Abstract
Purpose
Immune checkpoint inhibitor (ICI) therapy is often suspended because of immune-related enterocolitis (irEC). We examined the effect of resumption of ICIs with or without concurrent selective immunosuppressive therapy (SIT) on rates of symptom recurrence and survival outcomes.
Methods
This retrospective, multicenter study examined patients who were treated with ICI and developed irEC requiring SIT (infliximab or vedolizumab) for initial symptom control or to facilitate steroid tapering between May 2015 and June 2020. After symptom resolution, patients were restarted either on ICI alone or on concurrent ICI and SIT at the discretion of the treating physicians. The associations between irEC recurrence and treatment group were assessed via univariate analyses and multivariate logistic regression. Cox proportional hazards model was used for survival analysis.
Results
Of the 138 included patients who required SIT for initial irEC symptom control, 61 (44.2%) patients resumed ICI without concurrent SIT (control group) and 77 (55.8%) patients resumed ICI therapy with concurrent SIT: 33 with infliximab and 44 with vedolizumab. After symptom resolution, patients in the control group were more commonly restarted on a different ICI regimen (65.6%) compared with those receiving SIT (31.2%) (p<0.001). The total number of ICI doses administered after irEC resolution and ICI resumption was similar in both groups (four to five doses). Recurrence of severe colitis or diarrhea after ICI resumption was seen in 34.4% of controls compared with 20.8% of patients receiving concurrent SIT. Concurrent SIT was associated with reduced risk of severe irEC recurrence after ICI resumption in a multivariate logistic regression model (OR 0.34; 95% CI 0.13 to 0.92; p=0.034). There was no difference in survival outcomes between patients in the control group and patients concurrently treated with SIT.
Conclusion
After resolution of irEC symptoms, reinitiation of ICI with concurrent SIT is safe, reduces severe irEC recurrence, and has no negative impact on survival outcomes.
Keywords: Immune Checkpoint Inhibitors, Autoimmunity, Immunotherapy, Inflammation
WHAT IS ALREADY KNOWN ON THIS TOPIC
Whether and how to reintroduce immune checkpoint inhibitor (ICI) therapy after the development of immune-related enterocolitis (irEC) is a common clinical problem. Small case series have suggested that concurrent use of selective immunosuppressive therapies (SIT) including vedolizumab and infliximab may reduce the risk of recurrent irEC after restarting ICI therapy.
WHAT THIS STUDY ADDS
In a retrospective analysis, we found that use of concurrent SIT was safe and associated with reduced risk of recurrent severe irEC in patients who restarted ICI therapy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These findings suggest that concurrent SIT may be appropriate to reduce the risk of recurrent irEC in patients who restart ICI therapy; this possibility should be directly tested in randomized, protective trials.
Introduction
Immune checkpoint inhibitors (ICIs), monoclonal antibodies that block the key immune regulatory ‘checkpoint’ receptors cytotoxic T lymphocyte-associated protein (CTLA)-4, programmed death (PD)-1, or its ligand (PD-L1), have revolutionized the treatment of solid and liquid malignancies over the last decade.1–4 The success of these agents derives from their ability to harness and augment spontaneous antitumor immune responses held in check by the CTLA-4 and or PD-1/PD-L1 pathways.5
Since ICIs cause immune activation, they can elicit mild to life-threatening inflammatory toxicities, collectively referred to as immune-related adverse events (irAEs). IrAEs frequently involve barrier surfaces (eg, gastrointestinal (GI) tract and skin) and vary with respect to ICI class, time to presentation, severity, and response to therapy.5–7 GI toxicities represent the majority of severe irAEs and are responsible for most ICI therapy discontinuations for irAEs.2 6 8–10
Guidelines for management of irAEs are based on clinical consensus and extrapolation from similar autoimmune syndromes such as inflammatory bowel disease (IBD) as there have been no successful prospective trials to test particular treatment strategies.9 11–14 For example, for the management of immune-related enterocolitis (irEC), current guidelines recommend initiation of glucocorticoids and continuation for at least 4–6 weeks after resolution of symptoms.9 Complications of extended steroid use as well as the potential impact of steroids on cancer survival outcomes suggest that glucocorticoids may not be the ideal strategy for management of all irAEs.15–18
In patients with glucocorticoid-refractory disease, selective immunosuppressive therapy (SIT), including inhibitors of tumor necrosis factor alpha (anti-TNFα), such as infliximab, as well as vedolizumab, an inhibitor of the α4β7 integrin, have been used to treat irEC.19 20 Retrospective analyses of patients who received anti-TNFα agents or vedolizumab for severe irEC have shown that, compared with glucocorticoids, these agents led to earlier symptomatic improvement.8 19 21–23
