Abstract
Objectives
To describe the incidence of and patterns of ‘escalated care’ (care in addition to standard treatment with systemic corticosteroids and inhaled bronchodilators) for children receiving prehospital treatment for asthma.
Design
Retrospective observational study.
Setting
State-wide ambulance service data (Ambulance Victoria in Victoria, Australia, population 6.5 million)
Participants
Children aged 1–17 years and given a final diagnosis of asthma by the treating paramedics and/or treated with inhaled bronchodilators from 1 July 2019 to 30 June 2020.
Primary and secondary outcome measures
We classified ‘escalation of care’ as parenteral administration of epinephrine, or provision of respiratory support. We compared clinical, demographic and treatments administered between those receiving and not receiving escalation of care.
Results
Paramedics attended 1572 children with acute exacerbations of asthma during the 1 year study period. Of these, 22 (1.4%) had escalated care, all receiving parenteral epinephrine. Patients with escalated care were more likely to be older, had previously required hospital admission for asthma and had severe respiratory distress at initial assessment.
Of 1307 children with respiratory status data available, at arrival to hospital, the respiratory status of children had improved overall (normal/mild respiratory distress at initial assessment 847 (64.8%), normal/mild respiratory distress at hospital arrival 1142 (87.4%), p<0.0001).
Conclusions
Most children with acute exacerbations of asthma did not receive escalated therapy during their pre-hospital treatment from ambulance paramedics. Most patients were treated with inhaled bronchodilators only and clinically improved by the time they arrived in hospital.
Keywords: Asthma, ACCIDENT & EMERGENCY MEDICINE, Paediatric A&E and ambulatory care
Strengths and limitations of this study.
Highly generalisable, with the use of a comprehensive electronic state-wide ambulance database.
Most ambulance cases were concentrated in metropolitan regions; this may limit generalisability to rural and regional settings.
Bias was minimised by direct download from electronic medical record, rather than abstraction by reviewers.
It is possible that a small number of critically ill cases were misclassified due to an ambulance diagnosis other than asthma.
Introduction
Asthma is a frequent reason for children to attend the emergency department (ED),1 2 and one of the most common reasons for paediatric hospitalisation after an ED visit.3 In the USA, the rate of paediatric ED visits for asthma increased by 13.3% between 2001 and 2010,4 while in the UK, it is estimated that a child is admitted to hospital with an asthma attack every 20 min.5
Most children with asthma have mild or moderate exacerbations, and respond to first-line treatment with inhaled bronchodilator therapy and systemic steroids.6–9 However, some children with severe asthma require more intensive therapies including intravenous medications, endotracheal intubation and/or admission to intensive care.9–11 Management of acute severe asthma is complicated by a number of problems, including a large number of treatment options, wide variation in self-reported and actual physician practice,12–15 and a weak evidence base.16 17
Early initiation of therapy in the prehospital setting may abort an asthma attack and prevent further escalation on arrival to the ED. This in turn may prevent the need for more invasive treatment and potential complications or side effects of medications used in escalation. The introduction of a new treatment protocol emphasising early use of systemic corticosteroids in a large Emergency Medical Services system was associated with reduced rates of hospitalisation, less need for critical care and shortened hospital length of stay.18 Systemic corticosteroid administration has been the subject of successful improvement projects in the prehospital setting.19 However, a separate study identified high rates of paramedic non-compliance with prehospital treatment protocols recommending parenteral epinephrine for children with high-severity respiratory distress.20
There are little data available on treatment patterns or prehospital outcomes for children with acute asthma in the Australian setting. This study aimed to extract information from the electronic medical records of Ambulance Victoria (AV), Australia, on all children treated for asthma to understand the incidence of and patterns of ‘escalated’ care (care in addition to standard treatment with systemic corticosteroids and inhaled bronchodilators).
Methods
Study design
This was a retrospective cohort study of all children who were either given a final diagnosis of asthma by the treating AV paramedics or treated with inhaled bronchodilators from 1 July 2019 to 30 June 2020. The project is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.21 The study was approved by the Royal Children’s Hospital Research Ethics and Governance Office, Melbourne, Australia (60707) and the Ambulance Victoria Research Governance Committee, Melbourne, Australia.
Study setting
AV is the single public emergency medical service for the state of Victoria, Australia (population of 6.5 million over 227 000 km2).
