, Holzer P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics. 2018;15(1):5‐22. doi: 10.1007/s13311-017-0600-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levi Mortera S, Soggiu A, Vernocchi P, et al. Metaproteomic investigation to assess gut microbiota shaping in newborn mice: A combined taxonomic, functional and quantitative approach. J Proteomics. 2019;15(203):103378. doi: 10.1016/j.jprot.2019.103378 [DOI] [PubMed] [Google Scholar]
- 27. Yeşilyurt N, Yılmaz B, Ağagündüz D, Capasso R. Involvement of probiotics and postbiotics in the immune system modulation. Biol Theory. 2021;1(2):89‐110. [Google Scholar]
- 28. Dai C, Zheng CQ, Meng FJ, Zhou Z, Sang LX, Jiang M. VSL#3 probiotics exerts the anti‐inflammatory activity via PI3k/Akt and NF‐κB pathway in rat model of DSS‐induced colitis. Mol Cell Biochem. 2013;374(1–2):1‐11. doi: 10.1007/s11010-012-1488-3 [DOI] [PubMed] [Google Scholar]
- 29. Bermúdez‐Humarán LG, Salinas E, Ortiz GG, Ramirez‐Jirano LJ, Morales JA, Bitzer‐Quintero OK. From Probiotics to Psychobiotics: Live Beneficial Bacteria Which Act on the Brain‐Gut Axis. Nutrients. 2019;11(4):890. doi: 10.3390/nu11040890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome‐brain‐gut axis communication. Adv Exp Med Biol. 2014;817:115‐133. doi: 10.1007/978-1-4939-0897-4_5 [DOI] [PubMed] [Google Scholar]
- 31. Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci. 2011;108(38):16050‐16055. doi: 10.1073/pnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bercik P, Park AJ, Sinclair D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut‐brain communication. Neurogastroenterol Motil. 2011;23(12):1132‐1139. doi: 10.1111/j.1365-2982.2011.01796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Lartigue G, de La Serre CB, Raybould HE. Vagal afferent neurons in high fat diet‐induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol Behav. 2011;105(1):100‐105. doi: 10.1016/j.physbeh.2011.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharma R, Gupta D, Mehrotra R, Mago P. Psychobiotics: The Next‐Generation Probiotics for the Brain. Curr Microbiol. 2021;78(2):449‐463. doi: 10.1007/s00284-020-02289-5 [DOI] [PubMed] [Google Scholar]
- 35. Kunze WA, Mao YK, Wang B, et al. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium‐dependent potassium channel opening. J Cell Mol Med. 2009;13(8B):2261‐2270. doi: 10.1111/j.1582-4934.2009.00686.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ma X, Mao YK, Wang B, Huizinga JD, Bienenstock J, Kunze W. Lactobacillus reuteri ingestion prevents hyperexcitability of colonic DRG neurons induced by noxious stimuli. Am J Physiol Gastrointest Liver Physiol. 2009;296(4):G868‐G875. doi: 10.1152/ajpgi.90511.2008 [DOI] [PubMed] [Google Scholar]
- 37. Cryan JF, Dinan TG. Mind‐altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701‐712. doi: 10.1038/nrn3346 [DOI] [PubMed] [Google Scholar]
- 38. Wang HX, Wang YP. Gut Microbiota‐brain Axis. Chin Med J (Engl). 2016;129(19):2373‐2380. doi: 10.4103/0366-6999.190667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yeşilyurt N, Yılmaz B, Ağagündüz D, Capasso R. Microbiome‐based personalized nutrition as a result of the 4.0 technological revolution: A mini literature review. Process Biochem. 2022;121:257‐262. [Google Scholar]
- 40. Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574‐581. doi: 10.1002/bies.201100024 [DOI] [PubMed] [Google Scholar]
- 41. Barrett E, Ross RP, O'Toole PW, Fitzgerald GF, Stanton C. γ‐Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113(2):411‐417. doi: 10.1111/j.1365-2672.2012.05344.x [DOI] [PubMed] [Google Scholar]
- 42. Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1‐9. doi: 10.1016/j.jpsychires.2015.02.021 [DOI] [PubMed] [Google Scholar]
