Highlights
-
•
Extrusion enhanced anti-hardening in high-protein nutrition bars (HPNBs).
-
•
Selection of optimal extrusion temperatures of HPNBs by TOPSIS.
-
•
WPI extruded at 150 °C was the best way for anti-hardening and performance of HPNBs.
Keywords: Whey protein isolate (WPI), Casein (CN), Extrusion, High-protein nutrition bars (HPNBs)
Abstract
Model high-protein nutrition bars (HPNBs) were formulated by incorporating whey protein isolate (WPI) and casein (CN) at various extrusion temperatures (50, 75, 100, 125, and 150 °C) with a protein content of 45 g per 100 g. The free sulfhydryl groups, amino groups, hardness, and microstructures of HPNBs were analyzed periodically at 37 °C over a storage period of 45 days. Specifically, sulfhydryl group, amino group and surface hydrophobicity of extruded whey protein isolate (WPE) and extruded casein (CE) were significantly reduced (P < 0.05) compared to those of unextruded protein. HPNBs formulated with WPE (HWPE) and CE (HWCE) exhibited a slower hardening rate compared to those formulated with unmodified protein. Moreover, the color difference, hardness and sensory score of HPNBs after 45 days of storage were used as indicators, and the results of the TOPSIS multiple index analysis indicated that HPNB formulated with WPI extruded at 150 °C possessed the best quality characteristics.
1. Introduction
Casein (CN) is the main component of milk protein, accounting for approximately 80%∼82%, and is a phosphorous acid protein (Madende & Osthoff, 2019). Whey protein isolate (WPI) is a compact globular protein with high biological activity and antibacterial properties (Janjarasskul et al., 2017, Huang et al., 2023). CN and WPI are the main ingredients in high protein nutrition bars (HPNBs). As a new type of health food with high protein, the protein content of HPNBs is 20–50% (Meng et al., 2019). Commercial HPNBs are usually medium moisture foods with a moisture content of 10–30%, and their shelf life is 6–12 months at room temperature (Li et al., 2008). However, prolonged storage can result in a decline in the flavor and texture of HPNBs, accompanied by a noticeable increase in hardness. This greatly limits their shelf life and consumer acceptance (Sherwin & Labuza, 2010). The hardening of HPNBs can be attributed to various factors, including the crystallization of sugars, migration of water, self-aggregation of proteins, phase separation, and glycosylation (Rao et al., 2013). Similarly, in the casein-based HPNB system, protein molecules hydrate and enlarge, forming a network structure through non-covalent interactions and accelerating hardening. Furthermore, protein aggregation caused by the Maillard reaction in high protein products usually occurs after storage for several months (Ismarti et al., 2020).
Numerous studies have explored the anti-hardening properties of HPNBs. Researchers have fabricated soft HPNBs by mixing a whey protein with two milk protein concentrates (Imtiaz et al., 2012). In addition, Li et al. (2017) found that the addition of polysaccharide molecules could effectively compete with protein molecules for water molecules, reducing protein molecular hydration and delaying the hardening of HPNBs. Moreover, the addition of hydrolyzed protein produces HPNBs with a soft texture (Pathania et al., 2019). Protein hydrolysates contain peptides and amino acids, which makes HPNBs bitter and not easily accepted.
Extrusion is a mechanical process that integrates the operations of mixing, heating, and pressurization and has been widely applied in the transformation of protein, starch, and cellulose. Extrusion is superior to other processing approaches due to its versatile attributes, high productivity, short processing duration, low cost and energy savings (Kim et al., 2017). Thomas Fischer (2004) concluded that intermolecular cross-linking by disulfide bonding through extrusion cooking occurred in wheat protein. Chaiyakul et al. (2009) found that extruded milk protein concentrates (MPCs) could lessen the hardening of HPNBs and extend their textural shelf-life. However, a comparison and investigation of the anti-hardening effects of extruded casein and whey protein in HPNBs has not been examined in depth.
Therefore, in our work, WPI and CN were modified by extrusion technology, and the extruded WPI and CN were made into high protein nutrition bars. The aim of this study was to compare and investigate the effects of extruded CN and WPI on the hardening, color, and sensory quality of HPNBs during storage. Additionally, the sensory quality of HPNBs was calculated comprehensively by the fuzzy mathematical method, and the optimum extrusion temperature of WPI and CN was determined through normalized Technique for Order Preference by Similarity to Ideal Solution (TOPSIS) analysis. This work potentially provides an effective anti-hardening method for HPNBs.
2. Materials and method
2.1. Materials and reagents
WPI (protein 91.3%, fat 1.31%), CN (protein 92.6%, fat 1.75%), and fructose syrup were obtained from Henan Qian Weiyuan Food Additive Co. LTD. Glycerin purchased from Tianjin Zhonghe Shengtai Chemical Co., LTD. Trimethylol aminomethane (Tris), ethylenediamine tetraacetic acid (EDTA), phthalaldehyde (OPA), 2-nitrobenzoic acid (DTNB), sodium dodecyl sulfate (SDS) and glycine were purchased from Beijing Boaotuo Technology Co., LTD. β-mercaptoethanol and 8-aniline naphthalene-sulfo1-ate (ANS) were obtained from the Sigma company. Phosphate, glutaraldehyde, ethanol, methanol, boric acid and sodium chloride (NaCl) were purchased from Tianjin Fuyu Fine Chemicals Co., LTD.
2.2. Preparation of extruded proteins
Extruded casein (CE) and whey protein isolate (WPE) were prepared by a twin-screw extruder (Process 11, Thermo Fisher Scientific Inc., Germany). The screw extruder was an inter-meshing corotating device with a screw diameter of 29 mm and an L/D ratio of 40:1, which comprised the feeding, extruding, and temperature controlling systems. During the extrusion, the feed moisture was 40% and the feed rate was 60 r/min. It had eight heating zones. The temperature from the fourth zone to the sixth zone was changed, and the corresponding extrusion temperature was set at 50, 75, 100, 125 and 150 °C. The temperatures in other heating zones were kept constant (the first zone: 25 °C, the second zone: 35 °C, the third zone: 45 °C, the seventh zone: 45 °C, and the eighth zone: 25 °C).
2.3. Model HPNBs manufacture
Model HPNBs were formulated to contain 45 g of protein per 100 g. Extruded or unextruded proteins, glycerol and fructose syrup were mixed in a ratio of 9:3:8, and then the obtained mixture was blended with water. A dough of HPNBs was uniformly packed into cylindrical molds, covered with a lid, and then sealed with parafilm. Samples were sealed into separate plastic bags and were left at room temperature for 1 h. After storage for 0, 3, 7, 14, 21, 28 or 45 days at 37 °C, the samples were removed for the corresponding analysis. HPNBs prepared with extruded CE and WPE were labeled HPCE and HWPE, respectively. HPNBs obtained with unextruded CN and WPI were marked as HPCN and HWPI, respectively.
