Abstract
Background
Pragmatic trials provide decision-oriented, real-world evidence that is highly applicable and generalizable. The interest in real-world evidence is fueled by the assumption that effects in the “real-world” are different to effects obtained under artificial, controlled, research conditions as often used for traditional explanatory trials. However, it is unknown which features of pragmatism, generalizability, and applicability would be responsible for such differences. There is a need to provide empirical evidence and promote meta-research to answer these fundamental questions on the pragmatism of randomized trials and real-world evidence. Here, we describe the rationale and design of the PragMeta database which pursues this goal (www.PragMeta.org).
Methods
PragMeta is a non-commercial, open data platform and infrastructure to facilitate research on pragmatic trials. It collects and shares data from published randomized trials that either have a specific design feature or other characteristic related to pragmatism or they form clusters of trials addressing the same research question but having different aspects of pragmatism. This lays the foundation to determine the relationship of various features of pragmatism, generalizability, and applicability with intervention effects or other trial characteristics.
The database contains trial data actively collected for PragMeta but also allows to import and link existing datasets of trials collected for other purposes, forming a large-scale meta-database. PragMeta captures data on (1) trial and design characteristics (e.g., sample size, population, intervention/comparison, outcome, longitudinal structure, blinding), (2) effects estimates, and (3) various determinants of pragmatism (e.g., the use of routinely collected data) and ratings from established tools used to determine pragmatism (e.g., the PRagmatic–Explanatory Continuum Indicator Summary 2; PRECIS-2).
PragMeta is continuously provided online, inviting the meta-research community to collaborate, contribute, and/or use the database. As of April 2023, PragMeta contains data from > 700 trials, mostly with assessments on pragmatism.
Conclusions
PragMeta will inform a better understanding of pragmatism and the generation and interpretation of real-world evidence.
Keywords: Pragmatic clinical trial [MeSH], Real-world clinical trials, Naturalistic randomized clinical trial, Databases, Bibliographic [MeSH]
Background
Pragmatic RCTs have been proposed to merge the advantages of using real-world data and following routine care (maximizing external validity) with the scientific rigor of RCTs (maximizing internal validity) [1]. Their main purpose is to inform decision-making in routine care, which requires high applicability and generalizability. Non-pragmatic, so-called explanatory, trials aim primarily at explaining the underlying mechanisms of treatment effects than to directly inform health care decisions. Pragmatic trials, their design, and their assessment are getting increasing attention with the creation of tools and initiatives such as the GetReal Trial Tool by the GetReal Initiative [2, 3]), a living textbook of pragmatic clinical trials by the National Institutes of Health [4], and the PRagmatic–Explanatory Continuum Indicator Summary 2 (PRECIS-2) to measure the pragmatism of a trial [5]. However, many trials labeled as “pragmatic” have features that are not compatible with routine care (e.g., the use of placebo control or double blinding), and the label “pragmatic” does not guarantee pragmatism and applicability of results [6, 7]. Many trials have several pragmatic features, but few have all or even most of them, and so it remains crucial to have a more in-depth understanding of these factors to better determine the impact of pragmatism on the estimation of treatment effects and evidence-based decisions.
Part of the emphasis on “real-world evidence” is the underlying assumption that studies with high generalizability and applicability would provide different treatment effect estimates than other studies. There is often the assumption that trials with non-pragmatic features that are typically done under artificial “ideal” and controlled settings with highly selected patients show stronger treatment effects on the desired endpoints [8, 9]. It is often argued this may result from better adherence [8], but we recently showed that adherence-adjusted effects are often similar to other effects [10]. It is also possible that including fewer patients with comorbidities leads to smaller differences with regard to adverse effects and harms. However, some empirical evaluations indicate that the overall degree of pragmatism can influence treatment effects estimates in meta-analyses [11, 12] and may also increase between-study heterogeneity [11, 13], which can limit the usefulness of meta-analyses for health technology assessments (and reimbursement decisions) [14] or clinical guidelines (and clinical decisions) [15]. Overall, the impact of features of pragmatism, generalizability, and applicability on treatment effects is unknown, and there is no large-scale systematic empirical evidence on this issue.
