Abstract
Background
With the recent success of immunotherapy, there is a growing interest in combining radiation with immunotherapy to boost abscopal response rates. Several challenges exist in determining how to synergize these two modalities in the treatment of metastatic NSCLC.
Methods
References for this review were identified through searches of MEDLINE/PubMed and Clinicaltrials.gov databases with the search terms “abscopal”, “radiation OR radiotherapy,” “NSCLC”, and “lung” on the index date of July 2022 from 2000-2022. This systematic review focuses primarily on clinical papers.
Discussion
Early work combining radiotherapy with immunotherapy show promise in unlocking the abscopal effect. Preliminary evidence suggests that radiotherapy regimens with <5 fractions and smaller fields may be superior to regimens with 15 fractions and larger fields. There does not appear to be enough evidence to draw conclusions about the optimal timing of radiotherapy in relation to immunotherapy or the optimal anatomical location of radiation to induce the abscopal effect. Several studies suggest selecting patients with a higher absolute lymphocyte count (ALC) and lower neutrophil-to-lymphocyte ratio (NLR) may help to further boost abscopal response rates. Furthermore, selecting tumors with programmed death ligand-1 (PD-L1) expression, mismatch repair deficiency, and higher tumor mutational burden may similarly achieve this goal. Lastly, additional work is needed to minimize and predict for severe toxicity associated with combination therapy.
Keywords: Radiation, Radiotherapy, Immunotherapy, Synergy, Challenges, Combination therapy
Introduction
The abscopal effect is a phenomenon in which irradiation of a single tumor causes a regression of tumor(s) outside the field of irradiation [1]. This phenomenon was first described by Mole in 1953 [2]. While the mechanism remains incompletely characterized, it is believed to be immune-related. Based on several in vivo studies [3], [4], [5], [6], [7], radiation is hypothesized to cause the release of tumor antigens that subsequently activate dendritic cells, which migrate to the draining lymph nodes and prime antigen-specific effector T cells that then attack tumor cells distant to the site of irradiation.
Despite advancements in our understanding of the immunological effects of radiation, and the promise of unlocking the abscopal effect when combining radiation with immunotherapy, abscopal responses are rarely seen in the clinic and several challenges remain in how to optimize the synergy between these two modalities. Several of challenges are radiation-related, e.g., determining the optimal dose and fractionation, the optimal timing of radiation in relation to immunotherapy, and the optimal anatomical location of radiation. Furthermore, optimizing patient and tumor selection in order to best augment the abscopal effect remains challenging. Finally, managing the toxicities associated with combination therapy is another obstacle for clinicians to overcome. The purpose of this narrative review is to summarize the challenges in synergizing radiation with immunotherapy, specifically in the setting of metastatic non-small cell lung cancer (NSCLC).
Methods
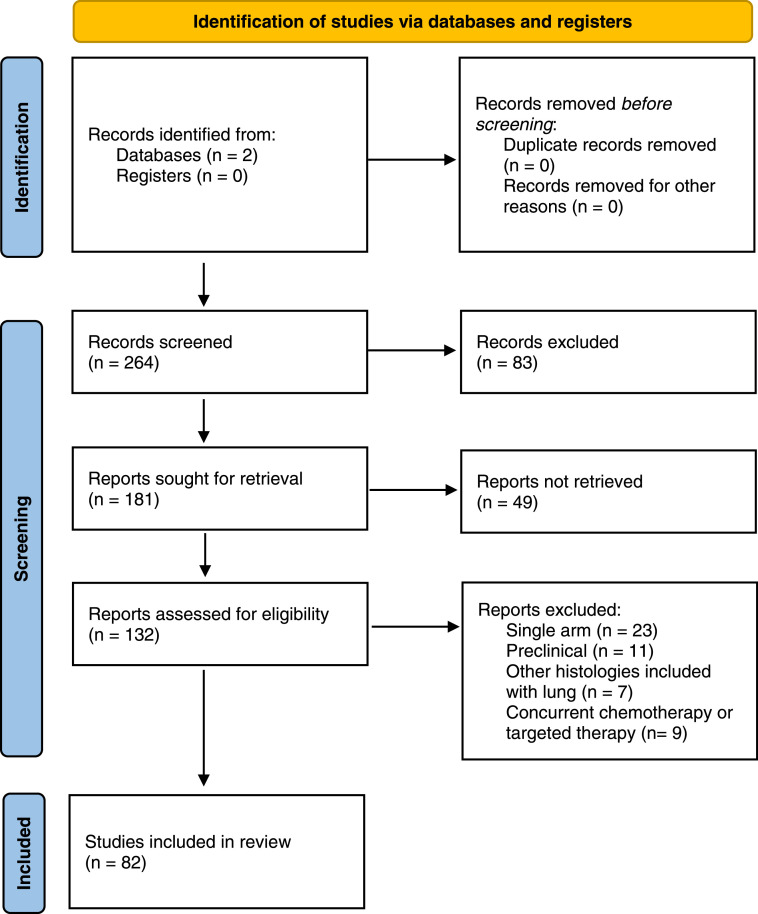
References for this review were identified through searches of MEDLINE/PubMed and Clinicaltrials.gov databases with the search terms “abscopal”, “radiation OR radiotherapy,” “NSCLC”, and “lung” on the index date of July 2022 from 2000-2022. Clinical papers were included only if they had two arms, one involving immunotherapy plus radiation and the other involving immunotherapy alone, in order to evaluate the effect of adding radiation to a systemic immunotherapy. Single arm studies were omitted because it is challenging to elucidate whether radiation has additive or synergistic benefit with single arm radiation-immunotherapy studies since immune checkpoint inhibitors have variable monotherapy response rates that depend on the tumor type, patient and tumor characteristics, as well as patients’ germline polymorphisms. Abstracts and case reports were also omitted. Papers were omitted if whole body irradiation was used or if patients were concurrently treated with cytotoxic chemotherapy or targeted therapy. References of selected clinical and preclinical papers were also screened for additional papers that met the predetermined selection criteria. A PRISMA flow diagram for our systematic review is shown in Figure 1.
