Summary
Background
18F-FDG-PET/CT is the current standard technique to define minimal residual disease (MRD) outside the bone marrow (BM) in multiple myeloma (MM), recently standardised applying the Deauville scores (DS) to focal lesions (FS) and bone marrow uptake (BMS) and defining the complete metabolic response (CMR) as uptake below the liver background (DS <4).
Methods
In this analysis, we aimed at confirming the role of CMR, and complementarity with BM multiparameter flow cytometry (MFC) at 10−5, in an independent cohort of newly diagnosed transplant-eligible MM patients previously enrolled in the phase II randomised FORTE trial. 109 of the 474 global patients enrolled in the trial between February 23, 2015, and April 5, 2017, who had paired PET/CT (performed at baseline [B] and preceding maintenance therapy [PM]) and MFC evaluation, were included in this analysis.
Findings
At B, 93% of patients had focal lesions within the bones (FS ≥4 in 89%) and 99% increased BM uptake (BMS ≥4 in 61%). At PM, CMR was achieved in 63% of patients, which was a strong predictor for prolonged PFS in univariate analysis at landmark time PM (HR 0.40, P = 0.0065) and in Cox multivariate analysis (HR 0.31, P = 0.0023). Regarding OS, a trend in favour of CMR was present in univariate (HR 0.44, P = 0.094), and Cox multivariate model (HR 0.17, P = 0.0037). Patients achieving both PET/CT CMR and MFC negativity at PM showed significantly extended PFS in univariate (HR 0.45, P = 0.020) and multivariate analysis (HR 0.41, P = 0.015).
Interpretation
We herein confirm the applicability and validity of DS criteria to define CMR and its prognostic relevance and complementarity with MFC at the BM level.
Funding
Amgen, Celgene/Bristol Myers Squibb, Italian Ministry of Health (RC-2022-2773423).
Keywords: Minimal residual disease (MRD), FDG-PET/CT, Newly diagnosed multiple myeloma (NDMM), Complete metabolic response (CMR), Multiparameter flow cytometry (MFC)
Research in context.
Evidence before this study
Minimal residual disease (MRD) evaluation has become a standard in most clinical trials and its use in routine clinical practice is progressively spreading, used as outcomes’ prognosticator and as a treatment end-point. Despite in the past most data relied on bone marrow MRD, more and more trials are currently requiring also functional imaging to guarantee the complete eradication of the disease after therapy, in light of the inhomogeneous PCs infiltration of the BM, making false negatives more likely, of the possible existence of extramedullary disease, due to metastatic spread, and of the possibility of spatial heterogeneity. IMWG response criteria include and recommend imaging-MRD category. PET/CT is an optimal technique for studying and monitoring the treatment efficacy, being able to distinguish active from inactive lesions, and it is widely used among many haematological centres. Several studies have demonstrated the prognostic value of negative FDG-PET after completion of therapy and some of them the complementarity with BM evaluation by flow cytometry with a sensitivity of 10−4 and 10−5. In a previous joint analysis of 2 prospective randomised studies, the first attempt to standardise the definition of PET negativity after therapy was proposed, applying the Deauville criteria in use for lymphomas to MM and allowing the definition of complete metabolic response (CMR), helping in avoiding false positive PET results and having an easy and practical reference to define PET-MRD.
Added value of this study
In the present analysis, we proved for the first time the applicability of the recently defined standardised criteria for FDG PET negativity in an independent prospective series of newly diagnosed MM patients eligible for ASCT. In particular, we confirmed that complete metabolic response after therapy can be identified as the reduction of every area of FDG uptake in the BM and in the focal lesions below the liver uptake. We believe that the reproducibility of the results is important to recommend a new established standardised referral for a broader use. We also confirmed the role of FDG-PET as an important prognostic tool in evaluating patients’ outcomes after therapy and the complementarity between imaging and BM MRD, at a higher sensitivity level, with the best outcomes belonging to those patients achieving MRD negativity by both techniques.
Implications of all the available evidence
Based on our results, we propose to consider the adoption of hepatic uptake as a simple and reproducible reference for defining the negativity of PET after therapy, both in the context of clinical trials and in daily clinical practice. This will allow to uniform PET interpretation among different haematological centres and will encourage the use of this imaging technique for MRD evaluation after therapy. In light of the future use of MRD to tailor patients’ management, we believe that the availability of reliable data is highly important.
Introduction
New treatment options for newly diagnosed transplant eligible multiple myeloma (NDTEMM) patients, including the 3 main pillars proteasome inhibitors (PIs), immune modulatory agents (IMiDs) and anti-CD38 monoclonal antibodies (mAbs), have expanded rapidly in the last decade, leading to deeper responses, up to the minimum detectable level.1
Minimal residual disease (MRD), detected with specialised standardised methods at the bone marrow (BM) level, such as next generation multiparameter flow cytometry (MFC) or next generation sequencing (NGS), with sensitivity threshold up to 10−5 and 10−6, became the most important predictors of long-term outcomes and survival and is currently extensively applied in prospective clinical trials, sometimes as an early clinical end-point.2
The patchy bone marrow plasma cell infiltration (BMPCs), the possible extramedullary disease escape, and the well-recognised genomic spatial heterogeneity of MM3, 4, 5 support the need to assess the whole extramedullary compartment and BM, to ensure complete tumour eradication as recommended by the International Myeloma Working Group (IMWG).6 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography integrated with computed tomography (PET/CT), assessing tumor metabolic activity in the context of CT evaluation, is currently the preferred imaging technique among others for evaluating response to therapy and monitoring patients after treatment, being able to distinguish between active and inactive (e.g., fibrotic) disease,7,8 and being validated in several prospective trials. The prognostic value of PET/CT during or after therapy have been demonstrated by several prospective studies9, 10, 11 and in real-life,12 even in patients achieving complete remission,13 and has proved complementary to MRD evaluation within BM by next generation flow (NGF).14,15
As with BM techniques, standardisation of imaging criteria and definition of cut-offs for positivity/negativity is of great importance, to ensure data reproducibility and harmonisation of results among different clinical trials. A joint analysis of 2 prospective randomised trials in NDTEMM patients applied for the first time the Deauville scores (DS) to focal lesions (FS) and bone marrow uptake (BMS), showing the liver background (DS <4) as the best cut-off to define PET/CT negativity after therapy and complete metabolic response (CMR).16 Validation of these new standardised criteria in an independent prospective series of NDTEMM patients is highly required to recommend their use both in clinical trials and in daily routine practice.
In this analysis, we aimed at demonstrating the applicability and validity of DS criteria to define PET CMR and showing their impact on patient outcomes in the multicentre phase II randomised FORTE trial for NDTEMM patients. We also looked at the complementarity with MFC at higher sensitivity levels, confirming the importance of MRD evaluation inside and outside the BM.
Patients and methods
Patients and treatment protocol
Four hundred and seventy-four patients ≤65 years were randomised in the phase II randomised FORTE trial to receive carfilzomib, lenalidomide, dexamethasone (KRD) induction-autologous stem cell transplantation (ASCT) intensification-KRd consolidation (arm A); KRd12 (arm B) and carfilzomib, cyclophosphamide, dexamethasone (KCd) induction-ASCT intensification-KCd consolidation (arm C), as described elsewhere.17 Thereafter, patients were randomised to maintenance with lenalidomide alone or plus 2-years carfilzomib until disease progression.
At enrolment, patients were randomly assigned (1:1:1) to one of the three induction–intensification–consolidation groups. A block randomisation (block size 12), stratified according to International Staging System (ISS) stage (I vs II or III) and age (<60 years vs 60–65 years), was generated at enrolment by a computer program and implemented into a web-based procedure by the investigator or designated research staff. Patients who did not experience unacceptable toxicity or progression during the induction, intensification, and consolidation phases were eligible for maintenance treatment. Maintenance randomisation was balanced with a permuted block (block size 8) and was stratified according to induction–intensification–consolidation treatment in a 1:1 ratio.
