Summary
A large interventional trial, the Systolic Blood Pressure Intervention Trial sub-study termed Memory and Cognition in Decreased Hypertension (SPRINT-MIND), found reduced risk of cognitive impairment in older adults with intensive, relative to standard, blood-pressure-lowering targets (systolic BP < 120 vs. <140 mm Hg). In this perspective, we discuss key questions and make recommendations for clinical practice and for clinical trials, following SPRINT-MIND.
Future trials should embody cognitive endpoints appropriate to the participant group, ideally with adaptive designs that ensure robust answers for cognitive and cardiovascular endpoints. Reliable data from diverse populations, including the oldest-old (age > 80 years), will maximize external validity and global implementation of trial findings. New biomarkers will improve phenotyping to stratify patients to optimal treatments. Currently no antihypertensive drug class stands out for dementia risk reduction. Multi-domain interventions, incorporating lifestyle change (exercise, diet) alongside medications, may maximize global impact. Given the low cost and wide availability of antihypertensive drugs, intensive BP reduction may be a cost-effective means to reduce dementia risk in diverse, aging populations worldwide.
Keywords: hypertension, blood pressure, dementia, cognitive impairment, clinical trials, aging, vascular disease, prevention
Graphical abstract
SPRINT-MIND was a landmark clinical trial, suggesting that intensive blood pressure lowering with cardiovascular drugs can ameliorate late-life cognitive impairment. Elahi et al. discuss challenges and opportunities for future clinical trials and for clinical management of older people at risk of dementia.
Introduction
Vascular disease is now recognized as a major contributing factor in dementia, usually as a comorbid condition with Alzheimer’s disease (AD).1,2,3 Vascular risk factors contribute to cognitive impairment4 and are recognized risk factors for dementia.5 Acknowledging the major contributions of vascular disease to cognitive impairment has led to the concept of vascular contributions to cognitive impairment and dementia (VCID).6 The importance of VCID and the heterogeneity of dementia pathology3 has implications for prevention and precision medicine. This is especially important in diverse populations where ethnic differences affect the prevalence of vascular comorbidities.7,8
Recently observed declines in the age-specific incidence of dementia in North America and Europe have shown that dementia risk is modifiable, likely due to better cardiovascular risk management.9 While these trends temper the increasing burden of dementia, prevalence is still expected to rise substantially over the next decades,9,10 particularly in low- and middle-income countries (LMICs). Targeted treatments are therefore important, but few licensed drug treatments are available. For 20 years, these were limited to three acetylcholinesterase inhibitors and a non-selective N-methyl-d-aspartate (NMDA) receptor antagonist, all with modest clinical effects. Amyloid-depleting antibodies aducanumab and lecanemab were recently approved by the US Food and Drug Administration (FDA) for Alzheimer-type dementia.11,12 Given the sparse treatment options, therapeutic strategies for prevention of dementia are much needed. Treatment of vascular risk factors may offer novel therapeutic targets for dementia prevention and treatment.13,14
The Systolic Blood Pressure Intervention Trial sub-study termed Memory and Cognition in Decreased Hypertension (SPRINT-MIND) was a large multi-center study comparing intensive blood pressure (BP) lowering (target, systolic BP [SBP] <120 mm Hg) with a standard BP target (SBP < 140 mm Hg). There was significant 20%–30% reduction in risk for major cardiovascular events with intensive BP lowering (the primary outcome of the parent study, SPRINT).15,16 This led to early termination of the trial by the sponsor at 3.3 years, rather than the planned 6 years. The intensive intervention showed a null effect on the SPRINT-MIND primary endpoint of dementia diagnosis, possibly due to the reduced duration of the study. Nevertheless, intensive BP lowering significantly reduced mild cognitive impairment (MCI) and a combined adjudicated cognitive endpoint of MCI or probable dementia.15
These positive findings of a cognitive benefit in a large interventional trial are the basis for our emphasis on SPRINT-MIND in this perspective. Evidence for cognitive treatment benefits in the dementia field are few. That said, there have been several previous large trials in the area of BP lowering for cognitive benefit, previously reviewed.17 Individually, these trails were not decisive in demonstrating significant benefit in terms of reducing dementia risk.17 A recent meta-analysis of individual participant data from five large double-blinded studies, ADVANCE, PROGRESS, SHEP, SYST-EUR, and HYVET (data from N = 28,000 participants in total) detected a significant reduction in dementia risk with late-life BP lowering.18
A major strength of SPRINT was that it was not designed to test any particular drug class but rather the hypothesis that BP lowering affects end-organ dysfunction. The results were consistent with recent large meta-analyses of BP-lowering studies, concluding that the cognitive benefits of BP control are not strongly dependent on a specific class of medication.19,20 The results also demonstrated that generic, and therefore low-cost, formulations of standard BP-lowering drugs are adequate to achieve the cognitive preservation reported in SPRINT-MIND. Population modeling predicts that substantial global cardiovascular disease (CVD) prevention could be achieved with generic medications at an average cost of approximately US$1 per year.21 Furthermore, a recent review suggests that the majority of published studies on cost-effectiveness of hypertension interventions (both pharmaceutical only and combination programs) in LMIC demonstrate cost-effectiveness when evaluated based on cost per averted disability-adjusted life-year (DALY).22 Thus, intensive BP reduction may be a cost-effective means to reduce dementia risk in older people from diverse economic backgrounds worldwide.
There are caveats to this encouraging concept. Intensive BP lowering requires careful monitoring by physicians and other healthcare professionals. Also, despite clinical trial evidence to the contrary,23 many geriatricians, internists, and primary care physicians are reluctant to lower BP intensively in older persons, fearing to compromise cerebral blood flow (CBF). In addition, because most older patients—particularly vascular patients—have a high prevalence of multiple comorbidities (chronic renal disease, diabetes mellitus, pulmonary disease, rheumatologic and neurological conditions)24 and the attendant co-medications, there is reluctance to support intensive BP control requiring additional medication. Thus, managing prevention of VCID in older people will require a multifactorial approach. Finally, we cannot assume that a US-based treatment regimen (as in SPRINT-MIND) will translate straightforwardly to diverse populations in other global healthcare settings.
In this perspective, we review the implications of SPRINT-MIND for current clinical practice, and for future trials, by discussing 10 outstanding questions. Can SPRINT-MIND findings translate into frontline clinical practice? Do they apply in LMIC? Should inclusion criteria be broad or narrow? Who needs to be treated and when? Is cerebral hypoperfusion or orthostatic hypotension a concern? Are particular drug classes most beneficial? Would a combined approach including lifestyle interventions be preferable? Can genetics and biomarkers improve risk stratification? Should we include the oldest-old in future trials? Is it ethical to include a control group without intensive BP lowering? We offer suggestions for future trial design and practical implementation.
SPRINT-MIND: Design and outcomes
Design
The parent trial SPRINT randomized participants to a cardiovascular drug regimen of either intensive or standard BP lowering, standard being the American Heart Association guideline goal at the time of trial initiation (SBP < 140 mm Hg). The primary hypothesis was that CVD event rates would be lower in the intensive arm. The design is summarized in Table 1.
Table 1.
Overview of the SPRINT trial design
| Sponsor | National Heart, Lung, and Blood Institute |
|---|---|
| Start date; completion date | October 2010; July 2016 (for primary outcome measure) |
| Primary outcome measures | number of participants with first occurrence of a myocardial infarction, acute coronary syndrome, stroke, heart failure, or CVD death |
| Secondary outcome measures | (1) Number of participants with all-cause mortality (2) Number of CKD Participants with at least 50% decline from baseline eGFR (3) Participants who developed end-stage renal disease (4) Number of patients with all-cause dementia. Basic Cognition Screening Battery; then, Extended Cognitive Assessment Battery plus the Functional Assessment Questionnaire for daily living skills. All data were adjudicated by a central panel of dementia experts. (5) Small Vessel Cerebral Ischemic Disease. Change in total white matter lesion volume from baseline; change in total brain volume from baseline |
| Study design | allocation: randomized intervention model: parallel assignment masking: single (outcomes assessor) primary purpose: treatment |
| Intervention | Arm 1: intensive control of SBP goal of SBP < 120 mm Hg. Use of once-daily antihypertensive agents was encouraged unless alternative frequency is indicated/necessary. One or more medications from the following classes: ACE inhibitors, ARBs, direct vasodilators, thiazide-type diuretics, loop diuretics, potassium-sparing diuretics, beta-blockers, sustained-release CCBs, alpha1-receptor blockers, sympatholytics arm 2: standard control of SBP goal of SBP < 140 mm Hg. Same medications as Arm 1 |
| Actual enrolment | 9,361 (target: 9,250) |
| Eligibility criteria | inclusion criteria: at least 50 years old, female/male SBP of: • 130–180 mm Hg on 0 or 1 medication • 130–170 mm Hg on up to two medications • 130–160 mm Hg on up to three medications • 130–150 mm Hg on up to four medications risk (one or more of the following): (1) Presence of clinical or subclinical cardiovascular disease, other than stroke (2) CKD, defined as eGFR 20–59 mL/min/1.73m2 (3) Framingham Risk Score for 10-year CVD risk ≥15% (4) Age greater than 75 years exclusion criteria include: • known secondary cause of hypertension, causing concern regarding safety of the protocol. • one-minute standing SBP <110 mm Hg. • proteinuria (within the past 6 months) • arm circumference too large or too small to allow accurate BP measurement • history of stroke (not cardioembolic or stenting), or cardiovascular event or procedure, or hospitalization for unstable angina within last 3 months, or diabetes mellitus • polycystic kidney disease; or glomerulonephritis, with immunosuppressive therapy; or end-stage renal disease • symptomatic heart failure within the past 6 months, or left ventricular ejection fraction <35% • living in the same household as a SPRINT participant |
For full details of the SPRINT-MIND trial design, see ClinicalTrials.gov Identifier: NCT01206062 (from clinicaltrials.gov). CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
Interventions
SPRINT was an open-label trial. Use of once-daily antihypertensive agents was encouraged unless alternative frequency was indicated/necessary. One or more medications from the classes listed in Table 1 were provided by the study for use in managing participants in both randomization groups. Preferred regimens included a thiazide diuretic (drug of choice, chlorthalidone), plus a calcium channel blockers (CCB) (drug of choice, amlodipine), plus an angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blocker (ARB) (Table 1). The order in which agents were selected was left to the investigator. It was expected that many patients would need at least three antihypertensive drugs to achieve SBP < 120 mm Hg. Most (90%) of the medications used in SPRINT were generic drugs.