Here, we describe the rationale and design of the PragMeta database. The PragMeta database is a non-commercial, open data platform and infrastructure which contains information on randomized trials with various degrees of pragmatic features and it is designed to facilitate research on the pragmatism of randomized trials. These research projects may analyze the characteristics of pragmatic trials, assess their pragmatism, and/or empirically evaluate the meta-epidemiology of trial characteristics related to pragmatism, generalizability, applicability, and their impact on treatment effect estimates.
Methods
In this methodological outline, we focus on the data infrastructure, processes, and general methods that are applied to feed the PragMeta database. PragMeta is managed using Directus, a free and open-source collaborative app to set up databases [16] that has been applied in other meta-research projects also lead by us [17, 18]. A data scientist (PD) developed the data infrastructure and website (www.PragMeta.org).
Eligibility criteria
PragMeta contains trials that are randomized (excluding trials described as “quasi-randomized” or “controlled before and after design”) and fulfill one of the following conditions: ratings from established tools used to determine pragmatism available (e.g., overall or per domain PRECIS-2 score) or presence of a key determinant of pragmatism (e.g., self-labeled as “pragmatic”).
There are no restrictions on publication type and year.
Data sources and organizational structure of PragMeta
Modules
The trials in PragMeta come from various sources, provided in a modular fashion. Modules are constructed around a specific research question and may have trial data that are provided via linkage and import of existing trials not identified and collected for the purpose of PragMeta or actively identified and collected for PragMeta (details on both types are provided in the following chapter).
The modules fall into two major categories with possible overlap: (a) collections of randomized trials that all share a specific design feature or other characteristic related to pragmatism and (b) collections of systematically identified randomized trials that may share a common design feature or a common clinical question and have information available on features related to pragmatism. Examples for (a) may be a collection of randomized trials that are self-labeled as “pragmatic” or that use routinely collected health data and examples for (b) may be a collection of randomized trials in a systematic review that were assessed with a tool such as PRECIS-2 for pragmatism or randomized trials in a disease/population.
First modules to actively collect data for PragMeta are underway and the description of specific underlying methods is published on Open Science Framework (OSF) [19]. These modules (as of April 2023) are shown in Table 1. All present modules, except PragSurgery, have been designed by the PragMeta team and are influenced by the clinical topics and research interest of our team. Additional modules may be initiated by the PragMeta team and/or collaborators in the future.
Table 1.
Title and topic of ongoing modules that feed the PragMeta database (as of April 2023)
| Actively identified and collected for PragMeta: |
| PragCOVID: Overview of COVID-19 randomized trials self-labeled as pragmatic. It included 37 trials |
| PragMS: Overview of pragmatic trials in multiple sclerosis [20]. All multiple sclerosis randomized trials likely to be pragmatic have been identified systematically and were assessed using PRECIS-2 for pragmatism. It includes 48 trials |
| PragQoL: Impact of pragmatism on the assessment of patient-reported outcomes (pain, fatigue, and quality of life) compared with objective clinical outcomes [21]. Cochrane reviews which include a pragmatic labeled trial in meta-analyses assessing pain, fatigue or quality of life and an objective outcome have been systematically identified. It includes 52 trials from 9 clusters |
| Linkage and import of existing trials not identified and collected for the purpose of PragMeta: |
| PragEpi: Association of pragmatism with treatment effect estimates [22]. This project collects trials from systematic review and meta-analyses citing and assessing PRECIS-2. It includes 185 trials from 18 clusters |
| PragSurgery: Collection of PRECIS-2 assessment in surgery trials. This module is the first large actively shared data collection integrated in PragMeta |
Except from PragSurgery, all modules have been designed by the PragMeta team
Cluster
For modules that refer to category (b), systematically identified trials that share a common clinical question form a cluster; hence, such a module is fed by multiple clusters. While not all the trials in a cluster may share a specific design feature or other characteristic related to pragmatism (e.g., are labeled as “pragmatic”), they all assess effects in the same population, intervention, comparator, and outcome, but with different aspects of pragmatism (from none to all) or differences in other features related to pragmatism. Such trials may be very non-pragmatic and are included in PragMeta to determine the relationship of features of pragmatism, generalizability, and applicability with intervention effects or other characteristics.