Fig. 1.
PRISMA flow diagram.
Discussion
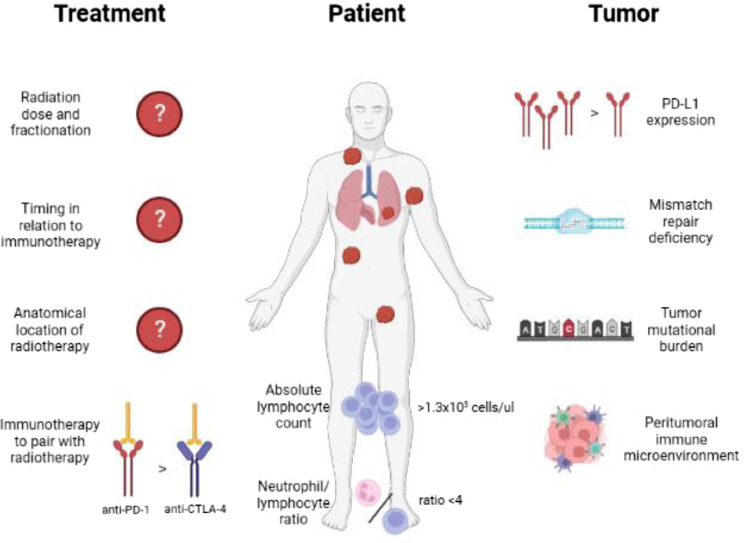
A summary of pivotal trials discussed in this review is shown in Table 1. A graphical representation of variables influencing the abscopal effect is shown in Figure 2.
Table 1.
Summary of pivotal studies combining radiation and immunotherapy in metastatic NSCLC.
| Design | Patients | Treatment | Results | |
|---|---|---|---|---|
| Theelen et al. [8] | Pooled analysis of two phase I/II trials | 148 | Pembrolizumab ± RT | Higher ARR with pembrolizumab + RT vs. pembrolizumab alone (42% vs. 20%, p<0.01); higher ARR with 24 Gy/3 fx and 50 Gy/4 fx vs. 45 Gy/15 fx (47% vs. 56% vs. 20%, p<0.05) |
| Pevzner et al. [29] | Metanalysis of 35 studies | 51 | RT to metastatic site | Highest incidence of abscopal response in lung (41%) > lymph nodes (31%) > liver (16%) |
| Chen et al. [36] | Pooled analysis of two phase I/II trials | 33 | Pembrolizumab + RT vs. ipilimumab + RT | Higher ARR with pembrolizumab + RT vs. ipilimumab + RT (37% vs. 24%, p=0.05); higher PFS with pembrolizumab + RT vs. ipilimumab + RT (63% vs. 23% at 18 months, p=0.02) |
| KEYNOTE-598 [39] | Phase III | 568 | Pembrolizumab ± ipilimumab | No PFS benefit with pembrolizumab + ipilimumab vs. pembrolizumab alone (8.2 vs. 8.4 months, p=0.72); worse grade 3-5 toxicity (62% vs. 50%, p<0.05) |
| Chen et al. [41] | Pooled analysis of three phase I/II trials | 165 | Immunotherapy + RT | Higher ARR with baseline ALC >1.3 × 103 cells/μl vs. <1.3 × 103 cells/μl (30% vs. 8%, p<0.01); higher ARR with post-treatment ALC >0.56 × 103 cells/μl vs. <0.56 × 103 cells/μl (34% vs. 4%, p<0.01) |
| Golden et al. [43] | Phase II | 41 | GM-CSF + RT | Patients who had abscopal response had lower baseline NLR vs. patients who did not (2.29 vs. 4.24) |
| Formenti et al. [50] | Phase II | 21 | Ipilimumab + RT | Higher proportion of patients with progressive disease had EGFRm tumors vs. patients who had better disease control (50% vs. 0%, p=0.03) |
ARR=abscopal response rate, PFS=progression free survival, ALC=absolute lymphocyte count, GM-CSF=granulocyte-macrophage colony-stimulating factor, NLR=neutrophil-to-lymphocyte ratio, EGFRm=epidermal growth factor receptor mutated
Fig. 2.
Variables influencing the abscopal effect.
Dose and fractionation
There is limited prospective evidence examining the optimal radiotherapy schedule when combined with immunotherapy in metastatic NSCLC. Recently, a pooled analysis [8] of two phase I/II trials [9,10] found a significant increase in the abscopal (out-of-field) response rate (ARR) in 148 patients with metastatic NSCLC treated with pembrolizumab plus radiation vs. pembrolizumab alone (42% vs. 20%, p<0.01). Patients treated with radiation also had a significant improvement in progression free survival (PFS) and overall survival (OS), likely due to improved systemic disease control. Radiation was directed to up to 4 lesions and directed primarily to intrathoracic disease, with doses and fractionations ranging from 45 Gy in 15 fractions, 24 Gy in 3 fractions, to 50 Gy in 4 fractions. Although the combined analysis was not powered to compare radiotherapy schedules, there was a striking difference in ARR in the 45 Gy in 15 fraction arm compared to the 24 Gy in 3 fraction and 50 Gy in 4 fraction arms with 20% vs. 47% vs. 56%, respectively.
It remains unknown if the difference in ARR observed in the 45 Gy in 15 fraction arm was due to differences in the absolute lymphocyte count or lymphocyte subsets, radiation fractionations causing variable antigen release, unforeseen clinical factors associated with the choice of radiotherapy schedule, or other patient and tumor factors that were unaccounted for. Lymphocytes such as T cells play an important role in an effective antitumor immune response, but they are also exquisitely radiosensitive and likely to undergo radiation-induced lymphocyte apoptosis [11]. Patients treated with 45 Gy in 15 fractions had a significant drop in absolute lymphocyte count compared to patients in the other two arms, suggesting a detrimental effect of this fractionation. Prior evidence has suggested that patients treated with protracted regimens are less likely to have an abscopal response due to the decreased availability of effector and memory T cells [12]. Even with smaller, more conformal treatment fields, protracted regimens have been shown to deliver lymphotoxic doses and exhaust Tcells [13], hindering their ability to produce an abscopal response. It is likely that patients in the 24 Gy in 3 fraction and the 50 Gy in 4 arm had less T cell exhaustion from shorter courses and smaller fields compared to patients in the 45 Gy in 15 fraction arm, resulting in improved ARR rates.
