Abstract
Aims
Evaluate the impact of a quality improvement programme on the reduction of feeding tube obstruction frequencies, analyse the predictive variables of this safety incident, and estimate the economic costs related to the quality improvement programme during the period from 2014 to 2019.
Methods
Plan–Do–Study–Act cycles were performed to test the changes in drug preparation and administration processes via a nasoenteral feeding tube and to evaluate the outcome, process and balance measures. Statistical control charts were elaborated, and the bottom-up direct costing methodology was used to estimate the costs of the improvement programme. The impact of the programme on the monitoring measures was evaluated using logistic regression analysis.
Interventions
The following changes were tested in the hospital participating in the study: acquisition of the Easy Crush equipment for tablet crushing, use of appropriate packaging to crush hard tablets, standardise procedures for scheduling administration times and/or substitution of the pharmaceutical form, educational activities for the nursing team and elaboration and availability of infographics for the nursing team, patients and/or family/caregivers.
Results
There was a significant improvement in the frequency of tube obstructions, from 41.1% in 2014 to 57.9% in 2015–2017 and 9.6% in 2018–2019 (p=0.0010). After the execution of the improvement programme, it was estimated that the cost of dose preparation was reduced from R$1067.50 in 2014 to R$719.80 in 2015–2017 and R$433.10 in 2015–2019.
Conclusion
By re-establishing the processes of drug preparation and administration via a nasoenteral feeding tube, through the acquisition of appropriate equipment for crushing hard tablets, together with educational activities for the nursing team, we could observe a reduction in tube obstructions and the cost of processes.
Keywords: Healthcare quality improvement, PDSA, Quality improvement, Medication safety
Introduction
In Brazil, the accidental loss and obstruction of nasoenteral tubes are considered the fifth most common type of incident reported by the Brazilian health services to the Health Surveillance Reporting System.1
A multicentre survey conducted in seven hospitals across four regions of Brazil found that accidental loss and tube obstruction were the most common mechanical incidents in adult patients receiving enteral nutrition.2 These incidents are often attributed to professionals’ lack of knowledge about best practices in dose preparation and administration, as well as inadequate training and equipment for correct medication preparation.3
Reducing tube obstruction rates should be a priority for healthcare institutions, as such incidents increase costs and the risk of negative outcomes for patients.4 5 Additionally, this event can be considered a key indicator of the quality of care. In a study conducted in a private hospital in the interior of São Paulo, a quality improvement programme (QIP) was implemented to reduce the number of obstructions in nasoenteral tubes after four Plan–Do–Study–Act (PDSA) cycles. The results showed a significant decrease in this indicator, from 33.3% to 7.4%, indicating an improvement in the process.6
In a survey conducted in 2014 across three Brazilian hospitals, errors were identified in the preparation and administration of medications via nasoenteral tubes. One of these institutions was selected for a QIP to address these issues (baseline—2014).7 Between 2015 and 2017,8 9 PDSA cycles were conducted to evaluate the processes. Factors that influenced a deterioration of the process were identified, including the lack of appropriate equipment to crush hard tablets into a fine and homogeneous powder, and the lack of standardisation of techniques for preparing and administering medications through a feeding tube. These process failures contributed to an increased number of obstructed feeding tubes between the baseline (2014) and change tests (2015–2017), which can be observed in online supplemental file 1.7–9
bmjoq-2022-002183supp001.pdf (560.9KB, pdf)
The hospital purchased new equipment (Easy Crush) which became available in the wards in August 2018. At that time, the improvement team (IT) had three key questions: (1) Would the frequency of tube obstructions decrease with the use of the new equipment?; (2) Would the process of administering medications via nasoenteral tube be safe for the patient? and (3) Would the process costs be maintained after the execution of the QIP? To address these hypotheses, the objective of the study was to evaluate the impact of a QIP in reducing the frequency of tube obstructions during the periods of 2014, 2015–2017 and 2018–2019. Additionally, predictor variables of this safety incident were analysed, and an economic estimate of costs related to the QIP during the same periods was conducted.
Methods
Design
The study was an intervention study, and the Standards for Quality Improvement Reporting Excellence guidelines were used to describe the intervention and its outcomes.10
Setting
The study was conducted in a secondary-complexity medical clinic ward within a public hospital located in the municipality of Ribeirão Preto, São Paulo.