Linkage and import of existing datasets of trials
Existing datasets of randomized trials with common features or assessing the same clinical question which report the ratings of pragmatism using PRECIS-2 or another tool per trial (see category b above) can be systematically imported into the PragMeta database. Currently, we have systematically identified all systematic reviews and meta-analyses that have cited and assessed the PRECIS-2 (see Table 1 module PragEpi). We are also importing datasets non-systematically identified and/or provided by collaborators and research partners.
Active data collection: Index trial approach
To identify clusters of trials reflecting a broad spectrum of aspects of pragmatism, an “index trial approach” can be used. This approach allows an efficient collection of relevant trials with various degrees of pragmatism on the same clinical question. Since most trials conducted thus far are not pragmatic, this approach increases the chance that identified collections of trials answering the same research questions include at least one pragmatic trial and therefore reflect a broader spectrum of aspects of pragmatism. Index trials are such trials, presenting one or more features that indicate potential pragmatism (e.g., are labeled as “pragmatic”). They are used as starting point to obtain multiple other trials presenting variable aspects of pragmatism and addressing the same topics. Such trials are referred to as corresponding trials. It is assumed that trials that are all included in the same meta-analysis of a clinical systematic review address the same topic. This approach is efficient as it relies on available systematic searches from clinical systematic reviews (e.g., Cochrane reviews), ensuring a comprehensive set of multiple studies on the same research question.
Identification of index trials
A sample of index trials can be obtained by searches in literature databases using a search component focusing on pragmatic trials. Either a simplified search to identify RCTs self-labeled as “pragmatic” in title and/or abstracts (i.e., “(pragmatic$ or naturalistic) and trial).ab,ti.”) or a more complex search string designed for, e.g., Ovid MEDLINE may be used [23]. Depending on the module of interest, other relevant components may be added to the search strategy.
Identification of corresponding trials
To identify corresponding trials, automatic citation-based searches are used (e.g., with the iCiteR package for R [24]) to search for systematic reviews (e.g., Cochrane reviews) citing the index trial of interest. The focus is on Cochrane reviews due to their high methodological standards, and transparent and standardized reporting [25], but using other original systematic reviews is possible.
Once relevant citing systematic reviews are identified, it needs to be determined if the index trial is actually included with other trials on the same clinical question. For Cochrane reviews, this can be assumed if the index trial is meta-analytically combined with other trials, the procedure would then be as follows: (1) check if the index trials are listed in the section “References to studies included in this review” and if it is included in a meta-analysis. If multiple meta-analyses within a Cochrane review or across multiple reviews include the index trial, the selection of the eligible meta-analysis may be guided by the following hierarchy of rules:
Meta-analysis reporting the outcome of interest of our module (if not defined, next level or if more than one, go to level d)
Meta-analysis reporting the primary outcome of our index RCT (if not available or more than one, next level)
Meta-analysis reporting the primary outcome as identified by the Cochrane review in which it is included (if does not include the identified index RCTs or more than one, next level)
Meta-analysis reporting the largest number of RCTs (if more than one, next level)
Meta-analysis reporting the largest total sample size
This process may be adapted for types of evidence synthesis other than Cochrane reviews.
