Abstract
Background
Dendrobium nobile and Dendrobium chrysotoxum are important species of the genus Dendrobium and have great economic and medicinal value. However, the medicinal properties of these two plants remain poorly understood. This study aimed to investigate the medical properties of D. nobile and D. chrysotoxum by conducting a comprehensive chemical profiling of the two plants. Additionally, active compounds and predictive targets for anti-hepatoma activity in D. chrysotoxum extracts were identified using Network Pharmacology.
Results
Chemical profiling showed that altogether 65 phytochemicals were identified from D. nobile and D. chrysotoxum, with major classes as alkaloids, terpenoids, flavonoids, bibenzyls and phenanthrenes. About 18 compounds were identified as the important differential metabolites in D. nobile and D. chrysotoxum. Furtherly, CCK-8 results showed that the extracts of stems and leaves of D. nobile and D. chrysotoxum could inhibit the growth of Huh-7 cells, and the anti-hepatoma activity of extracts were dose-dependent. Among the extracts, the extract of D. chrysotoxum showed significant anti-hepatoma activity. In order to find the potential mechanism of anti-hepatoma activity of D. chrysotoxum, five key compounds and nine key targets were obtained through constructing and analyzing the compound-target-pathway network. The five key compounds were chrysotobibenzyl, chrysotoxin, moscatilin, gigantol and chrysotoxene. Nine key targets, including GAPDH, EGFR, ESR1, HRAS, SRC, CCND1, HIF1A, ERBB2 and MTOR, could be considered as the core targets of the anti-hepatoma activity of D. chrysotoxum.
Conclusions
In this study, the chemical composition difference and anti-hepatoma activity of stems and leaves of D. nobile and D. chrysotoxum were compared, and the potential anti-hepatoma mechanism of D. chrysotoxum was revealed in a multi-target and multi-pathway manner.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-023-04048-y.
Keywords: D. nobile, D. chrysotoxum, Metabolomics, Network pharmacology, Hepatoma, Biomarkers
Introduction
Hepatoma is the major cause of cancer associated mortality. In fact, since cancer treatment is often accompanied by side effects, there is a growing demand for innocuous and more effective anticancer drugs [1]. Phytochemicals isolated from medicinal plants have been largely neglected in this context, although their pharmacological activities have been well investigated in the past, and they may have considerable medicinal potential [2]. In particular, medicinal plants could be and more effective in the treatment of various diseases, including cancer [3]. Various biomolecules presented in the plant extract such as alkaloids, terpenoids, polyphenols, polysaccharides, flavonoids, tannins, saponins, phenolics, amino acids, and proteins are natural sources of therapeutic drugs [4–8].
With approximately 1,400 native species, Dendrobium is one of the largest families of orchids and is widely distributed around the world [9]. Recent pharmacological studies have shown that Dendrobium has a wealth of medicinal effects, such as hepatoprotective, anti-inflammatory, anti-angiogenic and anti-oxidative properties [9, 10]. The stems of D. nobile and D. chrysotoxum have great economic and medicinal value and are listed in the Chinese Pharmacopoeia of 2020 (Fig. 1A, B and C) [11]. The chemical constituents of D. nobile and D. chrysotoxum are mainly alkaloids, terpenoids, polyphenols, flavonoids, phenanthrenes, bibenzyls and amino acids [12–14]. Recent pharmacological studies have shown that components of the stems of Dendrobium, possess a broad range of activities, encompassing hepatoprotective, anti-proliferative activity toward cancer cells, immunostimulating, anti-diabetic activity, cataractogenesis-inhibiting activity, neuroprotective activity, anti-inflammatory activity, anti-platelet aggregation activity and hemagglutininating activities and also exert beneficial actions on colonic health and alleviate symptoms of hyperthyroidism [15–17]. Because only the stems of D. nobile and D. chrysotoxum are permitted for use according to the Chinese Pharmacopoeia, their leaves are largely discarded [18]. However, the leaves of these Dendrobium plants possess chemical constituents similar to those found in the stems [19–21].
Fig. 1.
Plants and metabolites. (A, B, C) Dendrobium species planting base in Wenshan, Yunnan
Plant extracts act on certain molecular targets due to the synergistic effects of their chemical compounds and the fact that they could interact with many targets simultaneously [3, 22]. In recent years, network pharmacological analysis has been effectively applied to predict the relationship between protein targets, active compounds and related diseases. This approach attempts to explore disease-related, multiple active compounds and targets, thus allowing to characterize the multi-target mechanism of bioactive compounds [23, 24]. This analysis can provide insights into the link between Traditional Chinese Medicine (TCM) bioactive compounds and diseases, possibly revealing the pharmacological properties of multiple biomolecules found in medicinal plants [25].
Screening and identification of active components from TCM is rather challenging due to the diversity and complexity of chemical components. Herein, comprehensive metabolite profiling of the D. nobile and D. chrysotoxum were conducted by LC-MS using an untargeted metabolomics approach. The anti-hepatoma activities of the extracts from the stems and leaves of D. Nobile and D. chrysotoxum were compared by CCK-8 assay. A network pharmacology approach was applied to characterize the possible mechanism of action of D. chrysotoxum compounds on the human hepatoma Huh-7 cell line. Active compounds and potential targets of D. chrysotoxum, as well as related genes of hepatoma were obtained from the public databases, while the potential targets and signalling pathways were determined by protein-protein interaction (PPI), gene ontology (GO) and pathway enrichment analyses. Ultimately, the compound-target and target-pathway networks were constructed. This study compared the chemical composition and anti-hepatoma activity of D. nobile and D. chrysotoxum extracts, and revealed the potential anti-hepatoma action mechanism of D. chrysotoxum using a multi-target and multi-pathway approach.
Materials and methods
Reagents and materials
Methanol (MeOH), acetonitrile and ethanol were obtained from Thermo Fisher Scientific (Waltham, MA, USA). 5-Fluorouracil was bought from Macklin Biochemica Co., Ltd.(Shanghai, China). CCK-8 kit was bought from Beyotime Biotechnology(Shanghai, China); Phosphate Buffered Saline (PBS) was bought from Biological Industries (The State of Israel); DMEM medium; Fetal bovine serum (FBS); Antibiotics; Trypsin were bought from Thermo Fisher Scientific (Massachusetts, US).