Sample
This study considered the doses of oral medications prepared and administered to adult patients using nasoenteral tubes. A total of 366 doses were determined, with 122 per period (2014, 2015–2017 and 2018–2019), according to the sample calculation performed in previous research.7 9
Data collection
Data were collected through direct and participant observation of the processes of preparation and administration of oral medications via a nasoenteral feeding tube. We used a form developed in previous research,7 and a panel of five experts validated the data for face and content. A previously trained researcher collected the data during the periods (2014, 2015–2017 and 2018–2019, from Monday to Sunday, including holidays, in the morning, afternoon and evening periods, through direct and participatory observation of nursing professionals during the preparation and administration of oral medications via nasoenteral tube, according to previous research.7 8
An improvement strategy to assess the impact of acquiring new equipment on reducing the frequency of tube obstructions
An interactive approach, based on three PDSA cycles, was used to test and to implement changes in medication preparation and administration processes via a nasoenteral tube. The changes included: the acquisition of the Easy Crush equipment for grinding the tablets, use of appropriate packaging for grinding the hard tablets, standardise procedures for scheduling administration times and/or substitution of the pharmaceutical form, educational activities for the nursing team and elaboration and availability of infographics for the nursing team, patients and/or family/caregivers.
For the purposes of this study, an ‘obstructed tube’ was defined as the occlusion of the tube lumen with a consequent increase in internal resistance, preventing the infusion of medication and/or enteral nutrition.2
To assess the impact of acquiring new equipment, the established target was to reduce the frequency of tube obstruction in adult patients, which was achieved with a reduction from 57.9% to 10% in 5 months. For this, three PDSA cycles were necessary, which occurred in the period from December 2018 to April 2019. A multicriteria decision was used as a starting point for the first cycle; the second and third cycles were based on sequential knowledge construction. The three cycles were performed based on the sample of 122 doses collected in 2018–2019 and compared with the previous samples (2014 and 2015–2017).7–9
First PDSA cycle
This cycle was conducted from 2 December 2018 to 5 December 2018 to evaluate the use of the Easy Crush equipment in the grinding of tablets. The equipment was made available in the hospital medication room in August 2018. The continuing education nurse trained the nursing team to correctly use the equipment according to following the manufacturer’s recommendations in the same period: to grind the tablets individually in the specific plastic container of the product and to reconstitute it in the same container with drinking water.
A member of the IT identified that the packaging recommended by the manufacturer was not available at the hospital. Therefore, the tablets were crushed in the package provided by the hospital pharmacy, reconstituted with distilled water, and placed in a 50 mL disposable cup. Furthermore, a search for articles related to the correct use of the Easy Crush equipment was performed in the PubMed/MEDLINE, CINAHL, LILACS and EMBASE databases; however, no articles on this theme were identified. In a meeting, the IT decided that the manufacturer’s recommendations should be followed, which allowed for reflection on the unnecessary costs related to purchasing disposable cups for medication preparation. Such information was passed on to the hospital management team, and it was agreed that the tablets should be crushed and reconstituted in the appropriate packaging as recommended by the manufacturer. In addition, it was decided that the administrative assistant would be responsible for requesting the purchase of packaging, making it available in the medication preparation rooms and controlling the product stock.
Second PDSA cycle
The second PDSA cycle was performed from 8 December 2018 to 20 December 2018 to train the nursing team on how to correctly use the Easy Crush equipment in the preparation of doses. Initially, the strategy was to gather the nursing professionals in a previously organised room in the hospital itself, in groups of up to six people and at times of lower demand. However, due to the difficulty of the release of the team members by the shift nurses, a decision was made to provide information on the correct use of the equipment during the shift and/or personally during the work shift and with the help of an infographic. It is noteworthy that the infographic was made available in the medication room for professionals to consult when in doubt about the preparation of medications.
During the dialogical exposure, the number of oral medications prescribed for the same patient and scheduled for the same time was discussed. Based on this situational diagnosis, the team decided to change the standardise procedures for scheduling administration times of oral medication administration and, when possible, to request the medical professional to replace the medication or pharmaceutical form. Among the 56 nursing professionals working in the medical clinic of the hospital, 16 were not trained during the study period because they were on leave or vacation or because they refused to participate in the training. The hospital’s continuing education nurse was the professional responsible for conducting frequent training sessions.
