Abstract
Objective
Restrictive diets, forced starvation or voluntary weight loss are attracting more and more attention from scientists. Overall trends show that about 80% of combat sports athletes use specific methods of reducing body mass. Rapid weight loss could be a risk factor for kidney-related adverse outcomes. This study aimed to examine the impact of high-intensity specific training combined with rapid weight loss in the first and without rapid weight loss in the second phases on body composition and biochemical markers of kidney function.
Methods
The study was conducted on 12 male wrestlers. Kidney function markers were measured, including blood urea nitrogen, serum creatinine, uric acid and serum Cystatin-C. Alterations in analysed markers were noted in both phases of the research.
Results
According to the data, a significant increase was noted in blood urea nitrogen (p=0.002), uric acid (p=0.000) and serum creatinine (p=0.006) during the first phase in comparison with the second phase. The levels of serum Cystatin-C were slightly elevated after both phases compared with the initial measurement.
Conclusion
It is evident that high-intensity specific training combined with rapid weight loss significantly affects the increase in kidney function markers compared with identical training without rapid weight loss. The findings in this study suggest that rapid body mass reduction is associated with an increased risk of acute kidney injury in wrestlers.
Keywords: dehydration, martial arts, eating disorders
WHAT IS ALREADY KNOWN ON THIS TOPIC
Fluid restriction is considered one of the most commonly used methods to reduce body mass in weight-classified sports by competitors.
WHAT THIS STUDY ADDS
High-intensity sport-specific training accompanied by rapid weight reduction increases the risk of acute kidney injury in combat sports athletes.
There is evidence that a certain percentage of body mass reduction affects an alteration in kidney function biomarkers.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The knowledge provided in this research should encourage combat sports athletes to carefully approach the weight reduction process as it could negatively affect the individual’s health.
Introduction
The benefits of weight loss through lifestyle interventions are well documented regarding health outcomes. However, kidney alterations induced by rapid weight loss (RWL) are still incompletely understood. Previous studies have reported that body mass fluctuation is associated with an increased risk of rapid kidney function decline in participants with normal kidney function.1 Many weight-class athletes try to lose weight rapidly before a competition.2 RWL is described as a rapid weight reduction over a short period. Abrupt reduction of body mass refers to methods an athlete uses to reduce body mass in the last week before a competition, with an average loss of 2%–10%.3–6 RWL methods are generally intended to give the athlete a certain advantage, but there are also potential negative aspects of losing weight rapidly. It was documented that RWL practice negatively impacts athletes' health, with noted dead cases as a consequence of RWL.7 8 Still, evidence shows that around 80% of combat sports athletes use specific methods of reducing body mass.9–15 Usually, RWL techniques involve more intensive exercise, using a sauna and rubber or plastic suits, reducing energy intake, fasting, laxatives and primarily dehydration.16–18
The kidney is an organ that can tolerate exposure to various factors, whereby a predisposing factor such as dehydration may represent an additional risk for the development of acute kidney injury (AKI). Although dehydration was thought not to be associated with long-term adverse effects on kidney function, this opinion has been disproved.19 The possible factor causing acute kidney failure is dehydration, and even mild dehydration can be a risk factor in the progression of kidney disease. Since athletes in combat sports reduce their body mass up to 10 times yearly,20 frequent dehydration can affect kidney function. There is no evidence that sport practising can lead to chronic kidney problems. However, early detection of AKI in athletes who regularly use RWL methods is of utmost importance for preventing more severe kidney disease. Dehydration and soft drink intake during and following exercise may lead to acute kidney dysfunction.21 Also, repeated episodes of acute kidney failure may lead to chronic kidney disease.22 Several classification systems have been developed to streamline research and clinical practice concerning AKI.23 Changes in serum creatinine (CRE) levels, blood urea nitrogen (BUN), uric acid (URCA) and Cystatin-C (CysC) levels are indicators of kidney function.24 However, CRE and BUN are influenced by many renal and nonrenal factors independent of kidney function.25 26 Unlike CRE and BUN, CysC levels are much less influenced by factors such as gender, age and muscle mass.27–29 CysC is a suitable marker for assessing kidney functions during and after exercise.30 Elevating URCA levels are also associated with strenuous exercise.31 32
Athletes are usually healthy by definition, but the combination of RWL and high training workload may modify their homeostasis, inducing pathological biochemical kidney marker values. This study examined the impact of high-intensity specific training combined with RWL and without RWL on body composition and biochemical markers of kidney function.