AV clinical practice guidelines22 provide recommendations for asthma management according to severity (Box 1), which include: inhaled salbutamol via a pressurised metered dose inhaler (pMDI) as initial treatment for mild/moderate asthma; nebulised salbutamol and ipratropium reserved for severe or critical illness, or failure of moderate asthma to respond to treatment after 20 min; corticosteroids (intravenous or oral dexamethasone) for critical asthma in children and for severe and critical asthma in adults; parenteral epinephrine (intramuscular (IM), intravenous infusion or titrated boluses) for critical asthma and assisted ventilation and/or intubation for unconsciousness or respiratory arrest. Children aged 12 years or more are managed according to an ‘adult’ algorithm, which has a lower threshold for corticosteroids compared with the paediatric algorithm (recommended for all severe cases, rather than only in critical illness).22
Box 1. Asthma severity assessment and treatment according to Ambulance Victoria clinical practice guidelines.
Mild/moderate: normal conscious state, some increased work of breathing, tachycardia, speaking in phrases/sentences
Salbutamol pMDI and spacer:
6 or more years: 4–12 doses
2–5 years: 2–6 doses
Severe: agitated/distressed, markedly increased work of breathing, including accessory muscle use/retraction, tachycardia, speaking in words.
Salbutamol nebulised (repeated at 20 min if required)
2–4 years: 2.5 mg
5–11 years: 2.5–5 mg
Ipratropium bromide nebulised 250 mcg
Critical: altered conscious state, maximal work of breathing, marked tachycardia and unable to talk.
Salbutamol nebulised 10 mg (repeated at 5 min if required)
Ipratropium bromide nebulised 250 mcg
Epinephrine 10 mcg/kg IM (repeated at 5 min if required)
Dexamethasone 0.6 mg/kg intravenous or oral (max 12 mg)
Epinephrine intravenous boluses and infusion (for Mobile Intensive Care Paramedics)
IM, intramuscular; pMDI, pressurised metered dose inhaler.
Selection of participants
We searched the AV electronic patient care system for presentations of children aged more than 1 year and less than 18 years matching the following criteria: final primary assessment of asthma or cough or shortness of breath. We excluded children with a paramedic diagnosis of cough or shortness of breath if they were not administered any inhaled bronchodilator (salbutamol or ipratropium). Records of cases assessed by multiple ambulance teams during the same incident were unified as a single paramedic attendance. Interhospital transports and patients managed for cardiac arrest were excluded.
Data collection
Data were extracted directly from the AV medical record database into a purpose-designed spreadsheet and analysed. Exact medication doses were not extracted, as treatment is highly protocolised (box 1).
We defined ‘respiratory support’ as the use of continuous positive airway pressure, bi-level positive airway pressure, assisted ventilation, intubation and mechanical ventilation, or application of a bag-valve-mask device.
We defined ‘escalation’ of care as parenteral administration of epinephrine, or provision of respiratory support. Although AV protocols recommend oral (or parenteral) corticosteroids for severe and critical asthma, corticosteroids are usually considered part of routine asthma care (rather than reserved for critical illness). We did not include nebulised epinephrine for suspected croup/upper airway obstruction. The case notes were reviewed and verified by a second paramedic abstractor (BD) for all patients where escalation was identified through electronic medical record data.
Analysis
Descriptive statistics were used to summarise patient characteristics, clinical features and treatments administered. Non-parametric data are reported using median and IQR, while categorical data are presented as count and percentage. We did not impute any missing data.
Comparisons were made between those requiring escalation of care to those not requiring escalation of care. Categorical data are compared using χ2 test or Fisher’s exact test as appropriate. Non-parametric data are compared using Mann-Whitney U test.
All analyses were performed using SPSS for Windows (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, V. 28.0. Armonk, NY: IBM Corp.).
Patient and public involvement
Patients were not involved in the design of this study.
Results
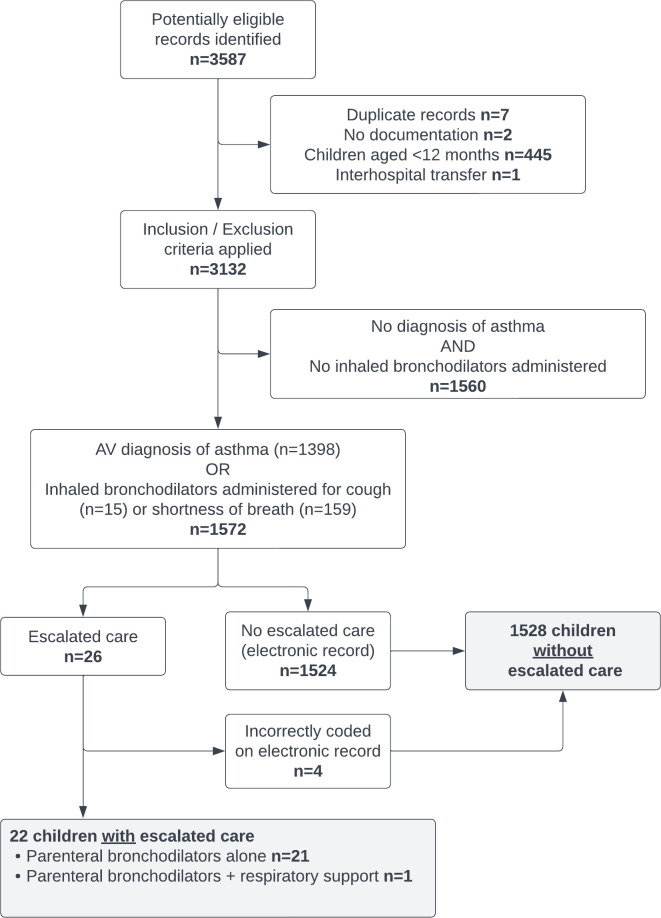
Over the study period, the service responded to 633 950 on-road emergency cases,23 mainly using advanced life support or mobile intensive care ambulance paramedics. We identified 3587 children who had been assessed by AV with a primary assessment diagnosis of asthma, cough or shortness of breath, 1520 were excluded, leaving 1572 children managed by AV with asthma (figure 1).
