Summary
Background
VLA1553 is a live-attenuated vaccine candidate for active immunisation and prevention of disease caused by chikungunya virus. We report safety and immunogenicity data up to day 180 after vaccination with VLA1553.
Methods
This double-blind, multicentre, randomised, phase 3 trial was done in 43 professional vaccine trial sites in the USA. Eligible participants were healthy volunteers aged 18 years and older. Patients were excluded if they had history of chikungunya virus infection or immune-mediated or chronic arthritis or arthralgia, known or suspected defect of the immune system, any inactivated vaccine received within 2 weeks before vaccination with VLA1553, or any live vaccine received within 4 weeks before vaccination with VLA1553. Participants were randomised (3:1) to receive VLA1553 or placebo. The primary endpoint was the proportion of baseline negative participants with a seroprotective chikungunya virus antibody level defined as 50% plaque reduction in a micro plaque reduction neutralisation test (μPRNT) with a μPRNT50 titre of at least 150, 28 days after vaccination. The safety analysis included all individuals who received vaccination. Immunogenicity analyses were done in a subset of participants at 12 pre-selected study sites. These participants were required to have no major protocol deviations to be included in the per-protocol population for immunogenicity analyses. This trial is registered at ClinicalTrials.gov, NCT04546724.
Findings
Between Sept 17, 2020 and April 10, 2021, 6100 people were screened for eligibility. 1972 people were excluded and 4128 participants were enrolled and randomised (3093 to VLA1553 and 1035 to placebo). 358 participants in the VLA1553 group and 133 participants in the placebo group discontinued before trial end. The per-protocol population for immunogenicity analysis comprised 362 participants (266 in the VLA1553 group and 96 in the placebo group). After a single vaccination, VLA1553 induced seroprotective chikungunya virus neutralising antibody levels in 263 (98·9%) of 266 participants in the VLA1553 group (95% CI 96·7–99·8; p<0·0001) 28 days post-vaccination, independent of age. VLA1553 was generally safe with an adverse event profile similar to other licensed vaccines and equally well tolerated in younger and older adults. Serious adverse events were reported in 46 (1·5%) of 3082 participants exposed to VLA1553 and eight (0·8%) of 1033 participants in the placebo arm. Only two serious adverse events were considered related to VLA1553 treatment (one mild myalgia and one syndrome of inappropriate antidiuretic hormone secretion). Both participants recovered fully.
Interpretation
The strong immune response and the generation of seroprotective titres in almost all vaccinated participants suggests that VLA1553 is an excellent candidate for the prevention of disease caused by chikungunya virus.
Funding
Valneva, Coalition for Epidemic Preparedness Innovation, and EU Horizon 2020.
Introduction
Chikungunya is a mosquito-transmitted disease that occurs with sporadic, unpredictable outbreaks. At least 5 million chikungunya virus infections were reported over the past 15 years, underscoring it as a global health threat.1, 2, 3 With increasing international travel and spread of potential vectors, infections caused by chikungunya virus have been identified in over 100 countries worldwide.4, 5 Signs and symptoms of chikungunya virus disease usually appear 4–7 days post-infection and can include rapid onset of fever, viraemia, severe joint pain, recurring mild joint pain, and maculopapular rash, affecting all sex and age groups.1, 6, 7, 8 Previous studies have shown persistent disease in more than half of patients, causing disabling polyarthritis.9 The case-fatality ratio is estimated to be 0·3–1 per 1000, with most deaths reported in neonates, adults with underlying conditions, and older people.7, 10 There is an urgent medical need for prophylaxis against chikungunya virus infection, since neither specific treatment nor vaccine is available.
VLA1553 is a live-attenuated, single dose vaccine candidate developed for prophylaxis and is aimed to target all circulating chikungunya virus strains, with comprehensive cross-neutralisation to the rapidly spreading Asian lineage.11 VLA1553 is based on the La Reunion strain (LR2006-OPY1) of east central South African genotype and is characterised by a 61 amino acid deletion in its non-structural protein 3, encoded by a viral replicase complex gene, which attenuates the virus in vivo. VLA1553 is intended to rapidly trigger a protective, durable antibody titre. In a phase 1 study, three dose levels of VLA1553 were assessed in healthy adults.12 All dose groups showed excellent immunogenicity with 100% seroconversion (a chikungunya virus-specific neutralising antibody titre [μNT50] ≥20) from day 14. Antibodies persisted in all dose groups after a single dose of vaccine, with seroconversion rates of 100% after 12 months and stable neutralising antibody titres. VLA1553 was generally safe in all dose groups, was well tolerated in the low and medium dose groups after a single vaccination, but showed higher adverse event rates and viraemia in the high dose group. The live-attenuated vaccine VLA1553 showed a strong reduced viraemia in humans as compared with wild-type chikungunya virus, which was cleared by all participants by day 14 post-vaccination. The transient viremia peak levels are considered too low to transmit VLA1553 virus from a vaccinated human (unpublished data). Due to its safety profile in combination with high immunogenicity, the medium dose of VLA1553 was selected for further development, ie, a final dose of 1 × 104 TCID50 per 0·5 mL (50% Tissue Culture Infectious Dose [TCID50]). As the dose had been identified and no schedule optimisation was necessary, the vaccine candidate progressed directly into phase 3 trials. As chikungunya virus epidemiology and outbreaks are unpredictable, partly also due to inadequate surveillance, and efficacy trials are considered unfeasible,13 regulators from the US Food and Drug Administration (FDA) and European Medicines Agency agreed to a pivotal study using a surrogate of protection as immunogenicity endpoint, later defined as μPRNT50 150 or higher, based on passive transfer studies of human post-vaccination sera to non-human primates before a wild-type challenge in combination with results from seroepidemiology.14
Research in context.
Evidence before this study
Chikungunya virus vaccine candidates currently under clinical development include inactivated whole virus vaccines, virus-like-particle vaccines, and RNA and DNA vaccines. A live-attenuated vaccine candidate developed by the Walter Reed Army Institute of Research advanced to phase 2. Although the vaccine proved to be safe and immunogenic in alphavirus-naive volunteers, development was terminated in 1998. A measles-virus-based vaccine developed by the Merck subsidiary Themis Bioscience completed phase 1 and 2 trials with favourable safety results and a durable immune response, but its development was discontinued. Emergent BioSolutions developed a virus-like particle (VLP) vaccine for prophylaxis. First-in-human and phase 2 studies in non-endemic and endemic regions demonstrated that the VLP vaccine candidate is well-tolerated and immunogenic after two vaccinations inducing a robust serum neutralising antibody immune response for up to 2 years. Emergent BioSolutions completed a phase 3 trial in April, 2023, enrolling 3258 healthy adolescents and adults. The International Vaccine Institute in partnership with Bharat Biotech International is developing an inactivated vaccine (BBV87) with a phase 2/3 multi-country trial enrolling 3210 healthy participants aged 12–65 years with an estimated study completion date in December, 2023.