D. nobile and D. chrysotoxum were harvested from Wenshan, Yunnan Province, China. The experiment was conducted using at 3-year-old wild-cultured D. nobile and D. chrysotoxum, which were collected in February.
Human hepatocellular carcinoma (Huh7 cell line) was purchased from Hangzhou Qiannuo Biotechnology Co., Ltd (Yuanlong Commercial Building, Baiyang Street, Qiantang District, Hangzhou, China). Normal human hepatocytes (Lo2 cells line) was purchased from Shanghai Cell Bank, Chinese Academy of Sciences (No. 320 Yueyang Road, Xuhui District, Shanghai, China). ( Note:We used only Huh-7 and Lo2 (normal human hepatocytes) cell lines in our experiments, and no other human samples were used.)
Extraction procedure
The stems and leaves of the D. nobile and D. chrysotoxum were cut into small segments completely dried at 50–60 ℃. Then crushed by a pulverizer and put into a sealed bag for storage. 10 g of crushed stems and leaves were extracted with 500 mL of 70% methanol aqueous solution with ultrasonic for 45 min respectively, the filtrate was collected, concentrated by a rotary evaporator, and freeze-dried [26].
Liquid chromatography tandem mass spectrometry (LC–MS/MS) analysis for metabolite identification
Sample preparation for metabolomics
The tissue samples were dried at 55℃ and ground into a fine powder. Approximately 0.01 g of root was homogenized with 10 mL of MeOH/H2O (70:30, v/v), centrifuged for 5 min and subjected to ultrasonic treatment for 20 min. The sample was then centrifuged at 10,000 rpm for 10 min, and the resulting supernatant solution was passed through a 0.22-µm pore-size membrane. The filtrate was transferred to sample vials for LC-MS/MS analysis [26].
UPLC-Q-TOF-MS/MS analysis method
Chromatographic separation was achieved using an Waters ACQUITY UPLC HSS T3 column (1.8 μm, 2.1 mm × 100.0 mm; Waters Corporation, Milford, MA, USA). The column temperature and flow rate were set at 40℃ and 0.4 mL·min− 1, respectively. The injection volume and detection wavelength of the chromatographic column were set at 1 µL and 254 nm, respectively. The mobile phase was composed of water (A) and acetonitrile (B), with a gradient elution program of 4% B (0–1 min), 4–50% B (1–7 min), 50–70% B (7–8 min), 70–95% B (8–12 min), 95% B (12–15 min), 95 − 4% B (15–16 min) and 4% B (16–18 min).
Mass spectrometric detection was performed in electrospray mode using a Xevo G2-XS Q-Tof mass spectrometer detector (Waters Corporation, Milford, MA, USA). Argon was used as the desolvation and collision gas. The full-scan data range was 50 − 1,200 Da, the source temperature was 100 °C, the desolvation temperature was 400 °C and the scanning frequency was 1.000 s. The locking spray standard was 400 mg·mL− 1 with a collision energy of 6.000 V for the low collision energy scan and 30 to 70 V for the high collision energy scan of the mass spectrometer, and the UPLC system was controlled by MassLynx4.1 software (Waters Corporation, Milford, MA, USA).
CCK-8 assay of plant extracts and cytotoxicity tests
Human hepatoma Huh-7 cells were maintained routinely in DMEM supplemented with 10% FBS and 1% antibiotics in a humidified atmosphere of 5% CO2/50% air [27]. The anti-hepatoma activity of extracts from the stems and leaves of the D. nobile and D. chrysotoxum were determined by CCK-8 assay. 100 µL cell suspension were cultured in a 96-well plate for 24 h, then the medium was removed, and the cells were incubated with different concentrations of the extracts, the extracts were dissolved in DMEM medium containing 10% FBS and 1% antibiotics for 48 h. 10 µL CCK-8 solution was added to each well for 2 h, the absorbance was measured at 450 nm using a microplate reader. The absorbance of blank group without cells was Abs blank. 5-Fluorouracil was used as a positive control [28]. The cell inhibition rate of extract solution was calculated according to the following formula.
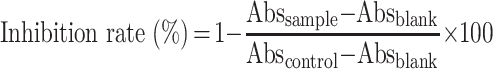 |
To evaluate the effect of D. nobile and D. chrysotoxum on the viability of Lo2 cells (normal human hepatocytes), cytotoxicity assays were performed using the Cell Counting Kit-8. We used the method of Xia et al. as a reference for experimental design, briefly, cells in the logarithmic growth phase were subjected to the cell passage cultivation and finally 100 µL of cell suspension (cell density of 1 × 10 5 /mL) was added to each well of the 96-well plate. The blank control was cell-free cell culture medium. The final concentrations of the experimental group were 0, 50, 100, 150, 200, 250, 500 µg/mL, and 6 replicate wells were added for each concentration, and each group was repeated 3 times. For the control group, only 100 µL of cell culture medium was added. After incubation for 24 h, 10 µL of CCK-8 was added to each well and incubated for 2 h. The absorbance (OD) was measured at 450 nm on an enzyme marker [29].
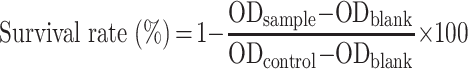 |
Network pharmacology analysis
Database construction and prediction of potential targets
The SIMILES and InChIKey of the chemical components of aerial part of D. chrysotoxum were collected from the PubChem database. The GI absorption and bioavailability score of the compounds were calculated by SwissADME, and the compounds with GI absorption were high and bioavailability score > 0.3 were screened. The Swiss Target Prediction database was used to obtain the target corresponding of the components [30]. Use “Liver cancer” as the key words, the liver cancer related genes were screened out by DisGeNET [31], GeneCards [32], OMIM [33]. The obtained targets of the constituents and the disease targets of liver cancer were unified as UniProt ID through the UniProt database [34].
Network construction
Cytoscape software was used to construct the “drug components-target” network to explain the interactions between the core chemical components of D. chrysotoxum and potential targets in liver cancer therapy. In order to better analyze the protein-protein interaction, the PPI network of potential targets was constructed by using the STRING database using the common target of the liver cancer related genes and target corresponding of the components, the species was defined as Homo sapiens [35]. The “drug components-target” network and PPI network was imported into Cytoscape software for topological attribute analysis, and the key nodes and degree values in the network map were analyzed to obtain the core components and targets of D. chrysotoxum.
