Abstract
Background
Reducing Salmonella infection in broiler chickens by using effective and safe alternatives to antibiotics is vital to provide safer poultry meat and minimize the emergence of drug-resistant Salmonella and the spread of salmonellosis to humans. This study was to first evaluate the protective efficacy of feeding coated essential oils and organic acids mixture (EOA) on broiler chickens infected with Salmonella Enteritidis (S. Enteritidis, SE), and then its action mechanism was further explored.
Methods
A total of 480 1-day-old Arbor Acres male chickens were randomly assigned into five treatments with six replicates, including non-challenged control fed with basal diet (A), SE-challenged control (B), and SE-infected birds fed a basal diet with 300 mg/kg of EOA (BL), 500 mg/kg of EOA (BM) and 800 mg/kg of EOA (BH), respectively. All birds on challenged groups were infected with Salmonella Enteritidis on d 13.
Results
Feeding EOA showed a reversed ability on negative effects caused by SE infection, as evidenced by decreasing the feed conversion rate (FCR) and the ratio of villus height to crypt depth (VH/CD) (P < 0.05), obviously decreasing intestinal and internal organs Salmonella load along with increasing cecal butyric acid-producing bacteria abundance (P < 0.05). Moreover, supplemental different levels of EOA notably up-regulated claudin-1 (CLDN-1), occludin (OCLN), zonula occludens-1 (ZO-1), mucin-2 (MUC-2), fatty acid binding protein-2 (FABP-2), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), myeloid differential protein-88 (MyD88) and interleukin-6 (IL-6) mRNA levels in the ileum of the infected chickens after challenge, whereas down-regulated toll-like receptor-4 (TLR-4) mRNA levels (P < 0.05). Linear discriminant analysis combined effect size measurements analysis (LEfSe) showed that the relative abundance of g_Butyricicoccus, g_Anaerotruncus and g_unclassified_f_Bacillaceae significantly was enriched in infected birds given EOA. Also, phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) analysis showed that alpha-linolenic acid metabolism, fatty acid metabolism and biosynthesis of unsaturated fatty acids were significantly enriched in the EOA group.
Conclusion
Our data suggest that the essential oils and organic acids mixture can be used as an effective strategy to ameliorate and alleviate Salmonella Enteritidis infection in broilers.
Keywords: Broiler chickens, Essential oils and organic acids mixture, Gut health, Salmonella Enteritidis
Introduction
Salmonella enterica serotype Enteritidis (S. Enteritidis, SE) is one of the remarkable foodborne pathogens that endanger the health of broiler chickens and poultry products safety. Infection of SE in chickens can destroy the balance of intestinal flora, adhere to intestinal epithelial cells, induce intestinal inflammation and damage intestinal barrier, resulting in diarrhea and growth loss of infected chickens [1–3]. In addition, Salmonella which breaks through the intestinal mucosal barrier and invades into the body can be colonized in internal organs such as spleen and liver, resulting in bacteremia and chicken death [4]. Traditionally, the addition of antibiotics to feed or water have been the main strategy of preventing and controlling salmonellosis in animal production [5]. However, many countries, including China, have gradually begun to ban the use of antibiotics in livestock production due to the continued emergence of antibiotic resistant strains and drug residues in poultry products [6]. Additionally, consumption of contaminated eggs or chicken meat is one of the leading causes of Salmonella food poisoning in humans [7]. Therefore, it is becoming more important to search effective and safe antibiotic substitute incorporation into feed and/or drinking water as a pre-harvest strategy to reduce Salmonella incidence and prevalence in poultry at the farm level.
In recent years, many relevant studies have reported that natural plant extracts such as essential oils, acidifiers, probiotics and their metabolites can effectively inhibit or kill Salmonella, improve the growth performance of livestock and poultry, reduce morbidity and mortality, and have the potential to become a substitute for antibiotics [6, 8, 9]. Poultry’s trials found that some essential oils, such as carvacrol, thymol, trans-cinnamaldehyde and eugenol have antibacterial effects against Salmonella in chickens [10–14], and could improve performance and reduce mortality and morbidity in broilers [15, 16]. Plant essential oils can exert their biological functions by affecting bacterial biofilms and destroying ion gradients [17], effectively scavenging nitric oxide [18], inhibiting the oxidation of low density lipoprotein and the expression of cyclooxygenase-2 and activating peroxisome proliferator activated receptors α and γ [19, 20]. Additionally, organic acids such as formic acids, butyric acid, medium chain fatty acid caprylic acid and benzoic acid have gained wide application in livestock production due to their possessing a variety of functions such as antibacterial (such as Salmonella, Campylobacter, Clostridium perfringens and other pathogens), immune-regulation, barrier-protection, health-promotion and/or growth promoters in chickens [8, 21–23].
Interestingly, previous studies have indicated that dietary essential oils combined with organic acids supplementation not only showed synergistic beneficial effects on growth performance and gut health, but also exhibited higher efficacy in controlling harmful intestinal bacterial infection such as Escherichia coli, Salmonella spp. and Clostridium perfringens [24–28], compared with individual addition. In addition, our previous studies have demonstrated that dietary supplementation with a blend product of essential oils and organic acids (4% carvacrol, 4% thyme, 0.5% hexanoic, 3.5% benzoic, and 0.5% butyric acid) could improve growth performance and intestinal health in broilers challenged with necrotic enteritis, and could be used as in-feed antibiotic alternative in broiler production [27, 28]. However, the efficacy of a blend of essential oils and organic acids for chicken growth performance and gut health was influenced by many factors, such as, the properties of essential oils (EOs) or organic acids (OAs), essential oils and organic acids (EOA) formula composition, protected EOA or not, EOA dosage, chicken health status, diet composition, and housing environment hygienic conditions [29]. A commercial blend product of coated essential oils and organic acids which contains thymol > 8.0%, carvacrol > 8.0%, cinnamaldehyde > 5%, caprylic acid > 1.0%, benzoic acid > 6.0%, butyric acid > 1.0% and carrier was used in the current study. In vitro studies have confirmed that the EOA product exhibited strong antibacterial activity against Salmonella, and the lowest minimum inhibitory concentration and minimum bactericidal concentration values against SE of this EOA was 2.35 and 4.69 mg/mL, respectively (unpublished data). The purpose of this study was to assess the effect of dietary inclusion of the EOA on growth performance and intestinal health of Salmonella-infected broilers chickens, and then action mechanism was explored.
Materials and methods
Animal ethics statement
All animal experiments were approved by the China Agricultural University Animal Care and Use Committee, Beijing, P. R. China (approval number: AW51112202-1–2).
Experimental design and diets
Four hundred and eighty (n = 480) 1-day-old Salmonella-free male Arbor Acres (AA) broiler chickens were purchased from a local supplier (Beijing Arbor Acres Poultry Breeding Company, China). These birds were randomly divided into five treatments according to their initial body weight including: negative control group (A, neither EOA treatment nor SE infection), positive control group (B, SE infection but without EOA treatment) and infected birds given the basal diets with three levels of EOA-treated groups, respectively. Namely, BL, SE with 300 mg/kg EOA treatment; BM, SE with 500 mg/kg EOA treatment; and BH, SE with 800 mg/kg EOA treatment. Each treatment group had six replicates with 16 birds per replicate. Each replicate was housed in a separate cage (240 cm × 60 cm × 60 cm) to avoid direct physical contact of the birds and minimize cross-contamination among isolators. The un-medicated pelleted basal diet was formulated according to the American National Research Council (NRC) (1994) [30] broiler feeding standard. The composition and nutrient levels of the basal diet is shown in Table 1.
Table 1.
Composition and nutrient levels of the basal diets
| Items | d 1–21 | d 22–42 |
|---|---|---|
| Ingredient, % | ||
| Corn (CP 8.0%) | 51.30 | 53.25 |
| Soybean meal (CP 44%) | 37.00 | 33.50 |
| Wheat powder (CP 13.5%) | 4.20 | 5.00 |
| Soybean oil | 4.20 | 5.00 |
| DL-Methionine, 99% | 0.25 | 0.15 |
| L-Lysine HCl, 78% | 0.25 | 0.20 |
| Limestone | 1.12 | 1.00 |
| Dicalcium phosphate | 1.00 | 1.20 |
| Sodium chloride | 0.35 | 0.35 |
| Choline chloride, 50% | 0.12 | 0.15 |
| Vitamin premixa | 0.02 | 0.02 |
| Mineral premixb | 0.10 | 0.10 |
| NSP enzymec | 0.02 | 0.02 |
| Phytase | 0.02 | 0.01 |
| Antioxidantd | 0.05 | 0.05 |
| Total | 100.00 | 100.00 |
| Nutrient levelse | ||
| Metabolizable energy, Mcal/kg | 3.03 | 3.10 |
| Crude protein, % | 21.45 | 20.05 |
| Total calcium, % | 0.77 | 0.76 |
| Total phosphorus, % | 0.57 | 0.59 |
| Available phosphorus, % | 0.27 | 0.30 |
| Lysine, % | 1.33 | 1.21 |
| Methionine, % | 0.56 | 0.44 |
| Methionine + Cystine, % | 0.90 | 0.77 |
| Threonine, % | 0.80 | 0.75 |
| Tryptophan, % | 0.25 | 0.23 |
aVitamin premix provided per kilogram of complete diet: vitamin A, 10,000 IU; vitamin D3, 2,400 IU; vitamin E, 20 IU; vitamin K3, 2.0 mg; vitamin B1, 1.6 mg; vitamin B2, 6.4 mg; vitamin B6, 2.4 mg; vitamin B12, 0.020 mg; nicotinic acid, 30 mg; pantothenic acid, 9.2 mg; folic acid, 1.0 mg; biotin, 0.10 mg
bMineral premix provided per kilogram of complete diet: iron, 40 mg; copper, 8 mg; manganese, 60 mg; zinc, 55 mg; iodine, 0.75 mg; selenium, 0.15 mg
cNSP enzyme: non-starch polysaccharide enzyme
dAntioxidant: 33% ethoxyquinoline
eCalculated value based on the analyzed data of experimental diets
All chickens were kept in an environmentally controlled house and had free access to feed and water throughout the entire experimental period. In accordance with the AA Broiler Management Guide, room temperature was maintained at 32 to 34 °C during d 1 to 5, and then gradually decreased by 2 °C weekly to reach a final room temperature of 22 to 24 °C. Artificial light was provided in a 23 h light/1 h dark program during the whole period of the study. In addition, the chickens were vaccinated against Newcastle disease virus and infectious bronchitis virus vaccines on d 7 and 21, and against infection bursa disease virus by drinking water on d 12 and 26, respectively.
Salmonella Enteritidis culture and challenge protocol
Salmonella Enteritidis serotype CVCC3379 (China Veterinary Culture Collection Center, China Institute of Veterinary Drug Control, Beijing, China) was cultured in nutrient broth (CM106, NB, Beijing Land Bridge Technology Co., Ltd., Beijing, China) at 37 °C with orbital shaking for 16 h. The concentration of viable SE in the culture was counted on Salmonella Shigella agar (CM206, SS, Beijing Land Bridge Technology Co., Ltd., Beijing, China) at 37 °C for 24 h and the stock culture was adjusted to a final concentration of 1 × 109 CFU/mL SE. On d 13, birds in the SE-challenged groups were administered 1.0 mL of bacterial suspension containing approximately 1 × 109 CFU/mL of SE suspension by gavage. Unchallenged groups received 1.0 mL of phosphate buffered saline (PBS) without SE on the same date. Feed was withdrawn from all birds for 10 h before challenge.
Growth performance
Dead birds were recorded daily and the mortality rate of each replicate was calculated through the experiment. Body weight (BW) and feed of the birds were weighed on a per cage basis on d 0, 23 and 39. Average body weight (ABW), average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratios (FCR) were calculated and corrected for mortality rate for each feeding stage at different experimental period.
Sample collection
On 3 days post infection (DPI) and 10 DPI, one bird from each replicate pen was randomly selected, weighed, blood samples were collected from the wing vein and centrifuged (3,000 × g, 10 min) at 4 °C, and then the serum was harvested and stored at –20 °C until analysis. The birds were euthanized by cervical dislocation. The middle intestinal sections of the ileum were cut out (approximately 200 mg), gently washed with ice-cold sterile saline, then put into a sterile tube and immediately snap-frozen in liquid nitrogen solution and stored at –80 °C for mRNA expression determination. Another ileal sample (approximately 1 cm) was rinsed in 0.9% (w/vol) physiological saline and fixed in 4% (w/vol) paraformaldehyde buffer solution for later morphological analysis. Liver and spleen samples (approximately 2 g, respectively) from each killed bird were aseptically collected into sterile tubes, then immediately snap-frozen in liquid nitrogen, stored at –40 °C for the determination of Salmonella translocation. The cecal contents of each killed bird were aseptically collected, put into three sterile tubes, then immediately snap-frozen in liquid nitrogen and transferred to –80 °C for microbial culture, microbial 16S rRNA analysis and the measurement of short-chain fatty acids (SCFA) contents. Ileal mucosa was collected and homogenized in ice-cold PBS (pH 7.2), and centrifuged, then the supernatant was collected and stored at –20 °C for anti-Salmonella specific IgA determination.
Determination of bacteria in cecal contents and internal organs
Salmonella enumeration in the cecal contents and internal organ were determined as described previously [31]. Briefly, liver, spleen and cecal samples were weighed, tenfold diluted with sterile saline (w/v) and homogenized for 1 min using a stomacher respectively. The homogenate was further serially diluted tenfold (1:10) with sterile PBS to appropriate levels, then 100 μL of each dilution was plated onto selective ager plates for bacterial quantification, respectively. Salmonella and Escherichia coli were counted with Salmonella Shigella agar (CM206, SS, Beijing Land Bridge Technology Co., Ltd., China) and Eosin-Methylene Blue Agar (CM105, EMB, Beijing Land Bridge Technology Ltd., China) by aerobical incubation at 37 °C for 24 h respectively. Lactobacillus spp. were determined with Man Rogosa Sharpe Medium (HB0384, MRS, Qingdao HopeBio Technology Co., Ltd., Shandong Province, China) by anaerobical culture for 24–48 h at 37 °C. Campylobacter were incubated by using modified Charcoal Cefoperazone Deoxycholate agar (HB0274, mCCDA, Qingdao HopeBio Technology Co., Ltd., Shandong Province, China) supplemented with CCDA selective supplement, and incubated microaerobically at 42 °C for 48–96 h using Anaero Jars (AG0025A, Thermo Fisher Scientific, Waltham, MA, United States). The number of colony-forming units in spleen, liver and cecal digesta was expressed as a logarithmic transformation per gram. Subsequently, the liver and spleen samples of all unchallenged chickens were enriched in tetrathionate broth base (HB4086, TTB, Qingdao HopeBio Technology Co., Ltd., Shandong Province, China) and further incubated at 37 °C for 24 h. Enrichment samples were confirmed negative for Salmonella spp. by streak plating on Salmonella Shigella agar selective media.
