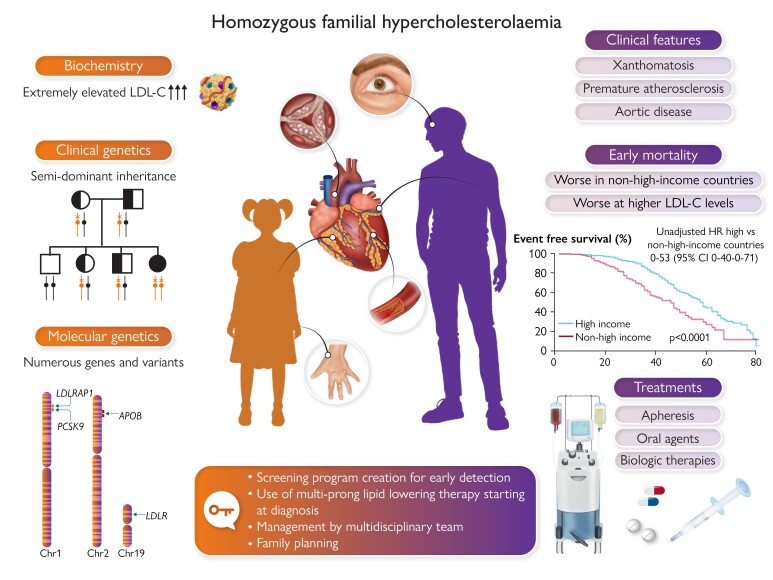
Graphical Abstract
Graphical Abstract.
Homozygous familial hypercholesterolaemia is a rare autosomal semi-dominant disease affecting males and females equally, characterized by markedly elevated levels of low-density lipoprotein cholesterol (LDL-C) from conception and accelerated atherosclerotic cardiovascular disease, often resulting in early death. Homozygous familial hypercholesterolaemia is diagnosed late and undertreated; both established and novel therapies offer hope to patients, but treatment inequity exacerbates poorer outcomes in less affluent countries.12APOB, apolipoprotein B gene; CI, confidence interval; HR, hazard ratio; LDLR, low-density lipoprotein receptor gene; LDLRAP1, low-density lipoprotein adaptor protein 1 gene; PCSK9, proprotein conertase subtilisin/kexin type 9 gene.
Keywords: Homozygous familial hypercholesterolaemia, Clinical guidance, Diagnosis, Genetics, Treatment, Women
Homozygous familial hypercholesterolaemia is a rare autosomal semi-dominant disease affecting males and females equally, characterized by markedly elevated levels of low-density lipoprotein cholesterol (LDL-C) from conception and accelerated atherosclerotic cardiovascular disease, often resulting in early death. Homozygous familial hypercholesterolaemia is diagnosed late and undertreated; both established and novel therapies offer hope to patients, but treatment inequity exacerbates poorer outcomes in less affluent countries (reference 12). APOB, apolipoprotein B; CI, confidence interval; HR, hazard ratio; LDLR, low-density lipoprotein receptor; LDLRAP1, low-density lipoprotein receptor adaptor protein 1; PCSK9, proprotein convertase subtilisin/kexin type 9.
Abstract
This 2023 statement updates clinical guidance for homozygous familial hypercholesterolaemia (HoFH), explains the genetic complexity, and provides pragmatic recommendations to address inequities in HoFH care worldwide. Key strengths include updated criteria for the clinical diagnosis of HoFH and the recommendation to prioritize phenotypic features over genotype. Thus, a low-density lipoprotein cholesterol (LDL-C) >10 mmol/L (>400 mg/dL) is suggestive of HoFH and warrants further evaluation. The statement also provides state-of-the art discussion and guidance to clinicians for interpreting the results of genetic testing and for family planning and pregnancy. Therapeutic decisions are based on the LDL-C level. Combination LDL-C-lowering therapy—both pharmacologic intervention and lipoprotein apheresis (LA)—is foundational. Addition of novel, efficacious therapies (i.e. inhibitors of proprotein convertase subtilisin/kexin type 9, followed by evinacumab and/or lomitapide) offers potential to attain LDL-C goal or reduce the need for LA. To improve HoFH care around the world, the statement recommends the creation of national screening programmes, education to improve awareness, and management guidelines that account for the local realities of care, including access to specialist centres, treatments, and cost. This updated statement provides guidance that is crucial to early diagnosis, better care, and improved cardiovascular health for patients with HoFH worldwide.
Introduction
In 2014, the European Atherosclerosis Society (EAS) statement on homozygous familial hypercholesterolaemia (HoFH)1 focused attention on this rare and life-threatening disease, characterized by markedly elevated plasma low-density lipoprotein cholesterol (LDL-C) levels from birth and accelerated atherosclerotic cardiovascular disease (ASCVD). At that time, HoFH had limited therapeutic options. Expert-led call-to-action statements and guidance2–9 catalysed enormous progress, particularly in understanding the genetic complexity of HoFH and in developing new treatments. However, persistent questions regarding screening, diagnosis, and management require a new consensus statement (Box 1).
Box 1. What is new in this EAS Consensus Statement on HoFH?
Updated diagnostic criteria
New insights into the genetics of HoFH
Updated recommendations on screening strategies for early identification
Updated recommendations for cardiac work-up at diagnosis and follow-up
Updated treatment algorithm incorporating novel treatments
Updated guidance on family planning for HoFH women of childbearing potential and heterozygous FH parents
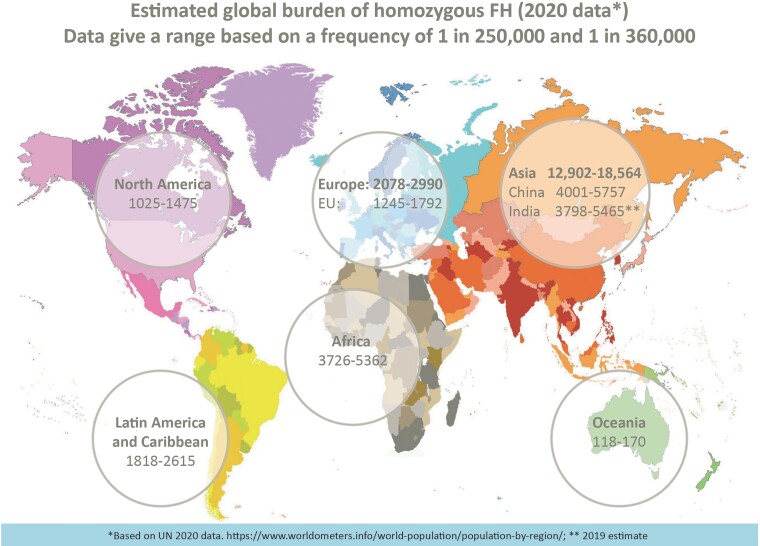
Underdiagnosis and undertreatment of HoFH are major issues impacting HoFH care. Recent estimates indicate that ∼30 000 people worldwide have HoFH (Figure 1),10,11 but <5% are identified.12 Inadequate awareness and ascertainment efforts, and a disconnect between clinical diagnosis and interpretation of genetic results by health providers and payers contribute to underdiagnosis. HoFH care is suboptimal, as shown by the HoFH International Clinical Collaborators (HICC) global registry,12 a clinician-founded initiative supported by the EAS including over 750 individuals from 38 countries (both high- and non-high-income regions). Diagnosis is made too late (median age 12 years), with ∼10% of patients showing clinical coronary atherosclerosis or aortic valve disease, and only 5% attain LDL-C goals.12 Treatment inequity is a major issue impacting patient cardiovascular health in less affluent countries.12 Urgent action is clearly needed to (i) improve awareness, and (ii) implement pragmatic clinical guidance for HoFH care, recognizing practical issues in different world regions. This second EAS statement updates evidence and addresses practical constraints impacting equity in care. How to balance advances in scientific knowledge with the realities of care globally is key to improving HoFH diagnosis and management.
Figure 1.
Estimated global prevalence of homozygous familial hypercholesterolaemia by United Nations world region, based on 2020 population data and estimates of homozygous familial hypercholesterolaemia prevalence ranging from 1:250 000 to 1:360 000.10,11
Diagnosis of homozygous familial hypercholesterolaemia
Clinical criteria
Plasma LDL-C is the critical discriminator for clinical diagnosis of HoFH. The 2014 EAS consensus statement recommended LDL-C diagnostic criteria of >13 mmol/L (>500 mg/dL) for untreated patients or >8 mmol/L (>300 mg/dL) for patients treated with conventional therapy (i.e. statin plus ezetimibe), together with evidence of cutaneous or tendon xanthomas before 10 years, or untreated elevated LDL-C levels consistent with heterozygous FH in both parents.1 However, LDL-C criteria are not the sole guide to diagnosis, given the genetic complexity of HoFH (see below) and variability in LDL-C levels and clinical phenotype; indeed, LDL-C levels <13 mmol/L were reported in several cohorts of genetically confirmed HoFH.12–14 Therefore, this panel proposes that HoFH should be suspected if untreated LDL-C levels are >10 mmol/L (>∼400 mg/dL), requiring further evaluation, including a detailed medical and family history and/or genetic testing (Box 2). The multiplicity of lipid-lowering treatments that these patients typically receive means that historic cut-offs for treated LDL-C are probably obsolete.
Box 2. Updated criteria for the diagnosis of homozygous familial hypercholesterolaemia.
Clinical criteria
• LDL-C criteria: Untreated LDL-C >10 mmol/L (>∼400 mg/dL) is suggestive of HoFH requiring further investigation to confirm the diagnosis.
• Additional criteria: Cutaneous or tendon xanthomas before age of 10 years and/or untreated elevated LDL-C levels consistent with heterozygous FH in both parents* *In digenic form, one parent may have normal LDL-C levels and the other may have LDL-C levels consistent with HoFH.