2.4. Free sulfhydryl measurement
The free sulfhydryl content of each protein ingredient and HPNB was determined by Ellman’s assay with modifications (Jiang et al., 2023). Fifteen milligrams of protein ingredient or HPNBs was dissolved in Tris-Gly buffer solution (0.086 mol/L Tris, 0.09 mol/L Gly and 0.04 mol/L EDTA, pH 8.0). Sample supernatants (0.5 mL) were vortexed with 50 μL of 10 mmol DTNB/L, which was held at room temperature for 15 min and the absorbance was read at 412 nm. Sample and standard blanks were prepared by substituting DTNB with buffer, and then calculated according to Formula (1).
| (1) |
where A412 nm is the absorbance at 412 nm, ρ is the sample mass concentration mg/mL, and D is the dilution factor.
2.5. Free amine measurement
The free amine content was determined by the OPA method (Frister et al., 1988). OPA (40 mg) was dissolved in 1 mL methanol. Then, 12.5 mL of 20% SDS solution, 25 mL of 0.1 mol/L borax and 500 μL β-mercaptoethanol were added, and the volume was brought to 250 mL with distilled water. The 100 μL solution sample (0.2 mg/mL) was mixed with 3 mL OPA reagent, reacted in the dark for 5 min, and then determined at 340 nm. The standard curves were obtained using l-leucine (0.1–0.5 mg/mL) as the standard: Y = 1.362–0.0094, R2 = 0.9997.
2.6. Surface hydrophobicity (H0) measurement
The surface hydrophobicity of the samples was measured by ANS (Li et al., 2022). The diluted 0.1, 0.05, 0.025 and 0.0125 mg/mL samples were added to 20 μL ANS (8 mmol/L, dissolved in 0.01 mol/L phosphate buffer, pH 7.0), mixed well, and then tested after 15 min in the dark. The excitation wavelength and emission wavelength were 390 nm and 470 nm, respectively. The surface hydrophobicity index was the slope of the regression curve between fluorescence intensity and protein solution quality.
2.7. Color
Color values for the HPNBs were acquired with a WSC-S colorimeter (Shanghai Precision Scientific Instrument Inc., China). Total color change (DE) was calculated with the Formula (2) for each HPNB preparation with the reference color values (i.e., a0*, b0*, and L0*) set to the values of HPNB at 0 d.
| (2) |
2.8. Hardness
The HPNB sample was placed into a cylinder with a diameter of 1 cm and a height of 1 cm. A cylindrical probe with a diameter of 36 mm (P/36R) was selected to measure the hardness of the sample by using texture profile analysis (TPA) mode. The determination was repeated at least 3 times for each sample.
2.9. Microstructure analysis
The microstructure was observed by the glutaraldehyde fixation method. The HPNBs were prepared in sheets and fixed in 2.5% glutaraldehyde fixative. The microstructure of the samples was observed by scanning electron microscopy (Model JSM-5310LV; JEOL Ltd., Tokyo, Japan; Ma et al., 2023). The SEM micrographs of the surface and interior samples were obtained at 500× magnification.
2.10. Sensory evaluation method
Ten qualified evaluators were selected as members of the sensory panel. Members randomly evaluated HPNB samples simultaneously and individually, and transitioned from ‘very good’ (value = 10) to ‘poor’ (value = 0) (Supplementary Material) depending on the performance of HPNBs.
Specifically, fuzzy mathematics evaluation was superior to the average system (Liu et al., 2012), and it was required to select the evaluation domain = (color, flavor, fragrance, hardness), comment domain= (9, 7, 5, 3), fuzzy weight vector = (0.22, 0.31, 0.23, 0.24), and sensory evaluation indices (See Table 1). In this study, we adopted fuzzy mathematics comprehensive evaluation in three steps: (i) the results of each evaluation domain were calculated, and the evaluation matrix was acquired; (ii) the comprehensive grading vector, and the specific operations of (i) and (ii) were carried out according to the literature (Gao and Fu, 2011, Liu et al., 2014); and (iii) the comprehensive evaluation score of each sample was calculated, which was applied to rank competition of HPNBs.
2.11. TOPSIS multi-index comprehensive evaluation
TOPSIS was applied to thoroughly assess each index of HPNBs under unconventional conditions. Its principle was to determine the best solution by calculating the ideal alternative and negative ideal alternative of each solution (Hwang & Yoon, 1981).
The TOPSIS program consists of the following steps:
-
(1)
Development of the ranking decision matrix
-
(2)
Index normalized deformation
-
(3)
Calculation of weighted normalized decision matrix
-
(4)
Determining positive and negative ideal solutions
-
(5)
Calculating the distance between different evaluation objects and positive and negative ideal solutions
-
(6)
Computing solutions that are relatively close to ideal, comparing Ci values and ranking alternatives
2.12. Statistical analyses
SPSS 16.0 was used for one-way analysis of variance and Duncan's multiple comparisons. P < 0.05 was considered that the difference was significant.
3. Results and discussion
3.1. Surface hydrophobicity of extruded proteins
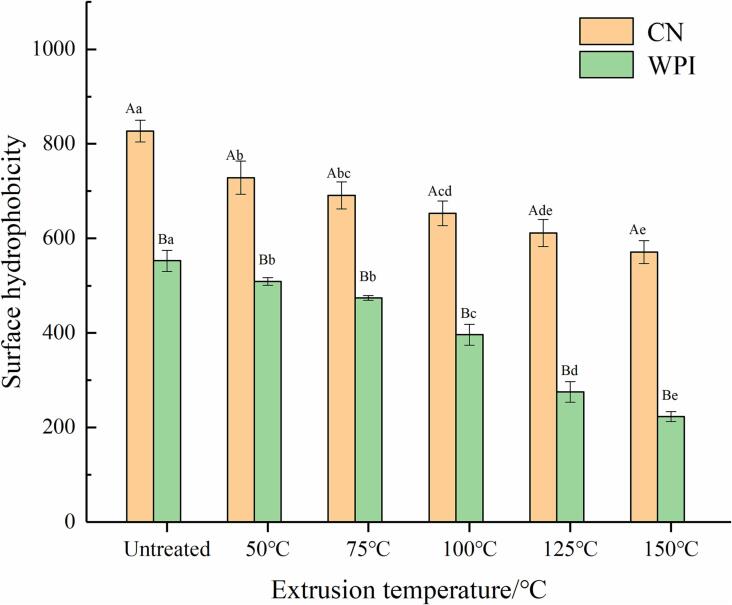
Fig. 1 shows the change in the surface hydrophobicity of CN and WPI with extrusion temperature (50–150 °C, interval 25 °C). WPI is a water-soluble protein with the lowest hydrophobicity index. CN exposed a more hydrophobic region than WPI. The surface hydrophobicity of the two proteins was significantly different (P < 0.05). The hydrophobicity of CE and WPE (extrusion at 150 °C) decreased by 30.99% and 59.65%, respectively. These results indicated that extrusion treatment caused a decrease in H0 for protein, as also reported by Jung et al. (2010). The possible reason for this may be that the amount of cysteine in the protein decreases after extrusion. Cysteine residues in proteins are moderately hydrophobic amino acid residues, and their reduction will reduce protein surface hydrophobicity (Yu et al., 2010).