- 43. Ağagündüz D, Kocaadam‐Bozkurt B, Bozkurt O, et al. Microbiota alteration and modulation in Alzheimer's disease by gerobiotics: The gut‐health axis for a good mind. Biomed Pharmacother. 2022;153:113430. doi: 10.1016/j.biopha.2022.113430 [DOI] [PubMed] [Google Scholar]
- 44. Juárez Olguín H, Calderón Guzmán D, Hernández García E, Barragán MG. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxid Med Cell Longev. 2016;2016:9730467. doi: 10.1155/2016/9730467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brunello N, Blier P, Judd LL, et al. Noradrenaline in mood and anxiety disorders: basic and clinical studies. Int Clin Psychopharmacol. 2003;18(4):191‐202. doi: 10.1097/00004850-200307000-00001 [DOI] [PubMed] [Google Scholar]
- 46. Müller B, Rasmusson AJ, Just D, et al. Fecal Short‐Chain Fatty Acid Ratios as Related to Gastrointestinal and Depressive Symptoms in Young Adults. Psychosom Med. 2021;83(7):693‐699. doi: 10.1097/PSY.0000000000000965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang HJ, Chen JL, Liao JF, et al. Lactobacillus plantarum PS128 prevents cognitive dysfunction in Alzheimer's disease mice by modulating propionic acid levels, glycogen synthase kinase 3 beta activity, and gliosis. BMC Complement Med Ther. 2021;21(1):259. doi: 10.1186/s12906-021-03426-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O'Hagan C, Li JV, Marchesi JR, Plummer S, Garaiova I, Good MA. Long‐term multi‐species Lactobacillus and Bifidobacterium dietary supplement enhances memory and changes regional brain metabolites in middle‐aged rats. Neurobiol Learn Mem. 2017;144:36‐47. doi: 10.1016/j.nlm.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 49. Kobayashi Y, Sugahara H, Shimada K, et al. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer's disease. Sci Rep. 2017;7(1):13510. doi: 10.1038/s41598-017-13368-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nimgampalle M, Kuna Y. Anti‐Alzheimer Properties of Probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer's Disease induced Albino Rats. J Clin Diagn Res. 2017;11(8):10428. doi: 10.7860/JCDR/2017/26106.10428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bonfili L, Cecarini V, Berardi S, et al. Microbiota modulation counteracts Alzheimer's disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 2017;7(1):2426. doi: 10.1038/s41598-017-02587-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Athari Nik Azm S, Djazayeri A, Safa M, et al. Lactobacilli and bifidobacteria ameliorate memory and learning deficits and oxidative stress in β‐amyloid (1‐42) injected rats. Appl Physiol Nutr Metab. 2018;43(7):718‐726. doi: 10.1139/apnm-2017-0648 [DOI] [PubMed] [Google Scholar]
- 53. Lee HJ, Hwang YH, Kim DH. Lactobacillus plantarum C29‐Fermented Soybean (DW2009) Alleviates Memory Impairment in 5XFAD Transgenic Mice by Regulating Microglia Activation and Gut Microbiota Composition. Mol Nutr Food Res. 2021;65(12):e2170024. doi: 10.1002/mnfr.202170024 [DOI] [PubMed] [Google Scholar]
- 54. Akbari E, Asemi Z, Daneshvar Kakhaki R, et al. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer's Disease: A Randomized, Double‐Blind and Controlled Trial. Front Aging Neurosci. 2016;10(8):256. doi: 10.3389/fnagi.2016.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Agahi A, Hamidi GA, Daneshvar R, et al. Does Severity of Alzheimer's Disease Contribute to Its Responsiveness to Modifying Gut Microbiota? A Double Blind Clinical Trial. Front Neurol. 2018;15(9):00662. doi: 10.3389/fneur.2018.00662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leblhuber F, Steiner K, Schuetz B, Fuchs D, Gostner JM. Probiotic Supplementation in Patients with Alzheimer's Dementia ‐ An Explorative Intervention Study. Curr Alzheimer Res. 2018;15(12):1106‐1113. doi: 10.2174/1389200219666180813144834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tamtaji OR, Heidari‐Soureshjani R, Mirhosseini N, et al. Probiotic and selenium co‐supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer's disease: A randomized, double‐blind, controlled trial. Clin Nutr. 2019;38(6):2569‐2575. doi: 10.1016/j.clnu.2018.11.034 [DOI] [PubMed] [Google Scholar]