Added value of this study
Valneva's live-attenuated chikungunya virus vaccine candidate (VLA1553) is based on the La Reunion strain of east central South African genotype with a 61 amino acid deletion in the non-structural protein 3, which leads to an attenuation of the virus in vivo. In this trial, a single dose of VLA1553 was generally safe, well tolerated, and highly immunogenic compared with placebo in healthy adults from the USA. VLA1553 induced chikungunya virus neutralising antibody levels predicted to be protective in almost all participants, irrespective of age. The adverse event profile of VLA1553 was similar to other licensed vaccines and equally well tolerated in younger and older adults. Although chikungunya virus neutralising antibody titres declined beyond 28 days post-vaccination, seroprotection persisted at rates of more than 96% throughout the study period of 180 days.
Implications of all the available evidence
VLA1553 is an excellent candidate for prevention of disease caused by chikungunya virus. This could be the first chikungunya virus vaccine candidate for active immunisation of travellers to and residents of endemic areas or areas at risk for an upcoming outbreak. Good antibody persistence after vaccination, as expected from a live-attenuated vaccine, is an important feature given the disease's unstable epidemiology. As age is a risk factor for severity and mortality of chikungunya disease, the strong immune response observed in older participants might be particularly beneficial in protecting those in need.
We report safety and immunogenicity data up to day 180 post-vaccination from our phase 3 trial assessing VLA1553 and placebo in 4115 healthy participants.
Methods
Study design
In this randomised, placebo-controlled, double-blind, multicentre, phase 3 trial, we assessed the immunogenicity and safety of the final dose of the live-attenuated chikungunya virus vaccine candidate (VLA1553) in healthy male and female participants. The study was done at 43 professional vaccine trial sites in the USA in compliance with International Council on Harmonisation Good Clinical Practice guidelines and in accordance with the principles set forth in the Declaration of Helsinki. Ethical approval was obtained before any study-related assessment (Chesapeake IRB; Reference number Pro00045587; approval date Aug 06, 2020).
Participants
Participants were considered eligible if they were generally healthy. Female participants of childbearing potential had to agree to use an adequate method of contraception for the first 3 months after vaccination as live-attenuated vaccines are generally contraindicated during pregnancy. The main exclusion criteria were history of chikungunya virus infection or immune-mediated or chronic arthritis or arthralgia, known or suspected defect of the immune system, any inactivated vaccine received within 2 weeks before vaccination with VLA1553, or any live vaccine received within 4 weeks before vaccination with VLA1553. A full list of exclusion criteria is provided in the appendix (pp 1, 2). All participants provided written informed consent before study-related procedures.
Randomisation and masking
Participants were randomly assigned (3:1) to receive VLA1553 or placebo. Participants were stratified by age (stratum A: 18–64 years and stratum B: 65 years or older). The first 501 participants comprised the immunogenicity subset of participants who were enrolled at 12 preselected study sites across the USA. Participants, investigators, and sponsor staff were masked to the assignment into study arms. Randomisation was done via an interactive response system or interactive web response system (IXRS). Each participant was assigned a unique individual screening number obtained from IXRS at the screening visit and was randomly assigned to treatment at visit 1 (day 1). The vaccine was prepared by unmasked study staff in accordance with the IXRS information. Syringe content was masked before administration by use of prefilled syringes of identical appearance to conceal content.
Procedures
Participants received a single intramuscular vaccination in the deltoid region of the arm on day 1. Vaccinated individuals were assessed during on-site study visits for safety and immunogenicity 7 days, 28 days, 84 days (3 months), and 179 days (6 months) after vaccination. Blood was drawn for safety and immunogenicity assessments (clinical chemistry, coagulation panel, and haematology for safety and neutralisation assay for immunogenicity). Safety laboratory samples were analysed at baseline from all study participants; participants from the immunogenicity subset also had safety laboratory assessments at subsequent visits. Safety laboratory parameters were assessed with the FDA's toxicity grading scale15 and captured as adverse events if considered clinically relevant.
Safety data were collected via electronic diary for daily oral body temperature, solicited injection site adverse events, and solicited systemic adverse events for the first 10 days after vaccination. From 11 days after vaccination until study end, participants were provided with an electronic memory aid. Safety data were entered into the electronic case report form. Participants were monitored for symptoms that could suggest an acute stage of chikungunya virus-associated event when presenting with sudden onset of fever and arthralgia, back pain, neurological symptoms, cardiac symptoms, rash, or oedema until 21 days after vaccination. These symptoms lasting for 3 days or longer were monitored separately as adverse events of special interest (AESI). Adverse events that met the seriousness criteria were reported as serious adverse events (SAEs). An independent Data Safety Monitoring Board (DSMB) regularly reviewed accruing safety information until the last participant had completed the end-of-study visit (day 180).
Immune response after vaccination was measured for chikungunya virus-specific neutralising antibodies and assessed by use of a validated micro plaque reduction neutralisation test (μPRNT) with a heterologous chikungunya virus strain providing a readout for cross-neutralising coverage. Briefly, serial dilutions of serum samples were prepared, added sequentially to the live-attenuated chikungunya virus vaccine (TSI-GSD-218 or 181/clone 25, developed by the Walter Reed Army Institute of Research, Washington, DC, USA) followed by incubation for 60 min. The TSI-GSD-218 chikungunya virus vaccine strain is a derivative of the Asian lineage harbouring two point mutations in the E2 glycoprotein that mediate attenuation.16 The virus strain was originally derived from the serum isolate chikungunya virus strain AF15561 obtained from an infected patient during the 1962 outbreak of chikungunya in Thailand.17, 18 Serum-virus mixtures were then transferred onto Vero cells and incubated for 60 min. Then, the serum-virus mixtures were removed and a virus maintenance medium containing methyl cellulose was added to the wells, followed by an incubation for 17 h. The following day, cells were fixed, permeabilised with Triton X-100, and indirect immunostaining was performed. Images from each well were acquired by an automated microscope system (ScanLab reader). The number of resulting plaque-forming units in the wells was inversely proportional to the concentration of functional antibodies present in the serum, which was directly proportional to the immunological response. With a lower assay limit of quantitation of μPRNT50=20 seroconversion (ie, presence of chikungunya virus neutralising antibodies) had been defined as μPRNT50 20 or more for baseline negative participants. Seroconversion for baseline positive participants was defined as a more than 4-fold increase over baseline. Seroprotection was defined as μPRNT50 150 or more.14 Negative samples with a μPRNT50 titre less than 20 were imputed with 10. Immunogenicity samples were taken from all participants in case of need but analysed only from a statistically representative immunogenicity subset.