Genetic criteria
-
• Genetic confirmation of bi-allelic pathogenic/likely pathogenic variants on different chromosomes at the LDLR, APOB, PCSK9, or LDLRAP1 genes or ≥2 such variants at different loci (Box 3); for abbreviations for genetic nomenclature see below. ABCG5, ABCG8: Genes encoding ATP-binding cassette subfamily G members 5 and 8
APOB: Gene encoding apolipoprotein B LDLR: Gene encoding the low-density lipoprotein receptor LDLRAP1: Gene encoding low-density lipoprotein receptor adaptor protein 1 LIPA: Gene encoding lysosomal acid lipase PCSK9: Gene encoding proprotein convertase subtilisin/kexin type 9 protein (PCSK9)
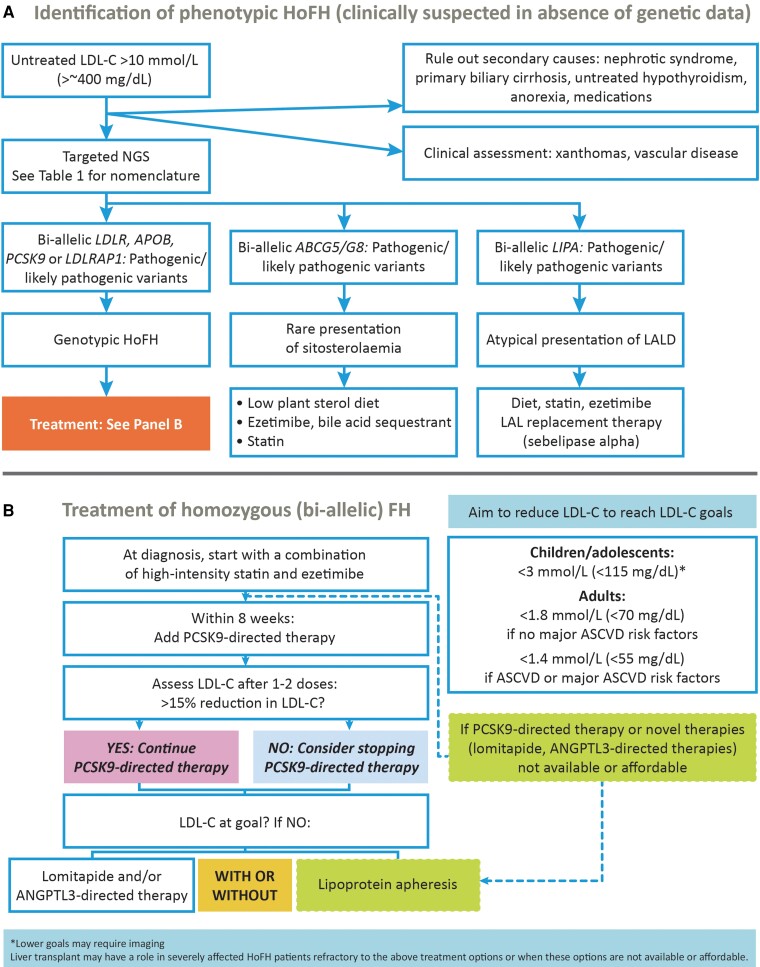
Clinicians should exclude other conditions associated with elevated LDL-C levels before making a diagnosis of HoFH (Figure 2A). These include sitosterolaemia (or ‘phytosterolaemia’), caused by bi-allelic rare pathogenic variants in two ATP-binding cassette transporter genes, ABCG5 and/or ABCG8 (or at least one such variant in each gene), with elevated LDL-C levels that respond well to dietary recommendations, ezetimibe and/or resins,15–17 and lysosomal acid lipase deficiency (i.e. cholesterol ester storage disease), caused by bi-allelic pathogenic variants in the LIPA gene, and for which enzyme replacement treatment is available.18 Patients with cerebrotendinous xanthomatosis may have xanthomas resembling HoFH but plasma cholesterol levels are normal to mildly elevated (with elevated cholestanol levels) associated with neurological, cognitive, and ophthalmic symptoms.19,20
Key recommendation: An untreated LDL-C level >10 mmol/L (>∼400 mg/dL) suggests HoFH and requires further evaluation.
Figure 2.
Identification and treatment of homozygous familial hypercholesterolaemia. (A) Algorithm for identification of phenotypic homozygous familial hypercholesterolaemia when clinically suspected in the absence of genetic data. (B) Treatment algorithm for homozygous familial hypercholesterolaemia (bi-allelic). ANGPTL3, angiopoietin-like 3; ASCVD, atherosclerotic cardiovascular disease; LALD, lysosomal acid lipase deficiency; LDL-C, low-density lipoprotein cholesterol; NGS, next-generation DNA sequencing; PCSK9, proprotein convertase subtilisin/kexin type 9.
Genetic criteria and interpreting genetic test results
The term ‘HoFH’ dates from the pre-genomic era when scientists and clinicians envisioned a simple scenario in which the identical pathogenic variant was present on both alleles of an affected individual, as sometimes observed in consanguineous matings. Pathogenic variants were mainly in three main genes, LDLR (loss-of-function), APOB (receptor-binding defective), and PCSK9 (gain-of-function), accounting for 85%–90%, 5%–10%, and 1%–3%, respectively, of all variants causing semi-dominant FH.21 More recently, a rare autosomal recessive form of FH [autosomal recessive hypercholesterolaemia (ARH)] accounting for <1% of cases was identified, caused by bi-allelic loss-of-function variants in LDLRAP1 (see abbreviations in Box 2); heterozygotes are true ‘carriers’: with normal LDL-C levels.22
As an autosomal semi-dominant condition,23 individuals with one copy of a pathogenic DNA variant—i.e. mono-allelic or heterozygous FH (HeFH)—express an abnormal biochemical phenotype, sometimes with characteristic clinical features. Individuals with bi-allelic pathogenic variants (one pathogenic variant inherited from each parent, each on a different chromosome, i.e. genotypic HoFH) have more extreme LDL-C deviation and more severe clinical features from a younger age. Mono-allelic and bi-allelic forms—i.e. HeFH and HoFH—have a prevalence of ∼1 in 250–30010,11 and ∼1 in 250 000–360 000, respectively.24 In some populations, such as French Canadians, Afrikaners in South Africa, or Christian Lebanese, HoFH prevalence may be at least 10-fold higher due to founder effects.10,11,24
The molecular complexity initially described in the 2014 consensus statement1 was reaffirmed by next-generation DNA sequencing (NGS). Most patients have two different pathogenic variants either in the same (i.e. monogenic) or two different (i.e. digenic) causative genes. Permutations (shown in Table 1) include bi-allelic variants (identical or different) from a single gene, as well as different bi-allelic variants from two different genes (digenic inheritance). This resulted in confusing descriptions such as ‘compound heterozygous’ or ‘double heterozygous’ form of HoFH. To avoid misinterpretation, the panel suggests retaining the operational term ‘phenotypic HoFH’ as a useful clinical shorthand in the absence of a genetic diagnosis, while acknowledging the underlying genetic complexity, as reported by genetic diagnostic laboratories (Box 3, Table 1). This more precise genetic terminology supersedes traditional terms such as ‘simple’ or ‘true homozygote’, ‘compound heterozygote’ and ‘double heterozygote’.
Table 1.
Modern genetic nomenclature for the clinical homozygous familial hypercholesterolaemia phenotype
| Traditional clinical terminology | Precise nomenclature based on genetics | Comments |
|---|---|---|
| Homozygous familial hypercholesterolaemia (HoFH): an umbrella term that encompasses a spectrum of genetic diagnoses | 1. Bi-allelic semi-dominant hypercholesterolaemia: monogenic (single gene) | ‘Semi-dominant’ indicates that mono-allelic (i.e. heterozygote) relatives have a less severe phenotype |
| (a) defined by causative genes: | ||
| • LDLR-related | Accounts for 85%–90% of all variants | |
| • APOB-related | Accounts for 5%–10% of all variants | |
| • PCSK9-related | Accounts for 1%–3% of all variants | |
| (b) defined by nature of variants: | ||
| · 2 copies of the identical variant | Formerly called ‘true’ or ‘simple’ HoFH | |
| · 1 copy each of 2 different variants | Formerly called ‘compound heterozygous FH’ | |
| 2. Bi-allelic semi-dominant hypercholesterolaemia: digenic (two different causal genes) | ‘Semi-dominant’ indicates that mono-allelic (i.e. heterozygote) relatives have a less severe phenotype | |
| (a) defined by causative genes: | ||
| • LDLR-related plus APOB-related | Accounts for 90%–95% of cases with digenic variants | |
| • LDLR-related plus PCSK9-related | Accounts for 2%–5% of cases with digenic variants | |
| • APOB-related plus PCSK9-related | Accounts for <2% of cases with digenic variants | |
| (b) defined by nature of variants: | ||
| · 1 copy each of 2 different variants | Formerly called ‘double heterozygous FH’ | |
| 3. Bi-allelic recessive hypercholesterolaemia: single gene | Heterozygous parents are true carriers with normal clinical and biochemical phenotypes | |
| (a) defined by causative gene: | ||
| • LDLRAP1-related | Accounts for all patients with this subtype;<1% of all cases of ‘HoFH’. | |
| (b) defined by nature of variants: | ||
| · 2 copies of the identical variant | Formerly called ‘true’ or ‘simple’ ARH | |
| · 1 copy each of 2 different variants | Formerly called ‘compound heterozygous’ ARH |
Box 3. Sources of genetic complexity of phenotypic homozygous FH.