Methods
Study population
Twelve male Greco-Roman wrestlers (mean body mass 73.48±4.52 kg, mean age 24.3 ± 5.1 years, mean height 175.22±3.68 cm) participated in this study. The sample size was calculated using the power analysis (effect size 0.4, α 0.05, power 0.80) for the primary outcome measures (G-Power 3, Heinrich-Heine-Universität Düsseldorf, Germany). All participants were currently active athletes with at least 5 years of competitive experience and at least 10 hours of practising sport per week. Only those who had already implemented RWL techniques over the last 2 years were selected for the study.
Study design
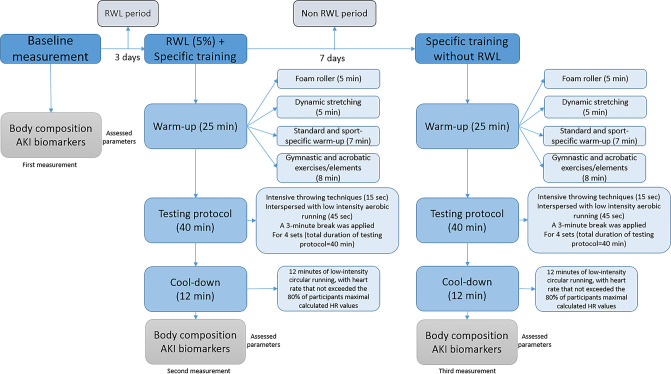
This open-label, repeated-measure pilot study was designed to investigate the impact of high-intensity specific training combined with RWL and without RWL on body composition and AKI markers. After the initial measurement (IM), the experiment consisted of two phases (figure 1). The first phase (P1) of the research was conducted with the implementation of high-intensity sport-specific training (HISST) with a 5% reduction in body mass. In the above-mentioned phase of the experiment, lasting 3 days, the subjects had the usual training and independently applied different methods of reducing body mass. All participants used methods such as increased physical activity, plastic suit training, caloric deficit, reduced fluid intake and sauna to lose weight rapidly. On the last day of the first phase (day 3), all athletes performed a HISST in the morning, followed by a second measurement (P1). The second phase of the experimental treatment was conducted 7 days after the P1 and included the same athletes who participated in the first phase. The research’s second phase (P2) was carried out with an identical training protocol without the mandatory reduction of body mass. This phase consists of a single training episode (HISST) without RWL. Testing and blood sampling were conducted immediately after HISST in the morning.
Figure 1.
Flow chart of the study procedure. AKI, acute kidney injury; RWL, rapid weight loss.
The experiment took place in the sports hall of the wrestling club. Both HISST protocol and weight loss procedures were supervised by two experienced wrestling coaches who had been training the athletes (respondents) for 5 years at the time of the experiment, along with three senior researchers with expertise in combat sports, who conducted weight measurements and body composition assessments in all phases. Blood sampling was performed by trained laboratory personnel.
Anthropometric measures
Body composition characteristics were measured before and after the first and second phases simultaneously during each study day (08:00 local time.) Body mass, body fat percentage, amount of fat mass and muscle mass were measured using the bioelectrical impedance (Omron 511BF, OMRON HEALTHCARE, Kyoto, Japan). Participants’ body mass was measured to the nearest 0.1 kg. Subjects were instructed not to eat after 21:00 local time.
Blood sample collection
Venous blood was collected from the antecubital vein in a fasted state before and after each phase. Biochemical parameters were determined routinely using standard methods. Commercially available routine assays were used to determine BUN, URCA and CRE (Sysmex America, Lincolnshire, Illinois, USA). Serum CysC was measured with a Particle-Enhanced Turbidimetric Immunoassay for in vitro diagnostic testing of CysC in human plasma and serum samples.