- 58. Hsieh TH, Kuo CW, Hsieh KH, et al. Probiotics Alleviate the Progressive Deterioration of Motor Functions in a Mouse Model of Parkinson's Disease. Brain Sci. 2020;10(4):206. doi: 10.3390/brainsci10040206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Srivastav S, Neupane S, Bhurtel S, et al. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone‐induced neurotoxicity. J Nutr Biochem. 2019;69:73‐86. doi: 10.1016/j.jnutbio.2019.03.021 [DOI] [PubMed] [Google Scholar]
- 60. Castelli V, d'Angelo M, Lombardi F, et al. Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson's disease models. Aging (Albany NY). 2020;12(5):4641‐4659. doi: 10.18632/aging.102927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Perez Visñuk D, Savoy de Giori G, LeBlanc JG, de Moreno de LeBlanc A. Neuroprotective effects associated with immune modulation by selected lactic acid bacteria in a Parkinson's disease model. Nutrition. 2020;79–80:110995. doi: 10.1016/j.nut.2020.110995 [DOI] [PubMed] [Google Scholar]
- 62. Georgescu D, Ancusa OE, Georgescu LA, Ionita I, Reisz D. Nonmotor gastrointestinal disorders in older patients with Parkinson's disease: is there hope? Clin Interv Aging. 2016;11(11):1601‐1608. doi: 10.2147/CIA.S106284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tan AH, Lim SY, Chong KK, et al. Probiotics for Constipation in Parkinson Disease: A Randomized Placebo‐Controlled Study. Neurology. 2021;96(5):e772‐e782. doi: 10.1212/WNL.0000000000010998 [DOI] [PubMed] [Google Scholar]
- 64. Cassani E, Privitera G, Pezzoli G, et al. Use of probiotics for the treatment of constipation in Parkinson's disease patients. Minerva Gastroenterol Dietol. 2011;57(2):117‐121. [PubMed] [Google Scholar]
- 65. Borzabadi S, Oryan S, Eidi A, et al. The Effects of Probiotic Supplementation on Gene Expression Related to Inflammation, Insulin and Lipid in Patients with Parkinson's Disease: A Randomized, Double‐blind PlaceboControlled Trial. Arch Iran Med. 2018;21(7):289‐295. [PubMed] [Google Scholar]
- 66. Barichella M, Pacchetti C, Bolliri C, et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology. 2016;87(12):1274‐1280. doi: 10.1212/WNL.0000000000003127 [DOI] [PubMed] [Google Scholar]
- 67. Guo Y, Xie JP, Deng K, et al. Prophylactic Effects of Bifidobacterium adolescentis on Anxiety and Depression‐Like Phenotypes After Chronic Stress: A Role of the Gut Microbiota‐Inflammation Axis. Front Behav Neurosci. 2019;18(13):126. doi: 10.3389/fnbeh.2019.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wei CL, Wang S, Yen JT, et al. Antidepressant‐like activities of live and heat‐killed Lactobacillus paracasei PS23 in chronic corticosterone‐treated mice and possible mechanisms. Brain Res. 2019;15(1711):202‐213. doi: 10.1016/j.brainres.2019.01.025 [DOI] [PubMed] [Google Scholar]
- 69. Barros‐Santos T, Silva KSO, Libarino‐Santos M, et al. Effects of chronic treatment with new strains of Lactobacillus plantarum on cognitive, anxiety‐ and depressive‐like behaviors in male mice. PLoS One. 2020;15(6):e0234037. doi: 10.1371/journal.pone.0234037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kochalska K, Oakden W, Słowik T, et al. Dietary supplementation with Lactobacillus rhamnosus JB‐1 restores brain neurochemical balance and mitigates the progression of mood disorder in a rat model of chronic unpredictable mild stress. Nutr Res. 2020;82:44‐57. doi: 10.1016/j.nutres.2020.06.019 [DOI] [PubMed] [Google Scholar]