Outcomes
A total of 9,361 older Americans were enrolled, including 35.6% women, 30% African American, and 10.5% Hispanic, and 1,167 (12.5%) were aged 80 years or more at entry.16 Early termination of the trial (at 3.3 years) resulted in limited follow-up time to observe the development of dementia. Follow-up for cognitive and kidney outcomes continues during the post-intervention phase.
In terms of cognitive outcomes, intensive treatment did not lead to decreased risk of probable dementia over a median follow-up of 5.1 years, although it reduced the occurrence of MCI and a composite measure combining MCI or probable dementia (Table 2).15 In a non-random subgroup of participants that received comprehensive neuropsychological testing at each cognitive assessment, intensive treatment showed no difference on a composite measure of memory function, but slighter larger decreases on a composite measure of processing speed (driven by small differences on the Trail Making Test, Part B).25 Somewhat contrasting results were observed in an MRI sub-study. Intensive treatment was associated with a smaller increase in the volume of white matter lesions (WMLs), an MRI marker for cerebral small vessel disease (SVD),26 and with increased CBF.27,28,29 By contrast, intensive treatment was associated with larger decreases in total brain volume and hippocampal volume.27,28,29 Smaller increases in WML volume and larger decreases in total brain volume were similarly observed with intensive treatment in another large trial (ACCORD).30
Table 2.
What SPRINT-MIND showed: Cognitive and MRI outcomes
| Intensive treatment | Standard treatment | – | – | – | |
|---|---|---|---|---|---|
| Adjudicated cognitive impairment (N = 8563, median follow-up = 5.1 years) | |||||
| – | Cases per | Cases per | Hazard ratio | – | – |
| Outcome | 1,000 person-years | 1,000 person-years | (95% CI)a | p value | Reference |
| Probable dementia | 7.2 | 8.6 | 0.83 (0.67–1.04) | 0.10 | Williamson et al.15 |
| Mild cognitive impairment | 14.6 | 18.3 | 0.81 (0.69–0.95) | 0.007 | |
| Composite of MCI or probable dementia | 20.2 | 24.1 | 0.85 (0.74–0.97) | 0.01 | |
| Cognitive decline outcomes (N = 2921, median follow-up = 4.1 years) | |||||
| Yearly | Yearly | Difference | – | – | |
| Outcome | Slope (95% CI)b | Slope (95% CI)b | (95% CI) | p value | Reference |
| Memory domainc | −0.005 | −0.001 | −0.004 | 0.33 | Rapp et al.25 |
| (−0.01 to 0.001) | (−0.006 to 0.005) | (−0.012 to 0.004) | – | ||
| Processing domaind | −0.025 | −0.015 | −0.01 | 0.02 | |
| (−0.03 to −0.019) | (−0.021 to −0.009) | (−0.017 to −0.002) | – | ||
| MRI outcomes (baseline N = 670, median follow-up = 4.0 years) | |||||
| Mean change | Mean change | Difference | – | – | |
| Outcome | (95% CI)e | (95% CI)e | (95% CI) | p value | Reference |
| White matter lesion volume, cm3 | 0.92 | 1.45 | −0.54 | <0.001 | Williamson et al. 201915; Nasrallah et al. 202128; Dolui et al. 2022.27 |
| (0.69–1.14) | (1.21–1.70) | (-0.87 to −0.20) | – | ||
| Total brain volume, cm3 | −30.60 | −26.9 | −3.7 | 0.006 | |
| (−32.3 to −28.8) | (−28.8 to −24.9) | (−6.3 to −1.1) | – | ||
| Hippocampal volume, cm3 | −0.06 | −0.02 | −0.033 | 0.03 | |
| (−0.08 to −0.04) | (−0.05 to 0.00) | (−0.062 to −0.003) | – | ||
| Whole brain CBF, mL/100 g/min | 1.46 | −0.84 | 2.3 | 0.02 | |
| (0.08–2.83) | (−2.30 to 0.61) | (0.30–4.30) | – | ||
Intensive treatment versus standard treatment based on stratified Cox proportional hazards regression model.
Yearly slope assuming a linear trend based on a linear mixed model.
Includes the Logical Memory I and II, Modified Rey-Osterrieth Complex Figure (immediate recall), and the Hopkins Verbal Learning Test, Revised (delayed recall).
Includes the Trail Making Test (parts A and B) and Digit Symbol Coding.
For MRI outcomes, change estimates at 3.98 years post randomization based on a linear mixed model minimally adjusting for intracranial volume and days since randomization, including random effects for participant and imaging facility. Estimates for white matter lesion volume are based on a robust mixed model formulation given the skewed distribution of that outcome. Estimates for hippocampal volume and CBF also adjusted for age and sex.
What are the lessons learned from SPRINT-MIND? What should be done differently in future trials?
Adaptive design
Early termination of SPRINT due to the success in reducing major cardiovascular outcomes of death, myocardial infarction, and stroke reduced the power to detect the impact of treatment on cognitive outcomes. This is compounded by gradual return of BP to pre-enrolment levels following trial completion.31 With hindsight, the design of SPRINT should have included an alternative design trigger that facilitated continued assessment of cognitive outcomes beyond the finding of a beneficial effect on CVD. Effects on cognitive function require longer-term follow-up than CVD endpoints. For this reason, it will be advantageous to include adaptive trial designs in future studies assessing cognitive impact, especially if there is CVD assessment in the trial. For example, study participants may be enrolled in an extended open-label treatment protocol to assess the impact of both short- and longer-term treatment on cognitive outcomes.
Additional endpoints
Other endpoints more sensitive to the impact of CVD on brain structure and function could be included, to support the biological plausibility of treatment efficacy. Such surrogate markers could include the burden of SVD and white matter microstructure. Additionally, assessment of potential intermediates and effect-modifying characteristics (pulse wave velocity, BP variability) may help to understand which BP lowering interventions are most efficacious, and in which patients.
Several recent prospective cohort studies (Diverse-VCID; MESA-MIND)32 were designed to examine pathways by which vascular risk factors contribute to cognitive decline in diverse groups of at-risk individuals. These studies will likely identify new biomarkers and intervention targets. MarkVCID33,34 and the HARNESS Initiative35 are consortia currently examining and harmonizing various blood-based and imaging outcome biomarkers to identify the best measures for clinical trials related to VCID. Advanced diffusion-based MRI techniques are increasingly being employed to image white matter microstructural injury.35,36
Greater diversity
SPRINT-MIND was a US-based study. Greater geographic diversity in trials will be advantageous, not only to understand intervention impacts in diverse populations7 but also because countries with high prevalence of hypertension stand to benefit most at the population level. This is critical in LMICs given their increasing dementia incidence. The PROGRESS study included some diversity37 but did not specifically target diverse individuals at highest risk for hypertension, such as African Americans. Risk stratification, therefore, should be considered along with diverse inclusion in designing future trials.
Lessons learned: Summary
The lessons learned from SPRINT-MIND include adaptive trial protocols; integration of cognitive outcomes in cardiovascular treatment trials; proposed lengthening of such trials for cognitive outcomes, possibly through open-label extension, when the primary cardiovascular outcomes are attained; increasing participant diversity; and risk stratification in study design. We welcome development of better vascular biomarkers for assessment of brain injury and for use as secondary measures in treatment trials.
Is there a need for earlier interventions and longer trials?