Of the entire population, one hundred and nine NDTEMM patients enrolled in the imaging sub-study of the FORTE trial formed part of the present analysis. The imaging PET/CT sub-study was offered to all the centres willing to participate, provided they had a nuclear medicine facility in line with the European Association of Nuclear Medicine (EANM) guidelines and consented to apply the recently proposed standardised criteria to all the PET scan.
All patients gave signed informed consent, in accordance with the Helsinki Declaration. The study was approved by ethics committees and registered at ClinicalTrials.gov, number NCT02203643.
Imaging sub-study, relative end-points and Deauville scores application
Twelve centres, who met the above characteristics, participated in the ancillary imaging sub-study, aimed at prospectively evaluating the prognostic significance of FDG-PET/CT, evaluated at baseline (B) and after therapy (PM). The second end-point was to validate the recently established standardised PET/CT interpretative criteria, applying Deauville scores as described elsewhere,16 in an independent series of patients, with particular attention to complete metabolic response (CMR). Whole-body PET/CT scans (including skull, upper limbs, and femurs) were performed at each participating centre using standard procedures,18 at the following time-points: baseline (before starting any treatment) and within 20 days after consolidation therapy, prior to the start of maintenance.17
All PET/CT scans were acquired according to the EANM PET procedures guidelines for FDG studies18 and reported following the lines of Italian Myeloma criteria for PET Use (IMPeTUs), as previously described.19 Particularly, the following characteristics had to be checked and reported: bone marrow metabolic state (BM), focal lesions within the bones (FLs) (number and metabolic state), extramedullary disease (EMD) (sites, number, and metabolic state). The metabolic state of BM outside FLs, EMD and FLs was visually quantified according to the five-point Deauville scale adopted for PET scan in lymphomas (focal lesion score, FS and bone marrow score, BMS).16 Furthermore, semi-quantitative measures were obtained in reference organs (liver, and blood mediastinal pool (BMP)) using a spherical volume of interest (VOI). The liver VOI with a 3 cm diameter was drawn in the central portion of the organ avoiding the edge. The BMP VOI was entirely within the aortic arch, taking care to avoid the vessel wall or areas of wall calcification. The following semi-quantitative parameters were annotated and used to reinforce the visual analysis interpretation intrapatient, especially in borderline cases: liver and BMP standardised uptake value (SUV) maximum (max) and mean, BM SUVmax of the hottest lesion per macro-area. No other pre-defined criteria for PET positivity in FLs, BM or EMD were applied to either B or PM, but a DS >1 (=no uptake). CMR was defined as DS <4 both in the FLs and BM.16 MRD evaluation in the BM was performed by 8-color second-generation flow cytometry (sensitivity 10−5), as described elsewhere,20 in patients who achieved at least VGPR before maintenance.21,22
Statistical analysis
All patients with double PET assessment (at B and PM) were included in this analysis, focused on the standardisation and prognostic value of CMR (prognostic relevance of baseline PET/CT will be discussed in a separate analysis).
A multivariate (MV) Cox proportional hazards regression analysis was performed to compare the impact of CMR achievement on progression-free survival (PFS), calculated from the date of randomisation to the date of first observation of PD/death from any cause, and overall survival (OS), calculated from the date of randomisation to the date of death from any cause.
Sub-group analyses were performed to determine the role of CMR, using interaction terms between baseline prognostic factors and CMR. The null hypothesis examined with the interaction test was that the hazard ratio (HR) of CMR-versus CMR+ was the same in each sub-group. Forest plots were used to display it.
Time-to-event analysis were carried out using a 12-month landmark point (PM) to ensure that CMR status could be considered a fixed factor and avoid “time immortal bias”.
Time-to-event data were analysed using the Kaplan–Meier method. The Cox proportional hazards model was used to estimate the adjusted hazard ratios (HRs) and the 95% CIs. To account for potential confounders, the Cox models were also adjusted for well-known prognostic factors (International Staging System [ISS] stage, cytogenetic abnormalities by fluorescence in situ hybridisation [FISH], and levels of lactate dehydrogenase [LDH]) and first randomisation (R1, induction/intensification/consolidation treatment) and Age (≥60 years vs < 60 years). In addition, to explore the effect by centre/hospital, a random intercept term for centre was added to the model (using the R package coxme) and tested using the likelihood ratio test (LRT).
The association between baseline features (and response) and CMR was evaluated by Fisher's Exact Test or Kruskal–Wallis test, as appropriate.
The association between CMR, MRD by flow and IMWG response was assessed by Fisher's exact test and Cramér's V (φc).
The statistical analysis was performed using R (v.4.1.0). Data cut-off was 2022-02-17.
Role of the funding source
The funders of the UNITO-MM-01/FORTE had no role in study design, data collection, data analysis, data interpretation, or writing of this report.
Results
Patient characteristics (Table 1)
Table 1.
Patient characteristics at baseline, randomisation arm and response to therapy in the PET population in comparison with the general population.
| Characteristic | All FORTE (n = 474) | All pet (n = 109) | Excluded from subanalysis (n = 365) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Median age (IQR) | 57 (51–62) | 56 (51–61) | 57 (51–62) | 0.74 |
| Sex, female (%) | 212 (45) | 52 (48) | 160 (44) | 0.51 |
| Sex, male (%) | 262 (55) | 57 (52) | 205 (56) | |
| Disease characteristics, n (%) | ||||
| Isotype, BJ | 71 (15) | 18 (17) | 53 (15) | 0.62 |
| Isotype, IgA | 81 (17) | 17 (16) | 64 (18) | |
| Isotype, IgD | 3 (1) | 1 (1) | 2 (1) | |
| Isotype, IgG | 298 (63) | 66 (61) | 232 (64) | |
| Ns | 21 (4) | 7 (6) | 14 (4) | |
| Light chain, kappa | 295 (64) | 73 (68) | 222 (63) | 0.42 |
| Light chain, lambda | 165 (36) | 35 (32) | 130 (37) | |
| ISS, n (%) | ||||
| Stage I | 240 (51) | 55 (50) | 185 (51) | 0.93 |
| Stage II | 152 (32) | 34 (31) | 118 (32) | |
| Stage III | 82 (17) | 20 (18) | 62 (17) | |
| R–ISS, n (%) | ||||
| Stage I | 127 (31) | 40 (39) | 87 (28) | 0.08 |
| Stage II | 247 (59) | 52 (51) | 195 (62) | |
| Stage III | 42 (10) | 10 (10) | 32 (10) | |
| Missing | 58 | 7 | 51 | |
| LDH > ULN | 61 (13) | 12 (11) | 49 (14) | 0.62 |
| High-risk cytogenetics, n (%) | ||||
| del (13) | 204 (51) | 54 (56) | 150 (49) | 0.30 |
| del (17p13.1) | 61 (15) | 15 (15) | 46 (15) | 1 |
| del (1p32.3) | 44 (11) | 12 (13) | 32 (11) | 0.58 |
| t (4; 14) | 65 (16) | 8 (8) | 57 (18) | 0.017 |
| t (14; 16) | 21 (5) | 5 (5) | 16 (5) | 1 |
| t (11; 14) | 92 (23) | 15 (16) | 77 (25) | 0.069 |
| amp (1q21) | 185 (46) | 38 (40) | 147 (48) | 0.20 |
| High-risk cytogeneticsa | 133 (33) | 26 (26) | 107 (35) | 0.14 |
| Induction treatment randomisation (R1), n (%) | ||||
| KCd plus ASCT | 159 (34) | 29 (27) | 130 (36) | 0.15 |
| KRd12 | 157 (33) | 43 (39) | 114 (31) | |
| KRd plus ASCT | 158 (33) | 37 (34) | 121 (33) | |
| Pre-maintenance best response, n (%) | ||||
| sCR | 192 (41) | 69 (63) | 123 (34) | – |
| CR | 48 (10) | 7 (6) | 41 (11) | |
| VGPR | 157 (33) | 29 (24) | 128 (35) | |
| PR | 48 (10) | 4 (4) | 44 (12) | |
| SD | 9 (2) | 0 | 9 (2) | |
| PD | 6 (1) | 0 | 6 (2) | |
| Not evaluable | 14 (3) | 0 | 14 (4) | |
| Pre-maintenance MFC MRD status, n (%) | ||||
| Positive | 134 (28) | 23 (21) | 111 (30) | 0.0012 |
| Negative | 255 (54) | 83 (76) | 172 (47) | |
| Not evaluable | 85 (18)b | 3 (3) | 82 (22) | |
Abbreviations. amp, Amplification; ASCT, Autologous stem-cell transplantation; BJ, Bence–Jones; CR, Complete response; del, Deletion; EMD, Extra-medullary disease; Ig, Immunoglobulin; IQR, Interquartile range; ISS, International staging system; KCd, carfilzomib, cyclophosphamide and dexamethasone; KRd, carfilzomib, lenalidomide, and dexamethasone; MEL200, melphalan at 200 mg/m2; KRd plus ASCT, 4 KRd induction cycles, MEL200-ASCT, 4 KRd consolidation cycles; KRd12, 12 KRd cycles without ASCT; KCd plus ASCT, 4 KCd induction cycles, MEL200-ASCT, 4 KCd consolidation cycles; LDH, Lactate dehydrogenase; ULN, Upper limit of normal; MFC, Multiparameter flow cytometry; MRD, Minimal residual disease; N, n, number; Ns, Not specified; PD, Progression disease; PET, Positron emission tomography; PR, Partial response; R1, randomisation 1; R–ISS, Revised international staging system; sCR, stringent complete response; SD, Stable disease; t, translocation; VGPR, Very good partial response.