After initial irEC symptom resolution, the decision to restart ICI is often complicated and guidelines are based largely on expert consensus. Broadly, we consider three approaches to restarting ICI after the development of an irAE. One is to rechallenge the patient with the same agent after near complete symptom resolution, the second is to change to a different class of ICI that is approved for the same malignancy, and the third is to continue the index or alternative ICI with concurrent irAE-directed therapy. We have recently reported a case series of five patients with various malignancies treated with single-agent or combined ICI therapy who developed glucocorticoid-refractory irEC, were treated with infliximab, and then restarted on concurrent ICI and infliximab therapy. Concurrent therapy with infliximab resulted in improved colitis symptoms in all patients and all but one patient experienced overall cancer stability.24 Similarly, vedolizumab was used in a small cohort concurrently with ICI resumption after initial irEC symptom resolution with good outcomes.19
Here we present our multicenter, retrospective experience in treating patients with advanced malignancies with concurrent SIT and ICI. We evaluate the safety and outcome data with concurrent SIT and ICI therapy. Our findings show evidence of the safety and efficacy of this approach and highlight the need for prospective clinical trials examining the impact of concurrent SIT and ICI therapy on both irAEs and antitumor immunity.
Materials and methods
Study design and patient population
This retrospective, case–control, multicenter study was approved by the Institutional Review Boards of the three participating institutions (online supplemental table 1). Patients treated between May 2015 and June 2020 were identified using pharmacy and institutional databases, followed by detailed chart review. The inclusion criteria included adult patients with cancer who received an ICI, developed irEC, and received SIT treatment (infliximab or vedolizumab) for initial symptom control or to facilitate steroid tapering. For the concurrent treatment groups, irEC was treated with SIT, and ICI was then resumed with concurrent SIT at the discretion of the treating physician. A control group was identified, comprising patients who received an ICI, developed irEC, and received SIT treatment (infliximab or vedolizumab) and then resumed ICI without concurrent SIT. Exclusion criteria were if patients received SIT for reasons other than irEC and if patients did not resume ICI therapy following irEC. Infliximab was used at the 5 mg/kg intravenous dose while vedolizumab was used at the 300 mg intravenous dose.
jitc-2023-007195supp001.pdf (364.3KB, pdf)
Clinical characteristics
Demographic data, medical and oncologic history, and data related to ICI therapy were extracted from the electronic medical record. Charlson Comorbidity Index score was calculated based on recorded comorbid conditions.25 Variables related to oncologic history included cancer type and stage at time of ICI initiation, presence of metastatic disease, and occurrence of non-GI irAEs as documented in patients’ charts. Types of ICI therapy, number of infusions, and days on treatment before and after irEC were recorded.
IrEC and SIT characteristics
GI adverse events recorded were diarrhea, abdominal pain, bleeding, mucous in stool, fever, and nausea/vomiting. IrEC was diagnosed with a variety of methods, including clinical characteristics, imaging, and endoscopic evaluation (online supplemental table 2). We recorded peak grade of diarrhea and colitis according to Common Terminology Criteria for Adverse Events version (CTCAE) V.5.0 and need for hospitalization.13 The primary outcome of this study was the recurrence of severe irEC which was defined as CTCAE grades 3 and 4 colitis and diarrhea. Secondary outcomes included the duration of ICI hold and days to symptom remission, as defined by presence of CTCAE grade ≤1 symptoms. Secondary outcomes related to irEC treatment included total days of steroid treatment and number of attempts at steroid taper prior to SIT, defined as a planned decrease in steroid dose given improvement in symptoms, as documented in the medical record. SIT toxicities, including infection, nasopharyngitis, arthralgias, and infusion reactions are also reported. Information regarding SIT, including type administered, doses before and after ICI resumption, and days on concurrent therapy with SIT and ICI were recorded. Where available, last endoscopy prior to irEC diagnosis was noted and endoscopic evaluation was categorized as mild, moderate, or severe according to the Mayo endoscopic score.26 Clinical outcome methods are included in online supplemental material.