Outcomes
The primary endpoint was the proportion of participants with a seroprotective chikungunya virus antibody level (defined as 50% plaque reduction in a μPRNT) with a μPRNT50 titre of at least 150 for baseline negative participants 28 days after vaccination.14 Immunogenicity analyses were done in the preselected subset representative for the entire study population, using the placebo group as negative control.
Secondary immunogenicity endpoints included the immune response as measured by chikungunya virus-specific neutralising antibody titres 7 days, 28 days, 84 days (3 months), and 179 days (6 months) post-vaccination. The proportion of participants with seroprotective chikungunya virus antibody levels (defined as μPRNT50 ≥150 for baseline negative participants based on passive transfer studies of human sera to non-human primates)14 and with seroconversion was assessed. Fold increases of chikungunya virus-specific neutralising antibody titres compared with baseline were measured along with the proportion of participants reaching an at least 4-fold, 8-fold, 16-fold, or 64-fold increase in chikungunya virus-specific neutralising antibody titre compared with baseline.
Safety was assessed in all participants who received vaccination (safety population) or placebo. Secondary endpoints included the frequency and severity of solicited injection site and systemic adverse events for 10 days post-vaccination and unsolicited adverse events for 28 days post-vaccination. Safety endpoints included the frequency and severity of AESIs from 2–21 days after vaccination and of all adverse events and SAEs for the study duration.
Statistical analysis
The sample size of approximately 3000 VLA1553-vaccinated participants was calculated to allow for the detection of at least one vaccine-attributable uncommon event (incidence rate 1/1000) with a probability of 95%.
The immunogenicity subset of 375 participants vaccinated with VLA1553 was calculated to allow for sufficient statistical power when applying a one-sided exact binomial test with a significance level of 2·5% against a non-acceptance threshold of 70% on the proportion of participants with a seroprotective level (defined as μPRNT50 titre ≥150 for baseline negative participants) 28 days post-vaccination. A seroprotection rate of 80% was assumed on the basis of previous results,12 and 200 VLA1553-vaccinated participants were therefore necessary for a statistical power of 90%. With an expected dropout and major protocol deviations rate of approximately 10%, 225 VLA1553 vaccinees needed to be allocated to the immunogenicity subset. To account for placebo participants, to achieve a meaningful number of participants in both age strata, and for long-term follow-up in a subsequent trial, 501 participants were required for enrolment into the immunogenicity subset.
The primary immunogenicity analysis was a comparison of the observed proportion of participants with a seroprotective chikungunya virus antibody level at 28 days post-vaccination against a non-acceptance threshold of 70%. An exact binomial test for the null-hypothesis H0 (seroprotection rate ≤70%) against the alternative H1 (seroprotection rate >70%) with a one-sided significance level of 2·5% was applied and exact (Clopper-Pearson) two-sided 95% CIs were calculated. In addition, both treatment arms were compared by use of Fisher's exact test for seroprotection and seroconversion rates. Difference in proportion between the two treatment groups, and exact (Clopper-Pearson) two-sided 95% CIs for the difference in treatment groups were presented. Geometric mean antibody titre (GMT) was compared between VLA1553 and placebo groups by an analysis of covariance (ANCOVA) model including treatment group and age stratum as factors. Estimates of treatment differences in GMT and associated 95% CIs were presented.
All participants vaccinated at day 1 were included in the safety population. For immunogenicity analyses, all vaccinated participants who were chikungunya virus seronegative at baseline (defined as μPRNT50 titre <20), part of the immunogenicity subset, and had no major protocol violations that could affect the immune response were included in the per-protocol population. The immunogenicity population contained all vaccinated subjects of the immunogenicity subset with at least one evaluable post-baseline titre measurement after vaccination. This population was used for sensitivity analyses.
The study is registered with ClinicalTrials.gov, NCT04546724.
Role of the funding source
Employees of Valneva were responsible for study design, data collection, data analysis, data interpretation, and writing of the report.
Results
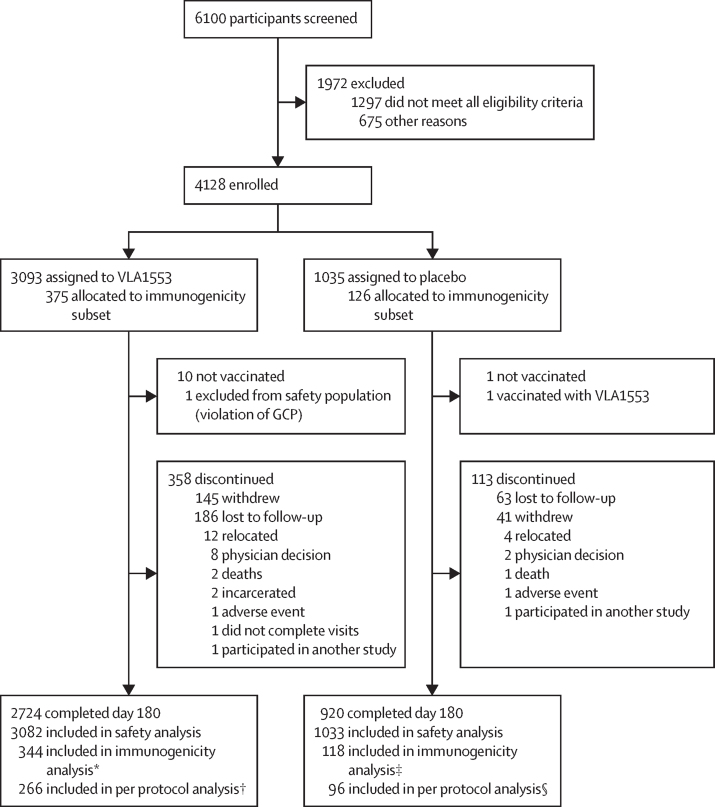
Between Sept 17, 2020, and April 10, 2021, 6100 participants were screened for eligibility. 1972 patients were excluded (1297 did not meet all eligibility criteria and 675 for other reasons) and 4128 participants were enrolled and randomly assigned to receive VLA1553 (n=3093) or placebo (n=1035; figure 1). Ten participants in the VLA1553 group and one in the placebo group did not receive vaccination (reason unknown). All vaccinated participants were included in the safety population, except one participant who was assigned and vaccinated twice with VLA1553 and was thus excluded from the safety population (non-compliance with GCP). One participant who was assigned to placebo was vaccinated with VLA1553 and was included in the VLA1553 group for safety analyses. The safety population consisted of 4115 participants (3082 in the VLA1553 group and 1033 in the placebo group). 471 participants discontinued the study (358 in the VLA1553 group and 113 in the placebo group). The per-protocol population was composed of 362 participants (266 in the VLA1553 arm [including 207 participants aged 18–64 years and 59 participants aged 65 years or older], and 96 in the placebo arm [including 73 participants aged 18–64 years and 23 participants aged 65 years or older]). 2251 (54·7%) of 4115 patients were female and 1864 (45·3%) were male. 3309 (80·4%) of patients were White and 573 (13·9%) were Black or African American. Median age was 45·0 years (IQR 32·0–57·0). 463 (11·3%) of participants were aged 65 years or older. Baseline characteristics in the VLA1553 arm and the placebo arm were similar (table 1). The immunogenicity subset was representative of the whole study population in terms of demographics (appendix p 4).