Fig.1.
Influence of extrusion temperature on surface hydrophobicity of CN and WPI.
3.2. Free sulfhydryl content of extruded proteins and HPNBs
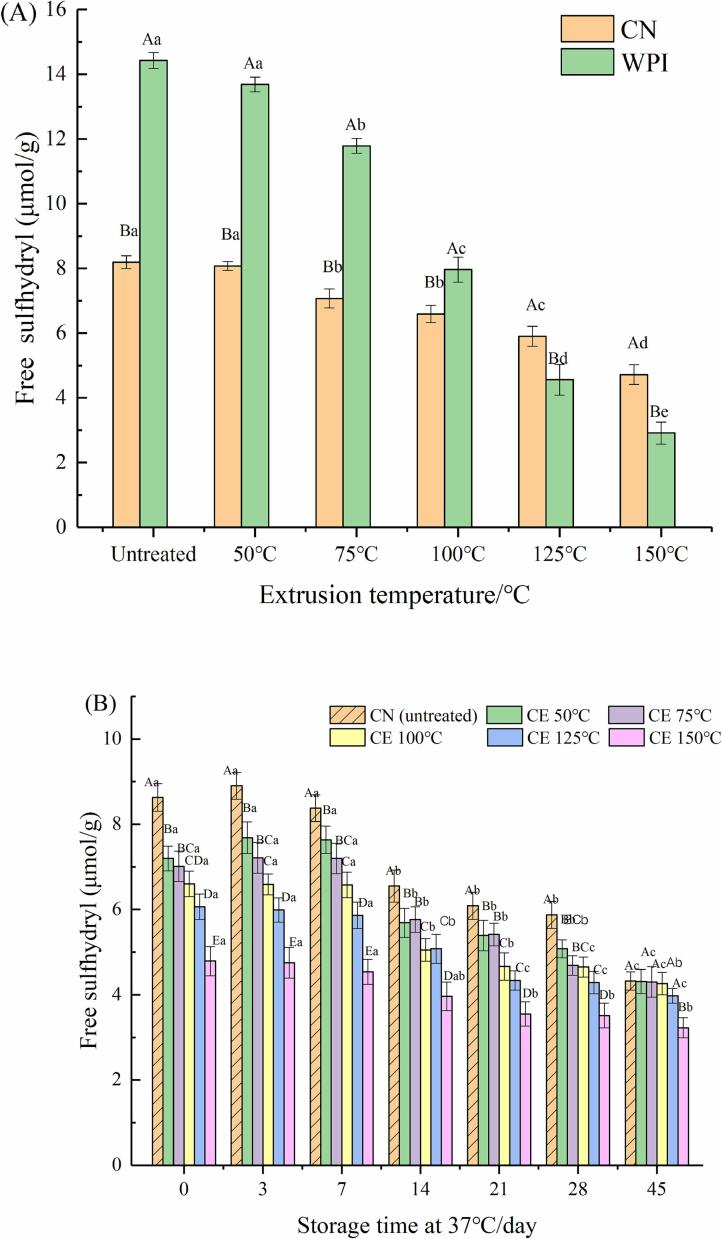
The free sulfhydryl content of extruded CN and WPI with extrusion temperature (50-150 °C, interval 25 °C) is depicted in Fig. 2(A). The sulfhydryl content in CN and WPI decreased with increasing extrusion temperature. There was a significant difference in the content of sulfhydryl between CE (150 °C) and CN (P < 0.05). The content of sulfhydryl in the WPE group was significantly decreased (P < 0.05), and there was a significant difference in the content of sulfhydryl between WPE and WPI (P < 0.05). The sulfhydryl content of CE (150 °C) and WPE (150 °C) was reduced by 42.37% and 79.81%, respectively. The mechanical shearing caused by extrusion exposes more sulfhydryl groups of protein molecules, leading to oxidation reactions and promoting the formation of disulfide bonds (Qi & Onwulata, 2011). Moreover, with increasing temperature, the mercapto-disulfide bond exchange reaction became more intense, and the content of mercapto decreased, forming new small molecular aggregates (Su et al., 2021).
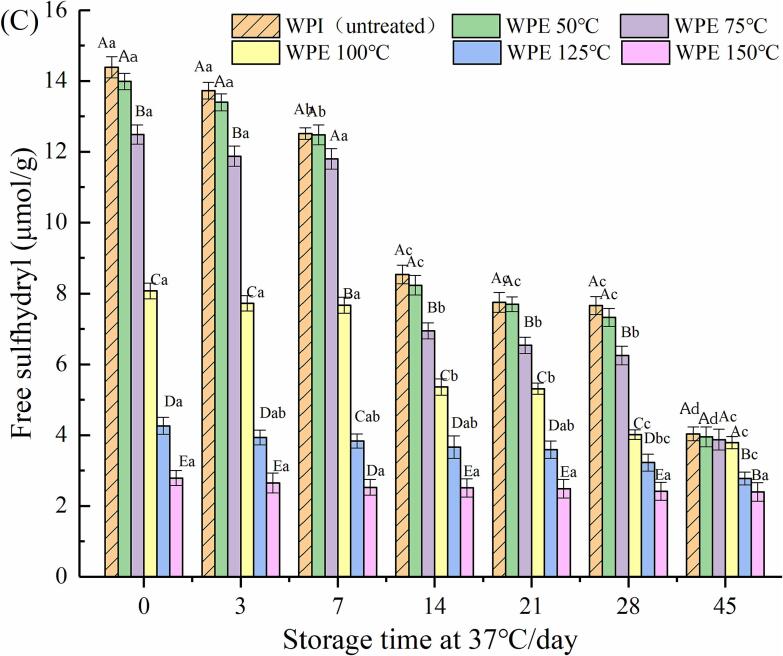
Fig. 2.