Ultimately, data regarding the optimal dose and fractionation to induce the abscopal effect in NSCLC are lacking. Additional studies are needed in order to clarify the optimal method in which radiation is delivered. Potential areas for further research include conventional fractionation vs. hypofractionation, daily vs. every other day (QOD) fractionation, using photons vs. protons, what total dose to prescribe to, and whether ablative doses (biological equivalent dose (BED) >100 Gy) are required to produce the abscopal effect [14], [15], [16].
Timing of radiation in relation to immunotherapy
The aforementioned pooled analysis [8] of two phase I/II trials [9,10] utilized different timing of radiotherapy and immunotherapy between the two trials. The first trial (PEMBRO-RT) [9] treated patients with 24 Gy in 3 fractions to a single lesion followed by pembrolizumab within 7 days of completion. The second trial (MDACC) [10] treated patients with either 45 Gy in 15 fractions or 50 Gy in 4 fractions to 1-4 lesions concurrent with pembrolizumab. The ARR was 36% in PEMBRO-RT (sequential timing) vs. 38% in the MDACC trial (concurrent timing). Given several differences in the design of the two trials, conclusions regarding the optimal timing of radiotherapy in relation to immunotherapy cannot be drawn.
The landmark PACIFIC [17,18] trial for patients with stage III unresectable NSCLC found that the addition of consolidation durvalumab significantly improved PFS and OS over placebo after definitive chemoradiation. While the study did not directly assess the difference between radiation and immunotherapy sequencing, there was an important trend of improved responses for patients who received durvalumab closer to the chemoradiation administration. In the latest 5-year update, there was a PFS and OS benefit for patients who were randomized <14 days from chemoradiation compared to >14 days. This indicates that starting immunotherapy closer to end of radiation may better harness an anti-tumor immune response. However, this may also be confounded by the fact that patients who received durvalumab later were also likely sicker and needed more time to recover from chemoradiation, which would be a confounding factor. Several recent phase II trials [19], [20], [21] have promising results for concurrent delivery of immunotherapy with chemoradiation in stage III disease, with a (nonsignificant) increase of PFS and OS rates in comparison to the PACIFIC trial. The PACIFIC-2 [22] trial is currently underway to examine the benefit of concurrent immunotherapy in stage III NSCLC.
Interestingly, sequential therapy may be preferred over concurrent therapy in tumors with negative (programmed death ligand-1) PD-L1 expression. In the PEMBRO-RT [9] trial, sequential therapy was delivered, with initiation of pembrolizumab <7 days after completion of stereotactic body radiation therapy (SBRT). Subgroup analysis showed the largest benefit from the addition of radiotherapy in patients with PD-L1 negative tumors with respect to PFS (HR 0.49, 95% CI 0.26-0.94, p=0.03). Mechanistically, the benefit may be due to the ability of radiation to lyse tumor cells releasing tumor antigens, raise intra-tumoral PD-L1 levels, and enhance the immune response in patients treated with PD-1/PD-L1 inhibitors [23,24]. The phase II/III Alliance A082002 [25] is currently underway to test whether the addition of SBRT to a single tumor site will enhance the anti-tumor activity of systemic immunotherapy or chemo-immunotherapy in patients with stage IV PD-L1(-) NSCLC. Further investigation is needed to better clarify the optimal timing of radiation in relation to immunotherapy in various clinical situations.
Location of radiotherapy
Patients in the combined analysis [8] of two phase II trials [9,10] received radiotherapy to various sites of disease. The most common location was lung metastasis (N=28), followed by intrathoracic or extrathoracic lymph node metastases (N=22), followed by other sites of metastases including adrenal, bone, skin, liver, and retroperitoneum (N=17), followed by lung primary (N=12). The ARR was not compared between the various sites of irradiation; however, PFS and OS were not significantly different between each subgroup. It remains unknown if location of radiotherapy matters when attempting to induce the abscopal effect.
It has been demonstrated that genomic and immune heterogeneity is common among metastatic sites, affecting antigenic composition which influences response to immunotherapy [26], [27], [28]. Inducing the abscopal effect is likely to be hindered by the fact that different metastases may not share common antigens. Despite this, certain metastatic sites may be more likely to respond via the abscopal effect than others. A metanalysis [29] of 35 clinical studies describing 51 cases of the abscopal effect found that the most common sites for its occurrence were lung (41%), lymph nodes (31%), and liver (16%). This suggests that radiation should be preferentially targeted to lesions in the lung and liver since these organs are inherently more immunogenic. This could be due to differences in systemic T cell and natural killer cell (NK cell) subsets in the peripheral blood after stereotactic body radiation therapy to the lung and liver as compared to the brain and bone [30]. However, this study was conducted in patients who did not received immunotherapy, and additional work is needed to define the optimal anatomical location to irradiate in order to enhance immune response in combination with immunotherapy.
An alternative approach to inducing the abscopal effect may be to irradiate as many sites of disease as feasible to release the greatest tumor antigen burden. This is based on a landmark translational study showing that the amount of reinvigoration of circulating exhausted T CD8+ cells vs. pretreatment tumor burden correlated with clinical response [31]. Delivering SBRT to as many as 5 lesions was demonstrated to be safe and feasible in patients with oligometastatic disease in the phase II SABR-COMET [32,33] trial. Further studies are warranted to determine whether irradiating >5 lesions is safe and effective. SABR-COMET 10 [34] is currently underway to examine the safety and efficacy of SBRT to 4-10 sites of disease using a broader definition of oligometastatic cancers, and the ARREST [35] trial is currently underway to examine the safety and feasibility of SBRT to all sites of disease in patients with polymetastatic cancers (>10 lesions).