Genetic heterogeneity
LDLR gene loss-of-function variants: >3000 reported, accounting for 85%–90% of cases
APOB gene-receptor-binding-impaired variants accounting for 5%–10% of cases
PCSK9 gene gain-of-function variants accounting for 1%–3% of cases
LDLRAP1 gene loss-of-function variants accounting for <1% of cases
Variable inheritance patterns
Semi-dominant (i.e. LDLR, APOB, and PCSK9 genes)
True autosomal recessive (i.e. LDLRAP1 gene)
Variant types
Usually interpreted as a ‘null’ variant of LDLR (≤ 2% functional activity)
Copy number variants (i.e. large insertion or deletion of genetic material)
Nonsense variant resulting in premature termination
• small insertion or deletion that shifts the reading frame
• single nucleotide variant introducing a stop codon
Splicing variant
A few missense variants (defined below) are proven experimentally to be ‘null’
Usually interpreted as a ‘defective’ variant of LDLR (2%–70% functional activity)
Typically, a missense (i.e. single nucleotide) variant altering a single amino acid residue
See abbreviations in Box 2 and Berberich and Hegele (2019).21
Targeted NGS diagnostic panels predominantly used today will eventually be replaced by high-pass exome or genome methods.21 Results from all methods are reported similarly but interpretation can present challenges for the care provider.25 Genetic reports describe variants as benign/likely benign, variant of uncertain or unknown significance (VUS), or pathogenic/likely pathogenic.26 Ascribing pathogenicity to DNA variants is a challenge faced by the entire genetics field. This panel recommends that clinicians only consider variants reported as ‘pathogenic/likely pathogenic’ according to criteria from the American College of Medical Genetics and Genomics modified for FH when communicating a confirmed genetic diagnosis to patients.25,26 VUS are not diagnostic, but potentially could be reclassified as pathogenic in the future.
Key recommendation: Consider only variants reported as ‘pathogenic/likely pathogenic’ according to recognized criteria as a confirmed genetic diagnosis of HoFH.
Variants in LDLR are often classified as ‘null’ (≤2% activity) or ‘receptor defective’ (2%–70% activity) based on their predicted impact on receptor function. Yet only ∼10% of all variants have been directly functionally tested; instead, most have been classified based on predictive algorithms, particularly missense variants.25 There are general patterns of genotype-phenotype correlation, with LDLR null variants having the most extreme LDL-C deviations and most blunted response to pharmacologic interventions.27 However, the genotype cannot definitively predict the precise phenotypic expression in an individual patient.
On balance, the benefits outweigh the limitations of genetic testing in HoFH (Box 4), with increased certainty of diagnosis and access to, use of, and compliance with appropriate treatment. For example, genetic testing occasionally reveals causal variants in genes that normally cause non-FH dyslipidaemias such as ABCG5 or ABCG8 in sitosterolaemia, with distinct treatment implications, i.e. elimination of dietary sterols and use of ezetimibe rather than statins plus apheresis (Figure 2A).18 However, genetic results can be potentially misunderstood or miscommunicated.28 A few patients with phenotypic HoFH have only one identified pathogenic variant because of technical reasons, interactions with undiscovered causal genes, or an unmeasured background polygenic effect, although the latter is currently not felt to be an important genetic mechanism underlying phenotypic HoFH.29 Furthermore, NGS has identified some individuals with phenotypic HeFH and an appropriate response to statins, who have two pathogenic variants on opposite chromosomes that would be expected to cause phenotypic HoFH.30,31 In cases of discordance, this panel recommends that a phenotypic diagnosis, HoFH or HeFH, takes priority over the genetic results. Patients and families with genetic results that are either difficult to understand or complex should be referred to a genetic counsellor or clinician with extensive experience in human genetics and lipidology.32
Box 4. Benefits and limitations of genetic testing in HoFH.
Benefits
Permits a definitive diagnosis
Allows for objective visualization of the underlying genetic complexity
Enables access to appropriate current and emerging therapies and clinical trials
Can predict disease severity and treatment response in populations or cohorts, within limits
Can rule out similar conditions that require other treatments (e.g. sitosterolaemia)
Triggers cascade screening to find new patients with HeFH or phenotypic HoFH
Can facilitate prenatal genetic counselling
Knowledge of a definitive genetic basis can promote adherence to optimal therapy
Limitations
Accessibility and cost
Predicting individual phenotype and clinical response from genotype is not straightforward
Pathogenicity for many detected DNA variants cannot be definitively established
Some patients with phenotypic HoFH have only one or even no pathogenic variant detected
Some patients with bi-allelic pathogenic variants express HeFH but not HoFH phenotypically
The healthcare provider must be cautious not to overinterpret genetic results
Professional pre- and post-test genetic counselling is desirable but usually unavailable
Screening recommendations: key to early identification of homozygous familial hypercholesterolaemia
Early detection of HoFH is essential, but in practice is too late, as shown by the HICC registry.12 Lack of awareness about HoFH and poor guideline implementation are important barriers. Current guidelines recommend screening in children at ≤2 years if there is a positive family history of premature ASCVD or hypercholesterolaemia, and universal cholesterol screening at ages varying from 5 to 11 years.33–35 These guidelines are hardly followed, emphasizing the need for improved implementation.36
This panel strongly recommends (i) screening for HoFH whenever suspected clinically and/or with premature ASCVD; (ii) expansion of paediatric guidelines to include newborn lipid screening when both parents are known to have HeFH or hypercholesterolaemia without a confirmed diagnosis of HeFH, or in regions with a strong founder effect; and (iii) creation of national screening programmes in countries where these are lacking. This panel joins FH advocacy groups worldwide in calling for paediatric universal FH screening, taking into consideration specific conditions, needs and resources of individual countries, to improve identification of FH, including HoFH.7,8,37,38
Education programmes to increase awareness among health providers likely to have first contact with possible patients with HoFH due to signs such as xanthomas1 is another priority to reduce time to diagnosis. Collaboration between paediatricians, primary healthcare providers, lipid specialists, cardiologists, and geneticists is highly desired. Use of electronic medical records and strategies to flag laboratory levels suggestive of HoFH with prompts to guidelines would increase awareness and link providers to regional specialist centres for shared management of patients with HoFH.
Finally, as HoFH is a disease of the family, identification of a patient with LDL-C levels compatible with phenotypic HoFH must prompt evaluation of lipids and medical history of family members via ‘reverse cascade’ testing.39 Ideally, this is centrally co-ordinated and undertaken by staff skilled in genetic and family counselling. Although genetic testing may facilitate identification of family members with FH, screening of lipid levels is acceptable, especially with privacy concerns among probands or parents and limited access to testing. For the autosomal semi-dominant subtype of HoFH (i.e. pathogenic variants in LDLR, PCSK9, and APOB genes), parents are obligate heterozygotes for single copy pathogenic variants in the same or different genes and are therefore at risk for elevated LDL-C levels (frequently >95th percentile by country-specific age and gender criteria) and a family history of premature ASCVD (<55 years in men and <60 years in women among first-degree relatives). For ARH, however, LDL-C levels in parents are in the normal range.18
Key recommendations
Paediatric guidelines should include newborn lipid screening when both parents are known to have HeFH or simply hypercholesterolaemia.
The panel advocates for paediatric universal FH screening that will also improve the detection of HoFH.
Education programmes are needed to increase awareness among all healthcare providers.
Management plan
Referral of the patient with HoFH to a specialist centre for appropriate LDL-lowering therapy and reverse cascade screening is essential (see Supplementary data online, Table S1). Initial laboratory analysis should be comprehensive and include lipoprotein(a) [Lp(a)], which enhances cardiovascular risk.40 Whether elevated Lp(a) also predisposes to accelerated aortic valve stenosis in HoFH warrants further study.
Because most patients with HoFH are identified in childhood, paediatricians have a key role in the management plan, to implement lifestyle measures and treat other ASCVD-risk factors.1 Cardiologists (paediatric or adult) are integral to the multidisciplinary management team. Initial assessment should include physical examination and auscultation, particularly valuable where there is limited access to imaging. Accelerated atherosclerosis, typically affecting the aortic root and coronary ostia, is characteristic of HoFH,1 and calcific valvular aortic disease has become very prevalent with increasing longevity.41
Given diagnostic delay and disease severity, detailed cardiovascular imaging at baseline is strongly advised where available (Box 5). An echocardiogram and low-dose computed tomography (CT) angiography are recommended to identify disease burden with special emphasis on high-risk lesions in the coronary ostia. Low-dose CT is preferable for detection of subclinical coronary and aortic root atherosclerosis41 and may help to guide therapy, as valvular and supravalvular aortic diseases may progress even when LDL-C levels are reduced. Tendon imaging is not recommended as part of routine clinical assessment. Different imaging protocols are required in adults vs. children, both requiring defined referral pathways and involving multidisciplinary discussion.
Box 5. Cardiovascular imaging in HoFH management.
Polyvascular subclinical atherosclerosis may be present in coronary, femoral, and carotid vascular territories in HoFH. Where available and easily accessible, imaging is an important tool in HoFH management.
Carotid plaque rather than increased carotid intima-medial thickness is a marker of early stage atherosclerosis. Quantitative carotid plaque measurement by three-dimensional ultrasound is a reliable measure of disease progression and response to therapy. High-resolution magnetic resonance imaging (MRI) has been shown to detect thrombosis and lipid-rich carotid plaques.
Patients with HoFH should receive echocardiographic evaluation of the heart and aorta at baseline and annually thereafter. CT angiography should be performed at least once after age 3 as it also allows for differentiation of coronary ostial stenosis (which may be asymptomatic) and aortic stenosis. A stress electrocardiogram should only be performed after exclusion of coronary ostial or tight aortic stenosis.
Follow-up CT angiography should be performed as clinically indicated. The follow-up interval in children depends on baseline disease, extent of LDL-C lowering, radiation risk, and cost. Radiation may be less of an issue with newer CT devices and imaging protocols. Early imaging can help to identify soft plaque to target for intervention and asymptomatic atherosclerosis.
Coronary calcium scoring is less predictive in young patients since extensive calcification may not yet have developed in the plaques.
Invasive coronary angiography is indicated in patients with symptoms and/or signs suggestive of ischaemia or valve malfunction.
The atherosclerotic burden of the aorta can also be evaluated by MRI or transoesophageal echocardiography.
Careful electrocardiographic or nuclear stress testing may be used to determine the presence of ischaemia. Coronary flow velocity reserve determined noninvasively with echocardiography has also been used to determine risk.