Statistical analysis
The association between irEC recurrence and treatment group as well as other characteristics were assessed via univariate analyses and multivariate logistic regression with significance level of 0.05. Analyses were carried out using SAS V.9.4. Full statistical methods are included in online supplemental material.
Results
Patients demographics and cancer-related characteristics
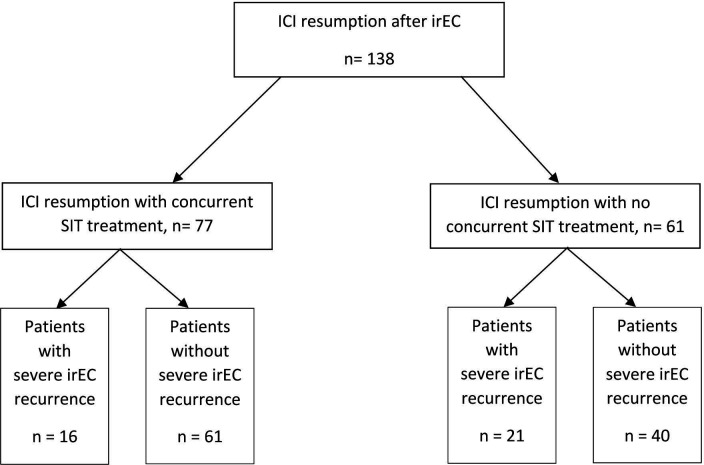
Patients with cancer treated with ICI and SIT were reviewed for inclusion in this study. A total of 138 patients restarted ICI after the development of irEC (table 1). Among this cohort, 77 patients received SIT (either infliximab or vedolizumab) for initial control of irEC and were subsequently continued on ICI with concurrent infliximab (33 patients) or vedolizumab (44 patients). Patients who received SIT for initial control or subsequent maintenance of irEC and were restarted on ICI therapy without concurrent SIT served as controls (61 patients) (figure 1). Of the control patients, 46 (75.4%) received infliximab, 11 (18.0%) received vedolizumab, 4 (6.6%) received infliximab and vedolizumab sequentially prior to but not after restarting ICI. Male patients constituted 72.1% of the control group compared with 54.5% of the concurrent SIT group (p=0.051). Advanced melanoma was the most common malignancy across the groups and combination of anti PD-(L)1 and CTLA-4 therapy was the most common regimen used prior to irEC.
Table 1.