Optimal immunotherapy to combine with radiotherapy
There is currently no randomized study comparing the combination of radiotherapy with various immunotherapy agents. A retrospective analysis [36] of 2 single-institution prospective studies [37,38] compared the response rates and outcomes of combining radiotherapy with pembrolizumab or ipilimumab. A total of 16 patients were treated with SBRT + pembrolizumab and a total of 17 patients were treated with SBRT + ipilimumab. Response rates for out-of-field lesions were similar between pembrolizumab and ipilimumab (37% vs. 24%, p=0.054). The PFS for pembrolizumab vs. ipilimumab was 94% vs. 76% at 3 months, 87% vs. 52% at 6 months, 80% vs. 31% at 12 months, and 63% vs. 23% at 18 months (p=0.02). Respective OS values were 87% vs. 76% at 6 months, 80% vs. 47% at 12 months, and 66% vs. 39% at 18 months (p=0.08). Authors concluded that both pembrolizumab and ipilimumab prompt a similar degree of in-field and out-of-field response after SBRT, although the global response rate and PFS were significantly higher with pembrolizumab than ipilimumab after SBRT. Randomized prospective evidence is needed to validate these findings. One benefit of PD-1/PD-L1 inhibitors over cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors is the lower toxicity profile of PD-1/PD-L1 inhibitors, with lower rates of colitis but higher rates of pneumonitis.
Further studies are also needed to determine what type of PD-1/PD-L1 inhibitor is best combined with radiotherapy (e.g., pembrolizumab, nivolumab, atezolizumab) to induce the abscopal effect. Currently, there are no studies comparing various PD-1/PD-L1 inhibitors with radiation, and this study is unlikely because each PD-1/PD-L1 inhibitor is made by a different pharmaceutical company. Further studies are needed to determine whether a combination of PD-1/PD-L1 inhibitors with CTLA-4 inhibitors is able to further boost response rates when paired with radiation. The recently published phase III KEYNOTE-598 [39] trial showed no clinical benefit and greater toxicity when combining ipilimumab with pembrolizumab vs. pembrolizumab alone in patients with metastatic NSCLC with PD-L1 expression ≥50%, so it is unlikely that we will see such a trial take place. A recent phase II study [40] combining PD-L1 and CTLA-4 inhibition with targeted low dose or hypofractionated radiation for patients with metastatic MSS (microsatellite stable) colorectal cancer did show that combined treatment is safe and feasible, although 84% of patients did experience toxicity, of which 42% had grade 3-4 toxicity. A summary of ongoing studies examining the combination of radiation and immunotherapy in metastatic NSCLC is shown in Table 2.
Table 2.
Summary of ongoing studies combining radiation and immunotherapy in metastatic NSCLC.
| Phase | Patients | Treatment | Primary outcome | |
|---|---|---|---|---|
| NCT03158883 [67] | I | 8 | Avelumab + SBRT 50 Gy/5 fx | ORR |
| NCT03224871 [68] | I | 3 | Nivolumab + pembrolizumab + intratumor IL-2 + SBRT 24 Gy/3 fx | MTD |
| NCT03436056 (PRIMING) [69] | I | 2 | Pembrolizumab + SBRT 30 Gy/3 fx or 54 Gy/3 fx | MTD |
| NCT03812549 [70] | I | 29 | Sintilimab + SBRT 30 Gy/3 fx + low dose RT 2 Gy/1 fx or 4 Gy/2 fx or 10 Gy/5 fx | MTD |
| NCT03223155 (COSINR) [71] | I | 80 | Ipilimumab + nivolumab + SBRT 3-5 fx, 2-4 sites | MTD |
| NCT02639026 [72] | I | 30 | Durvalumab + tremelimumab + SBRT 24 Gy/3fx or 17 Gy/1 fx | MTD |
| NCT03275597 [73] | I | 17 | Durvalumab + tremelimumab + SBRT 30-50 Gy/5 fx | MTD |
| NCT02444741 [37] | I/II | 104 | Pembrolizumab + SBRT 4 fx or HFRT 15 fx | MTD, ORR |
| NCT02239900 [38] | I/II | 143 | Ipilimumab + SBRT 50 Gy/4 fx or HFRT 60 Gy/10 fx | MTD |
| NCT03168464 [74] | I/II | 15 | Ipilimumab + nivolumab + SBRT 30 Gy/5 fx | ORR |
| NCT02221739 [75] | I/II | 39 | Ipilimumab + SBRT 30 Gy/5 fx | ORR |
| NCT03176173 (RRADICAL) [76] | II | 44 | Nivolumab + pembrolizumab + atezolizumab + SBRT or HFRT 1-10 fx | PFS |
| NCT03965468 (CHESS) [77] | II | 48 | Durvalumab + chemotherapy + SBRT or HFRT 1-10 fx | PFS |
| NCT03044626 (FORCE) [78] | II | 101 | Nivolumab + RT 20 Gy/5 fx | ORR |
| NCT02658097 [79] | II | 13 | Pembrolizumab + RT 8 Gy/1 fx | ORR |
| NCT04929041 (Alliance A082002) [25] | II/III | 100 | Nivolumab ± chemotherapy ± SBRT | PFS, OS |
| NCT03391869, (LONESTAR) [80] | III | 360 | Ipilimumab + nivolumab ± SBRT | OS |
| NCT03867175 [81] | III | 112 | Pembrolizumab ± SBRT or HFRT 3-10 fx | PFS |
| NCT03774732 (NIRVANA-LUNG) [82] | III | 460 | Pembrolizumab + chemotherapy ± SBRT 81 Gy/3 fx | OS |
SBRT=stereotactic body radiation therapy, HFRT=hypofractionated RT, ORR=overall response rate, MTD=maximum tolerated dose, PFS=progression free survival, OS=overall survival
Optimizing patient selection
Determining which patients will exhibit an abscopal effect and which patients will not has proven difficult. Given that the abscopal effect is based on immune activation, patients with immunosuppression or lymphopenia are less likely to achieve an abscopal response. A pooled analysis [41] of three phase I/II [10,36,42] of 153 patients with predominantly metastatic NSCLC found that absolute lymphocyte count (ALC) when analyzed as a continuous variable was significantly associated with abscopal responses on multivariate analysis (p<0.01). The ARR was 30.3% in patients with pre-radiation ALC above the median (1.3 × 103 cells/μl) vs. 7.8% in patients with ALC below the median (p<0.01). The ARR was 34.2% for patients with post-radiation ALC above the median (0.56 × 103 cells/μl), vs. 3.9% for patients whose post-radiation ALC was below the median (p<0.01).