High risk was defined as the presence of one or more of the following cytogenetic abnormalities: del (17p) and/or t (4; 14) and/or t (14; 16).
45 patients were not evaluable because best response was either SD or PR, or because they were in PD; 40 patients were not evaluable because, despite VGPR response, their MRD status was unknown (MFC MRD assessment not available).
Baseline PET was performed in 126 patients with a median age of 57 years [52–62]. Risk stratification was assessed using ISS and R–ISS; according to ISS, 47% of patients (n = 86) were rated to ISS-1, 35% (n = 63) to ISS-2 and 18% (n = 33) to ISS-3, whereas, according to R–ISS, 33% of patients (n = 60) were rated to R–ISS–1, 52% (n = 91) to R–ISS–2 and 7% (n = 16) to R–ISS–3, 8% (n = 15) non evaluable.
In 109 patients both B and PM PET were performed; the remaining 17 patients did not proceed to final PET either for patients' or physicians’ decision or for study abandonment. Clinical characteristics were reflecting those of the general population (see Table 1 for details). According to ISS, 50% of patients (n = 55) were rated to ISS-1, 31% (n = 34) to ISS-2 and 18% (n = 20) to ISS-3, whereas, according to R–ISS, 39% (n = 40) were rated to R–ISS–1, 51% (n = 52) to R–IS–2 and 10% (n = 10) to R–ISS–3 (R–ISS not available for 7 patients). High-risk cytogenetics was found in 26% of patients.
Out of 109 patients described above, 27% received KCd plus ASCT (n = 29) as first line therapy, 39% (n = 43) KRd, and 34% (n = 37) KRd plus ASCT. At PM, 63% of patients (n = 69) achieved a stringent complete response (sCR), 6% (n = 7) a complete response (CR), 24% (n = 29) a very good partial response (VGPR), and 4% (n = 4) a partial response (PR), with no significant difference for treatment arm. According to MFC MRD assessment (10−5 sensitivity), 76% patients (n = 83) achieved MRD negativity, whereas 21% (n = 23) maintained a MFC positivity, and in 3% (n = 3) MCF was not evaluable.
Similar MRD negativity rates were registered among patients randomised in the KRd (OR 0.90, CI 0.30–2.72, P = 0.86) and KRd plus ASCT (OR 1.26, CI 0.37–4.26, P = 0.71) arms of the trial than in KCd-ASCT arm.17
PET Deauville scores at baseline and pre-maintenance (Table 2)
Table 2.
Baseline (B) and pre-maintenance (PM) PET characteristics.
| All PET pts (n = 109) | B-PET_FLs | B-PET_BMs | PM-PET_FLs | PM-PET_BMs |
|---|---|---|---|---|
| Positivea, n (%) | 98 (93) | 106 (99) | 43 (40) | 97 (89) |
| Negative, n (%) | 7 (7) | 1 (1) | 65 (60) | 12 (11) |
| Missing, n | 4 | 2 | ||
| EMD, n (%) | 8 (7) | |||
| Deauville scores | ||||
| DS 1, n (%) | 7 (7) | 1 (1) | 65 (60) | 12 (11) |
| DS 2, n (%) | 1 (1) | 34 (32) | 2 (2) | 52 (48) |
| DS 3, n (%) | 4 (4) | 7 (7) | 14 (13) | 28 (26) |
| DS 4, n (%) | 93 (89) | 65 (61) | 27 (25) | 16 (15) |
| Missing, n | 4 | 2 | 1 | 1 |
| SUV max, median (IQR) | 6.04 (4.31–8.29) | 3.5 (2.8–4.37) | 3.04 (2.2–5.39) | 2.8 (2.3–3.44) |
| CMRb, n (%) | 69 (63) | |||
Abbreviations. B, Baseline; BM, Bone marrow; CMR, Complete metabolic response; DS, Deauville scores; EMD, Extramedullary disease; FLs, Focal lesions; IQR, Interquartile range; n, Number; PET, Positron emission tomography; PM, Pre-maintenance; pts, Patients; SUV, Standardised uptake value.
Defined as DS >1.
Defined as DS <4 both in the FLs and BM.
In the 109 patients with double PET available, baseline PET resulted positive for FS in 93% (n = 98) of patients, with 1% DS2 (n = 1), 4% DS3 (n = 4), and 89% DS4 (n = 93) (DS was missing in 4 patients), and for BMS in 99% (n = 106) of patients, with 32% DS2 (n = 34), 7% DS3 (n = 7) and 61% DS4 (n = 65). Median SUVmax at baseline was 6.04 [IQR 4.31–8.29] and 3.5 [IQR 2.8–4.37] for FLs and BM, respectively. Baseline PET characteristics of this population were similar to the above mentioned 126 PET baseline evaluated patients. Extramedullary disease (defined as soft tissue plasmacytomas) was found at baseline in 7% of patients (n = 8). Moreover, FS and BMS positivity or negativity were not significantly different among the 3 arms of treatment. No significant correlation between FS and BMS and other patients’ baseline characteristics was found.
PM- PET showed FS and BMS negativity (DS ≤1) in 60% (n = 65) and 11% (n = 12) of the patients, respectively. The remaining 43 (40%) and 97 (89%) patients retained some FDG uptake in the FLs and BM, respectively, with 25% and 15% of them showing DS4, respectively, that according to previous reported standardised criteria should be considered truly positive, as related to survival outcomes (Table 2). CMR, as defined above, was achieved in 63% (n = 69) of the overall population. Association between haematological response, in terms of CR and VGPR rates, and PM CMR was assessed without significant result (CR: P = 0.13, φc = 0.16; VGPR: P = 0.14, φc = 0.16). Moreover, MFC MRD negativity rate was higher among PM PET negative (91%, n = 63) than PET positive patients (50%, n = 20) (P < 0.0001, φc = 0.47).