High-intensity sport-specific training
The total duration of training (test protocol) was 90 min. Before performing specific training, all subjects warmed up. The total duration of the warm-up was 25 min. After a warm-up, working in pairs, the subjects started a specific training session consisting of four series of the maximum number of throws. Each set lasted 10 min with a ratio of work (throwing) lasting 15 s and low-intensity jogging lasting 45 s. The subjects had a break of 3 min between each set. Wrestlers performed arm throw as a throwing technique for this test. The test started with a low-intensity circular run (45 s). Five seconds before the throwing part, the subject was instructed to take place at a point 9 m away from the sparring partner and prepares to perform the throw. At the sound signal of the coach, the athlete runs to the sparring partner as fast as possible and performs the throwing technique. Immediately after the throw, he had to return to the starting point of 9 m. The subject performs this cycle continuously for 15 s, intending to make as many throws as possible. At the end of 15 s, the subject starts a new set of low-intensity running for 45 s. After the subject completes the 10 described sequences, a 3 min break was applied. The main part of the training ends after the athlete has completed four series of intense throwing and jogging. The total duration of this part (test protocol) was 40 min. The cool-down phase consisted of 12 min low-intensity circular running, with heart rate not exceeding 80% of participants’ maximal calculated HR values. A heart rate was monitored (Polar-H10 heart rate sensor, Polar Pro chest strap) from the beginning to the end of the training session for all athletes. The heart rate monitoring was performed using an iPad Pro 10 Polar Team application.
Statistical analysis
Basic descriptive statistics were calculated for all variables, and values were presented as arithmetic mean (M) and SD. Statistical analysis was performed using the one-way RM analysis of variance and post hoc Bonferroni analysis of the Statistical Package for the Social Sciences—IBM SPSS Statistics for Windows, V.20.0 (IBM). Statistical significance level was set at p˂0.05.
Equity, diversity and inclusion statement
Our authors comprised four women and six men, including senior and less experienced researchers from various research areas such as exercise physiology, biomedical science, sports science and sports medicine. Nevertheless, the authors of the research group are from two different countries. The current study included white male junior and senior Greco-Roman wrestlers from different socioeconomic backgrounds. Each respondent performed the experimental protocol equally regardless of educational and socioeconomic differences.
Results
Twelve participants completed and fulfilled the study requirements. Significant differences were observed in the body composition characteristics in both phases. There was a significant decrease in body mass, body mass index (BMI), and body fat compared with the initial measurement. Furthermore, an increase in muscle mass (%) in both phases was observed. Differences are also visible when P1 and P2 values are compared. Body mass, BMI and fat percentage were significantly higher in the second phase, while the percentage of muscle was lower compared with P1.
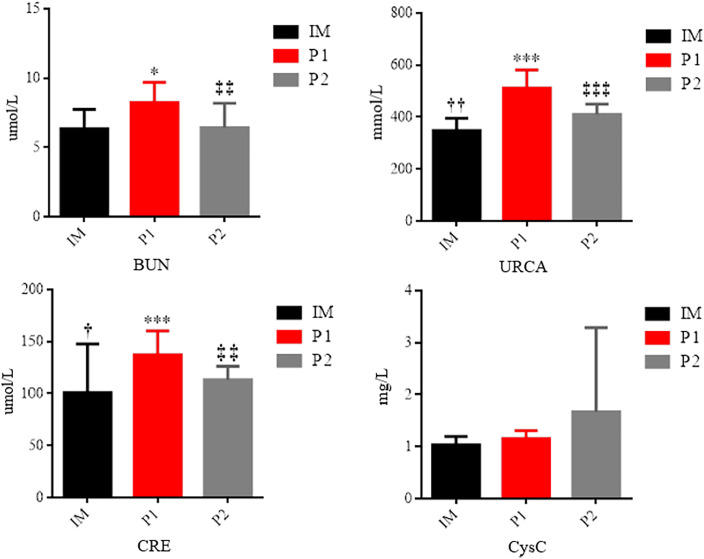
Changes in analysed markers of AKI are presented in figure 2. All analysed markers were elevated during the P1 compared with the initial measurement. The level of BUN (p=0.011), URCA (p=0.000) and creatinine (p=0.001) increased significantly. No significant changes were observed in CysC values during P1, but elevated values in relation to IM are evident. When we compared P2 with IM, significantly higher values were noted in the second phase for URCA (p=0.003) and creatinine (p=0.011). Comparing the second phase with the first, significantly higher values were observed during P1 in the levels of urea (p=0.002), URCA (p=0.000) and creatinine (p=0.006).
Figure 2.
Changes in the analysed markers of AKI. ***Statistically significant difference in relation to the baseline values IM, p≤0.001; *statistically significant difference in relation to the baseline values IM, p≤0.05; ††statistically significant difference in relation to P2, p<0.01; †statistically significant difference in relation to P2, p≤0.05; ‡‡‡statistically significant difference in relation to P1, p≤0.001; ‡‡statistically significant difference in relation to P1, p<0.01. BUN, blood urea nitrogen; CRE, creatinine; CysC, Cystatin C; HISST, high-intensity sport-specific training; IM, initial measurement; P1, phase 1: RWL, rapid weight loss; URCA, uric acid.