Biological function and pathway analysis
In order to illustrate the role of the core target in the gene function and signal pathway of the active ingredient in the treatment of liver cancer. David database was used to perform gene ontology (GO) function and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis to explore the mechanism of anti-hepatoma activity of D. chrysotoxum [36–39].
Data analysis
We used the method of Xia et al. as a reference for data analysis, mass spectrometry data were collected using MassLynx V4.2 software (Waters Corporation, Milford, MA, USA) for automatic peak identification, peak matching, peak alignment, peak extraction, peak integration and normalization. The metabolites and their possible cleavage modes were identified using secondary mass spectrometry data, Unifi (Waters Corporation, Milford, MA, USA), online databases (SciFinder, Chemspider and PubMed) and data from previous studies. Unsupervised principal component analysis (PCA) and supervised orthogonal partial least squares discriminant analysis (OPLS-DA) were performed using SIMCA-P 14.1 (Umetrics Corporation, Umea, Sweden) software [29].
Results
Overview of the metabolomes of different tissues
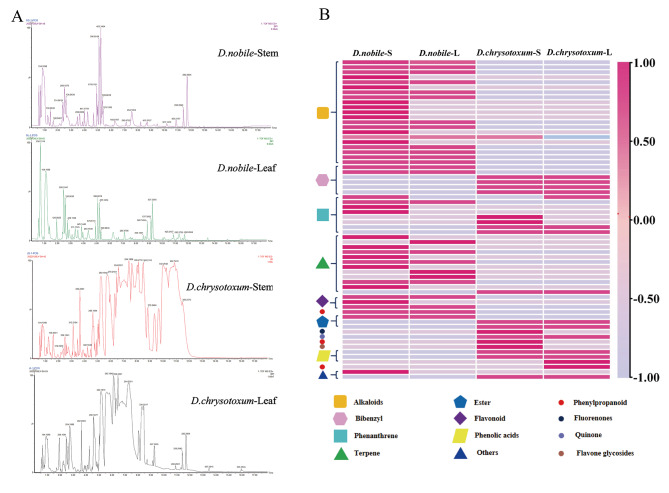
Comprehensive metabolite profiling of the D. nobile and D. chrysotoxum was conducted by LC-MS using an untargeted metabolomics approach. By observing the trend of ion peaks in BPI and the clustering of metabolite species between samples, it was indicated that there was a high similarity in the accumulation of metabolites between different tissues of the same Dendrobium species (Fig. 2A and B). In order to analyze the differences of accumulated metabolites between different samples, the identified metabolite types were assigned to different samples (Fig. 2B). In D. nobile, the major metabolite types were alkaloids (21 metabolites), terpenoids (8 metabolites), flavonoids (3 metabolites) and phenanthrenes (3 metabolites); in D. chrysotoxum, the largest number of metabolite types belonged to bibenzyls (4 metabolites), phenanthrenes (3 metabolites) and phenolic acids (2 metabolites) (Figure S1). Notably, As for the metabolites specifically enriched in D. nobile, alkaloids were abundant in stems but also were observed in leaves (Fig. 2B). In both plants, the predominant differential metabolites were alkaloids and bibenzyls.
Fig. 2.
Differentially accumulated metabolites among the four tissues of two Dendrobium species. (A)The base peak ion chromatogram (BPI) of different samples; (B) A heatmap of the abundance of metabolites in the four tissues of two Dendrobium species
Metabolomic multivariate statistical analysis of D. nobile and D. chrysotoxum
Metabolic profiling of D. nobile and D. chrysotoxum
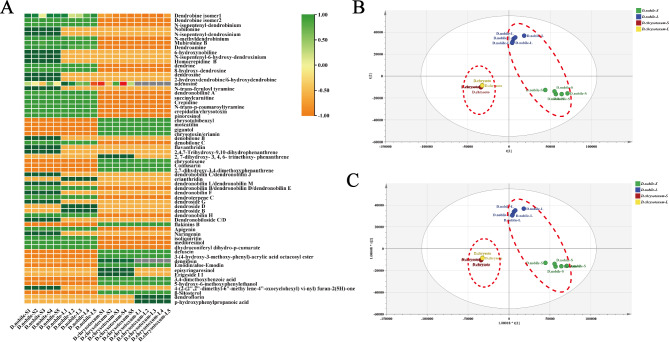
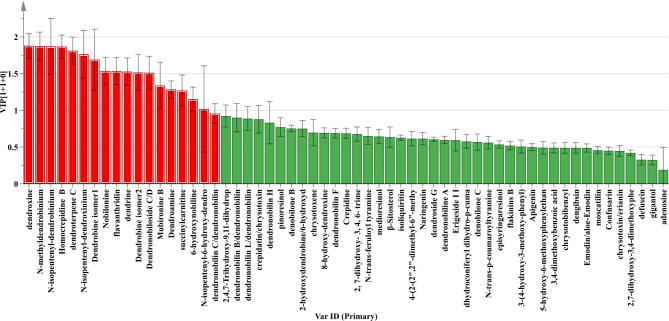
Using our in-house and public databases, we tentatively identified 65 metabolites of negative and positive ion modes, and thus gave a comprehensive metabolome for the stems and leaves of D. nobile and D. chrysotoxum (Tables S1, S2, S3 and S4). The majority of metabolite types were alkaloids, terpenoids, bibenzyls, phenanthrenes, phenolic acids, flavonoids, carboxylic acids. Figure 3A presents two heat-maps corresponding to the relative contents of different metabolites in stems and leaves of different Dendrobium species. As shown in Fig. 3A, alkaloids had significantly greater metabolite diversity, especially in stems and leaves of D. nobile, such as dendrobine, N-isopentenyl-dendrobinium, N-isopentenyl-dendroxinium, N-methyldendrobinium, homocrepidine B and dendroxine. Different content of phenanthrene, as the main metabolite, was detected in the stems of the two Dendrobium species. Chemometric analysis was applied to determine metabolites abundance of D. nobile and D. chrysotoxum. The results of PCA and OPLS-DA analysis showed that D. nobile and D. chrysotoxum species were clearly separated, with significant differences in chemical profiles between the two species. In addition, the metabolites in the stems and leaves of D. nobile differed more markedly (Fig. 3B-C). The VIP value ≥ 1.0 derived from the OPLS-DA analysis revealed the differences in metabolite profiles between D. nobile and D. chrysotoxum (Fig. 4). Notably, 18 compounds were identified as important differential metabolites between D. nobile and D. chrysotoxum, including alkaloids, phenanthrenes and terpenoids.