Ileum morphology analysis
Gut morphology analysis was performed as previously described [32]. The fixed tissue samples were dehydrated in a tissue processor (Leica Microsystems K. K., Tokyo, Japan), and embedded in paraffin wax. Paraffin sections. (5 μm) were sliced serially using a microtome (Leica Microsystems K. K., Tokyo, Japan) and mounted on glass slides. The paraffin was removed by xylene (2 times for 5 min each), followed by rehydration in 95% alcohol (5 min) and 50% alcohol (5 min). Sections were stained with haematoxylin and eosin (HE) for villous morphology measurement. The villi height (VH) and crypt depth (CD) of the stained sections were measured using image processing and analyzing system (at 40 × combined magnification, Inverted microscope: NIKON CI-S, Tokyo, Japan; Imaging system: NIKON DS-U3, Tokyo, Japan; CaseViewer 2.3, JAVS, Inc.). Ten intact villi were selected for measurement.
Determination of gene expression in the ileum using quantitative real-time polymerase chain reaction (RT-PCR)
Extraction of total RNA in ileum (50–100 mg) was performed by using Trizol reagent (Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer’s instructions. The purity and concentration of total RNA were measured using a NanoDrop-2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States). Then, cDNA was synthesized by using PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) kit (Takara BioTechnology Co., Ltd., Beijing, China). Quantitative real-time PCR (qRT-PCR) reactions were performed in the Applied Biosystems’ 7500 Fast Real-Time PCR system by using SYBR Premix Ex Taq diagnostic kit (Takara BioTechnology Co., Ltd., Beijing, China) and each sample was measured in duplicate. β-actin gene was used as housekeeping control to normalize variations in the mRNA amount for the target genes including OCLN, ZO-1, MUC-2, CLDN-1, FABP-2, NF-κB, TLR-4, MyD88, IL-6, interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ). The sequences of gene primers used in this study are shown in Table 2. Relative target gene expression level of each target gene was normalized by the comparative cycle threshold (CT) 2−ΔΔCT method [33].
Table 2.
Sequences of the oligonucleotide primers used for quantitative real-time PCRa
| Gene | Primer sequence (5′→3′) | GenBank ID |
|---|---|---|
| Barrier-related genes | ||
| CLDN-1 | F: CATACTCCTGGGTCTGGTTGGT | NM_001013611.2 |
| R: GACAGCCATCCGCATCTTCT | ||
| OCLN | F: ACGGCAGCACCTACCTCAA | NM_205128.1 |
| R: GGGCGAAGAAGCAGATGAG | ||
| ZO-1 | F: CTTCAGGTGTTTCTCTTCCTCCTC | XM_040706827.1 |
| R: CTGTGGTTTCATGGCTGGATC | ||
| MUC-2 | F: TTCATGATGCCTGCTCTTGTG | XM_421035 |
| R: CCTGAGCCTTGGTACATTCTTGT | ||
| FABP-2 | F: GAAGCAATGGGCGTGAATGTGATG | NM_001007923.1 |
| R: TTCGATGTCGATGGTACGGAAGTTG | ||
| Immune-related genes | ||
| NF-κB | F: TGGAGAAGGCTATGCAGCTT | NM_205134.1 |
| R: CATCCTGGACAGCAGTGAGA | ||
| TLR-4 | F: CCACTATTCGGTTGGTGGAC | NM_001030693.1 |
| R: ACAGCTTCTCAGCAGGCAAT | ||
| MyD88 | F: TGCAAGACCA TGAAGAACGA | NM_001030962.3 |
| R: TCACGGCAGCAAGAGAGATT | ||
| IL-6 | F: GATCCGGCAGATGGTGATAA | NM_204628.1 |
| R: AGGATGAGGTGCATGGTGAT | ||
| IL-1β | F: TCATCTTCTACCGCCTGGAC | NM_204524.1 |
| R: GTAGGTGGCGATGTTGACCT | ||
| TNF-α | F: GAGCGTTGACTTGGCTGTC | NM_204267.1 |
| R: AAGCAACAACCAGCTATGCAC | ||
| IFN-γ | F: CTTCCTGATGGCGTGAAGA | NM_205149.1 |
| R: GAGGATCCACCAGCTTCTGT | ||
| β-actin | R: GAGAAATTGTGCGTGACATCA | NM_205518.1 |
| F: CCTGAACCTCTCATTGCCA | ||
aPrimers were designed and synthesized by Sangon Biotech (Shanghai) Co., Ltd. (China). F Forward, R Reverse
CLDN-1 Claudin-1, FABP-2 Fatty acid binding protein, IFN-γ Interferon-γ, IL-1β Interleukin-1β, IL-6 Interleukin-6, MUC-2 Mucin-2, MyD88 Myeloid differential protein-88, NF-κB Nuclear factor kappa-light-chain-enhancer of activated B cells, OCLN Occludin, TLR-4 Toll-like receptor-4, TNF-α Tumor necrosis factor-α, ZO-1 Zonula occludens-1
Measurement of anti-Salmonella specific antibody in the serum and ileal content
Serum anti–SE specific immunoglobulin G (IgG) and specific immunoglobulin A (IgA) in ileal content were measured using an indirect enzyme-linked immunosorbent assay (ELISA) as described previously [32]. Briefly, SE (1 × 108 CFU/mL) cells were washed 3 times with sterile PBS (pH 7.2) and lysed by an ultrasonic processor JY96-IIN (Ningbo Xinzhi Biotechnology Co., Ltd., China) at 85 Watts and 30 s intervals on ice for 5 min. The lysed cells were centrifuged at 10,000 × g for 10 min, and the resultant supernatant was collected and stored at –70 °C until use. Protein concentration of the lytic supernatant of Salmonella bacteria was determined by bicinchoninic acid kit (G2026-200 T, Wuhan ServiceBio Technology, Co., Ltd., China). Flat-bottomed 96-well ELISA microtiter plates (Corning Costar, Corning, NY, USA) were incubated with 100 μL/well of the prepared Salmonella lytic supernatant (20 μg/mL) dissolved in 0.1 mol/L carbonate-bicarbonate buffer (15 mmol/L Na2CO3, 35 mmol/L NaHCO3, 0.3 mmol/L NaN3) overnight at 4 °C. Antigen-coated plate was then washed 3 times with PBST (phosphate buffered saline pH 7.2 containing 0.05% Tween X-100), 200 μL of blocking solution (PBST containing 1% bovine serum albumin) was added to each well and incubated at 37 °C for 2 h for blocking nonspecific binding. After washing 3 times with PBST, 100 μL of diluted serum samples or intestinal mucosa supernatant were added to each well, respectively, and incubated for 1 h at 37 °C. After washing, 100 μL of diluted horseradish peroxidase (HRP)-conjugated goat anti-chicken IgG (A30-104P, Bethyl Laboratories Inc., Montgomery, TX) or HRP- conjugated goat anti-chicken IgA-Fc (A30-103P, Bethyl Laboratories Inc., Montgomery, TX) were added to each well, and incubated at 37 °C for 1 h. The plates were washed 3 times with PBST and incubated with 3,3′,5,5′-tetra-methylbenzidine solution for 30 min at room temperature in the dark. Finally, the reaction was stopped with 2 mol/L sulfuric acid, and the absorbance was measured at 450 nm using an automatic ELISA reader (Bio-Tek EL311SX autoreader, Bio-Tek, USA). The result is presented as an optical density (OD) value.
Determination of short chain fatty acids concentration in cecal content
A total of 100 mg of frozen cecal digesta sample of each replicate was dissolved and homogenized in 1.5 mL of pre-cold sterile ultra-pure water, and then centrifuged (12,000 × g, 10 min at 4 °C). Then, 1 mL of the supernatant was diluted with 0.2 mL of 25% (w/v) metaphosphoric acid solution containing crotonic acid. The mixture was incubated at –20 °C for 24 h and then centrifuged (10,000 × g, 10 min at 4 °C) to remove protein precipitates. The extracted solution was filtered with a 0.22-μm syringe filter, and then analyzed short chain fatty acids (SCFAs) using a gas chromatograph (Shimadzu GC-2014 ATF instrument) equipped with a capillary column (30 m × 0.25 mm × 0.5 μm). The N2 was used for carrier gas (12.5 Mpa, 18 mL/min). The temperature of the injector and detector was 180 °C, and the column was gradually heated from 80 °C to 170 °C at a rate of 5 °C/min. The results of SCFAs were expressed as milligrams per kilogram of digesta.
Microbial DNA extraction, 16S rRNA gene amplification, sequencing and bioinformatics analysis
Microbial genomic DNA was extracted from about 250 mg cecal digesta samples taken from all groups, respectively, using E.Z.N.A.® Soil DNA Kit (Omega Bio-Tek, GA, USA) according to the manufacturers’ instructions. The concentration and purity of total DNA were detected by NanoDrop 2000 spectrophotometer (Thermo Scientific, MA, USA), and the integrity of DNA was detected by 1% agarose gel electrophoresis (voltage 5 V/cm, time 20 min). The V3–V4 regions of bacterial 16S rDNA sequences were amplified using primer 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) according to the method described previously [28]. The PCR product was purified using AxyPrep DNA Gel Extraction Kit (Axygen, Union City, USA), quantified, homogenized, and then constructed the Miseq library. The library was sequenced by the Illlumina MiSeq PE250 platform (Illumina, Santa Clara, CA, USA) using a MiSeq Reagent Kit at Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China).
Raw pair-end sequences were demultiplexed and quality-filtered using Quantitative Insights Into Microbial Ecology (QIIME, version 1.17) [34]. The effective reads were clustered into operational taxonomic units (OTUs) based on the 97% similarity. Classification of OTUs at various taxonomic levels was carried out using the Greengenes database. For rarefaction curves and α-diversity (Chao 1 index, Simpson index, ACE index, Shannon index) analysis were calculated using QIIME software [35]. β-diversity was estimated using principal coordinate analysis (PCoA) and partial least squares discriminant analysis. The results were plotted using “vegan” and “ggplot2” package in R software (Version 3.4.4). The significance of microbial community differences among groups was assessed using ANOSIM with R package “vegan” [36]. Linear discriminant analysis (LDA) combined effect size (LEfSe) analysis (LDA score > 2.0, P < 0.05) estimated the impact of the abundance of bacteria on the difference effect of bacteria from phylum to genus among different groups. Non-parametric factorial Kruskal–Wallis sum-rank test was employed to explore the differences in the relative abundances of bacteria among groups [37]. Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt 1.0.0) was used to predict metagenome functions associated with bacterial communities based on high-quality 16S rRNA sequencing data [38]. The functions were deduced using Kyoto Encyclopedia of Genes and Genomes annotations for level 3 pathways. Differentially represented functional pathways were analyzed with two-sided Welch’s t-test. The obtained biome file was processed by STAMP (Halifax, Nova Scotia, Canada) version 2.1.3 [39].
Statistical analysis
Linear and quadratic relationship analysis and one-way analysis of variance (one-way ANOVA) followed by Duncan’s multiple comparison test (SPSS, version 21.0, Chicago, IL, USA) was employed to analyze the difference in growth performance, intestinal morphology, bacterial population, gene expression, specific antibody levels and SCFAs content. P < 0.05 was considered significant, while 0.05 ≤ P < 0.10 was considered a trend. Data were expressed as mean and pooled standard error of mean (SEM). Correlations were analyzed using spearman correlation with the p-heatmap package (P < 0.05).
Results
Growth performance
The growth performance results are summarized in Table 3. From d 1 to 23, SE-infected control group had the lowest ADG and the highest FCR compared with the other four groups, but there was no statistical difference (P > 0.05). From d 24 to 39, ADFI and FCR of BH group were significantly lower than those of other groups (P < 0.05), and both indexes exhibited a quadratic change with increasing levels of EOA (P < 0.05). Moreover, the FCR of B and BL group was the highest among the five groups. During the overall period, although there were no significant differences in ABW, ADG and MOT among all groups from d 1 to 39 (P > 0.05), ADFI and FCR exhibited a quadratic change with increasing levels of EOA (P < 0.05). In addition, SE-induced increase in FCR was significantly inhibited by the addition of EOA into broiler diets compared to that in SE-infected control group.
Table 3.
Effects of dietary EOA supplementation on growth performances of broiler chickens infected with Salmonella Enteritidis (n = 6)
| Time | Items | Groups | SEM1 | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | BL | BM | BH | P12 | Linear3 | Quadratic4 | |||
| d 1–23 | ADG, g/bird/d | 45.68 | 44.34 | 45.82 | 45.93 | 45.62 | 0.325 | 0.544 | 0.120 | 0.490 |
| ADFI, g/bird/d | 61.06 | 60.22 | 60.78 | 62.91 | 61.55 | 0.479 | 0.524 | 0.132 | 0.027 | |
| FCR5 | 1.34 | 1.38 | 1.32 | 1.36 | 1.34 | 0.011 | 0.371 | 0.120 | 0.985 | |
| d 24–39 | ADG, g/bird/d | 88.55 | 86.23 | 82.04 | 83.80 | 81.35 | 1.349 | 0.420 | 0.349 | 0.607 |
| ADFI, g/bird/d | 143.34ab | 144.58b | 137.08b | 138.47ab | 130.79c | 1.285 | 0.001 | 0.023 | 0.009 | |
| FCR | 1.61b | 1.67a | 1.67a | 1.65ab | 1.61b | 0.009 | 0.021 | 0.582 | 0.008 | |
| d 39 | ABW, g/bird | 2,512.00 | 2,448.33 | 2,410.05 | 2,440.78 | 2,394.75 | 21.358 | 0.471 | 0.859 | 0.993 |
| d 1–39 | ADG, g/bird/d | 63.29 | 61.67 | 60.67 | 61.45 | 60.29 | 0.732 | 0.398 | 0.679 | 0.775 |
| ADFI, g/bird/d | 94.49a | 94.87a | 92.89ab | 93.66ab | 89.15b | 0.747 | 0.090 | 0.388 | 0.027 | |
| FCR | 1.49b | 1.54a | 1.53a | 1.52a | 1.48b | 0.008 | 0.015 | 0.817 | 0.020 | |
| MOT, % | 1.62 | 1.39 | 2.58 | 1.25 | 1.18 | 0.638 | 0.891 | 0.612 | 0.543 | |
1SEM Standard error of the mean
2P1-value represent the difference comparison between group A, B, BL, BM and BH groups
3Linear regression analysis among B, BL, BM and BH groups
4Quadratic curve analysis among B, BL, BM and BH groups
5FCR = feed conversion ratio = g of feed intake/g of body weight gain, g/g
a–cMeans within the same row without a common superscript differ significantly (P < 0.05)
ADG Average daily gain, ADFI Average daily feed intake, ABW Average body weight, MOT Mortality. A: broiler chickens with basal diet and no infection, B: broiler chickens with basal diet and SE infection, BL: broiler chickens with basal diet supplemented with 300 mg/kg EOA and SE infection, BM: broiler chickens with basal diet supplemented with 500 mg/kg EOA and SE infection, BH: broiler chickens with basal diet supplemented with 800 mg/kg EOA and SE infection. EOA Coated essential oils and organic acids mixture
Ileal morphology
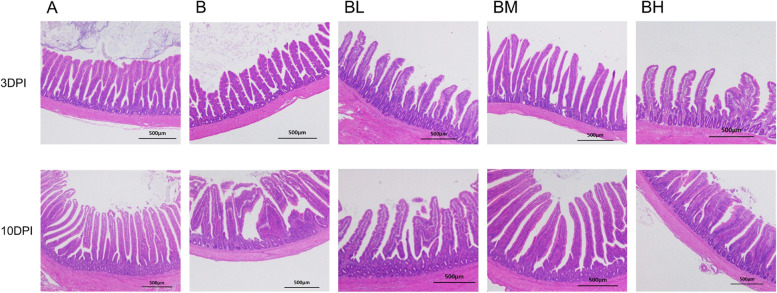
Figure 1 shows that the height of ileal villi was short and there was severe rupture of intestinal villi in the positive group. The addition of EOA can prevent ileal injury and improve the condition of ileal villi to some extent. As shown in Table 4, the VH/CD values in SE-infected B group was significantly lower than that in negative group and BM group at 3 DPI (P < 0.05). At 10 DPI, the CD of ileum in B group was significantly higher than that in the other four groups (P < 0.05), while the VH/CD in group B was significantly lower than that in negative group and BM group (P < 0.05). What's more, CD and VH/CD showed a linear change with increasing levels of EOA supplementation (P < 0.05).