- 71. Tian P, O'Riordan KJ, Lee YK, et al. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress‐induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol Stress. 2020;20(12):100216. doi: 10.1016/j.ynstr.2020.100216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhao Y, Yang G, Zhao Z, et al. Antidepressant‐like effects of Lactobacillus plantarum DP189 in a corticosterone‐induced rat model of chronic stress. Behav Brain Res. 2020;1(395):112853. doi: 10.1016/j.bbr.2020.112853 [DOI] [PubMed] [Google Scholar]
- 73. Savignac HM, Couch Y, Stratford M, et al. Prebiotic administration normalizes lipopolysaccharide (LPS)‐induced anxiety and cortical 5‐HT2A receptor and IL1‐β levels in male mice. Brain Behav Immun. 2016;52:120‐131. doi: 10.1016/j.bbi.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Burokas A, Arboleya S, Moloney RD, et al. Targeting the Microbiota‐Gut‐Brain Axis: Prebiotics Have Anxiolytic and Antidepressant‐like Effects and Reverse the Impact of Chronic Stress in Mice. Biol Psychiatry. 2017;82(7):472‐487. doi: 10.1016/j.biopsych.2016.12.031 [DOI] [PubMed] [Google Scholar]
- 75. Chi L, Khan I, Lin Z, et al. Fructo‐oligosaccharides from Morinda officinalis remodeled gut microbiota and alleviated depression features in a stress rat model. Phytomedicine. 2020;67:153157. doi: 10.1016/j.phymed.2019.153157 [DOI] [PubMed] [Google Scholar]
- 76. Maehata H, Kobayashi Y, Mitsuyama E, et al. Heat‐killed Lactobacillus helveticus strain MCC1848 confers resilience to anxiety or depression‐like symptoms caused by subchronic social defeat stress in mice. Biosci Biotechnol Biochem. 2019;83(7):1239‐1247. doi: 10.1080/09168451.2019.1591263 [DOI] [PubMed] [Google Scholar]
- 77. Warda AK, Rea K, Fitzgerald P, et al. Heat‐killed lactobacilli alter both microbiota composition and behaviour. Behav Brain Res. 2019;19(362):213‐223. doi: 10.1016/j.bbr.2018.12.047 [DOI] [PubMed] [Google Scholar]
- 78. Kambe J, Watcharin S, Makioka‐Itaya Y, et al. Heat‐killed Enterococcus fecalis (EC‐12) supplement alters the expression of neurotransmitter receptor genes in the prefrontal cortex and alleviates anxiety‐like behavior in mice. Neurosci Lett. 2020;720:134753. doi: 10.1016/j.neulet.2020.134753 [DOI] [PubMed] [Google Scholar]
- 79. Romijn AR, Rucklidge JJ, Kuijer RG, Frampton C. A double‐blind, randomized, placebo‐controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust N Z J Psychiatry. 2017;51(8):810‐821. doi: 10.1177/0004867416686694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chong HX, Yusoff NAA, Hor YY, et al. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: a randomised, double‐blind, placebo‐controlled study. Benef Microbes. 2019;10(4):355‐373. doi: 10.3920/BM2018.0135 [DOI] [PubMed] [Google Scholar]
- 81. Lew LC, Hor YY, Yusoff NAA, et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double‐blind, placebo‐controlled study. Clin Nutr. 2019;38(5):2053‐2064. doi: 10.1016/j.clnu.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 82. Murata M, Kondo J, Iwabuchi N, et al. Effects of paraprobiotic Lactobacillus paracasei MCC1849 supplementation on symptoms of the common cold and mood states in healthy adults. Benef Microbes. 2018;9(6):855‐864. doi: 10.3920/BM2017.0197 [DOI] [PubMed] [Google Scholar]
- 83. Nishida K, Sawada D, Kuwano Y, Tanaka H, Rokutan K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double‐Blind, Placebo‐Controlled Study. Nutrients. 2019;11(8):1859. doi: 10.3390/nu11081859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rudzki L, Ostrowska L, Pawlak D, et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double‐blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213‐222. doi: 10.1016/j.psyneuen.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 85. Ghorbani Z, Nazari S, Etesam F, Nourimajd S, Ahmadpanah M, Jahromi SR. The effect of synbiotic as an adjuvant therapy to fluoxetine in moderate depression: a randomized multicenter trial. Arch Neurosci. 2018;5(2):60507. [Google Scholar]