Prognostic relevance of pre-maintenance PET Deauville scores and correlation with bone marrow flow cytometry
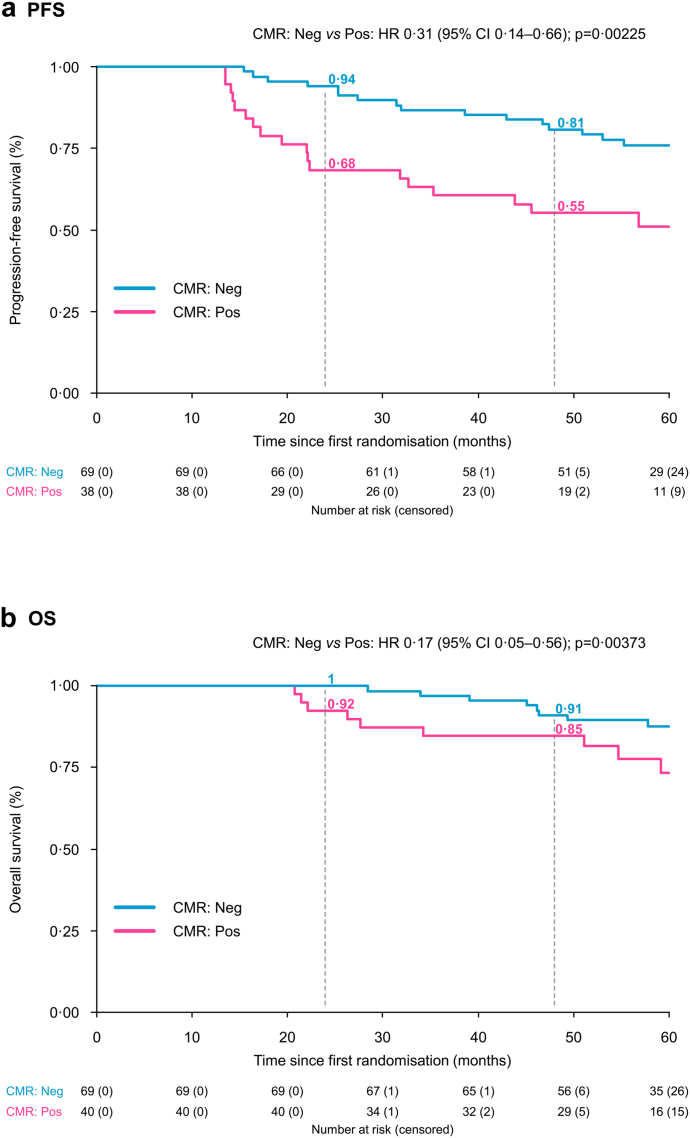
With a median follow-up of 61 months for the study population, in univariate analysis (Table 3) from the time of first randomisation to initial therapy, at landmark time PM, achievement of CMR was a strong predictor for prolonged PFS (survival probability at 24 and 48 months 94% and 81%, respectively, vs 68% and 55%, HR 0.40, CI 0.20–0.77, P = 0.0065) (Fig. 1). FS and BMS <4, separately, predicted for extended PFS (data not shown).
Table 3.
Univariate analysis and multivariable Cox regression analysis of pre-maintenance PET/CT parameters predicting for prolonged progression-free survival and overall survival.
| PFS (107 patients) | C-index | HR | 95% CI | P-value |
|---|---|---|---|---|
| CMR—Univariate analysis | 0.6244 | |||
| CMR: Neg vs Pos | 0.40 | (0.2–0.77) | 0.00654 | |
| CMR—Multivariate analysis | 0.7444 | |||
| CMR: Neg vs Pos | 0.31 | (0.14–0.66) | 0.00225 | |
| ISS: II/III vs 1 | 0.76 | (0.34–1.67) | 0.48,743 | |
| LDH: ≤ULN vs > ULN | 0.54 | (0.22–1.34) | 0.18,460 | |
| CA by FISH: Standard vs High riska | 0.31 | (0.13–0.70) | 0.00480 | |
| CA by FISH: NE vs High riska | 0.09 | (0.01–0.69) | 0.02081 | |
| R1: KRd12 vs KCd-ASCT | 1.77 | (0.71–4.43) | 0.22,255 | |
| R1: KRd plus ASCT vs KCd plus ASCT | 1.22 | (0.42–3.55) | 0.71,659 | |
| MFC MRD by ITT: Neg vs Pos | 1.04 | (0.45–2.38) | 0.92,713 | |
| Age: ≥60 vs < 60 | 0.80 | (0.36–1.80) | 0.59254 | |
| PET and MFC—Univariate analysis | 0.6125 | |||
| PET and MFC: Neg vs at least one Pos | 0.45 | (0.23–0.88) | 0.01979 | |
| PET and MFC—Multivariate analysis | 0.7302 | |||
| PET and MFC: Neg vs at least one Pos | 0.41 | (0.20–0.84) | 0.01516 | |
| ISS: II/III vs I | 0.88 | (0.42–1.86) | 0.73,879 | |
| LDH: ≤ULN vs > ULN | 0.50 | (0.20–1.23) | 0.13,185 | |
| LDH: NE vs > ULN | 1.05 | (0.11–10.54) | 0.96,431 | |
| CA by FISH: Standard vs High riska | 0.38 | (0.17–0.83) | 0.01516 | |
| CA by FISH: NE vs High riska | 0.11 | (0.01–0.90) | 0.03909 | |
| R1: KRd12 vs KCd plus ASCT | 1.79 | (0.71–4.50) | 0.21,936 | |
| R1: KRd plus ASCT vs KCd plus ASCT | 1.21 | (0.42–3.46) | 0.72,191 | |
| Age: ≥60 vs < 60 | 0.76 | (0.35–1.68) | 0.50093 | |
| OS (109 patients) | HR | 95% CI | p-value | |
| CMR—Univariate analysis | 0.6006 | |||
| CMR: Neg vs Pos | 0.44 | (0.17–1.15) | 0.09387 | |
| CMR—Multivariate analysis | 0.8200 | |||
| CMR: Neg vs Pos | 0.17 | (0.05–0.56) | 0.00373 | |
| ISS: II/III vs I | 0.57 | (0.16–2.08) | 0.39,713 | |
| LDH: ≤ULN vs > ULN | 0.18 | (0.06–0.61) | 0.00548 | |
| LDH: NE vs > ULN | 1.09 | (0.10–12.24) | 0.94,684 | |
| CA by FISH: Standard vs High riska | 0.10 | (0.03–0.41) | 0.00114 | |
| CA by FISH: NE vs High riska | 0.17 | (0.02–1.60) | 0.12,193 | |
| R1: KRd12 vs KCd plus ASCT | 1.06 | (0.25–4.53) | 0.93,899 | |
| R1: KRd plus ASCT vs KCd plus ASCT | 0.70 | (0.13–3.76) | 0.67,646 | |
| MFC MRD by ITT: NEG vs POS | 1.01 | (0.28–3.64) | 0.98,354 | |
| Age: ≥60 vs < 60 | 2.47 | (0.69–8.88) | 0.16669 | |
| PET and MFC—Univariate analysis | 0.574 | |||
| PET and MFC (Neg vs at least one Pos) | 0.56 | (0.21–1.44) | 0.22,786 | |
| PET and MFC—Multivariate analysis | 0.8072 | |||
| PET and MFC (Neg vs at least one Pos) | 0.29 | (0.09–0.96) | 0.04239 | |
| ISS: II/III vs I | 0.71 | (0.21–2.40) | 0.58,667 | |
| LDH: ≤ULN vs > ULN | 0.19 | (0.06–0.63) | 0.00658 | |
| LDH: NE vs > ULN | 0.95 | (0.08–10.60) | 0.96,542 | |
| CA by FISH: Standard vs High riska | 0.17 | (0.05–0.53) | 0.00247 | |
| CA by FISH: NE vs High riska | 0.29 | (0.03–2.40) | 0.24,893 | |
| R1: KRd12 vs KCd plus ASCT | 1.03 | (0.24–4.44) | 0.96,423 | |
| R1: KRd plus ASCT vs KCd plus ASCT | 0.79 | (0.16–3.89) | 0.77,375 | |
| Age: ≥60 vs < 60 | 2.04 | (0.61–6.84) | 0.25015 | |
Abbreviations. ASCT, Autologous stem-cell transplantation; CA, Cytogenetic abnormalities; CI, Confidence interval; CMR, Complete metabolic response; FISH, Fluorescence in situ hybridisation; HR, Hazard ratio; ISS, International staging system stage; KCd, carfilzomib-cyclophosphamide-dexamethasone; MEL200, melphalan at 200 mg/m2; KCd plus ASCT, 4 KCd induction cycles, MEL200-ASCT, 4 KCd consolidation cycles; KRd, carfilzomib-lenalidomide-dexamethasone; KRd12, 12 KRd cycles without ASCT; KRd plus ASCT, 4 KRd induction cycles, MEL200-ASCT, 4 KRd consolidation cycles; LDH, Lactate dehydrogenase; NE, Not estimable; Neg, Negative; MFC, Multiparameter flow cytometry; OS, Overall survival; PET/CT, Positron emission tomography/computed tomography; PFS, Progression-free survival; Pos, Positive; R1, first randomisation (induction-intensification-consolidation treatment); ULN, Upper limit of normal.