Treatment and low-density lipoprotein cholesterol goals
Given their very high risk, the panel proposes the same LDL-C goals in HoFH as recommended by guidelines for high- and very high-risk patients (Figure 2B). Thus, in adult patients with HoFH (≥18 years), the LDL-C goal is <1.8 mmol/L (<70 mg/dL), and <1.4 mmol/L (<55 mg/dL) with additional ASCVD-risk factors (elevated Lp(a), diabetes mellitus) or established ASCVD.42 In children and adolescents, an LDL-C goal of <3 mmol/L (<115 mg/dL) is recommended if treatment is initiated before 18 years and imaging assessment does not indicate ASCVD, with a lower goal in those with established ASCVD. The panel acknowledges that these goals are extrapolated from expert recommendations which have not been tested in clinical trials, and that attainment is challenging in real-world practice. In the HICC registry, only 12% of adult patients in high-income countries attained an LDL-C value <1.8 mmol/L.12 The panel believes, however, that with continued progress in lipid-lowering treatment and access, goal attainment may improve in the future.
The LDL-C level (i.e. the phenotype) and not the presence of a genetic diagnosis drives therapeutic decisions (Figure 2B). Combination lipid-lowering therapy, both pharmacologic intervention and lipoprotein apheresis (LA), is foundational,43 together with lifestyle measures (diet and smoking cessation). Treatment should be started as soon as possible, ideally at diagnosis. These recommendations also apply to children/adolescents, subject to local approval of pharmacotherapeutics, aiming to ensure adherence from an early age (Box 6).
Box 6. Treatment pathway for homozygous familial hypercholesterolaemia in childhood. Adapted from Reijman et al.50.
Initiate lifestyle changes, statins, and ezetimibe from diagnosis
If LDL-C >8 mmol/L (300 mg/dL), consider lipoprotein apheresis (LA)
If LDL-C >3 mmol/L (115 mg/dL), consider novel therapies if available and affordable
If LA or novel therapies are not available, consider liver transplantation
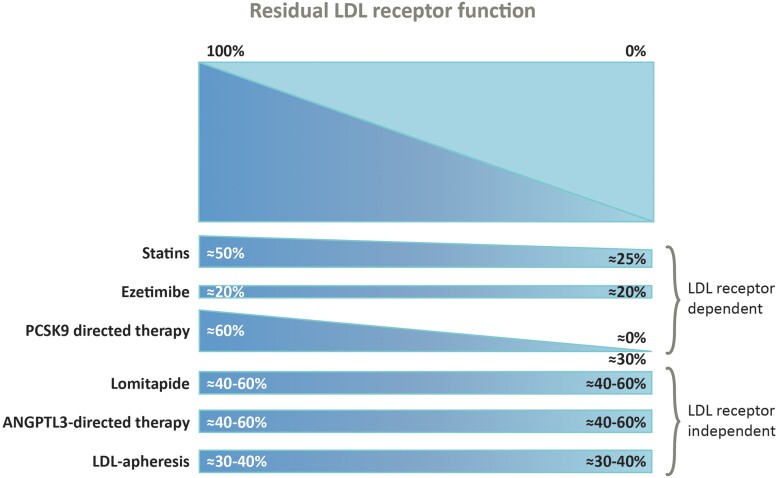
Patients should start on a high-intensity statin and ezetimibe rather than statin monotherapy, but most will require additional therapies to attain goal. Within 8 weeks proprotein convertase subtilisin/kexin type 9 (PCSK9)-directed therapy should be considered where available. Response to these treatments is dependent on the degree of residual LDL receptor activity (Figure 3).44 PCSK9 monoclonal antibody therapy (evolocumab or alirocumab at approved doses for HoFH) is effective in many patients with HoFH.45–49 If patients show >15% additional LDL-C reduction, PCSK9-directed therapy may be continued, but if response is poor, clinicians should consider stopping this therapy. While the average LDL-C lowering of ∼30% with PCSK9-directed therapy is likely to reduce the risk of ASCVD events, LDL-C levels will remain substantially above recommended goals for most patients. Subsequent options include LDL receptor–independent therapies and/or LA.
Figure 3.
Response to statin, ezetimibe, and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors is dependent on the degree of residual low-density lipoprotein receptor activity; however, for lomitapide, angiopoietin-like protein 3 (ANGPLT3)-directed therapy and lipoprotein apheresis, response is independent of low-density lipoprotein receptor function. Reproduced with permission from Mohamed et al.44.
Novel therapies independent of residual low-density lipoprotein receptor function
Lomitapide is an oral inhibitor of the microsomal triglyceride transfer protein affecting the production of very low-density lipoproteins, the precursor of LDL.51 In real-world practice, lomitapide added to standard of care treatment reduced plasma LDL-C levels by 60%, albeit with some variability in response,52 and Lp(a) by ∼15% at 26 weeks.53 Lomitapide also provided better control of LDL-C than lipoprotein apheresis (LA).54 Preliminary findings from the Pan-European Project in HoFH including 75 patients with HoFH showed that lomitapide treatment for up to 9 years (median 19 months) resulted in more than half attaining at least 50% reduction from baseline in LDL-C at last visit, with less need for apheresis in a substantial proportion of patients.55 However, hepatic steatosis remains a concern and liver imaging has shown a moderate increase in hepatic fat with normal liver stiffness during longer term real-world treatment, consistent with clinical trial data.56
Another approach targets angiopoietin-like protein 3 (ANGPTL3), which modulates lipid-lipoprotein metabolism and has pleiotropic functions. The ANGPTL3 monoclonal antibody evinacumab is licensed for patients with HoFH aged at least 12 years, based on results from the ELIPSE HoFH trial, in which evinacumab (15 mg/kg intravenously every 4 weeks) reduced LDL-C by ∼50% on top of maximally tolerated lipid-lowering therapy with or without LA, with comparable response during open-label treatment.57 Long-term follow-up (median 53 weeks, maximum 132 weeks) showed durable and similar LDL-C lowering in adults and adolescents (43% and 52%, respectively).58,59 Importantly, response to evinacumab did not depend on LDLR genotype and was similar in patients with bi-allelic null variants or with predicted residual LDL receptor function.57
Data with these newer agents are, however, limited in children with HoFH. To address this, the panel strongly supports creation of a fast-tracked regulatory pathway for approval of novel treatments in young children with HoFH; e.g. evinacumab was recently approved for children aged 5–11 years by the US Food and Drug Administration. As most countries do not have access to these newer agents, and, where available, cost may limit their use, other approaches such LA are a viable alternative.
Lipoprotein apheresis
Lipoprotein apheresis is foundational in children and adults with HoFH, adjunctive to other lipid-lowering therapy,60 and is therefore vital in countries without access to newer therapies. Limitations include variable access, cost, and a time-consuming procedure impacting patient’s quality of life.61 Treatment should be started as soon as possible, in children ideally by age 3 and not later than 8 years, depending on appropriate venous access. LA is usually performed fortnightly or even weekly. If not available, plasma exchange may be considered. Registry data provide strong evidence for the efficacy and clinical benefit of LA in adults, with resolution of clinical manifestations such as xanthomas and no major safety concerns to date.62,63 Rebound effects are slower in HoFH than in HeFH (up to 30 days) with an average LDL-C reduction of 55%, as well as over 50% reduction in Lp(a) concentration.64,65 The frequency or requirement for apheresis may be reduced with novel therapies such as lomitapide and evinacumab.
Liver transplantation
Liver transplantation may be an option for a small subset of patients with HoFH, particularly severely affected young children with bi-allelic null variants. It may be considered a last resort, despite maximal therapy, with (i) evidence of progression of coronary artery disease (CAD) and LDL-C levels >1.8 mmol/L (>70 mg/dL), or (ii) minimal or stable CAD but LDL-C >13 mmol/L (>500 mg/dL). Combined liver/heart transplantation may be indicated for rapidly progressive disease or extensive heart damage.2,66 Multiple case reports demonstrate normalization of LDL-C levels within a few weeks after transplantation of a normal donor liver, persisting up to 28 years.67–70 Effects on CAD are less well-documented, although regression has been reported,68,71–73 and the effect on aortic stenosis is uncertain.67,68,74 The benefits of liver transplantation need to be balanced against the risks, which include surgical complications, acute and chronic rejection of the donor liver, and side effects of immunosuppressive therapy, although these have decreased since first report in 1984 and will vary by institution.2 Access to an experienced paediatric or adult liver transplant centre and lack of contraindications for transplant surgery and immunosuppressive regimens, including the possibility of poor compliance, are essential. This panel advocates for the creation of a global registry of patients with liver transplantation to facilitate more comprehensive assessment of this approach.
Emerging therapeutic approaches
Novel approaches to PCSK9 and ANGPTL3 blockade, including small interfering (si)RNA therapies, are in development. Advances in biotechnology offer the future possibility of liver-directed gene transfer and gene editing for HoFH.75,76 Adeno-associated virus (AAV)-mediated gene transfer is a viable approach to long-term transgene expression in pre-clinical models of HoFH. A first-in-human phase 1/2 trial of an AAV-mediated human LDLR gene transfer in nine patients with HoFH was recently completed (NCT02651675).75 In preclinical models, CRISPR (clustered regularly interspaced short palindromic repeats)-based gene editing has been successful in modifying both ANGPTL377 and PCSK978 and reducing LDL-C levels. A clinical trial with the first CRISPR PCSK9 treatment (VERVE-101) is ongoing in the UK and New Zealand and pending in the USA.79 Although promising, these approaches require careful evaluation of intermediate and long-term safety and efficacy.
Key recommendations for managing HoFH
Lipid-lowering treatment should start at diagnosis using multiple treatments and/or LA to reach LDL-C goal.
A fast-track path for approval of novel treatments in children is needed to ensure their use from a young age, decreasing cumulative LDL-C exposure and improving outcomes.
Gene therapy and CRISPR-based gene editing are promising approaches, but clinical trials are needed to evaluate efficacy and safety.
Multidisciplinary management in specialist centres integrating imaging, treatment, and comprehensive support of these patients is needed.
