Abstract
Background
In the randomized, controlled, phase III KEYNOTE-061 trial, second-line pembrolizumab did not significantly prolong overall survival (OS) versus paclitaxel in patients with PD-L1-positive (combined positive score ≥1) advanced gastric/gastroesophageal junction (G/GEJ) cancer but did elicit a longer duration of response and offered a favorable safety profile. This prespecified exploratory analysis was conducted to evaluate associations between tumor gene expression signatures and clinical outcomes in the phase III KEYNOTE-061 trial.
Methods
Using RNA sequencing data obtained from formalin-fixed, paraffin-embedded baseline tumor tissue samples, we evaluated the 18-gene T-cell-inflamed gene expression profile (TcellinfGEP) and 10 non-TcellinfGEP signatures (angiogenesis, glycolysis, granulocytic myeloid-derived suppressor cell (gMDSC), hypoxia, monocytic MDSC (mMDSC), MYC, proliferation, RAS, stroma/epithelial-to-mesenchymal transition/transforming growth factor-β, WNT). The association between each signature on a continuous scale and outcomes was analyzed using logistic (objective response rate (ORR)) and Cox proportional hazards regression (progression-free survival (PFS) and OS). One-sided (pembrolizumab) and two-sided (paclitaxel) p values were calculated for TcellinfGEP (prespecified α=0.05) and the 10 non-TcellinfGEP signatures (multiplicity-adjusted; prespecified α=0.10).
Results
RNA sequencing data were available for 137 patients in each treatment group. TcellinfGEP was positively associated with ORR (p=0.041) and PFS (p=0.026) for pembrolizumab but not paclitaxel (p>0.05). The TcellinfGEP-adjusted mMDSC signature was negatively associated with ORR (p=0.077), PFS (p=0.057), and OS (p=0.033) for pembrolizumab, while the TcellinfGEP-adjusted glycolysis (p=0.018), MYC (p=0.057), and proliferation (p=0.002) signatures were negatively associated with OS for paclitaxel.
Conclusions
This exploratory analysis of tumor TcellinfGEP showed associations with ORR and PFS for pembrolizumab but not for paclitaxel. TcellinfGEP-adjusted mMDSC signature was negatively associated with ORR, PFS, and OS for pembrolizumab but not paclitaxel. These data suggest myeloid-driven suppression may play a role in resistance to PD-1 inhibition in G/GEJ cancer and support a strategy of considering immunotherapy combinations which target this myeloid axis.
Trial registration number
Keywords: Gastrointestinal Neoplasms, Gene Expression Profiling, Genetic Markers, Immunotherapy, Programmed Cell Death 1 Receptor
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Despite multiple systemic therapeutic options, prognosis for patients with advanced gastric or gastroesophageal junction (G/GEJ) cancer remain poor globally. One potential explanation for the poor prognosis may be the constantly evolving molecular expression patterns and interactions between tumor cells and immune cells in the tumor microenvironment. As a result, there is a need to identify molecular determinants that may predict response to systemic therapy in patients with advanced G/GEJ cancer.
WHAT THIS STUDY ADDS
This exploratory study from the randomized, controlled, phase III KEYNOTE-061 trial of pembrolizumab versus paclitaxel as second-line therapy in patients with advanced G/GEJ cancer found that tumor 18-gene T-cell-inflamed gene expression profile (TcellinfGEP) as a continuous variable showed a positive association with objective response rate (ORR) and progression-free survival (PFS) in patients treated with pembrolizuamb, and that the TcellinfGEP-adjusted monocytic myeloid-derived suppressor cell (mMDSC) signature when analyzed as a continuous variable showed a negative association with ORR, PFS, and overall survival.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY.
Together, these data indicate a potential role for TcellinfGEP and mMDSC signatures in predicting anti-PD-1 therapy outcomes in patients with advanced G/GEJ cancer. Furthermore, the negative associations between mMDSC and outcomes supports a strategy of considering immunotherapy combinations intended to target the myeloid axis in advanced G/GEJ cancer.
Introduction
Clinical outcomes remain poor for patients with advanced gastric or gastroesophageal junction (G/GEJ) cancer globally despite multiple systemic therapeutic options.1–3 An estimated 769,000 deaths due to gastric cancer occurred in 2020, and the 5-year relative survival rate for advanced or metastatic disease in the USA is 6%.4 5 Such poor prognosis for patients with G/GEJ cancer may result from the constantly evolving molecular expression patterns and interactions between tumor cells and immune cells in the tumor microenvironment (TME).6–8 As a result, there is a need to identify biomarkers that may predict response to systemic therapy in patients with advanced G/GEJ cancer.
Pembrolizumab is a programmed cell death protein 1 (PD-1) inhibitor that is recommended for patients with microsatellite instability-high (MSI-H)/deficient mismatch repair tumors and tumor mutational burden (TMB) high (TMB-H; ≥10 mutations/megabase) tumors when no other satisfactory alternative therapeutic options exist.3 9 It was recently suggested that programmed death ligand 1 (PD-L1) expression using Combined Positive Score (CPS), TMB, and MSI-H are predictors of response to pembrolizumab in patients with previously treated advanced G/GEJ cancer.1 10 11 However, given the complexity of the TME, a greater understanding of the TME beyond TMB is needed for a more robust and reliable prediction of patient response to pembrolizumab. Exploratory studies showed that certain other molecular determinants in the TME such as an interferon gamma (IFN-γ)-related 18-gene T-cell-inflamed gene expression profile (TcellinfGEP) signature and other non-TcellinfGEP consensus signatures associated with key cell types, certain biological processes, or oncogenic pathways are associated with pan-tumor response to pembrolizumab.12–16 In one such pan-tumor study involving seven tumor types, it was found that TcellinfGEP along with 10 other TME-associated non-TcellinfGEP consensus RNA expression signatures for angiogenesis, glycolysis, granulocytic myeloid-derived suppressor cells (gMDSC), hypoxia, monocytic MDSCs (mMDSC), MYC, proliferation, RAS, stromal/epithelial-to-mesenchymal transition (EMT)/transforming growth factor-β (TGFβ), and WNT had a high concordant coexpression pattern across tumor types; some of these consensus gene expression signatures were significantly associated with response to pembrolizumab.12 At present, the association of these gene expression signatures with clinical response to pembrolizumab has not been specifically explored in the G/GEJ cancer setting.
The randomized, open-label, phase 3 KEYNOTE-061 trial was designed to evaluate the efficacy and safety of pembrolizumab versus paclitaxel as second-line therapy in patients with advanced G/GEJ cancer that progressed on platinum and fluoropyrimidine-based chemotherapy.17 18 Overall survival (OS) and progression-free survival (PFS) in the primary population (PD-L1 CPS≥1) were not significantly prolonged with pembrolizumab versus paclitaxel; however, pembrolizumab showed more durable responses and had a manageable adverse event profile compared with paclitaxel.18 We evaluated the association between tumor gene expression signatures and clinical outcomes of pembrolizumab versus paclitaxel in previously treated patients with advanced G/GEJ cancer from the KEYNOTE-061 trial.