High risk was defined as the presence of one or more of the following cytogenetic abnormalities: del (17p) and/or t (4; 14) and/or t (14; 16).
Fig. 1.
Progression-free survival (a) and overall survival (b) according to pre-maintenance PET/CT complete metabolic response Abbreviations. CI, Confidence interval; CMR, Complete metabolic response; HR, Hazard ratio; Neg, Negativity; P, P-value; PET/CT, positron emission tomography/computed tomography; PFS, Progression-free survival; Pos, Positivity.
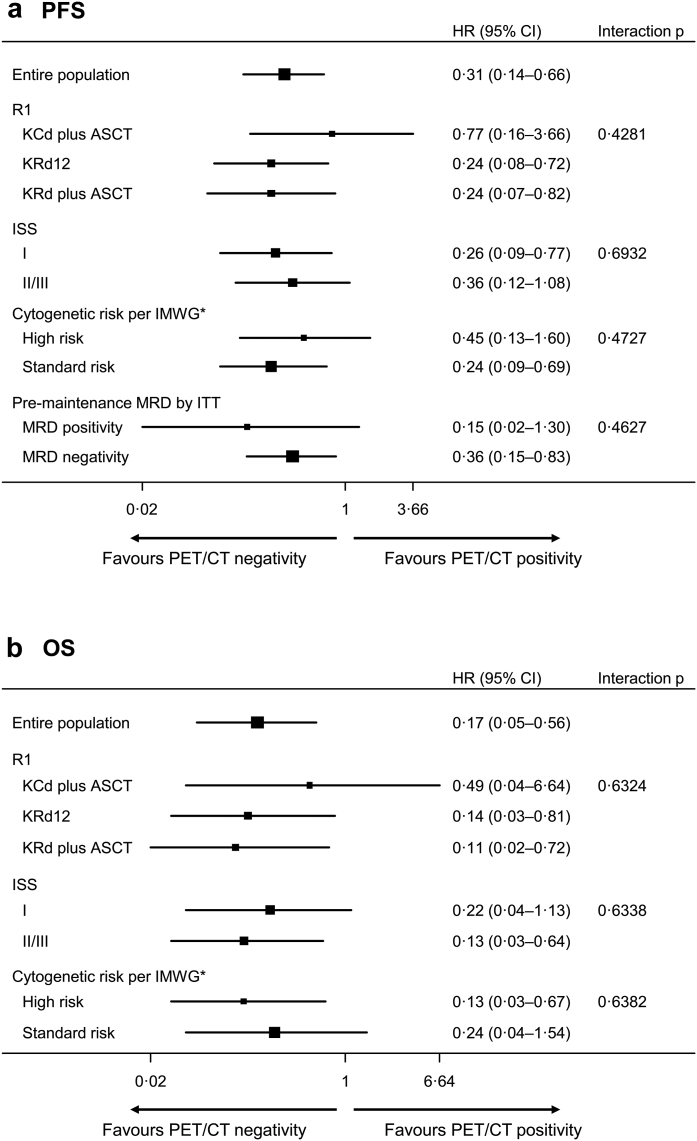
In the sub-group analysis, PFS advantage for patients achieving CMR was present irrespective of randomisation arm, ISS stage, cytogenetic and MFC status PM (Fig. 2). In Cox multivariate analysis, achievement of CMR and absence of high-risk cytogenetic abnormalities were independent predictors of PFS (HR 0.31, CI 0.14–0.66, P 0.0023 and HR 0.31, CI 0.13–0.70, P = 0.0048, respectively) (Table 3). No improvement in the model fit was observed when the random intercept for the centre was added (LRT P = 0.88).
Fig. 2.
Forest plots for PET/CT pre maintenance for PFS (a) and OS (b). ∗High-risk cytogenetics were defined in accordance with the International Myeloma Working Group (IMWG) criteria: presence of t (4; 14) and/or t (14; 16) and/or del (17p). Abbreviations. ASCT, Autologous stem-cell transplantation; CI, Confidence interval; HR, Hazard ratio; IMWG, International myeloma working group; ISS, International staging system stage; ITT, Intention to treat; MRD, Minimal residual disease; KCd, carfilzomib-cyclophosphamide-dexamethasone; MEL200, melphalan at 200 mg/m2; KCd plus ASCT, 4 KCd induction cycles, MEL200-ASCT, 4 KCd consolidation cycles; KRd, carfilzomib-lenalidomide-dexamethasone; KRd12, 12 KRd cycles without ASCT; KRd plus ASCT, 4 KRd induction cycles, MEL200-ASCT, 4 KRd consolidation cycles; OS, Overall survival; P, P-value; PET/CT, positron emission tomography/computed tomography; PFS, Progression-free survival; R1, first randomisation (induction-intensification-consolidation treatment).
Regarding OS, with a median follow-up of 61 months, and median values not reached in either group, at landmark time PM, patients with CMR negative status had a 2,4-years OS rate of 100% and 91%, respectively, vs 92% and 85% for CMR positive status (HR 0.44, CI 0.17–1.15, P = 0.094) (Fig. 1), and maintained in all sub-groups (Fig. 2). In the Cox multivariate model, achievement of CMR, absence of high-risk cytogenetic abnormalities and normal LDH values were independent predictors of OS (HR 0.17, CI 0.05–0.56, P 0.0037, HR 0.10, CI 0.03–0.41, P = 0.0011 and HR 0.18, CI 0.06–0.61, P 0.0055, respectively) (Table 3). No improvement in the model fit was observed when the random intercept for the centre was added (LRT P = 0.96).