The neutrophil-to-lymphocyte ratio (NLR) also appears to correlate with abscopal responses. A prospective study by Golden and colleagues [43] treated 41 patients with radiotherapy plus granulocyte-macrophage colony-stimulating factor (GM-CSF) and found that patients who had an abscopal response presented with a lower baseline NLR compared to patients who did not (2.29 vs. 4.24). This finding was confirmed by other studies, including one by Zucker and colleagues [44] which found that a lower baseline NLR <4 was associated with improved ARR and OS. Future studies investigating the combination of radiotherapy with immunotherapy should tailor the eligibility criteria to select patients with pre-treatment ALC >1.3 × 103 cells/μl and baseline NLR <4 in order to maximize the chances of an abscopal response.
Optimizing tumor selection
There is strong evidence that tumors with PD-L1 expression [45] and mismatch repair deficiency [46] have improved response to immunotherapy and may be more likely to induce an abscopal effect. Both PD-L1 expression and mismatch repair deficiency are imperfect markers that do not absolutely confirm or preclude a favorable response. Tumor mutational burden is usually considered as the primary predictor of neoantigen load, which is directly associated with tumoral immunoreactivity, and may influence the chances of achieving an abscopal effect include [47]. Tumors with epidermal growth factor receptor (EGFR) mutations have been shown to have a lower tumor mutational burden [48] and poorer response to immunotherapy [49] compared to tumors without these mutations. A prospective study by Formenti and colleagues [50] treated 21 patients with metastatic NSCLC with concurrent ipilimumab and SBRT and found that a higher proportion of patients with progressive disease had EGFR mutated tumors compared to patients who had better disease control (50% vs. 0%, p=0.03). Additional evidence is needed to determine whether tumors with EGFR mutations are poorer candidates for combination radiotherapy and immunotherapy.
Tumoral immunogenicity is not solely dependent on tumor mutational burden, but also on antigen presenting cells (like dendritic cells) and activated cytotoxic CD8+ T cells in the tumoral immune microenvironment. A tumor immune microenvironment with a higher degree of cytotoxic T cell infiltration and T cell activation has been associated with favorable response to immunotherapy, even in the absence of high tumor mutational burden [51]. Distinct orthogonal signatures, like chemokine expression, can be used as complementary proxies of the degree of tumoral T cell infiltration and activation. Further studies aiming to optimize tumor selection when inducing the abscopal effect should consider identifying tumors not only based on their tumor mutational burden, but also by using specific signatures indicative of tumor lymphocytic infiltration.
Toxicity associated with combined treatment
One concern with combining radiotherapy with immunotherapy is the additional toxicity associated with combined modality therapy. However, multiple early phase studies [43,52,53] have examined the safety of combining SBRT with immunotherapy, overall demonstrating favorable toxicity profiles. There were no grade 4 or higher toxicities observed in any of these early trials, and the most common grade 3 toxicity was anemia, unlikely to have been related to the effects of SBRT.
Several pooled analyses have also shown no significant increase in toxicities when adding radiotherapy to immunotherapy [54,55], while others have shown a marginal increase in toxicities [56]. The main overlapping toxicity with radiotherapy and pembrolizumab is pneumonitis [57]. The rate of grade 3 or higher pneumonitis associated with modern SBRT series is <5% [58], and the rate of grade 3 or higher pneumonitis with pembrolizumab in the KEYNOTE-001 trial was 2% [45]. A post-hoc analysis of the KEYNOTE-001 trial [59] found that patients who had preceding thoracic radiation prior to pembrolizumab had a higher rate of all pulmonary toxicities (13% vs. 1%, p<0.05), although there was no difference in the rate of grade 3 or higher severe pneumonitis (4% vs. 1%, p=0.44).
Despite this, providers should still be cautious to “do no harm”, especially in patients with metastatic disease not receiving curative therapy. There is evidence to support the use of intensity-modulated radiation therapy (IMRT) to reduce toxicity [60]. Other strategies such as deep inspiratory breath hold (DIBH) and image guidance (IGRT) may considerably reduce pulmonary toxicities and facilitate combination treatment [61]. Potential areas of further investigation include identifying biomarkers that can predict the occurrence of severe toxicity after the combination of radiotherapy and immunotherapy [62]. The ongoing PREMIS [63] study aims to discover the underlying mechanisms responsible for severe immune related adverse events and identify predictive biomarkers. Biomarkers currently under investigation include cytokines, immune-cell subsets, autoantibodies, human leukocyte antigen haplotype, and radiomic characterization [64]. Other ongoing studies are investigating the reduction of immunotherapy-related side effects through the use immunosuppressive drugs such as rituximab and tocilizumab [65]. Radiation can also cause adverse events similar to immunotherapy when non-tumor specific antigens are released into the tissue microenvironment, and taken up by antigen presenting cells that prime auto-reactive T cells to attack normal tissue [66]. Therefore, further studies are needed to predict adverse events related to the combination of immunotherapy and radiotherapy.
Conclusion
Early clinical work combining radiotherapy with immunotherapy shows promise in unleashing the abscopal effect. Preliminary evidence suggests that radiotherapy regimens with <5 fractions and smaller fields may be superior to regimens with 15 fractions and larger fields. There does not appear to be enough evidence to draw conclusions about the optimal timing of radiotherapy in relation to immunotherapy or the optimal anatomical location of radiation to induce the abscopal effect. Several studies suggest selecting patients with a higher ALC and lower NLR may help to further boost abscopal response rates. Furthermore, selecting tumors with PD-L1 expression, mismatch repair deficiency, and higher tumor mutational burden may similarly achieve this goal. Additional work is needed to minimize and predict which patients will develop severe toxicity associated with combination therapy. These challenges must be overcome in order to help convert the abscopal effect from a rare phenomenon to more common entity.