Figure 1.
Flow chart—prehospital management of acute asthma in children. AV, Ambulance Victoria.
The median age of the cohort was 6 years (IQR 4–10 years) and 888 (56.5%) were male. Most (87.6%) patients had a documented history of asthma, 115 (7.3%) had been hospitalised, 63 (4%) had required intensive care admission and 19 (1.2%) had been intubated for a previous asthma exacerbation. Information on usual asthma medications was not available. The median initial respiratory rate was 32 breaths/min (IQR 24–40 breaths/min). Of the 1460 patients who had initial work of breathing documented, 978 (67.0%) had normal or mild work of breathing, and 166 (7.7%) had severe work of breathing.
Ambulance response time was a median of 11.9 min (IQR 8.2 to 15.2 min); paramedics were on the scene with the patient for a median of 17 min (IQR 12.7 to 25.1 min). Patients were transported by ambulance in 90% (n=1419) of attendances.
Paramedics administered inhaled bronchodilators in 946 (60.2%) of cases. Of those, 493 (52.1%) received salbutamol alone, 13 (1.4%) received ipratropium alone and 440 (46.5%) received salbutamol and ipratropium. For those receiving bronchodilators, a median (IQR) of 1 (1–2) administrations were recorded. Oxygen administration was documented in 306 (19.4%) patients, most commonly by nebuliser mask, nasal cannulae or an oxygen mask; however, 514 (32.6%) received nebulised medication, driven by oxygen. Oral corticosteroids were administered to 141 (9.0%) patients.
Twenty-six records were reviewed for escalation of care; in four patients, the electronic record was incorrectly coded, due to inadvertent selection of intravenous salbutamol (used by AV for preterm labour) instead of nebulised salbutamol, leaving 22 (1.4%) patients with escalated care (figure 1). Patients with escalated care were more likely to be older, had previously required hospital admission for asthma and had severe respiratory distress at initial assessment (table 1). Those receiving escalated care were more likely to be treated with inhaled bronchodilators, corticosteroids and oxygen (table 2). With increasing severity of illness, children were more likely to be administered nebulised salbutamol, less likely to be administered salbutamol by a pMDI, more likely to receive ipratropium and more likely to receive systemic corticosteroids (online supplemental table).
Table 1.