Third PDSA cycle
The third PDSA cycle was performed from 20 February 2019 to 15 April 2019, aiming to include patients and/or family members in the process of medication administration via a nasoenteral tube. At the study hospital, patients who were discharged home with a tube were oriented by the dietitian about the necessary care for the tube. The IT decided to elaborate and to make available an infographic containing information on the technique of drug administration by this route. The infographic was placed at the patients’ bedside. In addition, the study researcher guided the patients and/or family members on how to perform the technique and the importance of correctly administering medications through the nasoenteral tube. A nurse was selected by the hospital’s IT team for the study to receive training from the researcher and continue the education of patients and/or family members.
Measures
The following measures were selected for the study.
Process: ‘Number of medications prescribed via tube in 24 hours’ and ‘number of medications scheduled for the same time’.
Result: ‘Tube obstruction’.
Balance: ‘Compromising of biopharmaceutical aspects during crushing’ and ‘time elapsed between preparation and administration of the drug’.
Seven meetings with the IT were necessary to achieve the proposed goal for the programme. The meetings took place at the hospital and lasted 15 min each. The same researcher in all of the study periods performed data collection, and 122 doses of medications via a nasoenteral tube were observed.
Estimate of the effect of the QIP on the cost of preparation and administration of medications via a nasoenteral tube
To estimate the possible effects of the QIP on the cost of preparation and administration of medications via a nasoenteral tube, a total of 366 doses in the years 2014 (122 doses), from 2015 to 2017 (122 doses), and from 2018 to 2019 (122 doses) were analysed.
The literature recommends11 the use of the bottom-up direct costing methodology for intervention studies such as this one. This methodology is more detailed and has greater rigour in the evaluation of the cost components.
The direct costing methodology was used to calculate the dose administration costs, which is a variant of the bottom-up microcosting methodology, since the cost object has a restricted scope12 . In the case of this research, one dose.
Operationally, the analyses included the direct costs associated with dose preparation and administration: the salaries of the different nursing professional categories, materials used in this process (such as crepe tape, distilled water, 50 mL disposable cup, 70% alcohol, 20 mL syringe, cotton, procedure glove and gases), and depreciation of the Easy Crush equipment.
The cost of medications was not part of the scope of observation, given that the possible effects of adopting a QIP on the cost of preparation and administration of medications via a nasoenteral tube were being evaluated.
Data analysis
Data were entered into EpiData V.3.1 and transferred to the statistical program R. In the descriptive analysis, the absolute and relative frequencies of the measures ‘tube obstruction’ and ‘compromising of biopharmaceutical aspects during crushing’ were evaluated. The following process measures were analysed in terms of averages: ‘number of medications prescribed in 24 hours’, ‘number of medications scheduled for the same time’ and ‘time elapsed between preparation and administration of the drug’. The statistical tests χ2 of Pearson and Kruskal-Wallis were used for these analyses. In addition, the measurements ‘tube obstruction’, ‘number of medications prescribed in 24 hours’ and ‘number of medications scheduled for the same administration time’ were monitored and followed up with the help of the trend graph and statistical control U. The graphs were prepared using the Minitab V.19 program.
To analyse the predictor variables of tube obstruction, logistic regression analysis was performed for this variable. The explanatory variables were as follows: crushing the tablet to a fine and homogeneous powder (yes/no); washing the tube before administering the medication (yes/no); washing the tube between one medication and another (yes/no); washed the tube after the end of the medication (yes/no); the number of medications prescribed via tube in 24 hours; the number of medications scheduled for the same time; time elapsed between preparation and administration of the drug (in minutes); and study period, namely baseline (2014) and PDSA cycles (2015–2017 and 2018–2019). The χ2 statistical tests of Pearson and Ranksum were performed for these analyses.
The likelihood ratio test was also applied to verify the interaction between the outcome variable ‘tube obstruction’ during hospitalisation (yes/no) and the following explanatory variables: washed the tube after the end of the drug (yes/no) and PDSA cycle period (2014, 2015–2017 and 2018–2019). In these analyses, the likelihood ratio and Wald tests were applied. Variables with p≤0.05 values were included in the final regression model.