Figure 1.
Trial profile
The immunogenicity subset was recruited from 12 pre-selected study sites enrolling the first 501 participants entering the study. *31 of 375 participants were excluded from the immunogenicity subset for the following reasons: seropositive or unknown at baseline (n=17), no evaluable post-baseline titre (n=10), not vaccinated (n=4). †78 participants in the immunogenicity population were excluded from the per protocol subset for the following reasons: major protocol deviations (one or more of: out of visit windows [n=42], investigational product issue [n=20], missing endpoint data [n=15], exclusion criteria met [n=1], safety assessment issue [n=1], prohibited medication taken [n=1]). ‡Eight of 126 participants were excluded from the immunogenicity subset for the following reasons: no evaluable post-baseline titre (n=4), seropositive at baseline (n=4). §22 participants in the immunogenicity population were excluded from the per protocol subset for the following reasons: major protocol deviations (one or more of the following categories: out of visit windows [n=15], investigational product issue [n=4], missing endpoint data [n=3], prohibited medication taken [n=1]).
Table 1.
Baseline characteristics (safety population)
| VLA1553 (n=3082) | Placebo (n=1033) | Total (n=4115) | |
|---|---|---|---|
| Sex | |||
| Female | 1682 (54·6%) | 569 (55·1%) | 2251 (54·7%) |
| Male | 1400 (45·4%) | 464 (44·9%) | 1864 (45·3%) |
| Race | |||
| American Indian or Alaska Native | 27 (0·9%) | 5 (0·5%) | 32 (0·8%) |
| Asian | 51 (1·7%) | 17 (1·6%) | 68 (1·7%) |
| Black or African American | 451 (14·6%) | 122 (11·8%) | 573 (13·9%) |
| Native Hawaiian or other Pacific Islander | 13 (0·4%) | 5 (0·5%) | 18 (0·4%) |
| White | 2456 (79·7%) | 853 (82·6%) | 3309 (80·4%) |
| Other | 84 (2·7%) | 31 (3·0%) | 115 (2·8%) |
| Ethnicity | |||
| Hispanic or Latino | 545 (17·7%) | 177 (17·1%) | 722 (17·5%) |
| Not Hispanic or Latino | 2498 (81·1%) | 840 (81·3%) | 3338 (81·1%) |
| Not reported | 34 (1·1%) | 14 (1·4%) | 48 (1·2%) |
| Unknown | 5 (0·2%) | 2 (0·2%) | 7 (0·2%) |
| Age (years) | |||
| N | 3082 | 1033 | 4115 |
| Median (IQR) | 45·0 (32·0–57·0) | 45·0 (32·0–58·0) | 45·0 (32·0–57·0) |
| Min–max | 18–88 | 18–94 | 18–94 |
| Age group | |||
| 18–64 years (stratum A) | 2736 (88·8) | 916 (88·7) | 3652 (88·7) |
| ≥65 years (stratum B) | 346 (11·2) | 117 (11·3) | 463 (11·3) |
| Weight (kg) | |||
| N | 3078 | 1031 | 4109 |
| Median (IQR) | 85·0 (71·8–100·3) | 83·6 (70·3–98·9) | 84·6 (71·6–100·0) |
| Min–max | 38·7–247·7 | 43·8–197·8 | 38·7–247·7 |
| Height (cm) | |||
| N | 3079 | 1030 | 4109 |
| Mean (SD) | 169·8 (9·9) | 169·9 (9·9) | 169·8 (9·9) |
| Min–max | 128·8–199·4 | 133·9–204·2 | 128·8–204·2 |
| BMI (kg/m2) | |||
| n | 3078 | 1029 | 4107 |
| Median (IQR) | 29·4 (25·3–34·3) | 28·9 (24·7–33·9) | 29·3 (25·1–34·2) |
| Min–max | 14·1–102·3 | 16·6–63·1 | 14·1–102·3 |
Data are n (%) unless otherwise specified.
The study met its primary immunogenicity endpoint. In the per-protocol population, chikungunya virus neutralising antibody titres above the protective threshold were induced in 263 (98·9%) of 266 participants 28 days after receiving VLA1553 (95% CI for seroprotection rate 96·7–99·8%; p<0·0001). No significant difference in the seroprotection rate was observed between patients aged 18–64 years (204 [98·6%] of 207 participants) and 65 years or older (59 [100%] of 59 participants; table 2). At day 180, 233 (96·3%) of 242 participants remained with titres above the seroprotective level of antibodies in the VLA1553 arm.
Table 2.
Seroprotection rate for chikungunya virus-specific neutralising antibodies on day 29
|
18–64 years (stratum A) |
≥65 years (stratum B) |
Total |
||||
|---|---|---|---|---|---|---|
| VLA1553 (n=207) | Placebo (n=73) | VLA1553 (n=59) | Placebo (n=23) | VLA1553 (n=266) | Placebo (n=96) | |
| Total* (n) | 207 | 73 | 59 | 23 | 266 | 96 |
| Participants with seroprotection, n (%) | 204 (98·6%) | 0 | 59 (100%) | 0 | 263 (98·9%) | 0 |
| 95% CI for seroprotection rate | 95·8–99·7 | 0·0–4·9 | 93·9–100·0 | 0·0–14·8 | 96·7–99·8 | 0·0–3·8 |
| p value† | <0·0001 | >0·9999 | <0·0001 | >0·9999 | <0·0001 | >0·9999 |
| Difference in seroprotection rate‡ | 98·6 | .. | 100·0 | .. | 98·9 | .. |
| 95% CI | 96·9–100·0 | .. | 100·0–100·0 | .. | 97·6–100·0 | .. |
| p value§ | <0·0001 | .. | <0·0001 | .. | <0·0001 | .. |
Data are in the per-protocol population. Percentages are based on the number of baseline negative participants with non-missing titres at the visit. Seroprotection was defined as μPRNT50 titre ≥150 for μPRNT baseline negative participants (<20). Two-sided 95% exact (Clopper-Pearson) CIs are presented. Where the upper bound of the CI would be greater than 100%, the upper confidence limit is restricted to 100. μPRNT50 titre=serum dilution with 50% plaque reduction in a micro plaque reduction neutralisation test.