Fig. 1.
Effects of dietary EOA supplementation on gut morphological structure (× 40 magnification; scale bar: 500 μm) of the SE-infected broiler chickens. A: broiler chickens with basal diet and no infection, B: broiler chickens with basal diet and SE infection, BM: broiler chickens with basal diet supplemented with 500 mg/kg EOA and SE infection, BH: broiler chickens with basal diet supplemented with 800 mg/kg EOA and SE infection. DPI: days post infection. EOA: coated essential oils and organic acids mixture. SE: Salmonella Enteritidis
Table 4.
Effects of dietary EOA supplementation on ileal morphology of broiler chickens infected with Salmonella Enteritidis (n = 6)
| Items | Groups | SEM1 | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | BL | BM | BH | P12 | Linear3 | Quadratic4 | ||
| 3 DPI | |||||||||
| Villus height, μm | 621.18 | 539.93 | 594.39 | 590.57 | 580.15 | 10.236 | 0.107 | 0.067 | 0.209 |
| Crypt depth, μm | 101.08 | 105.56 | 105.10 | 95.26 | 100.29 | 1.919 | 0.477 | 0.183 | 0.920 |
| VH/CD | 6.20a | 5.15b | 5.70ab | 6.21a | 5.79ab | 0.132 | 0.040 | 0.012 | 0.326 |
| 10 DPI | |||||||||
| Villus height, μm | 699.76 | 695.11 | 672.05 | 761.26 | 696.20 | 19.022 | 0.714 | 0.650 | 0.982 |
| Crypt depth, μm | 104.75b | 139.75a | 109.94b | 111.91b | 109.27b | 3.435 | 0.001 | 0.001 | 0.162 |
| VH/CD | 6.73a | 5.11b | 6.11ab | 6.77a | 6.36ab | 0.209 | 0.043 | 0.003 | 0.364 |
1SEM Standard error of the mean
2P1-value represent the difference comparison between group A, B, BL, BM and BH groups
3Linear regression analysis among B, BL, BM and BH groups
4Quadratic curve analysis among B, BL, BM and BH groups
a,bMeans within the same row without a common superscript differ significantly (P < 0.05)
DPI: days post infection, VH/CD: villus height to crypt depth ratio. A: broiler chickens with basal diet and no infection, B: broiler chickens with basal diet and SE infection, BL: broiler chickens with basal diet supplemented with 300 mg/kg EOA and SE infection, BM: broiler chickens with basal diet supplemented with 500 mg/kg EOA and SE infection, BH: broiler chickens with basal diet supplemented with 800 mg/kg EOA and SE infection. EOA: coated essential oils and organic acids mixture
Caecal bacterial colonization and internal organs bacteria invasion
The results of plate count method showed that Salmonella and Escherichia coli were not detected in liver and spleen in negative group at 3 and 10 DPI. At 3 DPI, Salmonella was detected only in the liver (0.58 lgCFU/g) and spleen (0.33 lgCFU/g) of SE-infected control group and in the liver (0.40 lgCFU/g) in the BM group. Besides, Escherichia coli was detected in the livers of the four groups and the concentration of Escherichia coli in SE-infected control group was the highest (2.04 lgCFU/g) at 3 DPI. Notably, no Salmonella was detected in the liver and spleen of the four groups at 10 DPI. Similarly, the content of Escherichia coli was the highest in the spleen of SE-infected control group (1.17 lgCFU/g) at 10 DPI.
As summarized in Table 5, the numbers of Salmonella and Lactobacillus in the infected positive group were significantly higher than that in other groups at 3 DPI (P < 0.05). Dietary supplementation of EOA exhibited a significant linear decrease in the number of Salmonella and Lactobacillus in cecal digesta at 3 DPI (P < 0.05). At 10 DPI, Salmonella and Campylobacter counts in positive group were significantly higher than those in negative group (P < 0.05). Moreover, the number of Salmonella, Escherichia coli and Campylobacter in BL, BM and BH groups was lower than that in the positive group at 3 and 10 DPI. Therefore, the addition of EOA could suppress the increase of harmful bacteria in cecum of broilers caused by Salmonella challenge to some extent.
Table 5.
Effects of EOA on microbial concentration (lgCFU/g)1 in the cecum contents of broilers infected with Salmonella Enteritidis (n = 6)
| Items | Time | Groups | SEM2 | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | BL | BM | BH | P13,4 | Linear5 | Quadratic6 | |||
| Salmonella | 3 DPI | 0.00 | 5.30a | 4.00b | 4.24b | 3.82b | 0.181 | 0.018 | 0.004 | 0.310 |
| 10 DPI | 0.00 | 5.46 | 5.06 | 4.75 | 4.53 | 0.203 | 0.481 | 0.140 | 0.770 | |
| Escherichia coli | 3 DPI | 4.70 | 5.62 | 4.98 | 4.75 | 4.72 | 0.138 | 0.168 | 0.024 | 0.734 |
| 10 DPI | 6.31 | 6.44 | 5.85 | 5.97 | 5.80 | 0.123 | 0.360 | 0.111 | 0.643 | |
| Lactobacillus | 3 DPI | 10.38b | 11.28a | 10.28b | 9.78b | 10.10b | 0.139 | 0.001 | 0.001 | 0.207 |
| 10 DPI | 9.33c | 11.02a | 9.64bc | 10.29ab | 10.55ab | 0.177 | 0.002 | 0.097 | 0.025 | |
| Campylobacter | 3 DPI | 5.61 | 6.45 | 6.12 | 6.17 | 6.07 | 0.106 | 0.142 | 0.164 | 0.796 |
| 10 DPI | 5.35b | 6.76a | 6.62a | 6.25a | 6.29a | 0.146 | 0.006 | 0.138 | 0.779 | |
1lgCFU/g log10 colony-forming units per gram of cecal digesta
2SEM Standard error of the mean
3P-value between B, BL, BM and BB groups in Salmonella content
4P-value represent the difference of other bacteria content among A, B, BL, BM and BB groups
5Linear regression analysis among B, BL, BM and BH groups
6Quadratic curve analysis among B, BL, BM and BH groups
a–cMeans within the same row without a common superscript differ significantly (P < 0.05)
DPI: days post infection, A: broiler chickens with basal diet and no infection, B: broiler chickens with basal diet and SE infection, BL: broiler chickens with basal diet supplemented with 300 mg/kg EOA and SE infection, BM: broiler chickens with basal diet supplemented with 500 mg/kg EOA and SE infection, BH: broiler chickens with basal diet supplemented with 800 mg/kg EOA and SE infection. EOA: coated essential oils and organic acids mixture
Gene expression of tight junction protein genes and immune-related genes in the ileum
Table 6 presents the results of ileal barrier-related gene expression in broilers. At 3 DPI, the mRNA levels of CLDN-1, OCLN, ZO-1 and MUC-2 in B, BL, BM and BH groups were significantly lower than those in non-infected A group (P < 0.05), indicating that SE infection damage intestinal barrier function. At 10 DPI, the gene expression of CLDN-1, OCLN and MUC-2 in negative group was significantly higher than those in SE-infected B group (P < 0.05). Furthermore, our data showed that the gene expression of CLDN-1, OCLN, ZO-1, MUC-2 and FABP-2 in the ileum of BL, BM and BH groups was significantly higher than those in SE-infected B group (P < 0.05) and exhibited a quadratic change with increasing levels of EOA (P < 0.05). These data suggest that dietary supplementation of EOA can improve the expression of tight junction protein in the ileum of broilers challenged by SE.
Table 6.
Effect of dietary EOA on mRNA expression of ileal tight junction proteins of broiler chickens infected with Salmonella Enteritidis (n = 6)
| Items | Groups | SEM1 | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | BL | BM | BH | P12 | Linear3 | Quadratic4 | ||
| 3 DPI | |||||||||
| CLDN-1 | 2.72a | 1.00b | 1.35b | 1.17b | 1.03b | 0.157 | 0.001 | 0.679 | 0.255 |
| OCLN | 1.83a | 1.00b | 1.05b | 1.07b | 0.60b | 0.109 | 0.002 | 0.372 | 0.100 |
| ZO-1 | 3.18a | 1.00b | 1.41b | 1.30b | 1.01b | 0.193 | 0.001 | 0.356 | 0.025 |
| MUC-2 | 1.83a | 1.00b | 1.22b | 1.31b | 1.19b | 0.088 | 0.022 | 0.180 | 0.571 |
| FABP-2 | 1.46 | 1.00 | 1.26 | 1.17 | 1.44 | 0.090 | 0.481 | 0.219 | 0.743 |
| 10 DPI | |||||||||
| CLDN-1 | 2.25bc | 1.00d | 2.46b | 3.49a | 1.68c | 0.207 | 0.001 | < 0.001 | < 0.001 |
| OCLN | 1.80b | 1.00c | 2.78a | 2.98a | 1.32bc | 0.188 | < 0.001 | 0.002 | < 0.001 |
| ZO-1 | 1.32bc | 1.00c | 1.84a | 1.70ab | 1.66ab | 0.083 | 0.002 | 0.001 | 0.033 |
| MUC-2 | 3.17b | 1.00d | 4.99a | 5.69a | 2.07c | 0.393 | < 0.001 | 0.002 | < 0.001 |
| FABP-2 | 1.61dc | 1.00d | 5.68a | 4.04b | 1.93c | 0.417 | < 0.001 | < 0.001 | < 0.001 |
1SEM Standard error of the mean
2P1-value represent the difference comparison between group A, B, BL, BM and BH groups
3Linear regression analysis among B, BL, BM and BH groups
4Quadratic curve analysis among B, BL, BM and BH groups
a–dMeans within the same row without a common superscript differ significantly (P < 0.05)
DPI: days post infection, A: broiler chickens with basal diet and no infection, B: broiler chickens with basal diet and SE infection, BL: broiler chickens with basal diet supplemented with 300 mg/kg EOA and SE infection, BM: broiler chickens with basal diet supplemented with 500 mg/kg EOA and SE infection, BH: broiler chickens with basal diet supplemented with 800 mg/kg EOA and SE infection. EOA: coated essential oils and organic acids mixture
The results of immune-related gene expression were listed in Table 7. The mRNA levels of NF-κB, IL-1β and TNF-a in BM group were significantly lower than those in SE-infected B group at 3 DPI (P < 0.05). The expression of inflammatory genes (TLR4, MyD88, IL-6 and IFN-γ) in the four groups was also lower than that in SE-infected B group, but the difference was not significant. At 10 DPI, dietary supplementation of EOA showed a significant linear decreasing effect on TLR4 mRNA level, displayed a quadratic effect on NF-κB and MyD88 mRNA levels and had a significant linear and quadratic influence on IL-6 mRNA levels (P < 0.05). Moreover, dietary different dosage of EOA administration all significantly reduced TLR4 mRNA levels in the ileum (P = 0.002).
Table 7.