Results
Evaluation of the impact of a QIP in reducing the frequency of tube obstruction
In the periods (2014, 2015–2017 and 2018–2019), significant improvement was observed in the outcome measure ‘tube obstruction’ (p=0.0010), process measures ‘number of medications prescribed via tube in 24 hours’ (p=0.0010) and ‘number of medications scheduled for the same time’ (p=0.0010) and equilibrium measure ‘compromising of biopharmaceutical aspects during crushing’ (p=0.0010) (online supplemental file 2).
bmjoq-2022-002183supp002.pdf (11.6KB, pdf)
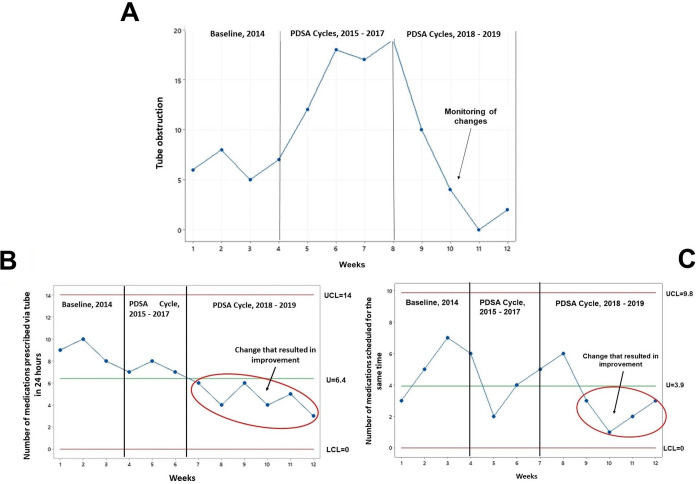
The measures ‘tube obstruction’, ‘number of medications prescribed via tube in 24 hours’ and ‘number of medications scheduled for the same time’ were monitored and followed up weekly (figure 1).
Figure 1.
Trend (A) and statistical control U chart (B, C) of the outcome and process measures monitored over the years. (A) The trend graph shows the weekly monitoring conducted 2014–2019 and improvement in the outcome measure ‘probe obstructed’. (B) The U-chart shows the weekly monitoring conducted in 2014–2019. From the seventh week, we observed a special cause that resulted in a variation that significantly changed the ‘numbers of medications prescribed within 24 hours’. (C) Chart U shows the weekly monitoring conducted in 2014–2019. From the eighth week, we observed a special cause that resulted in a variation that significantly changed and improved the process measure ‘number of medications scheduled for the same time’. LCL, lower control line; LIC, middle line-U; PDSA, Plan–Do–Study–Act; UCL, upper control line.
Analysis of predictor variables of tube obstruction
A total of 366 doses were observed between the years 2014, 2015–2017 and 2018–2019. The doses were prepared and administered by 60 nursing professionals in 54 patients with a mean age of 69 (41±97) years. In the association analysis between the outcome (tube obstruction) and explanatory measures (number of medications prescribed in 24 hours, crushed the tablet to a fine and homogeneous powder, washed the tube before administering the drug and washed the tube between one medication and another) were statistically significant (table 1).
Table 1.
Association analysis between the outcome (tube obstruction) and explanatory measures in patients using nasoenteral tubes (N=366)
| Measures | Yes | No | P value | ||
| n | % | n | % | ||
| No of medications prescribed via tube in 24 hours | 6 | 4.6 | 5 | 4.6 | 0.0010* |
| No of medications scheduled for the same time | 2 | 1.3 | 3 | 1.3 | 0.8170* |
| Time elapsed between preparation and administration of the drug | 15 | 8.23 | 11 | 5.2 | 0.0020* |
| Compromising of biopharmaceutical aspects during crushing | 73 | 57.5 | 172 | 72 | 0.0040† |
| Crushing the tablet to a fine and homogeneous powder | |||||
| Yes | 46 | 56.1 | 149 | 85.6 | 0.0010† |
| No | 36 | 43.9 | 25 | 14.4 | |
| Washing the tube before administering the drug | |||||
| Yes | 27 | 21.3 | 94 | 39.3 | 0.0010* |
| No | 100 | 78.7 | 145 | 60.7 | |
| Washing the tube between one medication and another | |||||
| Yes | 27 | 36 | 106 | 67.5 | 0.0010* |
| No | 48 | 64 | 51 | 32.5 | |
| Washed the tube after the end of the medication | |||||
| Yes | 117 | 92.1 | 215 | 90.3 | 0.5700* |
| No | 10 | 7.9 | 23 | 9.7 | |
*Kruskal-Wallis.