Number of μPRNT baseline negative participants (<20) with non-missing titres on day 29.
p value from an exact binomial test for the null-hypothesis H0: seroprotection rate ≤70% against the alternative H1: seroprotection rate >70% with a one-sided significance level of 2·5%.
Differences, p values, and associated CIs are presented for the VLA1553 group minus the placebo group.
p value from Fisher's exact test.
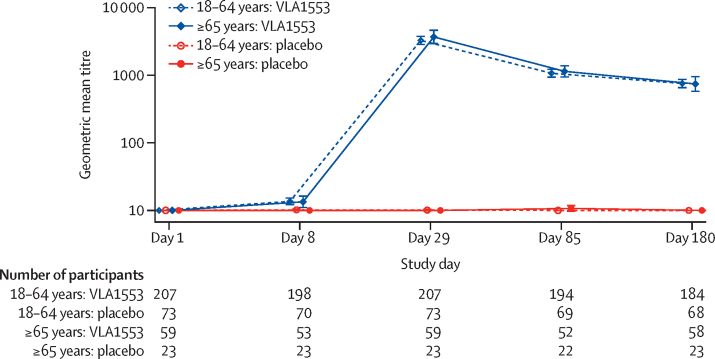
The seroconversion rate (titre of at least 20 in participants seronegative at baseline) was 99·2% on day 29 and remained stable throughout, with 238 (98·3%) of 242 participants maintaining seroconversion (p<0·0001; appendix p 5). In two participants, chikungunya virus neutralising antibodies could not be detected after exposure to VLA1553; one participant met the seroconversion but not the seroprotection criteria. GMT was modest on day 8 (negligible) and peaked on day 29 at 3362. Titres were 752 at day 180 with similar titres and titre kinetics observed for both age strata (ANCOVA: p<0·0001 for both age strata comparing VLA1553 with placebo; figure 2, appendix pp 3, 12). Although titres decreased during the observation period of 180 days, they remained high in most participants (appendix p 12). Chikungunya virus-specific neutralising antibody titres showed a 471-fold increase compared with baseline at day 29 and persisted with a 107-fold increase at day 180 compared with baseline. No difference in the immune response was observed over time between the two age strata (appendix pp 6, 11).
Figure 2.
Assessment of neutralising antibodies after vaccination
Line plot of chikungunya virus-specific neutralising antibodies geometric mean titres by study day and age stratum. Days shown in the figure refer to study days; day 1=day of vaccination. Error bars indicate 95% CIs. Neutralising antibodies to the vaccine were evaluated from clinical specimen (human serum) using a micro plaque reduction neutralisation test (μPRNT). A μPRNT50 titre was defined as the dilution with 50% plaque reduction in the μPRNT.
VLA1553 was generally well tolerated across all age groups with most events being mild or moderate. The safety profile in adults aged 65 years and older was similar to that of adults younger than 65 years (data not shown). Almost all adverse events occurred within 4 weeks after vaccination. Up to day 180 after vaccination, adverse events were reported more frequently for VLA1553 than placebo (1926 [62·5%] of 3082 vs 463 [44·8%] 1033 participants; p<0·0001). 1575 (51·1%) of 3082 participants in the VLA1553 group and 322 (31·2%) of 1033 participants in the placebo group experienced at least one adverse event that was considered related to vaccination (p<0·0001; table 3). 1547 (50·2 %) of 3082 AEs were solicited systemic adverse events (appendix pp 7, 9).
Table 3.
Overall Summary of adverse events (safety population)
| VLA1553 (n=3082) | Placebo (n=1033) | Total (n=4115) | ||
|---|---|---|---|---|
| Any adverse events | 1926 (62·5%, 60·8–64·2) 6415 | 463 (44·8%, 41·8–47·9) 1071 | 2389 (58·1%, 56·5–59·6) 7486 | |
| Any related adverse events | 1575 (51·1%, 49·3–52·9) 4621 | 322 (31·2%, 28·4–34·1) 647 | 1897 (46·1%, 44·6–47·6) 5268 | |
| Any related severe adverse events | 62 (2·0%, 1·5–2·6) 70 | 1 (0·1%, 0·0–0·5) 3 | 63 (1·5%, 1·2–2·0) 73 | |
| Any serious adverse events | 46 (1·5%, 1·1–2·0) 73 | 8 (0·8%, 0·3–1·5) 10 | 54 (1·3%, 1·0–1·7) 83 | |
| Any related serious adverse events | 2 (0·1%, 0·0–0·2) 2 | 0 (0%, 0·0–0·4) 0 | 2 (0·0%, 0·0–0·2) 2 | |
| Any adverse events of special interest | 10 (0·3%, 0·2–0·6) 26 | 1 (0·1%, 0·0–0·5) 2 | 11 (0·3%, 0·1–0·5) 28 | |
| Any adverse event with a frequency ≥10% in at least one study arm | ||||
| Headache | 986 (32·0%, 30·3–33·7) 1028 | 160 (15·5%, 13·3–17·8) 178 | 1146 (27·8%, 26·5–29·2) 1206 | |
| Fatigue | 886 (28·7%, 27·2–30·4) 893 | 137 (13·3%, 11·3–15·5) 139 | 1023 (24·9%, 23·5–26·2) 1032 | |
| Myalgia | 750 (24·3%, 22·8–25·9) 758 | 82 (7·9%, 6·4–9·8) 84 | 832 (20·2%, 19·0–21·5) 842 | |
| Arthralgia | 554 (18·0%, 16·6–19·4) 589 | 63 (6·1%, 4·7–7·7) 70 | 617 (15·0%, 13·9–16·1) 659 | |
| Injection site pain | 413 (13·4%, 12·2–14·7) 519 | 101 (9·8%, 8·0–11·8) 122 | 514 (12·5%, 11·5–13·5) 641 | |
| Pyrexia | 427 (13·9%, 12·7–15·1) 429 | 13 (1·3%, 0·7–2·1) 13 | 440 (10·7%, 9·8–11·7) 442 | |
| Nausea | 359 (11·6%, 10·5–12·8) 364 | 63 (6·1%, 4·7–7·7) 64 | 422 (10·3%, 9·3–11·2) 428 | |
| Any serious adverse event with a frequency ≥0·2% in at least one study arm by system organ class | ||||
| Infections and infestations | 9 (0·3%, 0·1–0·6) 9 | 3 (0·3%, 0·1–0·8) 3 | 12 (0·3%, 0·2–0·5) 12 | |
| Injury, poisoning, and procedural complications | 8 (0·3%, 0·1–0·5) 15 | 1 (0·1%, 0·0–0·5) 1 | 9 (0·2%, 0·1–0·4) 16 | |
| Psychiatric disorders | 7 (0·2%, 0·1–0·5) 8 | 2 (0·2%, 0·0–0·7) 4 | 9 (0·2%, 0·1–0·4) 12 | |
| Cardiac disorders | 5 (0·2%, 0·1–0·4) 7 | 0 (0%, 0·0–0·4) 0 | 5 (0·1%, 0·0–0·3) 7 | |
Data are n (%, 95% CI) N. For each category, participants were included only once, even if they experienced multiple events in that category. Related adverse events are those recorded as probably related or possibly related on the eCRF. Adverse events of special interest counts are for the overall event and the adverse event of special interest symptom count includes a count of all symptoms contributing to the event. Two-sided exact Clopper-Pearson 95% CIs are presented. eCRF=electronic case report form. n=number of participants. N=number of events.