Fig. 3.
The results of non-targeted metabolomics and chemometric analysis of D. nobile and D. chrysotoxum. (A) Heat map of metabolomics and chemometrics clustering of D. nobile and D. chrysotoxum; (B) Principal component analysis of D. nobile and D. chrysotoxum; (C) OPLS-DA analysis of D. nobile and D. chrysotoxum
(D. nobile-S: D. nobile stem;D. nobile-L: D. nobile leaf; D. chrysotoxum-S: D. chrysotoxum stem; D. chrysotoxum-L: D. chrysotoxum leaf; No.1–5: Number of repetitions)
Fig. 4.
The values of VIP of the D. nobile and D. chrysotoxum
Evaluations of in vitro anti-hepatoma activity
CCK-8 results showed that all four extracts could inhibit the growth of Huh-7 cells, and the anti-hepatoma activity of extracts were dose-dependent (Figure S2). According to the Table 1, among the extracts, the extract of leaves of D. chrysotoxum showed the strongest anti-hepatoma activity, while the stems extract of D. nobile showed the weakest anti-hepatoma activity. In addition, the inhibitory activity of ethanolic extract of D. chrysanthum leaves against HeLa human cervical cancer cells was evaluated by in vitro and in vivo assays with an IC50 value of 450 µg/mL [18, 40], and this result was in excellent agreement with our experimental data. As shown in Fig. S3, the Lo2 cells (normal human hepatocytes) survival rate was above 90% at all concentration gradients of Dendrobium samples. The results showed that all Dendrobium extracts with the same concentration are nontoxicity to normal cells.
Table 1.
Anti-hepatoma activity of four extracts determined by CCK-8 (Each lower case indicates significant differences(P < 0.05))
| Sample |
|
|---|---|
| Anti-hepatoma activity IC50(µg/mL) | |
| 5-Fluorouracil | 30.04 ± 1.16a |
| D. Nobile-Stem | 1315.67 ± 37.50e |
| D. Nobile-Leaf | 815.00 ± 70.73c |
| D. chrysotoxum-Stem | 1051.67 ± 48.09d |
| D. chrysotoxum-Leaf | 493.50 ± 28.56b |
Network pharmacology analysis
PPI network analysis
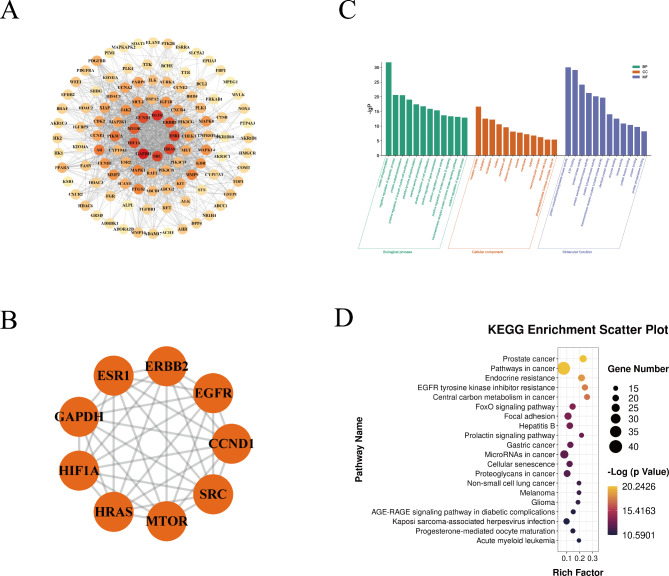
Because the stems and leaves of D. chrysotoxum have greater anti-hepatoma activity than that observed in D. nobile, the former species was selected. About 1526 liver cancer related targets were obtained by DisGeNET, GeneCards and OMIM. Finally, 112 potential targets for anti-hepatoma activity of D. chrysotoxum were obtained, the PPI network of the 112 potential targets were constructed by using the STRING database (Fig. 5A). The required interaction score was set to 0.400. Protein-protein interaction (PPI) network topological features, which have been widely used in bioinformatics to predict disease-related gene and drug targets [41]. According to the topological attribute analysis of PPI network, GAPDH, EGFR, ESR1, HRAS, SRC, CCND1, HIF1A, ERBB2 and MTOR were the protein nodes with high degree and could be considered as the core-targets of the anti-hepatoma activity of D. chrysotoxum (Fig. 5B).
Fig. 5.
Construction of PPI network and identification of core targets. (A) The PPI network of potential targets was constructed. The darker the color, the higher the degree of the node; (B) According to the degree of the nodes, the top 9 targets were selected as the core targets. (C) The top 12 items in the GO biological process, cellular component and molecular function; (D) The top 20 items in Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathway. (Note: The PPI network contained 112 nodes, 1192 edges, an average node degree of 21.3. Each node in the figure represents a protein, and the connections between the nodes represent the interaction between the proteins)
Pathway enrichment analysis
DAVID is a biological information database that can provide systematic and comprehensive biological function annotation information for large-scale gene or protein lists, helping users to extract biological information from them [42]. Biological function analysis of anti-hepatoma activity of chemical components were performed by David database. The GO enrichment results for the first 12 terms of biological process, cellular component and molecular function are shown in Fig. 5C. According to statistical analysis, the results of the first 20 KEGG pathways were shown in Fig. 5D, including prostate cancer, pathways in cancer, endocrine resistance, EGFR tyrosine kinase inhibitor resistance, central carbon metabolism in cancer, FoxO signaling pathway, etc.
Herbs-chemicals-targets-pathways-therapeutic effects network analysis
22 components were identified by UPLC-Q-TOF-MS, 6 non-target proteins were eliminated and 454 targets were obtained by Pubchem, SwissADME and Swiss target prediction. Chrysotobibenzyl, chrysotoxin, moscatilin, gigantol and chrysotoxene were identified as core components by correlation analysis of “drug components-target”.