†χ2.
Table 2 reveals that the variables ‘number of medications scheduled for the same time’, ‘time elapsed between preparation and administration of the drug’, ‘compromising of biopharmaceutical aspects during crushing’ ‘washed the tube before administering the medication’ and ‘washed the tube after the end of the medication’ exerted a statistically significant contribution to the model.
Table 2.
Explanatory variables included in the final logistic regression model for the analysis of predictor variables of tube obstruction
| Measures | Estimate | IF | Z-value | Pr(>|z|) |
| No of medications prescribed via tube in 24 hours | −1.0821 | 0.8351 | −1.2958 | 0.1950 |
| No of medications scheduled for the same time | 0.2920 | 0.0989 | 2.9508 | 0.0032 |
| Time elapsed between preparation and administration of the drug | −4.0249 | 1.2122 | −3.3203 | 0.0009 |
| Compromising of biopharmaceutical aspects during crushing | 1.3243 | 0.6397 | 2.0703 | 0.0384 |
| Crushing the tablet to a fine and homogeneous powder | −0.6011 | 0.5492 | −1.0944 | 0.2738 |
| Washed the tube before administering the medication | 1.1056 | 0.5251 | 2.1053 | 0.0353 |
| Washed the tube between one medication and another | 0.0547 | 0.4421 | 0.1236 | 0.9016 |
| Washed the tube after the end of the medication | −2.9022 | 0.5831 | −4.9771 | 0.0000 |
Values of Pr(>|z|). The statistically significant variables are in bold.
Standard error of estimate
The washing of the nasoenteral tube after administration presented a chance 94.7% lower chance of obstruction (1–0.0530). In the comparison between the years 2015–2017 and 2018–2019 with the baseline (reference: 2014), it was found that the nasoenteral tube washed after the end of drug administration presented a 94.8% less chance of tube obstruction (1–0.0549) compared with those that were not washed (table 3).
Table 3.
Logistic regression model of the measure ‘tube obstruction’
| Measures | Gross RC (95% CI) | P value | Adjusted RC (95% CI) | P Wald’s test |
P LR test |
| No of medications prescribed via tube in 24 hours | 1.27 (1.1 to 1.47) | 0.0010 | 1.34 (1.1 to 1.63) | 0.0030 | 0.0020 |
| Time elapsed between preparation and administration of the drug | 1.03 (1.01 to 1.05) | 0.0150 | 1.06 (1.02 to 1.1) | 0.0040 | 0.0010 |
| Reference no versus yes | |||||
| Washed the tube after finishing the medication | 0.44 (0.15 to 1.36) | 0.0530 | 0.02 (0 to 0.19) | 0.0010 | 1.0000 |
| Reference year 2014 | |||||
| Year 2015–2017 | 1.97 (1.01 to 3.84) | 0.0450 | 1.06 (0.44 to 2.51) | 0.9020 | 1.0000 |
| Year 2018–2019 | 0.15 (0.07 to 0.33) | 0.0010 | 0.05 (0.02 to 0.17) | 0.0010 | 1.0000 |
| Reference ‘washed the tube after finishing the medication’ in the year 2014 | |||||
| Year 2015–2017 | 1.13 (0.49 to 2.63) | 7.9920 | 0 (0) | 0.9910 | 1.0000 |
| Year 2018–2019 | 2.42 (1.1 to 5.34) | 0.0549 | 1055.45 (44.89 to 24814.45) | 0.0010 | 1.0000 |
The statistically significant results are in bold.
Odds ratio
LR, likelihood ratio test.
Economic estimate of the costs related to the 2014–2019 improvement programme
In 2014, 122 doses were prepared and administered by a total of 22 nursing professionals, 18 (81.1%) assistants, 3 (15.6%) technicians and 1 (3.3%) nurse. Moreover, the doses were administered to 16 patients, 7 (43.7%) of whom were female individuals and 9 (56.2%), male individuals. The cost of the doses was R$1067.50.