Results from SPRINT-MIND emphasize the need for timely intervention, in line with the stronger link of mid-life CVD risk factors (especially BP) with dementia incidence, compared with late-life risk factors.38 Although in SPRINT-MIND only 3.3 years of treatment were needed to show cognitive benefit, it seems likely that benefits will increase with more prolonged treatment (5 years or more). Reductions in BP from mid-life onward, either through individualized or public health interventions, may therefore help to maintain cognitive health into old age.
Because dementia reflects end-organ damage to the brain, trials should focus on the inclusion of participants who are at risk and monitor events over longer periods of time. This has financial implications requiring commitment from governments and funders, particularly if trials are to be conducted in younger populations, where dementia incidence is low and cognitive testing can be hampered by ceiling effects. Sensitive and well-validated surrogate outcome measures may aid in detecting treatment effects in individuals prior to the onset of cognitive deficits. Detection of early cognitive impairment is a priority, with a focus on its prevention in the phase of subjective complaints (or even before). This requires increased public awareness about the benefits of treatment before the onset of cognitive impairment,38 which, in turn, has implications for developing methods for managing inclusion of trial populations. Recognition of VCID should be highlighted across different settings, including primary care and public health campaigns within the community.
Intervention timing: Summary
The optimal approach to dementia prevention through BP lowering starts in mid-life and will require individual as well as population interventions. Feasibility of individual participant trials will depend on prolonged duration of observation and treatment, and employment of more sensitive (possibly subclinical) outcome measures.
Is orthostatic hypotension a concern?
Orthostatic hypotension (OH) refers to the acute fall in BP that results from standing up suddenly from a sitting or lying position. OH is often due to slowing of the autonomic reflexes that maintain adequate perfusion pressure and is frequently seen in older persons. A salient concern with OH is the risk of insufficient brain perfusion and possible loss of consciousness. There is a theoretical concern that intensive BP lowering may compromise CBF, and many medical personnel are apprehensive about intensive treatment at older ages. Observational evidence has suggested adverse outcomes of a lower BP, including cognitive decline and mortality.39 In patients with impaired CBF autoregulation, intensive BP lowering could indeed be a cause for concern; however, research in older people shows that, in fact, autoregulation remains largely intact with aging,40,41 even in persons with MCI and dementia.42 Measuring CBF before and after BP lowering in people with hypertension, including older adults, as a rule revealed no reductions in cerebral perfusion.41 This was confirmed in a recent systematic review, which included MCI and dementia.23 In SPRINT-MIND, intensive treatment was associated with a small but significant increase (4%) in whole-brain CBF (Table 2).27 Smaller studies confirm that there is no concern for a reduction in CBF with intensive BP lowering.23,43
In SPRINT, incidence of OH was not more common in the intensive BP-lowering group, nor was baseline OH associated with incidence of adverse events. There was more self-reported or clinician-reported syncope in the intensive group relative to the control group (3.5% vs. 2.4%)44 but with the caveat of possible bias due to “open-label” treatment allocation. There was no increase in electrolyte abnormalities, acute renal failure, or injurious falls in the intensive group. A careful examination of OH in SPRINT and meta-analysis of several randomized controlled trials (RCTs) of antihypertensive therapy concluded that “symptomless OH during hypertension treatment should not be viewed as a reason to down-titrate therapy even in the setting of a lower BP goal.”45,46 Limitations of these studies were that OH was measured only using the transition from sit to stand, which is less sensitive than supine to stand, and only at 1 min. Future studies should include more detailed measures of OH at initiation and discontinuation of antihypertensive therapy.
While SPRINT did not include persons with prevalent dementia, it is important to recall that concerns of autonomic dysfunction in dementia are mainly based on Lewy body dementia and Parkinson’s disease dementia, with limited evidence of autonomic dysfunction in AD and vascular dementia. In the NILVAD trial testing the CCB nilvadipine in AD patients,47 and in several smaller studies, there was also no evidence of increased risk of OH in patients with dementia, also with prolonged standing (up to 5 min).42
Orthostatic hypotension: Summary
For older people without dementia, OH appears not to be a major concern in BP lowering, and the prevalence of autonomic dysfunction is low (in view of physiological data from patients with AD, as well as SPRINT-MIND). High BP is itself an important cause of OH, and persons exhibiting OH may benefit from antihypertensive treatment. Overall, current evidence suggests that BP lowering does not lead to CBF reduction in older patients, with or without cognitive impairment or dementia.
How to translate the SPRINT-MIND findings into a frontline clinical setting? Who needs to be treated, when do they need to be treated, and to what target?
From a clinical perspective, early recognition of vascular risk factors is key, as is the need to identify and prioritize treatment of risk factors with the largest health effect. For instance, the decrease in BP from baseline (the “delta”) may be more important for cognition than an absolute BP target (such as SBP < 120 mm Hg). Rather than one BP fits all, individuals with diverse risk factors, comorbidities, and biological characteristics may require different BP thresholds. After termination of SPRINT-MIND, SBP in the intensively treated group increased back toward previous guideline levels within 4–5 years.31 Not surprisingly, maintaining lower SBP, closer to 120 mm Hg, requires ongoing monitoring and management.
Despite the demonstrated benefits of BP lowering, mechanisms underpinning this remain to be elucidated. Understanding the molecular pathways modified will provide an opportunity to target these pathways, with a larger effect size. There remains an outstanding question of whether the benefits of BP lowering on cognition are mediated through neurodegenerative molecular dysfunctions, such as loss of neuro-glial proteostasis, or through a blood vessel delimited process. Implementation of molecular biomarkers in future trials will shed light on these important unknowns, with great relevance to preventive therapeutics for dementia.
Currently the only vascular and neurodegenerative risk stratification performed in clinical practice is through structural brain imaging, vital signs, and some basic laboratory tests. While more sensitive imaging and molecular biomarkers will improve clinical risk stratification, the feasibility of implementing these within large healthcare systems is a considerable challenge. Routine assessment of cerebral autoregulation, blood-brain barrier function, and neurovascular coupling, all important contributions from vascular dysfunction to cognitive impairment, are not currently feasible at scale in clinical practice. While such measurements are performed in research groups, thresholds and clinical interpretation at n-of-1 levels, needed for implementation in clinical practice, remain to be determined.
Implementing sensitive and specific vascular biomarkers that can be quantified non-invasively (for example, in blood) and are interpretable on an n-of-1 level will change clinical practice and prove instrumental for identifying persons at high risk and for guiding personalized, impactful interventions. Akin to their use in cancer and heart disease, biomarkers (imaging and molecular) in VCID can help to (1) stratify persons at increased risk of VCID who are most suited for interventions, (2) guide the selection and tailoring of the intervention to the individual (“n = 1 medicine”), (3) monitor the response to treatment, and (4) minimize side effects. The MarkVCID consortium is developing biomarkers of VCID for future clinical trials. MarkVCID initially evaluated 11 novel fluid34 and neuroimaging-based33 biomarkers of SVD, several of which progressed to the second round of clinical validations. Three novel fluid biomarkers for VCID were identified: plasma vascular endothelial growth factor (VEGF), placental growth factor (PlGF) and fibroblast growth factor (FGF)-2.34,48 In addition, CSF concentrations of PlGF and two endothelial inflammation markers (C3b and Bb measured from endothelial-derived extracellular vesicles) were deemed to be too early in development to be validated for clinical trials.34,48 These promising biomarkers continue to be investigated, with potential to give invaluable specificity for vascular disease. The initial neuroimaging-based candidate biomarkers include WML volume, WML growth/regression, peak width of skeletonized mean diffusivity, arteriolosclerosis, MRI free water, cerebrovascular reactivity, and optical coherence tomography angiography (OCTA) for retinal capillaries.33 From this list, WML volume and OCTA were eliminated from further validations in MarkVCID. Subcortical CBF49 and cerebral vasoreactivity50 have recently been used as outcome measures in clinical trials. Overall, vascular biomarkers have the potential to facilitate the early identification of SVD pathology and offer better monitoring of disease progression or intervention efficacy.
Translation to practice: Summary
Effective BP treatment for cognitive health is likely to require some clinical phenotyping for patient stratification. Better biomarkers, especially reliable, cost-effective, non-invasive biomarkers with proven specificity for VCID, could be implemented into clinical trials and eventually clinical practice. They will leverage risk stratification and fine-tuning of therapies. Plasma biomarkers, such as some investigated in MarkVCID, are promising candidates.
Are SPRINT-MIND findings applicable in LMIC healthcare systems?