Data entry
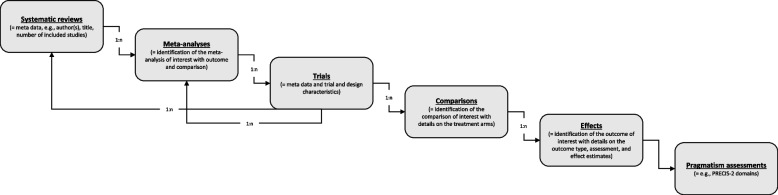
PragMeta data may come from data import or active extraction as described above. The database feeding procedure is organized in six hierarchical collections: systematic reviews, meta-analyses (included in the systematic reviews), trials (included in the meta-analyses or individual), comparisons (in the trials), effects (of the comparisons), pragmatism assessments. Thus, to an entry of a superordinated collection (e.g., systematic reviews or meta-analyses), one or more entries of a subordinated collection (e.g., trials) may be assigned. For example, a systematic review may contain several meta-analyses, or a meta-analysis contains several trials, and, specifically, a trial may be assigned to multiple systematic reviews and/or meta-analyses.
The data source for extractions at the systematic reviews, meta-analyses, comparisons, and effects level is the systematic review (e.g., Cochrane review); for extractions at the trials and pragmatism assessments level, the data source is the original trial report.
An overview of core variables per collection is given in Table 2. How the collections are related is shown in Fig. 1. A comprehensive list of variables and explanations can be found in the procedure document on OSF [19].
Table 2.
Core variables per collection
| Variable | Data type | Description |
|---|---|---|
| Systematic reviews | ||
| Digital objective identifier (DOI) | Alphanumeric | Unique Digital Object Identifier |
| Publication year | Categorical | Year of publication |
| First author | Alphanumeric | Name of the first author |
| Title | Alphanumeric | Title of the publication |
| Number of studies included | Numeric | Number of studies included in the quantitative synthesis of the systematic review |
| Meta-analyses | ||
| Number of trials included | Numeric | Number of trials included in the meta-analysis regardless if they are eligible for PragMeta or not |
| Comparison | Alphanumeric | Details on the comparison assessed in the meta-analysis, e.g., “Shared decision-making versus usual care” |
| Outcome | Alphanumeric | Outcome used in the meta-analysis |
| Trials | ||
| DOI | Alphanumeric | Unique Digital Object Identifier |
| Publication year | Categorical | Year of publication |
| First author | Alphanumeric | Name of the first author |
| Title | Alphanumeric | Title of the publication |
| Trial category | Categorical | Index or corresponding trial |
| Trial registration | Alphanumeric | Any identification number that can be traced back to an official trial registry |
| Country of conduct | Alphanumeric | Country or countries of conduct |
| Trial purpose | Categorical | E.g., treatment, prevention, supportive care |
| Funding | Categorical | E.g., funded by industry/for profit or public/not-for-profit |
| Patient representatives | Categorical | Indicates if author mention the participation of patients’ representatives at any level of conceptualization, conduct, or reporting of the trial |
| Disease | Alphanumeric | Brief description of the disease |
| Therapeutic area | Categorical | E.g., cardiology, neurology, dermatology |
| Patient type | Categorical | Population or setting, e.g., outpatients, healthy volunteers, general population |
| Randomization | Categorical | Assessment of the randomization process |
| Blindinga | Categorical | None, single, double, or unclear |
| Longitudinal structure | Categorical | Parallel, crossover, factorial, or other |
| Advanced design features | Categorical | E.g., platform, adaptive, remote |
| Number of sites | Numeric | Number of trials sites/study centers |
| Number of arms | Numeric | Number of trial arms |
| Methods to collect informed consenta | Categorical | Waived, written, orally, in person, online, unclear, not reported (multiple choices) |
| Use of routinely collected dataa | Categorical | Yes, no, unclear |
| Comparisons | ||
| Intervention type | Categorical | E.g., care management, drug, lifestyle |
| Comparison typea | Categorical | E.g., care management, drug, lifestyle |
| Backbone | Categorical | If there is a common backbone therapy |
| Effects | ||
| Outcome | Alphanumeric | Brief description of the outcome |
| Outcome typea | Categorical | E.g., clinical, surrogate, biomarker |
| Outcome reported by | Categorical | E.g., investigator, patient, carer |
| Outcome assessor blinded | Categorical | Yes, no, partly, unclear |
| Length of follow-up in months | Numeric | Number of months as the patient being followed during the trial until the latest measure of the outcomes |
| Metric | Alphanumeric | Metric of the estimated mean difference or of the estimated ratio, e.g., odds ratio, mean change |
| Dispersion variable | Alphanumeric | E.g., standard deviation, standard error |