In 2015–2017, 122 doses were prepared and administered by 18 nursing professionals, 4 (22.2%) assistants and 14 (77.7%) technicians. The drugs were administered to 16 patients, of whom 8 (50%) were female individuals and 8 (50%), male individuals. The cost of the doses was R$719.80.
In 2018–2019, 122 doses of oral medications were prepared and administered by 20 nursing professionals, 5 (22.2%) nursing assistants and 15 (77.7%) nursing technicians. The doses were administered to 15 patients with enteral nutrition, of whom 9 (60%) were female individuals and 6 (40%), male individuals. The cost of the doses was R$433.10. The cost analysis can be seen in table 4.
Table 4.
Protocol for cost analysis of drug preparation and administration via a nasoenteral tube
| Year 2014 | Professionals | Time* 287 |
Value R$1.24 |
| Nurse, nursing auxiliary and nursing technician | |||
| Materials used for the preparation and administration of doses and equipment | Value† R$7.51 |
||
| Crepe tape, distilled water (20 mL ampoule), 50 mL disposable cup, 70% alcohol, 20 mL syringe, cotton and procedure glove. Porcelain gral and pistil and stethoscope. | |||
| Total cost per dose | Value R$8.75 |
||
| Period 2015–2017 | Professionals | Time* 227 |
Value R$0.83 |
| Nursing assistant and nursing technician | |||
| Materials used for the preparation and administration of doses and equipment | Value† R$5.07 |
||
| Crepe tape, distilled water (20 mL ampoule), 50 mL disposable cup, 70% alcohol, 20 mL syringe, cotton and procedure glove. The tablets were crushed in the package itself with the help of scissors or a glass bottle (for example, a syrup bottle) or reconstituted in a disposable cup or in the 20 mL syringe itself, along with a stethoscope. | |||
| Total cost per dose | Value R$5.90 |
||
| Period 2018–2019 | Professionals | Time* 227 |
Value R$0.92 |
| Nursing assistant and nursing technician | |||
| Materials used for the preparation and administration of doses and equipment | Value† R$2.63 |
||
| Crepe tape, distilled water (20 mL ampoule), 50 mL disposable cup, 70% alcohol, 20 mL syringe, cotton and procedure glove. Easy Crush and stethoscope. | |||
| Total cost per dose | Value R$3.55 |
||
Value in Reais
*Average time, in s.
†Current values applied in November 2019 (Source: Human Resources of Ribeirao Preto State Hospital/Foundation for the Support of Teaching, Research and Assistance).
Discussion
The results of this study revealed that over time, the changes tested improved the processes of medication preparation and administration via a nasoenteral tube and that such changes implied a reduction in the frequency of tube obstruction and in the time spent by nursing professionals to prepare and to administer medications. Moreover, the changes resulted in cost reduction for the institution. Thus, these results point in the same direction of previous research that also demonstrated the impact of the QIP in improving care provided to hospitalised adult patients.13–15 To improve healthcare processes, it is fundamental to thoroughly know all of the stages of the systems and to use methods that will help leaders improve complex healthcare processes.16
The results also revealed that via PDSA cycles, the changes tested in this study improved the aforementioned processes and resulted in the achievement of the proposed goal for the QIP, corroborating research previously conducted (the PDSA cycles in 2015–2017; online supplemental file 1).
In an investigation conducted in a medical clinic of an American hospital, changes related to equipment repair and qualification of healthcare professionals were tested to reduce medication administration errors. After several PDSA cycles, a decrease of 20% in errors was observed, and the project’s proposed goal was achieved.17 Unlike what occurred in that study, the changes tested in this study additionally involved reducing the number of medications scheduled for the same time and within 24 hours. This is an essential factor for the reduction in the frequency of tube obstruction since the concomitant grinding and administration of several medications can result in drug interactions and subsequent bezoar formation. Moreover, such interactions may result in significant adverse reactions in patients, especially in older individuals.18
The prevention of obstructions secondary to errors in the preparation and administration of medications through tubes requires safe practices that include the following: checking the compatibility of the medication with the route of administration; grinding solid medications until a fine and homogeneous powder is obtained; preparing and administering medications separately; stopping the enteral diet before administering the medication; and systematically washing the tube before administration, between the administration of one medication and another, and after finishing the medication.7 9
In this study, a new piece of equipment intended for tablet crushing was acquired by the hospital. The decision for choosing the device was collaborative and involved the team responsible for executing the technique. Such a strategy can contribute to the team’s adherence to the changes tested through the PDSA cycles. According to the literature on this subject, this action can contribute to improving the processes of preparation and administration of medications via a nasoenteral tube. It is also noteworthy that the decision-making process is in line with what Deming (1997) advocates: people need to be included and trained in the processes to be improved to develop a critical look at the systems and to act towards a common goal. In this context, the people responsible must know the processes, continuously analyse the data, and, from the lessons learnt, invest in changes that generate improvements continuously and sustainably.19 20
Tube obstruction result in additional costs for healthcare services.21 This is a consequence of errors in the preparation and administration of medications through nasoenteral tubes. In this study, a reduction in process costs was detected over time (in the years 2014, 2015–2017 and 2018–2019) after the implementation of the PMQ. It is noteworthy that in the literature, no previous studies were found that evaluated the costs related to the implementation of the QIP in the process of preparation and administration of medications via a nasoenteral tube.