Demographics and clinical characteristics of children treated or assessed for asthma by AV
| Total (n=1572) |
Escalation of care (n=22) |
No escalation of care (n=1550) |
P value (escalation vs no escalation) | |
| Age, years, n (%) | ||||
| 1–4 | 561 (36.3) | 6 (27.3) | 555 (35.8) | 0.38 |
| 5–11 | 690 (43.9) | 9 (40.9) | 681 (43.9) | |
| 12–17 | 321 (20.4) | 7 (31.8) | 314 (20.3) | |
| Median age, years (IQR) | 6 (4–10) | 10.5 (3.8–14.3) | 6 (3.8–10) | 0.045 |
| Female sex, n (%) | 684 (43.5) | 11 (50) | 877 (43.4) | 0.54 |
| Pre-existing conditions, n (%) | ||||
| Asthma | 1377 (87.6) | 20 (90.9) | 1357 (87.5) | 0.64 |
| Requiring hospital admission | 115 (7.3) | 5 (22.7) | 110 (7.1) | 0.005 |
| Requiring intensive care | 63 (4) | 1 (4.5) | 62 (4) | 0.89 |
| Requiring intubation | 19 (1.2) | 1 (4.5) | 18 (1.2) | 0.15 |
| With cardiac/respiratory arrest | 5 (0.3) | 0 (0) | 5 (0.3) | 0.79 |
| Other respiratory illness | ||||
| Croup | 94 (6) | 1 (4.5) | 93 (6) | 0.78 |
| Bronchiolitis | 80 (5.1) | 1 (4.5) | 79 (5.1) | 0.91 |
| Pneumonia | 44 (2.8) | 1 (4.5) | 43 (2.8) | 0.62 |
| Chest infection | 32 (2) | 1 (4.5) | 31 (2) | 0.40 |
| Other | 8 (0.5) | 0 (0) | 8 (0.5) | 0.64 |
| Initial physiological parameters | ||||
| Respiratory rate (breaths/min), median (IQR) | 32 (24–40) | 35.5 (28–48.5) | 32 (24–40) | 0.09 |
| Pulse rate (beats/min), median (IQR) | 130 (112–146) | 134.5 (120–150.5) | 130 (112–146) | 0.24 |
| Initial respiratory status, n (%) | ||||
| Normal | 615 (39.1) | 3 (13.6) | 612 (39.5) | <0.001 |
| Mild respiratory distress | 363 (23.1) | 1 (4.5) | 362 (23.4) | |
| Moderate respiratory distress | 315 (20) | 2 (9.1) | 313 (20.2) | |
| Severe respiratory distress | 166 (10.6) | 16 (72.7) | 150 (9.7) | |
| Depressed respirations | 1 (0.1) | 0 (0) | 1 (0.1) | |
| Final physiological parameters | ||||
| Respiratory rate (breaths/min), median (IQR)* | 28 (22–36) | 28 (22–36) | 30 (27–40) | 0.06 |
| Pulse rate (beats/min), median (IQR)* | 126 (108–142) | 126 (108–142) | 126 (112–162) | 0.29 |
| Final respiratory status, n (%)† | ||||
| Normal | 742 (56.8) | 4 (18.2) | 738 (57.4) | <0.001 |
| Mild respiratory distress | 400 (30.6) | 4 (18.2) | 396 (30.8) | |
| Moderate respiratory distress | 127 (9.7) | 6 (27.3) | 121 (9.4) | |
| Severe respiratory distress | 38 (2.9) | 8 (36.4) | 30 (2.4) | |
All P values calculated using χ2 tests, except for continuous variables where Mann-Whitney U tests* were used.
*Data were not available for final pulse rate and respiratory rate for 54 patients in the ‘No escalation of care’ group.
†Data were not available for final respiratory status for 265 patients in the ‘No escalation of care’ group.
AV, Ambulance Victoria.
Table 2.
Treatment provided by AV paramedics
| Total (n=1572) |
Escalation of care (n=22) |
No escalation of care (n=1550) |
P value (escalation vs no escalation) | |
| Respiratory support, n(%) | ||||
| Bag-valve-mask applied | 1 (0.1) | 1 (4.5) | 0 (0) | <0.001 |
| Oxygen delivery | ||||
| Nasal cannulae | 46 (2.9) | 4 (18.2) | 42 (2.7) | <0.001 |
| Nebuliser mask | 258 (16.4) | 10 (45.5) | 248 (16) | <0.001 |
| Oxygen mask | 48 (3.1) | 0 (0) | 48 (3.1) | 0.40 |
| Non-rebreather mask | 8 (0.5) | 0 (0) | 8 (0.5) | 0.74 |
| Other oxygen therapy (not otherwise specified) |
2 (0.1) | 0 (0) | 2 (0.1) | 0.87 |
| Parenteral bronchodilator | ||||
| Epinephrine IM injection | 20 (1.3) | 20 (90.9) | 0 (0) | <0.001 |
| Epinephrine infusion | 4 (0.3) | 4 (18.2) | 0 (0) | <0.001 |
| Dexamethasone | ||||
| Intravenous injection | 25 (1.6) | 4 (18.2) | 21 (1.4) | <0.001 |
| Oral | 141 (9) | 11 (50) | 130 (8.4) | <0.001 |
| Inhaled bronchodilator | ||||
| Any inhaled bronchodilator | 946 (60.2) | 21 (95.5) | 925 (59.7) | <0.001 |
| Any Ipratropium bromide nebulisation | 453 (28.8) | 17 (77.3) | 436 (28.1) | <0.001 |
| Any salbutamol pMDI | 465 (29.6) | 3 (13.6) | 462 (29.8) | 0.10 |
| Any salbutamol nebulisation | 513 (32.6) | 20 (90.9) | 493 (31.8) | <0.001 |
| Single administration of inhaled salbutamol | 348 (22.1) | 3 (13.6) | 345 (22.3) | |
| Single administration of inhaled ipratropium bromide | 13 (0.8) | 1 (4.5) | 12 (0.8) | |
| Single administration of inhaled salbutamol and single administration of inhaled iptratropium bromide | 280 (17.8) | 6 (27.3) | 274 (17.7) | |
| Two administrations of inhaled salbutamol alone | 114 (7.3) | 1 (4.5) | 113 (7.3) | |
| Two administrations of inhaled salbutamol and at least one administration of ipratropium bromide | 112 (7.1) | 3 (13.6) | 109 (7) | |
| Three or more administrations of inhaled salbutamol alone | 31 (2.0) | 0 (0) | 31 (2) | |
| Three or more administrations of inhaled salbutamol and at least one administration of ipratropium bromide | 48 (3.1) | 7 (31.8) | 41 (2.6) | |
| Total instances of inhaled bronchodilator administration, median (IQR) | 1 (0–2) | 2 (1.8–4) | 1 (0–2) | <0.001 |
| Intravenous access | ||||
| Intravenous access attempt | 39 (2.5) | 7 (31.8) | 32 (2.1) | <0.001 |
| Successful intravenous attempt | 34 (2.2) | 7 (31.8) | 27 (1.7) | <0.001 |
No patients received any of: BiPAP, manual ventilation, mechanical ventilation, intravenous salbutamol infusion, IM dexamethasone.