| Outcome direction | Categorical | Whether the outcome occurs more or is improved in participants in the intervention arm compared with comparison arm or in participants in the comparison arm compared with intervention arm |
| Outcome scale | Categorical | Whether the outcome translate a positive (e.g., clinical improvement) or negative effect (e.g., death) for the participant |
| Participants in intervention arm | Numeric | Number of participants in the intervention arm |
| Participants in comparison arm | Numeric | Number of participants in the comparison arm |
| Events intervention arm | Numeric | Number of events in the intervention arm |
| Events comparison arm | Numeric | Number of events in the comparison arm |
| Estimated ratio | Numeric | Estimated ratio of intervention and comparison arm |
| Estimated ratio lower limit | Numeric | Estimated ratio lower limit of 95% confidence interval |
| Estimated ratio upper limit | Numeric | Estimated ratio upper limit of 95% confidence interval |
| Pragmatism assessment | ||
| PRECIS-2 Domain | ||
| Eligibilityb | Alphanumeric | Who is/which clusters are selected to participate in the trial? |
| Recruitmentb | Alphanumeric | How are participants/clusters recruited into the trial? |
| Setting | Alphanumeric | Where is the trial being done? |
| Organization | Alphanumeric | What experience and resources are needed to deliver the intervention? |
| Flexibility: delivery | Alphanumeric | How should the intervention be delivered? |
| Flexibility: adherence | Alphanumeric | What measures are in place to make sure participants adhere to the intervention? |
| Follow-up | Alphanumeric | How closely are participants followed-up? |
| Primary outcome | Alphanumeric | How relevant is it to participants? |
| Primary analysis | Alphanumeric | To what extent are all data included? |
aPotential determinants of pragmatism
bIn case of cluster-RCTs, this domain is assessed on the individual participant level and on the cluster level, respectively
Fig. 1.
Structure of the PragMeta database used for data collection and hierarchical management of collections (figure design inspired by Ladanie et al. [26]). n, number; PRECIS-2, PRagmatic–Explanatory Continuum Indicator Summary 2
The level of data entry may vary between specific modules and imported existing datasets, and thus missing data may occur. For imported existing datasets, datasets are matched to our database infrastructure, but no additional data quality controls are carried out. For modules conducted by us, one reviewer enters/extracts and assesses the included trials. This procedure is complemented through entry/extraction and assessment by a second reviewer (independently in duplicate or not, depending on the module). The backend infrastructure of the database enables to indicate the entry/extraction status as well as the possibility to keep track of verifications within each of the six collections. PRECIS-2 assessments done by the PragMeta team are performed by one reviewer or by two independent reviewers (depending on the module). The infrastructure of the database allows to compare multiple PRECIS-2 assessments and to designate a consented version that is shown on the PragMeta website. Furthermore, as indicators of data quality, the data source, and who extracted the data and/or rated pragmatism using PRECIS-2 or another tool (e.g., PragMeta team, publication team of the original dataset, trial investigators) is always recorded. The frequency for updating the search, screening, and data entry may vary between specific modules.
The PragMeta database is publicly available and can be downloaded. For contributions and collaborations including active data entry and data import, we invite research groups to contact us.
Data on pragmatism
The information collected in PragMeta about pragmatism, for now, includes the assessment of PRECIS-2 which comprises 9 domains addressing features of a RCT that might impact the pragmatic-explanatory continuum (Table 2). Each domain receives a score based on a 5-point Likert scale (1 = very explanatory; 3 = equally pragmatic and explanatory; and 5 = very pragmatic; or no information). We also collect specific trial characteristics or determinants, not captured by PRECIS-2, that may be applicable to more pragmatic or explanatory approaches. Such determinants include, for example, (i) the use of routine collected data and how they are used (e.g., to recruit and/or to collect outcome data), (ii) how is informed consent collected, (iii) use of blinding (e.g., double-blind), (iv) type of control (e.g., placebo or standard of care), and (vi) type of outcome (e.g., patient-reported or surrogate outcome). PragMeta can be expanded by further variables, reflecting for example further determinants of pragmatism identified in due course.