SAEs were reported in 46 (1·5%) of 3082 participants exposed to VLA1553 and eight (0·8%) of 1033 participants in the placebo arm (non-significant). Two SAEs in two (0·1%) of 3082 participants were deemed related to treatment; both participants received VLA1553. A 58-year-old woman experienced mild myalgia, which led to hospital admission from day 4 to day 9 after vaccination for monitoring and diagnostic procedures. Medical history included fibromyalgia. No other trigger for myalgia could be identified and the event was assessed by the investigator as probably related to vaccination. Creatine kinase values were within normal range on day 4, and the event was resolved on day 31. The second SAE (verbatim: syndrome of inappropriate antidiuretic hormone secretion [SIADH]) was reported for a 66-year-old man who experienced several solicited adverse events starting 3 days after vaccination, including severe fever (highest temperature 39·6°C [103·3°F] on day 10 and 38·9°C [102·1°F] on day 11), which resolved on day 12. On day 11, he experienced severe atrial fibrillation and severe hyponatraemia, which led to hospital admission from day 11 to day 14. The SIADH was diagnosed by a nephrologist, although the sponsor would have considered a hypovolemic hyponatraemia a more likely diagnosis. No other cause for SIADH was identified or mentioned by the treating physicians. The event was assessed by the investigator as probably related to vaccination due to prolonged fever and symptoms post-vaccination. Both participants recovered completely.
Signs and symptoms potentially indicative of an acute chikungunya virus infection were closely monitored as AESIs. AESIs were reported in ten (0·3%) of 3082 participants in the VLA1553 group and one (0·1%) of 1033 participants in the placebo group. Five (50%) of ten participants with AESI in the VLA1553 group experienced fever 39°C or higher. Most AESI events were self-limiting and resolved after 2–4 days. These events were mostly combinations of solicited adverse events such as fever with arthralgia or back pain. During the study, 15 participants got pregnant, 13 of whom received VLA1553. The following outcomes were reported: nine healthy babies, one lost to follow-up and three miscarriages earlier than gestation week 20 (none deemed related to treatment).
Solicited injection site adverse events were reported in 463 (15·0%) of 3082 participants after receiving VLA1553. Tenderness was the most common event, and local reactions showed a marginally higher frequency in the VLA1553 arm by comparison with placebo (appendix pp 7, 8). Solicited systemic adverse events were significantly more common in the VLA1553 group than in the placebo group (1547 [50·2%] of 3082 participants in the VLA1553 arm and 278 [26·9%] of 1033 participants in the placebo arm, p<0·0001; appendix pp 7, 9). Adverse events in the VLA1553 group were mostly mild or moderate; 58 (1·9%) of 3082 participants experienced related severe adverse events (mainly fever [39 (1·3%) of 3082], followed by arthralgia [nine (0·3%) of 3082] and myalgia [eight (0·3%) of 3082; appendix p 7). Mean duration of solicited adverse events after receiving VLA1553 ranged from 1·9 days (induration) to 6·0 days (rash), and most solicited adverse events resolved within 2–3 days. 303 (9·8%) of 3082 participants who received VLA1553 had unsolicited adverse events deemed related to VLA1553 treatment up to day 180, compared with 48 (4·6%) of 1033 participants who received placebo (appendix p 10). There were no clinically relevant changes in laboratory parameters over time (haematology, clinical chemistry, or coagulation). Transient, minor changes in haematology parameters were considered expected and consistent with a physiologic response to a live-attenuated viral vaccine, and were not considered clinically significant.
Discussion
The study met its primary endpoint. 28 days post-vaccination, VLA1553 induced seroprotective levels of antibodies in 98·9% of participants, and high seroprotection rates were sustained up to 180 days after vaccination, with GMT indicating high immunogenicity in adults of all age groups. The generation of protective titres in virtually all vaccinated participants independent of age positions VLA1553 as an excellent candidate for the prevention of chikungunya. Despite some centres located in Florida, USA, where sporadic infections with chikungunya virus have been reported and a few participants with chikungunya virus positive antibody titres at baseline in the study population, negative serology results in the entire immunogenicity subset excluded bias through natural infection during the trial.
In this trial, different antibody kinetics were observed in comparison to phase 1 results in which titres seemed to remain stable from day 29 to day 180 post-vaccination.12 This observation is probably due to differences in assays (μPRNT versus μNT). Similar antibody kinetics as observed in this phase 3 trial were also reported for yellow fever vaccine (17DD-YFV),19 which has long lasting immunity.20 Further data are being generated to monitor seroprotection beyond this period as we expect this vaccine to induce long-lasting immunity.
VLA1553 was generally safe and well tolerated in all populations after a single vaccination. An independent DSMB evaluated safety data for the study duration and did not identify any safety concerns after evaluating all classes of reported adverse events. The two related SAEs reported during the study both recovered fully and were reviewed by the DSMB who did not raise concerns or consider serious risks caused by the vaccination in general.