AV, Ambulance Victoria; BiPAP, bi-level positive airway pressure; IM, intramuscular; pMDI, pressurised metered dose inhaler.
bmjopen-2023-073029supp001.pdf (49.8KB, pdf)
All patients who received escalated care received parenteral epinephrine. No patients received non-invasive ventilation, assisted ventilation or intubation. Four children (aged 2, 14, 16 and 17 years) received an epinephrine infusion. One patient who received IM epinephrine also had a bag-valve-mask applied, however, did not receive positive pressure ventilation. They were a 2 year-old child who had difficulty in breathing and cough that was not improving with salbutamol administered at home. They became unresponsive after a coughing episode and bystander cardiopulmonary resuscitation was initiated. They were breathing spontaneously and responsive on initial paramedic assessment.
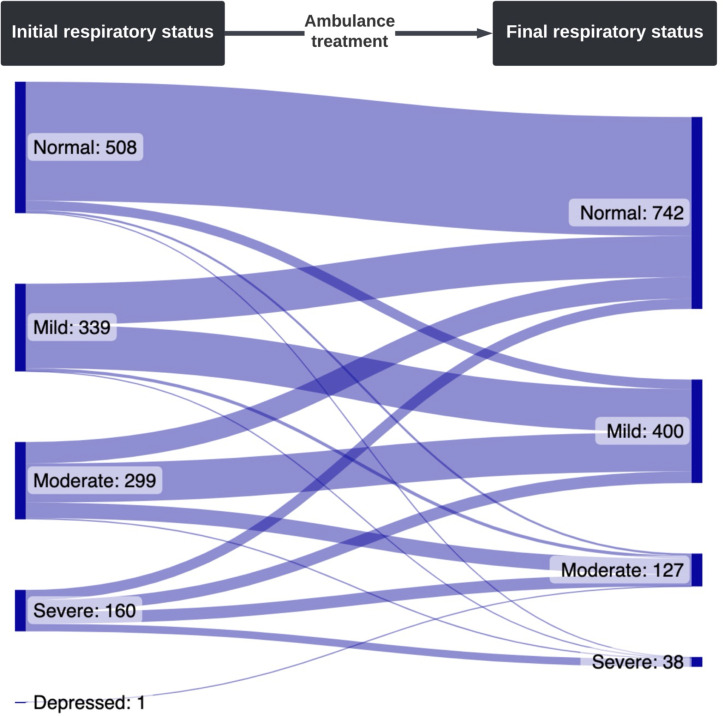
Reports of respiratory status at initial assessment and hospital arrival were available for 1307 (85.5%) of the cohort. On arrival to hospital, the respiratory status of children had improved overall (normal/mild respiratory distress at initial assessment 847 (64.8%), normal/mild respiratory distress at hospital arrival 1142 (87.4%), p<0.0001). One hundred and thirty-one (81.2%) of the 160 children with severe respiratory distress at initial assessment had improved. Of the 847 children with normal/mild respiratory distress at initial assessment, only 24 (2.8%) were documented as having moderate or severe respiratory distress at hospital arrival; and only 9 (0.8%) of the 1146 children with normal/mild/moderate respiratory distress at initial assessment were documented as having severe respiratory distress at hospital arrival (figure 2).
Figure 2.
Initial and final respiratory status documented by AV paramedics. 111 patients missing initial respiratory status, and 265 missing final respiratory status. AV, Ambulance Victoria.