Effect of dietary EOA on mRNA expression of ileal inflammatory genes of broiler chickens infected with Salmonella Enteritidis (n = 6)
| Items | Groups | SEM1 | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | BL | BM | BH | P12 | Linear3 | Quadratic4 | ||
| 3 DPI | |||||||||
| TLR4 | 0.76 | 1.00 | 0.66 | 0.65 | 0.74 | 0.062 | 0.418 | 0.086 | 0.363 |
| NF-κB | 0.68ab | 1.00a | 0.58ab | 0.51b | 0.76ab | 0.064 | 0.129 | 0.066 | 0.139 |
| MyD88 | 1.08 | 1.00 | 0.96 | 0.81 | 0.89 | 0.063 | 0.711 | 0.509 | 0.973 |
| IL-6 | 0.63 | 1.00 | 0.50 | 0.33 | 0.64 | 0.115 | 0.462 | 0.167 | 0.418 |
| IL-1β | 0.69ab | 1.00a | 0.56ab | 0.42b | 0.74ab | 0.069 | 0.078 | 0.046 | 0.137 |
| TNF-a | 0.66ab | 1.00a | 0.94a | 0.55b | 0.92a | 0.059 | 0.043 | 0.183 | 0.621 |
| IFN-γ | 0.88 | 1.00 | 0.50 | 0.33 | 0.64 | 0.094 | 0.281 | 0.597 | 0.822 |
| 10 DPI | |||||||||
| TLR4 | 0.89ab | 1.00a | 0.48c | 0.66bc | 0.36c | 0.066 | 0.002 | 0.001 | 0.559 |
| NF-κB | 0.94b | 1.00b | 1.47a | 1.20ab | 0.96b | 0.058 | 0.027 | 0.941 | 0.005 |
| MyD88 | 1.35b | 1.00b | 2.69a | 2.89a | 1.35b | 0.206 | 0.001 | 0.091 | 0.001 |
| IL-6 | 0.86c | 1.00c | 2.59a | 2.32b | 1.70b | 0.175 | < 0.001 | 0.003 | 0.002 |
| IL-1β | 0.72 | 1.00 | 1.07 | 1.10 | 0.92 | 0.071 | 0.413 | 0.871 | 0.470 |
| TNF-a | 0.95 | 1.00 | 0.94 | 0.94 | 0.50 | 0.071 | 0.189 | 0.108 | 0.136 |
| IFN-γ | 0.55 | 1.00 | 0.94 | 1.06 | 1.15 | 0.101 | 0.330 | 0.711 | 0.648 |
1SEM Standard error of the mean
2P1-value represent the difference comparison between group A, B, BL, BM and BH groups
3Linear regression analysis among B, BL, BM and BH groups
4Quadratic curve analysis among B, BL, BM and BH groups
a–cMeans within the same row without a common superscript differ significantly (P < 0.05)
DPI: days post infection, A: broiler chickens with basal diet and no infection, B: broiler chickens with basal diet and SE infection, BL: broiler chickens with basal diet supplemented with 300 mg/kg EOA and SE infection, BM: broiler chickens with basal diet supplemented with 500 mg/kg EOA and SE infection, BH: broiler chickens with basal diet supplemented with 800 mg/kg EOA and SE infection. EOA: coated essential oils and organic acids mixture
Anti-Salmonella specific IgA and IgG concentrations
As presented in Table 8, OD value of the serum anti-SE IgG in BM group was significantly higher than that in SE-infected B group at 3 DPI (P < 0.05). In addition, OD value of specific IgA against Salmonella in the ileum digesta in BH group was significantly higher than that in BL group at 3 DPI (P < 0.05). Notably, no significant difference in the concentration of ileal IgA and serum IgG was observed among the five groups at 10 DPI.
Table 8.
Effect of dietary coated essential oils and organic acids mixture (EOA) on anti-Salmonella specific IgG and IgA of broiler chickens (n = 6)
| Items | Groups | SEM1 | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | BL | BM | BH | P12 | Linear3 | Quadratic4 | ||
| Serum anti-Salmonella IgG (OD450) | |||||||||
| 3 DPI | 2.59b | 2.62b | 2.59b | 2.78a | 2.72ab | 0.023 | 0.017 | 0.038 | 0.302 |
| 10 DPI | 2.80 | 2.80 | 2.77 | 2.72 | 2.73 | 0.014 | 0.338 | 0.142 | 0.923 |
| Intestinal anti-Salmonella IgA (OD450) | |||||||||
| 3 DPI | 2.76ab | 2.77ab | 2.68b | 2.75ab | 2.82a | 0.015 | 0.037 | 0.329 | 0.002 |
| 10 DPI | 2.75 | 2.80 | 2.78 | 2.71 | 2.75 | 0.014 | 0.286 | 0.138 | 0.875 |
1SEM Standard error of the mean
2P1-value represent the difference comparison between group A, B, BL, BM and BH groups
3Linear regression analysis among B, BL, BM and BH groups
4Quadratic curve analysis among B, BL, BM and BH groups
a,bMeans within the same row without a common superscript differ significantly (P < 0.05)
DPI: days post infection, A: broiler chickens with basal diet and no infection, B: broiler chickens with basal diet and SE infection, BL: broiler chickens with basal diet supplemented with 300 mg/kg EOA and SE infection, BM: broiler chickens with basal diet supplemented with 500 mg/kg EOA and SE infection, BH: broiler chickens with basal diet supplemented with 800 mg/kg EOA and SE infection. EOA: coated essential oils and organic acids mixture
Concentration of short-chain fatty acids in cecal content
As illustrated in Table 9, the concentration of isobutyric acid in the cecum digesta of negative group, BL and BH group were significantly higher than that in SE-infected group (P < 0.05), and adding EOA in the diet linearly increased iso-butyric acid concentration in cecal digesta of infected broilers (P < 0.05).
Table 9.
Effects of dietary EOA on volative fatty acids concentration (mg/kg) in the cecal contents of broilers infected with Salmonella Enteritidis at 10 days post infection (n = 6)
| Items | Groups | SEM1 | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | BL | BM | BH | P12 | Linear3 | Quadratic4 | ||
| Acetic acid | 141.45 | 147.19 | 134.28 | 125.09 | 125.35 | 10.136 | 0.954 | 0.503 | 0.978 |
| Propionic acid | 29.02 | 37.22 | 26.41 | 27.30 | 31.64 | 2.174 | 0.511 | 0.271 | 0.246 |
| Isobutyric acid | 76.49a | 44.37b | 83.43a | 63.12ab | 75.60a | 4.567 | 0.040 | 0.026 | 0.164 |
| Butyric acid | 85.70 | 63.53 | 76.07 | 61.30 | 96.77 | 5.429 | 0.217 | 0.231 | 0.414 |
| Isovaleric acid | 56.25 | 55.78 | 58.87 | 67.78 | 65.29 | 2.430 | 0.432 | 0.144 | 0.893 |
| Valeric acid | 63.54 | 65.47 | 63.51 | 63.20 | 65.60 | 2.670 | 0.998 | 0.943 | 0.792 |
1SEM Standard error of the mean
2P1-value represent the difference comparison between group A, B, BL, BM and BH groups
3Linear regression analysis among B, BL, BM and BH groups
4Quadratic curve analysis among B, BL, BM and BH groups
a,bMeans within the same row without a common superscript differ significantly (P < 0.05)
A: broiler chickens with basal diet and no infection, B: broiler chickens with basal diet and SE infection, BL: broiler chickens with basal diet supplemented with 300 mg/kg EOA and SE infection, BM: broiler chickens with basal diet supplemented with 500 mg/kg EOA and SE infection, BH: broiler chickens with basal diet supplemented with 800 mg/kg EOA and SE infection. EOA: coated essential oils and organic acids mixture
Cecal microbiome analysis by 16S rRNA sequencing and bioinformatics
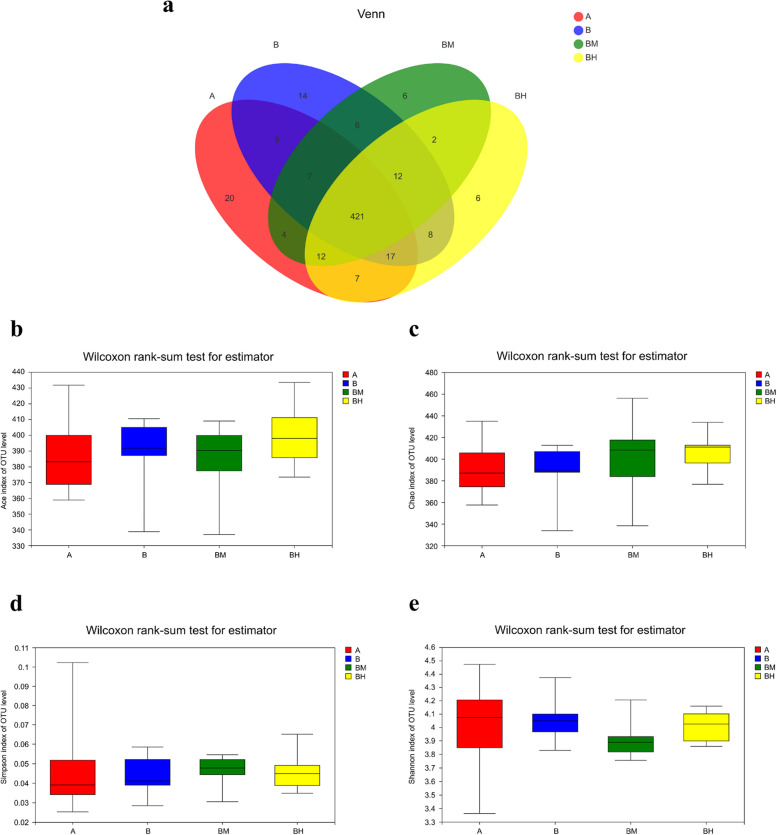
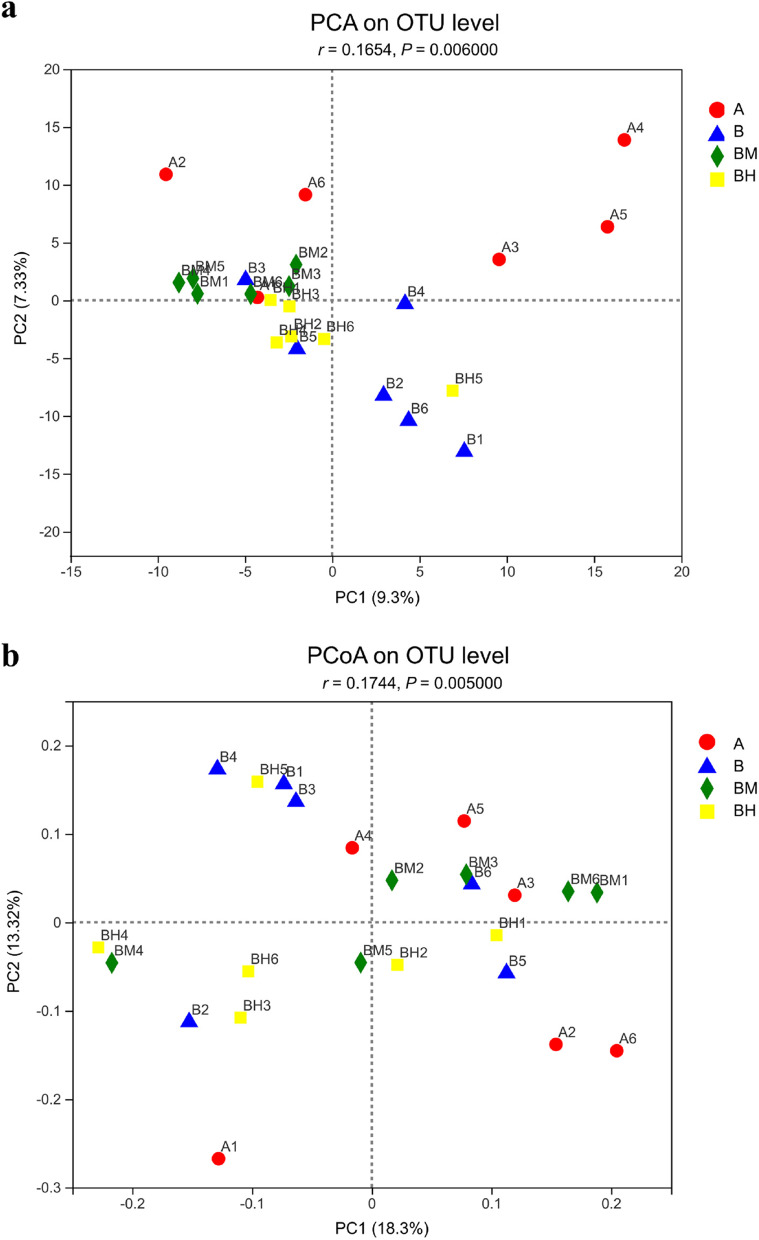
In this study, 551 OTUs were obtained from ceca contents samples of the four groups based on 97% sequence similarity level. Venn diagram (Fig. 2a) indicated 421 common core OTUs were shared by the four groups, while 20, 14, 6 and 6 OTUs were unique to groups A, B, BM and BH, respectively. There were no significant differences (P > 0.05) in ACE index, Chao1 index, Simpson index and Shannon index among all dietary treatments (Fig. 2b–e), indicating that cecum microbial α-diversity was not influenced by EOA treatment or Salmonella challenge. In order to study the similarity or difference of cecum microbial community structure in different samples, the β-diversity of cecal microorganisms was assessed by PCA analysis and PCoA analysis. PCA analysis showed that there was significant separation in cecal microbial community structure among the four groups (P = 0.006) (Fig. 3a and b), especially between the infected control and non-infected control, and between the infected control and the BM group.
Fig. 2.
Effects of dietary supplementation EOA on the α-diversity indices of cecal microbiota communities of the SE-infected broiler chickens at 23 days of age. (a) Venn diagram showing the shared OTUs by groups, (b) Ace index, (c) Chao index, (d) Simpson index, (e) Shannon index. A: broiler chickens with basal diet and no infection, B: broiler chickens with basal diet and SE infection, BM: broiler chickens with basal diet supplemented with 500 mg/kg EOA and SE infection, BH: broiler chickens with basal diet supplemented with 800 mg/kg EOA and SE infection. EOA: coated essential oils and organic acids mixture. SE: Salmonella Enteritidis
Fig. 3.