The observed rate of miscarriages (23·1% of reported pregnancies) is slightly higher than those which typically occur in the general population (about 11–16%)21, 22, 23 or in women vaccinated with mRNA COVID-19 vaccine (14·1%),24 but this could be due to the small sample size. Two of the miscarriages were explained by a genetic disorder of the fetus (Turner syndrome 45 X) or the participant's predisposition (BMI of 60 and two previous miscarriages); in one case no reason could be identified. The DSMB discussed all reported miscarriages in detail and could not identify any safety concerns. Miscarriage after chikungunya virus infection is rarely reported in literature. By contrast with infection with Zika virus, for example, chikungunya virus infection is not associated with miscarriage or negative pregnancy outcomes on an epidemiological level.25
The safety profile was consistent with phase 1 trial results,12 results from this study, and lot-to-lot bioequivalence trial day 29 analyses (NCT04786444). The yellow fever vaccine YF-VAX reported almost identical rates of headache (31·4%; VLA1553 31·4%), fatigue (29·5%; VLA1553 28·5%), myalgia (25·1%, VLA1553 23·8%), and fever (15·0%; VLA1553 13·4%), but a higher incidence of nausea (22·9%; VLA1553 11·2%). Severe solicited adverse events were reported in less than 1% of participants receiving YF-VAX (VLA1553 2·1%).26 The profile of solicited injection site reactions of VLA1553 compares favourably with mRNA vaccines such as COMIRNATY, with similar frequencies of redness and swelling but significantly lower injection site pain (88·6%; VLA1553 6·0%), which also applies to common solicited systemic adverse events such as headache, fatigue, or chills, but not to fever. Even the frequency of arthralgia differs marginally (21·5–27·5% in COMIRNATY; VLA1553 16·9%).27, 28 Although yellow fever and COVID-19 are more lethal diseases than chikungunya, these comparisons might help contextualise the safety profile of VLA1553.
Chikungunya virus is regarded as one of the viruses most likely to spread globally, thus raising an urgent demand for efficient prophylaxis.2, 5, 13 Given the unpredictable epidemiology of chikungunya, the conduct of a typical vaccine efficacy trial to demonstrate disease prevention is regarded as unfeasible both economically and logistically (the FDA reached this conclusion after the VRBPAC meeting in November, 2019).29 For the licensure pathway, a serological surrogate of protection was agreed with regulators, based on data derived from passive human sera transfer studies in non-human primates, to allow evaluation of vaccine effectiveness against chikungunya virus.14 A very conservative titre (μPRNT50 ≥150) was defined based on passive transfer studies,14 further supported by the protective levels of chikungunya virus neutralising antibodies obtained in a field study from the Philippines.14, 30 The convalescent serum samples obtained from this cohort study measured with our μPRNT assay (GMT 1341, min–max 170–5297; n=39)14 were in a similar range as titres observed following VLA1553 vaccination.
Our study had several limitations. First, the study did not take place in an endemic region and therefore the effect of pre-existing immunity on VLA1553 immunogenicity is unknown, as is the full safety profile in this population. Second, the vaccine is based on a live, attenuated virus platform and therefore will probably not be usable in severely immunocompromised groups; any use during pregnancy will have to weigh the serious risks to newborn babies from perinatal chikungunya transmission against the general practice of avoiding or contraindicating live-attenuated vaccines during pregnancy. Both pregnant and severely immunocompromised people were excluded from the trial as a precautionary measure. Third, immunogenicity was determined in a small subset of participants. However, the per-protocol population analysed for the immunogenicity endpoints was representative of the whole study population when comparing demographic data. Despite the small immunogenicity subset, the very narrow 95% CI was sufficient to meet the primary study endpoint and the findings have since been confirmed in a lot-to-lot consistency study in 408 adults aged 18–45 years (NCT04786444). Fourth, to be highly effective in controlling endemic disease, a chikungunya vaccine must also be administered to children. As a first step in this direction, a study in adolescents has been initiated including endemic areas of Brazil (NCT04650399). Fifth, the follow-up period of 6 months does not allow durability of the immune response to be predicted. A follow-up study is underway to monitor the immune response over 5 years, with encouraging results 1 year after vaccination (NCT04838444).31 Finally, this study does not include clinical endpoints, which will only be attainable in large post-marketing settings.
No treatment or vaccine is available against chikungunya virus-induced debilitating disease, its various symptoms, or long-term sequelae. This live-attenuated vaccine is intended to be used for active immunisation to protect from chikungunya. As age is a risk factor for severity and mortality after chikungunya virus infection, the strong immune response and favourable safety profile observed in older adults could be of relevance. A live-attenuated chikungunya virus vaccine holds the promise of providing long-term protection after a single immunisation, an important feature due to limitations of compliance with booster doses, in light of repeated exposure (eg, through repeat travel), and for intervention in sporadic endemic outbreaks.
Data sharing
All data that underlie the results reported in this article (including study protocol) on individual participants will be made available after publication to researchers who provide a methodologically sound proposal by application to the corresponding author.
Declaration of interests
All authors (except RMa and JLS) are Valneva employees and own stock and share options of Valneva. RH is an inventor in a patent relevant to the work. RMa and JLS are consultants of Valneva and have received payments.
Acknowledgments
Acknowledgments
This study was funded by Valneva Austria. In addition, we acknowledge the Coalition for Epidemic Preparedness Innovation and EU Horizon 2020 for partially funding the VLA1553 development programme. We thank the independent data safety monitoring board and its members (Herwig Kollaritsch, Lin Chen, Eva-Maria Poellabauer) for their role in this study. We also want to acknowledge the very important contributions made to our VLA1553 programme by Prof Fabrice Simon and Prof Frank Sonnenburg, whose valuable input we will miss greatly. We thank the team at Nexelis, a Q2 Solutions company, especially Luc Gagnon, Julien St-Jean, and Chantal Arguin that did the analysis of neutralising antibodies for this study. Valneva thanks all investigators, site staff, and participants for contributing to this study.