The burden of hypertension is rapidly rising in LMICs and the prevalence is higher compared with high-income countries (HICs). There is a wide treatment gap for hypertension and only a minority of hypertensives in LMICs receive treatment, due to low awareness, poor socioeconomic status, limited access to healthcare services, and poor treatment adherence.51 Therefore, there is a large population at risk of VCID, reflected in the higher prevalence of vascular dementia diagnoses in LMICs. Robust evidence for the benefits of BP lowering and reducing risk of VCID in this large vulnerable population is lacking. There is observational evidence regarding natural history of hypertension and other vascular diseases in LMICs, and some well-characterized longitudinal cohorts of persons with vascular disease have been established. The majority of trials examining vascular risk factor control on dementia risk—including SPRINT-MIND—are conducted in HICs and may not represent global diverse populations.7 While there is increasing government support and clinical research to reduce the burden of hypertension through public health programs52 and multi-center studies, there are still challenges for implementation of clinical trials comparable with SPRINT-MIND in LMICs53,54 (see Table 3). Nevertheless, management of hypertension does appear cost-effective even in resource-poor settings across various LMICs22 and therefore seems feasible to test as a dementia prevention strategy.
Table 3.
Challenges for blood-pressure-lowering studies in LMICs
| Category | Challenge |
|---|---|
| Risk stratification | well-characterized cohorts of individuals with hypertension and other vascular risk factors are needed across LMIC |
| Socio-demographics | differences in sociodemographic profiles between LMIC and HIC populations are likely to affect design and outcomes of clinical trials (Alladi et al., 2018)8 |
| Life expectancy | life expectancy is lower in LMIC compared with HIC, changing the age profile of participants;, see World Bank data: https://data.worldbank.org/indicator/SP.DYN.LE00.IN |
| High comorbidity burden | the high burden of coexistent vascular risk factors, including untreated diabetes, metabolic syndrome, dietary factors, and cigarette smoking, must be considered in developing trial-specific cohorts |
| Cognitive assessment | trials that evaluate cognitive outcomes also require a uniform set of cognitive tests that are validated across diverse populations. Cognitive testing is challenging due to cultural, educational, and linguistic diversity. Harmonization efforts are underway to fill this gap, and validated neuropsychological batteries are now available in multiple languages and for different educational levels (Akinyemi et al.53; Iyer et al.)54 |
| Biomarker standardization | imaging and plasma biomarkers of dementia and vascular disease also need to be standardized for diverse populations |
| Genetic studies | genetic factors, notably APOE genotype, may affect cognitive outcomes and should be systematically incorporated into study design |
| Infrastructure | infrastructure to implement trials is needed, including training of clinicians and researchers |
The LMIC context: Summary
Clinical trials such as SPRINT-MIND demonstrating the impact of BP control have enormous potential to benefit cognitive outcomes in the context of LMICs. First, they may be fundamental to developing evidence to influence policy. Second, they may lead to population-based strategies to reduce the burden of VCID and dementia globally.
Are particular classes of antihypertensive drugs more beneficial for brain health and cognitive outcomes?
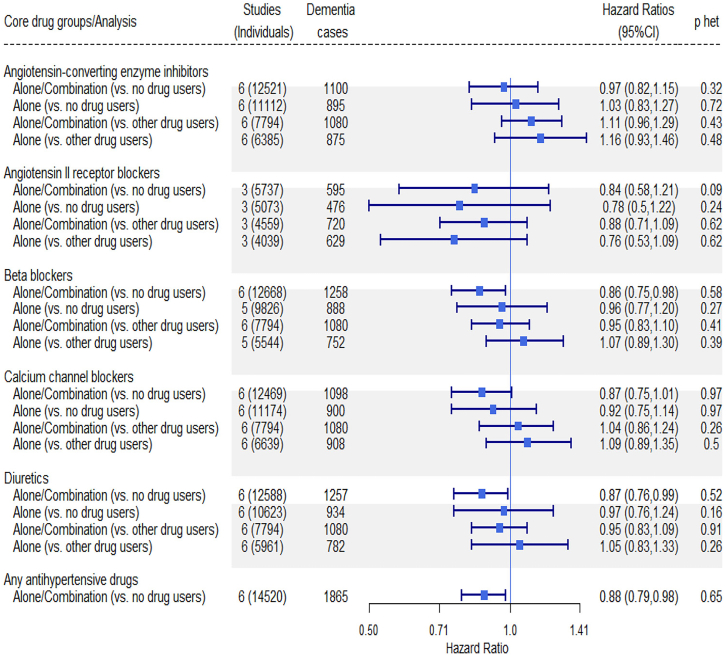
Meta-analyses of data from large observational cohorts19,20 support the notion that antihypertensive therapy may help prevent cognitive decline. One meta-analysis of individual patient data of six prospective community-based cohort studies, including over 31,000 participants, showed that antihypertensive treatment significantly reduced dementia risk but found no evidence for one drug class being more efficient than others (Figure 1).19 Another, larger meta-analysis including 27 studies and over 50,000 participants also found no consistent pattern to support any one antihypertensive drug class in cognitive decline or incident dementia.20
Figure 1.
Associations of specific antihypertensive medication use with incident dementia in persons with high BP
Pooled data from six population cohorts of prospectively recruited community-dwelling adults (N = 31,090). Horizontal symbols show hazard ratio (HR) (mean, 95% CI). The p values for heterogeneity (p-het) are listed. Adapted from Figure 1 of Ding et al.19 by permission from the publisher (Elsevier).
Single study evidence has suggested that CCBs or ARBs may particularly reduce dementia risk. The Systolic Hypertension in Europe (SYST-EUR) trial achieved the biggest reduction in incidence of dementia (by 50%) with a CCB,55 while trials with other drug types showed no or only modest benefits.56,57,58,59 An observational study suggested that healthy older adults and MCI patients taking ARBs had larger hippocampal volume, less atrophy, and better cognition compared with patients treated with ACE inhibitors,60 although these beneficial effects were not echoed by a trial in mild to moderate AD showing no cognitive benefits of ARB treatment.58 ARBs have a good blood-brain barrier penetration and may enhance the catabolism and clearance of Aβ. CCBs have a good blood-brain barrier penetration as well, have neuroprotective effects,61 and may reduce BP variability.62 The emerging importance of BP variability in subclinical SVD63 and dementia onset may reveal preferential class effects of certain BP-lowering medications over others. As the benefit of BP lowering is likely to reflect several causal factors (as in other diseases; e.g., chronic heart failure, chronic kidney disease) a holistic view of multiple pathways appears warranted.
Since SVD progression is likely to underlie dementia risk, a post hoc analysis of SPRINT-MIND drugs and SVD was carried out.64 Use of ACE inhibitors (β, −0.14 [95% confidence interval [CI], −0.23 to −0.04]; p = 0.002) and ARBs (β, −0.14 [95% CI, −0.263 to −0.05]; p = 0.003) had a small negative association with WML progression, while dihyropyridine CCBs showed mixed effects in logistic and linear models.64 These observational findings suggest there may be a modest class-specific pleiotropic effect of antihypertensive therapy on SVD progression.
Particular drug classes: Summary
Currently there is no convincing evidence that one antihypertensive drug class affords a larger reduction of dementia risk over another. These findings support clinical freedom in the selection of type of antihypertensive drugs to achieve BP goals. The possible protective effects of antihypertensive drugs in prodromal stages of neurodegeneration merit further exploration in prospective outcome-based studies.
Should a VCID trial recruit the oldest-old (age 80+ years)?
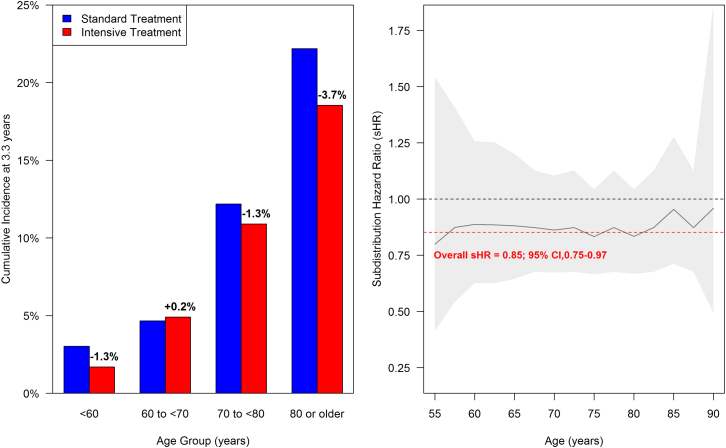
The significant treatment effect detected in SPRINT-MIND was particularly evident in older participants (age > 70 years), although this positive finding may be driven by higher event rates in older people (Figure 2). Older adults without pre-existing MCI did well in SPRINT-MIND, and their overall adverse event rate did not differ between the treatment groups. This included careful monitoring of kidney function. Specifically, most cases of incident MCI or dementia (and most of the risk reduction) occurred in patients aged >75 years,15,65 supporting the safety of this intervention in older people. We speculate that, in older patients at risk of dementia, cardiovascular events (stroke, myocardial infarction, vascular surgery, or other cardiovascular interventions) may trigger delirium, and this in turn could progress to cognitive decline.66,67 Prevention of cardiovascular events may therefore contribute to prevention of dementia in this indirect pathway. Prevalence of hypertension, as well as incidence of CVD and dementia, is highest in older adult populations, so treatment is especially important for them.
Figure 2.