Effects of dietary supplementation EOA on the β-diversity indices of cecal microbiota communities of the SE-infected broiler chickens at 23 days of age. (a) Principal component analysis (PCA) plot of the caecal microbiota based on weighted unifrac distance, (b) Principal co-ordinates analysis (PCoA) plot of the caecal microbiota based on weighted unifrac distance. A: broiler chickens with basal diet and no infection, B: broiler chickens with basal diet and SE infection, BM: broiler chickens with basal diet supplemented with 500 mg/kg EOA and SE infection, BH: broiler chickens with basal diet supplemented with 800 mg/kg EOA and SE infection. EOA: coated essential oils and organic acids mixture. SE: Salmonella Enteritidis
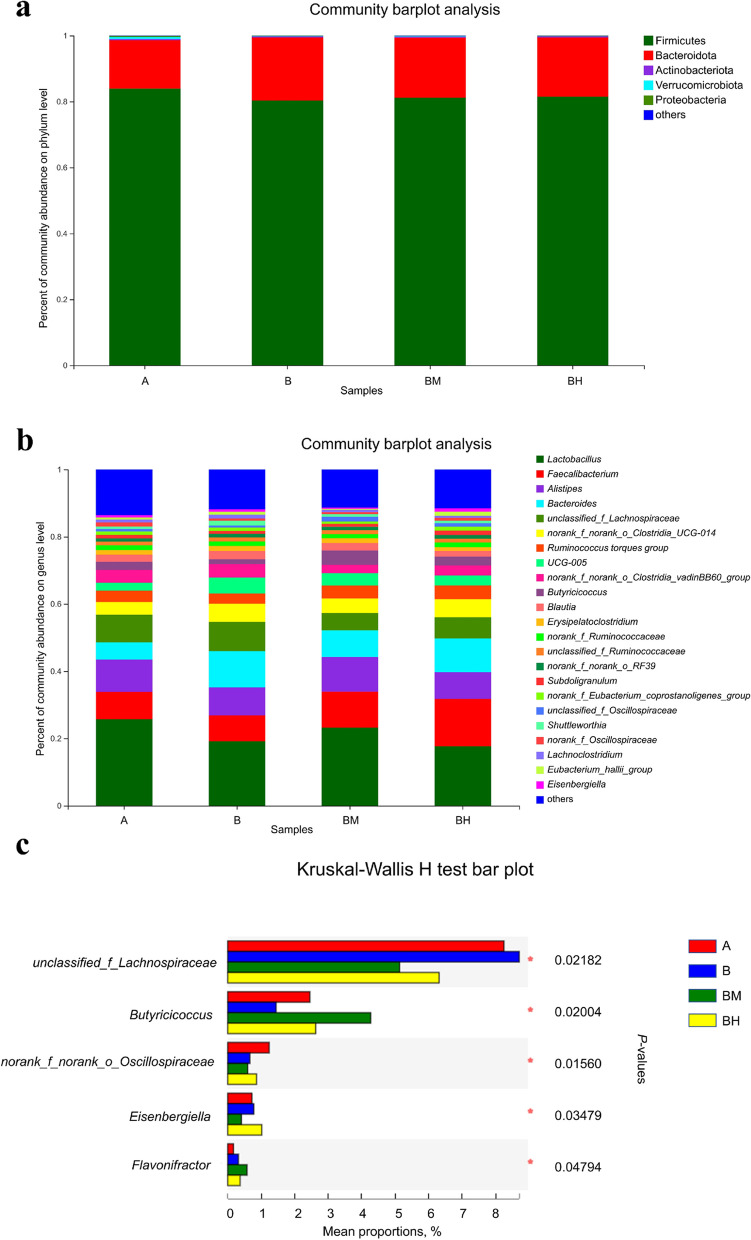
As presented in Fig. 4a, at the phyla level, ceca microbiota was dominated by Firmicutes (81.72%), Bacteroidota (17.50%), Actinobacteriota (0.34%), followed by Verrucomicrobiota (0.23%) and Proteobacteria (0.16%) for all treatments, with no significant differences in the relative abundance among four treatment groups (P > 0.05). At the genus taxa, the top 10 genera in abundance were Lactobacillus (21.44%), Faecalibacterium (10.17%), Alistipes (9.05%), Bacteroides (8.44%), unclassified_f_Lachnospiraceae (7.10%), norank_f_norank_o_Clostridia_UCG-014 (4.72%), Ruminococcus torques group (3.59%), UCG-005 (3.42%), followed by norank_f_norank_o_Clostridia_vadinBB60_group (3.32%) and Butyricicoccus (2.70%) (Fig. 4b). The comparison of cecal bacterial compositions among four groups showed that the relative abundance of unclassified_f_Lachnospiraceae was significantly (P < 0.05) increased in the single SE-infected group, while the relative abundance of Butyricicoccus was significantly (P < 0.05) increased in BM group. In addition, the relative abundances of norank_f_Oscillospiraceae, Eisenbergiella and Flavonifractor were significantly (P < 0.05) increased in the non-infected group, BH group and BM group respectively (Fig. 4c). Salmonella infection also significantly (P < 0.05) decreased the relative abundance of norank_f_norank_o_Oscillospiraceae, norank_f_norank_o_Rhodospirillales and Eggerthella. However, dietary EOA treatment significantly (P < 0.05) increased relative abundance of Butyricicoccus, unclassified_f_Oscillospiraceae, Anaerotruncus, unclassified_f_Bacillaceae and Enterococcus, whereas decreased relative abundance of unclassified_f_Lachnospiraceae, norank_f_norank_o_Clostridia_vadinBB60_group, Eisenbergiella, UCG-009 and Merdibacter (P < 0.05).
Fig. 4.
Relative abundance of cecal microbial composition of broiler chickens from different treatment groups. (a) Composition of caecal microbiota of the broilers at the phylum level, (b) Composition of caecal microbiota of the broilers at the genus level, (c) Differential cecal microbiota at different taxa levels among different groups. Asterisks (∗P < 0.05, ∗∗P < 0.01) indicate statistical differences between the treatment group. A: broiler chickens with basal diet and no infection, B: broiler chickens with basal diet and SE infection, BM: broiler chickens with basal diet supplemented with 500 mg/kg EOA and SE infection, BH: broiler chickens with basal diet supplemented with 800 mg/kg EOA and SE infection. EOA: coated essential oils and organic acids mixture. SE Salmonella Enteritidis
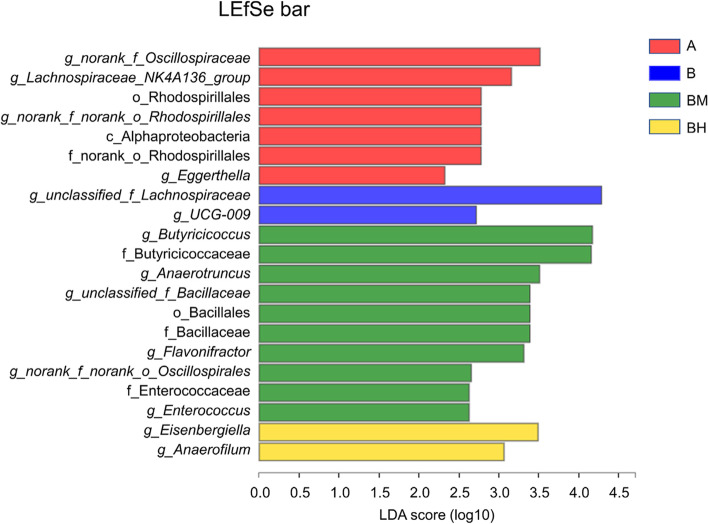
LEfSe analysis (Fig. 5) showed that g_norank_f_Oscillospiraceae, g_Lachnospiraceae_NK4A136_group, g_Eggerthella, f_norank_o_Rhodospirillales, g_norank_f_norank_o_Rhodospirillales, o_Rhodospirillales and c_Alphaproteobacteria were significantly (P < 0.05) enriched in the non-infected group, while g_unclassified_f_Lachnospiraceae and g_UCG-009 were significantly (P < 0.05) enriched in the positive B group. Moreover, g_Butyricicoccus, f_Butyricicoccaceae, g_Anaerotruncus, g_norank_f_norank_o_Oscillospirales, g_unclassified_f_Bacillaceae, o_Bacillales, f_Bacillaceae, g_Flavonifractor, f_Enterococcaceae and g_Enterococcus were significantly (P < 0.05) enriched in the BM group, and g_Eisenbergiella and g_Anaerofilum were significantly (P < 0.05) enriched in the BH group.
Fig. 5.
Histogram of the Linear Discriminant Analysis (LDA) score computed for differentially abundant taxa with cut-off LDA score > 2.0. The different colors represent microbial groups that play a significant role in groups A, B, BM and BH. A: broiler chickens with basal diet and no infection, B: broiler chickens with basal diet and SE infection, BM: broiler chickens with basal diet supplemented with 500 mg/kg EOA and SE infection, BH: broiler chickens with basal diet supplemented with 800 mg/kg EOA and SE infection. EOA: coated essential oils and organic acids mixture. SE: Salmonella Enteritidis
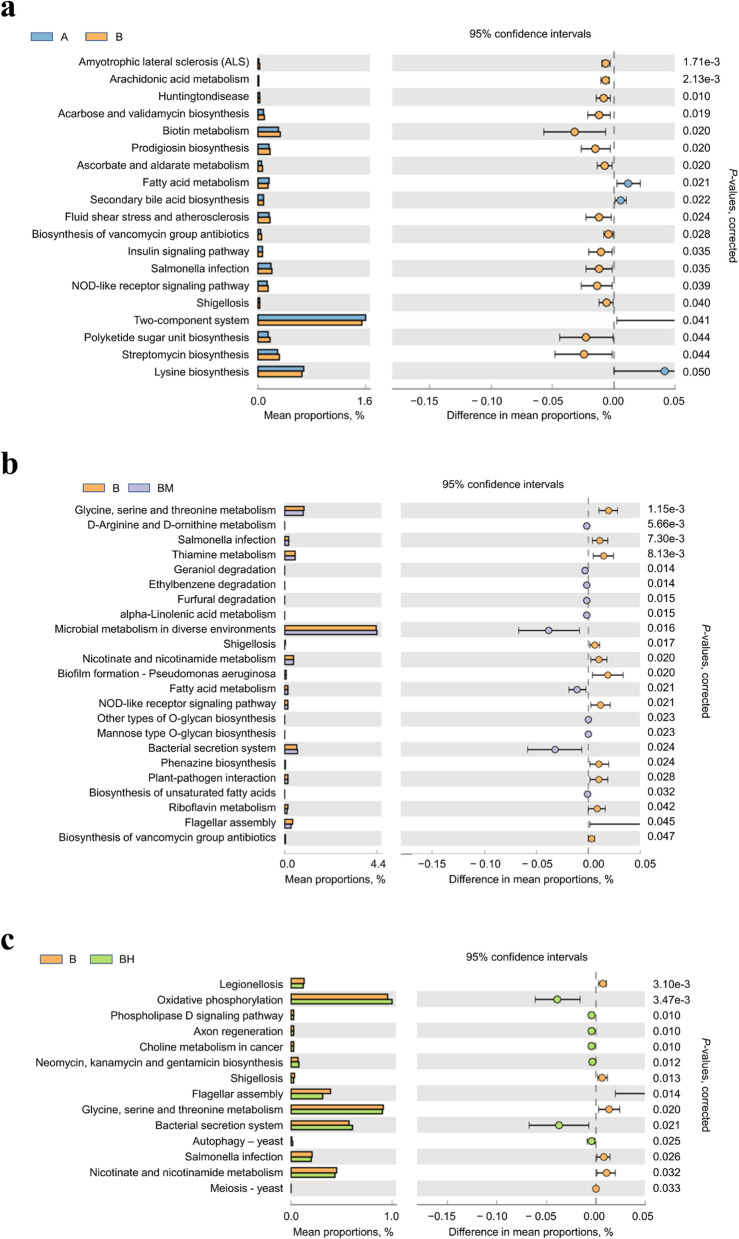
PICRUSt analysis exhibited that functions related to microbial infection and anti-infection such as Salmonella infection, Shigellosis, nucleotide oligomerization domain-like (NOD-like) receptor signaling pathway, streptomycin biosynthesis, prodigiosin biosynthesis, acarbose and validamycin biosynthesis, biotin metabolism, ascorbate and aldarate metabolism, biosynthesis of vancomycin group antibiotics and insulin signaling pathway, were significantly enhanced in single SE-infected B group compared with the non-infected A group (P < 0.05) (Fig. 6a). Comparing with the single SE-infected B group, D-arginine and D-ornithine metabolism, ethylbenzene degradation, furfural degradation, alpha-linolenic acid metabolism, microbial metabolism in diverse environments, fatty acid metabolism, bacterial secretion system and biosynthesis of unsaturated fatty acids were significantly enhanced in EOA-treated group (P < 0.05), while Salmonella infection, thiamine metabolism, Shigellosis, NOD-like receptor signaling pathway, flagellar assembly and biosynthesis of vancomycin group antibiotics were significantly enriched in single SE-infected B group (P < 0.05) (Fig. 6b and c).
Fig. 6.
PICRUSt metagenome inference analysis based on 16S rRNA dataset: (a) A vs. B, (b) B vs. BM, and (c) B vs. BH. (a–c) Prediction of significant KEGG pathways (level 3) that were differentially regulated in SE-infected group compared to non-infected group (P < 0.05). Mean proportion of functional pathways is illustrated with bar plots and dot plots indicate the differences in mean proportions between two groups based on P-values obtained from two-sided Welch’s t-test. A: broiler chickens with basal diet and no infection, B: broiler chickens with basal diet and SE infection, BM: broiler chickens with basal diet supplemented with 500 mg/kg EOA and SE infection, BH: broiler chickens with basal diet supplemented with 800 mg/kg EOA and SE infection. EOA: coated essential oils and organic acids mixture, KEGG: Kyoto encyclopedia of genes and genomes, SE: Salmonella Enteritidis
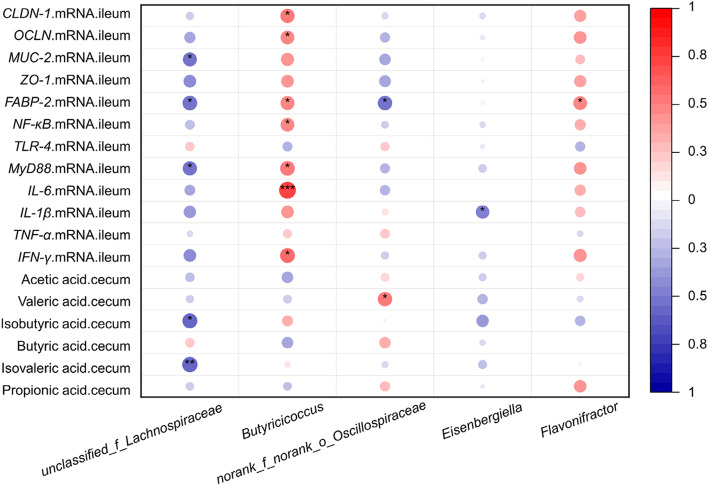
It is vital to construct a network between the differential microbiota and the expressions of intestinal tight junction protein genes and immune-related genes together with SCFA concentration of cecal content to understand how the intestinal host-microbial relationship regulates host defense and inflammation (Fig. 7). Results of the Spearman’s correlation coefficients showed that the relative abundances of unclassified_f_Lachnospiraceae (significantly enriched in Salmonella-infected chickens) was markedly negatively correlated with the relative mRNA expression levels of MUC-2, FABP-2 and MyD88, and concentration of isobutyric acid and isovaleric acid in cecal content (P < 0.05 or P < 0.01). The Butyricicoccus showed a positive regulatory effect on the mRNA expression of CLDN-1, OCLN, FABP-2, NF-κB, MyD88, IL-6 and IFN-γ (P < 0.05 or P < 0.01), while the relative abundance of g_norank_f_Oscillospiraceae had a negative correlation with the relative mRNA expression of FABP-2, but displayed a positive correlation with the concentration of valeric acid. In addition, the significant positive correlation between the relative abundances of g_Flavonifractor and the relative mRNA expression of FABP-2 was observed (P < 0.05).
Fig. 7.