Materials and methods
Trial design and patients
Details of the KEYNOTE-061 (ClinicalTrials.gov, NCT02370498) trial design and eligibility criteria have been published.18 Briefly, key eligibility criteria included age ≥18 years, histologically or cytologically confirmed unresectable advanced or metastatic gastric or GEJ adenocarcinoma that progressed after first-line therapy with a platinum agent and fluoropyrimidine or after first-line trastuzumab for human epidermal growth factor receptor 2-positive tumors, measurable disease per Response Evaluation Criteria in Solid Tumors, V.1.1 (RECIST V1.1), by investigator assessment, and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients with squamous cell or undifferentiated gastric cancer histology, previous therapy with an anti-PD-1/L1 agent, and active autoimmune disease that necessitated systemic treatment were excluded.
Patients were randomly assigned (1:1) to receive pembrolizumab 200 mg intravenously every 3 weeks or paclitaxel 80 mg/m² intravenously on days 1, 8, and 15 of 4-week cycles for 35 cycles (approximately 2 years; pembrolizumab only) or until disease progression, unacceptable toxicity, physician decision, or patient withdrawal of consent. The trial protocol and all amendments were approved by the institutional review board or ethics committee at each participating institution. The name of each ethics committee/institutional review board at each participating center including approval numbers are shown in online supplemental table 1. The trial was conducted in accordance with the protocol, its amendments, the ethical principles originating from the Declaration of Helsinki, and Good Clinical Practice guidelines. Written informed consent was provided by all patients before enrollment.
Outcomes
In this exploratory post hoc analysis, prespecified objectives included: (1) assessment of whether TcellinfGEP (as a continuous variable) is associated with clinical efficacy with pembrolizumab or paclitaxel, (2) assessment of whether the 10 non-TcellinfGEP signatures (angiogenesis, glycolysis, gMDSC, hypoxia, mMDSC, MYC, proliferation, RAS, stroma/EMT/TGFβ, and WNT) adjusted for the TcellinfGEP (as continuous variables) are associated with clinical efficacy with pembrolizumab or paclitaxel, (3) estimation of the relevant treatment effects of pembrolizumab versus paclitaxel in TcellinfGEP subgroups based on a prespecified cut-off of the first tertile, and (4) estimation of the relevant treatment effects of pembrolizumab versus paclitaxel in non-TcellinfGEP signatures that showed an association with OS when analyzed as continuous variables via a prespecified cut-off of the median.
Procedures
RNA sequencing was performed on formalin-fixed, paraffin-embedded (FFPE) tumor tissue samples provided at screening using the HiSeq 4000 platform (Illumina, California, USA). Of note, the gene expression signatures performed similarly in gene expression data obtained from freshly frozen and FFPE samples.12 The RNA-sequencing raw reads were processed using a customized RNA-sequencing data analysis pipeline in OmicSoft ArraySuite version 9 (Qiagen, Hilden, Germany) as previously described.12 First, the raw reads were quality-filtered and then aligned to the reference genome Human.B37.3 using the Omicsoft sequence aligner.19 Thereafter, gene expression levels, defined as raw read counts and fragments per kilobase of exon per million mapped fragments, were quantified using the RNA sequencing by expectation maximization algorithm with the Ensembl.R75 gene model.20
TcellinfGEP score was calculated as the weighted sum of normalized expression values for the 18 genes determined as predictors of response in the pan-tumor setting on the NanoString platform.12 13 The 10 non-TcellinfGEP signature scores were calculated as the average of the genes (on the logarithmic scale) in each signature gene set as previously described.12 13
TMB was determined via whole exome sequencing (mut/exome) of tumor samples and matched DNA. TMB was calculated as the number of somatic non-synonymous single nucleotide variants and indels that met prespecified criteria as previously described.16 21
Radiologic tumor imaging was performed every 6 weeks and survival follow-up assessed every 12 weeks. Objective response rate (ORR) was defined as the proportion of patients in the analysis population who had a confirmed complete response or partial response per RECIST V.1.1 by blinded independent central review. PFS was defined as the time from randomization to the first documented disease progression per RECIST V.1.1 by investigator assessment or death due to any cause, whichever occurred first. OS was defined as the time from randomization to death due to any cause.
Statistical analysis
This prespecified exploratory analysis included all treated patients in KEYNOTE-061 who had available RNA sequencing data.
The association between continuous scores for the TcellinfGEP and 10 non-TcellinfGEP signatures and clinical outcomes was evaluated using logistic regression (ORR) and Cox proportional hazards regression (PFS and OS), with adjustments for ECOG performance status; the 10 non-TcellinfGEP scores were also adjusted for TcellinfGEP. Adjustment for the TcellinfGEP was performed to understand the additional explanatory value that any non-TcellinfGEP signatures had for clinical outcome, an approach equivalent to evaluating the association between clinical outcome and the residuals of consensus signatures after detrending for their relationship with the TcellinfGEP. For the TcellinfGEP, one-sided (pembrolizumab; positive association hypothesized) and two-sided (paclitaxel; no direction hypothesized) p values were calculated; these hypotheses were informed by prior evidence in other tumor types that the TcellinfGEP may be positively associated with response to pembrolizumab22 23 and because the association between TcellinfGEP and paclitaxel has not been substantiated. P values were calculated (prespecified significance level, α=0.05). For the 10 non-TcellinfGEP signatures, one-sided (pembrolizumab; negative association hypothesized except for proliferation) and two-sided (paclitaxel; no direction hypothesized) multiplicity-adjusted p values were calculated (prespecified significance level, α=0.10).
Descriptive subgroup analyses to estimate efficacy of pembrolizumab versus paclitaxel and understand potential clinical utility were performed using prespecified cutoffs for the TcellinfGEP (≥ first tertile (TcellinfGEPnonlow) and <first tertile (TcellinfGEPlow) as previously defined and validated13 and non-TcellinfGEP signatures (≥ median and <median, where median is signature specific, TcellinfGEP-detrended). Within each subgroup, the exact binomial method was used to estimate difference in ORR whereas the Cox proportional hazards regression model, with adjustment for ECOG performance status (TcellinfGEP and non-TcellinfGEP signatures) and TcellinfGEP (non-TcellinfGEP signatures only), was used to estimate the OS and PFS HRs) and corresponding 95% CIs for pembrolizumab versus paclitaxel. OS was also evaluated by dual cutoffs of the TcellinfGEP signature and TMB (<175 mut/exome [TMBnonhigh] and ≥175 mut/exome [TMBhigh]; this cut-off of 175 mut/exome had been determined to be optimal for predicting response to pembrolizumab across several tumor types and is concordant with 10 mut/Mb via FoundationOneCDx.24–26
Results
Patients
Between June 4, 2015, and July 26, 2016, 592 patients were randomly assigned to receive either pembrolizumab or paclitaxel. The median follow-up duration, defined as time between randomization and the October 26, 2017, data cut-off, in the total KEYNOTE-061 population was 7.9 months (IQR 3.4–14.6 months).18 Of those treated, 274 patients (pembrolizumab, n=137; paclitaxel, n=137) had evaluable RNA sequencing data and were included in this analysis. Baseline characteristics in the RNA sequencing analysis population were generally well balanced between treatment groups (table 1) and similar to those of the total KEYNOTE-061 trial population.18 Clinical outcomes for patients with evaluable RNA sequencing data are presented in table 1.