Discussion
This study provides a population-based state-wide assessment of prehospital asthma management in children. Most children with acute exacerbations of asthma in Victoria, Australia, did not receive escalated therapy during their prehospital treatment from ambulance paramedics. Although more than 60% had either mild or no respiratory distress, over 90% of all patients were transported to hospital. Overall, the respiratory status of children improved from ambulance arrival to hospital arrival in all severity categories.
The overall rate of parenteral bronchodilator (epinephrine) administration was 1.6%. No patients received non-invasive ventilation, assisted ventilation or intubation and most patients were treated with inhaled bronchodilators and clinically improved by the time they arrived in hospital. Those receiving escalated care were older, were more likely to have a history of asthma requiring hospital admission and/or intubation and have severe respiratory distress on ambulance arrival.
A recent large study described in-hospital management of acute asthma exacerbations in Australia and New Zealand. In 14 029 children, there was a higher overall rates of escalated therapy (7.3% overall, with 4.2% receiving parenteral bronchodilators and 4.3% respiratory support).12 A common indication for escalation of care is failure to adequately respond to first-line therapy. The relatively low rates of treatment escalation in the prehospital setting (1.6%) suggest that a small proportion of children are seriously ill, while most are early in their treatment, and may not have had sufficient time to demonstrate improvement (or lack of improvement) prior to hospital arrival.
There is little evidence to guide escalated therapy for asthma. A recent Overview of Cochrane reviews of clinical trials on escalated therapy for asthma16 assessed the evidence for parenteral bronchodilators, Heliox, respiratory support and inhaled magnesium. The review found that the majority of comparisons involved between one and three trials and fewer than 100 participants, making it difficult to assess the balance between benefits and potential harms. The authors were unable to make firm practice recommendations.16
There is little evidence to support IM epinephrine as first-line treatment for seriously ill children with asthma,24 although it has a number of advantages, including ease of administration and paramedic familiarity. Parenteral epinephrine is also used for anaphylaxis, cardiac arrest and management of hypotension, while nebulised epinephrine is used for severe upper airway obstruction in croup. In addition, it can be easily and rapidly administered as there is no need for dilution prior to administration, and no requirement for a prolonged infusion.22
Prehospital treatment of asthma rarely results in escalation of therapy beyond inhaled bronchodilators and systemic corticosteroids. In addition, the use of parenteral bronchodilators is often reserved for those who do not improve after initial inhaled bronchodilators, and is administered relatively late in the course of an ED visit.15 Given that most children with asthma will improve with prehospital treatment, and/or will not have sufficient time to ‘fail to improve’ with standard therapy, it appears that any comparative clinical trials to determine the superiority of one parenteral bronchodilator over another should be reserved for the in-hospital rather than prehospital setting.
Limitations
Inclusion in the study was based on a combination of paramedic diagnosis of asthma and administration of inhaled bronchodilators. While only 89% had a diagnosis of asthma recorded in the ambulance notes, it seems that the cohort is reflective of the asthma population as over 87% of cases had a previous diagnosis of asthma.
Due to state-wide data collection and large numbers of patients, our study is likely to be generalisable to other settings with similar prehospital care systems. However, most ambulance cases within Victoria are concentrated in the metropolitan area of Melbourne (the capital city), which may limit generalisability to rural and regional settings. Approximately 10% of children were not transported to hospital; this is similar to the rate identified in a study of children with seizures from the same ambulance service.25
This study is a retrospective review of a comprehensive electronic database. We optimised data extraction and minimised bias through the collection of variables using a piloted data collection instrument, and application of predefined inclusion and exclusion criteria.26 27 Due to the nature of record-keeping within the ambulance service (all cases are documented using the electronic system), it is unlikely that any cases of escalated care were missed. As we downloaded fields directly from the electronic medical record system, we did not independently abstract any variables. However, we verified all instances of documented escalation of care through consultation with a second (paramedic) reviewer and identified four cases of misclassification. It is possible that we missed some children who were not classified as asthma, were critically ill, not given inhaled bronchodilators and only given parenteral epinephrine. However, this is likely to be a very small number of cases. There was some missing data on final observations on arrival to hospital, however, this was not a primary objective of our study.
Conclusions
Most children with acute exacerbations of asthma did not receive escalated therapy during their prehospital treatment from ambulance paramedics. Most patients were treated with inhaled bronchodilators only and clinically improved by the time they arrived in hospital. Due to the very low incidence of treatment escalation or clinical deterioration, any comparative clinical trials to determine the superiority of one parenteral bronchodilator over another should be reserved for the in-hospital rather than prehospital setting.