Effect of extrusion treatment on the free sulfhydryl groups of CN and WPI (A), HPCE (B) and HWPE system (C). Capital letters (A, B, C) indicate significant differences in different extrusion temperature (P < 0.05), and lowercase letters (a, b, c) indicate significant differences in different storage days (P < 0.05). The same letter indicates no significant difference (P > 0.05). The same below.
Fig. 2(B) and (C) shows the free sulfhydryl in HPCE or HWPE (37 °C, 0, 3, 7, 14, 21, 28 and 45 d). HPNBs prepared with untreated protein were used as blank samples. On the 45th day, the content of free sulfhydryl groups in HPCN and HPCE (50–150 °C, interval 25 °C) decreased more significantly than that in HWPI and HWPE (50–150 °C, interval 25 °C). The free sulfhydryl group in HPNBs decreased during storage. It was speculated that during storage, protein self-aggregation occurs, and the thiol-disulfide bond exchange reaction causes protein aggregation in the system, leading to a continuous decrease in thiol content (Faria et al., 2018). The content of free sulfhydryl groups lost the most in the WPE (150 °C) extrusion process, and when it was added to the protein bar system, there were fewer free sulfhydryl groups that could participate in the thiol-disulfide reaction.
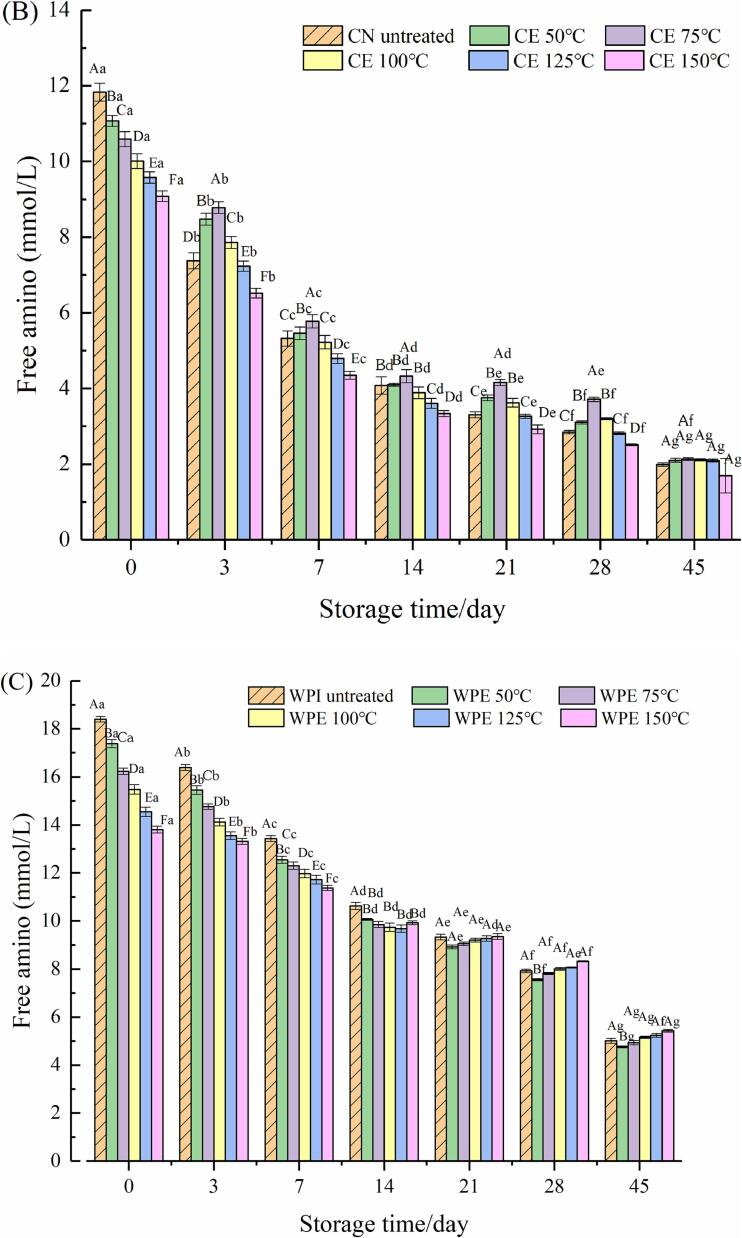
3.3. Free amino of extruded protein and HPNBs
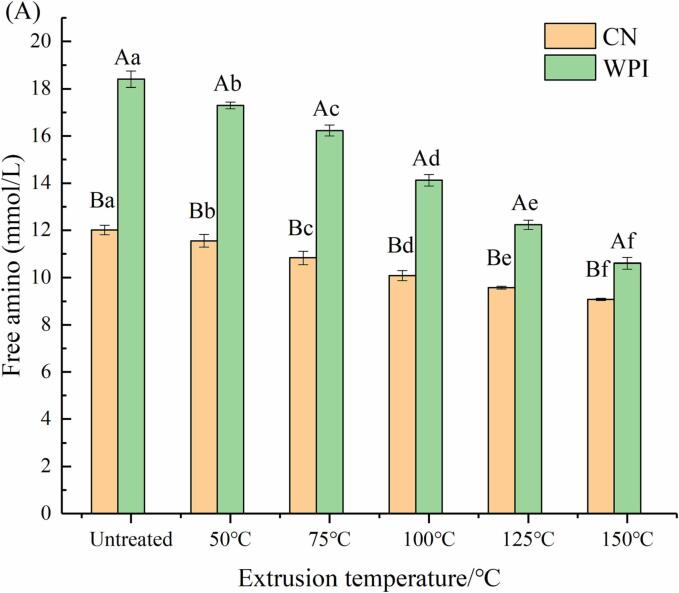
The free amino content of extruded CN and WPI with extrusion temperature (50–150 °C, interval 25 °C) is depicted in Fig. 3(A). The amino content in CN and WPI decreased with increasing extrusion temperature. Compared to CN and WPI, the amino content of CE (150 °C) and WPE (150 °C) was reduced by 24.4% and 42.35%, respectively.
Fig. 3.
Effect of extrusion treatment on free amino groups of CN and WPI (A), HPCE (B) and HWPE system (C).