Family planning and pregnancy
With advances in treatment, HoFH is becoming a more chronic, manageable disease, and individuals are increasingly likely to survive well into adulthood.27 For HoFH women of child-bearing potential, timely discussion of adequate contraception is essential to prevent unplanned pregnancy. Safe methods with minimal risk of ASCVD are preferred, although the panel recognizes the need to respect personal preference in an informed context. Family planning discussions are essential to management in both men and women, incorporating a model of care with cholesterol testing of the partner and genetic counselling, as children will have obligate HeFH.
A multidisciplinary team and an integrated care approach before, during, and after pregnancy are particularly important for HoFH women. Cardiovascular evaluation is recommended before pregnancy for risk assessment, including echocardiographic assessment of the pressure gradient across the aortic valve and root, and if possible, CT coronary angiography to evaluate CAD. Female patients should be counselled that pregnancy is associated with an increased cardiovascular risk and is potentially life-threatening; those with established CAD and/or aortic valve pathology should be referred to a high-risk obstetric clinic. Serum LDL-C levels can increase by 25%–50% during the second and particularly the third trimester of pregnancy. Although the relative increases are similar in individuals with and without FH, the absolute increases are much greater in FH, especially HoFH.80 An individual management plan is needed from the patient’s first wish to conceive to resumption of lipid-lowering medication.
Treatment during pregnancy and lactation is challenging as most lipid-lowering drugs, including statins until recently, are contraindicated. Although bile sequestering resins such as colesevelam are not associated with an increased risk of congenital abnormalities, data in pregnancy are scarce, their tolerability may be poor, and efficacy limited. Thus, HoFH women should be offered weekly or fortnightly LA during pregnancy.81,82 If unavailable, continuation of statin therapy or reintroduction of statin plus other lipid-lowering therapy from the second trimester onwards appears to be safe.83 Indeed, following recent comprehensive review, the FDA recommended changes in statin contraindications for pregnant women, recognizing the favourable risk/benefit ratio in high-risk women such those with HoFH.84 Reintroduction of lipid-lowering therapy should be postponed in lactating HoFH women due to possible transfer to the breast milk; if prolonged, discussion with the mother regarding the risks is needed. Finally, heterozygous parents of an HoFH child considering another child should receive additional counselling and care.
Key recommendations
HoFH women should receive integrated care from a multidisciplinary team, including cardiovascular assessment, before, during, and after pregnancy.
To minimize LDL-C exposure, LA should be offered during pregnancy, and statin plus other lipid-lowering therapy restarted from the second trimester.
Quality of life
Few studies have assessed the quality of life of individuals living with HoFH. A recent meta-analysis showed overall impairment in quality of life, mostly in general health, and physical and social functioning.61 Impact on quality of life may depend on disease status and treatment, especially for patients undergoing LA given significant treatment burden affecting education and employment.61,85,86 In contrast, a Dutch study87 in which 90% of patients with HoFH were treated with pharmacological interventions without LA, showed that quality of life was only slightly below the national average. Transient anxiety was reported when confronted with the consequences of HoFH, such as the onset or worsening of ASCVD. Family planning delivered by a dedicated HoFH centre helped patients cope with their disease. These findings underline the importance of comprehensive support for patients with HoFH and their family in specialized lipid centres.
Global perspectives
HoFH is a major challenge as it is underdiagnosed and undertreated in every world region. As shown by the HICC Registry, the implications for cardiovascular health are depressing; the first major adverse cardiovascular event occurred at a median age of 31 years, and before 18 years in 4% of patients.12 Treatment inequity exacerbates this; in the HICC registry, individuals in less affluent countries have higher treated LDL-C levels and experienced their first cardiovascular event about a decade earlier than those in high-income countries (mean age 24.5 vs. 37 years). Limited or no access to newer highly effective LDL-lowering therapies, and genetic testing to facilitate identification of less severe or extreme phenotypes, are important contributors (Box 7).12 These findings underpin the call to action to improve HoFH care globally.7–9
Box 7. Why we need a global perspective on HoFH: insights from the HICC Registry.
Poor awareness
Underdiagnosis: lack of national cholesterol screening programmes, including universal paediatric screening for FH, and limited access to genetic testing
Lack of systematic diagnostic criteria (genetics, variability in LDL-C levels)
Barriers to treatment: newer therapies or LA not available or limited due to variable reimbursement
Consideration of founder effects in certain settings
These disparities will increase assuming the use of newer treatments continues to be restricted to more affluent countries. Changes to health policy at global and regional levels are urgently needed to ensure that all patients with HoFH receive effective treatment as soon as possible to improve cardiovascular outcome.
Key recommendations
Creation of national screening programmes tailored to the healthcare system and culture of individual countries. These underpin the universal recommendation for early identification of HoFH via universal screening or systematic cascade screening. The panel advocates genetic testing to facilitate cascade screening; where not yet possible or privacy concerns limit implementation, screening based on LDL-C cut-offs are equally acceptable.
Creation of education programmes to improve awareness, both among clinicians, starting from medical school, and patients.
Creation at the local (institutional), regional, and government level of management guidelines that take account of resources, including access to specialist centres and effective lipid-lowering treatments. These should address costs associated with treatment and access to care.
Implementing clinical guidance
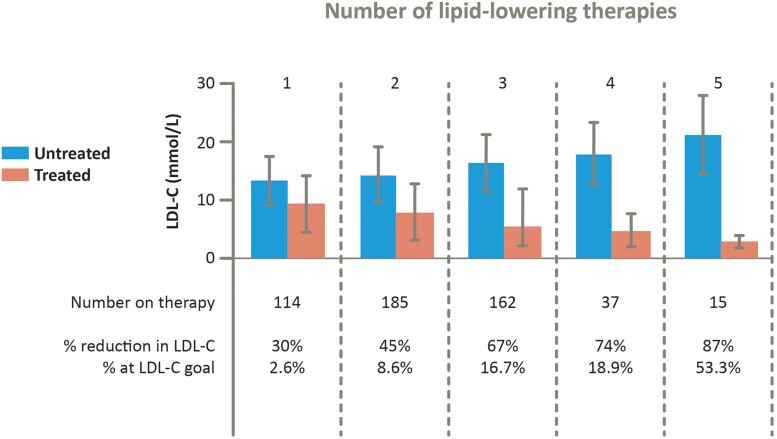
Systematic implementation of guideline recommendations is integral to better HoFH care, but difficult. In real world practice, lack of access to imaging and use of an adequate multi-drug therapeutic strategy are major issues (Figure 4). While goal attainment will likely improve with novel therapies, increasing awareness of HoFH among clinicians and improving access to imaging and treatment are paramount. New thinking on delivery of optimal management is needed, especially in less affluent countries where novel drugs are not approved or affordable and sophisticated clinical approaches may be lacking. Such approaches could include the use of telemedicine to help maintain adherence, especially during late adolescence/early adulthood,88 web-based education, and case discussion, such as the global EAS Lipid Clinics Network,89 together with other initiatives supported by the EAS and the International Atherosclerosis Society. Patient advocacy groups are crucial for patient empowerment, and advocating for improved care, access to new therapies, and influencing health policy.
Figure 4.
As shown for high-income countries in the HoFH International Clinical Collaborators registry, multidrug therapy substantially improved low-density lipoprotein cholesterol- lowering. More than 50% of the few patients on five lipid-lowering therapies attained low-density lipoprotein cholesterol goal (defined as <2.5 mmol/L in primary prevention or <1.8 mmol/L in secondary prevention). Adapted with permission from Tromp et al.12
The panel recommends a multifaceted approach including education of all stakeholders, training and upskilling of healthcare providers, creation of a multidisciplinary team, promotion of participatory medicine, creation of academic service partnerships, and provision of adequate funding of health services.90,91 Health policy, as in Japan,92 should focus on country-specific needs and leverage funding for what is a very rare condition with special needs, aiming to improve and sustain best care for patients with HoFH, focusing on promotion of effective detection and funding of advanced therapies. Registry data linked to patient outcomes should be used to inform health policy. Quality clinical registries should also be used as a network of collaborative centres of clinical excellence, able to provide guidance to providers.12
Conclusions
HoFH is still underdiagnosed and/or identified too late and remains undertreated despite its severe adverse impact on cardiovascular health (Graphical abstract). The last decade has seen much progress, particularly with new highly efficacious LDL-C-lowering therapies which offer the prospect of LDL-C goal attainment, leading to improved survival and quality of life. Yet important challenges remain. The priorities are improving education, early diagnosis, and treatment, as well as addressing inequities in access to all therapies in less affluent regions. This updated EAS consensus statement provides pragmatic guidance crucial to driving better care and improved cardiovascular health and quality of life for patients with HoFH worldwide.
Supplementary data
Supplementary data are available at European Heart Journal online.
Supplementary Material
Acknowledgements
European Atherosclerosis Society (EAS) Consensus Panel—Co-chairs: M.C., F.J.R., and R.A.H. The panel convened two virtual meetings; at the first meeting updates to the evidence and issues in HoFH care were discussed and the draft manuscript was discussed at the second meeting.
Contributor Information
Marina Cuchel, Division of Translational Medicine and Human Genetics, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, 9017 Maloney Building, 3600 Spruce Street, Philadelphia, PA 19104, USA.
Frederick J Raal, Carbohydrate and Lipid Metabolism Research Unit, Division of Endocrinology and Metabolism, Department of Medicine, Faculty of Health Sciences, University of the Witwatersrand Parktown, Johannesburg, South Africa.
Robert A Hegele, Department of Medicine and Robarts Research Institute, Schulich School of Medicine and Dentistry, Western University, London, Ontario, Canada.
Khalid Al-Rasadi, Department of Biochemistry, College of Medicine & Health Sciences, Medical Research Center, Sultan Qaboos University, Muscat, Oman.
Marcello Arca, Department of Translational and Precision Medicine, Sapienza University of Rome, Rome, Italy.
Maurizio Averna, Department of Health Promotion Sciences Maternal and Infantile Care, Internal Medicine and Medical Specialities, University of Palermo, Palermo, Italy; Istituto di Biofisica, Consiglio Nazionale delle Ricerche, Genova, Italy.