Discussion
In this study, HISST with RWL among wrestlers was associated with an increased risk of AKI. This study demonstrated changes in the analysed markers of AKI in both phases of the research. However, during P1, some values of AKI markers exceeded the reference range (BUN: 2.5–7.5 mmol/L; URCA: 155–428 mmol/L; CRE: 49–115 mmol/L). Kidney injury begins by inducing biological and molecular changes, which biomarkers can detect early.33–35 The incidence of AKI is likely due to a combination of different factors.36 Of these factors, hypohydration may be particularly interesting, as it is commonly seen during RWL in combat athletes.37 According to the literature, dehydration appeared to be the primary RWL strategy practised by a number of combat sports athletes.38 39 Dehydration has multiple effects on the kidney. Moreover, the current literature recognises that even mild dehydration may be a risk factor in the progression of all types of chronic kidney diseases.19 According to Lakicevic et al,40 RWL caused dehydration and subsequent acute kidney damage despite various degrees of weight loss. Based on obtained results, high-intensity specific training combined with RWL significantly affects the increase of kidney function markers compared with identical acute training without RWL. This study showed that RWL affects CRE, BUN and URCA but not CysC. Conversely, the mean serum CysC remained remarkably stable during the RWL phase. Also, serum CysC did not differ significantly between the phases. A study conducted on a sample of judokas reached similar results.41 After a rapid reduction in body mass (6%) within 7 days, a significant increase in creatinine concentration was recorded (p≤0.05). A significant increase in creatinine level was also recorded in wrestlers after reducing a greater percentage of body mass (~8%) over a longer period (2–3 weeks).42 One study reported high CRE levels during the final phase of the weight cutting, consistent with AKI.43 Also, previous studies demonstrated that CRE concentrations might increase due to exercise-induced muscle breakdown.44 Similar to creatinine, kidney injury biomarkers such as BUN and URCA were significantly increased. There is a greater trend of rise in the mentioned marker’s concentration during RWL compared with acute training without RWL. Elevated BUN and creatinine concentrations often serve as biomarkers of AKI.45 URCA concentration also increased significantly during P1 compared with the initial measurement. In addition, URCA concentration exceeded the reference range after RWL. Significantly higher values were also noted after the acute training session (HISST) measurement (P2) compared with the initial measurement but remained within the normal reference range. Similar results were obtained in a study where an increase in the concentration of URCA was recorded in judokas after 7 days of a restrictive diet and a 5% reduction in body mass.46 47 The kidney is the main excretory organ for URCA. The high URCA concentration after P1 and P2 can be due to increased purine nucleotide degradation and damage to fast-twitch fibres under conditions of high energy demand. Also, the elevated concentration of this marker may be due to insufficient hydration as a consequence of RWL. It is critical to point out that the biomarkers (BUN, URCA and CRE) that changed significantly throughout the experimental phases surpassed the reference range only in the phase when RWL was applied together with HISST.
These results suggest that RWL with regular exercise has detrimental effects on kidney function. Moreover, acute exercise (HISST) barely impacts kidney injury biomarkers, following the previous study.48 Thus, HISST in relation to RWL leads to abnormalities observed on biochemical screening for the factors related to kidney functions such as BUN, URCA and CRE. Contrary, HISST without RWL induces alteration in mentioned markers but with no clinical significance. Therefore, obtained data confirm reports of the harmful effects of RWL in combat sports athletes. This is the first study to monitor kidney function biomarkers during the weight reduction and training phases in wrestlers. Also, further research is needed to understand better and elucidate alterations in kidney function biomarkers related to RWL.
Limitations
The study has limitations. HISST presents a non-standardised protocol developed to imitate the intensity of actual training sessions wrestlers often engage in during the season. Additionally, this study examined only male participants, so the response of female athletes to RWL and vigorous training remains to be determined. Moreover, the dietary plan of participants was not monitored throughout the study. In particular, athletes did not report precise food and beverage intake in the phase when RWL methods were implemented.