- 86. Ullah H, Di Minno A, Esposito C, et al. Efficacy of a food supplement based on S‐adenosyl methionine and probiotic strains in subjects with subthreshold depression and mild‐to‐moderate depression: A monocentric, randomized, cross‐over, double‐blind, placebo‐controlled clinical trial. Biomed Pharmacother. 2022;156:113930. doi: 10.1016/j.biopha.2022.113930 [DOI] [PubMed] [Google Scholar]
- 87. Tian P, Chen Y, Zhu H, et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav Immun. 2022;100:233‐241. doi: 10.1016/j.bbi.2021.11.023 [DOI] [PubMed] [Google Scholar]
- 88. Kao AC, Chan KW, Anthony DC, Lennox BR, Burnet PW. Prebiotic reduction of brain histone deacetylase (HDAC) activity and olanzapine‐mediated weight gain in rats, are acetate independent. Neuropharmacology. 2019;15(150):184‐191. doi: 10.1016/j.neuropharm.2019.02.014 [DOI] [PubMed] [Google Scholar]
- 89. Huang D, Gao J, Li C, et al. A potential probiotic bacterium for antipsychotic‐induced metabolic syndrome: mechanisms underpinning how Akkermansia muciniphila subtype improves olanzapine‐induced glucose homeostasis in mice. Psychopharmacology (Berl). 2021;238(9):2543‐2553. doi: 10.1007/s00213-021-05878-9 [DOI] [PubMed] [Google Scholar]
- 90. Savignac HM, Corona G, Mills H, et al. Prebiotic feeding elevates central brain derived neurotrophic factor, N‐methyl‐D‐aspartate receptor subunits and D‐serine. Neurochem Int. 2013;63(8):756‐764. doi: 10.1016/j.neuint.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Janik R, Thomason LAM, Stanisz AM, Forsythe P, Bienenstock J, Stanisz GJ. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N‐acetyl aspartate and glutamate. Neuroimage. 2016;15(125):988‐995. doi: 10.1016/j.neuroimage.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 92. Burokas A, Arboleya S, Moloney RD, et al. Targeting the Microbiota‐Gut‐Brain Axis: Prebiotics Have Anxiolytic and Antidepressant‐like Effects and Reverse the Impact of Chronic Stress in Mice. Biol Psychiatry. 2017;82(7):472‐487. doi: 10.1016/j.biopsych.2016.12.031 [DOI] [PubMed] [Google Scholar]
- 93. Gronier B, Savignac HM, Di Miceli M, et al. Increased cortical neuronal responses to NMDA and improved attentional set‐shifting performance in rats following prebiotic (B‐GOS®) ingestion. Eur Neuropsychopharmacol. 2018;28(1):211‐224. doi: 10.1016/j.euroneuro.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo‐controlled trial. Prim Care Companion CNS Disord. 2014;16(1):01579. doi: 10.4088/PCC.13m01579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tomasik J, Yolken RH, Bahn S, Dickerson FB. Immunomodulatory Effects of Probiotic Supplementation in Schizophrenia Patients: A Randomized, Placebo‐Controlled Trial. Biomark Insights. 2015;1(10):47‐54. doi: 10.4137/BMI.S22007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Severance EG, Gressitt KL, Stallings CR, et al. Probiotic normalization of Candida albicans in schizophrenia: A randomized, placebo‐controlled, longitudinal pilot study. Brain Behav Immun. 2017;62:41‐45. doi: 10.1016/j.bbi.2016.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Eslami Shahrbabaki M, Sabouri S, Sabahi A, et al. The Efficacy of Probiotics for Treatment of Bipolar Disorder‐Type 1: A Randomized, Double‐Blind, Placebo Controlled Trial. Iran J Psychiatry. 2020;15(1):10‐16. [PMC free article] [PubMed] [Google Scholar]
- 98. Reininghaus EZ, Wetzlmair LC, Fellendorf FT, et al. The Impact of Probiotic Supplements on Cognitive Parameters in Euthymic Individuals with Bipolar Disorder: A Pilot Study. Neuropsychobiology. 2018;18(1–8):63‐70. doi: 10.1159/000492537 [DOI] [PubMed] [Google Scholar]
- 99. Weaver DF. Alzheimer's disease as an innate autoimmune disease (AD2): A new molecular paradigm. Alzheimers Dement. 2022:1‐13. doi: 10.1002/alz.12789 [DOI] [PubMed] [Google Scholar]