Eric Bruckert, Pitié-Salpêtrière Hospital and Sorbonne University, Cardio metabolic Institute, Paris, France.
Tomas Freiberger, Centre for Cardiovascular Surgery and Transplantation, and Medical Faculty, Masaryk University, Brno, Czech Republic.
Daniel Gaudet, Clinical Lipidology and Rare Lipid Disorders Unit, Community Genomic Medicine Center, Department of Medicine, Université de Montréal, ECOGENE, Clinical and Translational Research Center, and Lipid Clinic, Chicoutimi Hospital, Chicoutimi, Québec, Canada.
Mariko Harada-Shiba, Cardiovascular Center, Osaka Medical and Pharmaceutical University, Osaka, Japan.
Lisa C Hudgins, Rogosin Institute, Weill Cornell Medical College, New York, NY, USA.
Meral Kayikcioglu, Department of Cardiology, Faculty of Medicine, Ege University, Izmir, Turkey.
Luis Masana, Vascular Medicine and Metabolism Unit, Research Unit on Lipids and Atherosclerosis, Sant Joan University Hospital, Universitat Rovira i Virgili, IISPV CIBERDEM, Reus, Spain.
Klaus G Parhofer, Medizinische Klinik und Poliklinik IV, Ludwigs-Maximilians University Klinikum, Munich, Germany.
Jeanine E Roeters van Lennep, Department of Internal Medicine, Erasmus MC, Medical Center, Rotterdam, The Netherlands.
Raul D Santos, Lipid Clinic, Heart Institute (InCor), University of São Paulo Medical School Hospital, São Paulo, Brazil; Academic Research Organization Hospital Israelita Albert Einstein, Sao Paulo, Brazil.
Erik S G Stroes, Department of Vascular Medicine, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, The Netherlands.
Gerald F Watts, Medical School, University of Western Australia, and Department of Cardiology, Lipid Disorders Clinic, Royal Perth Hospital, Perth, Australia.
Albert Wiegman, Department of Pediatrics, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, The Netherlands.
Jane K Stock, European Atherosclerosis Society, Gothenburg, Sweden.
Lale S Tokgözoğlu, Department of Cardiology, Hacettepe University Faculty of Medicine, Ankara, Turkey.
Alberico L Catapano, IRCCS MultiMedica, and Department of Pharmacological and Biomolecular Sciences, Università degli Studi di Milano, Milan, Italy.
Kausik K Ray, Imperial Centre for Cardiovascular Disease Prevention, Department of Primary Care and Public Health, School of Public Health, Imperial College London, London, UK.
Author contributions
All authors contributed to the manuscript, and the complete manuscript was revised by M.C., R.A.H., F.J.R., A.L.C., and J.K.S. All panel members agreed to the conception and design, contributed to interpretation of available data, reviewed drafts, and approved the final document before submission.
Data availability
No data were generated or analysed for this manuscript.
Funding
All authors declare no funding for this contribution.
References
- 1. Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, et al. . Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014;35:2146–2157. 10.1093/eurheartj/ehu274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. France M, Rees A, Datta D, Thompson G, Capps N, Ferns G, et al. . HEART UK statement on the management of homozygous familial hypercholesterolaemia in the United Kingdom. Atherosclerosis 2016;255:128–139. 10.1016/j.atherosclerosis.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 3. Watts GF, Gidding S, Wierzbicki AS, Toth PP, Alonso R, Brown WV, et al. . Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Int J Cardiol 2014;171:309–325. 10.1016/j.ijcard.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 4. Harada-Shiba M, Arai H, Ishigaki Y, Ishibashi S, Okamura T, Ogura M, et al. . Guidelines for diagnosis and treatment of familial hypercholesterolemia 2017. J Atheroscler Thromb 2018;25:751–770. 10.5551/jat.CR003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brunham LR, Ruel I, Aljenedil S, Riviere JB, Baass A, Tu JV, et al. . Canadian Cardiovascular Society Position Statement on Familial Hypercholesterolemia: update 2018. Can J Cardiol 2018;34:1553–1563. 10.1016/j.cjca.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 6. Watts GF, Sullivan DR, Hare DL, Kostner KM, Horton AE, Bell DA, et al. . Integrated guidance for enhancing the care of familial hypercholesterolaemia in Australia. Heart Lung Circ 2021;30:324–349. 10.1016/j.hlc.2020.09.943 [DOI] [PubMed] [Google Scholar]
- 7. Groselj U, Wiegman A, Gidding SS. Screening in children for familial hypercholesterolaemia: start now. Eur Heart J 2022;43:3209–3212. 10.1093/eurheartj/ehac224 [DOI] [PubMed] [Google Scholar]
- 8. Representatives of the Global Familial Hypercholesterolemia Community, Wilemon KA, Patel J, Aguilar-Salinas C, Ahmed CD, Alkhnifsawi M, et al. . Reducing the clinical and public health burden of familial hypercholesterolemia: a global call to action. JAMA Cardiol 2020;5:217–229. 10.1001/jamacardio.2019.5173 [DOI] [PubMed] [Google Scholar]
- 9. Vallejo-Vaz AJ, Kondapally Seshasai SR, Cole D, Hovingh GK, Kastelein JJ, Mata P, et al. . Familial hypercholesterolaemia: a global call to arms. Atherosclerosis 2015;243:257–259. 10.1016/j.atherosclerosis.2015.09.021 [DOI] [PubMed] [Google Scholar]
- 10. Hu P, Dharmayat KI, Stevens CAT, Sharabiani MTA, Jones RS, Watts GF, et al. . Prevalence of familial hypercholesterolemia among the general population and patients with atherosclerotic cardiovascular disease: a systematic review and meta-analysis. Circulation 2020;141:1742–1759. 10.1161/CIRCULATIONAHA.119.044795 [DOI] [PubMed] [Google Scholar]
- 11. Beheshti SO, Madsen CM, Varbo A, Nordestgaard BG. Worldwide prevalence of familial hypercholesterolemia: meta-analyses of 11 million subjects. J Am Coll Cardiol 2020;75:2553–2566. 10.1016/j.jacc.2020.03.057 [DOI] [PubMed] [Google Scholar]
- 12. Tromp TR, Hartgers ML, Hovingh GK, Vallejo-Vaz AJ, Ray KK, Soran H, et al. . Worldwide experience of homozygous familial hypercholesterolaemia: retrospective cohort study. Lancet 2022;399:719–728. 10.1016/S0140-6736(21)02001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sjouke B, Kusters DM, Kindt I, Besseling J, Defesche JC, Sijbrands EJ, et al. . Homozygous autosomal dominant hypercholesterolaemia in The Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J 2015;36:560–565. 10.1093/eurheartj/ehu058 [DOI] [PubMed] [Google Scholar]
- 14. Bertolini S, Calandra S, Arca M, Averna M, Catapano AL, Tarugi P, et al. . Homozygous familial hypercholesterolemia in Italy: clinical and molecular features. Atherosclerosis 2020;312:72–78. 10.1016/j.atherosclerosis.2020.08.027 [DOI] [PubMed] [Google Scholar]
- 15. Salen G, von Bergmann K, Lutjohann D, Kwiterovich P, Kane J, Patel SB, et al. . Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation 2004;109:966–971. 10.1161/01.CIR.0000116766.31036.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kidambi S, Patel SB. Sitosterolaemia: pathophysiology, clinical presentation and laboratory diagnosis. J Clin Pathol 2008;61:588–594. 10.1136/jcp.2007.049775 [DOI] [PubMed] [Google Scholar]
- 17. Hansel B, Carrie A, Brun-Druc N, Leclert G, Chantepie S, Coiffard AS, et al. . Premature atherosclerosis is not systematic in phytosterolemic patients: severe hypercholesterolemia as a confounding factor in five subjects. Atherosclerosis 2014;234:162–168. 10.1016/j.atherosclerosis.2014.02.030 [DOI] [PubMed] [Google Scholar]
- 18. Hegele RA, Boren J, Ginsberg HN, Arca M, Averna M, Binder CJ, et al. . Rare dyslipidaemias, from phenotype to genotype to management: a European Atherosclerosis Society task force consensus statement. Lancet Diabetes Endocrinol 2020;8:50–67. 10.1016/S2213-8587(19)30264-5 [DOI] [PubMed] [Google Scholar]
- 19. Bjorkhem I. Cerebrotendinous xanthomatosis. Curr Opin Lipidol 2013;24:283–287. 10.1097/MOL.0b013e328362df13 [DOI] [PubMed] [Google Scholar]
- 20. Koyama S, Sekijima Y, Ogura M, Hori M, Matsuki K, Miida T, et al. . Cerebrotendinous xanthomatosis: molecular pathogenesis, clinical spectrum, diagnosis, and disease-modifying treatments. J Atheroscler Thromb 2021;28:905–925. 10.5551/jat.RV17055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berberich AJ, Hegele RA. The complex molecular genetics of familial hypercholesterolaemia. Nat Rev Cardiol 2019;16:9–20. 10.1038/s41569-018-0052-6 [DOI] [PubMed] [Google Scholar]
- 22. D'Erasmo L, Di Costanzo A, Arca M. Autosomal recessive hypercholesterolemia: update for 2020. Curr Opin Lipidol 2020;31:56–61. 10.1097/MOL.0000000000000664 [DOI] [PubMed] [Google Scholar]
- 23. Biesecker LG. Correspondence on: “homozygous familial hypercholesterolemia in Italy: clinical and molecular features”. Atherosclerosis 2021;326:63–64. 10.1016/j.atherosclerosis.2021.03.015 [DOI] [PubMed] [Google Scholar]
- 24. Defesche JC, Gidding SS, Harada-Shiba M, Hegele RA, Santos RD, Wierzbicki AS. Familial hypercholesterolaemia. Nat Rev Dis Primers 2017;3:17093. 10.1038/nrdp.2017.93 [DOI] [PubMed] [Google Scholar]
- 25. Brown EE, Sturm AC, Cuchel M, Braun LT, Duell PB, Underberg JA, et al. . Genetic testing in dyslipidemia: a scientific statement from the National Lipid Association. J Clin Lipidol 2020;14:398–413. 10.1016/j.jacl.2020.04.011 [DOI] [PubMed] [Google Scholar]