Patient characteristics
| Characteristic | Subgroup | Control (N=61) |
Concurrent SIT (N=77) |
P value |
| Age (years), median (IQR), n=138 | 59.8 (51.1–70.4) | 61.3 (49.7–71.9) | 0.921 | |
| Sex, n (%) | Male | 44 (72.1) | 42 (54.5) | 0.051 |
| Charlson Comorbidity Index, median (IQR), n=138 | 8.0 (7.0–9.0) | 8.0 (7.0–9.0) | 0.137 | |
| Malignancy type, n (%) | 0.031 | |||
| Melanoma | 40 (65.6) | 35 (45.5) | ||
| GU | 12 (19.7) | 14 (18.2) | ||
| Lung/head/neck | 7 (11.5) | 15 (19.5) | ||
| GI | 0 (0.0) | 6 (7.8) | ||
| Endocrine | 1 (1.6) | 1 (1.3) | ||
| Other* | 1 (1.6) | 6 (7.8) | ||
| Stage at initiation of ICI, n (%) | 0.789 | |||
| III | 6 (9.8) | 9 (11.7) | ||
| IV | 55 (90.2) | 68 (88.3) | ||
| Metastatic sites, n (%) | None | 6 (9.8) | 6 (7.8) | 0.765 |
| GI tract | 5 (8.2) | 8 (10.4) | 0.774 | |
| Lung | 37 (60.7) | 40 (51.9) | 0.388 | |
| Liver | 15 (24.6) | 22 (28.6) | 0.700 | |
| Peritoneal | 5 (8.2) | 11 (14.3) | 0.298 | |
| History of prior ICI use, n (%) | Yes | 18 (29.5) | 19 (24.7) | 0.565 |
| ICI before irEC, n (%) | 0.001 | |||
| Anti-CTLA-4 | 13 (21.3) | 3 (3.9) | ||
| Anti-PD-1 | 14 (23.0) | 31 (40.3) | ||
| Anti-PD-L1 | 4 (6.6) | 1 (1.3) | ||
| Combination | 30 (49.2) | 42 (54.5) | ||
| Duration of ICI treatment prior to irEC onset (days), median (IQR), n=136 | 51.0 (28.0–84.0) | 86.0 (42.0–231.0) | 0.019 | |
| Doses of ICI prior to irEC, median (IQR), n=137 | 4.0 (3.0–7.0) | 6.0 (3.0–11.0) | 0.018 |
Combination denotes therapy with both anti-CTLA-4 and anti-PD-(L)1 therapy.
*Other malignancy types include: cutaneous squamous cell carcinoma, meningioma, and ovarian cancer.
CTLA-4, cytotoxic T lymphocyte-4; GI, gastrointestinal; GU, genitourinary; ICI, immune checkpoint inhibitor; irEC, immune-related enterocolitis; PD-1/L1, programmed cell death 1 or ligand 1; SIT, selective immunosuppressive therapy.
Figure 1.
Patient selection. ICI, immune checkpoint inhibitor; irEC, immune-related enterocolitis; SIT, selective immunosuppressive therapy.
The patients in the concurrent SIT group had a longer median duration of ICI treatment prior to irEC onset (86 days vs 51 day in the control group, (table 1, p=0.019) and received a median of six doses of ICI prior to irEC symptom onset compared with four doses in the control group (table 1, p=0.018). Other irAEs while on isolated ICI therapy or on concurrent therapy with SIT are reported in online supplemental table 3.
Diagnosis and management of irEC
Diarrhea was the presenting symptom in over 98% of patients (table 2). The diagnosis of irEC was confirmed through a combination of clinical, imaging, and endoscopic tools (online supplemental table 2) with histologic confirmation in patients undergoing endoscopic evaluation (online supplemental table 4). The endoscopic findings on presentation with irEC and on follow-up after resumption of ICI with or without concurrent therapy are demonstrated in online supplemental figure 1. More patients in the control group (63.9%) received a combination of oral and intravenous steroids compared with patients treated concurrently with SIT (37.7%, p=0.003).
Table 2.