Data sharing
PragMeta is an open data platform. The cleaned datasets are continuously provided online and can be downloaded in various formats.
Conclusions
The PragMeta database serves as a collection of trials that are used for multiple and diverse research projects on the generalizability, applicability, and pragmatism of clinical trials; including, but not limited to, meta-epidemiological studies on the comparison of treatment estimates between trials with different levels of pragmatic and explanatory features. PragMeta is, to our knowledge, the first freely available, large-scale, collaborative database with information on pragmatism, other design features, and treatment effects.
Overall, with the PragMeta database, we aim at providing a platform and an infrastructure for meta-research on pragmatic trials, and we invite other research groups to contribute to and/or use the PragMeta database. By following this collaborative idea to generate a large sample of pragmatic trials that assess health interventions, we aim to better understand the determinants, characteristics, and impact of pragmatic trial design features.
PragMeta aims to better understand and to quantify the relationship of trial pragmatism and treatment effects and to determine which trial features are key drivers for differences between treatment effects in more artificial research settings versus more practical real-world settings and hopefully result into a more standardized methodological framework that can be used by researchers, clinicians, and regulators and to better generate, use, and apply clinical research results.
Acknowledgements
We thank Kinga Dembowska and Thao Vy Thi Nguyen, University of Basel, for testing the PragMeta database and providing data to populate the database.
Abbreviations
- OSF
Open Science Framework
- PRECIS-2
PRagmatic Explanatory Continuum Indicator Summary tool
- RCD
Routinely collected health data
- RCT
Randomized clinical trial
Authors’ contributions
All authors made substantial contributions to the conception and design of the work; all authors have drafted the work or substantively revised it; all authors have approved the submitted version; all authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
Open access funding provided by University of Basel. The PragMeta project is supported by the Swiss National Science Foundation, project ID 188675. The funder had no role in the design of the project; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Julian Hirt and Perrine Janiaud have co-first authorship.
Contributor Information
Julian Hirt, Email: julian.hirt@usb.ch.
Perrine Janiaud, Email: perrine.janiaud@usb.ch.
Pascal Düblin, Email: pascal.dueblin@usb.ch.
Lars G. Hemkens, Email: lars.hemkens@usb.ch
References
- 1.Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Clin Epidemiol. 2009;62:499–505. doi: 10.1016/j.jclinepi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 2.GetReal Initiative. GetReal Trial Tool: Navigating RWE options in clinical trials. 2022. https://getrealtrialtool.eu/. Accessed 21 Nov 2022.
- 3.GetReal Initiative. PragMagic: Pragmatic trial resources. https://www.pragmagic.eu/. Accessed 21 Nov 2022.
- 4.National Institutes of Health. Rethinking clinical trials: a living textbook of pragmatic clinical trials. 2022. https://rethinkingclinicaltrials.org/. Accessed 21 Nov 2022.