Table 1.
Baseline characteristics and clinical outcomes in patients with evaluable RNA sequencing data
| Pembrolizumab n=137 |
Paclitaxel n=137 |
|
| Age, median (IQR), years | 63 (16.0) | 60 (16.0) |
| Male | 94 (68.6) | 95 (69.3) |
| ECOG performance status | ||
| 0/1 | 61 (44.5)/76 (55.5) | 63 (46.0)/74 (54.0) |
| Histology* | ||
| Adenocarcinoma | 106 (77.4) | 107 (78.1) |
| Tubular adenocarcinoma | 8 (5.8) | 13 (9.5) |
| Poorly cohesive carcinoma | 6 (4.4) | 6 (4.4) |
| Signet-ring cell carcinoma, diffuse type | 5 (3.6) | 6 (4.4) |
| Histological subtype† | ||
| Diffuse | 46 (33.6) | 33 (24.1) |
| Intestinal | 21 (15.3) | 31 (22.6) |
| Mixed | 3 (2.2) | 7 (5.1) |
| Primary location | ||
| Stomach | 95 (69.3) | 92 (67.2) |
| Gastroesophageal junction | 42 (30.7) | 45 (32.8) |
| Previous gastrectomy | ||
| Total | 26 (19.0) | 36 (26.3) |
| Subtotal | 19 (13.9) | 21 (15.3) |
| Partial | 13 (9.5) | 11 (8.0) |
| None | 79 (57.7) | 69 (50.4) |
| PD-L1 CPS | ||
| ≥1 | 90 (65.7) | 105 (76.6) |
| <1 | 47 (34.3) | 32 (23.4) |
| MSI status‡ | ||
| MSI-H | 8 (5.8) | 9 (6.6) |
| Non-MSI-H | 124 (90.5) | 122 (89.1) |
| TTP on first therapy | ||
| <6 months | 87 (63.5) | 82 (59.9) |
| ≥6 months | 50 (36.5) | 55 (40.1) |
| HER2 positive | 28 (20.4) | 26 (19.0) |
| Current disease stage | ||
| Metastatic | 30 (21.9) | 46 (33.6) |
| Locally advanced | 107 (78.1) | 91 (66.4) |
| Peritoneal metastasis | 30 (21.9) | 46 (33.6) |
| Presence of ascites | 18 (13.1) | 15 (10.9) |
| Clinical outcomes | ||
| Responders§ | 16 (11.7) | 20 (14.6) |
| PFS,¶ median (95% CI), months | 1.5 (1.4 to 1.9) | 4.0 (3.0 to 4.2) |
| OS, median (95% CI), months | 6.1 (4.6 to 10.1) | 8.4 (7.8 to 8.9) |
Values are n (%) unless stated otherwise.
*There were 12 patients (8.8%) and 5 patients (3.6%) with other histological types in the pembrolizumab and paclitaxel groups, respectively.
†There were 67 patients (48.9%) and 66 patients (48.2%) with unknown histological subtypes in the pembrolizumab and paclitaxel groups, respectively.
‡There were five patients and six patients with unknown MSI tumor status in the pembrolizumab and paclitaxel groups, respectively.
§Based on confirmed complete response or partial response per RECIST V.1.1 by BICR.
¶Per RECIST V.1.1 by investigator assessment.
BICR, blinded independent central review; CPS, combined positive score; ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; MSI-H, microsatellite instability-high; OS, overall survival; PD-L1, programmed death ligand 1; PFS, progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumors; TTP, time to progression.
Association between gene expression signatures and clinical outcomes
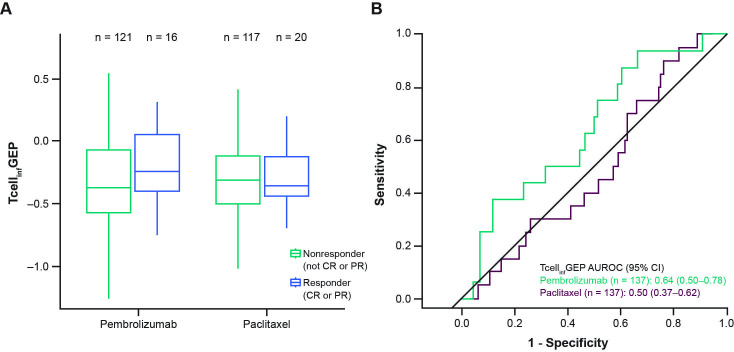
TcellinfGEP as a continuous variable was positively associated with ORR and PFS for pembrolizumab (one-sided p=0.041 and 0.026, respectively); no significant associations were observed between TcellinfGEP and clinical outcomes for paclitaxel (table 2). The distribution of TcellinfGEP scores trended higher in responders (n=16) than nonresponders (n=121) for pembrolizumab, but no difference was observed between responders (n=20) and nonresponders (n=117) for paclitaxel (figure 1A). The area under the receiver operating characteristics curve for discriminating TcellinfGEP as a predictor of objective response was 0.64 (95% CI 0.50 to 0.78) for pembrolizumab and 0.50 (95% CI 0.37 to 0.62) for paclitaxel (figure 1B).
Table 2.
Association p values of gene expression signatures with clinical outcomes
| Pembrolizumab n=137 |
Paclitaxel n=137 |
|||||
| ORR | PFS | OS | ORR | PFS | OS | |
| TcellinfGEP* | 0.041 | 0.026 | 0.178 | 0.822 | 0.207 | 0.644 |
| Adjusted for TcellinfGEP† | ||||||
| Angiogenesis | 0.077‡ | 0.298 | 0.623 | 0.220 | 0.462 | 0.263 |
| Glycolysis | 0.934 | 0.861 | 0.956 | 0.223 | 0.097‡ | 0.018‡ |
| gMDSC | 0.626 | 0.573 | 0.623 | 0.694 | 0.352 | 0.263 |
| Hypoxia | 0.934 | 0.861 | 0.956 | 0.223 | 0.220 | 0.440 |
| mMDSC | 0.077‡ | 0.057‡ | 0.033‡ | 0.694 | 0.932 | 0.263 |
| MYC | 0.934 | 0.861 | 0.956 | 0.223 | 0.327 | 0.057‡ |
| Proliferation | 0.934 | 0.861 | 0.956 | 0.223 | 0.071‡ | 0.002‡ |
| RAS | 0.934 | 0.861 | 0.956 | 0.223 | 0.327 | 0.440 |
| Stroma/EMT/TGFβ | 0.306 | 0.366 | 0.522 | 0.694 | 0.462 | 0.263 |
| WNT | 0.934 | 0.861 | 0.956 | 0.097‡ | 0.327 | 0.440 |
All models include additional covariates of ECOG performance status.
