Highlights
-
•
We examine the burden of lifetime healthcare expenses among disadvantaged individuals.
-
•
Black men have higher lifetime healthcare expenses compared to other demographic groups.
-
•
Lifetime healthcare expenditure tends to increase with higher cardiovascular risk factor prevalence.
Keywords: Healthcare expenses, Social determinants of health
Abstract
Objective
To understand the burden of healthcare expenses over the lifetime of individuals and evaluate differences among those with cardiovascular risk factors and among disadvantaged groups based on race/ethnicity and sex.
Methods
We linked data from the longitudinal multiethnic Dallas Heart Study, which recruited participants between 2000 and 2002, with inpatient and outpatient claims from all hospitals in the Dallas-Fort Worth metroplex through December 2018, capturing encounter expenses. Race/ethnicity and sex, as well as five risk factors, hypertension, diabetes, hyperlipidemia, smoking, and overweight/obesity, were defined at cohort enrollment. For each individual, expenses were indexed to age and cumulated between 40 and 80 years of age. Lifetime expenses across exposures were evaluated as interactions in generalized additive models.
Results
A total of 2184 individuals (mean age, 45±10 years; 61% women, 53% Black) were followed between 2000 and 2018. The mean modeled lifetime cumulative healthcare expenses were $442,629 (IQR, $423,850 to $461,408). In models that included 5 risk factors, Black individuals had $21,306 higher lifetime healthcare spending compared with non-Black individuals (P < .001), and men had modestly higher expenses than women ($5987, P < .001). Across demographic groups, the presence of risk factors was associated with progressively higher lifetime expenses, with significant independent association of diabetes ($28,075, P < .001), overweight/obesity ($8816, P < .001), smoking ($3980, P = .009), and hypertension ($528, P = .02) with excess spending.
Conclusion
Our study suggests Black individuals have higher lifetime healthcare expenses, exaggerated by the substantially higher prevalence of risk factors, with differences emerging in older age.
Healthcare expenses pose a major burden on patients and our health system. These challenges are increasingly recognized for patients with cardiovascular disease, particularly those with socioeconomic disadvantage. However, the understanding of healthcare spending has focused on describing spending around healthcare events or annual spending in those with established disease [1], [2], [3], [4]. This understanding of cross-sectional burden from healthcare expenses presents only a partial assessment of the challenges from chronic disease, especially in the setting of substantial deferral of care in early-to-mid life. Moreover, it does not identify avenues for prevention.
The assessment of healthcare spending among individuals has found that more than half of asymptomatic individuals enrolled in longitudinal cohorts developed cardiovascular risk factors over 10 years of follow-up, and up to a third require healthcare interventions [5]. However, these assessments are limited to a 10-year time horizon. These are also based on simulated expenses assigned to self-reported healthcare events without confirmatory data on actual healthcare costs spanning inpatient and outpatient spending [6]. The challenges with self-reported healthcare events limits the ability to assess the entirety of the healthcare burden [6].
The challenges with healthcare expenses are more acute in racial and ethnic minorities, particularly Black individuals, and among women [2,7], who have lower healthcare spending in early life but more frequently self-report deferral of healthcare due to expenses [8], [9], [10]. A comprehensive assessment of lifetime healthcare spending among historically underserved groups across patients with modifiable cardiovascular risk factors has the potential to better define the scope of the burden patients face and potential avenues to intervention.
In the current study, we followed a racially and ethnically diverse cohort of individuals enrolled in the Dallas Heart Study (DHS) for all healthcare events over a 15-year period across all hospitals and outpatient practices in the Dallas-Fort Worth Metroplex and assessed their healthcare spending on cardiovascular and non-cardiovascular care based on billing data from these healthcare events. We specifically evaluated differences across racial/ethnic groups and sex, and the association of cardiovascular risk factors on long term healthcare spending.
1. Methods
The study was based on the longitudinal cohort of individuals enrolled in the DHS. The DHS has been described previously [11]. Briefly, it is a cohort of community-dwelling individuals enrolled between 2000 and 2002, with oversampling of African American and Hispanic individuals. The data includes a detailed phenotypic assessment of participants at baseline based on in-depth interviews and laboratory testing to define their cardiometabolic risk profile. Healthcare events for the cohort were obtained from the Dallas-Fort Worth (DFW) Hospital Council Consortium, which includes 77 healthcare institutions in the DFW Metroplex, along with ambulatory practices across 28 counties and 97% of all healthcare encounters in the region [12]. The records for cohort participants were linked using direct identifiers, including a combination of name, address, medical record number, and date of birth. The research dataset represented deidentified data constructed from the baseline DHS enrollment visit and healthcare events from the DFW healthcare council.