- 100. Cho J, Park YJ, Gonzales‐Portillo B, et al. Gut dysbiosis in stroke and its implications on Alzheimer's disease‐like cognitive dysfunction. CNS Neurosci Ther. 2021;27(5):505‐514. doi: 10.1111/cns.13613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rutsch A, Kantsjö JB, Ronchi F. The Gut‐Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front Immunol. 2020;10(11):604179. doi: 10.3389/fimmu.2020.604179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kowalski K, Mulak A. Brain‐Gut‐Microbiota Axis in Alzheimer's Disease. J Neurogastroenterol Motil. 2019;25(1):48‐60. doi: 10.5056/jnm18087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lynch MA. Long‐term potentiation and memory. Physiol Rev. 2004;84(1):87‐136. doi: 10.1152/physrev.00014.2003 [DOI] [PubMed] [Google Scholar]
- 104. Hu X, Wang T, Jin F. Alzheimer's disease and gut microbiota. Sci China Life Sci. 2016;59(10):1006‐1023. doi: 10.1007/s11427-016-5083-9 [DOI] [PubMed] [Google Scholar]
- 105. Wang R, Reddy PH. Role of Glutamate and NMDA Receptors in Alzheimer's Disease. J Alzheimers Dis. 2017;57(4):1041‐1048. doi: 10.3233/JAD-160763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Li Z, Zhu H, Guo Y, Du X, Qin C. Gut microbiota regulate cognitive deficits and amyloid deposition in a model of Alzheimer's disease. J Neurochem. 2020;155(4):448‐461. doi: 10.1111/jnc.15031 [DOI] [PubMed] [Google Scholar]
- 107. Doifode T, Giridharan VV, Generoso JS, et al. The impact of the microbiota‐gut‐brain axis on Alzheimer's disease pathophysiology. Pharmacol Res. 2021;164:105314. doi: 10.1016/j.phrs.2020.105314 [DOI] [PubMed] [Google Scholar]
- 108. Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife coffee and tea drinking and the risk of late‐life dementia: a population‐based CAIDE study. J Alzheimers Dis. 2009;16(1):85‐91. doi: 10.3233/JAD-2009-0920 [DOI] [PubMed] [Google Scholar]
- 109. Mancuso C, Santangelo R. Alzheimer's disease and gut microbiota modifications: The long way between preclinical studies and clinical evidence. Pharmacol Res. 2018;129:329‐336. doi: 10.1016/j.phrs.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 110. Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2013;9(1):13‐24. doi: 10.1038/nrneurol.2012.242 [DOI] [PubMed] [Google Scholar]
- 111. Cersosimo MG, Raina GB, Pecci C, et al. Gastrointestinal manifestations in Parkinson's disease: prevalence and occurrence before motor symptoms. J Neurol. 2013;260(5):1332‐1338. doi: 10.1007/s00415-012-6801-2 [DOI] [PubMed] [Google Scholar]
- 112. Chen H, Zhao EJ, Zhang W, et al. Meta‐analyses on prevalence of selected Parkinson's nonmotor symptoms before and after diagnosis. Transl Neurodegener. 2015;4(1):1. doi: 10.1186/2047-9158-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Scheperjans F, Aho V, Pereira PA, et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord. 2015;30(3):350‐358. doi: 10.1002/mds.26069 [DOI] [PubMed] [Google Scholar]
- 114. Unger MM, Möller JC, Mankel K, et al. Postprandial ghrelin response is reduced in patients with Parkinson's disease and idiopathic REM sleep behaviour disorder: a peripheral biomarker for early Parkinson's disease? J Neurol. 2011;258(6):982‐990. doi: 10.1007/s00415-010-5864-1 [DOI] [PubMed] [Google Scholar]
- 115. Tetz G, Brown SM, Hao Y, Tetz V. Parkinson's disease and bacteriophages as its overlooked contributors. Sci Rep. 2018;8(1):10812. doi: 10.1038/s41598-018-29173-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Santos SF, de Oliveira HL, Yamada ES, Neves BC, Pereira A Jr. The Gut and Parkinson's Disease‐A Bidirectional Pathway. Front Neurol. 2019;4(10):574. doi: 10.3389/fneur.2019.00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Unger MM, Spiegel J, Dillmann KU, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age‐matched controls. Parkinsonism Relat Disord. 2016;32:66‐72. doi: 10.1016/j.parkreldis.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 118. Knudsen K, Krogh K, Østergaard K, Borghammer P. Constipation in parkinson's disease: Subjective symptoms, objective markers, and new perspectives. Mov Disord. 2017;32(1):94‐105. doi: 10.1002/mds.26866 [DOI] [PubMed] [Google Scholar]