Discussion
Plants are unique in the richness and diversity of their secondary metabolism, the literatures suggest that the number of metabolites produced in the plant kingdom exceeds 200,000 [43]. More and more researchers are paying attention to the study of plant secondary metabolism and pharmacological efficacy [44–48]. Although there are hundreds of millions of phytochemicals, only a small number have been isolated and identified from plants [49, 50]. The advancement of metabolomics in terms of techniques for measuring small molecule composition has enabled the rapid assay and quantification of numerous the endogenous metabolites of an organism [51]. With the optimization of the analytics platform, such as gas or liquid chromatography mass spectrometry (GC-MS and LC-MS, respectively) and nuclear magnetic resonance spectroscopy (NMR), have enabled characterizing the dynamic of metabolites [52–54]. Based on this, UPLC-Q-TOF-MS was used to identify D. nobile and D. chrysotoxum comprehensive metabolome in different tissues. Research shows that the levels of compounds from different Dendrobium varied greatly. In D. nobile, the major metabolite types were alkaloids, terpenoids, flavonoids and phenanthrenes, however, in D. chrysotoxum, the largest number of metabolite types belonged to bibenzyls, phenanthrenes and phenolic acids, which was consistent with previous studies [55–57]. Notably, D. nobile stem is abundant and diversified in alkaloids, such as dendrobine, N-isopentenyl-dendrobinium and N-methyldendrobinium, in D. chrysotoxum, bibenzyls and phenanthrenes secondary metabolites were significantly accumulated, such as chrysotobibenzyl, chrysotoxin, moscatilin, and chrysotoxene. Therefore, these secondary metabolites can be used as quality markers for the two Dendrobium species, providing a reference for species identification and quality control of Dendrobium. Furthermore, chemometric analysis of the metabolites differences between the two species revealed that they are quite different in evolution [58, 59]. Therefore, integrated metabolomics based on UPLC-Q-TOF-MS and chemometric analysis provides new insights for quality-oriented identification of chemical profiles of traditional chinese herbal medicines.
Modern pharmacological studies have shown that D. nobile and D. chrysotoxum has had a strong anti-cancer effect [15, 60]. For example, D. chrysotoxum natural products caused moderate growth delay in xenografted human hepatoma Bel7402 and melanoma A375 and induced significant vascular shutdown within 4 h of administering 100 mg/kg of the drug [61]. In addition, D. nobile extracts down regulated the expression level of decoy receptor-3 and synergized with Fas ligand to bring about apoptotic cell death in pancreatic adenocarcinoma cells [62]. However, the inhibition rates of the two dendrobium species on hepatocellular carcinoma cells were different. The study showed that the anti-hepatoma effect of D. chrysotoxum was significantly better than that of D. nobile, and it was worth noting that D. chrysotoxum leaves had the strong anti-hepatoma effect. Due to the wide variation in the types and contents of secondary metabolites of different Dendrobium species, the differences in their active ingredients may lead to the differences in their pharmacological activities.
The result of KEGG enrichment indicated that the 112 potential targets were highly enriched in the EGFR tyrosine kinase inhibitor resistance and FoX O signaling pathway. It has been confirmed that EGFR targets have a significant impact on the proliferation and migration of liver cancer cells [63, 64], which is consistent with the results of network pharmacological analysis in this study. In addition, FoX O signaling pathway related to a variety of tumors. The research of Yang et al. and Wang et al. indicated that FOXO1 is weakly expressed in liver cancer tissue, which results in abnormal cell proliferation and cell apoptosis [65, 66]. The influence of D. chrysotoxum on hepatocellular carcinoma inhibition may depend on making an impact on EGFR tyrosine kinase inhibitor resistance pathway and FoX O signaling pathway.
The stems of D. nobile and D. chrysotoxum had a long history of medicinal use, but the leaves are often discarded during the production of medicinal materials. And the leaves on the Dendrobium stems are sometimes peeled for aesthetic reasons. However, the metabolite fingerprint results here indicated that there were still a large number of bioactive compounds in the leaves of D. nobile and D. chrysotoxum. Previous pharmacological studies also have shown that have also shown that Dendrobium leaves play an important role in dermatologic disorders, metabolic syndromes, nervous system disorders, and musculoskeletal system disorders [18]. Therefore, it is necessary to develop an approach for the secondary utilization of leaves. Moreover, comprehensive metabolomics results showed that leaves of D. nobile and D. chrysotoxum were rich in many bioactive components and had excellent pharmacological effects (Fig. 3; Table 1), indicating that the leaves should be preserved as much as possible in the process of crude drug production. However, in traditional processing of Dendrobiums, the leaves were removed after thoroughly washing. Therefore, Dendrobium leaves show potential for the research and development of pharmacological biomolecules.
Conclusions
In this study, mass spectrometry-based metabolomic and multivariate statistical analysis were conducted to screen the differential metabolites in D. nobile and D. chrysotoxum. We screened differential ions in the positive-ion and negative-ion model of UPLC-Q-TOF-MS/MS using OPLS-DA and PCA. CCK-8 results showed that D. nobile and D. chrysotoxum extracts could inhibit the growth of Huh-7 cells, and the anti-hepatoma activity of extracts were dose-dependent. Network pharmacology analysis revealed chrysotobibenzyl, chrysotoxin, moscatilin, gigantol and chrysotoxene as relevant compounds for D. chrysotoxum anti-hepatoma activity. Our research provided a effective method for rapid screening and identification of the differential metabolites in different Dendrobium species, and provided candidate chemical markers for herb quality screening of D. nobile and D. chrysotoxum.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 2: Review history.
Acknowledgements
We thank Tinggang Zhang, Jiahao Fang, and Jiani Yu for material collection and experimental assistance. Reviewers are acknowledged for their contribution to the improvement of the manuscript in the revision process.
Authors’ contributions
JX and FY were responsible for the experimental study, data analysis and manuscript writing, TGZ, FJH and JNY for the sample collection, GGL, ZYX and YY for the composition testing, corresponding authors XDZ, ZSL were responsible for the overall design, planning of the project and revision of the manuscript.
Funding
This work was financially supported by the Basic public welfare research program of Zhejiang Province (grant number LGN22H280004) and the key project at central government level of the ability establishment of sustainable use for valuable Chinese medicine resources (2060302).
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on request.