The baseline profile of individuals included their demographic features of age, sex, and race as well as 5 key cardiovascular risk factors. Race and ethnicity were self-reported in mutually exclusive groups of Hispanics, non-Hispanic Black, non-Hispanic white, and other race and ethnicity groups. These included hypertension, diabetes, hyperlipidemia, current or former smoking and obesity, and were defined based on established standards [13,14]. Hypertension was defined by a systolic blood pressure of 130 mmHg or higher, diastolic blood pressure of 85 mm Hg or higher, and/or the use of antihypertensive medications. Diabetes was defined by fasting serum glucose ≥126 mg/dL, or the use of glucose-lowering agents. Hyperlipidemia was defined by a low-density lipoprotein level of 160 or higher, or use of lipid-lowering therapy. Smoking status was based on self-report. Overweight/obesity was defined by a body mass index of 25 kg/m2 or higher [13].
Healthcare spending was based on healthcare billing claims submitted by hospitals and clinics in the DFW Hospital Council. The data represent charges submitted by the hospitals and healthcare institutions across all payers and are a representation of the burden of healthcare on our health system, as opposed to the payments by insurers or out-of-pocket spending on healthcare, both of which vary substantially across patients and insurance providers [15,16].
These expenses were further classified into cardiovascular and non-cardiovascular expenses based on the principal diagnosis codes of the encounters. The International Classification of Diseases Clinical Modification 9th Edition codes corresponding to cardiovascular conditions are tabulated in eTable 1.
We identified 3072 individuals at least 18 years of age, of whom 2311 had at least one healthcare event in the DFW database (eFigure 1). We further excluded 54 individuals who were missing information on baseline demographics or comorbidities and 73 individuals who did not receive any healthcare during the ages of 40 to 80, the age group of interest to assess longitudinal healthcare spending.
We developed a novel approach that used an overlapping panel design to evaluate healthcare spending at each year of life across all individuals. We landmarked our assessments at age 40 years, given the infrequent healthcare needs for individuals below that age. We assessed healthcare expenses for all individuals for each year from the age of 40 or the age of enrollment through their age at the end of the period of observation in the study. Among individuals who enrolled in the cohort after age 40 years, we imputed the healthcare spending from age 40 through their age at enrollment (eFigure 2). After the age of enrollment through the end of the observation period or death, healthcare expenses were drawn from the DFW database. The study did not impute any values during the study period or after the end of the study or death as our study aimed to evaluate observable healthcare expenses.
The imputation was based on chained random forest with predictive mean matching across all individuals with data at that age [17]. Healthcare expenses at a given age represented cumulative spending on healthcare from age 40 years to that age (Central Illustration).
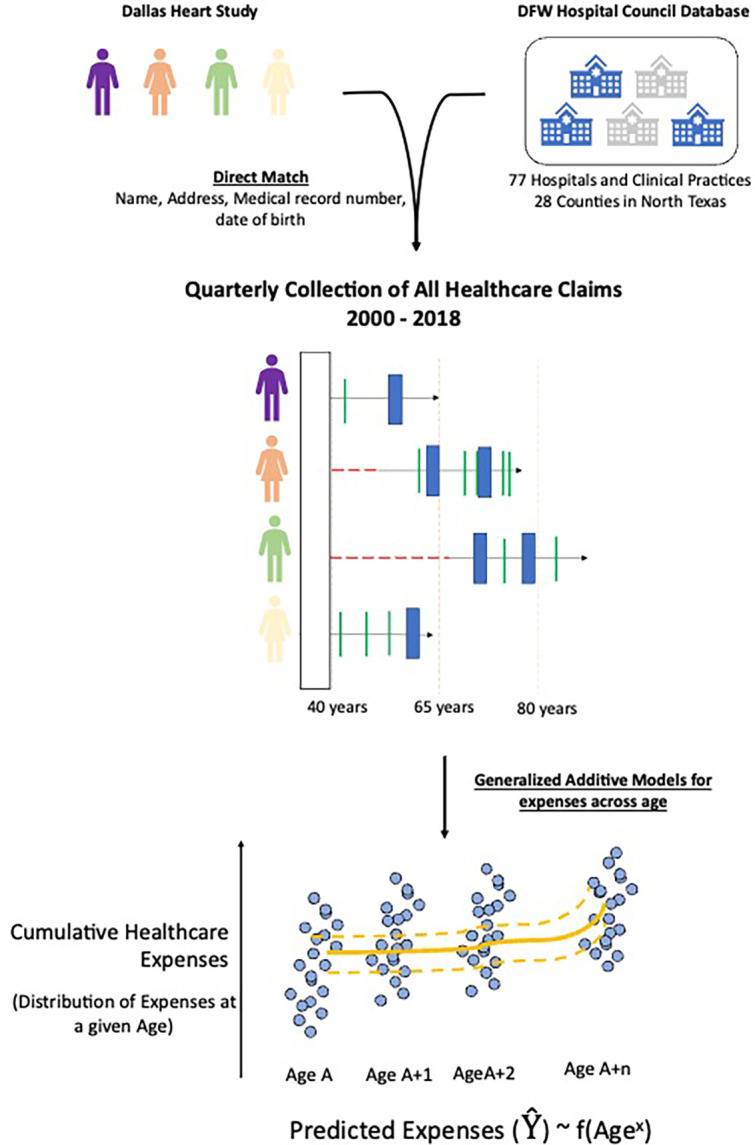
Central Illustration: Study Outline.