*Bolded p values (one sided for pembrolizumab with hypothesized positive association and two sided for paclitaxel with no hypothesized association) indicate nominal statistical significance (α=0.05); model includes additional covariates of ECOG performance status.
†Bolded p values (one sided for pembrolizumab with hypothesized negative association except for proliferation with hypothesized positive association and two sided for paclitaxel with no hypothesized association) indicate multiplicity-adjusted statistical significance (α=0.10); model includes additional covariates of ECOG performance status and TcellinfGEP.
‡Negative association observed.
ECOG, Eastern Cooperative Oncology Group; EMT, epithelial-to-mesenchymal transition; gMDSC, granulocytic myeloid-derived suppressor cells; mMDSC, monocytic MDSC; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; TcellinfGEP, T-cell-inflamed gene expression profile.
Figure 1.
Association between TcellinfGEP and response. (A) Response for TcellinfGEP in the pembrolizumab and paclitaxel groups. (B) Receiver operating characteristics curve for sensitivity and specificity. AUROC, area under the receiver operating characteristics curve; CR, complete response; PR, partial response; TcellinfGEP, T-cell-inflamed gene expression profile.
After adjusting for the TcellinfGEP, the angiogenesis and mMDSC signatures were negatively associated with outcomes with pembrolizumab (one-sided multiplicity-adjusted p values for angiogenesis: ORR 0.077; mMDSC: ORR 0.077; PFS 0.057; OS 0.033); none of the remaining signatures were statistically significantly associated with outcomes for pembrolizumab after multiplicity adjustment (table 1). After adjusting for the TcellinfGEP, the glycolysis, MYC, proliferation, and WNT signatures were associated with negative outcomes with paclitaxel (two-sided multiplicity-adjusted p values for glycolysis: PFS 0.097; OS, 0.018; MYC: OS 0.057; proliferation, PFS 0.071; OS 0.002; WNT: ORR 0.097; table 2).
Efficacy estimates by TcellinfGEP
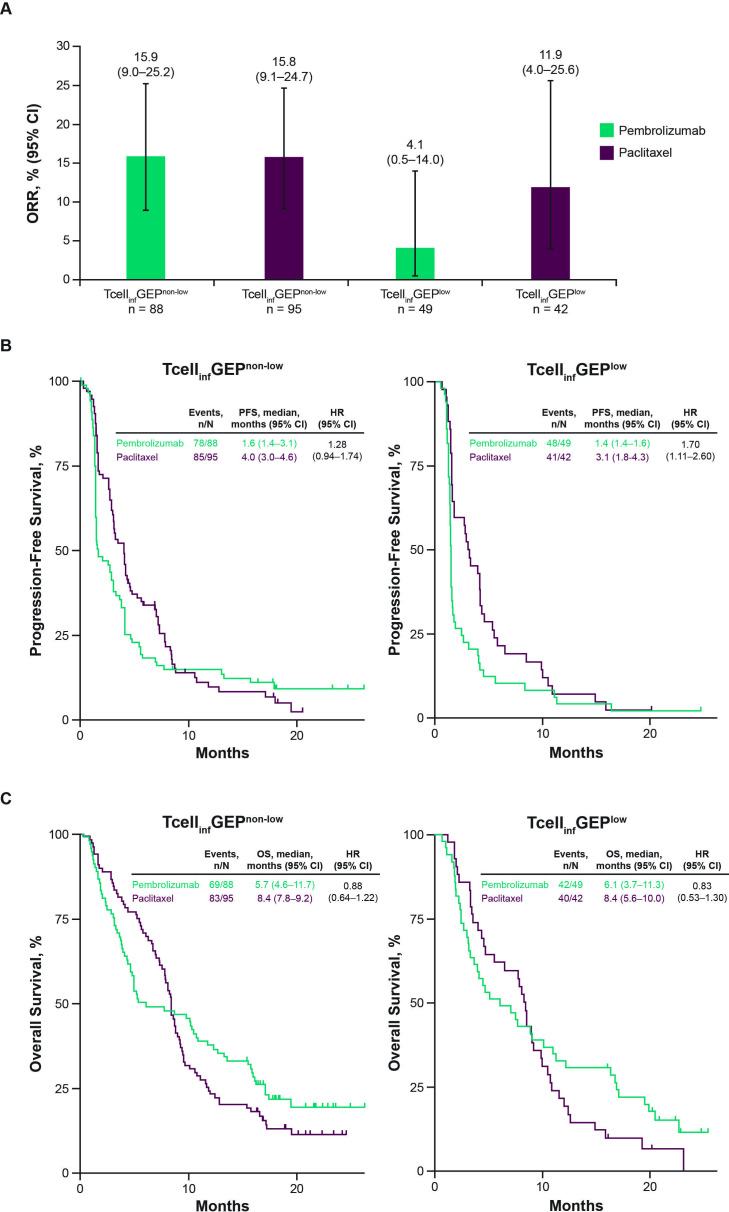
We assessed the clinical utility of the TcellinfGEP using a prespecified cut-off of the first tertile (TcellinfGEPnonlow, n=183; TcellinfGEPlow, n=91). ORRs were similar between pembrolizumab and paclitaxel in the TcellinfGEPnonlow subgroup (15.9% (95% CI 9.0% to 25.2%) vs 15.8% (95% CI 9.1% to 24.7%), respectively) and numerically lower with pembrolizumab versus paclitaxel in the TcellinfGEPlow subgroup (4.1% (95% CI 0.5% to 14.0%) vs 11.9% (95% CI 4.0% to 25.6%), respectively; figure 2A). The HR of pembrolizumab versus paclitaxel for PFS was lower in the TcellinfGEPnonlow subgroup compared with the TcellinfGEPlow subgroup (1.28 (95% CI 0.94 to 1.74) vs 1.70 (95% CI 1.11 to 2.60); figure 2B) and the HR of pembrolizumab versus paclitaxel for OS was similar between the TcellinfGEPnonlow and TcellinfGEPlow subgroups (0.88 (95% CI 0.64 to 1.22) vs 0.83 (95% CI 0.53 to 1.30); figure 2C). When OS was evaluated by dual TcellinfGEP and TMB cutoffs, survival probability was generally higher in patients with TcellinfGEPnonlow and TMBhigh tumors compared with other subgroups based on the dual TcellinfGEP and TMB cutoffs with pembrolizumab; OS probability was similar across subgroups based on dual TcellinfGEP and TMB cutoffs with paclitaxel (online supplemental figure 1).