- 119. Indrio F, Riezzo G, Raimondi F, et al. Lactobacillus reuteri accelerates gastric emptying and improves regurgitation in infants. Eur J Clin Invest. 2011;41(4):417‐422. doi: 10.1111/j.1365-2362.2010.02425.x [DOI] [PubMed] [Google Scholar]
- 120. Zhou L, Foster JA. Psychobiotics and the gut‐brain axis: in the pursuit of happiness. Neuropsychiatr Dis Treat. 2015;16(11):715‐723. doi: 10.2147/NDT.S61997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Luo Y, Zeng B, Zeng L, et al. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl Psychiatry. 2018;8(1):187. doi: 10.1038/s41398-018-0240-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Knudsen JK, Michaelsen TY, Bundgaard‐Nielsen C, et al. Faecal microbiota transplantation from patients with depression or healthy individuals into rats modulates mood‐related behaviour. Sci Rep. 2021;11:21869. doi: 10.1038/s41598-021-01248-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Liang S, Wu X, Hu X, Wang T, Jin F. Recognizing Depression from the Microbiota−Gut−Brain Axis. Int J Mol Sci. 2018;19(6):1592. doi: 10.3390/ijms19061592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Du Y, Gao XR, Peng L, Ge JF. Crosstalk between the microbiota‐gut‐brain axis and depression. Heliyon. 2020;6(6):e04097. doi: 10.1016/j.heliyon.2020.e04097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Minayo MS, Miranda I, Telhado RS. A systematic review of the effects of probiotics on depression and anxiety: an alternative therapy? Cien Saude Colet. 2021;26(9):4087‐4099. doi: 10.1590/1413-81232021269.21342020 [DOI] [PubMed] [Google Scholar]
- 126. Mika A, Day HE, Martinez A, et al. Early life diets with prebiotics and bioactive milk fractions attenuate the impact of stress on learned helplessness behaviours and alter gene expression within neural circuits important for stress resistance. Eur J Neurosci. 2017;45(3):342‐357. doi: 10.1111/ejn.13444 [DOI] [PubMed] [Google Scholar]
- 127. McVey Neufeld KA, O'Mahony SM, Hoban AE, et al. Neurobehavioural effects of Lactobacillus rhamnosus GG alone and in combination with prebiotics polydextrose and galactooligosaccharide in male rats exposed to early‐life stress. Nutr Neurosci. 2019;22(6):425‐434. doi: 10.1080/1028415X.2017.1397875 [DOI] [PubMed] [Google Scholar]
- 128. Cowen P, Harrison P, Burns T. Shorter Oxford Textbook of Psychiatry. Oxford University Press; 2017. doi: 10.1093/med/9780198747437.001.0001 [DOI] [Google Scholar]
- 129. Shen Y, Xu J, Li Z, et al. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: A cross‐sectional study. Schizophr Res. 2018;197:470‐477. doi: 10.1016/j.schres.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 130. Zhang X, Pan LY, Zhang Z, Zhou YY, Jiang HY, Ruan B. Analysis of gut mycobiota in first‐episode, drug‐naïve Chinese patients with schizophrenia: A pilot study. Behav Brain Res. 2020;379:112374. doi: 10.1016/j.bbr.2019.112374 [DOI] [PubMed] [Google Scholar]
- 131. Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP‐activated protein kinase in Caco‐2 cell monolayers. J Nutr. 2009;139(9):1619‐1625. doi: 10.3945/jn.109.104638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate‐producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275‐1283. doi: 10.1136/gutjnl-2013-304833 [DOI] [PubMed] [Google Scholar]