Risk reduction across the age range in SPRINT-MIND
The left panel shows the cumulative incidence of cognitive impairment (a composite outcome of MCI or probable dementia) by age in SPRINT-MIND comparing intensive with standard treatment, accounting for the competing risk of death. The absolute risk reduction is greater in older adults (age 70 years or older), likely due to higher event rates. The right panel shows estimated overall and age-specific sub-distribution HR from a Fine-Gray competing risks regression model for cognitive impairment (same composite outcome) in SPRINT-MIND comparing intensive with standard treatment. Relative risk reduction is quite consistent across the age range. Shaded areas denote 95% CIs. Overall subdistribution Hazard Ratio (sHR) = 0.85, 95% CI = 0.75–0.97 (N.M.P., J.D.W., unpublished data).
The oldest-old: Summary
Trials of VCID should be designed to include the oldest-old. This age group may be instrumental for detection of treatment effects of intensive BP lowering.
Should inclusion criteria be broad or narrow?
More crudely, should we lump or split? Lack of external validity of clinical trials can hamper the transportability of clinical trial results to practice. The perception by physicians that certain patient groups are under-represented in research studies is an important cause for under-use of effective treatments. Hence, there is a need to diversify the pool of individuals included in trials. Beneficial effects of BP lowering on cognition may be dependent on patient characteristics (race, comorbidities, cognitive status) or setting (primary care, memory, or stroke clinic). A typical older adult at risk of dementia has multiple comorbidities, all potentially modifying treatment efficacy. Hence larger samples are needed to confirm benefit in those most affected. In addition to age, sex, education, and APOE-ε4 status, trajectories of disease progression may differ by race/ethnicity and respond differently to treatment.68 Broad and proportional inclusion criteria would potentially avoid replicating findings in different populations. It may be more efficient to use the established machinery of a large trial (recruitment resources, trained staff, equipment, start-up funding) to address a question of diversity than to launch entirely new studies.
Nonetheless, in view of anticipated clinical benefit, there are valid reasons to exclude individuals who are not at risk for the outcome measure of interest. Examples include those with limited life expectancy (such as nursing home residents) or healthy young persons who have no probability of contributing data to the trial outcomes. Inclusion of participants with high BP or evidence of cerebral SVD at baseline will increase trial power (analogous to screening patients for β-amyloid-positive status for inclusion in trials of amyloid lowering therapy).
Several counter-arguments complicate this strategy. First, benefits of BP lowering on CVD outcomes are clear in persons within the normal range of BP,69 supporting potential benefits in a wider population at risk of cognitive decline. Second, the need for early intervention favors inclusion of individuals before symptoms of vascular disease become manifest. With the advent of blood tests for AD markers (Aβ peptides; ptau-181, 217, or 231), we may be able to detect nascent AD pathology, alongside vascular risk, in individuals while they are still cognitively normal. Third, randomized assessment of treatment efficacy may not be feasible or ethical in those with other strong indications for treatment (such as acute cardiovascular events). Broad inclusivity in the design of forthcoming trials will benefit external validity and facilitate subgroup analyses of diverse clinical profiles and racial/ethnic background. SPRINT-MIND did not detect differences between pre-specified ethnic subgroups and was not powered to do so.
Inclusion criteria: Summary
There is a need to diversify the pool of individuals included in clinical trials. Diversification based on age, sex, education, and APOE-ε4 status, alongside race, is critical as these factors may influence treatment response. Recruitment strategies should carefully consider the selection of participants in whom treatment efficacy can be demonstrated during the course of the trial. These should be weighed against effects of restricted inclusion on external validity and consequent lack of implementation of trial findings in routine clinical practice.
Would a combined approach that includes lifestyle (not just drugs) be more effective?
In high-risk populations, an intervention based on tailored exercise alone may be beneficial for SVD and cognitive outcomes, although the available data are mixed.70,71 Such lifestyle interventions may be enhanced by expanding to multi-domain approaches (physical activity, diet, cognitive engagement, and vascular risk factor management)72 and could potentially maximize the benefits of pharmacological interventions. Current guidelines for management of hypertension already favor such a combined approach,73 and this could be extended to brain-related outcomes.
For individuals with cognitive impairment and CVD risk factors, a multi-domain intervention may improve executive function, memory, and learning, with additional reduction in BP.74 In a small study of participants who already had an AD dementia diagnosis, a multi-domain intervention was associated with reduced WML progression.75 Among dementia-free older adults, a much larger trial of a multi-domain intervention (PreDIVA) showed no difference in all-cause dementia but detected a positive effect on non-AD dementia favoring the intervention group.76
Beyond direct effects on cognition, exercise interventions can improve other determinants of cognitive function, such as mood regulation and physical mobility,77,78 while pharmacological treatment could exert specific effects in managing chronic illness and reducing overall risk. This is important, as lifestyle interventions alone did not lead to significant changes in CVD risk markers in the FINGER trial (BP, serum total cholesterol, fasting plasma glucose).72
Implementation and long-term sustainability of combined interventions may be challenging, especially in ethnically diverse populations, due to differences in dietary patterns, socioeconomic status, geographical location, and accessibility. The ongoing World-Wide FINGERS (WW-FINGERS) trial (including its US-based component POINTER) will be critical, as it addresses the implementation barriers in diverse populations. WW-FINGERS is testing a multifactorial intervention to reduce the risk for cognitive decline, based on the original FINGERS trial (based in Finland),72 which showed cognitive benefit of a multifactorial intervention. General recommendations for multi-domain trials were reviewed in an expert perspective79).
Combined approaches: Summary
With the success of SPRINT-MIND and growing evidence for the efficacy of lifestyle interventions, a combined approach seems pragmatic in dementia prevention. This multifactorial approach holds promise for additive effects on brain function, ultimately reducing dementia risk. Significant effort and funding will be necessary to perform definitive trials of these combined approaches, considering the complex implementation logistics and long-term sustainability.
Is it unethical to include a control group without intensive BP lowering in future trials?
This is a challenging question. On one hand, a clinical trial should have a control group, and a randomization procedure to assign participants either to intervention or to control treatment. On the other hand, the beneficial effects of intensive BP lowering are clear in terms of mortality and major CVD (as shown by the main outcomes of SPRINT16,44). Hence investigators (and ethical review boards) may be reluctant to have participants assigned to an untreated control group. A control group treated with the national, accepted standard of care is more acceptable.
Control group: Summary
We agreed that it would be unethical to use a no-treatment control group and that intensive BP treatment can be appropriately compared with a control group treated with standard of care.
Concluding remarks
Hindsight gets paradoxically clearer with time. SPRINT-MIND gave justified optimism for future cognitive trials. Since 2010, when SPRINT opened, we have learned a lot from SPRINT and other BP-lowering studies. There are numerous points where a future trial would be designed differently. We offer the following take-home messages. (1) Intensive BP lowering with cardiovascular medicines can achieve substantial decline in MCI/dementia risk. This is a foundation to build on. (2) In real-world clinical management, there is a need for early intervention, before symptoms of cognitive decline become manifest. (3) Future study design should include flexibility to adapt to early termination, while maintaining statistical power to determine effects on cognitive endpoints (including dementia incidence). (4) Dementia biomarkers are a flourishing field. Improved biomarkers will provide better, quantifiable trial endpoints and also greater understanding of physiological effects and risk mediators. (5) Researchers should be encouraged to work toward local and global generalizability, across socioeconomic and ethnic spectra. This is already in progress in WW-FINGERS. (6) Future studies should investigate specific mechanistic targets and whether there are antihypertensive drug classes that afford the greatest cognitive protection. (7) Future trials should consider combined interventions, with medication alongside lifestyle change (e.g., exercise, diet, cognitive training, social stimulation, vascular risk monitoring).
Acknowledgments
Research in F.M.E.’s group is supported by National Institute on Aging and Department of Veterans Affairs (IK2CX002180), a Larry L. Hillblom start-up grant (2019A012SUP), and an American Academy of Neurology fellowship. J.M. is appointed at the Netherlands Consortium of Dementia Cohorts (NCDC), which is funded in the context of Deltaplan Dementie from ZonMW Memorabel (project nr 73305095005) and Alzheimer Nederland. C.D. is supported by NIH P30 AG 072972 U19 NS 120384, and UF1NS100608. N.M.P. is supported by R01 AG055606 and R01 AG065805 from the National Institute on Aging. L.S. was supported by a Swiss National Science Foundation award [P2GEP3_191584]. C.E.S. was supported by T32 AG055381 and is presently supported by K01AG071849, both from the National Institute on Aging of the US National Institutes of Health. J.D.W. is supported by R01 AG055606 from the National Institute on Aging. F.J.W. is supported by the Alzheimer's Association (AARF-22-924982) and the Netherlands Organisation for Health Research and Development (ZonMw) (Veni 09150162010108). Research in A.H.H.’s group is funded by the UK Medical Research Council (MR/R005567/1, MR/T033371/1), British Heart Foundation (PG/20/10397), UK Alzheimer’s Society, and Alzheimer's Drug Discovery Foundation (20140901). The graphical abstract was prepared using BioRender. The views expressed in this paper are those of the individual authors.