Conclusion
Although strong evidence points to the harmful health consequences of practising RWL, aggressive weight reduction methods are still prevalent among combat sports athletes. In this study, RWL was associated with an increased risk of AKI. Therefore, these results suggest that rapid weight reduction with regular exercise has detrimental effects on the kidney. All athletes should be advised of avoiding RWL and the importance of adequate hydration. There is also a need to increase awareness among coaches about the long-term risk of a repeated episode of AKI as a possible consequence of RWL. A better understanding of the pathophysiology in the progression of AKI related to RWL would help to identify and formulate weight management strategies for athletes. Also, future research could focus on biomarkers that more specifically reflect kidney dysfunction in combat athletes and identify those at greatest risk.
Acknowledgments
The authors would like to thank the wrestling team of Serbia and its national coaches.
Footnotes
Contributors: TT, RR and PD conceived the study idea. AM, AB and AC processed and interpreted the data. TT, RR, AM, ML-S, NM and PD prepared the first draft of the manuscript. TT, RR, NZ, TM, AM, ML-S, NM, AB, AC and PD all contributed substantially to the manuscript’s revision before submission. PD is a guarantor for the work.
Funding: The preparation of this paper was supported by the Provincial Secretariat for Higher Education and Scientific Research, grant number (142-451-3098); Ministry of Science, Technological Development and Innovation, Serbia.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All screened participants agreed to participate in the study voluntarily and provided written informed consent. The study was approved by the Institutional Review Committee of the University of Novi Sad, Serbia (Ref. No. 46-06-02/2020-1) and was conducted under the Declaration of Helsinki.
References
- 1.Joo YS, Nam KH, Jhee JH, et al. Body weight fluctuation is associated with rapid kidney function decline. Obesity (Silver Spring) 2022;30:257–67. 10.1002/oby.23326 [DOI] [PubMed] [Google Scholar]
- 2.Matthews JJ, Stanhope EN, Godwin MS, et al. The magnitude of rapid weight loss and rapid weight gain in combat sport athletes preparing for competition: a systematic review. Int J Sport Nutr Exerc Metab 2019;29:441–452. 10.1123/ijsnem.2018-0165 [DOI] [PubMed] [Google Scholar]
- 3.Franchini E, Brito CJ, Artioli GG. Weight loss in combat sports: physiological, psychological and performance effects. J Int Soc Sports Nutr 2012;9:52. 10.1186/1550-2783-9-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khodaee M, Olewinski L, Shadgan B, et al. Rapid weight loss in sports with weight classes. Curr Sports Med Rep 2015;14:435–41. 10.1249/JSR.0000000000000206 [DOI] [PubMed] [Google Scholar]
- 5.Reale R, Slater G, Burke LM. Individualised dietary strategies for Olympic combat sports: acute weight loss, recovery and competition nutrition. Eur J Sport Sci 2017;17:727–40. 10.1080/17461391.2017.1297489 [DOI] [PubMed] [Google Scholar]
- 6.Barley OR, Chapman DW, Guppy SN, et al. Considerations when assessing endurance in combat sport athletes. Front Physiol 2019;10:205. 10.3389/fphys.2019.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for disease control and prevention. hyperthermia and dehydration-related deaths associated with intentional rapid weight loss in three collegiate wrestlers-North Carolina, Wisconsin, and Michigan, November-December 1997. JAMA 1998;279:824. 10.1001/jama.279.11.824-JWR0318-3-1 [DOI] [PubMed] [Google Scholar]
- 8.Sansone RA, Sawyer R. Weight loss pressure on a 5 year old wrestler. Br J Sports Med 2005;39:e2. 10.1136/bjsm.2004.013136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brito CJ, Roas A FCM, Brito I SS, et al. Methods of body-mass reduction by combat sport athletes. Int J Sport Nutr Exerc Metab 2012;22:89–97. 10.1123/ijsnem.22.2.89 [DOI] [PubMed] [Google Scholar]
- 10.Crighton B, Close GL, Morton JP. Alarming weight cutting behaviours in mixed martial arts: a cause for concern and a call for action. Br J Sports Med 2016;50:446–7. 10.1136/bjsports-2015-094732 [DOI] [PubMed] [Google Scholar]