Given the expected non-linear association of healthcare spending over time, cumulative healthcare expenses were modeled in generalized additive models as a fourth-order polynomial function of continuous age between 40 and 80 years. To evaluate the association of race/ethnicity, sex, and comorbid health conditions, we constructed individual models across individuals with and without individual healthcare expenses stratified by subgroups of race/ethnicity and sex. We identified the association of race/ethnicity with lifetime spending by modeling its interaction with age. We parametrized race/ethnicity as Black and non-Black individuals, with the latter group including Hispanic, non-Hispanic White, and other racial and ethnic groups. This choice was driven by a similar pattern in healthcare expenses for Hispanic and non-Hispanic White individuals and the smaller size of these subpopulations individually relative to the Black population, representing over 50% of the study population.
We visualized patterns across subgroups using the component plots of the GAM functions. We repeated the analyses across spending for cardiovascular disease.
The study was approved by the UT Southwestern Institutional Review Board which waived the right to informed consent as the study uses previously collected deidentified data. All analyses were pursued using R 4.0, and statistical tests were two-sided with a level of significance of 0.05.
2. Results
A total of 2184 individuals, with a mean age, 45(±10) years at enrollment, were followed for a median of 17.7 (IQR 17.7–18.0) years. Of these, 1339 (61.3%) were women and 1034 (47.3%) were Non-Hispanic Black (Table 1). At enrollment, 797 (36.5%) individuals had hypertension, 271 (12.4%) had diabetes, 317(14.5%) had hyperlipidemia, 982 (45.0%) were smokers, and 1629 (74.6%) had obesity. The prevalence of cardiovascular risk factors was significantly higher among Black individuals compared with non-Black individuals, particularly for hypertension (46.3% vs 25.5%, P < .001) and diabetes (15.1% vs 9.4%, P < .001). Men, compared with women, had significantly higher prevalence of smoking (53.5% vs 39.6%, P < .001).
Table 1.
Baseline clinical characteristics of study participants.
| Overall | Female | Male | P value (sex) | Black | Non-Black |
P value (race) |
|
|---|---|---|---|---|---|---|---|
| N | 2184 | 1339 | 845 | 1150 | 1034 | ||
| Age at enrollment, mean (SD) | 45.4 (9.5) | 45.1 (9.7) | 45.8 (9.2) | 0.12 | 45.8 (9.58) | 44.9 (9.4) | 0.038 |
| Hypertension, (%) | 797 (36.5) | 495 (37.0) | 302 (35.7) | 0.59 | 533 (46.3) | 264 (25.5) | <0.001 |
| Diabetes, (%) | 271 (12.4) | 163 (12.2) | 108 (12.8) | 0.72 | 174 (15.1) | 97 (9.4) | <0.001 |
| Smoking, (%) | 982 (45.0) | 530 (39.6) | 452 (53.5) | <0.001 | 505 (43.9) | 477 (46.1) | 0.32 |
| Hyperlipidemia, (%) | 317 (14.5) | 179 (13.4) | 138 (16.3) | 0.06 | 164 (14.3) | 153 (14.8) | 0.77 |
| Overweight/Obesity (%) | 1629 (74.6) | 1002 (74.8) | 627 (74.2) | 0.78 | 889 (77.3) | 740 (71.6) | 0.002 |
| Income in US$ (%) | <0.001 | <0.001 | |||||
| <25K | 656 (30.0) | 433 (32.3) | 223 (26.4) | 436 (37.9) | 220 (21.3) | ||
| >25K | 615 (28.2) | 314 (23.5) | 301 (35.6) | 178 (15.5) | 437 (42.3) | ||
| 25K-50K | 603 (27.6) | 386 (28.8) | 217 (25.7) | 329 (28.6) | 274 (26.5) | ||
| Unknown | 310 (14.2) | 206 (15.4) | 104 (12.3) | 207 (18.0) | 103 (10.0) | ||
| Education, No College (%) | 1048 (48.0) | 656 (49.0) | 392 (46.4) | 0.25 | 638 (55.5) | 410 (39.7) | <0.001 |
Data presented as N with% for categorical variables and median with interquartile range for continuous variables. Abbreviations: CV, cardiovascular; SD, standard deviation.