Declarations
Ethics approval and consent to participate
The authors declared that experimental research works on the plants described in this paper comply with institutional, national and international guidelines. Collections of both D. nobile and D. chrysotoxum species have been permitted or licensed and comply with the relevant regulations for scientific research. Field studies were conducted in accordance with local legislation and get permissions from provincial department of forest and grass of Yunnan province. The two plants were collected with collection permits and identified by Professor Zongsuo Liang, College of Life Sciences and Medicine, Zhejiang Sci-Tech University. The samples were stored in the Key Laboratory of Plant Secondary Metabolism, Zhejiang Sci-Tech University (No. 20220228007 and 20220228008). In addition, we state that only Huh-7 and Lo2 (normal human hepatocytes) cell lines were used in the experiments, and no other human samples were used.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Review history
The review history is available as Supplementary Material 2.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xia Jie and Yin Feng contributed equally to this work.
Change history
7/13/2023
To include ‘Peer-Review’ in the article.
References
- 1.Burlacu E, Tanase C. Anticancer potential of natural bark products-a review. Plants (Basel) 2021;10(9):1895. doi: 10.3390/plants10091895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Efferth T, Oesch F. Repurposing of plant alkaloids for cancer therapy: Pharmacology and toxicology. Semin Cancer Biol. 2021;68:143–63. doi: 10.1016/j.semcancer.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Taha KF, Khalil M, Abubakr MS, Shawky E. Identifying cancer-related molecular targets of Nandina domestica Thunb. By network pharmacology-based analysis in combination with chemical profiling and molecular docking studies. J Ethnopharmacol. 2020;249:112413. doi: 10.1016/j.jep.2019.112413. [DOI] [PubMed] [Google Scholar]
- 4.Abotaleb M, Liskova A, Kubatka P, Büsselberg D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules. 2020;10(2):221. doi: 10.3390/biom10020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma L, Zhang M, Zhao R, Wang D, Ma Y, Li A. Plant natural products: promising resources for cancer chemoprevention. Molecules. 2021;26(4):933. doi: 10.3390/molecules26040933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei W, Rasul A, Sadiqa A, Sarfraz I, Hussain G, Nageen B, Liu X, Watanabe N, Selamoglu Z, Ali M, Li X, Li J. Curcumol: from plant roots to Cancer roots. Int J Biol Sci. 2019;15(8):1600–9. doi: 10.7150/ijbs.34716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S, Zhang X, Cao Z, Zhao K, Wang S, Chen M, Hu X. Growth-promoting Sphingomonas paucimobilis ZJSH1 associated with Dendrobium officinale through phytohormone production and nitrogen fixation. Microb Biotechnol. 2014;7:611–20. doi: 10.1111/1751-7915.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li RQ, Li JF, Zhou ZY, Guo Y, Zhang TT, Tao FF, Hu XF, Liu WH. Antibacterial and antitumor activity of secondary metabolites of endophytic Fungi Ty5 from Dendrobium officinale. J Biobased Mater Bioenergy. 2018;12:184–93. doi: 10.1166/jbmb.2018.1769. [DOI] [Google Scholar]
- 9.Teixeira da Silva JA, Ng TB. The medicinal and pharmaceutical importance of Dendrobium species. Appl Microbiol Biotechnol. 2017;101(6):2227–39. doi: 10.1007/s00253-017-8169-9. [DOI] [PubMed] [Google Scholar]
- 10.Shen YC, Korkor NL, Xiao R, Pu Q, Hu M, Zhang SS, Kong DD, Zeng GH, Hu XF. Antagonistic activity of combined bacteria strains against southern blight pathogen of Dendrobium officinale. Biol Control. 2020;151:1049–9644. doi: 10.1016/j.biocontrol.2020.104291. [DOI] [Google Scholar]
- 11.Committee NP. Pharmacopoeia of the People’s Republic of China, 2020 edn. Beijing: Chemical Industry Press.
- 12.Li Q, Liu C, Huang C, Wang M, Long T, Liu J, Shi J, Shi J, Li L, He Y, Xu DL. Transcriptome and metabonomics analysis revealed the molecular mechanism of differential metabolite production of Dendrobium nobile under different epiphytic patterns. Front Plant Sci. 2022;13:868472. doi: 10.3389/fpls.2022.868472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nie X, Chen Y, Li W, Lu Y. Anti-aging properties of Dendrobium nobile Lindl: from molecular mechanisms to potential treatments. J Ethnopharmacol. 2020;257:112839. doi: 10.1016/j.jep.2020.112839. [DOI] [PubMed] [Google Scholar]
- 14.He L, Su Q, Bai L, Li M, Liu J, Liu X, Zhang C, Jiang Z, He J, Shi J, Huang S, Guo L. Recent research progress on natural small molecule bibenzyls and its derivatives in Dendrobium species. Eur J Med Chem. 2020;204:112530. doi: 10.1016/j.ejmech.2020.112530. [DOI] [PubMed] [Google Scholar]
- 15.Ng TB, Liu J, Wong JH, Ye X, Wing Sze SC, Tong Y, Zhang KY. Review of research on Dendrobium, a prized folk medicine. Appl Microbiol Biotechnol. 2012 Mar;93(5):1795–803. 10.1007/s00253-011-3829-7 [DOI] [PubMed]
- 16.Pei C, Mi CY, Sun LH, Liu WH, Li O, Hu XF. Diversity of endophytic bacteria of Dendrobium officinale based on culture-dependent and culture-independent methods. Biotechnol Biotechnol Equip. 2017;31:112–9. doi: 10.1080/13102818.2016.1254067. [DOI] [Google Scholar]
- 17.Yang CW, Chuang TH, Wu PL, Huang WH, Lee SJ. Anti-inflammatory effects of 7-methoxycryptopleurine and structure-activity relations of phenanthroindolizidines and phenanthroquinolizidines. Biochem Biophys Res Commun. 2007;354(4):942–8. doi: 10.1016/j.bbrc.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 18.Wang YH. Traditional uses, chemical constituents, pharmacological activities, and toxicological effects of Dendrobium leaves: a review. J Ethnopharmacol. 2021;270:113851. doi: 10.1016/j.jep.2021.113851. [DOI] [PubMed] [Google Scholar]