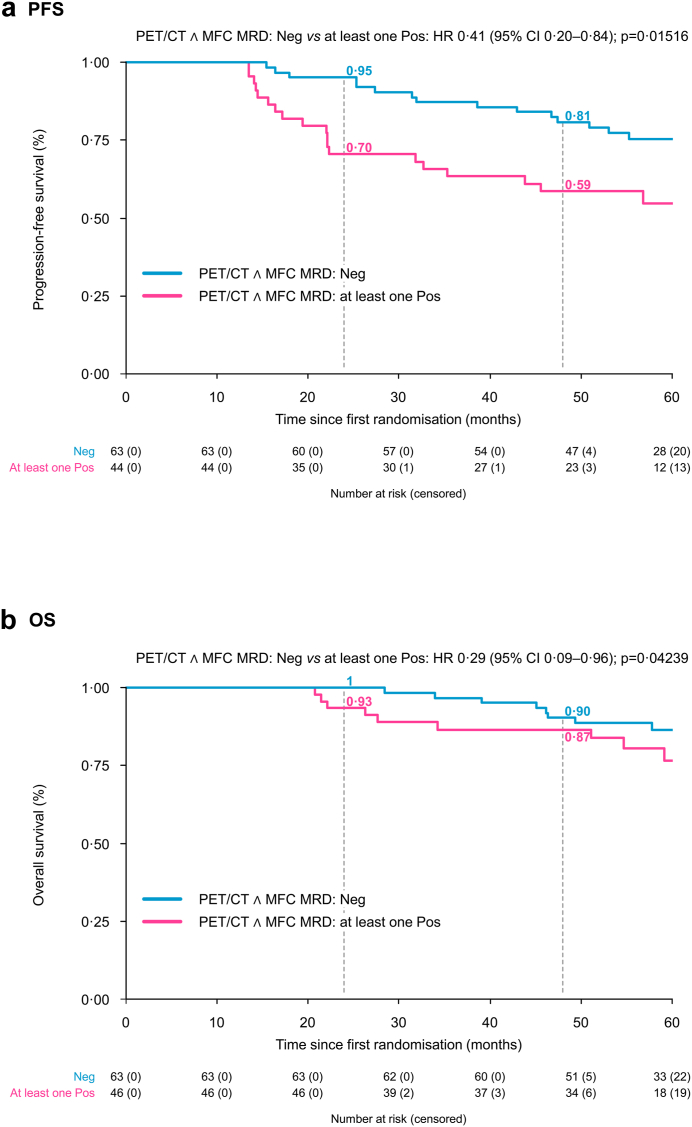
Agreement between CMR and MFC negativity was 0.76; in particular, it was 0.64 and 0.92 when considering FLs and BM, respectively. Patients achieving both PET/CT CMR and MFC negativity at PM showed significantly extended PFS (survival probability at 24 and 48 months 95% and 81% vs 70% and 59%, HR 0.45, CI 0.23–0.88, P 0.020) compared to all the others (Fig. 3); due to the low number of patients, we could not further split the outcomes of this latter category, according to either PET or MFC positivity.
Fig. 3.
Progression-free survival (a) and overall survival (b) according to pre-maintenance PET/CT complete metabolic response and multiparameter flow cytometry negativity Abbreviations. ∧, and; CI, Confidence interval; HR, Hazard ratio; MFC, Multiparameter flow cytometry; MRD, Minimal residual disease; Neg, Negativity; P, P-value; PET/CT, Positron emission tomography/computed tomography; PFS, Progression-free survival; Pos, Positivity.
In Cox multivariate analysis, achievement of both BM MFC and CMR (imaging MRD negativity as per IMWG criteria) and absence of high-risk cytogenetic abnormalities were independent predictors of PFS (HR 0.41, CI 0.20–0.84, P 0.015 and HR 0.38, CI 0.17–0.83, P = 0.015, respectively) (Table 3).
Discussion
In this prospective imaging sub-analysis on 109 NDTEMM patients enrolled in the phase II randomised FORTE clinical trial, evaluated with FDG-PET/CT at baseline and after treatment, we were able to confirm, in an independent prospective cohort of patients, the applicability of the new defined standardised interpretative criteria and the prognostic value of achieving a CMR, as well as to demonstrate its complementarity to BM MFC, when evaluated at the level of 10−5.
The importance of functional imaging to guarantee the complete eradication of the disease after therapy, established by the IMWG,6 relies on the inhomogeneous PCs infiltration of the BM, making false negatives more likely, on the possible existence of EMD, due to metastatic spread, associated with a worse prognosis5 and on the possibility of spatial heterogeneity.3,4 Prospective studies that have monitored with contextual biopsies the genomic profile of BM and FLs have demonstrated in some patients the coexistence of different disease clones. The larger the FL, the greater the heterogeneity.4 The discrepancy between BM and imaging MRD evaluation, with residual FLs in the context of NGS or NGF negativity (sensitivity 10−4 and 10−5; data are lacking about higher sensitivity levels) is higher in the patients with EMD or with para-skeletal plasmacytomas at diagnosis or in the relapsed/refractory setting.23
To assess response to therapy, the preferred imaging methods are functional rather than morphological ones.6, 7, 8 Particularly, PET/CT is an optimal technique for studying and monitoring the treatment efficacy, being able to distinguish active from inactive lesions7 and its use in several oncologic diseases ensure a wide range availability and access. Several studies, both within clinical trials and in routine clinical practice, have demonstrated the favourable prognostic value of negative 18 F-FDG-PET lesions after completion of therapy.9, 10, 11, 12,14 Furthermore, it has recently been shown that the prognosis of patients who achieve normalisation of PET during or after therapy is comparable to that of patients without metabolically active lesions at baseline, suggesting the importance of treatment until complete suppression of metabolism is achieved (CMR).24 Several studies demonstrated complementarity in defining patient prognosis between BM intramedullary evaluation by flow cytometry with a sensitivity of 10−4 and 10−5 and imaging techniques (FDG-PET/CT or WB-DWI-MRI).14,15,25
As with BM assessments, the standardisation of imaging criteria and the definition of cut-offs for positivity/negativity after treatment is very important while recommending PET/CT as the gold standard, allowing for reproducibility of data and harmonisation of results between different clinical studies and in routine clinical practice. In a previous joint analysis of 2 prospective randomised studies for NDTEMM patients, the applicability of the Deauville criteria to MM was demonstrated for the first time, proving the reproducibility of the scores, especially of the score 4, and the agreement between the reviewers in using these criteria.16 The definition of CMR helps in avoiding false positive PET results after therapy and to have an easy and practical reference to define PET-MRD.
In the present analysis, we proved the applicability of recently defined standardised criteria for FDG PET negativity and we confirmed the role of FDG-PET in the setting of NDTEMM receiving ASCT as an important prognostic tool in evaluating patients’ outcomes after therapy. The reduction of FDG uptake prior to the start of maintenance, both in FLs and BM, has been shown to be an independent prognostic factor in lasting disease control and prolonged OS. Applying the recently defined DS to both FLs and BM at PM PET, we found a different distribution of patients within the various categories for the 2 parameters: almost all patients (89%) showed an altered FDG uptake in the BM, with a low DS (2 and 3), not associated with survival outcomes, probably reflecting BM reconstitution after therapy; by contrast, 40% of patients showed residual uptake in FLs after therapy, mainly distributed between DS 3 and 4. In both BM and FLs, reducing FDG uptake < DS4 (with the liver as a reference) was the most significant independent factor predictive for PFS and OS. CMR was confirmed to be independently associated with PFS, with a trend on OS.
Moreover, the complementarity between BM and imaging MRD tools was confirmed, with the best outcomes belonging to those patients achieving MRD negativity by both techniques among all the rest of the population and the independent prognostic relevance of PET MRD in the multivariate analysis; however, due to the low number of discrepant patients, we were not able to further highlight differences in the prognosis of patients with both or either positive results among MFC and PET.
Different interpretative criteria may be applied to PET/CT, such as the Total Lesion Glycolysis (TLG), the Metabolic Tumor Volume (MTV) and a dynamic evaluation26, 27, 28; however, no prospective clinical trials have been run and no consensus on their applicability in clinical practice is yet available.
Regardless of the interpretation issue, it should be emphasised that FDG-PET/CT can lead to false negative results after therapy, for example in relation to hyperglycaemia or high-dose steroid administration, resulting in transient metabolic suppression.7 Furthermore, it has been reported that in a variable rate of patients ranging from 10 to 15% at diagnosis, but changing over the course of the disease, it is possible that PCs are not 18FDG avid, and this may be due to the low expression of hexokinase,29,30 an enzyme implicated in the metabolism of glucose and therefore of its modified analogue 18FDG, or to other unknown causes. In such patients, FDG-PET/CT is not an appropriate tool for assessing the metabolic response to therapy. New PET/CT tracers, exploiting different metabolic pathways or receptors expressed on PCs, and acting as potentially more sensitive and specific molecular imaging biomarkers than FDG, have been studied in small groups of MM patients and in mouse models.31 However, it has not yet been possible to draw definitive conclusions on their use due to limited availability, the heterogeneity of specific tumour targets among the various patients, and the lack of prognostic data and standard reporting. Alternatively, other imaging techniques can be used, such as DWI-MRI, which has demonstrated high sensitivity, especially in detecting diffuse BM infiltration, showing significant changes in patients in remission after therapy, both early and at the end of the treatment32; however, specificity problems are still unresolved. Recently, a group of expert radiologists, physicists and haematologists provided guidelines for the acquisition, interpretation, and reporting of DWI-MRI (MY-RADS imaging recommendations), to promote its standardisation and reduce the variability of interpretation between several studies.33 In a recent retrospective study on 100 NDTEMM, DWI-MRI demonstrated to be able to independently stratify patients with different outcomes.25 Validation of the MY-RADS criteria in prospective studies is on-going.32 To date, homogeneous and prospective data on the comparison between DWI-MRI and FDG-PET/CT in the evaluation of response to therapy are lacking.