- 11.Reale R, Slater G, Burke LM. Weight management practices of Australian Olympic combat sport athletes. Int J Sports Physiol Perform 2018;13:459–66. 10.1123/ijspp.2016-0553 [DOI] [PubMed] [Google Scholar]
- 12.Drid P, Figlioli F, Lakicevic N, et al. Patterns of rapid weight loss in elite sambo athletes. BMC Sports Sci Med Rehabil 2021;13:39. 10.1186/s13102-021-00267-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figlioli F, Bianco A, Thomas E, et al. Rapid weight loss habits before a competition in sambo athletes. Nutrients 2021;13:1063. 10.3390/nu13041063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranisavljev M, Kuzmanovic J, Todorovic N, et al. Rapid weight loss practices in grapplers competing in combat sports. Front Physiol 2022;13:842992. 10.3389/fphys.2022.842992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roklicer R, Rossi C, Bianco A, et al. Prevalence of rapid weight loss in Olympic style wrestlers. J Int Soc Sports Nutr 2022;19:593–602. 10.1080/15502783.2022.2119095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langan-Evans C, Close GL, Morton JP. Making weight in combat sports. Strength Cond J 2011;33:25–39. 10.1519/SSC.0b013e318231bb64 [DOI] [Google Scholar]
- 17.Kim JC, Park KJ. Injuries and rapid weight loss in elite Korean wrestlers: an epidemiological study. Phys Sportsmed 2021;49:308–15. 10.1080/00913847.2020.1824536 [DOI] [PubMed] [Google Scholar]
- 18.Baranauskas M, Kupčiūnaitė I, Stukas R. The association between rapid weight loss and body composition in elite combat sports athletes. Healthcare 2022;10:665. 10.3390/healthcare10040665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roncal-Jimenez C, Lanaspa MA, Jensen T, et al. Mechanisms by which dehydration may lead to chronic kidney disease. Ann Nutr Metab 2015;66 Suppl 3:10–3. 10.1159/000381239 [DOI] [PubMed] [Google Scholar]
- 20.Artioli GG, Gualano B, Franchini E, et al. Prevalence, magnitude, and methods of rapid weight loss among Judo competitors. Med Sci Sports Exerc 2010;42:436–42. 10.1249/MSS.0b013e3181ba8055 [DOI] [PubMed] [Google Scholar]
- 21.Chapman CL, Johnson BD, Sackett JR, et al. Soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. Am J Physiol Regul Integr Comp Physiol 2019;316:R189–98. 10.1152/ajpregu.00351.2018 [DOI] [PubMed] [Google Scholar]
- 22.Rangaswamy D, Sud K. Acute kidney injury and disease: long‐term consequences and management. Nephrology (Carlton) 2018;23:969–80. 10.1111/nep.13408 [DOI] [PubMed] [Google Scholar]
- 23.Lameire NH, Levin A, Kellum JA, et al. Harmonizing acute and chronic kidney disease definition and classification: report of a kidney disease: improving global outcomes (KDIGO) consensus conference. Kidney Int 2021;100:516–26. 10.1016/j.kint.2021.06.028 [DOI] [PubMed] [Google Scholar]
- 24.Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J 2013;6:8–14. 10.1093/ckj/sfs160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosten AO. BUN and creatinine. In: Walker HK, Hall WD, Hurst JW, eds. Clinical methods: the history, physical, and laboratory examinations. 1990: 874–8. [PubMed] [Google Scholar]
- 26.Rigalleau V, Lasseur C, Perlemoine C, et al. Cockcroft-Gault formula is biased by body weight in diabetic patients with renal impairment. Metabolism 2006;55:108–12. 10.1016/j.metabol.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 27.Vinge E, Lindergård B, Nilsson-Ehle P, et al. Relationships among serum Cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest 1999;59:587–92. 10.1080/00365519950185076 [DOI] [PubMed] [Google Scholar]
- 28.Finney H, Newman DJ, Price CP. Adult reference ranges for serum Cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem 2000;37 (Pt 1):49–59. 10.1258/0004563001901524 [DOI] [PubMed] [Google Scholar]
- 29.Shlipak MG. Cystatin C as a marker of glomerular filtration rate in chronic kidney disease: influence of body composition. Nat Clin Pract Nephrol 2007;3:188–9. 10.1038/ncpneph0404 [DOI] [PubMed] [Google Scholar]