Fig. 3(B) and (C) shows the free amino content of HPNBs prepared based on CE or WPE (37 °C, 0, 3, 7, 14, 21, 28 and 45 days). The amino content decreased with the extension of storage time. On the third day, the amino content of HPCN decreased to 55.58%. On Day 45, there was no significant difference in free amino content among all groups (P > 0.05). Likewise, on the 3rd day, the amino content of HWPI decreased to 87.9%. The free amino contents of HWPE (50 °C, 75 °C, 100 °C, 125 °C and 150 °C) were 87.67%, 89.86%, 90.06%, 92.16% and 95.94%, respectively. On Day 45 of the experiment, the loss of amino content in the HWPE (150 °C) was the lowest.
The free amino groups in the HPNBs of the two systems show a decreasing trend during storage. It is speculated that the addition of the reducing sugar syrup is responsible for the glycosylation reaction between the active carbonyl group in the reducing sugar and the amino group in the protein when stored at 37 °C, resulting in a continuous decrease in the amino content (Stadler et al., 2002). WPE (150 °C) extrusion process loss of free amino content. When it was added to the protein bar system, fewer amino groups could participate in the reaction, so the glycosylation reaction was effectively inhibited.
3.4. Color of HPNBs
Table 2 is shows the △E of HPNBs of two different systems (37 °C, 0, 3, 7, 14, 21, 28 and 45 days). There was no significant difference between treatment groups in the HPCN and HPCE when stored for 0–28 days (P > 0.05). Moreover, when stored for 45 days, the color of HWPE (150 °C) was the lightest. Fructose syrup consists primarily of glucose and fructose, which are both reducing sugars. During storage, the Maillard reaction occurs in the bars, which produces Maillard browning substances (Mottram et al., 2002). However, the free amino content of WPE decreased during the extrusion process, and the amino content decreased significantly at 150 °C. Therefore, in the later storage process, the glycosylation reaction of HWPE (150 °C) was the slowest, with a good sensory coloration.
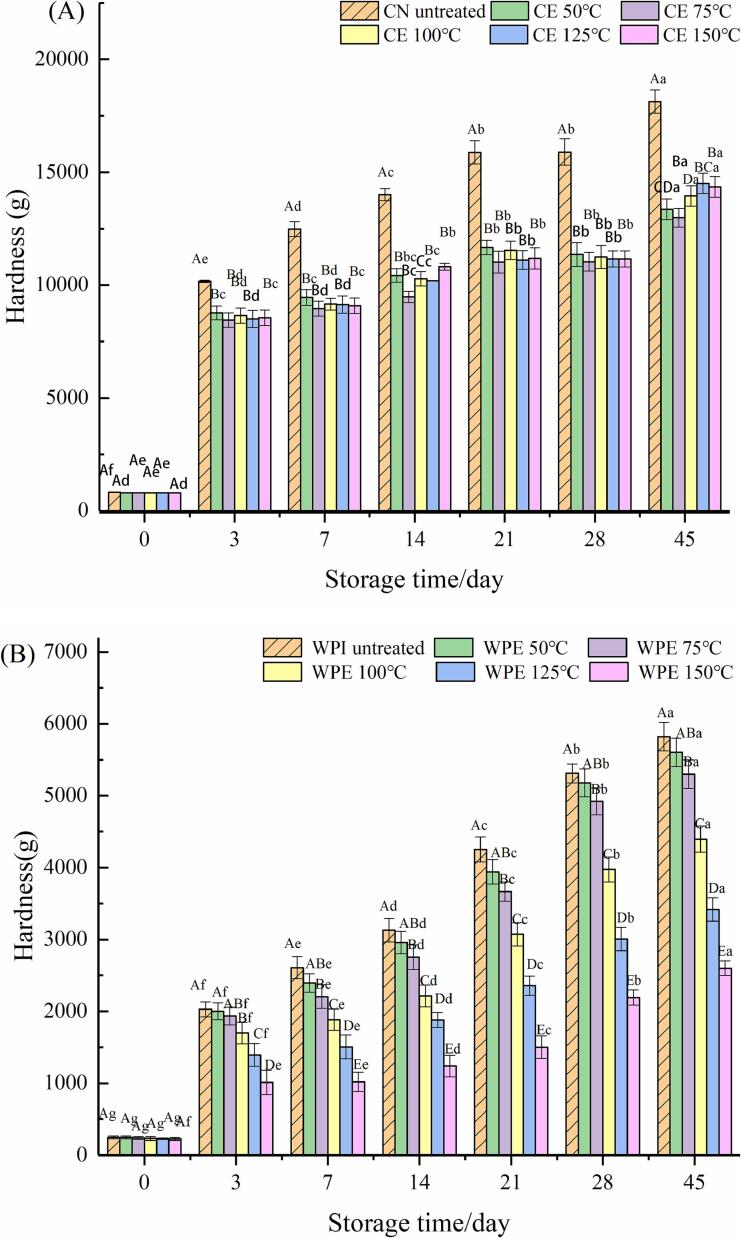
3.5. Hardness of HPNBs
Fig. 4(A) and (B) show the hardness of HWPE, HPCE, HWPI and HPCN during storage for 45 days at 37 °C. The hardness of the four HPNBs showed an increasing trend during storage. On the 45th day of storage, their hardness reached a maximum. At the initial Day 0, there was no significant difference between the HPCE and HPCN groups (P > 0.05). However, the hardness of HWPE and HPCE increased less than that of HWPI and HPCN. In all the groups, the hardness of HWPE (150 °C) was the lowest (P < 0.05).
Fig. 4.
Effect of extrusion treatment on the hardness of HPCE (A) and HWPE system (B).
The hardness changes of bars could be divided into two stages. In the first stage (0–3 days), the hardness of the HPNBs increased significantly, which was caused by the migration of water and glycerol in the system (Savitree et al., 2015). WPI has strong hydrophilicity and can be completely hydrated with water, but CN has a strong hydrophobic capacity, and only dissolves proteins on the surface of particles. The spherical structure of proteins still exists, and the system has a large viscosity, forming a gelatinous structure and producing a large hardness (Fishman et al., 2015). After extrusion, the content of hydrophobic residues on the protein surface decreased, which was beneficial for slowing phase separation during the initial storage (0–3 days).
In the second stage (3–45 days), the hardness of each system of bars increased with the extension of storage time at 37 °C. This was mainly caused by disulfide cross-linking and glycosylation reactions. The glycosylation reaction could lead to the formation of some protein aggregates, among which WPI is more prone to glycosylation reactions, and its hardness changes are the most obvious (Xi et al., 2020). Due to the decrease in sulfhydryl group content after extrusion treatment, the self-aggregation of protein through the sulfhydryl disulfide bond exchange reaction could be reduced to cause protein bar hardening. In addition, due to the reduction in amino content in the extrusion process, the glycosylation reaction of HPCE and HWPE was slowed down and the hardening of the storage period was reduced.