Medication errors with the use of a nasoenteral tube can result in severe and potentially fatal adverse patient outcomes.22 Furthermore, such errors increase healthcare costs and consume more material and human resources, in addition to increasing patients’ length of stay in healthcare institutions.22
Strengths and limitations of the study
Because this was a study that occurred over time (in the years 2014, 2015–2017 and 2018–2019), it was not possible to control the subjects of the interventions, and there was turnover among nursing professionals. To control this bias, the hospital’s continuing education nurse trained all new nursing team members as changes occurred. In addition, these nurses were members of the quality IT during the study period. Another limitation is regarding the acquisition of new equipment, we did not find other studies in the literature that reported improvement. More studies are needed to assess the effectiveness of the equipment to grind medicines to a fine and homogeneous powder. The strong point was the adherence of the hospital management team that recognised the importance and need to change the processes of preparation and administration of medications via a nasoenteral tube.
Conclusion
The results revealed that the changes made in medication preparation and administration processes through a QIP positively impacted the frequency of tube obstruction and financial costs. In addition, logistic regression analysis revealed that obstructed tubes were strongly influenced by the procedures adopted by the nursing team in washing the tube before, during and after the end of drug administration. Leaders of healthcare institutions must invest in improvements in healthcare processes, test changes and systematically monitor the results over time to improve health outcomes.
Footnotes
Contributors: RAP author guarantor and contributed to the study concept and design, acquisition of data, analysis of data and drafting of the manuscript. FREG contributed to the study concept and design, analysis of data and drafting of the manuscript. MCGR, LRMdC, FBdS, MMG and CAGB contributed to the analysis and interpretation of data and dissemination of the results of this study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The project was approved by the Research Ethics Committee of University of São Paulo at Ribeirão Preto College of Nursing (CAAE: 64957217.7.0000.5393), according to Resolution 466/12, of the National Research Ethics Council of the Ministry of Health, which addresses research ethics with human beings.
References
- 1.GVIMS . Boletim Segurança do Paciente E Qualidade em Serviços de Saúde N° 15: Incidentes Relacionados À Assistência À Saúde 2017. Available: https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/servicosdesaude/boletim-seguranca-do-paciente/boletim-seguranca-do-paciente-e-qualidade-em-servicos-de-saude-no-15.pdf
- 2.Gimenes FRE, Baracioli F, Medeiros A de, et al. Factors associated with mechanical device-related complications in tube Fed patients: a multicenter prospective cohort study. PLoS One 2020;15:e0241849. 10.1371/journal.pone.0241849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.dos Santos de Souza MRN, Contarine LM, Coutinho Barreto JB, et al. Obstrução do Cateter de Nutrição Enteral E a Administração de Fármacos Sólidos NA Unidade de Terapia Intensiva Adulto. Biológicas & Saúde 2018;26:8. [Google Scholar]
- 4.Costa LFRMd . Redução de Não Conformidades no Preparo E NA Administração de Medicamentos Orais via Sonda Nasogástrica/ Nasoentérica: Impacto de um Programa de Melhoria DA Qualidade. Universidade de São Paulo; 2021. [Google Scholar]