Presentation and management of irEC
| Characteristic | Subgroup | Control (N=61) |
Concurrent SIT (N=77) |
P value |
| IrEC symptoms, n (%) | Diarrhea | 61 (100.0) | 76 (98.7) | 1.000 |
| Abdominal pain | 27 (44.3) | 46 (59.7) | 0.087 | |
| GI bleeding | 10 (16.4) | 21 (27.3) | 0.153 | |
| Mucus in the stool | 13 (21.3) | 17 (22.1) | 1.000 | |
| Fever | 4 (6.6) | 4 (5.2) | 0.732 | |
| Nausea/vomiting | 10 (16.4) | 24 (31.2) | 0.049 | |
| CTCAE V.5.0 grade of diarrhea, n (%) | 0.216 | |||
| 1–2 | 19 (31.1) | 32 (42.1) | ||
| 3–4 | 42 (68.9) | 44 (57.9) | ||
| CTCAE V.5.0 grade of colitis, n (%) | 0.109 | |||
| 1–2 | 34 (55.7) | 53 (69.7) | ||
| 3–4 | 27 (44.3) | 23 (30.3) | ||
| Hospitalization, n (%) | Yes | 40 (65.6) | 47 (61.0) | 0.600 |
| Steroids, n (%) | 0.003 | |||
| None | 0 (0.0) | 3 (3.9) | ||
| PO | 22 (36.1) | 45 (58.4) | ||
| PO+intravenous | 39 (63.9) | 29 (37.7) | ||
| Total time on steroids (days), median (IQR), n=135 | 35 (27–41) | 32 (22–45) | 0.536 | |
| Number of steroid tapering attempts prior to IFX/VDZ use, median (IQR), n=132 | 2 (1−2) | 1 (1−2) | 0.203 | |
| SIT used before ICI resumption, n (%) | 0.003 | |||
| IFX | 46 (75.4) | 36 (46.8) | ||
| VDZ | 11 (18.0) | 30 (39.0) | ||
| Combination | 4 (6.6) | 11 (14.3) | ||
| Doses of SIT (IFX, VDZ, or combination) before ICI resumption, median (IQR), n=120 | 1 (1−2) | 2 (1−3) | 0.018 | |
| IrEC clinical remission† before ICI resumption, n (%) | Yes | 60 (98.4) | 70 (90.9) | 0.077 |
| Duration for which ICI was held due to irEC (days), median (IQR), n=135 | 159 (91–245) | 123 (73–224) | 0.132 | |
| Change to different ICI regimen, n (%)‡ | Yes | 40 (65.6) | 24 (31.2) | <0.001 |
| ICI regimen resumed, n (%) | 0.072 | |||
| Anti-CTLA-4 | 3 (4.9) | 5 (6.5) | ||
| Anti-PD-(L)1 | 48 (78.7) | 47 (61.0) | ||
| Combination | 10 (16.4) | 25 (32.5) | ||
| Doses of SIT administered from ICI resumption, median (IQR), n=87 | 0 (0–0.5) | 2 (2−4) | <0.001 | |
| Time on concurrent ICI therapy (days), median (IQR), n=74§ | NA | 57 (21–150) | NA | |
| Number of additional ICI doses given, median (IQR), n=137 | 5 (2−7) | 4 (2−8) | 0.658 | |
| Total ICI doses administered, median (IQR), n=138 | 9 (6−14) | 12 (8–17) | 0.082 | |
| Total SIT doses given during disease course, median (IQR), n=136 | 1 (1−2) | 4 (2−6) | <0.001 |
*SIT was used either for induction or facilitation of steroid tapering. Combination refers to patients who received both IFX and VDZ in succession to induce remission at symptom onset.
†Defined as symptom improvement to less than grade 1.
‡See online supplemental table 5 for details on regimen change.
§Defined as time receiving both ICI and SIT.
CTCAE V.5.0, Common Terminology Criteria for Adverse Events (V.5.0) collected at peak disease severity; CTLA-4, cytotoxic T lymphocyte-associated protein; ICI, immune checkpoint inhibitor; IFX, infliximab; irEC, immune related enterocolitis; PD-L1, programmed death ligand 1; PO, oral; SIT, selective immunosuppressive therapy; VDZ, vedolizumab.
Conversely more patients in the concurrent SIT group (58.4%) received only oral but not intravenous steroids compared with controls (36.1%, p=0.003) (table 2). Patients in the concurrent SIT group received a median of 2 SIT infusions prior to ICI resumption compared with one infusion in the control group. SIT-related toxicities were typically mild and were most common in patients receiving infliximab as concurrent therapy (18.9%) and included infusion reactions (three patients), Clostridioides difficile infection (one patient), and sinopulmonary infection (one patient). In patients who received vedolizumab as concurrent therapy, 11.4% (five patients) developed sinopulmonary infections, nasopharyngitis, or arthralgias (online supplemental table 5).