Figure 2.
Efficacy of pembrolizumab versus paclitaxel by TcellinfGEP cutoff. (A) Objective response rate. (B) Progression-free survival. (C) Overall survival. ORR, objective response rate; OS, overall survival; PFS, progression-free survival; TcellinfGEP, T-cell-inflamed gene expression profile.
jitc-2023-006920supp001.pdf (794.9KB, pdf)
Efficacy estimates by selected non-TcellinfGEP signatures
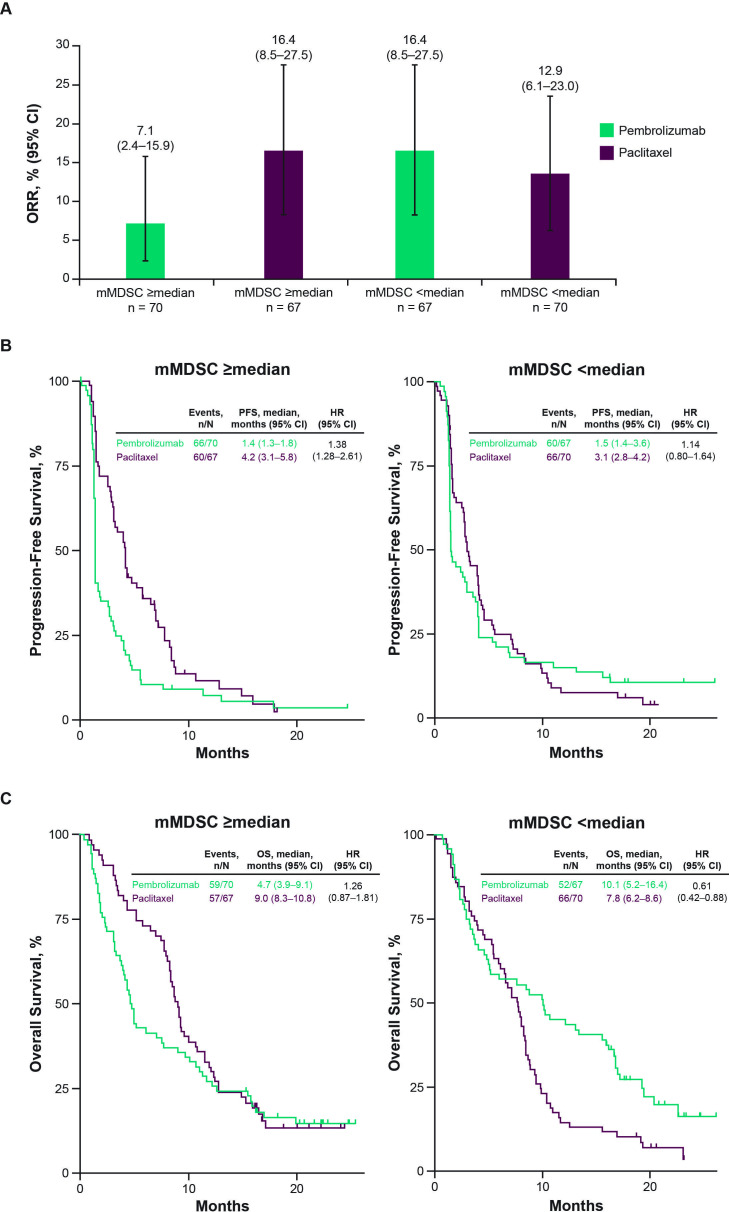
For illustrative purposes, we also evaluated the four non-TcellinfGEP signatures (mMDSC, glycolysis, proliferation, and MYC), which showed an association with OS for pembrolizumab or paclitaxel when analyzed as a continuous variable, using a prespecified cut-off of the signature specific, TcellinfGEP-adjusted median. When TcellinfGEP-adjusted mMDSC was evaluated using a prespecified cut-off of the median (mMDSC≥median, n=137; mMDSC<median, n=137), ORR was numerically lower for pembrolizumab versus paclitaxel (7.1% (95% CI 2.4% to 15.9%) vs 16.4% (95% CI 8.5% to 27.5%)), respectively) in the mMDSC≥median subgroup and higher with pembrolizumab versus paclitaxel in the mMDSC<median subgroup (16.4% (95% CI 8.5% to 27.5) vs 12.9% (95% CI 6.1% to 23.0%), respectively; figure 3A). The PFS and OS HRs for pembrolizumab versus paclitaxel were lower in the mMDSC<median subgroup compared with the mMDSC≥median subgroup (PFS: 1.14 (95% CI 0.80 to 1.64) vs 1.83 (95% CI 1.28 to 2.61); OS: 0.61 (95% CI 0.42 to 0.88) vs 1.26 (95% CI 0.87 to 1.81), figure 3B, C). When the evaluation of the TcellinfGEP-adjusted mMDSC signatures was restricted to patients with non-MSI-H tumors (mMDSC≥median, n=129; mMDSC<median, n=128), the OS HR for pembrolizumab versus paclitaxel was lower in the mMDSC<median subgroup versus the mMDSC≥median subgroup (0.66 (95% CI 0.45 to 0.96) vs 1.44 (95% CI 0.99 to 2.10), respectively; online supplemental figure 2), consistent with the MSI-H inclusive results.
Figure 3.
Efficacy of pembrolizumab versus paclitaxel by mMDSC cut-off after adjusting for TcellinfGEP. (A) Objective response rate. (B) Progression-free survival. (C) Overall survival. mMDSC, monocytic myeloid-derived suppressor cells; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; TcellinfGEP, T-cell-inflamed gene expression profile.
For the TcellinfGEP-adjusted glycolysis signature, the OS HR for pembrolizumab versus paclitaxel was lower in the glycolysis≥median subgroups compared with the <median subgroup (0.58 (95% CI 0.40 to 0.85) vs 1.24 (95% CI 0.86 to 1.81), respectively; online supplemental figure 3A).
For the TcellinfGEP-adjusted proliferation and MYC signatures, OS HRs for pembrolizumab versus paclitaxel were lower in the proliferation and MYC≥median subgroups compared with the respective <median subgroups (proliferation: 0.63 (95% CI 0.43 to 0.91) vs 1.12 (95% CI 0.77 to 1.63), respectively, and MYC: 0.63 (95% CI 0.43 to 0.92) vs 1.17 (95% CI 0.81 to 1.70), respectively; online supplemental figure 3B and 3C).