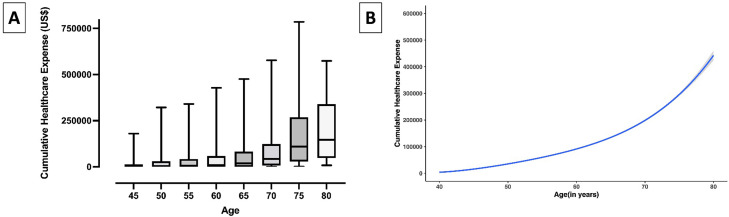
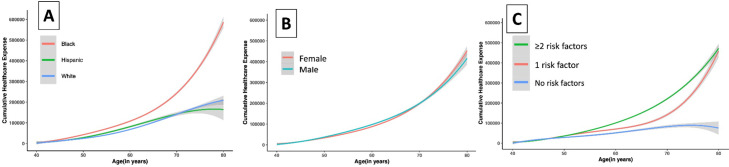
The median cumulative lifetime healthcare spending, defined as spending between ages 40 and 80 years, was $442,629 (25th to 75th percentile, $423,850 to $461,408) (Fig. 1). Lifetime healthcare spending was higher among Black compared with non-Black adults ($586,746 [95% CI, 562,867 to 610,625] vs $204,492 [175,262, 233,722]; P < .001), accelerating in advanced age (P, age-race interaction <0.001). Similarly, women had higher healthcare expenses compared with men ($452,957 [$430,514 – $475,400] vs $416,355 [$382,005 – $450,704); P = .01), manifested at a later age (P, age-sex interaction = 0.001) (Fig. 2). Individuals with cardiovascular risk factors also had higher lifetime spending with progressively higher spending among those with higher risk factor burden, overall and across demographic subgroups (Fig. 2). The predicted lifetime healthcare spending was $75,228 (95% CI, $4913 – $145,543) among patients without any risk factors but was several-fold higher for individuals with 1 cardiovascular risk factor ($459,039, 95% CI, $415,881 – $502,198), and 2 or more risk factors ($472,223, 95% CI, $450,548 – $493,897) (P < .001) (Fig. 2). The cumulative expenditure among patients with and without risk factors stratified by race and sex are shown in eFigures 3–7.
Fig. 1.
Lifetime cumulative healthcare expenses, (A) Observed cumulative expense distribution at a given age, (B) Generalized Additive model with expenses modeled as 4th order function of age.
Fig. 2.
Differences in Modeled Lifetime Healthcare Expenses Across Subgroups based on (A) Race/Ethnicity, (B) Sex, and (C) Risk factor profile.
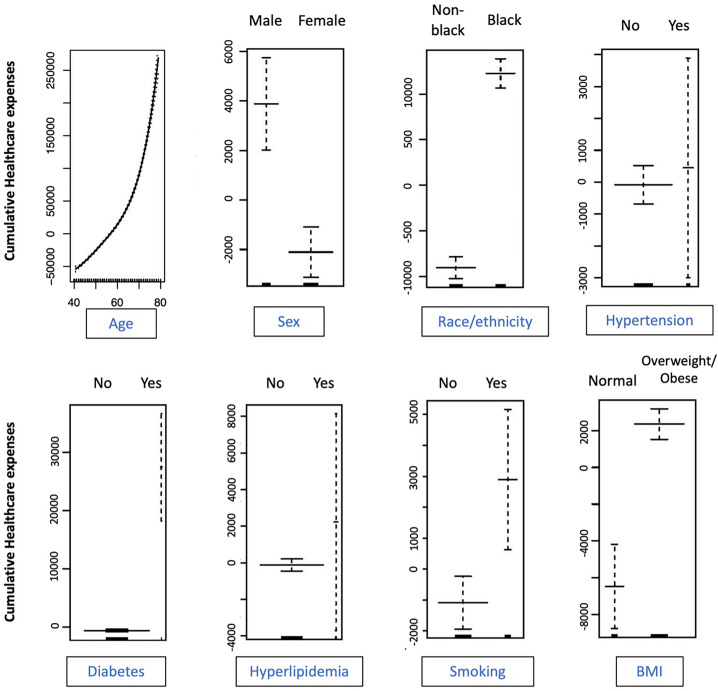
In multivariable generalized additive models that accounted for the non-linear association of expenses with age along with race/ethnicity, sex and the 5 cardiovascular risk factors, there was a significantly higher lifetime spending among Black individuals compared with other racial/ethnic groups (Fig. 3). Even after accounting for differences in risk factors, Black individuals had an excess spending of $21,306 (P < .001). In contrast with unadjusted associations, men had higher lifetime expenses in the 40-to-80-year age window, with adjusted excess spending of $5987 over women (P < .001). Among cardiovascular risk factors, the presence of diabetes ($28,075, P < .001), overweight/obesity ($8816, P < .001), and smoking ($3980, P = .009) were associated with significant excess spending. There was progressively higher healthcare spending with higher risk factor burden (eFigure 8),
Fig. 3.