- 19.Cao H, Ji Y, Li S, Lu L, Tian M, Yang W, Li H. Extensive metabolic profiles of Leaves and stems from the Medicinal Plant Dendrobiumofficinale Kimura et Migo. Metabolites. 2019;9(10):215. doi: 10.3390/metabo9100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Chen N, Ding Z, Gu Z, Zhang L, Shi G. Characterization and bioactivity analysis of Dendrobium officinale stem and leaf polysacchride. J Food Sci Biotechnol. 2017;36:959–65. [Google Scholar]
- 21.Zhou GF, Pang MX, Chen SH, Lv GY, Yan MQ. [Comparison on polysaccharide content and PMP-HPLC fingerprints of polysaccharide in stems and leaves of Dendrobium officinale] Zhongguo Zhong Yao Za Zhi. 2014;39(5):795–802. [PubMed] [Google Scholar]
- 22.Liu WJ, Jiang ZM, Chen Y, Xiao PT, Wang ZY, Huang TQ, Liu EH. Network pharmacology approach to elucidate possible action mechanisms of Sinomenii Caulis for treating osteoporosis. J Ethnopharmacol. 2020;15:112871. doi: 10.1016/j.jep.2020.112871. [DOI] [PubMed] [Google Scholar]
- 23.NamNam HH, Kim JS, Lee J, Seo YH, Kim HS, Ryu SM, Choi G, Moon BC, Lee AY. Pharmacological effects of Agastache rugosa against gastritis using a network pharmacology approach. Biomolecules. 2020;10(9):1298. doi: 10.3390/biom10091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Xue X, Yang X, Zhou S, Wang S, Meng J. A network pharmacology approach used to estimate the active ingredients of Moutan cortex charcoal and the potential targets in hemorrhagic diseases. Biol Pharm Bull. 2019;42(3):432–41. doi: 10.1248/bpb.b18-00756. [DOI] [PubMed] [Google Scholar]
- 25.Zhao J, Lv C, Wu Q, Zeng H, Guo X, Yang J, Tian S, Zhang W. Computational systems pharmacology reveals an antiplatelet and neuroprotective mechanism of Deng-Zhan-Xi-Xin injection in the treatment of ischemic stroke. Pharmacol Res. 2019;147:104365. doi: 10.1016/j.phrs.2019.104365. [DOI] [PubMed] [Google Scholar]
- 26.Lan Q, Liu C, Wu Z, Ni C, Li J, Huang C, Wang H, Wei G. Does the metabolome of wild-like Dendrobium officinale of different origins have regional differences? Molecules. 2022;27(20):7024. doi: 10.3390/molecules27207024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu X, Wang Y, Tao S, Sun S. Geniposide plays anti-tumor effects by down-regulation of microRNA-224 in HepG2 and Huh7 cell lines. Exp Mol Pathol. 2020;112:104349. doi: 10.1016/j.yexmp.2019.104349. [DOI] [PubMed] [Google Scholar]
- 28.Machon C, Catez F, Venezia ND, Vanhalle F, Guyot L, Vincent A, Garcia M, Roy B, Diaz JJ, Guitton J. Study of intracellular anabolism of 5-fluorouracil and incorporation in nucleic acids based on an LC-HRMS method. J Pharm Anal. 2021;11(1):77–87. doi: 10.1016/j.jpha.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia J, Li XY, Lin M, Yu JN, Zeng ZD, Ye F, Hu GJ, Miu Q, He QL, Zhang XD, Liang ZS. Screening out biomarkers of Tetrastigma hemsleyanum for anti-cancer and anti-inflammatory based on spectrum-effect relationship coupled with UPLC-Q-TOF-MS. Molecules. 2023;28(7):3021. doi: 10.3390/molecules28073021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47(W1):W357–64. doi: 10.1093/nar/gkz382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piñero J, Queralt-Rosinach N, Bravo À, Deu-Pons J, Bauer-Mehren A, Baron M, Sanz F, Furlong LI. DisGeNET: a discovery platform for the dynamical exploration of human diseases and their genes. Database (Oxford). 2015 Apr 15;2015:bav028. 10.1093/database/bav028 [DOI] [PMC free article] [PubMed]
- 32.Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, Kaplan S, Dahary D, Warshawsky D, Guan-Golan Y, Kohn A, Rappaport N, Safran M, Lancet D. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54:1. doi: 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- 33.Amberger JS, Bocchini CA, Scott AF, Hamosh A. OMIM.org: leveraging knowledge across phenotype-gene relationships. Nucleic Acids Res. 2019;47(D1):D1038–43. doi: 10.1093/nar/gky1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.UniProt Consortium UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49(D1):D480–9. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szklarczyk D, Morris JH, Cook H. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–8. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 37.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000 Jan 1;28(1):27–30. 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed]
- 38.Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019 Nov;28(11):1947–51. 10.1002/pro.3715 [DOI] [PMC free article] [PubMed]
- 39.Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49(D1):D545–51. doi: 10.1093/nar/gkaa970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prasad R, Rana NK, Koch B. Dendrobium chrysanthum ethanolic extract induces apoptosis via p53 up-regulation in HeLa cells and inhibits tumor progression in mice. J Complement Integr Med. 2017;14(2). 10.1515/jcim-2016-0070 [DOI] [PubMed]
- 41.Wu Y, Jing R, Jiang L, Jiang Y, Kuang Q, Ye L, Yang L, Li Y, Li M. Combination use of protein-protein interaction network topological features improves the predictive scores of deleterious non-synonymous single-nucleotide polymorphisms. Amino Acids. 2014;46(8):2025–35. doi: 10.1007/s00726-014-1760-9. [DOI] [PubMed] [Google Scholar]
- 42.Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki R. A. DAVID: database for annotation, visualization, and Integrated Discovery. Genome biology;2003, 4(5), P3. [PubMed]
- 43.Dixon RA, Strack D. Phytochemistry meets genome analysis, and beyond. Phytochemistry. 2003;62(6):815–6. doi: 10.1016/s0031-9422(02)00712-4. [DOI] [PubMed] [Google Scholar]
- 44.Hu W, Zheng Y, Xia P, Liang Z. The research progresses and future prospects of Tetrastigma hemsleyanum Diels et gilg: a valuable chinese herbal medicine. J Ethnopharmacol. 2021;271:113836. doi: 10.1016/j.jep.2021.113836. [DOI] [PubMed] [Google Scholar]