Limitations of the study include the relatively low number of patients studied by PET in comparison to the whole trial population and the possible positive selection of those patients being studied at baseline and pre-maintenance, that prevented us from further discriminating the outcomes of MRD positive patients (whether both or either). Moreover, the lack of a serial PET study during maintenance, prevented us to provide any information/recommendation on the role of “sustained-imaging MRD negativity”, similarly to what is recommended for BM sustained-MRD.
To conclude, our results confirm that FDG-PET/CT after therapy is a reliable predictor of long-term outcomes in NDTEMM patients. The Deauville scale, used for the first time in MM, proved to be applicable and representative of patient outcomes. Based on our results, we propose to consider the adoption of hepatic uptake as a simple and reproducible reference for defining the negativity of PET after therapy, both in the context of clinical trials and in daily clinical practice.
Contributors
All authors contributed to the acquisition, analysis, or interpretation of data for this work. All authors critically reviewed the work for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors had access to and verified the underlying data. EZ, SO, and CN contributed to conceptualisation and methodology. All authors contributed to data curation, investigation, validation, and writing in terms of review and editing. EZ, SO, AC, MD, TV, and CN contributed to formal analysis (CN reviewed all the PET scans, AC performed the statistical analysis, and SO, MD, and TV performed the flow cytometry analysis). DRS, AB, MGa, MR, RZ, BG, DA, AV, MGr, NS, FF, FP, AP, PZ, PT, SF, KM, PM, and MC contributed to resources. EZ and AC contributed to visualisation. EZ, PM, MC, and CN contributed to supervision. EZ, GDC, and SB contributed to writing of the original draft. All authors had access to all the data reported in the study and had final responsibility for the decision to submit this manuscript for publication.
Data sharing statement
After the publication of this article, data collected for this analysis and related documents will be made available to others upon reasonably justified request, which needs to be written and addressed to the attention of the corresponding author Dr. Elena Zamagni at the following e-mail address: e. zamagni [at]unibo.it. The sponsor of the trial, the University of Torino (Italy), via the corresponding author Dr. Elena Zamagni, is responsible to evaluate and eventually accept or refuse every request to disclose data and their related documents, in compliance with the ethical approval conditions, in compliance with applicable laws and regulations, and in conformance with the agreements in place with the involved subjects, the participating institutions, and all the other parties directly or indirectly involved in the participation, conduct, development, management and evaluation of this analysis.
Declaration of interests
EZ receives honoraria from Janssen, Bristol-Myers Squibb, Amgen, Takeda.
SO has received honoraria from Amgen, Celgene/Bristol Myers Squibb, and Janssen; has served on the advisory boards for Adaptive Biotechnologies, Janssen, Amgen, and Takeda.
FG has received honoraria from Amgen, Celgene, Janssen, Takeda, Bristol Myers Squibb, AbbVie, and GlaxoSmithKline; has served on the advisory boards for Amgen, Celgene, Janssen, Takeda, Bristol Myers Squibb, AbbVie, GlaxoSmithKline, Roche, Adaptive Biotechnologies, Oncopeptides, bluebird bio, and Pfizer.
MD has received honoraria for lectures from GlaxoSmithKline, Sanofi, and Janssen; has served on the advisory boards for GlaxoSmithKline, Sanofi, and Bristol Myers Squibb.
AB has served on the advisory board for Amgen, Janssen, Takeda, Celgene, GlaxoSmithKline.
MGa has received honoraria from Janssen, Bristol-Myers Squibb, Amgen, Takeda, GlaxoSmithKline.
RZ has served on the advisory boards for Amgen, Celgene, Janssen, Takeda, Bristol Myers Squibb, GlaxoSmithKline, and Pfizer.
BG has received honoraria from Amgen, Bristol Myers Squibb, Janssen, and Takeda; has served on the advisory boards for Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Sanofi, and Takeda.
AV received honoraria from Novartis, Advanced Accelerator Applications and GE Healthcare.
FP receives honoraria and travel accommodation support from Celgene, Janssen, Takeda and has served on the advisory boards for Celgene BMS, Janssen, Amgen, GlaxoSmithKline.
PT has received honoraria from Janssen, Celgene, Bristol Myers Squibb, Amgen, Takeda, AbbVie, Sanofi, GlaxoSmithKline, and Pfizer; has served on the data safety monitoring boards or advisory boards for Janssen, Celgene, Bristol Myers Squibb, and Amgen.
KM has received honoraria from Celgene, Takeda, Amgen, Sanofi, Janssen.
MB has received honoraria from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol Myers Squibb, and AbbVie; has served on the advisory boards for Janssen and GlaxoSmithKline; has received research funding from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol Myers Squibb, and Mundipharma.
PM has received honoraria from and/or served on scientific boards for AbbVie, Alexion, Amgen, AstraZeneca, Astellas, BeiGene, Bristol-Myers Squibb/Celgene, Gilead, GlaxoSmithKline, Incyte, Janssen, Jazz, Novartis, Pfizer, Roche, Sanofi, and Takeda.
MC has received honoraria from Janssen, Celgene, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Takeda, AbbVie, Sanofi, Pfizer, and Adaptive Biotechnologies; has served on the advisory boards for Janssen, Bristol Myers Squibb, Sanofi, Amgen, GlaxoSmithKline, and Pfizer; has served on the speakers’ bureaus for Janssen, Celgene, and Sanofi.
CN has been PET revisor for Keosys-Sanofi.
The other authors declare no competing financial interests.
Acknowledgements
The authors wish to thank all the study participants, referring clinicians, nurses, and data managers for their valuable contributions.
The UNITO-MM-01/FORTE trial was sponsored by the Università degli Studi di Torino (Italy), Department of Molecular Biotechnology and Health Sciences. Amgen and Celgene/Bristol Myers Squibb provided an unrestricted grant to conduct the trial, but had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or publication of this contribution. The work reported in this contribution was funded by the Italian Ministry of Health, RC-2022-2773423.
The authors were not precluded from accessing data in the study, and they accept responsibility to submit this manuscript for publication.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102017.