Multivariable generalized additive model assessing the association of demographic features and cardiovascular risk factors on lifetime healthcare expenses.
These patterns described above were consistent in sensitivity analyses where imputation of pre-enrollment healthcare spending over the age of 40 was not performed. This includes the association of Black race and male gender with higher healthcare expenses (eFigure 9). Similar to overall expenses, lifetime cardiovascular healthcare expenses were higher for Black and male DHS participants, independent of baseline cardiovascular risk profile, with $2838 higher expenses in Black over non-Black individuals and $4182 among men over women (eTable 2).
3. Discussion
In a multi-ethnic cohort followed longitudinally in the Dallas Heart Study, non-Hispanic Black individuals experienced more than 2-fold higher cumulative lifetime healthcare expenses between ages 40 and 80 compared with individuals of other racial and ethnic groups. We demonstrate a novel approach that allows the estimation of lifetime spending and the identification of subgroup differences. Of note, racial differences in lifetime healthcare expenses were driven by higher expenses among Black individuals compared with other races/ethnicities beyond the seventh decade of life. They were exaggerated by a substantially higher burden of cardiovascular risk factors among Black individuals compared with others. Notably, across subgroups, cardiovascular risk factors at baseline were associated with higher lifetime spending, with the addition of just one risk factor increasing expenses by more than 6-fold. Among cardiovascular risk factors, diabetes, and smoking were independently associated with the highest expenses. The cumulative burden of healthcare expenses among socially disadvantaged groups with cardiovascular risk factors remains an under-addressed challenge. Early intervention to improve healthcare access and preventive care among racial minorities represents an important avenue to reduce healthcare challenges and spending in later life [18].
Rising healthcare costs represent a key policy challenge in the US, driving research and policy initiatives to reduce spending on high-cost healthcare events [19,20]. However, the focus of policy has been on specific events and medical conditions [21], [22], [23], [24], obscuring larger trends in healthcare spending. Prior studies have demonstrated lower healthcare spending among Black vs. non-Black adults for specific conditions such as diabetes [25]. However, such studies are limited by short-term follow-up and their reliance on modeling approaches to determine long-term costs incurred. In the current study, we examine cumulative community-level expenditures among residents in the Dallas-Fort Worth metroplex over long-term follow-up and across common cardiovascular medical comorbidities. We observed that the longitudinal trajectory of healthcare use varies by race, with Black patients experiencing a higher risk factor burden earlier in life without corresponding excess healthcare utilization in middle age and an acceleration in healthcare spending later in life. This delay in care likely stems from a fractured preventive healthcare system that disproportionately disadvantages younger Black adults, who are less likely than other individuals of White race to have adequate access to health insurance [26], and are, therefore, prone to the longer-term effects of healthcare deprivation and deferred healthcare.
The excess healthcare expenses faced by Black adults may additionally be associated with the greater burden of cardiovascular risk factors observed in this population as observed in the in the Dallas Heart Study [27] and other community-based cohorts in the US [28,29]. However, in the present study, adjustment for risk factor prevalence and other individual-level characteristics failed to eliminate observed racial differences. Furthermore, the differences in expenditures by race begin to appear late in follow-up, during and after the seventh decade, suggesting that the financial toxicity experienced by Black adults cannot be explained by the presence of risk factors alone. After adjustment for risk factors, Black adults accrued $21,306 more in modeled healthcare expenses, amounting to 6% of the additional costs experienced in unadjusted analysis. Several factors may underlie this observation. First, prior studies point to higher utilization of high-cost and acute care services among Black patients, likely related to low-quality care leading to frequent rehospitalizations and repeat utilization [30,31]. Second, prior studies have attributed a large proportion (>50%) of the excess healthcare costs experienced by racial minorities has been linked to their disproportionate residence in population-dense, urban areas, where care expenses are often highest [32]. Finally, Black and Hispanic adults have been observed to accrue higher end-of-life expenses than White counterparts despite similar patterns in causes of mortality, driven by multiple factors, including missed opportunities for intensive care earlier in life, medical mistrust leading to high utilization of end-of-life therapies, and cultural differences around end-of-life [32]. Efforts to re-focus investment in prevention and treatment earlier in adulthood among Black populations may thus help to stymie the excess healthcare spending experienced by these groups later in life.