Author contributions
Conceptualization, F.M.E. and A.H.H.; ideas, all authors; writing – original draft, A.H.H.; writing – review & editing the final draft, all authors.
Declaration of interests
All authors are members of The Alzheimer’s Association International Society to Advance Alzheimer’s Research and Treatment (ISTAART). This perspective arose from a Roundtable discussion on 20/09/2021, hosted by the Vascular Cognitive Disorders Professional Interest Area within ISTAART. F.M.E. serves on the Scientific Advisory Board of Cordance Medical. S.E.B. has been an ad hoc consultant to Hoffman-LaRoche and Biogen and has participated in educational programs sponsored by Biogen. J.C. is Honorary Visiting Professor in the Department of Cardiovascular Sciences, University of Leicester, UK. C.D. advises Novartis on a safety trial of heart failure treatment and Nova Nordisk on two trials of GLP1 agonist treatment in AD. N.M.P. was a named investigator on the SPRINT-MIND study. B.R.P. is a full-time employee of Life Molecular Imaging Inc. N.C.B.S.S. is a post-doctoral fellow jointly funded by the Michael Smith Health Research BC, the Canadians for Leading Alzheimer Research, and the Canadian Institutes of Health Research. H.M.S. is a full-time employee of the Alzheimer’s Association. J.D.W. was a named investigator on the SPRINT-MIND study. A.H.H. has received honoraria from Eli-Lilly and from NIA, and chairs the Dementias Platform UK Vascular Experimental Medicine group.
Inclusion and diversity
One or more of the authors of this paper self-identifies as an under-represented ethnic minority in their field of research or within their geographical location. One or more of the authors of this paper self-identifies as a gender minority in their field of research. One or more of the authors of this paper self-identifies as living with a disability.
References
- 1.Esiri M.M., Nagy Z., Smith M.Z., Barnetson L., Smith A.D. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet. 1999;354:919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- 2.Kapasi A., DeCarli C., Schneider J.A. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134:171–186. doi: 10.1007/s00401-017-1717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamar M., Leurgans S., Kapasi A., Barnes L.L., Boyle P.A., Bennett D.A., Arfanakis K., Schneider J.A. Complex profiles of cerebrovascular disease pathologies in the aging brain and their relationship with cognitive decline. Stroke. 2022;53:218–227. doi: 10.1161/STROKEAHA.121.034814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debette S., Seshadri S., Beiser A., Au R., Himali J.J., Palumbo C., Wolf P.A., DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Exalto L.G., Quesenberry C.P., Barnes D., Kivipelto M., Biessels G.J., Whitmer R.A. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement. 2014;10:562–570. doi: 10.1016/j.jalz.2013.05.1772. [DOI] [PubMed] [Google Scholar]
- 6.Gorelick P.B., Scuteri A., Black S.E., DeCarli C., Greenberg S.M., Iadecola C., Launer L.J., Laurent S., Lopez O.L., Nyenhuis D., et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mooldijk S.S., Licher S., Wolters F.J. Characterizing demographic, racial, and geographic diversity in dementia research: a systematic review. JAMA Neurol. 2021;78:1255–1261. doi: 10.1001/jamaneurol.2021.2943. [DOI] [PubMed] [Google Scholar]
- 8.Alladi S., Hachinski V. World dementia: one approach does not fit all. Neurology. 2018;91:264–270. doi: 10.1212/WNL.0000000000005941. [DOI] [PubMed] [Google Scholar]
- 9.Wolters F.J., Chibnik L.B., Waziry R., Anderson R., Berr C., Beiser A., Bis J.C., Blacker D., Bos D., Brayne C., et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: the alzheimer cohorts consortium. Neurology. 2020;95:e519–e531. doi: 10.1212/WNL.0000000000010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruck C.C., Wolters F.J., Ikram M.A., de Kok I. Projected prevalence and incidence of dementia accounting for secular trends and birth cohort effects: a population-based microsimulation study. Eur. J. Epidemiol. 2022;37:807–814. doi: 10.1007/s10654-022-00878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budd Haeberlein S., Aisen P.S., Barkhof F., Chalkias S., Chen T., Cohen S., Dent G., Hansson O., Harrison K., von Hehn C., et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J. Prev. Alzheimers Dis. 2022;9:197–210. doi: 10.14283/jpad.2022.30. [DOI] [PubMed] [Google Scholar]
- 12.van Dyck C.H., Swanson C.J., Aisen P., Bateman R.J., Chen C., Gee M., Kanekiyo M., Li D., Reyderman L., Cohen S., et al. Lecanemab in early Alzheimer's disease. N. Engl. J. Med. 2023;388:9–21. doi: 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- 13.Smith E.E., Cieslak A., Barber P., Chen J., Chen Y.W., Donnini I., Edwards J.D., Frayne R., Field T.S., Hegedus J., et al. Therapeutic strategies and drug development for vascular cognitive impairment. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hainsworth A.H., Elahi F.M., Corriveau R.A. An introduction to therapeutic approaches to vascular cognitive impairment. Cereb. Circ. Cogn. Behav. 2021;2 doi: 10.1016/j.cccb.2021.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SPRINT MIND Investigators for the SPRINT Research Group. Williamson J.D., Pajewski N.M., Auchus A.P., Bryan R.N., Chelune G., Cheung A.K., Cleveland M.L., Coker L.H., Crowe M.G., et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553–561. doi: 10.1001/jama.2018.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SPRINT Research Group. Wright J.T., Jr., Williamson J.D., Whelton P.K., Snyder J.K., Sink K.M., Rocco M.V., Reboussin D.M., Rahman M., Oparil S., et al. A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters R., Beckett N., McCormack T., Fagard R., Fletcher A., Bulpitt C. Treating hypertension in the very elderly-benefits, risks, and future directions, a focus on the hypertension in the very elderly trial. Eur. Heart J. 2014;35:1712–1718. doi: 10.1093/eurheartj/eht464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters R., Xu Y., Fitzgerald O., Aung H.L., Beckett N., Bulpitt C., Chalmers J., Forette F., Gong J., Harris K., et al. Blood pressure lowering and prevention of dementia: an individual patient data meta-analysis. Eur. Heart J. 2022;43:4980–4990. doi: 10.1093/eurheartj/ehac584. [DOI] [PubMed] [Google Scholar]
- 19.Ding J., Davis-Plourde K.L., Sedaghat S., Tully P.J., Wang W., Phillips C., Pase M.P., Himali J.J., Gwen Windham B., Griswold M., et al. Antihypertensive medications and risk for incident dementia and Alzheimer's disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 2020;19:61–70. doi: 10.1016/S1474-4422(19)30393-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters R., Yasar S., Anderson C.S., Andrews S., Antikainen R., Arima H., Beckett N., Beer J.C., Bertens A.S., Booth A., et al. Investigation of antihypertensive class, dementia, and cognitive decline: a meta-analysis. Neurology. 2020;94:e267–e281. doi: 10.1212/WNL.0000000000008732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim S.S., Gaziano T.A., Gakidou E., Reddy K.S., Farzadfar F., Lozano R., Rodgers A. Prevention of cardiovascular disease in high-risk individuals in low-income and middle-income countries: health effects and costs. Lancet. 2007;370:2054–2062. doi: 10.1016/S0140-6736(07)61699-7. [DOI] [PubMed] [Google Scholar]
- 22.Kostova D., Spencer G., Moran A.E., Cobb L.K., Husain M.J., Datta B.K., Matsushita K., Nugent R. The cost-effectiveness of hypertension management in low-income and middle-income countries: a review. BMJ Glob. Health. 2020;5 doi: 10.1136/bmjgh-2019-002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Rijssel A.E., Stins B.C., Beishon L.C., Sanders M.L., Quinn T.J., Claassen J., de Heus R.A.A. Effect of antihypertensive treatment on cerebral blood flow in older adults: a systematic review and meta-analysis. Hypertension. 2022;79:1067–1078. doi: 10.1161/HYPERTENSIONAHA.121.18255. HYPERTENSIONAHA12118255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Licher S., Heshmatollah A., van der Willik K.D., Stricker B.H.C., Ruiter R., de Roos E.W., Lahousse L., Koudstaal P.J., Hofman A., Fani L., et al. Lifetime risk and multimorbidity of non-communicable diseases and disease-free life expectancy in the general population: a population-based cohort study. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapp S.R., Gaussoin S.A., Sachs B.C., Chelune G., Supiano M.A., Lerner A.J., Wadley V.G., Wilson V.M., Fine L.J., Whittle J.C., et al. Effects of intensive versus standard blood pressure control on domain-specific cognitive function: a substudy of the SPRINT randomised controlled trial. Lancet Neurol. 