- 133. Argou‐Cardozo I, Zeidán‐Chuliá F. Clostridium Bacteria and Autism Spectrum Conditions: A Systematic Review and Hypothetical Contribution of Environmental Glyphosate Levels. Med Sci (Basel). 2018;6(2):29. doi: 10.3390/medsci6020029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Munawar N, Ahsan K, Muhammad K, et al. Hidden Role of Gut Microbiome Dysbiosis in Schizophrenia: Antipsychotics or Psychobiotics as Therapeutics? Int J Mol Sci. 2021;22(14):7671. doi: 10.3390/ijms22147671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398‐403. doi: 10.1016/j.schres.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 136. Davey KJ, O'Mahony SM, Schellekens H, et al. Gender‐dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology (Berl). 2012;221(1):155‐169. doi: 10.1007/s00213-011-2555-2 [DOI] [PubMed] [Google Scholar]
- 137. Islam F, Mulsant BH, Voineskos AN, Rajji TK. Brain‐Derived Neurotrophic Factor Expression in Individuals With Schizophrenia and Healthy Aging: Testing the Accelerated Aging Hypothesis of Schizophrenia. Curr Psychiatry Rep. 2017;19(7):36. doi: 10.1007/s11920-017-0794-6 [DOI] [PubMed] [Google Scholar]
- 138. Flowers SA, Evans SJ, Ward KM, McInnis MG, Ellingrod VL. Interaction Between Atypical Antipsychotics and the Gut Microbiome in a Bipolar Disease Cohort. Pharmacotherapy. 2017;37(3):261‐267. doi: 10.1002/phar.1890 [DOI] [PubMed] [Google Scholar]
- 139. Nemani K, Ghomi RH, McCormick B, Fan X. Schizophrenia and the gut–brain axis. Prog Neuropsychopharmacol Biol Psychiatry. 2015;2(56):155‐160. [DOI] [PubMed] [Google Scholar]
- 140. Bengesser SA, Mörkl S, Painold A, et al. Epigenetics of the molecular clock and bacterial diversity in bipolar disorder. Psychoneuroendocrinology. 2019;101:160‐166. doi: 10.1016/j.psyneuen.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 141. Yolken R, Adamos M, Katsafanas E, et al. Individuals hospitalized with acute mania have increased exposure to antimicrobial medications. Bipolar Disord. 2016;18(5):404‐409. doi: 10.1111/bdi.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Evans SJ, Bassis CM, Hein R, et al. The gut microbiome composition associates with bipolar disorder and illness severity. J Psychiatr Res. 2017;87:23‐29. doi: 10.1016/j.jpsychires.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Painold A, Mörkl S, Kashofer K, et al. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019;21(1):40‐49. doi: 10.1111/bdi.12682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Aizawa E, Tsuji H, Asahara T, et al. Bifidobacterium and Lactobacillus Counts in the Gut Microbiota of Patients With Bipolar Disorder and Healthy Controls. Front Psych. 2019;18(9):730. doi: 10.3389/fpsyt.2018.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Watson S, Gallagher P, Ritchie JC, Ferrier IN, Young AH. Hypothalamic‐pituitary‐adrenal axis function in patients with bipolar disorder. Br J Psychiatry. 2004;184(6):496‐502. [DOI] [PubMed] [Google Scholar]
- 146. Fillman SG, Sinclair D, Fung SJ, Webster MJ, Shannon WC. Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Transl Psychiatry. 2014;4(2):e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Maletic V, Raison C. Integrated neurobiology of bipolar disorder. Front Psych. 2014;25(5):98. doi: 10.3389/fpsyt.2014.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Muneer A. Bipolar Disorder: Role of Inflammation and the Development of Disease Biomarkers. Psychiatry Investig. 2016;13(1):18‐33. doi: 10.4306/pi.2016.13.1.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Akkasheh G, Kashani‐Poor Z, Tajabadi‐Ebrahimi M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double‐blind, placebo‐controlled trial. Nutrition. 2016;32(3):315‐320. doi: 10.1016/j.nut.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 150. Dickerson F, Adamos M, Katsafanas E, et al. Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: A randomized controlled trial. Bipolar Disord. 2018;20(7):614‐621. doi: 10.1111/bdi.12652 [DOI] [PubMed] [Google Scholar]
- 151. Witkamp RF, van Norren K. Let thy food be thy medicine….when possible. Eur J Pharmacol. 2018;5(836):102‐114. doi: 10.1016/j.ejphar.2018.06.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