Funding
None
CRediT author statement
(I) Conception and design: All authors.
(II) Administrative support: NA.
(III) Provision of study materials or patients: NA.
(IV) Collection and assembly of data: NA.
(V) Data analysis and interpretation: NA.
(VI) Manuscript writing: All authors.
(VII) Final approval of manuscript: All authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Demaria S., Formenti S.C. The abscopal effect 67 years later: from a side story to center stage. Br. J. Radiol. 2020;93(1109) doi: 10.1259/bjr.20200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mole R. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953;26(305):234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 3.Walle T., Monge R.M., Cerwenka A., Ajona D., Melero I., Lecanda F. Radiation effects on antitumor immune responses: current perspectives and challenges. Ther. Adv. Med. Oncol. 2018;10 doi: 10.1177/1758834017742575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brix N., Tiefenthaller A., Anders H., Belka C., Abscopal L.K. immunological effects of radiotherapy: narrowing the gap between clinical and preclinical experiences. Immunol. Rev. 2017;280(1):249–279. doi: 10.1111/imr.12573. [DOI] [PubMed] [Google Scholar]
- 5.Formenti S.C., Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J. Natl. Cancer Inst. 2013;105(4):256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J., Mackley H.B. Combining immunotherapy with radiation therapy to induce the abscopal response: what clinical and treatment variables matter? Appl. Rad. Oncol. 2019;8(1):14–19. [Google Scholar]
- 7.Demaria S., Ng B., Devitt M.L., et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004;58(3):862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Theelen W.S.M.E., Chen D., Verma V., et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir. Med. 2021;9(5):467–475. doi: 10.1016/S2213-2600(20)30391-X. [DOI] [PubMed] [Google Scholar]
- 9.Theelen W.S.M.E., Peulen H.M., Lalezari F., et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welsh J., Menon H., Chen D., et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J. Immunother. Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnarr K., Boreham D., Sathya J., Julian J., Dayes I.S. Radiation-induced lymphocyte apoptosis to predict radiation therapy late toxicity in prostate cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2009;74(5):1424–1430. doi: 10.1016/j.ijrobp.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 12.Chi K.-H., Liu S.-J., Li C.-P., et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J. Immunother. 2005;28(2):129–135. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 13.Yovino S., Grossman S.A. Severity, etiology and possible consequences of treatment-related lymphopenia in patients with newly diagnosed high-grade gliomas. CNS Oncol. 2012;1(2):149–154. doi: 10.2217/cns.12.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onishi H., Shirato H., Nagata Y., et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2007;2(7 Suppl 3):S94–100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 15.Thames H.D., Bentzen S.M., Turesson I., Overgaard M. Van den Bogaert W. Time-dose factors in radiotherapy: a review of the human data. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1990;19(3):219–235. doi: 10.1016/0167-8140(90)90149-q. [DOI] [PubMed] [Google Scholar]
- 16.Fowler J.F. The linear-quadratic formula and progress in fractionated radiotherapy. Br. J. Radiol. 1989;62(740):679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 17.Antonia S.J., Villegas A., Daniel D., et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. 2017;377(20):1919–1929. doi: 10.1056/nejmoa1709937. [DOI] [PubMed] [Google Scholar]
- 18.Antonia S.J., Villegas A., Daniel D., et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018;379(24):2342–2350. doi: 10.1056/nejmoa1809697. [DOI] [PubMed] [Google Scholar]
- 19.Peters S., Felip E., Dafni U., et al. Progression-free and overall survival for concurrent nivolumab with standard concurrent chemoradiotherapy in locally advanced stage IIIA-B NSCLC: results from the european thoracic oncology platform NICOLAS phase II Trial (European Thoracic Oncology Platf. J. Thorac. Oncol. 2021;16(2):278–288. doi: 10.1016/j.jtho.2020.10.129. [DOI] [PubMed] [Google Scholar]
- 20.Jabbour S.K., Lee K.H., Frost N., et al. Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage III non-small cell lung cancer: the phase 2 KEYNOTE-799 nonrandomized trial. JAMA Oncol. 2021;7(9):1–9. doi: 10.1001/jamaoncol.2021.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin S.H., Lin Y., Yao L., et al. Phase II trial of concurrent atezolizumab with chemoradiation for unresectable NSCLC. J. Thorac. Oncol. 2020;15(2):248–257. doi: 10.1016/j.jtho.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Bradley J.D., Nishio M., Okamoto I., et al. PACIFIC-2: Phase 3 study of concurrent durvalumab and platinum-based chemoradiotherapy in patients with unresectable, stage III NSCLC. J. Clin. Oncol. 2019;37(15_suppl) doi: 10.1200/JCO.2019.37.15_suppl.TPS8573. TPS8573-TPS8573. [DOI] [Google Scholar]
- 23.Yoneda K., Kuwata T., Kanayama M., et al. Alteration in tumoural PD-L1 expression and stromal CD8-positive tumour-infiltrating lymphocytes after concurrent chemo-radiotherapy for non-small cell lung cancer. Br. J. Cancer. 2019;121(6):490–496. doi: 10.1038/s41416-019-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nabhan C., Jeune-Smith Y., Klinefelter P., Kelly R.J., Feinberg B.A. Challenges, perceptions, and readiness of oncology clinicians for the MACRA quality payment program. JAMA Oncol. 2018;4(2):252–253. doi: 10.1001/jamaoncol.2017.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schild S.E., Wang X., Bestvina C.M., et al. Alliance A082002 -a randomized phase II/III trial of modern immunotherapy-based systemic therapy with or without SBRT for PD-L1-negative, advanced non-small cell lung cancer. Clin. Lung Cancer. 2022;23(5):e317–e320. doi: 10.1016/j.cllc.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angelova M., Mlecnik B., Vasaturo A., et al. Evolution of metastases in space and time under immune selection. Cell. 2018;175(3):751–765. doi: 10.1016/j.cell.2018.09.018. e16. [DOI] [PubMed] [Google Scholar]
- 27.Reuben A., Spencer C.N., Prieto P.A., et al. Genomic and immune heterogeneity are associated with differential responses to therapy in melanoma. NPJ Genomic Med. 2017;2 doi: 10.1038/s41525-017-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Mattos-Arruda L., Sammut S.-J., Ross E.M., et al. The genomic and immune landscapes of lethal metastatic breast cancer. Cell Rep. 2019;27(9):2690–2708. doi: 10.1016/j.celrep.2019.04.098. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pevzner A.M., Tsyganov M.M., Ibragimova M.K., Litvyakov N.V. Abscopal effect in the radio and immunotherapy. Radiat. Oncol. J. 2021;39(4):247–253. doi: 10.3857/roj.2021.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGee H.M., Daly M.E., Azghadi S., et al. Stereotactic ablative radiation therapy induces systemic differences in peripheral blood immunophenotype dependent on irradiated site. Int. J. Radiat. Oncol. Biol. Phys. 2018;101(5):1259–1270. doi: 10.1016/j.ijrobp.2018.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang A.C., Postow M.A., Orlowski R.J., et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palma D.A., Olson R., Harrow S., et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 33.Palma D.A., Olson R., Harrow S., et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J. Clin. Oncol. 2020;38(25):2830–2838. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palma D. Stereotactic ablative radiotherapy for comprehensive treatment of 4-10 oligometastatic tumors (SABR-COMET 10). Clinicaltrials.gov.:NCT03721341. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02046389/full [DOI] [PMC free article] [PubMed]
- 35.Bauman G. ARREST - a phase I study of SABR for poly-metastatic disease. Clinicaltrials.gov.:NCT04530513.