Spearman’s correlation analysis between phenotypic variables and the relative abundance of microbial communities with significant differences (genus level, n = 6/group). The color and dot size represent the correlation coefficient within rows. *P < 0.05, **P < 0.01
Discussion
Since chickens serve as the reservoir of Salmonella, innovative on-farm non-antibiotic strategies for reducing pathogen colonization in birds are critical for reducing the contamination of poultry meat and eggs together with controlling human infections. Essential oils and short-chain fatty acids, used as potential antibiotics alternatives have received great attentions, in view of their potential antimicrobial properties and anti-inflammatory potential in broilers [40–42]. Herein, the present study assessed the efficacy of a new blend of coated essential oils and organic acids on growth performance, colonization and invasion of Salmonella as well as intestinal health of broilers infected with SE, and then the action mechanism was further explored.
Our results revealed that single Salmonella infection notably increased the feed to gain ratio during the later and the whole phase. Similar observations were obtained in some previous studies [43, 44]. Increased FCR induced by Salmonella challenge in our study might be due to a numerical reduce in ABW. Meanwhile, our study also indicated that dietary supplementation with 800 mg/kg of EOA remarkably improved feed efficiency of SE-infected chickens through numerically decreasing feed intake but without obvious effect on ABW during the later and the whole phase compared with the infected control birds, indicating that supplemental EOA could alleviate the negative effects caused by SE infection. Similarly, several studies have also reported an improvement in the body weight, and/or feed conversion rate in non-challenged broilers after feeding different EOA products. For example, Liu et al. [45] reported that dietary supplementation with protected essential oils and organic acids mixture containing citric acid, malic acid, sorbic acid, fumaric acid, thymol, vanillin and eugenol significantly improve FCR due to reducing average daily feed intake, but had no effects on ADG. Abdelli et al. [46] also showed that microencapsulated fumaric acid, thymol, or their mixture improved the overall FCR. Inversely, other studies suggested that dietary essential oils and organic acid blend inclusion had no obvious effects on growth performance in broiler chickens challenged with pathogens or not. For instance, a specific blend of EO based on a mixture of cinnamaldehyde and thymol alone or in combination with sodium butyrate did not affect growth performance of broiler chickens infected with SE, but significantly reduced Salmonella colonization in the cecum [25]. Adewole et al. [47] observed that dietary EOA treatments had no effect on ADFI and FCR at all phases in broiler chickens. Inconsistent results in growth performance across studies might be attributable to several factors, including the nature of essential oils and organic acids, chemical composition and dosage of EOA mixture used, protected EOA or unprotected, diet composition and digestibility, age and genetic background of the bird, health status, as well as characteristics of infection pathogen and challenge route. However, our findings suggested that the EOA supplementation could be effective in minimizing the negative impact on growth performance and FCR due to Salmonella infection.
Intestinal morphology, intestinal potential pathogens load and intestinal bacterial translocation together with intestinal immune responses are important indicators for assessing intestinal health, barrier integrity and functionality, and also be involved in the function of intestinal digestion and absorption [28, 48]. In this study, SE infection damaged the morphology of ileal villi, significantly reduced VH/CD ratio and promoted the growth of intestinal potential pathogens such as Salmonella, Escherichia coli, and Campylobacter at 3 and 10 DPI, which was in agreement with previous findings [49, 50]. Meanwhile, SE infection also induced intestinal inflammation by upregulating inflammatory-related cytokine TNF-α mRNA levels, pro-inflammatory cytokines IL-1β and NF-κB mRNA levels in the ileum at the early infection stage. Furthermore, SE infection also led to severe intestinal barrier function injury, as indicated by downregulated the expressions of intestinal tight junction proteins genes, such as CLDN-1, OCLN, ZO-1 together with MUC-2 obtained in our study. Totally, our observations indicated that SE infection caused intestinal inflammation and barrier dysfunction, resulting in damaged intestinal health in broiler chickens, which was in agreement with previous findings from chickens’ studies [51–55]. Nevertheless, SE-induced changes in the gut observed in the current study were reversed or mitigated by EOA administration, as evidenced by improved villus height and VH/CD in the ileum, and reduced Salmonella load in the cecum and internal organs. Meanwhile, dietary EOA treatment also upregulated CLDN-1, OCLN, ZO-1, MUC2 and FABP2 mRNA levels at the middle infection phase, as well as linearly reduced the gene expression level of TLR4, NF-κB and IL-1β at the early infection stage in infected broiler chickens. In accordance with our findings, plenty of studies have demonstrated that in-feed protected essential oils and organic acids blend administration could alleviate Salmonella-induced harmful effects on intestinal health through suppressing intestinal potential pathogen colonization and invasion [25, 26, 56, 57], reducing intestinal inflammatory responses, improving intestinal morphological structure [26, 28, 58, 59], and upregulating tight junction proteins genes expression [13, 60–62]. Additionally, increased amount of ileal anti-SE IgA and serum anti-SE IgG was also observed in the SE-infected chickens fed the medium and higher dose of EOA in our study. Nevertheless, different from the result of Zhang et al. [26], reported that dietary EOA administration had no difference in IgA content of the jejunum of SE-infected special pathogen-free birds. Several reports have indicated that cell mediated immunity is responsible for the clearance of S. Enteritidis from the tissue [63, 64], while humoral immunity such as intestinal IgA is critical for the limitation of intestinal pathogens such as Salmonella colonization, serum IgG directly contributes to an immune response including neutralization of toxins of pathogens [65, 66]. Based on above obtained findings, our data indicated that the EOA reduce Salmonella colonization and invasion in the gut, possibly related to more production of IgA in the gut of broiler chickens. Moreover, our results also suggested that dietary EOA addition improved FCR, possibly due to mitigating gut inflammation and gut injury caused by SE infection.
Surprisingly, in our study, SE infection enriched the relative level of Lactobacillus in the cecum compared with the non-infected control birds, which was similar to previous observation from Videnska [67]. Videnska et al. reported that SE infection increased cecal Lactobacillaceae relative abundance, but conflicted with other reports, which found the reduced beneficial bacteria such as Lactobacillus, Bifidobacterium numbers in the cecal contents following Salmonella infection [3, 49, 68, 69]. This pattern of changes would indicate serious dysbiosis in the composition of the intestinal microflora in Salmonella-infected chickens. The increase of Lactobacillus in the single SE-infected birds could be attributable to the microaerophilic growth of Lactobacilli, which may allow them to survive under conditions of increased redox potential due to the production of reactive oxygen species by granulocytes infiltrating the site of inflammation as occurs in a highly inflamed gut [67, 70]. Inversely, infected chickens fed diets supplemented with different concentrations of EOA exhibited a decrease in Lactobacillus counts in cecal digesta at whether 3 DPI or 10 DPI. Our data indicated that EOA could balance the intestinal ecosystem and reduce the dysbiosis, resulting in restoration of ecological balance of intestinal microflora. Likewise, a study also found that butyrate supplementation reduced intestinal Lactobacillus concentration in Salmonella-infected chickens [22, 71]. Lactobacillus spp. are one of the most abundant commensal bacteria in the gut. Decreased population of certain Lactobacillus spp. carrying gene encoding bile salt hydrolase in the cecal contents might explain the reason why the inclusion of EOA improved FCR of SE-infected chickens. Overall, a notable reduction in gut Salmonella load, along with gut morphological impairment induced by Salmonella. A remarkable increase in ileal specific IgA and intestinal TJ expression levels obtained from the Salmonella-infected chickens fed EOA, showing that the inclusion of the product EOA not only could alleviate SE-induced intestinal injury, but also is effective in providing protection against SE infection in broiler chickens. The findings also indirectly contribute to food safety together with reducing incidence of horizontal transmission of Salmonella infection. These observations obtained in our study may be directly associated with the antimicrobial and anti-inflammatory activity of EOs [72] or OAs in the gastrointestinal tract of chickens [41, 73–75] as well as downregulating Salmonella virulence genes expression capacity of EOs or OAs [41, 42] in the EOA product.
Numerous studies have confirmed that gut microbiota and their metabolites directly or indirectly by influence host’s immune and health [76, 77]. Additionally, many studies have showed that modifying the microflora balance in the gastrointestinal tract through nutritional strategies may improve gut barrier function and enhance bird’s resistance to colonization by Salmonella and other pathogens [28, 51]. In this study, SE infection alone remarkably reduced the concentration of isobutyric acids in the cecal digesta, whereas dietary supplementation of EOA tend to linearly increase isobutyric acid content in cecal digesta of broilers relative to the infected treatments, which was in agreement with previous study [78]. Moreover, the addition of high dose EOA also increased butyric acid content to some extent. Intestinal commensal microbes and SCFA, especially butyrate acid was reported to inhibit Salmonella colonization in the ceca [79] and downregulate the invasion and virulence genes expression of Salmonella in chickens [41, 42, 80]. Thus, our data suggested that EOA addition contribute to beneficial effects on gut health, possibly due to increasing the contents of isobutyric acid and butyric acid in the cecum of the Salmonella-infected broilers.
In the current study, neither SE infection nor EOA treatment altered α-diversity, while PCA analysis showed that SE infection obviously changed cecal microbial β-diversity relative to the negative non-infected control, indicating that SE infection notably disturbed microbial community structure of gut microbiota of chickens. In addition, interestingly, taxa analysis found that relative to the uninfected control, SE infection alone significantly expanded relative abundance of unclassified_f_Lachnospiraceae, which was similar to our observation from bacterial culture, whereas decreased the relative abundance of norank_f_norank_o_Oscillospiraceae, norank_f_norank_o_Rhodospirillales and Eggerthella. Although members of Lachnospiraceae are one of the major producers of short-chain fatty acids, different groups are positively correlated with gut health [81], extraintestinal diseases [82] and metabolic disorders [83]. Oscillospiraceae is a key bacterium in the pathogenesis of rheumatoid arthritis, which was be negatively associated with gut health [84]. Over-presentation of butyrate-producing Lachnospiraceae in the cecum showed that SE infection stimulates the immune system, allowing the proliferation of Lachnospiraceae as a biofilm to defend against pathogen infection and further confirming our observations from bacterial culture. Therefore, our data showed that SE infection altered the composition of cecal microbiome, resulting in inducing intestinal dysbiosis and intestinal inflammation, which was in similar with previous observations from chickens [3, 5, 49, 67]. While the medium-dose of EOA also notably altered cecal microbial β-diversity as compared with the infected control, which was similar to previous result [85], but was inconsistent with other previous observations [27, 58], possibly due to the differences in EOA formula or challenged model. Meanwhile, the inclusion of appropriate dose of EOA could alter cecal microbial community structure of the infected chickens. Taxa and LEfSe analysis found that dietary supplementation with suitable EOA significantly increased relative abundance of Firmicutes, g_Butyricicoccus, g_Anaerotruncus, g_unclassified_f_Bacillaceae, g_Enterococcus, whereas decreased relative abundance of Bacteroidetes, unclassified_f_Lachnospiraceae, norank_f_norank_o_Clostridia_vadinBB60_group, Eisenbergiella, UCG-009 and Merdibacter. Members of Bacteroidetes mainly produce acetic acid and propionic acid through hydrolysing a variety of indigestible dietary carbohydrates such as non-starch polysaccharides and resistant starch [86], which was associated with gut health and metabolism, while Firmicutes mainly produce butyric acid and was positively correlated with obesity, good FCR and gut health [28, 62]. Butyricicoccus is a Gram-positive, strictly anaerobic Clostridium cluster IV bacterium that produces high levels of butyrate and resists Salmonella infection, and its abundance is positively correlated with intestinal health [87]. Butyric acid-producing bacteria g_Anaerotruncus, is usually positively associated with obesity [88]. Some Enterococcus strains are normal resident commensal bacteria in the intestinal tract of food animals and human, which were positively associated with gut health and usually used as antibiotics alternatives in animal and poultry production, while other Enterococcus strains could invade into internal organs to cause malignant infection in humans and animals, especially when antibiotics are overused [89–91]. Eisenbergiella is a degrader of complex polysaccharides and producer of SCFA, which are involved in gut health and bile acid metabolism [92]. Thus, higher proportion of Firmicutes, Butyricicoccus, Anaerotruncus, and Enterococcus, accompanied by lower relative abundance of Bacteroidetes, unclassified_Lachnospiraceae, Eisenbergiella in the cecum of Salmonella-infected broiler chickens following EOA administration, suggesting that pretreatment with EOA control Salmonella infection and improve feed efficiency, possibly via improving gut microbiome and increasing the abundance of SCFA-producing bacteria. These results further confirmed our above observations. These data also implied that the health-improving effects of EOA on Salmonella-infected broiler chickens might be positively associated with the restoration of intestinal microbiota balance.
PICRUSt analysis indicated that compared with the non-infected group, SE infection increased abundances of cecal microbial functions involved in microbial infection and anti-infection such as Salmonella infection, Shigellosis, NOD-like receptor signaling pathway, flagella assembly, streptomycin biosynthesis, prodigiosin biosynthesis, acarbose and validamycin biosynthesis, biotin metabolism, ascorbate and aldarate metabolism, biosynthesis of vancomycin group antibiotics and insulin signaling pathway. NOD-like receptor signal pathway was involved in innate immune response, inflammation and apoptosis [93]. Ascorbate and aldarate metabolism were involved in antioxidant function. Hence, we speculated that Salmonella infection induced intestinal inflammatory responses and oxidative stress, increased metabolism of some nutrients and stimulated antibiotics biosynthesis in broiler chickens, all of these changes possibly contribute to reasonable reasons why Salmonella infection usually decreased feed efficiency of broiler chickens, caused gut damage and increased the occurrence of antibiotics-resistant bacteria. Fatty acid metabolism and biosynthesis of unsaturated fatty acids was involved in anti-oxidative, anti-inflammatory and anti-infective activities [94, 95]. These changes in functions of gut microbiota in the SE-infected chickens after feeding moderate dose of EOA group indicated that the coated essential oils and organic acid additives possesses anti-inflammatory and anti-infective capacities through modulating the functions of gut microbiota. Meanwhile, the enriched pathways related to neomycin, kanamycin and gentamicin biosynthesis in the high dose of EOA group, indicated that supplemental high level of EOA could effectively mobilize the bactericidal mechanism of gut microbiota, resulting in promoting the production of bacteriostatic substances, which may be one of the reasons for the effective reduction of intestinal Salmonella infection by adding EOA. Spearman’s correlation analysis found that the relative mRNA expression levels of MUC-2, FABP-2 and MyD88, together with concentration of isobutyric acid and isovaleric acid had a negative relationship with unclassified_f_Lachnospiraceae, while the relative mRNA expression of CLDN-1, OCLN, FABP-2, NF-κB, MyD88, IL-6 and IFN-γ had a positive relationship with Butyricicoccus. Some reports have showed that unclassified_f_Lachnospiraceae was associated with the destruction of tight junctions and aggravation of inflammation [96], whereas Butyricicoccus was positively related to the enhancement of epithelial barrier function and relief of colitis in rats [97]. Thus, we suggested that the health-improving effects of EOA on Salmonella-infected broiler chickens might attribute to increasing intestinal Butyricicoccus relative abundance. Further research is necessary to confirm our hypothesis.