Discussion
In this exploratory analysis of patients with previously treated advanced G/GEJ cancer from the KEYNOTE-061 trial, the tumor TcellinfGEP as a continuous variable showed some associations with clinical outcomes for pembrolizumab (ORR and PFS) and no associations with clinical outcomes for paclitaxel. For pembrolizumab, negative associations were observed between the TcellinfGEP-adjusted mMDSC signature as a continuous variable and all three clinical outcomes (ORR, PFS, and OS), and between the TcellinfGEP-adjusted angiogenesis signature as a continuous variable and ORR. For paclitaxel, the TcellinfGEP-adjusted glycolysis, MYC, and proliferation signatures as continuous variables were negatively associated with OS. Evaluation of the efficacy estimates of selected non-TcellinfGEP signatures, adjusted for TcellinfGEP, by prespecified cutoffs of the median showed improved OS HRs of pembrolizumab versus paclitaxel for the mMDSC<median, proliferation≥median, and MYC≥median subgroups compared with their respective alternative subgroups.
The significant positive association between TcellinfGEP and ORR with pembrolizumab is consistent with findings in the pan-tumor setting12 15 16 and suggests that TcellinfGEP may predict response to second-line pembrolizumab in patients with advanced G/GEJ cancer. Such a positive association between the TcellinfGEP and response to pembrolizumab could be expected given that the TcellinfGEP includes RNA expressions for PD-L1 (CD274 13), whose expression has been widely shown to correlate with response to anti-PD-1/L1 therapy in several tumors.27 A moderate but statistically significant correlation has also been demonstrated between the TcellinfGEP and PD-L1 CPS (via immunohistochemistry) in a pan-tumor dataset (Spearman ρ=0.40; p<0.001)15; however, the TcellinfGEP and PD-L1 CPS are regarded as nonequivalent biomarkers.12 Conversely, the lack of significant associations between TcellinfGEP as a continuous variable and clinical outcomes for paclitaxel is consistent with a previous report22 and suggests that the TcellinfGEP may not be a predictor of a patient’s response to systemic chemotherapy.
Similar to results obtained in the pan-tumor setting,16 our evaluation of the clinical utility of the TcellinfGEP in predicting response to pembrolizumab showed relatively higher response rates in the TcellinfGEPnonlow subgroup versus the TcellinfGEPlow subgroup, suggesting that the TcellinfGEP signature may be better suited in predicting response to second-line pembrolizumab in patients with advanced G/GEJ cancer with a TcellinfGEP score of at least or greater than the first tertile versus less than the first tertile, although formal testing is needed for verification. In contrast, the minimal difference in ORR between the TcellinfGEP cut-off subgroups for paclitaxel suggests a low sensitivity of the TcellinfGEP cut-off to predict response to paclitaxel consistent with testing results. Although pembrolizumab did not show PFS benefits versus paclitaxel in both TcellinfGEP subgroups, the HR for PFS was lower in the TcellinfGEPnonlow subgroup compared with the TcellinfGEPlow subgroup suggesting that TcellinfGEPnon-low may have clinical utility in this setting. We speculate that such clinical utility of the TcellinfGEP may be best when used in conjunction with other biomarkers such as TMB, which has been suggested to be a significant predictor of response to pembrolizumab in this patient population.1 In this regard, our subgroup analysis based on dual TcellinfGEP and TMB status showed highest OS probability in the subgroup of patients with TcellinfGEPnonlow and TMBhigh tumors.
Of all the non-TcellinfGEP signatures evaluated in this study, only the TcellinfGEP-adjusted mMDSC signature showed significant negative associations with all three clinical outcomes (ORR, PFS, and OS) of pembrolizumab treatment. This observation suggests that the mMDSC signature may be a strong negative predictor of clinical outcomes of pembrolizumab. Consistent with our findings, a negative association between TcellinfGEP-adjusted mMDSC signature and response to pembrolizumab has been observed in the pan-tumor setting.12 MDSCs are known to be associated with antigen-specific tolerance and suppression of T-cell response in the TME7 28; thus, the observed negative associations between the mMDSC signature and clinical outcomes suggests that myeloid-driven suppression may play a role in resistance to PD-1/L1 inhibition. Illustration of the potential clinical utility of the mMDSC signature for pembrolizumab treatment showed higher ORR and an OS benefit versus paclitaxel in the TcellinfGEP-adjusted mMDSC<median subgroup than the mMDSC≥median subgroup, with the OS benefit observed in the mMDSC<median subgroup robust to the exclusion of patients with MSI-H tumors. Conversely, trends in ORR and median OS between the TcellinfGEP-adjusted mMDSC subgroups for paclitaxel treatment suggest poor discriminatory potential of the mMDSC signature for paclitaxel in this setting consistent with testing results. Altogether, observations for the mMDSC signature for pembrolizumab suggest that immunotherapy targeting the myeloid axis may be an effective antitumor strategy to overcome anti-PD-1/L1 resistance across many tumor types, including advanced G/GEJ cancer. Such a strategy is currently being explored in a phase 1 trial (ClinicalTrials.gov, NCT03918278) of a novel humanized immunoglobulin G4 monoclonal antibody MK-0482 targeting the immunoglobulin-like transcript 3 receptor on MDSCs or tolerogenic dendritic cells.29 Preliminary results from this ongoing phase 1 trial have shown modest efficacy with MK-0482 in combination with pembrolizumab in patients with heavily pretreated advanced solid tumors.29
Furthermore, the negative association observed between the TcellinfGEP-adjusted angiogenesis signature as a continuous variable and ORR for pembrolizumab also supports the rationale for considering immunotherapy combinations that target the angiogenesis axis. At present, the LEAP program is being conducted to evaluate the safety and efficacy of the multikinase inhibitor lenvatinib in combination with pembrolizumab across several advanced solid tumors.30 In the population with advanced gastric cancer, the randomized phase 3 LEAP-015 trial (ClinicalTrials.gov, NCT04662710) is being conducted to evaluate the safety and efficacy of lenvatinib in combination with pembrolizumab and chemotherapy (CAPOX or mFOLFOX) as first-line therapy in patients with advanced/metastatic gastroesophageal adenocarcinoma.31 Preliminary results from the safety run-in phase of LEAP-015 have demonstrated antitumor activity.