- 36.Chen D., Menon H., Verma V., et al. Response and outcomes after anti-CTLA4 versus anti-PD1 combined with stereotactic body radiation therapy for metastatic non-small cell lung cancer: Retrospective analysis of two single-institution prospective trials. J. Immunother. Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welsh J. Pembrolizumab and stereotactic body radiation therapy or non-stereotactic wide-field radiation therapy in treating patients with non-small cell lung cancer. Clinicaltrials.gov.:NCT02444741. https://clinicaltrials.gov/ct2/show/NCT02599454
- 38.Ipilimumab and Stereotactic Body Radiation Therapy (SBRT) in advanced solid tumors. Clinicaltrials.gov. Published online 2014:NCT02239900. https://clinicaltrials.gov/show/NCT02239900
- 39.Boyer M., Şendur M.A., Rodríguez-Abreu D., et al. Pembrolizumab plus ipilimumab or placebo for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%: randomized, double-blind phase III KEYNOTE-598 study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021;39(21):2327–2338. doi: 10.1200/JCO.20.03579. [DOI] [PubMed] [Google Scholar]
- 40.Monjazeb A.M., Giobbie-Hurder A., Lako A., et al. A randomized trial of combined PD-L1 and CTLA-4 inhibition with targeted low-dose or hypofractionated radiation for patients with metastatic colorectal cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021;27(9):2470–2480. doi: 10.1158/1078-0432.CCR-20-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen D., Verma V., Patel R.R., Barsoumian H.B., Cortez M.A., Welsh J.W. Absolute lymphocyte count predicts abscopal responses and outcomes in patients receiving combined immunotherapy and radiation therapy: analysis of 3 phase 1/2 trials. Int. J. Radiat. Oncol. Biol. Phys. 2020;108(1):196–203. doi: 10.1016/j.ijrobp.2020.01.032. [DOI] [PubMed] [Google Scholar]
- 42.Welsh J.W., Heymach J.V., Chen D., et al. Phase I trial of pembrolizumab and radiation therapy after induction chemotherapy for extensive-stage small cell lung cancer. J. Thorac. Oncol. 2020;15(2):266–273. doi: 10.1016/j.jtho.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Golden E.B., Chhabra A., Chachoua A., et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16(7):795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 44.Zucker A., Winter A., Lumley D., Karwowski P., Jung M.-K., Kao J. Prognostic role of baseline neutrophil-to-lymphocyte ratio in metastatic solid tumors. Mol. Clin. Oncol. 2020;13(4):25. doi: 10.3892/mco.2020.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garon E.B., Rizvi N.A., Hui R., et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N. Engl. J. Med. 2015;372(21):2018–2028. doi: 10.1056/nejmoa1501824. [DOI] [PubMed] [Google Scholar]
- 46.Le D.T., Uram J.N., Wang H., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015;372(26):2509–2520. doi: 10.1056/nejmoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fabi F. Comment on “abscopal effect in the radio and immunotherapy”. Radiat. Oncol. J. 2022;40(1):86–87. doi: 10.3857/roj.2022.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spigel D.R., Schrock A.B., Fabrizio D., et al. Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. J. Clin. Oncol. 2016;34(15_suppl):9017. doi: 10.1200/JCO.2016.34.15_suppl.9017. [DOI] [Google Scholar]
- 49.Gainor J.F., Shaw A.T., Sequist L.V, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin. Cancer Res. 2016;22(18):4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Formenti S.C., Rudqvist N.-P., Golden E., et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018;24(12):1845–1851. doi: 10.1038/s41591-018-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gajewski T.F., Schreiber H., Fu Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Twyman-Saint Victor C., Rech A.J., Maity A., et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gulley J.L., Arlen P.M., Bastian A., et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin. Cancer Res. 2005;11(9):3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 54.Anscher M.S., Arora S., Weinstock C., et al. Association of radiation therapy with risk of adverse events in patients receiving immunotherapy: a pooled analysis of trials in the US food and drug administration database. JAMA Oncol. 2022;8(2):232–240. doi: 10.1001/jamaoncol.2021.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verma V., Cushman T.R., Tang C., Welsh J.W. Toxicity of radiation and immunotherapy combinations. Adv. Radiat. Oncol. 2018;3(4):506–511. doi: 10.1016/j.adro.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan H., Zhou Z., Hou X., Zhang F., Zhao J., Hu K. Safety and potential increased risk of toxicity of radiotherapy combined immunotherapy strategy. Asia Pac. J. Clin. Oncol. 2022;(May 2021):1–16. doi: 10.1111/ajco.13688. [DOI] [PubMed] [Google Scholar]