- 26. Chora JR, Iacocca MA, Tichy L, Wand H, Kurtz CL, Zimmermann H, et al. . The Clinical Genome Resource (ClinGen) Familial Hypercholesterolemia Variant Curation Expert Panel consensus guidelines for LDLR variant classification. Genet Med 2022;24:293–306. 10.1016/j.gim.2021.09.012 [DOI] [PubMed] [Google Scholar]
- 27. Belanger AM, Akioyamen L, Alothman L, Genest J. Evidence for improved survival with treatment of homozygous familial hypercholesterolemia. Curr Opin Lipidol 2020;31:176–181. 10.1097/MOL.0000000000000686 [DOI] [PubMed] [Google Scholar]
- 28. Lazarte J, Hegele RA. Editorial comment: hazards of interpreting genetic reports. Curr Opin Lipidol 2021;32:81–82. 10.1097/MOL.0000000000000738 [DOI] [PubMed] [Google Scholar]
- 29. Trinder M, Paquette M, Cermakova L, Ban MR, Hegele RA, Baass A, et al. . Polygenic contribution to low-density lipoprotein cholesterol levels and cardiovascular risk in monogenic familial hypercholesterolemia. Circ Genom Precis Med 2020;13:515–523. 10.1161/CIRCGEN.120.002919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang J, Dron JS, Ban MR, Robinson JF, McIntyre AD, Alazzam M, et al. . Polygenic versus monogenic causes of hypercholesterolemia ascertained clinically. Arterioscler Thromb Vasc Biol 2016;36:2439–2445. 10.1161/ATVBAHA.116.308027 [DOI] [PubMed] [Google Scholar]
- 31. D'Erasmo L, Minicocci I, Di Costanzo A, Pigna G, Commodari D, Ceci F, et al. . Clinical implications of monogenic versus polygenic hypercholesterolemia: long-term response to treatment, coronary atherosclerosis burden, and cardiovascular events. J Am Heart Assoc 2021;10:e018932. 10.1161/JAHA.120.018932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown EE. The genetic counselor's role in management of patients with dyslipidemia. Curr Opin Lipidol 2021;32:83–88. 10.1097/MOL.0000000000000732 [DOI] [PubMed] [Google Scholar]
- 33. Expert Panel on Integrated Guidelines for Cardiovascular Health, Risk Reduction in Children, Adolescents, National Heart Lung, Blood Institute . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011;128:S213–S256. 10.1542/peds.2009-2107C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Institute for Health and Care Excellence . Familial hypercholesterolaemia: identification and management. Updated 4 October 2019.https://www.nice.org.uk/guidance/cg71/chapter/Recommendations (14 August 2022, date last accessed). [PubMed]
- 35. Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, Cuchel M, et al. . Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J 2015;36:2425–2437. 10.1093/eurheartj/ehv157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gidding SS, Wiegman A, Groselj U, Freiberger T, Peretti N, Dharmayat KI, et al. . Paediatric familial hypercholesterolaemia screening in Europe—public policy background and recommendations. Eur J Prev Cardiol 2022;29:2301–2311. 10.1093/eurjpc/zwac200 [DOI] [PubMed] [Google Scholar]
- 37. Klancar G, Groselj U, Kovac J, Bratanic N, Bratina N, Trebusak Podkrajsek K, et al. . Universal screening for familial hypercholesterolemia in children. J Am Coll Cardiol 2015;66:1250–1257. 10.1016/j.jacc.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 38. Bedlington N, Abifadel M, Beger B, Bourbon M, Bueno H, Ceska R, et al. . The time is now: achieving FH paediatric screening across Europe—the Prague declaration. GMS Health Innov Technol 2022;16:Doc04. 10.3205/hta000136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ibarretxe D, Rodriguez-Borjabad C, Feliu A, Bilbao JA, Masana L, Plana N. Detecting familial hypercholesterolemia earlier in life by actively searching for affected children: the DECOPIN project. Atherosclerosis 2018;278:210–216. 10.1016/j.atherosclerosis.2018.09.039 [DOI] [PubMed] [Google Scholar]
- 40. Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, et al. . Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J 2022;43:3925–3946. 10.1093/eurheartj/ehac361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luirink IK, Kuipers IM, Hutten BA, Planken RN, Backx A, Groothoff JW, et al. . Coronary computed tomography angiography and echocardiography in children with homozygous familial hypercholesterolemia. Atherosclerosis 2019;285:87–92. 10.1016/j.atherosclerosis.2019.04.219 [DOI] [PubMed] [Google Scholar]
- 42. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. . 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 43. Ray KK, Reeskamp LF, Laufs U, Banach M, Mach F, Tokgozoglu LS, et al. . Combination lipid-lowering therapy as first-line strategy in very high-risk patients. Eur Heart J 2022;43:830–833. 10.1093/eurheartj/ehab718 [DOI] [PubMed] [Google Scholar]
- 44. Mohamed F, Seedat F, Raal FJ. Novel therapies for familial hypercholesterolemia. Curr Opin Endocrinol Diabetes Obes 2021;28:188–195. 10.1097/MED.0000000000000590 [DOI] [PubMed] [Google Scholar]
- 45. Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, et al. . Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA part B): a randomised, double-blind, placebo-controlled trial. Lancet 2015;385:341–350. 10.1016/S0140-6736(14)61374-X [DOI] [PubMed] [Google Scholar]
- 46. Blom DJ, Harada-Shiba M, Rubba P, Gaudet D, Kastelein JJP, Charng MJ, et al. . Efficacy and safety of alirocumab in adults with homozygous familial hypercholesterolemia: the ODYSSEY HoFH trial. J Am Coll Cardiol 2020;76:131–142. 10.1016/j.jacc.2020.05.027 [DOI] [PubMed] [Google Scholar]
- 47. Santos RD, Stein EA, Hovingh GK, Blom DJ, Soran H, Watts GF, et al. . Long-term evolocumab in patients with familial hypercholesterolemia. J Am Coll Cardiol 2020;75:565–574. 10.1016/j.jacc.2019.12.020 [DOI] [PubMed] [Google Scholar]
- 48. Raal FJ, Hovingh GK, Blom D, Santos RD, Harada-Shiba M, Bruckert E, et al. . Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol 2017;5:280–290. 10.1016/S2213-8587(17)30044-X [DOI] [PubMed] [Google Scholar]
- 49. Stein EA, Honarpour N, Wasserman SM, Xu F, Scott R, Raal FJ. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation 2013;128:2113–2120. 10.1161/CIRCULATIONAHA.113.004678 [DOI] [PubMed] [Google Scholar]
- 50. Reijman MD, Kusters DM, Wiegman A. Advances in familial hypercholesterolaemia in children. Lancet Child Adolesc Health 2021;5:652–661. 10.1016/S2352-4642(21)00095-X [DOI] [PubMed] [Google Scholar]
- 51. Hussain MM, Rava P, Walsh M, Rana M, Iqbal J. Multiple functions of microsomal triglyceride transfer protein. Nutr Metab (Lond) 2012;9:14. 10.1186/1743-7075-9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. D'Erasmo L, Cefalu AB, Noto D, Giammanco A, Averna M, Pintus P, et al. . Efficacy of lomitapide in the treatment of familial homozygous hypercholesterolemia: results of a real-world clinical experience in Italy. Adv Ther 2017;34:1200–1210. 10.1007/s12325-017-0531-x [DOI] [PubMed] [Google Scholar]
- 53. Cuchel M, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Hegele RA, et al. . Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet 2013;381:40–46. 10.1016/S0140-6736(12)61731-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. D'Erasmo L, Gallo A, Cefalu AB, Di Costanzo A, Saheb S, Giammanco A, et al. . Long-term efficacy of lipoprotein apheresis and lomitapide in the treatment of homozygous familial hypercholesterolemia (HoFH): a cross-national retrospective survey. Orphanet J Rare Dis 2021;16:381. 10.1186/s13023-021-01999-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. D'Erasmo L, Steward K, Cefalu AB, Di Costanzo A, Boersma E, Bini S, et al. . Efficacy and safety of lomitapide in homozygous familial hypercholesterolaemia: the pan-European retrospective observational study. Eur J Prev Cardiol 2022;29:832–841. 10.1093/eurjpc/zwab229 [DOI] [PubMed] [Google Scholar]
- 56. Blom DJ, Averna MR, Meagher EA, du Toit Theron H, Sirtori CR, Hegele RA, et al. . Long-term efficacy and safety of the microsomal triglyceride transfer protein inhibitor lomitapide in patients with homozygous familial hypercholesterolemia. Circulation 2017;136:332–335. 10.1161/CIRCULATIONAHA.117.028208 [DOI] [PubMed] [Google Scholar]
- 57. Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJP, Rubba P, et al. . Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med 2020;383:711–720. 10.1056/NEJMoa2004215 [DOI] [PubMed] [Google Scholar]
- 58. Gaudet D, Iannuzzo G, Stefanutti C, Stroes ES, Rosenson RS, Turner T, et al. . Long-term efficacy and safety of evinacumab in adult and adolescent patients with homozygous familial hypercholesterolemia. Circulation 2021;144:A12756. [Google Scholar]
- 59. Raal FJ, Reeskamp R, Kastelein JJ, Rubba P, Duell B, Koseki M, et al. . The long-term safety and efficacy of evinacumab in patients with homozygous familial hypercholesterolemia. Circulation 2021;144:A12066. 10.1161/circ.144.suppl_1.12066 [DOI] [Google Scholar]