- 45.Hu W, Xia P, Liang Z. Molecular cloning and structural analysis of key enzymes in Tetrastigma hemsleyanum for resveratrol biosynthesis. Int J Biol Macromol. 2021;190:19–32. doi: 10.1016/j.ijbiomac.2021.08.178. [DOI] [PubMed] [Google Scholar]
- 46.Xu L, Cao M, Wang Q, Xu J, Liu C, Ullah N, Li J, Hou Z, Liang Z, Zhou W, Liu A. Insights into the plateau adaptation of Salvia castanea by comparative genomic and WGCNA analyses. J Adv Res. 2022;42:221–35. doi: 10.1016/j.jare.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiang Q, Hu S, Ligaba-Osena A, Yang J, Tong F, Guo W. Seasonal Variation in Transcriptomic profiling of Tetrastigma hemsleyanum fully developed tuberous roots enriches candidate genes in essential metabolic pathways and Phytohormone Signaling. Front Plant Sci. 2021;12:659645. doi: 10.3389/fpls.2021.659645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ke L, Yu D, Zheng H, Xu Y, Wu Y, Jiao J, Wang X, Mei J, Cai F, Zhao Y, Sun J, Zhang X, Sun Y. Function deficiency of GhOMT1 causes anthocyanidins over-accumulation and diversifies fibre colours in cotton (Gossypium hirsutum) Plant Biotechnol J. 2022;20(8):1546–60. doi: 10.1111/pbi.13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh D, Chaudhuri PK. A review on phytochemical and pharmacological properties of Holy basil (Ocimum sanctum L) Ind Crops Prod. 2018;118:367–82. doi: 10.1016/j.indcrop.2018.03.048. [DOI] [Google Scholar]
- 50.Xiao J, Bai W. Bioactive phytochemicals. Crit Rev Food Sci Nutr. 2019;59(6):827–9. doi: 10.1080/10408398.2019.1601848. [DOI] [PubMed] [Google Scholar]
- 51.Fukushima A, Takahashi M, Nagasaki H, Aono Y, Kobayashi M, Kusano M, Saito K, Kobayashi N, Arita M. Development of RIKEN plant metabolome MetaDatabase. Plant Cell Physiol. 2022;63(3):433–40. doi: 10.1093/pcp/pcab173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou B, Xiao JF, Tuli L, Ressom HW. LC-MS-based metabolomics. Mol Biosyst. 2012;8(2):470–81. doi: 10.1039/c1mb05350g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia PG, Li QQ, Liang ZS, Zhang XM, Yan KJ. Spaceflight breeding could improve the volatile constituents of Andrographis paniculata. Ind Crops Prod. 2021;171:113967. doi: 10.1016/j.indcrop.2021.113967. [DOI] [Google Scholar]
- 54.Mahrous EA, Farag MA. Two dimensional NMR spectroscopic approaches for exploring plant metabolome: a review. J Adv Res. 2015;6(1):3–15. doi: 10.1016/j.jare.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen XM, Xiao SY, Guo SX. [Comparison of chemical compositions between Dendrobium candidum and Dendrobium nobile]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006 Aug;28(4):524–9. Chinese. [PubMed]
- 56.Lam Y, Ng TB, Yao RM. Evaluation of chemical constituents and important mechanism of pharmacological biology in dendrobium plants. Evid Based Complement Alternat Med. 2015;2015:841752. doi: 10.1155/2015/841752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Zhang JQ, Zhan R, Chen YG. Isopentenylated bibenzyls and phenolic compounds from Dendrobium chrysotoxum Lindl. Chem Biodivers. 2022;19(6):e202200259. doi: 10.1002/cbdv.202200259. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Zhang GQ, Zhang D, Liu XD, Xu XY, Sun WH, Yu X, Zhu X, Wang ZW, Zhao X, Zhong WY, Chen H, Yin WL, Huang T, Niu SC, Liu ZJ. Chromosome-scale assembly of the Dendrobium chrysotoxum genome enhances the understanding of orchid evolution. Hortic Res. 2021;8(1):183. doi: 10.1038/s41438-021-00621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takamiya T, Wongsawad P, Sathapattayanon A, Tajima N, Suzuki S, Kitamura S, Shioda N, Handa T, Kitanaka S, Iijima H, Yukawa T. Molecular phylogenetics and character evolution of morphologically diverse groups, Dendrobium section Dendrobium and allies. AoB Plants. 2014;6:plu045. doi: 10.1093/aobpla/plu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng S, Hu Y, Zhao R, Zhao T, Li H, Rao D, Chun Z. Quantitative assessment of secondary metabolites and cancer cell inhibiting activity by high performance liquid chromatography fingerprinting in Dendrobium nobile. J Chromatogr B Analyt Technol Biomed Life Sci. 2020;1140:122017. doi: 10.1016/j.jchromb.2020.122017. [DOI] [PubMed] [Google Scholar]
- 61.Gong YQ, Fan Y, Wu DZ, Yang H, Hu ZB, Wang ZT. In vivo and in vitro evaluation of erianin, a novel anti-angiogenic agent. Eur J Cancer. 2004;40(10):1554–65. doi: 10.1016/j.ejca.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 62.Yang LC, Deng H, Yi Y, Zhang XM, Wang YZ, Lin JQ. [Identification of medical Dendrobium herbs by ISSR marker] Zhong Yao Cai. 2010;33(12):1841–4. [PubMed] [Google Scholar]
- 63.Hindson J. Lenvatinib plus EGFR inhibition for liver cancer. Nat Reviews Gastroenterology&Hepatology. 2021;18:675. doi: 10.1038/s41575-021-00513-6. [DOI] [PubMed] [Google Scholar]
- 64.Yamada K, Kizawa R, Yoshida A, Koizumi R, Motohashi S, Shimoyama Y, Hannya Y, Yoshida S, Oikawa T, Shimoda M, Yoshida K. Extracellular PKCδ signals to epidermal growth factor receptor for tumor proliferation in liver cancer cells. Cancer Sci. 2022;113(7):2378–85. doi: 10.1111/cas.15386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang L, Peng F, Qin J, Zhou H, Wang B. Downregulation of microRNA-196a inhibits human liver cancer cell proliferation and invasion by targeting FOXO1. Oncol Rep. 2017;38:2148–54. doi: 10.3892/or.2017.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q, Yang X, Zhou X, Wu B, Zhu D, Jia W, Chu J, Wang J, Wu J, Kong L. MiR-3174 promotes proliferation and inhibits apoptosis by targeting FOXO1 in hepatocellular carcinoma. Biochem Biophys Res Commun. 2020;526:889–97. doi: 10.1016/j.bbrc.2020.03.152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 2: Review history.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on request.