- 57.Kalbasi A., Rengan R. Clinical experiences of combining immunotherapy and radiation therapy in non-small cell lung cancer: Lessons from melanoma. Transl. Lung Cancer Res. 2017;6(2):169–177. doi: 10.21037/tlcr.2017.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao J., Ling L., Yorke E.D., et al. Simple clinical factors associated with radiation-induced lung toxicity after stereotactic body radiation therapy in lung cancer: a pooled analysis of 70 studies. Int. J. Radiat. Oncol. 2014;90(1):S60. doi: 10.1016/j.ijrobp.2014.05.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaverdian N., Lisberg A.E., Bornazyan K., et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chun S.G., Hu C., Choy H., et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017;35(1):56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoeller U., Borgmann K., Oertel M., Haverkamp U., Budach V., Eich H.T. Late sequelae of radiotherapy—the effect of technical and conceptual innovations in radiation oncology. Dtsch Arztebl. Int. 2021;118(12):205–211. doi: 10.3238/arztebl.m2021.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang J.H., Bluestone J.A., Young A. Predicting and preventing immune checkpoint inhibitor toxicity: targeting cytokines. Trends Immunol. 2021;42(4):293–311. doi: 10.1016/j.it.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 63.A. Marabelle, Predictive markers of immune-related adverse events in patients treated with immune stimulatory drugs (PREMIS). Clinicaltrials.gov.:NCT03984318. doi:10.31525/ct1-nct03984318
- 64.von Itzstein M.S., Khan S., Gerber D.E. Investigational biomarkers for checkpoint inhibitor immune-related adverse event prediction and diagnosis. Clin. Chem. 2020;66(6):779–793. doi: 10.1093/clinchem/hvaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.B. Henick, Study of rituximab or tocilizumab for patients with steroid-dependent immune-related adverse events (irAEs) - full text view - ClinicalTrials.gov. Clinicaltrials.gov.:NCT04375228.
- 66.Tang C., Jiang W., Yap T.A. Efficacy and toxic effects of cancer immunotherapy combinations-a double-edged sword. JAMA Oncol. 2018;4(8):1116–1117. doi: 10.1001/jamaoncol.2017.4606. [DOI] [PubMed] [Google Scholar]
- 67.Daly M. UCDCC#270: avelumab and stereotactic ablative radiotherapy in non- responding and progressing NSCLC patients. Clinicaltrials.gov.:NCT03158883.
- 68.UCDCC#269: a pilot study of interlesional IL-2 and RT in patients with NSCLC. Clinicaltrials.gov. Published online 2019:NCT03224871. https://clinicaltrials.gov/show/NCT03224871
- 69.PembRolIzuMab and stereotactic body radiotherapy in metastatic non-small-cell lung cancer patients. Clinicaltrials.gov. Published online 2019:NCT03436056. https://clinicaltrials.gov/show/NCT03436056
- 70.Safety and tolerability evaluation of sintilimab in combination with radiation in stage IV NSCLC patients. Clinicaltrials.gov.:NCT03812549.
- 71.Chmura S. Concurrent or sequential immunotherapy and radiation therapy in patients with metastatic lung cancer (COSINR). Clinicaltrials.gov.:NCT03223155. https://clinicaltrials.gov/show/NCT03223155
- 72.Trial Of hypofractionated radiotherapy in combination with MEDI4736 and tremelimumab for patients with metastatic melanoma and lung, breast and pancreatic cancers. Clinicaltrials.gov. Published online 2015:NCT02639026. https://clinicaltrials.gov/show/NCT02639026
- 73.Bassetti M. Phase Ib study of stereotactic body radiotherapy (SBRT) in oligometastatic non-small lung cancer (NSCLC) with dual immune checkpoint inhibition. Clinicaltrials.gov.:NCT03275597. https://clinicaltrials.gov/show/NCT03275597
- 74.Radiation and immune checkpoints blockade in metastatic NSCLC (BMS # CA209-632). Clinicaltrials.gov.:NCT03168464.
- 75.Study of combined ionizing radiation and ipilimumab in metastatic non-small cell lung cancer (NSCLC). Clinicaltrials.gov.:NCT02221739.
- 76.Radical-dose image guided radiation therapy in treating patients with metastatic non-small cell lung cancer undergoing immunotherapy. Clinicaltrials.gov. Published online 2017:NCT03176173.
- 77.Immunotherapy, chemotherapy, radiotherapy and surgery for synchronous oligo-metastatic NSCLC. Case Med. Res. 2019 doi: 10.31525/ct1-nct03965468. Published online. [DOI] [Google Scholar]
- 78.Fostering Efficacy of Anti - PD-1 - Treatment: Nivolumab Plus Radiotherapy in Advanced NSCLC - Full Text View - ClinicalTrials.gov. Clinicaltrials.gov.:NCT03044626. https://clinicaltrials.gov/ct2/show/NCT03044626
- 79.A randomized two arm phase II trial of pembrolizumab alone or sequentially following single fraction non-ablative radiation to one of the target lesions, in previously treated patients with stage IV NSCLC. Clinicaltrials.gov. Published online 2016:NCT02658097. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01595773/full
- 80.J. Heymach, Nivolumab and ipilimumab with or without local consolidation therapy in treating patients with stage IV non-small cell lung cancer. ClinicaltrialsGov.:NCT03391869. https://www.clinicaltrials.gov/ct2/show/NCT03137771
- 81.M. Farris, Immunotherapy with or without SBRT in patients with stage IV non-small cell lung cancer. Clinicaltrials.gov.:NCT03867175. doi: 10.31525/ct1-nct03867175. [DOI]
- 82.PD-1 inhibitors and chemotherapy with concurrent irradiation at varied tumour sites in advanced non-small cell lung cancer - full text view - ClinicalTrials.gov. Clinicaltrials.gov.:NCT03774732. https://clinicaltrials.gov/ct2/show/NCT03774732.