IrEC outcomes after medical management
ICI therapy was held for a similar duration in both groups (median of 159 days in the control group and 123 days in the concurrent SIT group, p=0.132). In the control group, 65.6% of patients underwent a change in their ICI regimen on resumption of therapy while only 31.2% underwent an ICI regimen change in the concurrent SIT group (p<0.001). The most common ICI regimen change was combination anti-CTLA-4 and anti-PD-(L)1 to monotherapy with anti-PD-(L)1 alone (table 2 and online supplemental table 6). There was no significant difference between the groups with regards to the additional doses of ICI therapy administered.
Once patients were resumed on ICI therapy, patients who received concurrent SIT had an irEC recurrence rate of 29.9% compared with controls (37.7%) (table 3, p=0.367). When vedolizumab and infliximab were analyzed as separate treatments, we found vedolizumab was associated with 18.2% symptom recurrence compared with 45% with infliximab, although there was considerable heterogeneity in ICI regimens, duration of preceding ICI treatment, and SIT doses administered between the groups (online supplemental table 7,8). Next, we evaluated the rate of recurrence of severe irEC (CTCAE V.5.0 grade 3–4) as severe irEC is often the cause of ICI discontinuation.
Table 3.
Outcomes of irEC
| Characteristic | Subgroup | Control (N=61) |
Concurrent SIT (N=77) |
P value |
| Symptom recurrence on concurrent therapy, n (%) | Yes | 23 (37.7) | 23 (29.9) | 0.367 |
| Grade 3–4 diarrhea recurrence on concurrent therapy, n (%) | Yes | 21 (34.4) | 13 (16.9) | 0.028 |
| Grade 3–4 colitis recurrence on concurrent therapy, n (%) | Yes | 18 (29.5) | 14 (18.2) | 0.155 |
| Grade 3–4 diarrhea or colitis recurrence on concurrent therapy, n (%) | Yes | 21 (34.4) | 16 (20.8) | 0.084 |
| Time to recurrence after ICI resumption (days)*, median (IQR), n=36 | 33 (7–75) | 28 (8–63) | 0.987 | |
| Peak CTCAE V.5.0 grade of diarrhea on recurrence,* n (%) | 0.035 | |||
| 1 | 0 (0.0) | 4 (21.5) | ||
| 2 | 0 (0.0) | 2 (10.5) | ||
| 3 | 13 (61.9) | 7 (36.8) | ||
| 4 | 8 (38.1) | 6 (31.6) | ||
| Peak CTCAE V.5.0 grade of colitis on recurrence, n (%)* | 0.0516 | |||
| 1 | 2 (9.5) | 0 (0.0) | ||
| 2 | 1 (4.8) | 5 (26.3) | ||
| 3 | 15 (71.4) | 8 (42.1) | ||
| 4 | 3 (14.3) | 6 (31.6) | ||
| Endoscopic remission at last follow-up,† n (%) | Yes | 13 (39.4) | 27 (52.9) | 0.267 |
| Histologic remission at last follow-up,† n (%) | Yes | 7 (21.2) | 17 (33.3) | 0.323 |
| Any SIT toxicity, n (%) | Yes | 3 (4.9) | 11 (14.3) | 0.091 |
| Reason for discontinuation of ICI therapy, n(%) | Therapy complete | 0 (0.0) | 4 (6.2) | 0.124 |
| IrEC recurrence | 21 (38.2) | 15 (23.1) | 0.109 | |
| New irAE | 4 (7.3) | 9 (13.9) | 0.378 | |
| Infection | 0 (0.0) | 2 (3.1) | 0.499 | |
| Progressive disease | 31 (56.4) | 35 (53.9) | 0.855 | |
| Other‡ | 1 (1.8) | 4 (6.2) | 0.373 |
*For patients with symptom recurrence on concurrent therapy and non-excluded values.
†Endoscopic remission and histologic remission at last follow-up is calculated as the percentage of patients who had endoscopic/histologic remission divided by the percentage of patients who underwent endoscopic evaluation.
‡Other patients denote one patient who was lost to care (in the control group) and four patients who had complete irAE resolution prompting therapy discontinuation (in the SIT groups).
CTCAE V.5.0, Common Terminology Criteria for Adverse Events (V.5.0); irAE, immune-related adverse event; irEC, immune-related enterocolitis; SIT, selective immunosuppressive therapy.