- 5.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 6.Dal-Ré R, Janiaud P, Ioannidis JPA. Real-world evidence: how pragmatic are randomized controlled trials labeled as pragmatic? BMC Med. 2018;16:49. doi: 10.1186/s12916-018-1038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janiaud P, Dal-Ré R, Ioannidis JPA. Assessment of pragmatism in recently published randomized clinical trials. JAMA Intern Med. 2018;178:1278–1280. doi: 10.1001/jamainternmed.2018.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Usman MS, van Spall HGC, Greene SJ, Pandey A, McGuire DK, Ali ZA, et al. The need for increased pragmatism in cardiovascular clinical trials. Nat Rev Cardiol. 2022;19:737–750. doi: 10.1038/s41569-022-00705-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Troxel AB, Asch DA, Volpp KG. Statistical issues in pragmatic trials of behavioral economic interventions. Clin Trials. 2016;13:478–483. doi: 10.1177/1740774516654862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewald H, Speich B, Ladanie A, Bucher HC, Ioannidis JPA, Hemkens LG. Marginal structural models and other analyses allow multiple estimates of treatment effects in randomized clinical trials: meta-epidemiological analysis. J Clin Epidemiol. 2019;107:12–26. doi: 10.1016/j.jclinepi.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Yoong SL, Wolfenden L, Clinton-McHarg T, Waters E, Pettman TL, Steele E, Wiggers J. Exploring the pragmatic and explanatory study design on outcomes of systematic reviews of public health interventions: a case study on obesity prevention trials. J Public Health. 2014;36:170–176. doi: 10.1093/pubmed/fdu006. [DOI] [PubMed] [Google Scholar]
- 12.Sajobi TT, Li G, Awosoga O, Wang M, Menon BK, Hill MD, Thabane L. A comparison of meta-analytic methods for synthesizing evidence from explanatory and pragmatic trials. Syst Rev. 2018;7:19. doi: 10.1186/s13643-017-0668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aves T, Allan KS, Lawson D, Nieuwlaat R, Beyene J, Mbuagbaw L. The role of pragmatism in explaining heterogeneity in meta-analyses of randomised trials: a protocol for a cross-sectional methodological review. BMJ Open. 2017;7:e017887. doi: 10.1136/bmjopen-2017-017887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Allgemeine Methoden: Version 6.1. 2022.
- 15.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 16.GitHub. Directus. 2022. https://github.com/directus/directus. Accessed 21 Nov 2022.
- 17.COVID-evidence Database. Planned, ongoing and completed trials to treat and prevent COVID-19. 2022. https://covid-evidence.org/database. Accessed 24 Nov 2022.
- 18.CEIT-Cancer Project. The CEIT-Cancer Project: comparative effectiveness of innovative treatments for cancer. 2022. https://ceit-cancer.org/. Accessed 24 Nov 2022.
- 19.Janiaud P, Hirt J, Düblin P, Dembowska K, Nguyen TV, Hemkens LG. PragMeta: generalizability, applicability and pragmatism of clinical trials and their impact on treatment effect estimates: a metaepidemiological study. 2022. https://osf.io/cdbhn/.
- 20.Hirt J, Janiaud P, Hemkens LG. PragMS: Pragmatic trials in multiple sclerosis. 2022. https://osf.io/a7hvb/.
- 21.Dembowska K, Nguyen TV, Hirt J, Janiaud P, Hemkens LG. PragQoL: Impact of pragmatism on the assessment of pain, fatigue, and quality of life outcomes. 2022. https://osf.io/bj5gr/.
- 22.Nguyen TV, Dembowska K, Hirt J, Janiaud P, Hemkens LG. PragEpi: a meta-epidemiological study on pragmatism of randomized clinical trials. 2022. https://osf.io/6zn4y/.
- 23.Taljaard M, McDonald S, Nicholls SG, Carroll K, Hey SP, Grimshaw JM, et al. A search filter to identify pragmatic trials in MEDLINE was highly specific but lacked sensitivity. J Clin Epidemiol. 2020;124:75–84. doi: 10.1016/j.jclinepi.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 24.iCiteR. 2019. https://github.com/riddlet/iCiteR. Accessed 24 Nov 2022.
- 25.Higgins JPT, Thomas J. Cochrane Handbook for Systematic Reviews of Interventions: Version 6. 2. Hoboken: Wiley Online Library; 2019. [Google Scholar]
- 26.Ladanie A, Speich B, Naudet F, Agarwal A, Pereira TV, Sclafani F, et al. The Comparative Effectiveness of Innovative Treatments for Cancer (CEIT-Cancer) project: rationale and design of the database and the collection of evidence available at approval of novel drugs. Trials. 2018;19:505. doi: 10.1186/s13063-018-2877-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.