- 30.Mingels A, Jacobs L, Kleijnen V, et al. Cystatin C a marker for renal function after exercise. Int J Sports Med 2009;30:668–71. 10.1055/s-0029-1220733 [DOI] [PubMed] [Google Scholar]
- 31.Ascensão A, Rebelo A, Oliveira E, et al. Biochemical impact of a soccer match—analysis of oxidative stress and muscle damage markers throughout recovery. Clin Biochem 2008;41:841–51. 10.1016/j.clinbiochem.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 32.Ghoul N, Tabben M, Miarka B, et al. Mixed martial arts induces significant fatigue and muscle damage up to 24 hours post-combat. J Strength Cond Res 2019;33:1570–9. 10.1519/JSC.0000000000002078 [DOI] [PubMed] [Google Scholar]
- 33.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 2012;380:756–66. 10.1016/S0140-6736(11)61454-2 [DOI] [PubMed] [Google Scholar]
- 34.Devarajan P, Murray P. Biomarkers in acute kidney injury: are we ready for prime time Nephron Clin Pract 2014;127:176–9. 10.1159/000363206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wasung ME, Chawla LS, Madero M. Biomarkers of renal function, which and when? Clin Chim Acta 2015;438:350–7. 10.1016/j.cca.2014.08.039 [DOI] [PubMed] [Google Scholar]
- 36.Hoffman MD, Weiss RH. Does acute kidney injury from an ultramarathon increase the risk for greater subsequent injury Clin J Sport Med 2016;26:417–22. 10.1097/JSM.0000000000000277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ceylan B, Barley OR, Balci SS. Changes in body mass and hydration status in Judo athletes before and after a top-level competition: a descriptive case study. Phys Sportsmed 2023;51:228–33. 10.1080/00913847.2022.2026200 [DOI] [PubMed] [Google Scholar]
- 38.Berkovich BE, Eliakim A, Nemet D, et al. Rapid weight loss among adolescents participating in competitive Judo. Int J Sport Nutr Exerc Metab 2016;26:276–84. 10.1123/ijsnem.2015-0196 [DOI] [PubMed] [Google Scholar]
- 39.Hillier M, Sutton L, James L, et al. High prevalence and magnitude of rapid weight loss in mixed martial arts athletes. Int J Sport Nutr Exerc Metab 2019;29:512–7. 10.1123/ijsnem.2018-0393 [DOI] [PubMed] [Google Scholar]
- 40.Lakicevic N, Paoli A, Roklicer R, et al. Effects of rapid weight loss on kidney function in combat sport athletes. Medicina (Kaunas) 2021;57:551. 10.3390/medicina57060551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drid P, Krstulovic S, Erceg M, et al. Rapid weight loss negatively affects body composition and serum creatinine in elite Judokas. Med Sci Sports Exerc 2019;51:863. 10.1249/01.mss.0000563079.77371.f7 [DOI] [Google Scholar]
- 42.Karila T, Sarkkinen P, Marttinen M, et al. Rapid weight loss decreases serum testosterone. Int J Sports Med 2008;29:872–7. 10.1055/s-2008-1038604 [DOI] [PubMed] [Google Scholar]
- 43.Kasper AM, Crighton B, Langan-Evans C, et al. Case study: extreme weight making causes relative energy deficiency, dehydration, and acute kidney injury in a male mixed martial arts athlete. Int J Sport Nutr Exerc Metab 2019;29:331–8. 10.1123/ijsnem.2018-0029 [DOI] [PubMed] [Google Scholar]
- 44.Junglee NA, Lemmey AB, Burton M, et al. Does proteinuria-inducing physical activity increase biomarkers of acute kidney injury Kidney Blood Press Res 2012;36:278–89. 10.1159/000343417 [DOI] [PubMed] [Google Scholar]
- 45.Ostermann M, Zarbock A, Goldstein S, et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: a consensus statement. JAMA Netw Open 2020;3:e2019209. 10.1001/jamanetworkopen.2020.19209 [DOI] [PubMed] [Google Scholar]
- 46.Finaud J, Degoutte F, Scislowski V, et al. Competition and food restriction effects on oxidative stress in Judo. Int J Sports Med 2006;27:834–41. 10.1055/s-2005-872966 [DOI] [PubMed] [Google Scholar]
- 47.Degoutte F, Jouanel P, Bègue RJ, et al. Food restriction, performance, biochemical, psychological, and endocrine changes in Judo athletes. Int J Sports Med 2006;27:9–18. 10.1055/s-2005-837505 [DOI] [PubMed] [Google Scholar]
- 48.Bongers C, Alsady M, Nijenhuis T, et al. Impact of acute versus prolonged exercise and dehydration on kidney function and injury. Physiol Rep 2018;6:e13734. 10.14814/phy2.13734 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.