We also find that among all study participants, cardiovascular risk factors were strongly associated with long-term healthcare expenses, including diabetes, smoking, and hyperlipidemia, which drive early excess healthcare spending that only worsens over time. These broad effects of preventable cardiovascular risk factors highlight the need for prioritizing primordial prevention, targeting the emergence of risk factors and the structural barriers to healthcare access through multi-level interventions. However, neither primordial prevention nor risk factor management is prioritized in the current healthcare landscape that focuses on healthcare spending in later life.
The present study has important implications for the public health and efforts to mitigate racial disparities in cardiovascular disease. Our findings demonstrate the overwhelming and unequal burden placed on Black adults by healthcare expenses [29]. This is particularly relevant when viewed in the context of larger societal inequalities faced by Black adults in US society—including higher levels of poverty and reduced access to education. However, it should be stated that lower socioeconomic status is not the only driver of worse health statistics in Black individuals. At least one recent study indicates that education and employment status is not necessarily associated with ideal cardiovascular health in Black men [33]. Thus, efforts to address disparities will require aggressive investment into preventing the development of risk factors and treating early manifestations of cardiovascular disease in early adulthood among all adults as well as policies to dismantle the lingering racial discrimination in our society that imparts a heavy allostatic load on many Black individuals, even those that are not impoverished. Additionally, policies designed to link reimbursement to quality of care among disadvantaged groups may help combat imbalances in care delivery across racial and ethnic groups [34]. Finally, national initiatives to ensure more equitable access to health insurance and outpatient care for chronic medical conditions and to limit regional differences in healthcare costs are needed to provide a safety net for patients with risk factors.
Our study has certain limitations. First, while it is the largest contemporary cohort with complete follow-up from nearly all hospitals in a large metroplex, we did not include information on all DHS participants. The DHS includes a representative sample of Dallas-Fort Worth residents with oversampling for Black and Hispanic individuals. However, those who moved elsewhere during the follow-up could not be distinguished from those that did not require any healthcare. Therefore, we focused on individuals with at least some healthcare use, defined as at least one healthcare encounter, but did not institute a requirement for recurrent healthcare encounters for inclusion. This ensures more comprehensiveness in our data capture, but our findings would represent the lower bound of healthcare spending in case there was care sought outside of the metroplex. Second, we focused on billed expenses by hospitals that may not represent the payment from insurers or out-of-pocket spending. Since the actual costs may vary by insurance type, this hospital charge is likely the most consistent measure of healthcare expenses across patients. Moreover, the specific categories of healthcare spending corresponding to these healthcare encounters would be expected to vary but were not explicitly defined. Third, we focused on health status at enrollment to define cardiovascular risk factors but did not define the development of risk factors over time based on reported comorbidities in health encounters. This avoids biasing the study due to differential healthcare access among the groups. Fourth, the study does not explicitly model the effects of differential mortality across the study population, and despite the potential effects of premature mortality in Black individuals on lower measured lifetime expenses, we find excess healthcare spending in this group. Finally, the study represents a single cohort study drawn from a single large metropolitan area and would require an assessment of generalizability in other studies that included large representative populations and complete capture of comorbidities and outcomes.
4. Conclusion
Black individuals have higher lifetime healthcare expenses, exaggerated by the substantially higher prevalence of risk factors, with differences emerging in older age. This unequal expense burden highlights the need to prevent risk factors among Black individuals in early life.
Funding
The work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under the award 1K23HL153775 (Dr. Khera) and the National Institute on Aging under the GEMSSTAR Grant 1R03AG067960 (Dr. Pandey). Dr. Powell-Wiley is funded by the Division of Intramural Research of the National, Heart, Lung, and Blood Institute and the Intramural Research Program of the National Institute on Minority Health, and Health Disparities of the NIH. The Dallas Heart Study was funded by the Donald W. Reynolds Foundation and was partially supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number UL1TR001105.
Disclosures
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institute on Minority Health and Health Disparities, the National Institutes of Health; or the U.S. Department of Health and Human Services. None of the authors report any disclosures relevant to the current work.