- 60. Stefanutti C, Julius U, Watts GF, Harada-Shiba M, Cossu M, Schettler VJ, et al. . Toward an international consensus-integrating lipoprotein apheresis and new lipid-lowering drugs. J Clin Lipidol 2017;11:858–871.e3. 10.1016/j.jacl.2017.04.114 [DOI] [PubMed] [Google Scholar]
- 61. Alothman L, Belanger AM, Ruel I, Brunham LR, Hales L, Genest J, et al. . Health-related quality of life in homozygous familial hypercholesterolemia: a systematic review and meta-analysis. J Clin Lipidol 2022;16:52–65. 10.1016/j.jacl.2021.11.014 [DOI] [PubMed] [Google Scholar]
- 62. Kayikcioglu M, Tokgozoglu L, Yilmaz M, Kaynar L, Aktan M, Durmus RB, et al. . A nation-wide survey of patients with homozygous familial hypercholesterolemia phenotype undergoing LDL-apheresis in Turkey (A-HIT 1 registry). Atherosclerosis 2018;270:42–48. 10.1016/j.atherosclerosis.2018.01.034 [DOI] [PubMed] [Google Scholar]
- 63. Pottle A, Thompson G, Barbir M, Bayly G, Cegla J, Cramb R, et al. . Lipoprotein apheresis efficacy, challenges and outcomes: a descriptive analysis from the UK lipoprotein apheresis registry, 1989–2017. Atherosclerosis 2019;290:44–51. 10.1016/j.atherosclerosis.2019.09.006 [DOI] [PubMed] [Google Scholar]
- 64. Kroon AA, van't Hof MA, Demacker PN, Stalenhoef AF. The rebound of lipoproteins after LDL-apheresis. Kinetics and estimation of mean lipoprotein levels. Atherosclerosis 2000;152:519–526. 10.1016/S0021-9150(00)00371-3 [DOI] [PubMed] [Google Scholar]
- 65. Thompson GR, Barbir M, Davies D, Dobral P, Gesinde M, Livingston M, et al. . Efficacy criteria and cholesterol targets for LDL apheresis. Atherosclerosis 2010;208:317–321. 10.1016/j.atherosclerosis.2009.06.010 [DOI] [PubMed] [Google Scholar]
- 66. Ibrahim M, El-Hamamsy I, Barbir M, Yacoub MH. Translational lessons from a case of combined heart and liver transplantation for familial hypercholesterolemia 20 years post-operatively. J Cardiovasc Transl Res 2012;5:351–358. 10.1007/s12265-011-9311-1 [DOI] [PubMed] [Google Scholar]
- 67. El-Rassi I, Chehab G, Saliba Z, Alawe A, Jebara V. Fatal cardiac atherosclerosis in a child 10 years after liver transplantation: a case report and a review. J Clin Lipidol 2011;5:329–332. 10.1016/j.jacl.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 68. Martinez M, Brodlie S, Griesemer A, Kato T, Harren P, Gordon B, et al. . Effects of liver transplantation on lipids and cardiovascular disease in children with homozygous familial hypercholesterolemia. Am J Cardiol 2016;118:504–510. 10.1016/j.amjcard.2016.05.042 [DOI] [PubMed] [Google Scholar]
- 69. Al Dubayee M, Kayikcioglu M, van Lennep JR, Hergli N, Mata P. Is liver transplant curative in homozygous familial hypercholesterolemia? A review of nine global cases. Adv Ther 2022;39:3042–3057. 10.1007/s12325-022-02131-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ishigaki Y, Kawagishi N, Hasegawa Y, Sawada S, Katagiri H, Satomi S, et al. . Liver transplantation for homozygous familial hypercholesterolemia. J Atheroscler Thromb 2019;26:121–127. 10.5551/jat.RV17029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cephus CE, Qureshi AM, Sexson Tejtel SK, Alam M, Moodie DS. Coronary artery disease in a child with homozygous familial hypercholesterolemia: regression after liver transplantation. J Clin Lipidol 2019;13:880–886. 10.1016/j.jacl.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 72. Schmidt HH, Tietge UJ, Buettner J, Barg-Hock H, Offner G, Schweitzer S, et al. . Liver transplantation in a subject with familial hypercholesterolemia carrying the homozygous p.W577R LDL-receptor gene mutation. Clin Transplant 2008;22:180–184. 10.1111/j.1399-0012.2007.00764.x [DOI] [PubMed] [Google Scholar]
- 73. Revell SP, Noble-Jamieson G, Johnston P, Rasmussen A, Jamieson N, Barnes ND. Liver transplantation for homozygous familial hypercholesterolaemia. Arch Dis Child 1995;73:456–458. 10.1136/adc.73.5.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Greco M, Robinson JD, Eltayeb O, Benuck I. Progressive aortic stenosis in homozygous familial hypercholesterolemia after liver transplant. Pediatrics 2016;138:e20160740. 10.1542/peds.2016-0740 [DOI] [PubMed] [Google Scholar]
- 75. Bajaj A, Cuchel M. Advancements in the treatment of homozygous familial hypercholesterolemia. J Atheroscler Thromb 2022;29:1125–1135. 10.5551/jat.RV17065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cesaro A, Fimiani F, Gragnano F, Moscarella E, Schiavo A, Vergara A, et al. . New frontiers in the treatment of homozygous familial hypercholesterolemia. Heart Fail Clin 2022;18:177–188. 10.1016/j.hfc.2021.07.008 [DOI] [PubMed] [Google Scholar]
- 77. Chadwick AC, Evitt NH, Lv W, Musunuru K. Reduced blood lipid levels with in vivo CRISPR-Cas9 base editing of ANGPTL3. Circulation 2018;137:975–977. 10.1161/CIRCULATIONAHA.117.031335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Musunuru K, Chadwick AC, Mizoguchi T, Garcia SP, DeNizio JE, Reiss CW, et al. . In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature 2021;593:429–434. 10.1038/s41586-021-03534-y [DOI] [PubMed] [Google Scholar]
- 79. Verve Therapeutics 2022. https://ir.vervetx.com/news-releases/news-release-details/verve-therapeutics-provides-regulatory-update-verve-101 (22 December 2022, date last accessed).
- 80. Amundsen AL, Khoury J, Iversen PO, Bergei C, Ose L, Tonstad S, et al. . Marked changes in plasma lipids and lipoproteins during pregnancy in women with familial hypercholesterolemia. Atherosclerosis 2006;189:451–457. 10.1016/j.atherosclerosis.2006.01.002 [DOI] [PubMed] [Google Scholar]
- 81. Ogura M, Makino H, Kamiya C, Yoshimatsu J, Soran H, Eatough R, et al. . Lipoprotein apheresis is essential for managing pregnancies in patients with homozygous familial hypercholesterolemia: seven case series and discussion. Atherosclerosis 2016;254:179–183. 10.1016/j.atherosclerosis.2016.10.018 [DOI] [PubMed] [Google Scholar]
- 82. Russi G. Severe dyslipidemia in pregnancy: the role of therapeutic apheresis. Transfus Apher Sci 2015;53:283–287. 10.1016/j.transci.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 83. Graham DF, Raal FJ. Management of familial hypercholesterolemia in pregnancy. Curr Opin Lipidol 2021;32:370–377. 10.1097/MOL.0000000000000790 [DOI] [PubMed] [Google Scholar]
- 84. US Food and Drug Administration . Drug Safety Communication 7-20-2021. FDA requests removal of strongest warning against using cholesterol-lowering statins during pregnancy; still advises most pregnant patients should stop taking statins. 2021.https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-removal-strongest-warning-against-using-cholesterol-lowering-statins-during-pregnancy#:∼:text=Statins%20are%20safe%20to%20prescribe%20in%20patients%20who%20are%20not,not%20generally%20necessary%20during%20pregnancy (14 August 2022, date last accessed).
- 85. Bruckert E, Saheb S, Bonte JR, Coudray-Omnes C. Daily life, experience and needs of persons suffering from homozygous familial hypercholesterolaemia: insights from a patient survey. Atheroscler Suppl 2014;15:46–51. 10.1016/j.atherosclerosissup.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 86. Kayikcioglu M, Kuman-Tuncel O, Pirildar S, Yilmaz M, Kaynar L, Aktan M, et al. . Clinical management, psychosocial characteristics, and quality of life in patients with homozygous familial hypercholesterolemia undergoing LDL-apheresis in Turkey: results of a nationwide survey (A-HIT1 registry). J Clin Lipidol 2019;13:455–467. 10.1016/j.jacl.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 87. Mulder J, Kranenburg LW, Treling WJ, Hovingh GK, Rutten JHW, Busschbach JJ, et al. . Quality of life and coping in Dutch homozygous familial hypercholesterolemia patients: a qualitative study. Atherosclerosis 2022;348:75–81. 10.1016/j.atherosclerosis.2022.03.015 [DOI] [PubMed] [Google Scholar]
- 88. Cesaro A, Gragnano F, Fimiani F, Moscarella E, Diana V, Pariggiano I, et al. . Impact of PCSK9 inhibitors on the quality of life of patients at high cardiovascular risk. Eur J Prev Cardiol 2020;27:556–558. 10.1177/2047487319839179 [DOI] [PubMed] [Google Scholar]
- 89. Alieva AS, Tokgozoglu L, Ray KK, Catapano AL. Lipid clinics network. Rationale and design of the EAS global project. Atheroscler Suppl 2020;42:e6–e8. 10.1016/j.atherosclerosissup.2021.01.002 [DOI] [PubMed] [Google Scholar]
- 90. Sarkies MN, Jones LK, Gidding SS, Watts GF. Improving clinical practice guidelines with implementation science. Nat Rev Cardiol 2022;19:3–4. 10.1038/s41569-021-00645-x [DOI] [PubMed] [Google Scholar]
- 91. Watts GF, Gidding SS, Mata P, Pang J, Sullivan DR, Yamashita S, et al. . Familial hypercholesterolaemia: evolving knowledge for designing adaptive models of care. Nat Rev Cardiol 2020;17:360–377. 10.1038/s41569-019-0325-8 [DOI] [PubMed] [Google Scholar]
- 92. Tada H, Kurashina T, Ogura M, Takegami M, Miyamoto Y, Arai H, et al. . Prospective Registry Study of Primary Dyslipidemia (PROLIPID): rationale and study design. J Atheroscler Thromb 2022;29:953–969. 10.5551/jat.63222 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data were generated or analysed for this manuscript.