Supplementary Material
Footnotes
Twitter: @DrSimonCraig
Contributors: SC, CW and FEB identified the research question. SC and CW were responsible for the study design and research protocol. BD and ZN obtained data and input into data cleaning and analysis. SC was responsible for statistical analysis. SC drafted the initial manuscript. SC, BD, ZN, CW, SD, GMN, CP, AG and FEB contributed equally to writing, reviewing and editing the manuscript. All authors provided comments on the drafts and have read and approved the final version of the article. All authors have full access to all of the data (including statistical reports and tables) at the conclusion of the study and take responsibility for the integrity of the data and the accuracy of the data analysis. SC is the guarantor for the paper, accepts full responsibility for the work and/or the conduct of the study, had access to the data and controlled the decision to publish.
Funding: This work is supported by the NHMRC Centre of Research Excellence in Paediatric Emergency Medicine (GNT1171228), Canberra, Australia. SC’s contribution was funded by the Thoracic Society of Australia and New Zealand and National Asthma Council Fellowship, 2020 and the Australasian College for Emergency Medicine Foundation Al Spilman Early Career Research Grant 2017. SRD's time was in part funded by Cure Kids New Zealand. FEB’s time was funded by an NHMRC Investigator Leadership grant and the Royal Children’s Hospital Foundation, Parkville, Australia.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Deidentified participant data will be available for sharing from 1 July 2024. Any data access requests should be sent to SC (simon.craig@monash.edu), and should include a proposal from the individual or organisation regarding their plan for use of the data.The study team will review the request and consider the scientific merit of the proposed use of the data, and the legal, regulatory and ethical issues pertinent to the request. Presuming all constraints are addressed, the data will be shared using a secure file transfer platform.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants. The project was approved by the Royal Children’s Hospital Research Ethics and Governance Office, Melbourne, Australia (60707), and the Ambulance Victoria Research Governance Committee, Melbourne, Australia. A waiver of consent for review of existing medical records was granted as per ethics approval in accordance with the National Statement on Ethical Conduct in Human Research (National Health and Medical Research Council, Australia).
References
- 1. Alpern ER, Stanley RM, Gorelick MH, et al. Epidemiology of a pediatric emergency medicine research network: the PECARN core data project. Pediatric Emergency Care 2006;22:689–99. 10.1097/01.pec.0000236830.39194.c0 [DOI] [PubMed] [Google Scholar]
- 2. Acworth J, Babl F, Borland M, et al. Patterns of presentation to the Australian and New Zealand Paediatric emergency research network. Emerg Med Australas 2009;21:59–66. 10.1111/j.1742-6723.2009.01154.x [DOI] [PubMed] [Google Scholar]
- 3. Weiss AJ, Wier LM, Stocks C, et al. Overview of Emergency Department Visits in the United States, 2011: Statistical Brief #174. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US), 2006. [PubMed] [Google Scholar]
- 4. Nath JB, Hsia RY. Children’s emergency Department use for asthma, 2001-2010. Academic Pediatrics 2015;15:225–30. 10.1016/j.acap.2014.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kantor DB, Phipatanakul W. Intravenous beta agonists and severe pediatric asthma exacerbation: time for a closer look at terbutaline Annals of Allergy, Asthma & Immunology 2014;112:187. 10.1016/j.anai.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 6. Giordano K, Rodriguez E, Green N, et al. Pulmonary function tests in emergency Department pediatric patients with acute wheezing/asthma exacerbation. Pulm Med 2012;2012:724139. 10.1155/2012/724139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kelly AM, Kerr D, Powell C. Is severity assessment after one hour of treatment better for predicting the need for admission in acute asthma. Respir Med 2004;98:777–81. 10.1016/j.rmed.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 8. Powell CV, Kelly AM, Kerr D. Lack of agreement in classification of the severity of acute asthma between emergency physician assessment and classification using the National asthma Council Australia guidelines. Emergency Medicine 2003;15:49–53. 10.1046/j.1442-2026.2003.00408.x [DOI] [PubMed] [Google Scholar]