Concurrent SIT therapy was associated with a significantly lower rate of grade 3–4 diarrhea recurrence compared with controls (34.4% vs 16.9%, p=0.028). The same trend was seen for grade 3–4 colitis (29.5% vs 18.2%, p=0.155). The composite outcome of severe colitis or diarrhea recurrence, a better predictor of ICI discontinuation than each component alone, was studied next. On multivariate analysis of factors associated with severe colitis and diarrhea recurrence, concurrent SIT use was associated with a lower risk of recurrence (table 4, OR 0.34; 95% CI 0.13 to 0.92; p=0.034). IrEC recurrence was the reason for discontinuation of ICI therapy in 38.2% of controls compared with 23.1% in the concurrent SIT group (p=0.109).
Table 4.
Multivariate logistic regression analysis of factors associated with high grade irEC recurrence
| Characteristic | High grade colitis and/or diarrhea | |
| OR* (95% CI) | P value | |
| SIT | ||
| Concurrent | 0.34 (0.13 to 0.92) | 0.034 |
| Control | ref | – |
| Total doses of SIT | 1.12 (0.98 to 1.27) | 0.091 |
| ICI before irEC | ||
| Anti-CTLA-4 | 0.63 (0.16 to 2.50) | 0.506 |
| Anti-PD-(L)1 | 1.47 (0.50 to 4.29) | 0.484 |
| Combination | ref | – |
| Duration for which ICI was held (weeks) | 1.00 (0.98 to 1.01) | 0.439 |
| ICI regimen changed | ||
| Yes | 1.31 (0.46 to 3.75) | 0.614 |
| No | ref | – |
| ICI at resumption | ||
| Anti-CTLA-4 | 3.32 (0.47 to 23.47) | 0.229 |
| Anti-PD-(L)1 | 0.99 (0.34 to 2.88) | 0.978 |
| Combination | ref | – |
*OR is the odds of irEC recurrence in the specified subgroup divided by odds of irEC recurrence in the reference (‘ref’) subgroup. For duration variables, the OR is the relative change in odds of irEC recurrence for each 1 week increase in duration.
CTLA-4, cytotoxic T lymphocyte-4; ICI, immune checkpoint inhibitor; irEC, immune-related enterocolitis; PD-(L)1, programmed cell death or ligand 1; combination denotes therapy with both anti CTLA-4 and anti PD-(L)1 therapy; SIT, selective immunosuppressive therapy.
Malignancy type, duration of steroid exposure for index irEC, duration for which ICI regimen was held, or ICI regimen changes were not associated with irEC recurrence (table 4 and online supplemental table 9). Patients who received concurrent SIT had less severe endoscopic findings on recurrence with a decrease in Mayo endoscopic score 2 and 3 findings from 72% to 35% in the concurrent SIT group compared with a decrease of 67% to 57% in the control group (p=0.093, online supplemental figure 1). At last follow-up, 53–57% of patients had discontinued ICI therapy due to progressive cancer across all three groups (table 3, p=0.855).