Limitations of the prespecified exploratory analysis from the KEYNOTE-061 trial reported here include the small sample sizes of the subgroups and lack of statistical power which hinders making definitive conclusions. Additionally, the low proportion of patients who responded to pembrolizumab (11.7%) or paclitaxel (14.7%) in this RNA sequencing population may be another limitation. Last, paclitaxel was used as the comparator arm in this analysis because it was the standard of care at the time the KEYNOTE-061 trial was designed and approved. Since then, combination therapy with the vascular endothelial growth factor receptor 2 inhibitor, ramucirumab, plus paclitaxel is now the approved standard-of-care second-line therapy for patients with advanced G/GEJ in many countries.32
In conclusion, this exploratory analysis from the phase 3 KEYNOTE-061 trial showed that tumor TcellinfGEP as a continuous variable was associated with clinical outcomes with second-line pembrolizumab in advanced G/GEJ cancer. The TcellinfGEP-adjusted mMDSC signature as a continuous variable was negatively associated with clinical outcomes with pembrolizumab. This analysis suggests that myeloid-driven suppression may play a role in resistance to anti-PD-1 therapy and supports a strategy of considering immunotherapy combinations intended to target the myeloid axis.
Acknowledgments
The authors thank the patients and their families and caregivers and the primary investigators and site personnel for participating in the trial. Medical writing and/or editorial assistance was provided by Obinna T. Ezeokoli, PhD, Lauren D’Angelo, PhD, and Holly C. Cappelli, PhD, CMPP, of ApotheCom (Yardley, Pennsylvania, USA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, New Jersey, USA.
Footnotes
Contributors: Conceptualization of study: KS, JK, RC, ZAC and AL. Data curation: KS, CC, HCC, KM, MN and RC. Formal analysis: CC, JK, MN and RC. Investigations: MM, M-HR, CC, TO, HCC, EG, RSM, ZW, C-SS, MN and MÖ. Methodology: JK, RC, ZAC and AL. Project administration: RC. Resources: KS, MM, HCC and RC. Software: MN. Supervision: KS, HCC, ZAC, AL and MÖ. Validation: CC, HCC and ZW. Visualization: MN. Writing—original draft: KS, CC and JK. Writing—review and editing: KS, MDB, MM, M-HR, CC, TO, HCC, KM, EG, RSM, WM, ZW, C-SS, JK, MN, RC, ZAC, AL and MÖ. Approval to submit for publication: all authors. KS accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This work was supported by Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc., Rahway, New Jersey, USA. The study sponsor, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, New Jersey, USA, funded the study and designed the study protocol.
Competing interests: KS: reports receiving research funding paid to his institution from Astellas Pharma, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharma, MSD, Amgen, Eisai, and Medi Science; consulting fees for advisory role from Eli Lilly and Company, Bristol Myers Squibb, Takeda Pharmaceuticals, Pfizer, Ono Pharmaceutical, Novartis, AbbVie, Daiichi Sankyo, Taiho Pharmaceutical, GlaxoSmithKline, Amgen, Boehringer Ingelheim, MSD, Astellas, Guardant Health Japan, and Janssen; and honoraria for lectures from Bristol Myers Squibb, Takeda Pharmaceuticals, and Janssen. MDB: reports participation on a data safety monitoring board/advisory board for MDS. MM: reports no disclosures. M-HR: reports receiving consulting fees from Ono Pharmaceutical, Bristol Myers Squibb, MSD, Eli Lilly, Taiho, Novartis, AstraZeneca, and Daiichi Sankyo. CC: reports receiving payment or honoraria from MSD and Roche, and other financial or nonfinancial interests paid to his institution for participating as a principal investigator or subinvestigator for MSD, Medivation, AstraZeneca, Roche, Astellas Pharma, Bristol Myers Squibb, GlaxoSmithKline, Athenex, Daiichi Sankyo, and Sanofi. TO: reports no disclosures. HCC: reports receiving study material and medical writing support for the present work from Merck; grants or contracts paid to his institution by Eli Lilly, GSK, MSD, Merck Serono, Bristol Myers Squibb/Ono Pharmaceutical, Taiho, Amgen, BeiGene, Incyte, and Zymeworks; consulting fees from Taiho, Celltrion Healthcare, MSD, Eli Lilly, Bristol Myers Squibb/Ono Pharmaceutical, Merck-Serono, Beigene/Amgen, and Zymeworks; and payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Merck Serono and Eli Lilly; and participation on a data safety monitoring board or advisory board for MSD. KM: reports receiving research funding paid to his institution from Solasia Pharma, Merck Serono, Daiichi Sankyo, Parexel International, Pfizer, MSD, Amgen, Ono Pharmaceutical, Astellas, Sanofi, Taiho, and Eisai; consulting fees from AstraZeneca, Ono Pharmaceutical, and Amgen; honoraria for lectures from Ono Pharmaceutical, Taiho, Bristol Myers Squibb, and Eli Lilly; and participation on an advisory board for Ono Pharmaceutical, Amgen, AstraZeneca, Eli Lilly, and Takeda. EG: reports no disclosures. RSM: reports no disclosures. WM: reports no disclosures. ZW: reports receiving support for the present work from Merck; grants paid to his institution from Novartis, Bristol Myers Squibb, Plexxikon, and Arcus; consulting fees from AstraZeneca, Arcus, Amgen, Bristol Myers Squibb, Novartis, Eli Lilly, Bayer, Daiichi, Merck, and Astellas; support for attending meetings and/or travel from Amgen; and participation on a data safety monitoring board or advisory board for Mirati and Pfizer. C-SS: employee of Merck Sharp & Dohme, a subsidiary of Merck & Co, Rahway, New Jersey, USA. JK: employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, New Jersey, USA, and has stock in Merck & Co., Inc., Rahway, NJ, USA. MN: employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, New Jersey, USA, and has stock in Merck & Co., Inc., Rahway, NJ, USA. RC: employee of Merck Sharp & Dohme, a subsidiary of Merck & Co., Rahway, New Jersey, USA, and has stock in Merck & Co., Rahway, New Jersey, USA; and reports a pending patent (WO 2020/167619) related to the application of Angiogenesis and mMDSC gene expression-based biomarker of tumor response to PD-1 antagonists. ZAC: employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, New Jersey, USA, and has stock in Merck & Co., Inc., Rahway, NJ, USA. AL: employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, New Jersey, USA, and has stock in Merck & Co., Inc., Rahway, NJ, USA. MÖ: reports no disclosures.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the USA and the European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The trial protocol and all amendments were approved by the institutional review board or ethics committee at each participating institution. The name of each ethics committee/institutional review board at each participating center including approval numbers are shown in online supplemental table 1. The trial was conducted in accordance with the protocol, its amendments, the ethical principles originating from the Declaration of Helsinki, and Good Clinical Practice guidelines. Written informed consent was provided by all patients before enrolment.