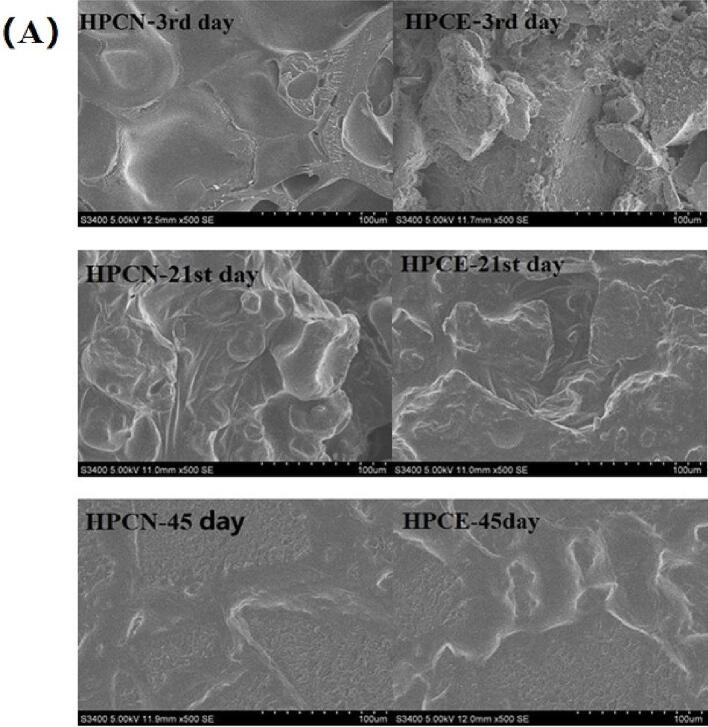
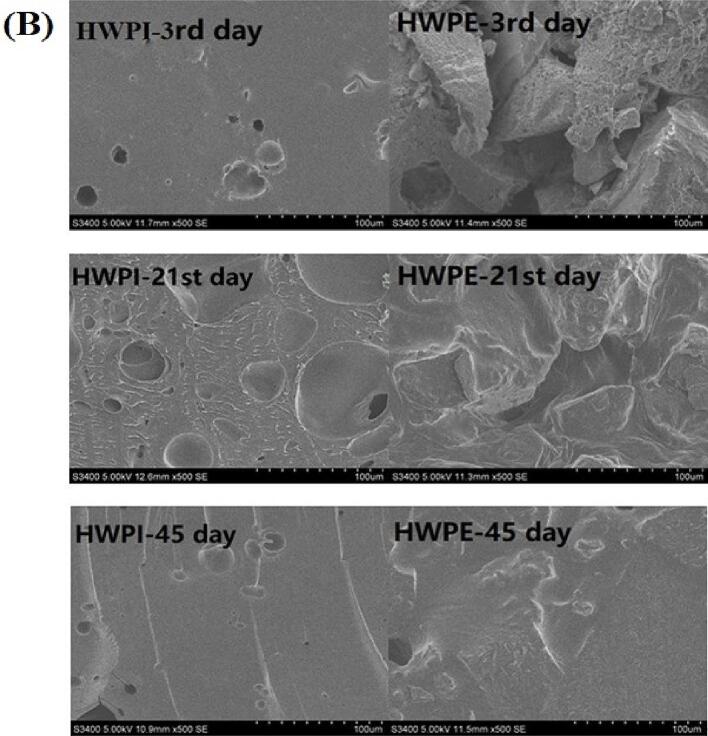
3.6. Microstructures of HPNBs
The effect of extrusion treatment on the microstructure of the HPNB system is pictured in Fig. 5. Fig. 5(A) shows that during the storage process of HPCN, protein particles absorbed water and swelled, forming a cross-linked network structure. Until fully crosslinked, it is difficult to observe distinct individual protein particles. At this point, Fig. 5(B) depicts that protein particles have formed aggregates. HWPI was evenly distributed and fused with bubbles to form a smooth and continuous internal structure. Compared with HWPE and HPCE, there were larger but fewer pores observed on the surface of HPCN and HWPI, which facilitated the interaction in the protein-glycerol-water ternary system. The morphology in the interior of the untreated HWPI and HPCN samples was relatively dense, partly due to the increasing hardness. The results indicated the physicochemical properties of proteins were closely related to the interaction between proteins and small molecules (Wang et al, 2023). Similar SEM morphology was reported previously after conjugation of polyhydroxy compounds with peanut protein (Fengchao et al., 2019).
Fig. 5.
Effect of extrusion treatment on microstructure of HPCE and HWPE system.
The extruded HWPE and HPCE samples showed the most plenty of pores on the surface and uniform honeycomb networks in the interior, which had almost no collapsed structure during the 21st day of storage. Compared with HPCN and HWPI, extruded HWPE and HPCE had an irregular surface with a few small cavities, while the organizational structure revealed multiple channels and large irregularities. It also provided a good way for the infiltration of water, thus increasing the solubility of proteins. After 45 days of storage, the Maillard reaction occurred in each group of HPNBs, which made the protein and water fuse and the whole system more uniform and compact. After 45 days of storage, there was no difference in microstructure among the HPNB samples.
3.7. Sensory properties of HPNBs
Traditional sensory evaluation is based on a single sensory evaluation index, without considering personal preference, personal experience, psychological factors and other direct effects on sensory evaluation. Fuzzy mathematics employs grading scales to assess samples (Zhang, 2015). In this evaluation process, the sensory team must scrutinize the elements that affect the performance of HPNBs, and determine the score of HPNBs by combining the weight of each factor and the membership degree. Fuzzy mathematics is immune to subjective factors and improves the accuracy of results. Therefore, the fuzzy mathematics comprehensive evaluation method was used for the sensory evaluation of HPNBs.
Table 3 indicates the sensory scores of HPNBs (37 °C, 3, 7, 14, 21, 28, and 45 days). The sensory scores of HWPE and HPCE decreased with storage time, flavor deteriorated, and hardness increased. Specifically, at Day 0, the samples were mushy and unconverted to solid, with low sensory scores. After short-term storage (0–3 days), the samples gradually formed solids and had good sensory scores. After 45 days of storage, HPCE (100 °C) had the highest sensory score of 6.09, and HWPE (150 °C) obtained the highest sensory score of 6.85. Moreover, the sensory scores of the HPCE and HWPE were higher than those of HPCN and HWPI, indicating that the extrusion-modified protein could improve the sensory characteristics of the HPNBs.