2020;19:899–907. doi: 10.1016/S1474-4422(20)30319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alber J., Alladi S., Bae H.J., Barton D.A., Beckett L.A., Bell J.M., Berman S.E., Biessels G.J., Black S.E., Bos I., et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): knowledge gaps and opportunities. Alzheimers Dement. 2019;5:107–117. doi: 10.1016/j.trci.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolui S., Detre J.A., Gaussoin S.A., Herrick J.S., Wang D.J.J., Tamura M.K., Cho M.E., Haley W.E., Launer L.J., Punzi H.A., et al. Association of intensive vs standard blood pressure control with cerebral blood flow: secondary analysis of the SPRINT MIND randomized clinical trial. JAMA Neurol. 2022;79:380–389. doi: 10.1001/jamaneurol.2022.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasrallah I.M., Gaussoin S.A., Pomponio R., Dolui S., Erus G., Wright C.B., Launer L.J., Detre J.A., Wolk D.A., Davatzikos C., et al. Association of intensive vs standard blood pressure control with magnetic resonance imaging biomarkers of alzheimer disease: secondary analysis of the SPRINT MIND randomized trial. JAMA Neurol. 2021;78:568–577. doi: 10.1001/jamaneurol.2021.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SPRINT MIND Investigators for the SPRINT Research Group. Nasrallah I.M., Pajewski N.M., Auchus A.P., Chelune G., Cheung A.K., Cleveland M.L., Coker L.H., Crowe M.G., Cushman W.C., et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322:524–534. doi: 10.1001/jama.2019.10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray A.M., Hsu F.C., Williamson J.D., Bryan R.N., Gerstein H.C., Sullivan M.D., Miller M.E., Leng I., Lovato L.L., Launer L.J., Action to Control Cardiovascular Risk in Diabetes Follow-On Memory in Diabetes ACCORDION MIND Investigators Accordion MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia. 2017;60:69–80. doi: 10.1007/s00125-016-4118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaeger B.C., Bress A.P., Bundy J.D., Cheung A.K., Cushman W.C., Drawz P.E., Johnson K.C., Lewis C.E., Oparil S., Rocco M.V., et al. Longer-term all-cause and cardiovascular mortality with intensive blood pressure control: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2022;7:1138–1146. doi: 10.1001/jamacardio.2022.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lockhart S.N., Schaich C.L., Craft S., Sachs B.C., Rapp S.R., Jung Y., Whitlow C.T., Solingapuram Sai K.K., Cleveland M., Williams B.J., et al. Associations among vascular risk factors, neuroimaging biomarkers, and cognition: preliminary analyses from the Multi-Ethnic Study of Atherosclerosis (MESA) Alzheimers Dement. 2022;18:551–560. doi: 10.1002/alz.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu H., Kashani A.H., Arfanakis K., Caprihan A., DeCarli C., Gold B.T., Li Y., Maillard P., Satizabal C.L., Stables L., et al. MarkVCID cerebral small vessel consortium: II. Neuroimaging protocols. Alzheimers Dement. 2021;17:716–725. doi: 10.1002/alz.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilcock D., Jicha G., Blacker D., Albert M.S., D'Orazio L.M., Elahi F.M., Fornage M., Hinman J.D., Knoefel J., Kramer J., et al. MarkVCID cerebral small vessel consortium: I. Enrollment, clinical, fluid protocols. Alzheimers Dement. 2021;17:704–715. doi: 10.1002/alz.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith E.E., Biessels G.J., De Guio F., de Leeuw F.E., Duchesne S., Düring M., Frayne R., Ikram M.A., Jouvent E., MacIntosh B.J., et al. Harmonizing brain magnetic resonance imaging methods for vascular contributions to neurodegeneration. Alzheimers Dement. 2019;11:191–204. doi: 10.1016/j.dadm.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baykara E., Gesierich B., Adam R., Tuladhar A.M., Biesbroek J.M., Koek H.L., Ropele S., Jouvent E., Chabriat H., et al. Alzheimer's Disease Neuroimaging Initiative A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms. Ann. Neurol. 2016;80:581–592. doi: 10.1002/ana.24758. [DOI] [PubMed] [Google Scholar]
- 37.Chalmers J., Neal B., MacMahon S., Committee P.M. PROGRESS (Perindopril Protection against Recurrent Stroke Study): regional characteristics of the study population at baseline. Heart Dis. 2000;2:S13–S17. [PubMed] [Google Scholar]
- 38.Cunningham E.L., Todd S.A., Passmore P., Bullock R., McGuinness B. Pharmacological treatment of hypertension in people without prior cerebrovascular disease for the prevention of cognitive impairment and dementia. Cochrane Database Syst. Rev. 2021;5:CD004034. doi: 10.1002/14651858.CD004034.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabayan B., Oleksik A.M., Maier A.B., van Buchem M.A., Poortvliet R.K.E., de Ruijter W., Gussekloo J., de Craen A.J.M., Westendorp R.G.J. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J. Am. Geriatr. Soc. 2012;60:2014–2019. doi: 10.1111/j.1532-5415.2012.04203.x. [DOI] [PubMed] [Google Scholar]
- 40.DeCarli C., Miller B.L., Swan G.E., Reed T., Wolf P.A., Garner J., Jack L., Carmelli D. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 41.Claassen J.A.H., Thijssen D.H.J., Panerai R.B., Faraci F.M. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol. Rev. 2021;101:1487–1559. doi: 10.1152/physrev.00022.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Heus R.A.A., de Jong D.L.K., Sanders M.L., van Spijker G.J., Oudegeest-Sander M.H., Hopman M.T., Lawlor B.A., Olde Rikkert M.G.M. Dynamic regulation of cerebral blood flow in patients with alzheimer disease. Hypertension. 2018;72:139–150. doi: 10.1161/HYPERTENSIONAHA.118.10900. [DOI] [PubMed] [Google Scholar]
- 43.Tryambake D., He J., Firbank M.J., O'Brien J.T., Blamire A.M., Ford G.A. Intensive blood pressure lowering increases cerebral blood flow in older subjects with hypertension. Hypertension. 2013;61:1309–1315. doi: 10.1161/HYPERTENSIONAHA.112.200972. [DOI] [PubMed] [Google Scholar]
- 44.SPRINT Research Group. Lewis C.E., Fine L.J., Beddhu S., Cheung A.K., Cushman W.C., Cutler J.A., Evans G.W., Johnson K.C., Kitzman D.W., et al. Final report of a trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 2021;384:1921–1930. doi: 10.1056/NEJMoa1901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Juraschek S.P., Hu J.R., Cluett J.L., Ishak A., Mita C., Lipsitz L.A., Appel L.J., Beckett N.S., Coleman R.L., Cushman W.C., et al. Effects of intensive blood pressure treatment on orthostatic hypotension : a systematic review and individual participant-based meta-analysis. Ann. Intern. Med. 2021;174:58–68. doi: 10.7326/M20-4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juraschek S.P., Taylor A.A., Wright J.T., Jr., Evans G.W., Miller E.R., 3rd, Plante T.B., Cushman W.C., Gure T.R., Haley W.E., Moinuddin I., et al. Orthostatic hypotension, cardiovascular outcomes, and adverse events: results from SPRINT. Hypertension. 2020;75:660–667. doi: 10.1161/HYPERTENSIONAHA.119.14309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Heus R.A.A., Donders R., Santoso A.M.M., Olde Rikkert M.G.M., Lawlor B.A., Nilvad Study Group Blood pressure lowering with nilvadipine in patients with mild-to-moderate alzheimer disease does not increase the prevalence of orthostatic hypotension. J. Am. Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elahi F.M., Harvey D., Altendahl M., Brathaban N., Fernandes N., Casaletto K.B., Staffaroni A.M., Maillard P., Hinman J.D., Miller B.L., et al. Elevated complement mediator levels in endothelial-derived plasma exosomes implicate endothelial innate inflammation in diminished brain function of aging humans. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-91759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pauls M.M., Binnie L.R., Benjamin P., Betteridge S., Clarke B., Dhillon M., Ghatala R., Hainsworth F., Howe F.A., Khan U., et al. The PASTIS trial. Testing tadalafil for possible use in vascular cognitive impairment. Alzheimer's Dementia. 2022;18:2393–2402. doi: 10.1002/alz.12559. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blair G.W., Appleton J.P., Law Z.K., Doubal F., Flaherty K., Dooley R., Shuler K., Richardson C., Hamilton I., Shi Y., et al. Preventing cognitive decline and dementia from cerebral small vessel disease: the LACI-1 Trial. Protocol and statistical analysis plan of a phase IIa dose escalation trial testing tolerability, safety and effect on intermediary endpoints of isosorbide mononitrate and cilostazol, separately and in combination. Int. J. Stroke. 2018;13:530–538. doi: 10.1177/1747493017731947. [DOI] [PubMed] [Google Scholar]