Conclusions
In summary, dietary supplementation with coated essential oils and organic acids mixture at 500 mg/kg and 800 mg/kg could alleviate the harmful effects caused by SE infection through improving intestinal morphology; reducing Salmonella load in liver and spleen and cecum; up-regulating ileal CLDN-1, OCLN, ZO-1, MUC-2 and FABP-2 mRNA levels whereas down-regulating TLR-4 and TNF-α mRNA levels; increasing cecal isobutyric acid concentration and the relative abundance of Butyricicoccus and Anaerotruncus in the cecum; along with enriching alpha-linolenic acid metabolism, fatty acid metabolism and biosynthesis of unsaturated fatty acids of gut microbiota. Overall, the inclusion of the compound preparation of coated essential oil and organic acids in the diets might be an effective strategy to alleviate the negative effects caused by SE infection.
Acknowledgements
The authors acknowledge Menon Animal Nutrition Technology Co., Ltd., Shanghai, China provided coated organic acids and organic acids product and research grant for this experiment. We also would like to extend our appreciation to the Zhuozhou Poultry Experimental Base with limited liability for animal management.
Abbreviations
- AA
Arbor Acres
- ABW
Average body weight
- ADFI
Average daily feed intake
- ADG
Average daily gain
- CD
Crypt depth
- CFU
Colony-forming unit
- CLDN-1
Claudin-1
- DPI
Days post infection
- ELISA
Enzyme-linked immune sorbent assay
- EOs
Essential oils
- EOA
Coated essential oils and organic acids mixtures
- FABP-2
Fatty acid binding protein
- FCR
Feed conversion rate
- HRP
Horseradish peroxidase
- IFN-γ
Interferon-γ
- IgA
Immunoglobulin A
- IgG
Immunoglobulin G
- IL-6
Interleukin-6
- IL-1β
Interleukin-1β
- LDA
Linear discriminant analysis
- LEfSe
Linear discriminant analysis combined effect size
- MOT
Mortality
- MUC-2
Mucin-2
- MyD88
Myeloid differential protein-88
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NOD
Nucleotide oligomerization domain
- NRC
National research council
- OAs
Organic acids
- OCLN
Occludin
- OD
Optical density
- OTUs
Operational taxonomic units
- PBS
Phosphate buffered saline
- PCA
Principal components analysis
- PCoA
Principal coordinate analysis
- PICRUSt
Phylogenetic investigation of communities by reconstruction of unobserved states
- QIIME
Quantitative insights into microbial ecology
- RT-PCR
Reverse transcription-rolymerase chain reaction
- SCFA
Short-chain fatty acids
- SEM
Standard error of the mean
- SE
Salmonella Enteritidis
- SPF
Special pathogen-free
- TLR-4
Toll-like receptor-4
- TNF-α
Tumor necrosis factor-α
- VH
Villus height
- VH/CD
Villus height to crypt depth ratio
- ZO-1
Zonula occludens-1
Authors’ contributions
ZW and JH conceived and designed the experiment; ZH, LL, FG, JH, JQ, RB, and KZ carried out the research; ZH and LL analyzed the data and wrote the manuscript; ZW, FG, LL and YG participate in the draft editing process manuscript. ZH and ZW had primary responsibility for final content. All authors read and approved the final manuscript.
Funding
This study was funded by Menon Animal Nutrition Technology Co., Ltd., Shanghai, China. The funders had no role in the study design, analysis, or writing of this article.
Availability of data and materials
The 16S rRNA gene sequencing data generated and analyzed during the current study are available in the NCBI primary data archive (PDA) with accession number PRJNA 915,671. This data can be found here: https://www.ncbi.nlm.nih.gov/bioproject/915671.
Declarations
Ethics approval and consent to participate
The experimental animal procedures were approved by the China Agricultural University Animal Care and Use Committee (Beijing, China). In this study, all experimental methods were performed following the China Agricultural University of Health Guide for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work. We confirm that the manuscript has been read and approved by all named authors. The manuscript has not been previously published.
References
- 1.Mountzouris KC, Dalaka E, Palamidi I, Paraskeuas V, Demey V, Theodoropoulos G, et al. Evaluation of yeast dietary supplementation in broilers challenged or not with Salmonella on growth performance, cecal microbiota composition and Salmonella in ceca, cloacae and carcass skin. Poult Sci. 2015;94:2445–2455. doi: 10.3382/ps/pev243. [DOI] [PubMed] [Google Scholar]
- 2.Song J, Li Q, Everaert N, Liu R, Zheng M, Zhao G, et al. Effects of inulin supplementation on intestinal barrier function and immunity in specific pathogen-free chickens with Salmonella infection. J Anim Sci. 2020;98:skz396. doi: 10.1093/jas/skz396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mon KK, Saelao P, Halstead MM, Chanthavixay G, Chang HC, Garas L, et al. Salmonella enterica serovars Enteritidis infection alters the indigenous microbiota diversity in young layer chicks. Front Vet Sci. 2015;2:61. doi: 10.3389/fvets.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adhikari P, Lee CH, Cosby DE, Cox NA, Kim WK. Effect of probiotics on fecal excretion, colonization in internal organs and immune gene expression in the ileum of laying hens challenged with Salmonella Enteritidis. Poult Sci. 2019;98:1235–1242. doi: 10.3382/ps/pey443. [DOI] [PubMed] [Google Scholar]
- 5.Eng SK, Pusparajah P, Mutalib NSA, Ser HL, Chan KG, Lee LH. Salmonella: a review on pathogenesis, pidemiology and antibiotic resistance. Front Life Sci. 2015;8:284–293. doi: 10.1080/21553769.2015.1051243. [DOI] [Google Scholar]
- 6.Tellez G, Latorre JD. Editorial: Alternatives to antimicrobial growth promoters and their impact in gut microbiota, health and disease. Front Vet Sci. 2017;4:196. doi: 10.3389/fvets.2017.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson BR, Griffin PM, Cole D, Walsh KA, Chai SJ. Outbreak-associated Salmonella enterica serotypes and food commodities, united states, 1998–2008. Emerg Infect Dis. 2013;19:1239–1244. doi: 10.3201/eid1908.121511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Saadony MT, Salem HM, El-Tahan AM, Abd El-Mageed TA, Soliman SM, Khafaga AF, et al. The control of poultry salmonellosis using organic agents: An updated overview. Poult Sci. 2022;101:101716. doi: 10.1016/j.psj.2022.101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruvalcaba-Gomez JM, Villagran Z, Valdez-Alarcon JJ, Martinez-Nunez M, Gomez-Godinez LJ, Ruesga-Gutierrez E, et al. Non-antibiotics strategies to control Salmonella infection in poultry. Animals (Basel) 2022;12:102. doi: 10.3390/ani12010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abd El-Hack ME, El-Saadony MT, Saad AM, Salem HM, Ashry NM, Abo Ghanima MM, et al. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: A comprehensive review. Poult Sci. 2022;101:101584. doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johny AK, Darre MJ, Donoghue AM, Donoghue DJ, Venkitanarayanan K. Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacrol, and thymol on Salmonella Enteritidis and Campylobacter jejuni in chicken cecal contents in vitro. J Appl Poultry Res. 2010;19:237–244. doi: 10.3382/japr.2010-00181. [DOI] [Google Scholar]
- 12.Kollanoor-Johny A, Upadhyay A, Baskaran SA, Upadhyaya I, Mooyottu S, Mishra N, et al. Effect of therapeutic supplementation of the plant compounds trans-cinnamaldehyde and eugenol on Salmonella enterica serovar Enteritidis colonization in market-age broiler chickens. J Appl Poultry Res. 2012;21:816–822. doi: 10.3382/japr.2012-00540. [DOI] [Google Scholar]
- 13.Abdelli N, Sola-Oriol D, Perez JF. Phytogenic feed additives in poultry: Achievements, prospective and challenges. Animals (Basel) 2021;11:3471. doi: 10.3390/ani11123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendary MM, Ibrahim D, Mosbah RA, Mosallam F, Hegazy WAH, Awad NFS, et al. Thymol nanoemulsion: A new therapeutic option for extensively drug resistant foodborne pathogens. Antibiotics (Basel) 2020;10:25. doi: 10.3390/antibiotics10010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S, Song M, Yun W, Lee J, Lee C, Kwak W, et al. Effects of oral administration of different dosages of carvacrol essential oils on intestinal barrier function in broilers. J Anim Physiol Anim Nutr (Berl) 2018;102:1257–1265. doi: 10.1111/jpn.12944. [DOI] [PubMed] [Google Scholar]
- 16.Irawan A, Hidayat C, Jayanegara A, Ratriyanto A. Essential oils as growth-promoting additives on performance, nutrient digestibility, cecal microbes, and serum metabolites of broiler chickens: A meta-analysis. Anim Biosci. 2021;34:1499–1513. doi: 10.5713/ab.20.0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burt S. Essential oils: Their antibacterial properties and potential applications in foods–a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Guimaraes AG, Oliveira GF, Melo MS, Cavalcanti SC, Antoniolli AR, Bonjardim LR, et al. Bioassay-guided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic Clin Pharmacol Toxicol. 2010;107:949–957. doi: 10.1111/j.1742-7843.2010.00609.x. [DOI] [PubMed] [Google Scholar]
- 19.Teissedre PL, Waterhouse AL. Inhibition of oxidation of human low-density lipoproteins by phenolic substances in different essential oils varieties. J Agric Food Chem. 2000;48:3801–3805. doi: 10.1021/jf990921x. [DOI] [PubMed] [Google Scholar]
- 20.Hotta M, Nakata R, Katsukawa M, Hori K, Takahashi S, Inoue H. Carvacrol, a component of thyme oil, activates pparalpha and gamma and suppresses COX-2 expression. J Lipid Res. 2010;51:132–139. doi: 10.1194/jlr.M900255-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Bansal M, Wagle B, Sun X, Rath N, Donoghue A, et al. Sodium butyrate reduces Salmonella Enteritidis infection of chicken enterocytes and expression of inflammatory host genes in vitro. Front Microbiol. 2020;11:553670. doi: 10.3389/fmicb.2020.553670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onrust L, Baeyen S, Haesebrouck F, Ducatelle R, Van Immerseel F. Effect of in feed administration of different butyrate formulations on Salmonella Enteritidis colonization and cecal microbiota in broilers. Vet Res. 2020;51:56. doi: 10.1186/s13567-020-00780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu JD, Bayir HO, Cosby DE, Cox NA, Williams SM, Fowler J. Evaluation of encapsulated sodium butyrate on growth performance, energy digestibility, gut development, and Salmonella colonization in broilers. Poult Sci. 2017;96:3638–3644. doi: 10.3382/ps/pex174. [DOI] [PubMed] [Google Scholar]
- 24.Aristimunha PC, Rosa AP, Boemo LS, Garcez DC, Rosa DP, Londero A, et al. A blend of benzoic acid and essential oil compounds as an alternative to antibiotic growth promoters in broiler diets. J Appl Poultry Res. 2016;25:455–463. doi: 10.3382/japr/pfw015. [DOI] [Google Scholar]
- 25.Cerisuelo A, Marin C, Sanchez-Vizcaino F, Gomez EA, de la Fuente JM, Duran R, et al. The impact of a specific blend of essential oil components and sodium butyrate in feed on growth performance and Salmonella counts in experimentally challenged broilers. Poult Sci. 2014;93:599–606. doi: 10.3382/ps.2013-03528. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Shen YR, Wu S, Xiao YQ, He Q, Shi SR. The dietary combination of essential oils and organic acids reduces Salmonella Enteritidis in challenged chicks. Poult Sci. 2019;98:6349–6355. doi: 10.3382/ps/pez457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pham VH, Abbas W, Huang J, He Q, Zhen W, Guo Y, et al. Effect of blending encapsulated essential oils and organic acids as an antibiotic growth promoter alternative on growth performance and intestinal health in broilers with necrotic enteritis. Poult Sci. 2022;101:101563. doi: 10.1016/j.psj.2021.101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pham VH, Kan L, Huang J, Geng Y, Zhen W, Guo Y, et al. Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis. J Anim Sci Biotechnol. 2020;11:18. doi: 10.1186/s40104-019-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhai H, Liu H, Wang S, Wu J, Kluenter AM. Potential of essential oils for poultry and pigs. Anim Nutr. 2018;4:179–186. doi: 10.1016/j.aninu.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Council NR. Nutrient requirements of poultry: Ninth revised edition, 1994. Washington, DC: The National Academies Press; 1994. p. 176. [Google Scholar]
- 31.Zhen W, Shao Y, Wu Y, Li L, Pham VH, Abbas W, et al. Dietary yeast beta-glucan supplementation improves eggshell color and fertile eggs hatchability as well as enhances immune functions in breeder laying hens. Int J Biol Macromol. 2020;159:607–621. doi: 10.1016/j.ijbiomac.2020.05.134. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y, Shao Y, Song B, Zhen W, Wang Z, Guo Y, et al. Effects of bacillus coagulans supplementation on the growth performance and gut health of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J Anim Sci Biotechnol. 2018;9:9. doi: 10.1186/s40104-017-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Edgar RC. Search and clustering orders of magnitude faster than blast. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 35.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. Qiime allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warton DI, Wright ST, Wang Y. Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol Evol. 2012;3:89–101. doi: 10.1111/j.2041-210X.2011.00127.x. [DOI] [Google Scholar]
- 37.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16s rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. Stamp: Statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brenes A, Roura E. Essential oils in poultry nutrition: Main effects and modes of action. Anim Feed Sci Technol. 2010;158:1–14. doi: 10.1016/j.anifeedsci.2010.03.007. [DOI] [Google Scholar]
- 41.Van Immerseel F, Russell JB, Flythe MD, Gantois I, Timbermont L, Pasmans F, et al. The use of organic acids to combat Salmonella in poultry: a mechanistic explanation of the efficacy. Avian Pathol. 2006;35:182–188. doi: 10.1080/03079450600711045. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez-Rubio C, Ordonez C, Abad-Gonzalez J, Garcia-Gallego A, Honrubia MP, Mallo JJ, et al. Butyric acid-based feed additives help protect broiler chickens from Salmonella Enteritidis infection. Poultry Sci. 2009;88:943–948. doi: 10.3382/ps.2008-00484. [DOI] [PubMed] [Google Scholar]
- 43.Chalghoumi R, Marcq C, Thewis A, Portetelle D, Beckers Y. Effects of feed supplementation with specific hen egg yolk antibody (immunoglobin γ) on Salmonella species cecal colonization and growth performances of challenged broiler chickens. Poult Sci. 2009;88:2081–2092. doi: 10.3382/ps.2009-00173. [DOI] [PubMed] [Google Scholar]