3.8. Selecting the optimum extrusion temperature of CN and WPI in HPNBs by TOPSIS
Table 4 shows the Ci ranking order of the six opportunities based on the color difference, hardness, and sensory score of HPNBs after 45 days of storage. Comparing HPCN and HWPI, the Ci values of all HPCE and HWPE samples were higher than those of HPCN and HWPI. Moreover, a larger Ci indicated that the alternative was relatively better. The largest Ci value was 0.9863. This indicated that WPE (150 °C) was the best ingredient for enhancing the anti-hardening and performance of HPNBs after taking all factors into consideration.
4. Conclusion
In this work, the anti-hardening and performance of HPNBs formulated with CE and WPE were evaluated for the first time, and it was found that extrusion pretreatment could significantly enhance the hardness of HPCE and HWPE during storage. In particular, after 45 days of storage, the hardness of the HPCE and HWPE was significantly lower than that of the HPCN and HWPI. Moreover, the color difference, hardness, and sensory score of HPNBs after 45 days of storage were used as indicators, and the results of the TOPSIS multiple index analysis indicated that HPNB formulated with WPI extruded at 150 °C possessed the best quality characteristics. Therefore, the HPNBs formulated with WPE (150 °C) exhibited the best anti-hardening and sensory characteristics. In conclusion, extrusion pretreatment of food protein is a highly effective approach to enhance the anti-hardening, sensory properties, and extend the shelf life of HPNBs.
CRediT authorship contribution statement
Kaili Wang: Methodology, Software, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. Xu Zhao: Software, Supervision, Validation. Munkh-Amgalan Gantumur: Writing – review & editing. Jinzhe Li: Software, Formal analysis. Yuxuan Huang: Software, Investigation. Narantuya Sukhbaatar: Investigation. Tian Bo: Resources, Supervision, Funding acquisition. Zhanmei Jiang: Conceptualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by project for Natural Science Foundation of China (No.32172164), Natural Key Science Foundation of Heilongjiang Province of China (No. ZD2021C007) and Cooperative Innovation Project of Heilongjiang Province Education Department (LJGXCG2022-029).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100719.
Contributor Information
Tian Bo, Email: tianbo@neau.edu.cn.
Zhanmei Jiang, Email: zhanmeijiang@neau.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Chaiyakul, S., K. Jangchud, A. Jangchud, P. Wuttijumnong and R. Winger (2009). Effect of extrusion conditions on physical and chemical properties of high protein glutinous rice-based snack. LWT-Food Science and Technology, 42(3):781-787. https://doi.org/ 10.1016/j.lwt.2008.09.011.
- Faria W., Giordani M.A., Arcas A.S., Cavenaghi D., Oliveira A.D., Santos J.D., et al. Novel soybean-based high protein bar rich in isoflavones improves insulin sensitivity in diabetic Wistar rats. Journal of Food Science & Technology. 2018:1–12. doi: 10.1007/s13197-017-2753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T. Effect of extrusion cooking on protein modification in wheat flour. European Food Research & Technology. 2004;218(2):128–132. doi: 10.1007/s00217-003-0810-4. [DOI] [Google Scholar]
- Fishman M.L., Chau H.K., Qi P.X., Hotchkiss A.T., Garcia R.A., Cooke P.H. Characterization of the global structure of low methoxyl pectin in solution. Food Hydrocolloids. 2015;46:153–159. doi: 10.1016/j.foodhyd.2014.12.021. [DOI] [Google Scholar]
- Frister H., Meisel H., Schlimme E. OPA method modified by use of N, N-dimethyl-2-mercaptoethylammonium chloride as thiol component. Fresenius Zeitschrift Für Analytische Chemie. 1988;330(7):631–633. doi: 10.1007/BF00473782. [DOI] [Google Scholar]
- Gao H., Fu S. Evaluation of jujube beverage prescriptions with fuzzy mathematics. Advances in Materials Research. 2011:573–576. doi: 10.4028/www.scientific.net/AMR.271-273.573. [DOI] [Google Scholar]
- Huang Y., Li J., Liu Y., Gantumur M.-A., Sukhbaatar N., Zhao P., et al. Improving gas-water interface properties and bioactivities of α-lactalbumin induced by three structurally different saponins. Food Hydrocolloids. 2023;138 doi: 10.1016/j.foodhyd.2023.108463. [DOI] [Google Scholar]
- Hwang C.L., Yoon K. Springer; Berlin Heidelberg: 1981. Methods for multiple attribute decision making; pp. 16–59. http://doi.org/10.1007/978-3-642-48318-9. [Google Scholar]
- Imtiaz S.R., Kuhn-Sherlock B., Campbell M. Effect of dairy protein blends on texture of high protein bars. Journal of Texture Studies. 2012;43(4):275–286. doi: 10.1111/j.1745-4603.2011.00337.x. [DOI] [Google Scholar]
- Ismarti I., Triyana K., Fadzilah N.A., Salleh H.M., Nordin N.F.H. Optimisation of the Maillard reaction of bovine gelatin-xylose model using response surface methodology. Food Research. 2020;4(S1):99–106. doi: 10.26656/fr.2017.4(S1).S13. [DOI] [Google Scholar]
- Janjarasskul T., Tananuwong K., Leuangsukrerk M., Phupoksakul T., Borompichaichartkul C. Effects of hasten drying and storage conditions on properties and microstructure of konjac glucomannan-whey protein isolate blend films. Food Biophysics. 2017;1–11 doi: 10.1007/s11483-017-9510-7. [DOI] [Google Scholar]
- Jiang Z., Meng Y., Hou C., Gantumur M.-A., Gao Y., Huang Y., et al. Extrusion for reducing malondialdehyde-induced whey protein isolate oxidation in relation with its physicochemical, functional and intro digestive properties. Food Hydrocolloids. 2023;142:108730. doi: 10.1016/j.foodhyd.2023.108730. [DOI] [Google Scholar]
- Jung S., Murphy P.A., Johnson L.A. Physicochemical and functional properties of soy protein substrates modified by low levels of protease hydrolysis. Journal of Food Science. 2010;70(2):C180–C187. doi: 10.1111/j.1365-2621.2005.tb07080.x. [DOI] [Google Scholar]