Appendix A. Supplementary data
References
- 1.Dimopoulos M.A., Moreau P., Terpos E., et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2021;32(3):309–322. doi: 10.1016/j.annonc.2020.11.014. Epub 2021 Feb 3. Erratum in: Ann Oncol. 2022 Jan;33(1):117. [DOI] [PubMed] [Google Scholar]
- 2.Munshi N.C., Avet-Loiseau H., Anderson K.C., et al. A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv. 2020;4(23):5988–5999. doi: 10.1182/bloodadvances.2020002827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasche L., Chavan S.S., Stephens O.W., et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat Commun. 2017;8(1):268. doi: 10.1038/s41467-017-00296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasche L., Angtuaco E.J., Alpe T.L., et al. The presence of large focal lesions is a strong independent prognostic factor in multiple myeloma. Blood. 2018;132(1):59–66. doi: 10.1182/blood-2018-04-842880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosiñol L., Beksac M., Zamagni E., et al. Expert review on soft-tissue plasmacytomas in multiple myeloma: definition, disease assessment and treatment considerations. Br J Haematol. 2021;194(3):496–507. doi: 10.1111/bjh.17338. Epub 2021 Mar 16. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S., Paiva B., Anderson K.C., et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 7.Cavo M., Terpos E., Nanni C., et al. Role of 18F-FDG positron emmission tomography/computed tomography in the diagnosis and management of multiple myeloma and other plasma cell dyscrasias: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017;18(4):e206–e217. doi: 10.1016/S1470-2045(17)30189-4. [DOI] [PubMed] [Google Scholar]
- 8.Zamagni E., Tacchetti P., Cavo M. Imaging in multiple myeloma: which? When? Blood. 2019;133(7):644–651. doi: 10.1182/blood-2018-08-825356. [DOI] [PubMed] [Google Scholar]
- 9.Bartel T.B., Haessler J., Brown T.L., et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114(10):2068–2076. doi: 10.1182/blood-2009-03-213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Usmani S.Z., Mitchell A., Waheed S., et al. Prognostic implications of serial 18-fluoro-deoxyglucose emission tomography in multiple myeloma treated with total therapy 3. Blood. 2013;121(10):1819–1823. doi: 10.1182/blood-2012-08-451690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamagni E., Patriarca F., Nanni C., et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118(23):5989–5995. doi: 10.1182/blood-2011-06-361386. [DOI] [PubMed] [Google Scholar]
- 12.Kaddoura M., Dingli D., Buadi F.K., et al. Prognostic impact of posttransplant FDG PET/CT scan in multiple myeloma. Blood Adv. 2021;5(13):2753–2759. doi: 10.1182/bloodadvances.2020004131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamagni E., Nanni C., Mancuso K., et al. PET/CT improves the definition of complete response and allows to detect otherwise unidentifiable skeletal progression in multiple myeloma. Clin Cancer Res. 2015;21(19):4384–4390. doi: 10.1158/1078-0432.CCR-15-0396. [DOI] [PubMed] [Google Scholar]
- 14.Moreau P., Attal M., Caillot D., et al. Prospective evaluation of magnetic resonance imaging and [18F]fluorodeoxyglucose positron emission tomography-computed tomography at diagnosis and before maintenance therapy in symptomatic patients with multiple myeloma included in the IFM/DFCI 2009 trial: results of the IMAJEM study. J Clin Oncol. 2017;35(25) doi: 10.1200/JCO.2017.72.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonso R., Cedena M.T., Gómez-Grande A., et al. Imaging and bone marrow assessments improve minimal residual disease prediction in multiple myeloma. Am J Hematol. 2019;94(8):853–861. doi: 10.1002/ajh.25507. [DOI] [PubMed] [Google Scholar]
- 16.Zamagni E., Nanni C., Dozza L., et al. Standardization of 18F-FDG-PET/CT according to Deauville criteria for metabolic complete response definition in newly diagnosed multiple myeloma. J Clin Oncol. 2021;39(2):116–125. doi: 10.1200/JCO.20.00386. [DOI] [PubMed] [Google Scholar]
- 17.Gay F., Musto P., Rota-Scalabrini D., et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): a randomised, open-label, phase 2 trial. Lancet Oncol. 2021;22(12):1705–1720. doi: 10.1016/S1470-2045(21)00535-0. [DOI] [PubMed] [Google Scholar]
- 18.Boellaard R., Delgado-Bolton R., Oyen W.J.G., et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanni C., Versari A., Chauvie S., et al. Interpretation criteria for FDG PET/CT in multiple myeloma (IMPeTUs): final results. IMPeTUs (Italian myeloma criteria for PET USe) Eur J Nucl Med Mol Imaging. 2018;45(5):712–719. doi: 10.1007/s00259-017-3909-8. [DOI] [PubMed] [Google Scholar]
- 20.Oliva S., Bruinink D.H.O., Rihova L., et al. Minimal residual disease assessment by multiparameter flow cytometry in transplant-eligible myeloma in the EMN02/HOVON 95 MM trial. Blood Cancer J. 2021;11(6):106. doi: 10.1038/s41408-021-00498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stetler-Stevenson M., Paiva B., Stoolman L., et al. Consensus guidelines for myeloma minimal residual disease sample staining and data acquisition. Cytometry B Clin Cytom. 2016;90(1):26–30. doi: 10.1002/cyto.b.21249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arroz M., Came N., Lin P., et al. Consensus guidelines on plasma cell myeloma minimal residual disease analysis and reporting. Cytometry B Clin Cytom. 2016;90(1):31–39. doi: 10.1002/cyto.b.21228. [DOI] [PubMed] [Google Scholar]
- 23.Paiva B., Puig N., Cedena M.T., et al. Measurable residual disease by next-generation flow cytometry in multiple myeloma. J Clin Oncol. 2020;38(8):784–792. doi: 10.1200/JCO.19.01231. [DOI] [PubMed] [Google Scholar]
- 24.Davies F.E., Rosenthal A., Rasche L., et al. Treatment to suppression of focal lesions on positron emission tomography-computed tomography is a therapeutic goal in newly diagnosed multiple myeloma. Haematologica. 2018;103(6):1047. doi: 10.3324/haematol.2017.177139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belotti A., Ribolla R., Cancelli V., et al. External validation of diffusion weighted whole body MRI (DW-MRI) response assessment category (RAC) criteria proposed by the myeloma response assessment and diagnosis System (MY-RADS) imaging recommendations: prognostic role of imaging response after transplant in multiple myeloma and comparison with MRD evaluation by flow cytometry. Blood. 2020;136(Supplement 1):41–42. [Abstract #145, ASH 2020 62nd Meeting] [Google Scholar]
- 26.Carlier T., Bailly C., Leforestier R., et al. Prognostic added value of PET textural features at diagnosis in multiple myeloma. J Nucl Med. 2017;58(supplement 1) abstract #111 [2017 SNMMI Annual Meeting] [Google Scholar]
- 27.McDonald J.E., Kessler M.M., Gardner M.W., et al. Assessment of total lesion Glycolysis by 18F FDG PET/CT significantly improves prognostic value of GEP and ISS in myeloma. Clin Cancer Res. 2017;23(8):1981–1987. doi: 10.1158/1078-0432.CCR-16-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachpekidis C., Merz M., Kopp-Schneider A., et al. Quantitative dynamic 18F-fluorodeoxyglucose positron emission tomography/computed tomography before autologous stem cell transplantation predicts survival in multiple myeloma. Haematologica. 2019;104(9) doi: 10.3324/haematol.2018.213041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasche L., Angtuaco E., McDonald J.E., et al. Low expression of hexokinase-2 is associated with false-negative FDG-positron emission tomography in multiple myeloma. Blood. 2017;130(1):30–34. doi: 10.1182/blood-2017-03-774422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kircher S., Stolzenburg A., Kortüm K.M., et al. Hexokinase-2 expression in 11C-Methionine-Positive, 18F-FDG-Negative multiple myeloma. J Nucl Med. 2019;60(3):348–352. doi: 10.2967/jnumed.118.217539. [DOI] [PubMed] [Google Scholar]
- 31.Jamet B., Bailly C., Carlier T., et al. Interest of pet imaging in multiple myeloma. Front Med. 2019;6:69. doi: 10.3389/fmed.2019.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaiser M.F., Porta N., Sharma B., et al. Prospective comparison of whole-body MRI and FDG PET/CT for detection of multiple myeloma and correlation with markers of disease burden: results of the iTIMM trial. J Clin Oncol. 2021;39(suppl. 15) abstract # 8012 [ASCO 2021 Annual Meeting] [Google Scholar]
- 33.Messiou C., Hillengass J., Delorme S., et al. Interpretation, and reporting of whole-body MRI in myeloma: myeloma response assessment and diagnosis system (MY-RADS) Radiology. 2019;291(1):5–13. doi: 10.1148/radiol.2019181949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.