- 9. O’Connor MG, Saville BR, Hartert TV, et al. Treatment variability of asthma exacerbations in a pediatric emergency Department using a severity-based management protocol. Clin Pediatr (Phila) 2014;53:1288–90. 10.1177/0009922813520071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biagini Myers JM, Simmons JM, Kercsmar CM, et al. Heterogeneity in asthma care in a statewide collaborative: the Ohio pediatric asthma repository. Pediatrics 2015;135:271–9. 10.1542/peds.2014-2230 [DOI] [PubMed] [Google Scholar]
- 11. Morris I, Lyttle MD, O’Sullivan R, et al. Which intravenous Bronchodilators are being administered to children presenting with acute severe Wheeze in the UK and Ireland? Thorax 2015;70:88–91. 10.1136/thoraxjnl-2014-206041 [DOI] [PubMed] [Google Scholar]
- 12. Craig S, Powell CVE, Nixon GM, et al. Treatment patterns and frequency of key outcomes in acute severe asthma in children: a Paediatric research in emergency departments International collaborative (PREDICT) Multicentre cohort study. BMJ Open Respir Res 2022;9:e001137. 10.1136/bmjresp-2021-001137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Monteverde-Fernandez N, Diaz-Rubio F, Vásquez-Hoyos P, et al. Variability in care for children with severe acute asthma in Latin America. Pediatr Pulmonol 2021;56:384–91. 10.1002/ppul.25212 [DOI] [PubMed] [Google Scholar]
- 14. Kalburgi S, Halley T. High-flow nasal Cannula use outside of the ICU setting. Pediatrics 2020;146:e20194083. 10.1542/peds.2019-4083 [DOI] [PubMed] [Google Scholar]
- 15. Johnson MD, Zorc JJ, Nelson DS, et al. Intravenous magnesium in asthma Pharmacotherapy: variability in use in the PECARN Registry. The Journal of Pediatrics 2020;220:165–174. 10.1016/j.jpeds.2020.01.062 [DOI] [PubMed] [Google Scholar]
- 16. Cochrane Airways Group, Craig SS, Dalziel SR, et al. Interventions for escalation of therapy for acute exacerbations of asthma in children: an overview of Cochrane reviews. Cochrane Database Syst Rev 2020;2020:Cd012977. 10.1002/14651858.CD012977.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gray CS, Powell CVE, Babl FE, et al. Variability of outcome measures in trials of intravenous therapy in acute severe Paediatric asthma: a systematic review. Emerg Med J 2019;36:225–30. 10.1136/emermed-2018-207929 [DOI] [PubMed] [Google Scholar]
- 18. Nassif A, Ostermayer DG, Hoang KB, et al. Implementation of a Prehospital protocol change for Asthmatic children. Prehosp Emerg Care 2018;22:457–65. 10.1080/10903127.2017.1408727 [DOI] [PubMed] [Google Scholar]
- 19. Riney LC, Schwartz H, Murtagh Kurowski E, et al. Improving administration of Prehospital corticosteroids for pediatric asthma. Pediatr Qual Saf 2021;6:e410. 10.1097/pq9.0000000000000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheetham AL, Navanandan N, Leonard J, et al. Impact of Prehospital pediatric asthma management protocol adherence on clinical outcomes. J Asthma 2022;59:937–45. 10.1080/02770903.2021.1881969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 22. Ambulance Victoria . Clinical practice guidelines for ambulance and MICA Paramedics. Doncaster: Ambulance Victoria, 2018. Available: https://www.ambulance.vic.gov.au/wp-content/uploads/2019/07/Clinical-Practice-Guidelines-2018-Edition-1.9-1.pdf [accessed 15 Sep 2022]. [Google Scholar]
- 23. Ambulance Victoria . Ambulance Victoria annual report 2019-20. Melbourne, 2020. Available: https://www.ambulance.vic.gov.au/about-us/our-performance/ [accessed 15 Sep 2022]. [Google Scholar]
- 24. Hasegawa K, Craig SS, Teach SJ, et al. Management of asthma exacerbations in the emergency Department. J Allergy Clin Immunol Pract 2021;9:2599–610. 10.1016/j.jaip.2020.12.037 [DOI] [PubMed] [Google Scholar]
- 25. Pfeiffer CK, Smith K, Bernard S, et al. Prehospital benzodiazepine use and need for respiratory support in Paediatric seizures. Emerg Med J 2022;39:608–15. 10.1136/emermed-2021-211735 [DOI] [PubMed] [Google Scholar]
- 26. Gilbert EH, Lowenstein SR, Koziol-McLain J, et al. Chart reviews in emergency medicine research: where are the methods Annals of Emergency Medicine 1996;27:305–8. 10.1016/S0196-0644(96)70264-0 [DOI] [PubMed] [Google Scholar]
- 27. Kaji AH, Schriger D, Green S. Looking through the Retrospectoscope: reducing bias in emergency medicine chart review studies. Ann Emerg Med 2014;64:292–8. 10.1016/j.annemergmed.2014.03.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-073029supp001.pdf (49.8KB, pdf)
Data Availability Statement
Data are available on reasonable request. Deidentified participant data will be available for sharing from 1 July 2024. Any data access requests should be sent to SC (simon.craig@monash.edu), and should include a proposal from the individual or organisation regarding their plan for use of the data.The study team will review the request and consider the scientific merit of the proposed use of the data, and the legal, regulatory and ethical issues pertinent to the request. Presuming all constraints are addressed, the data will be shared using a secure file transfer platform.