Author contributions
R.K. and A.P. conceived the study, A.P. accessed the data, R.K. and M.L. conducted the analyses, R.K. drafted the manuscript, and all authors provided critical revisions. R.K. and A.P. supervised the work and are the guarantors.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Dr Khera receives support from the National Heart, Lung, and Blood Institute of the National Institutes of Health (under award K23HL153775) and the Doris Duke Charitable Foundation (under award, 2022060). He also receives research support, through Yale, from Bristol-Myers Squibb and Novo Nordisk. He is a coinventor of U.S. Provisional Patent Applications 63/177,117, 63/428,569, and 63/346,610, unrelated to current work. He is also a founder of Evidence2Health, a precision health platform to improve evidence-based care. Dr. Pandey reports financial support was provided by GEMSSTAR Grant 1R03AG067960. Dr. Powell-Wiley reports financial support was provided by Division of Intramural Research of the National Heart, Lung, and Blood Institute and the Intramural Research Program of the National Institute on Minority Health, and Health Disparities of the NIH.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2023.100493.
Contributor Information
Rohan Khera, Email: rohan.khera@yale.edu.
Ambarish Pandey, Email: ambarish.pandey@utsouthwestern.edu.
Appendix. Supplementary materials
References
- 1.Khera R., Hong J.C., Saxena A., et al. Burden of catastrophic health expenditures for acute myocardial infarction and stroke among uninsured in the United States. Circulation. 2018;137(4):408–410. doi: 10.1161/CIRCULATIONAHA.117.030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khera R., Valero-Elizondo J., Okunrintemi V., et al. Association of out-of-pocket annual health expenditures with financial hardship in low-income adults with atherosclerotic cardiovascular disease in the United States. JAMA Cardiol. 2018;3(8):729–738. doi: 10.1001/jamacardio.2018.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khera R., Valero-Elizondo J., Nasir K. Financial toxicity in atherosclerotic cardiovascular disease in the united states: current state and future directions. J Am Heart Assoc. 2020;9(19) doi: 10.1161/JAHA.120.017793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S.Y., Valero-Elizondo J., Ali H.J., et al. Out-of-pocket annual health expenditures and financial toxicity from healthcare costs in patients with heart failure in the United States. J Am Heart Assoc. 2021 doi: 10.1161/JAHA.121.022164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw L.J., Goyal A., Mehta C., et al. 10-Year resource utilization and costs for cardiovascular care. Research-article. J Am Coll Cardiol. 2018;71(10):1078–1089. doi: 10.1016/j.jacc.2017.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caraballo C., Khera R., Jones P.G., et al. Rates and predictors of patient underreporting of hospitalizations during follow-up after acute myocardial infarction: an assessment from the TRIUMPH Study. Circ Cardiovasc Qual Outcomes. Jul 2020;13(7) doi: 10.1161/CIRCOUTCOMES.119.006231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S.Y., Valero-Elizondo J., Ali H.J., et al. Out-of-pocket annual health expenditures and financial toxicity from healthcare costs in patients with heart failure in the United States. J Am Heart Assoc. 2021;10(14) doi: 10.1161/JAHA.121.022164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khera R., Valero-Elizondo J., Das S.R., et al. Cost-related medication nonadherence in adults with atherosclerotic cardiovascular disease in the United States, 2013 to 2017. Circulation. 2019;140(25):2067–2075. doi: 10.1161/CIRCULATIONAHA.119.041974. [DOI] [PubMed] [Google Scholar]
- 9.Caraballo C., Valero-Elizondo J., Khera R., et al. Burden and consequences of financial hardship from medical bills among nonelderly adults with diabetes mellitus in the United States. Circ Cardiovasc Qual Outcomes. 2020;13(2) doi: 10.1161/CIRCOUTCOMES.119.006139. [DOI] [PubMed] [Google Scholar]
- 10.Valero-Elizondo J., Khera R., Saxena A., et al. Financial hardship from medical bills among nonelderly U.S. adults with atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2019;73(6):727–732. doi: 10.1016/j.jacc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 11.RG V., RW H., DL W., et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93(12) doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 12.Vigen R., Diercks D.B., Hashim I.A., et al. Association of a novel protocol for rapid exclusion of myocardial infarction with resource use in a US Safety Net Hospital. JAMA Netw Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathieu RAt, Powell-Wiley T.M., Ayers C.R., et al. Physical activity participation, health perceptions, and cardiovascular disease mortality in a multiethnic population: the Dallas Heart Study. Am Heart J. 2012;163(6):1037–1040. doi: 10.1016/j.ahj.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 15.Fronsdal T.L., Bhattacharya J., Tamang S. National Bureau of Economic Research; 2020. Variation in health care prices across public and private payers.https://www.nber.org/papers/w27490 Available at: Accessed February 13, 2022. [Google Scholar]