- 5.Nakagawa M, Sugihara K, Isobe K, et al. A case of tracheal obstruction caused by reflux and aspiration of semi-solid nutrients via the Nasogastric tube. Int J Surg Case Rep 2019;65:217–20. 10.1016/j.ijscr.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa LFRM da, Bonacim CAG, Pereira RA, et al. Programa de Melhoria DA Qualidade NA Administração de Medicamentos via Sonda Nasoenteral. Acta Paulista de Enfermagem 2022;35. 10.37689/acta-ape/2022AO000934 [DOI] [Google Scholar]
- 7.Fernanda REG, Rosana AP, Ana CPH, et al. Medication incidents related to feeding tube: a cross-sectional study. Afr J Pharm Pharmacol 2017;11:305–13. 10.5897/AJPP2017.4799 [DOI] [Google Scholar]
- 8.Rosana AP, Adriano MR, Andre AR, et al. Good practice guidance to support safe oral medication preparation and administration through feeding tubes. Afr J Pharm Pharmacol 2019;13:17–24. 10.5897/AJPP2018.4958 [DOI] [Google Scholar]
- 9.Pereira RA, de Souza FB, Rigobello MCG, et al. Quality improvement programme reduces errors in oral medication preparation and administration through feeding tubes. BMJ Open Qual 2020;9:e000882. 10.1136/bmjoq-2019-000882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Squire . Standards for quality improvement reporting Excellence. Promoting excellence in Helthcare improvement reporting. 2017. Available: http://www.squire-statement.org/index.cfm?fuseaction=page.viewpage&pageid=471
- 11.Moerer O, Plock E, Mgbor U, et al. A German national prevalence study on the cost of intensive care: an evaluation from 51 intensive care units. Crit Care 2007;11:R69. 10.1186/cc5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collier SA, Stockman LJ, Hicks LA, et al. Direct Healthcare costs of selected diseases primarily or partially transmitted by water. Epidemiol Infect 2012;140:2003–13. 10.1017/S0950268811002858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagle M, Dwyer D, Gettrust L, et al. Development and implementation of a model for research, evidence-based practice. J Nurs Care Qual 2020;35:102–7. 10.1097/NCQ.0000000000000422 [DOI] [PubMed] [Google Scholar]
- 14.Meny LM, Seiferlein MR, Chen AMH. Faculty perceptions of a town hall model for engagement in continuous quality improvement. Curr Pharm Teach Learn 2021;13:968–74. 10.1016/j.cptl.2021.06.004 [DOI] [PubMed] [Google Scholar]
- 15.Srivastava S, Datta V, Garde R, et al. Development of a Hub and spoke model for quality improvement in rural and urban Healthcare settings in India: a pilot study. BMJ Open Qual 2020;9:e000908. 10.1136/bmjoq-2019-000908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IHI [Institute for Healthcare Improvement] . Improving Healthand health care worldwide 2000. Available: http://www.ihi.org/regions/Pages/default.aspx
- 17.Cloete L. Reducing medication errors in nursing practice. Nurs Stand 2015;29:50–9. 10.7748/ns.29.20.50.e9507 [DOI] [PubMed] [Google Scholar]
- 18.Dutta AK, Goel A, Kirubakaran R, et al. Nasogastric versus nasojejunal tube feeding for severe acute pancreatitis. Cochrane Database Syst Rev 2020;3:CD010582. 10.1002/14651858.CD010582.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deming WE. A Nova Economia para A Industria, O Governo E A EducaçãO. 1997. [Google Scholar]
- 20.Langley G, Moen R, Nolan K, et al. Modelo de melhoria: uma abordagem prática para melhorar o desempenho organizacional. São Paulo: Mercado de Letras, 2011. [Google Scholar]
- 21.Larmené-Beld KHM, Spronk JT, Luttjeboer J, et al. A cost minimization analysis of ready-to-administer prefilled sterilized syringes in a Dutch hospital. Clin Ther 2019;41:1139–50. 10.1016/j.clinthera.2019.04.024 [DOI] [PubMed] [Google Scholar]
- 22.Milani RV, Wilt JK, Entwisle J, et al. Reducing inappropriate outpatient antibiotic prescribing: normative comparison using unblinded provider reports. BMJ Open Qual 2019;8:e000351. 10.1136/bmjoq-2018-000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjoq-2022-002183supp001.pdf (560.9KB, pdf)
bmjoq-2022-002183supp002.pdf (11.6KB, pdf)
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.