- 51.Anchala R., Kannuri N.K., Pant H., Khan H., Franco O.H., Di Angelantonio E., Prabhakaran D. Hypertension in India: a systematic review and meta-analysis of prevalence, awareness, and control of hypertension. J. Hypertens. 2014;32:1170–1177. doi: 10.1097/HJH.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian M., Ajay V.S., Dunzhu D., Hameed S.S., Li X., Liu Z., Li C., Chen H., Cho K., Li R., et al. A cluster-randomized, controlled trial of a simplified multifaceted management program for individuals at high cardiovascular risk (SimCard trial) in rural tibet, China, and Haryana, India. Circulation. 2015;132:815–824. doi: 10.1161/CIRCULATIONAHA.115.015373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akinyemi R.O., Yaria J., Ojagbemi A., Guerchet M., Okubadejo N., Njamnshi A.K., Sarfo F.S., Akpalu A., Ogbole G., Ayantayo T., et al. Dementia in Africa: current evidence, knowledge gaps, and future directions. Alzheimers Dement. 2022;18:790–809. doi: 10.1002/alz.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iyer G.K., Paplikar A., Alladi S., Dutt A., Sharma M., Mekala S., Kaul S., Saroja A.O., Divyaraj G., Ellajosyula R., et al. Standardising dementia diagnosis across linguistic and educational diversity: study design of the Indian Council of medical research-neurocognitive tool box (ICMR-NCTB) J. Int. Neuropsychol. Soc. 2020;26:172–186. doi: 10.1017/S1355617719001127. [DOI] [PubMed] [Google Scholar]
- 55.Forette F., Seux M.L., Staessen J.A., Thijs L., Birkenhäger W.H., Babarskiene M.R., Babeanu S., Bossini A., Gil-Extremera B., Girerd X., et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 56.Diener H.C., Sacco R.L., Yusuf S., Cotton D., Ounpuu S., Lawton W.A., Palesch Y., Martin R.H., Albers G.W., Bath P., et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol. 2008;7:875–884. doi: 10.1016/S1474-4422(08)70198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson C., Teo K., Gao P., Arima H., Dans A., Unger T., Commerford P., Dyal L., Schumacher H., Pogue J., et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011;10:43–53. doi: 10.1016/S1474-4422(10)70250-7. [DOI] [PubMed] [Google Scholar]
- 58.Kehoe P.G., Turner N., Howden B., Jarutyte L., Clegg S.L., Malone I.B., Barnes J., Nielsen C., Sudre C.H., Wilson A., et al. Safety and efficacy of losartan for the reduction of brain atrophy in clinically diagnosed Alzheimer's disease (the RADAR trial): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2021;20:895–906. doi: 10.1016/S1474-4422(21)00263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peters R., Dodge H.H., James S., Jicha G.A., Meyer P.F., Richards M., Smith A.D., Yassine H.N., Abner E., Hainsworth A.H., et al. The epidemiology is promising, but the trial evidence is weak. Why pharmacological dementia risk reduction trials haven't lived up to expectations, and where do we go from here? Alzheimers Dement. 2022;18:507–512. doi: 10.1002/alz.12393. [DOI] [PubMed] [Google Scholar]
- 60.Edwards J.D., Ramirez J., Callahan B.L., Tobe S.W., Oh P., Berezuk C., Lanctôt K., Swardfager W., Nestor S., Kiss A., et al. Antihypertensive treatment is associated with MRI-derived markers of neurodegeneration and impaired cognition: a propensity-weighted cohort study. J. Alzheimers Dis. 2017;59:1113–1122. doi: 10.3233/JAD-170238. [DOI] [PubMed] [Google Scholar]
- 61.Trompet S., Westendorp R.G.J., Kamper A.M., de Craen A.J.M. Use of calcium antagonists and cognitive decline in old age. The Leiden 85-plus study. Neurobiol. Aging. 2008;29:306–308. doi: 10.1016/j.neurobiolaging.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 62.Webb A.J.S., Fischer U., Mehta Z., Rothwell P.M. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 63.Ma Y., Blacker D., Viswanathan A., van Veluw S.J., Bos D., Vernooij M.W., Hyman B.T., Tzourio C., Das S., Hofman A. Visit-to-Visit blood pressure variability, neuropathology, and cognitive decline. Neurology. 2021;96:e2812–e2823. doi: 10.1212/WNL.0000000000012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldstein E.D., Wolcott Z., Garg G., Navarro K., Delic A., Yaghi S., Sederholm B., Prabhakaran S., Wong K.H., McLean K., et al. Effect of antihypertensives by class on cerebral small vessel disease: a post hoc analysis of SPRINT-MIND. Stroke. 2022;53:2435–2440. doi: 10.1161/STROKEAHA.121.037997. 101161STROKEAHA121037997. [DOI] [PubMed] [Google Scholar]
- 65.Pajewski N.M., Berlowitz D.R., Bress A.P., Callahan K.E., Cheung A.K., Fine L.J., Gaussoin S.A., Johnson K.C., King J., Kitzman D.W., et al. Intensive vs standard blood pressure control in adults 80 Years or older: a secondary analysis of the systolic blood pressure intervention trial. J. Am. Geriatr. Soc. 2020;68:496–504. doi: 10.1111/jgs.16272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pereira J.V.B., Aung Thein M.Z., Nitchingham A., Caplan G.A. Delirium in older adults is associated with development of new dementia: a systematic review and meta-analysis. Int. J. Geriatr. Psychiatry. 2021;36:993–1003. doi: 10.1002/gps.5508. [DOI] [PubMed] [Google Scholar]
- 67.Richardson S.J., Davis D.H.J., Stephan B.C.M., Robinson L., Brayne C., Barnes L.E., Taylor J.P., Parker S.G., Allan L.M. Recurrent delirium over 12 months predicts dementia: results of the Delirium and Cognitive Impact in Dementia (DECIDE) study. Age Ageing. 2021;50:914–920. doi: 10.1093/ageing/afaa244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rizvi B., Lao P.J., Chesebro A.G., Dworkin J.D., Amarante E., Beato J.M., Gutierrez J., Zahodne L.B., Schupf N., Manly J.J., et al. Association of regional white matter hyperintensities with longitudinal alzheimer-like pattern of neurodegeneration in older adults. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.25166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blood Pressure Lowering Treatment Trialists' Collaboration Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397:1625–1636. doi: 10.1016/S0140-6736(21)00590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bolandzadeh N., Tam R., Handy T.C., Nagamatsu L.S., Hsu C.L., Davis J.C., Dao E., Beattie B.L., Liu-Ambrose T. Resistance training and white matter lesion progression in older women: exploratory analysis of a 12-month randomized controlled trial. J. Am. Geriatr. Soc. 2015;63:2052–2060. doi: 10.1111/jgs.13644. [DOI] [PubMed] [Google Scholar]
- 71.Liu-Ambrose T., Best J.R., Davis J.C., Eng J.J., Lee P.E., Jacova C., Boyd L.A., Brasher P.M., Munkacsy M., Cheung W., Hsiung G.Y.R. Aerobic exercise and vascular cognitive impairment: a randomized controlled trial. Neurology. 2016;87:2082–2090. doi: 10.1212/WNL.0000000000003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ngandu T., Lehtisalo J., Solomon A., Levälahti E., Ahtiluoto S., Antikainen R., Bäckman L., Hänninen T., Jula A., Laatikainen T., et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 73.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Jr., Collins K.J., Dennison Himmelfarb C., DePalma S.M., Gidding S., Jamerson K.A., Jones D.W., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2018;138:e426–e483. doi: 10.1161/CIR.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 74.Blumenthal J.A., Smith P.J., Mabe S., Hinderliter A., Welsh-Bohmer K., Browndyke J.N., Doraiswamy P.M., Lin P.H., Kraus W.E., Burke J.R., Sherwood A. Longer term effects of diet and exercise on neurocognition: 1-year follow-up of the ENLIGHTEN trial. J. Am. Geriatr. Soc. 2020;68:559–568. doi: 10.1111/jgs.16252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Richard E., Gouw A.A., Scheltens P., van Gool W.A. Vascular care in patients with Alzheimer disease with cerebrovascular lesions slows progression of white matter lesions on MRI: the evaluation of vascular care in Alzheimer's disease (EVA) study. Stroke. 2010;41:554–556. doi: 10.1161/STROKEAHA.109.571281. [DOI] [PubMed] [Google Scholar]
- 76.Moll van Charante E.P., Richard E., Eurelings L.S., van Dalen J.W., Ligthart S.A., van Bussel E.F., Hoevenaar-Blom M.P., Vermeulen M., van Gool W.A. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. 2016;388:797–805. doi: 10.1016/S0140-6736(16)30950-3. [DOI] [PubMed] [Google Scholar]
- 77.Liu-Ambrose T., Davis J.C., Best J.R., Dian L., Madden K., Cook W., Hsu C.L., Khan K.M. Effect of a home-based exercise program on subsequent falls among community-dwelling high-risk older adults after a fall: a randomized clinical trial. JAMA. 2019;321:2092–2100. doi: 10.1001/jama.2019.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pahor M., Guralnik J.M., Ambrosius W.T., Blair S., Bonds D.E., Church T.S., Espeland M.A., Fielding R.A., Gill T.M., Groessl E.J., et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kivipelto M., Mangialasche F., Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018;14:653–666. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]