- Kim J.U., Kim B., Shahbaz H.M., Lee S.H., Park D., Park J. Encapsulation of probiotic Lactobacillus acidophilus by ionic gelation with electrostatic extrusion for enhancement of survival under simulated gastric conditions and during refrigerated storage. International Journal of Food Science & Technology. 2017;52(2):1–12. doi: 10.1111/ijfs.13308. [DOI] [Google Scholar]
- Li J., Fu J., Ma Y., He Y., Fu R., Qayum A. Low temperature extrusion promotes transglutaminase cross-linking of whey protein isolate and enhances its emulsifying properties and water holding capacity. Food Hydrocolloids. 2022;125 doi: 10.1016/j.foodhyd.2021.107410. [DOI] [Google Scholar]
- Li J., Wu Y., Ma Y., Lu N., Regenstein J.M., Zhou P. Effects of addition of hydrocolloids on the textural and structural properties of high-protein intermediate moisture food model systems containing sodium caseinate. Food & function. 2017;8(8):2897–2904. doi: 10.1039/c7fo00570a. [DOI] [PubMed] [Google Scholar]
- Li Y., Szlachetka K., Chen P., Lin X., Ruan R. Ingredient characterization and hardening of high-protein food bars: An NMR state diagram approach. Cereal Chemistry. 2008;85(6):780–786. doi: 10.1094/CCHEM-85-6-0780. [DOI] [Google Scholar]
- Liu C., Dong J., Wang J., Yin X., Qi L. A comprehensive sensory evaluation of beers from the Chinese market. Journal of the Institute of Brewing. 2012;118(3):325–333. doi: 10.12691/jfnr-3-1-1. [DOI] [Google Scholar]
- Liu Y., Fang P., Bian D., Zhang H., Wang S. Fuzzy comprehensive evaluation for the motion performance of autonomous underwater vehicles. Ocean Engineering. 2014;88:568–577. doi: 10.1016/j.oceaneng.2014.03.013. [DOI] [Google Scholar]
- Ma J., Li T., Wang Q., Xu C., Yu W., Yu H., et al. Enhanced viability of probiotics encapsulated within synthetic/natural biopolymers by the addition of gum arabic via electrohydrodynamic processing. Food Chemistry. 2023;413 doi: 10.1016/j.foodchem.2023.135680. [DOI] [PubMed] [Google Scholar]
- Madende M., Osthoff G. Comparative genomics of casein genes. The Journal of Dairy Research. 2019;1–8 doi: 10.1017/S0022029919000414. [DOI] [PubMed] [Google Scholar]
- Meng X., Ji J., Qi X., Nie X. Effect of anticaking agents on hardening and Maillard-induced protein aggregation in high-protein nutrition bars formulated with whey protein concentrate. Lebensmittel Wissenschaft Und Technologie. 2019;108:261–267. doi: 10.1016/j.lwt.2019.03.077. [DOI] [Google Scholar]
- Mottram, D. S., Wedzicha, B. L., & Dodson, A. T. (2002). Acrylamide is formed in the Maillard reaction. Nature, 419(6906): 448-449. https://doi.org/ 10.1038/419448a. [DOI] [PubMed]
- Pathania S., Parmar P., Tiwari B.K. Stability of proteins during processing and storage. Proteins: Sustainable Source, Processing and Applications. 2019:295–330. doi: 10.1016/B978-0-12-816695-6.00010-6. [DOI] [Google Scholar]
- Qi, P. X., & Onwulata, C. I. (2011). Physical properties, molecular structures, and protein quality of texturized whey protein isolate: Effect of EXTRUSION TEMPErature. Journal of Dairy Science, 94(5): 2231-2244. https://doi.org/ 10.3168/jds.2010-3942. [DOI] [PubMed]
- Rao, Q., Rocca-Smith, J. R., & Labuza, T. P. (2013). Storage stability of hen egg white powders in three protein/water dough model systems. Food Chemistry, 138(2-3): 1087-1094 https://doi.org/10.1016/j.foodchem.2012.11.082. [DOI] [PubMed]
- Savitree, R., Fukuoka, M., & Yada, S. (2015). The effect of sodium chloride on microstructure, water migration, and texture of rice noodle. LWT Food Science & Technology, 64(2), 1107-1113. https://doi.org/ 10.1016/j.lwt.2015.07.035.
- Sherwin, C. P., & Labuza, T. P. (2010). Role of moisture in Maillard browning reaction rate in intermediate moisture foods: comparing solvent phase and matrix properties. Journal of Food Science 68(2): 588-593. https://doi.org/ 10.1111/j.1365-2621.2003.tb05715.x.
- Stadler R.H., Blank I., Varga N., Robert F., Guy P.A. Acrylamide from Maillard reaction products. Nature. 2002;419:449–450. doi: 10.1038/419449a. [DOI] [PubMed] [Google Scholar]
- Su X., Li F., Gong Y., Dai M., Pan W., Zhang B. Physical and chemical properties of soy protein isolates treated with sodium sulphite under low temperature extrusion. International Journal of Food Science & Technology. 2021;1–9 doi: 10.1111/ijfs.15125. [DOI] [Google Scholar]
- Wang W., Shang H., Li J., et al. Four different structural dietary polyphenols, especially dihydromyricetin, possess superior protective effect on ethanol-induced ICE-6 and AML-12 cytotoxicity: The role of CYP2E1 and Keap1-Nrf2 pathways. Journal of Agricultural and Food Chemistry. 2023;71(3):1518–1530. doi: 10.1021/acs.jafc.2c06478. [DOI] [PubMed] [Google Scholar]
- Xi C., Kang N., Zhao C., Song H., Zhang T. Effect of reaction temperature on the protein structure and the ability to encapsulate β’arotene of WPI\extran conjugates. Journal of Food Science. 2020;85(2):1–10. doi: 10.1111/1750-3841.15141. [DOI] [PubMed] [Google Scholar]
- Yu, C., Xiong, Y. L. & Jie, C. (2010). Antioxidant and emulsifying properties of potato protein hydrolysate in soybean oil-in-water emulsions. Food Chemistry 120(1): 101-108. https://doi.org/10.1016/j.foodchem.2009.09.077.
- Zha, F., Shiyuan, D., Jiajia, R., & Chen, B. (2019). Pea protein isolate-gum Arabic Maillard conjugates improves physical and oxidative stability of oil-in-water emulsions. Food Chemistry, S0308-8146(19),1-42. https://doi.org/10.1016/j.foodchem.2019.01.151. [DOI] [PubMed]
- Zhang, H. (2015). Application on the entropy method for determination of weight of evaluating index in fuzzy mathematics for wine quality assessment. Advance Journal of Food Science & Technology, 7(3): 195-198. https://doi.org/ 10.19026/ajfst.7.1293.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.