- 16.Khera R., Valero-Elizondo J., Okunrintemi V., et al. Association of out-of-pocket annual health expenditures with financial hardship in low-income adults with atherosclerotic cardiovascular disease in the United States. JAMA Cardiol. 2018;3(8):729–738. doi: 10.1001/jamacardio.2018.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer M. missRanger: fast imputation of missing values. R Package Version. 2019;2(0) [Google Scholar]
- 18.Essien U.R., Dusetzina S.B., Gellad W.F. A policy prescription for reducing health disparities-achieving pharmacoequity. JAMA. 2021;326(18):1793–1794. doi: 10.1001/jama.2021.17764. [DOI] [PubMed] [Google Scholar]
- 19.Kahn C.N., 3rd, Ault T., Potetz L., et al. Assessing Medicare's hospital pay-for-performance programs and whether they are achieving their goals. Health Aff. 2015;34(8):1281–1288. doi: 10.1377/hlthaff.2015.0158. [DOI] [PubMed] [Google Scholar]
- 20.Ryan A.M., Krinsky S., Adler-Milstein J., et al. Association between hospitals' engagement in value-based reforms and readmission reduction in the hospital readmission reduction program. JAMA Intern Med. 2017;177(6):862–868. doi: 10.1001/jamainternmed.2017.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khera R., Krumholz H.M. Effects of the hospital readmissions reduction program. Circ Cardiovasc Qual Outcomes. 2018;11(12) doi: 10.1161/CIRCOUTCOMES.118.005083. [DOI] [PubMed] [Google Scholar]
- 22.Joynt Maddox K.E., Orav E.J., Zheng J., Epstein A.M. Evaluation of medicare's bundled payments initiative for medical conditions. N Engl J Med. 2018;379(3):260–269. doi: 10.1056/NEJMsa1801569. [DOI] [PubMed] [Google Scholar]
- 23.Cutler D.M., Ghosh K. The potential for cost savings through bundled episode payments. N Engl J Med. 2012;366(12):1075–1077. doi: 10.1056/NEJMp1113361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khera R., Wang Y., Bernheim S.M., et al. Post-discharge acute care and outcomes following readmission reduction initiatives: national retrospective cohort study of Medicare beneficiaries in the United States. BMJ. 2020;368:l6831. doi: 10.1136/bmj.l6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung M.Y., Pollack L.M., Colditz G.A., et al. Life years lost and lifetime health care expenditures associated with diabetes in the U.S., National Health Interview Survey, 1997-2000. Diabetes Care. 2015;38(3):460–468. doi: 10.2337/dc14-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace J., Jiang K., Goldsmith-Pinkham P., et al. Changes in racial and ethnic disparities in access to care and health among US adults at age 65 years. JAMA Intern Med. 2021;181(9):1207–1215. doi: 10.1001/jamainternmed.2021.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackler E., 3rd, Lew J., Gore M.O., et al. Racial differences in cardiovascular biomarkers in the general population. J Am Heart Assoc. 2019;8(18) doi: 10.1161/JAHA.119.012729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry J.D., Dyer A., Cai X., et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366(4):321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min Y.I., Anugu P., Butler K.R., et al. Cardiovascular disease burden and socioeconomic correlates: findings from the Jackson Heart Study. J Am Heart Assoc. 2017;6(8) doi: 10.1161/JAHA.116.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen H., Gordon S.H., Lee D., et al. Comparison of utilization, costs, and quality of medicaid vs subsidized private health insurance for low-income adults. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.32669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziaeian B., Heidenreich P.A., Xu H., et al. Medicare expenditures by race/ethnicity after hospitalization for heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6(5):388–397. doi: 10.1016/j.jchf.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanchate A., Kronman A.C., Young-Xu Y., et al. Racial and ethnic differences in end-of-life costs: why do minorities cost more than whites? Arch Intern Med. 2009;169(5):493–501. doi: 10.1001/archinternmed.2008.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azap R.A., Nolan T.S., Gray D.M., 2nd, et al. Association of socioeconomic status with ideal cardiovascular health in black men. J Am Heart Assoc. 2021;10(23) doi: 10.1161/JAHA.120.020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delaney S.W., Essien U.R., Navathe A. Disparate impact: how colorblind policies exacerbate black-white health inequities. Ann Intern Med. 2021;174(10):1450–1451. doi: 10.7326/M21-1748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.