- 44.El-Shall NA, Awad AM, El-Hack MEA, Naiel MAE, Othman SI, Allam AA, et al. The simultaneous administration of a probiotic or prebiotic with live salmonella vaccine improves growth performance and reduces fecal shedding of the bacterium in Salmonella-challenged broilers. Animals (Basel) 2019;10:70. doi: 10.3390/ani10010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu YL, Yang X, Xin HL, Chen S, Yang CB, Duan YL, et al. Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Anim Sci J. 2017;88:1414–1424. doi: 10.1111/asj.12782. [DOI] [PubMed] [Google Scholar]
- 46.Abdelli N, Francisco Perez J, Vilarrasa E, Melo-Duran D, Cabeza Luna I, Karimirad R, et al. Microencapsulation improved fumaric acid and thymol effects on broiler chickens challenged with a short-term fasting period. Front Vet Sci. 2021;8:686143. doi: 10.3389/fvets.2021.686143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adewole DI, Oladokun S, Santin E. Effect of organic acids-essential oils blend and oat fiber combination on broiler chicken growth performance, blood parameters, and intestinal health. Anim Nutr. 2021;7:1039–1051. doi: 10.1016/j.aninu.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Celi P, Cowieson AJ, Fru-Nji F, Steinert RE, Kluenter AM, Verlhac V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim Feed Sci Tech. 2017;234:88–100. doi: 10.1016/j.anifeedsci.2017.09.012. [DOI] [Google Scholar]
- 49.Juricova H, Videnska P, Lukac M, Faldynova M, Babak V, Havlickova H, et al. Influence of Salmonella enterica serovar Enteritidis infection on the development of the cecum microbiota in newly hatched chicks. Appl Environ Microb. 2013;79:745–747. doi: 10.1128/Aem.02628-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meijerink N, van den Biggelaar R, van Haarlem DA, Stegeman JA, Rutten V, Jansen CA. A detailed analysis of innate and adaptive immune responsiveness upon infection with Salmonella enterica serotype Enteritidis in young broiler chickens. Vet Res. 2021;52:109. doi: 10.1186/s13567-021-00978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhen W, Shao Y, Gong X, Wu Y, Geng Y, Wang Z, et al. Effect of dietary bacillus coagulans supplementation on growth performance and immune responses of broiler chickens challenged by Salmonella Enteritidis. Poult Sci. 2018;97:2654–2666. doi: 10.3382/ps/pey119. [DOI] [PubMed] [Google Scholar]
- 52.Chappell L, Kaiser P, Barrow P, Jones MA, Johnston C, Wigley P. The immunobiology of avian systemic salmonellosis. Vet Immunol Immunop. 2009;128:53–59. doi: 10.1016/j.vetimm.2008.10.295. [DOI] [PubMed] [Google Scholar]
- 53.Awad WA, Hess C, Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins (Basel) 2017;9:60. doi: 10.3390/toxins9020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matulova M, Varmuzova K, Sisak F, Havlickova H, Babak V, Stejskal K, et al. Chicken innate immune response to oral infection with Salmonella enterica serotype Enteritidis. Vet Res. 2013;44:37. doi: 10.1186/1297-9716-44-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rychlik I, Elsheimer-Matulova M, Kyrova K. Gene expression in the chicken caecum in response to infections with non-typhoid Salmonella. Vet Res. 2014;45:119. doi: 10.1186/s13567-014-0119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grilli E, Tugnoli B, Formigoni A, Massi P, Fantinati P, Tosi G, et al. Microencapsulated sorbic acid and nature-identical compounds reduced salmonella hadar and Salmonella Enteritidis colonization in experimentally infected chickens. Poultry Sci. 2011;90:1676–1682. doi: 10.3382/ps.2011-01441. [DOI] [PubMed] [Google Scholar]
- 57.Aljazzar A, Abd El-Hamid MI, El-Malt RMS, El-Gharreb WR, Abdel-Raheem SM, Ibrahim AM, et al. Prevalence and antimicrobial susceptibility of campylobacter species with particular focus on the growth promoting, immunostimulant and anti-Campylobacter jejuni activities of eugenol and trans-cinnamaldehyde mixture in broiler chickens. Animals (Basel) 2022;12:905. doi: 10.3390/ani12070905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdelli N, Perez JF, Vilarrasa E, Cabeza Luna I, Melo-Duran D, D'Angelo M, et al. Targeted-release organic acids and essential oils improve performance and digestive function in broilers under a necrotic enteritis challenge. Animals (Basel) 2020;10:259. doi: 10.3390/ani10020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ibrahim D, Eldemery F, Metwally AS, Abd-Allah EM, Mohamed DT, Ismail TA, et al. Dietary eugenol nanoemulsion potentiated performance of broiler chickens: Orchestration of digestive enzymes, intestinal barrier functions and cytokines related gene expression with a consequence of attenuating the severity of e. Coli o78 infection. Front Vet Sci. 2022;9:847580. doi: 10.3389/fvets.2022.847580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amer SA, Tolba SA, AlSadek DMM, Abdel Fattah DM, Hassan AM, Metwally AE. Effect of supplemental glycerol monolaurate and oregano essential oil blend on the growth performance, intestinal morphology, and amino acid digestibility of broiler chickens. BMC Vet Res. 2021;17:312. doi: 10.1186/s12917-021-03022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stefanello C, Rosa DP, Dalmoro YK, Segatto AL, Vieira MS, Moraes ML, et al. Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Front Vet Sci. 2019;6:491. doi: 10.3389/fvets.2019.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bortoluzzi C, Lahaye L, Oxford J, Detzler D, Eyng C, Barbieri NL, et al. Protected organic acid and essential oils for broilers raised under field conditions: Intestinal health biomarkers and cecal microbiota. Front Physiol. 2021;12:722339. doi: 10.3389/fphys.2021.722339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Babu U, Dalloul RA, Okamura M, Lillehoj HS, Xie H, Raybourne RB, et al. Salmonella Enteritidis clearance and immune responses in chickens following Salmonella vaccination and challenge. Vet Immunol Immunop. 2004;101:251–257. doi: 10.1016/j.vetimm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Beal RK, Wigley P, Powers C, Hulme SD, Barrow PA, Smith AL. Age at primary infection with Salmonella enterica serovar typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet Immunol Immunopathol. 2004;100:151–164. doi: 10.1016/j.vetimm.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 65.Baurhoo B, Ferket PR, Zhao X. Effects of diets containing different concentrations of mannanoligosaccharide or antibiotics on growth performance, intestinal development, cecal and litter microbial populations, and carcass parameters of broilers. Poultry Sci. 2009;88:2262–2272. doi: 10.3382/ps.2008-00562. [DOI] [PubMed] [Google Scholar]
- 66.Van Immerseel F, Methner U, Rychlik I, Nagy B, Velge P, Martin G, et al. Vaccination and early protection against non-host-specific Salmonella serotypes in poultry: Exploitation of innate immunity and microbial activity. Epidemiol Infect. 2005;133:959–978. doi: 10.1017/S0950268805004711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Videnska P, Sisak F, Havlickova H, Faldynova M, Rychlik I. Influence of Salmonella enterica serovar enteritidis infection on the composition of chicken cecal microbiota. BMC Vet Res. 2013;9:140. doi: 10.1186/1746-6148-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu L, Lin L, Zheng L, Tang H, Fan X, Xue N, et al. Cecal microbiome profile altered by Salmonella enterica serovar enteritidis inoculation in chicken. Gut Pathog. 2018;10:34. doi: 10.1186/s13099-018-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aljahdali NH, Sanad YM, Han J, Foley SL. Current knowledge and perspectives of potential impacts of Salmonella Enteritidis on the profile of the gut microbiota. BMC Microbiol. 2020;20:353. doi: 10.1186/s12866-020-02008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu W, Xiao Z, An W, Dong Y, Zhang B. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. Plos One. 2018;13:e0197762. doi: 10.1371/journal.pone.0197762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ibrahim D, Abdelfattah-Hassan A, Badawi M, Ismail TA, Bendary MM, Abdelaziz AM, et al. Thymol nanoemulsion promoted broiler chicken's growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella Typhimurium. Sci Rep. 2021;11:7742. doi: 10.1038/s41598-021-86990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Polycarpo GV, Andretta I, Kipper M, Cruz-Polycarpo VC, Dadalt JC, Rodrigues PHM, et al. Meta-analytic study of organic acids as an alternative performance-enhancing feed additive to antibiotics for broiler chickens. Poultry Sci. 2017;96:3645–3653. doi: 10.3382/ps/pex178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hovorkova P, Skrivanova E. Use of caprylic acid in broiler chickens: Effect on Campylobacter jejuni. Foodborne Pathog Dis. 2015;12:712–718. doi: 10.1089/fpd.2015.1978. [DOI] [PubMed] [Google Scholar]
- 75.Skrivanova E, Hovorkova P, Cermak L, Marounek M. Potential use of caprylic acid in broiler chickens: Effect on Salmonella Enteritidis. Foodborne Pathog Dis. 2015;12:62–67. doi: 10.1089/fpd.2014.1833. [DOI] [PubMed] [Google Scholar]
- 76.Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:223–237. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 77.Gasaly N, de Vos P, Hermoso MA. Impact of bacterial metabolites on gut barrier function and host immunity: A focus on bacterial metabolism and its relevance for intestinal inflammation. Front Immunol. 2021;12:658354. doi: 10.3389/fimmu.2021.658354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang X, Liu Y, Yan F, Yang C, Yang X. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poult Sci. 2019;98:2858–2865. doi: 10.3382/ps/pez031. [DOI] [PubMed] [Google Scholar]
- 79.Cox NA, Cason JA, Richardson LJ. Minimization of Salmonella contamination on raw poultry. Annu Rev Food Sci Technol. 2011;2:75–95. doi: 10.1146/annurev-food-022510-133715. [DOI] [PubMed] [Google Scholar]
- 80.Van Immerseel F, Fievez V, De Buck J, Pasmans F, Martel A, Haesebrouck F, et al. Microencapsulated short-chain fatty acids in feed modify colonization and invasion early after infection with Salmonella Enteritidis in young chickens. Poultry Sci. 2004;83:69–74. doi: 10.1093/ps/83.1.69. [DOI] [PubMed] [Google Scholar]
- 81.Stanley D, Hughes RJ, Geier MS, Moore RJ. Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: Challenges presented for the identification of performance enhancing probiotic bacteria. Front Microbiol. 2016;7:187. doi: 10.3389/fmicb.2016.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The controversial role of human gut lachnospiraceae. Microorganisms. 2020;8:573. doi: 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kameyama K, Itoii K. Intestinal colonization by a lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014;29:427–430. doi: 10.1264/jsme2.ME14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu Q, Wu C, Yu J, Luo J, Peng X. Angelica sinensis polysaccharide improves rheumatoid arthritis by modifying the expression of intestinal CLDN5, SLIT3 and RGS18 through gut microbiota. Int J Biol Macromol. 2022;209:153–161. doi: 10.1016/j.ijbiomac.2022.03.090. [DOI] [PubMed] [Google Scholar]
- 85.Qiao J, Shang Z, Liu X, Wang K, Wu Z, Wei Q, et al. Regulatory effects of combined dietary supplementation with essential oils and organic acids on microbial communities of cobb broilers. Front Microbiol. 2021;12:814626. doi: 10.3389/fmicb.2021.814626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, et al. A genomic view of the human-bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 87.Eeckhaut V, Wang J, Van Parys A, Haesebrouck F, Joossens M, Falony G, et al. The probiotic butyricicoccus pullicaecorum reduces feed conversion and protects from potentially harmful intestinal microorganisms and necrotic enteritis in broilers. Front Microbiol. 2016;7:1416. doi: 10.3389/fmicb.2016.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70:761–774. doi: 10.1136/gutjnl-2019-319664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ben Braiek O, Smaoui S. Enterococci: Between emerging pathogens and potential probiotics. Biomed Res Int. 2019;2019:5938210. doi: 10.1155/2019/5938210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shao Y, Zhen W, Guo F, Hu Z, Zhang K, Kong L, et al. Pretreatment with probiotics Enterococcus faecium NCIMB 11181 attenuated Salmonella Typhimurium-induced gut injury through modulating intestinal microbiome and immune responses with barrier function in broiler chickens. J Anim Sci Biotechnol. 2022;13:130. doi: 10.1186/s40104-022-00765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu Y, Zhen W, Geng Y, Wang Z, Guo Y. Pretreatment with probiotic Enterococcus faecium NCIMB 11181 ameliorates necrotic enteritis-induced intestinal barrier injury in broiler chickens. Sci Rep. 2019;9:10256. doi: 10.1038/s41598-019-46578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hylemon PB, Harris SC, Ridlon JM. Metabolism of hydrogen gases and bile acids in the gut microbiome. FEBS Lett. 2018;592:2070–2082. doi: 10.1002/1873-3468.13064. [DOI] [PubMed] [Google Scholar]
- 93.Platnich JM, Muruve DA. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch Biochem Biophys. 2019;670:4–14. doi: 10.1016/j.abb.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 94.Weldon SM, Mullen AC, Loscher CE, Hurley LA, Roche HM. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J Nutr Biochem. 2007;18:250–258. doi: 10.1016/j.jnutbio.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 95.Chen Y, Qiu X, Yang J. Comparing the in vitro antitumor, antioxidant and anti-inflammatory activities between two new very long chain polyunsaturated fatty acids, docosadienoic acid (DDA) and docosatrienoic acid (DTA), and docosahexaenoic acid (DHA) Nutr Cancer. 2021;73:1697–1707. doi: 10.1080/01635581.2020.1804949. [DOI] [PubMed] [Google Scholar]
- 96.Zhou Y, Zhang M, Zhao X, Feng J. Ammonia exposure induced intestinal inflammation injury mediated by intestinal microbiota in broiler chickens via TLR4/TNF-alpha signaling pathway. Ecotoxicol Environ Saf. 2021;226:112832. doi: 10.1016/j.ecoenv.2021.112832. [DOI] [PubMed] [Google Scholar]
- 97.Eeckhaut V, Machiels K, Perrier C, Romero C, Maes S, Flahou B, et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut. 2013;62:1745–1752. doi: 10.1136/gutjnl-2012-303611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The 16S rRNA gene sequencing data generated and analyzed during the current study are available in the NCBI primary data archive (PDA) with accession number PRJNA 915,671. This data can be found here: https://www.ncbi.nlm.nih.gov/bioproject/915671.