References
- 1. Shitara K, Özgüroğlu M, Bang Y-J, et al. Molecular determinants of clinical outcomes with pembrolizumab versus paclitaxel in a randomized, open-label, phase III trial in patients with gastroesophageal adenocarcinoma. Ann Oncol 2021;32:1127–36. 10.1016/j.annonc.2021.05.803 [DOI] [PubMed] [Google Scholar]
- 2. Hsu A, Raufi AG. Advances in systemic therapy for gastric cancer. Gastrointest Endosc Clin N Am 2021;31:607–23. 10.1016/j.giec.2021.03.009 [DOI] [PubMed] [Google Scholar]
- 3. Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:1005–20. 10.1016/j.annonc.2022.07.004 [DOI] [PubMed] [Google Scholar]
- 4. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 5. American Cancer Society . Stomach cancer survival rates. 2022. Available: https://www.cancer.org/cancer/stomach-cancer/detection-diagnosis-staging/survival-rates.html [Accessed 22 Sep 2022].
- 6. Fares CM, Van Allen EM, Drake CG, et al. Mechanisms of resistance to immune checkpoint blockade: why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ Book 2019;39:147–64. 10.1200/EDBK_240837 [DOI] [PubMed] [Google Scholar]
- 7. Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol 2018;19:108–19. 10.1038/s41590-017-0022-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018;24:1449–58. 10.1038/s41591-018-0101-z [DOI] [PubMed] [Google Scholar]
- 9. KEYTRUDA® (Pembrolizumab) injection, for intravenous use. 03/2022 . Merck Sharp & Dohme Corp. Rahway, NJ, USA, [Google Scholar]
- 10. Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med 2019;143:330–7. 10.5858/arpa.2018-0043-OA [DOI] [PubMed] [Google Scholar]
- 11. Chao J, Fuchs CS, Shitara K, et al. Assessment of Pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol 2021;7:895–902. 10.1001/jamaoncol.2021.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cristescu R, Nebozhyn M, Zhang C, et al. Transcriptomic determinants of response to Pembrolizumab monotherapy across solid tumor types. Clin Cancer Res 2022;28:1680–9. 10.1158/1078-0432.CCR-21-3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ayers M, Lunceford J, Nebozhyn M, et al. IFN-Γ–related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127:2930–40. 10.1172/JCI91190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ayers M, Nebozhyn M, Cristescu R, et al. Molecular profiling of cohorts of tumor samples to guide clinical development of Pembrolizumab as monotherapy. Clin Cancer Res 2019;25:1564–73. 10.1158/1078-0432.CCR-18-1316 [DOI] [PubMed] [Google Scholar]
- 15. Ott PA, Bang Y-J, Piha-Paul SA, et al. T-cell-inflamed Gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with Pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol 2019;37:318–27. 10.1200/JCO.2018.78.2276 [DOI] [PubMed] [Google Scholar]
- 16. Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018;362:eaar3593. 10.1126/science.aar3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fuchs CS, Özgüroğlu M, Bang Y-J, et al. Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer 2022;25:197–206. 10.1007/s10120-021-01227-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shitara K, Özgüroğlu M, Bang Y-J, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123–33. 10.1016/S0140-6736(18)31257-1 [DOI] [PubMed] [Google Scholar]
- 19. Hu J, Ge H, Newman M, et al. OSA: a fast and accurate alignment tool for RNA-Seq. Bioinformatics 2012;28:1933–4. 10.1093/bioinformatics/bts294 [DOI] [PubMed] [Google Scholar]
- 20. Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 2011;12:323. 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cristescu R, Aurora-Garg D, Albright A, et al. Tumor mutational burden predicts the efficacy of pembrolizumab monotherapy: a pan-tumor retrospective analysis of participants with advanced solid tumors. J Immunother Cancer 2022;10:e003091. 10.1136/jitc-2021-003091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bellmunt J, de Wit R, Fradet Y, et al. Putative biomarkers of clinical benefit with pembrolizumab in advanced urothelial cancer: results from the KEYNOTE-045 and KEYNOTE-052 landmark trials. Clin Cancer Res 2022;28:2050–60. 10.1158/1078-0432.CCR-21-3089 [DOI] [PubMed] [Google Scholar]
- 23. Haddad RI, Seiwert TY, Chow LQM, et al. Influence of tumor mutational burden, inflammatory gene expression profile, and PD-L1 expression on response to Pembrolizumab in head and neck squamous cell carcinoma. J Immunother Cancer 2022;10:e003026. 10.1136/jitc-2021-003026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aurora-Garg D, Albright A, Qiu P, et al. Large-scale evaluation of Concordance of Genomic scores in whole Exome sequencing and foundation medicine comprehensive Genomic platform across cancer types. J Immunother Cancer 2019;7:172. 10.1186/s40425-019-0763-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herbst RS, Lopes G, Kowalski DM, et al. Association between tissue TMB (tTMB) and clinical outcomes with Pembrolizumab monotherapy (Pembro) in PD-L1-positive advanced NSCLC in the KEYNOTE-010 and -042 trials. Annals of Oncology 2019;30:v916–7. 10.1093/annonc/mdz394.077 [DOI] [Google Scholar]
- 26. Paz-Ares L, Langer CJ, Novello S, et al. Pembrolizumab (Pembro) plus platinum-based chemotherapy (Chemo) for metastatic NSCLC: tissue TMB (tTMB) and outcomes in KEYNOTE-021, 189, and 407. Annals of Oncology 2019;30:v917–8. 10.1093/annonc/mdz394.078 [DOI] [Google Scholar]
- 27. Yi M, Jiao D, Xu H, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer 2018;17:129. 10.1186/s12943-018-0864-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh L, Muise ES, Bhattacharya A, et al. ILT3 (Lilrb4) promotes the immunosuppressive function of tumor-educated human monocytic myeloid-derived suppressor cells. Mol Cancer Res 2021;19:702–16. 10.1158/1541-7786.MCR-20-0622 [DOI] [PubMed] [Google Scholar]
- 29. Gutierrez M, Spreafico A, Wang D, et al. Phase 1 first-in-human study of anti–ILT3 mAb MK-0482 as monotherapy and in combination with Pembrolizumab in advanced solid tumors: dose escalation results. JCO 2022;40:2505. 10.1200/JCO.2022.40.16_suppl.2505 [DOI] [Google Scholar]
- 30. Taylor MH, Schmidt EV, Dutcus C, et al. The LEAP program: Lenvatinib plus Pembrolizumab for the treatment of advanced solid tumors. Future Oncol 2021;17:637–48. 10.2217/fon-2020-0937 [DOI] [PubMed] [Google Scholar]
- 31. Cohen DJ, Tabernero J, Van Cutsem E, et al. A randomized phase 3 study evaluating the efficacy and safety of first-line Pembrolizumab plus Lenvatinib plus chemotherapy versus chemotherapy in patients with advanced/metastatic gastroesophageal adenocarcinoma: LEAP-015. JCO 2022;40:TPS369. 10.1200/JCO.2022.40.4_suppl.TPS369 [DOI] [Google Scholar]
- 32. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35. 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-006920supp001.pdf (794.9KB, pdf)
Data Availability Statement
Data are available on reasonable request. Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the USA and the European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.