Contributors
MS, SH, RMM, RMa, KD, SE-L, and NW contributed to study concept and design. MS, MN-A, SH, ST, UF, and OZ supervised the study. RH, AB, and KK supervised the serological analyses. MS, MN-A, RMM, JL-S, RMa, KD, OZ, J-CJ, SE-L, and VB analysed and interpreted data. SH and VB verified the data presented in the manuscript. All authors had access to study data reported in the manuscript, contributed to the drafting and revision of the manuscript, approved the final version, and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Suhrbier A, Jaffar-Bandjee MC, Gasque P. Arthritogenic alphaviruses—an overview. Nat Rev Rheumatol. 2012;8:420–429. doi: 10.1038/nrrheum.2012.64. [DOI] [PubMed] [Google Scholar]
- 2.Simon F, Savini H, Parola P. Chikungunya: a paradigm of emergence and globalization of vector-borne diseases. Med Clin North Am. 2008;92:1323–1343. doi: 10.1016/j.mcna.2008.07.008. ix. [DOI] [PubMed] [Google Scholar]
- 3.Vairo F, Haider N, Kock R, Ntoumi F, Ippolito G, Zumla A. Chikungunya: epidemiology, pathogenesis, clinical features, management, and prevention. Infect Dis Clin North Am. 2019;33:1003–1025. doi: 10.1016/j.idc.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Hochedez P, Jaureguiberry S, Debruyne M, et al. Chikungunya infection in travelers. Emerg Infect Dis. 2006;12:1565–1567. doi: 10.3201/eid1210.060495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manzoor KN, Javed F, Ejaz M, et al. The global emergence of chikungunya infection: an integrated view. Rev Med Virol. 2022;32 doi: 10.1002/rmv.2287. [DOI] [PubMed] [Google Scholar]
- 6.Couderc T, Khandoudi N, Grandadam M, et al. Prophylaxis and therapy for Chikungunya virus infection. J Infect Dis. 2009;200:516–523. doi: 10.1086/600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabié A, Ledrans M, Abel S. Chikungunya virus infections. N Engl J Med. 2015;373:94. doi: 10.1056/NEJMc1505501. [DOI] [PubMed] [Google Scholar]
- 8.Simon F, Javelle E, Cabie A, et al. French guidelines for the management of chikungunya (acute and persistent presentations) Med Mal Infect. 2015;45:243–263. doi: 10.1016/j.medmal.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Sissoko D, Malvy D, Ezzedine K, et al. Post-epidemic Chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl Trop Dis. 2009;3:e389. doi: 10.1371/journal.pntd.0000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver SC, Osorio JE, Livengood JA, Chen R, Stinchcomb DT. Chikungunya virus and prospects for a vaccine. Expert Rev Vaccines. 2012;11:1087–1101. doi: 10.1586/erv.12.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roques P, Ljungberg K, Kümmerer BM, et al. Attenuated and vectored vaccines protect nonhuman primates against Chikungunya virus. JCI Insight. 2017;2 doi: 10.1172/jci.insight.83527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wressnigg N, Hochreiter R, Zoihsl O, et al. Single-shot live-attenuated chikungunya vaccine in healthy adults: a phase 1, randomised controlled trial. Lancet Infect Dis. 2020;20:1193–1203. doi: 10.1016/S1473-3099(20)30238-3. [DOI] [PubMed] [Google Scholar]
- 13.Bettis AA, L'Azou Jackson M, Yoon IK, et al. The global epidemiology of chikungunya from 1999 to 2020: a systematic literature review to inform the development and introduction of vaccines. PLoS Negl Trop Dis. 2022;16 doi: 10.1371/journal.pntd.0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roques P, Fritzer A, Dereuddre-Bosquet N, et al. Effectiveness of CHIKV vaccine VLA1553 demonstrated by passive transfer of human sera. JCI Insight. 2022;7 doi: 10.1172/jci.insight.160173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical [DOI] [PubMed]
- 16.Gorchakov R, Wang E, Leal G, et al. Attenuation of Chikungunya virus vaccine strain 181/clone 25 is determined by two amino acid substitutions in the E2 envelope glycoprotein. J Virol. 2012;86:6084–6096. doi: 10.1128/JVI.06449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison VR, Eckels KH, Bartelloni PJ, Hampton C. Production and evaluation of a formalin-killed Chikungunya vaccine. J Immunol. 1971;107:643–647. [PubMed] [Google Scholar]
- 18.Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE, Jr, Lupton HW. Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine. 1986;4:157–162. doi: 10.1016/0264-410x(86)90003-4. [DOI] [PubMed] [Google Scholar]
- 19.Martins RM, Maia ML, Farias RHG, et al. 17DD yellow fever vaccine: a double blind, randomized clinical trial of immunogenicity and safety on a dose-response study. Hum Vaccin Immunother. 2013;9:879–888. doi: 10.4161/hv.22982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roukens AHE, van Halem K, de Visser AW, Visser LG. Long-term protection after fractional-dose yellow fever vaccination: follow-up study of a randomized, controlled, noninferiority trial. Ann Intern Med. 2018;169:761–765. doi: 10.7326/M18-1529. [DOI] [PubMed] [Google Scholar]
- 21.Lang K, Nuevo-Chiquero A. Trends in self-reported spontaneous abortions: 1970–2000. Demography. 2012;49:989–1009. doi: 10.1007/s13524-012-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ. 2019;364:l869. doi: 10.1136/bmj.l869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossen LM, Ahrens KA, Branum AM. Trends in risk of pregnancy loss among US women, 1990–2011. Paediatr Perinat Epidemiol. 2018;32:19–29. doi: 10.1111/ppe.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA COVID-19 vaccines preconception and during pregnancy and risk of self-reported spontaneous abortions, CDC v-safe COVID-19 Vaccine Pregnancy Registry 2020–21. Res Sq. 2021 doi: 10.21203/rs.3.rs-798175/v1. published online Aug 9, 2022. (preprint). [DOI] [Google Scholar]
- 25.CDC Chikungunya. https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/chikungunya
- 26.Monath TP, Nichols R, Archambault WT, et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a phase III multicenter, double-blind clinical trial. Am J Trop Med Hyg. 2002;66:533–541. doi: 10.4269/ajtmh.2002.66.533. [DOI] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration Package insert - COMIRNATY (purple cap) https://www.fda.gov/media/151707/download
- 28.CHMP Comirnaty, INN-tozinameran, tozinameran/riltozinameran, tozinameran/famtozinameran. https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf
- 29.US Food and Drug Administration Vaccines and Related Biological Products Advisory Committee November 8, 2019 meeting announcement - 11/08/2019–11/08/2019. https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-november-8-2019-meeting-announcement
- 30.Yoon IK, Alera MT, Lago CB, et al. High rate of subclinical chikungunya virus infection and association of neutralizing antibody with protection in a prospective cohort in the Philippines. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valneva Valneva reports positive 12-month antibody persistence data for single-shot chikungunya vaccine candidate. https://valneva.com/press-release/valneva-reports-positive-12-month-antibody-persistence-data-for-single-shot-chikungunya-vaccine-candidate/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that underlie the results reported in this article (including study protocol) on individual participants will be made available after publication to researchers who provide a methodologically sound proposal by application to the corresponding author.