Overall survival and cancer outcome
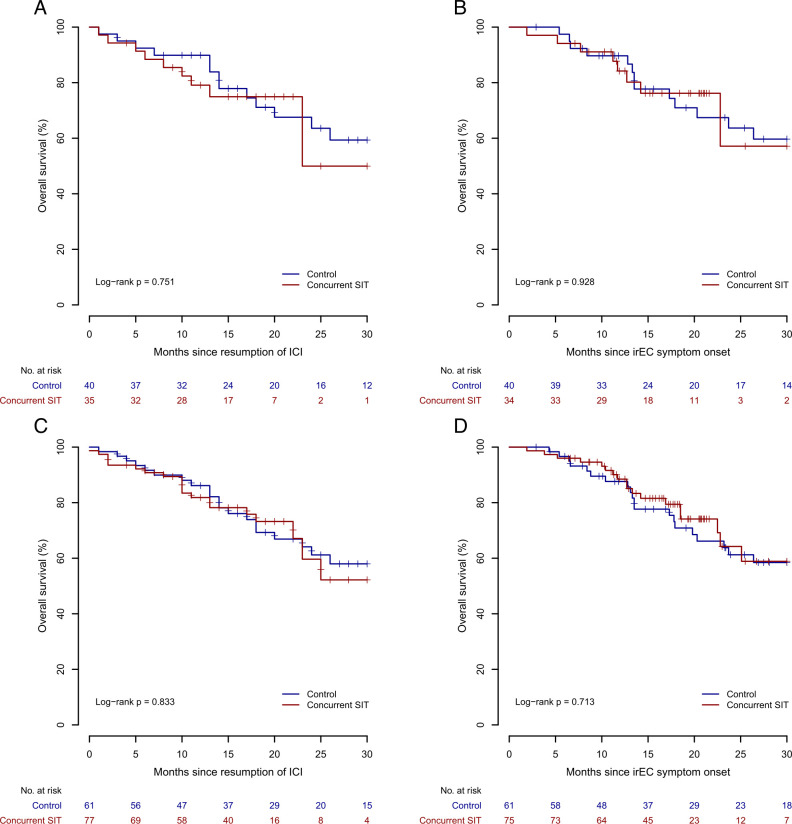
The overall survival of patients with stage III and IV melanoma who received concurrent SIT and ICI therapy was similar to patients in the control group when calculated from ICI resumption or irEC symptom onset (figure 2A,B). No difference in overall survival was seen in the pooled analysis of all patients irrespective of malignancy (figure 2C,D). Concurrent SIT use was not associated with a change in overall survival in a multivariate analysis of factors associated with overall survival from ICI resumption (online supplemental table 10). There was also no significant difference in cancer progression between both groups (online supplemental table 11). No differences in survival outcomes were seen when infliximab and vedolizumab were compared against each other and against the control group (data not shown).
Figure 2.
Overall survival from ICI resumption. Overall survival for patients with stage III and stage IV melanoma (A and B) or all patients (C and D) is displayed for each treatment group with number at risk from resumption of immunotherapy (A and C) or from irEC symptom onset (B and D). SIT denotes SIT, selective immunosuppressive therapy. ICI, immune checkpoint inhibitor; irEC, immune-related enterocolitis.
Discussion
The development of severe irAEs in the setting of ICI therapy is often followed by complex decision-making surrounding restarting ICI after symptom resolution. Clinical trials usually do not allow patients to resume ICI therapy if they have developed severe irAEs due to the risk of symptom recurrence, leading to potential morbidity and mortality. In real world practice, however, ICIs are resumed under some circumstances to allow patients to remain on antitumor therapy and potentially improve long-term outcomes.24 However, this decision is challenging due to the absence of prospective data that demonstrate a survival benefit associated with restarting therapy as well as the risk of serious toxicity.
We present the largest multicenter retrospective study to date that examines rechallenging patients with ICI and concurrent immunosuppression following an irAE. Patients concurrently treated with ICI and SIT after resolution of irEC tended to have less recurrence of severe irEA (CTCAE graded diarrhea or colitis) compared with control patients. We investigated multiple factors that may be associated with the recurrence of severe irEC in univariate and multivariate analyses; however, we identified concurrent treatment with SIT as the only factor associated with a significantly lower risk of recurrence (OR 0.34; 95% CI 0.13 to 0.92; p=0.034). In line with the reduction in severe symptoms, patients treated with concurrent SIT had less severe endoscopic findings on recurrence compared with control patients. The decreased risk of irEC recurrence associated with SIT was notable as control patients were more likely than SIT patients to switch from an anti-CTLA-4 containing regimen to anti-PD-(L)1 monotherapy, a transition previously associated with a lower risk of irEC recurrence.27
When we looked at the risk of overall irEC recurrence treating vedolizumab and infliximab as separate treatments, we saw that patients concurrently treated with ICI and vedolizumab after resolution of irEC had a significantly lower rate of irEC symptom recurrence (18.2%) when compared with patients treated with infliximab (45.5%) or controls (37.7%); however, the substantial heterogeneity among these groups, including in cancer type, ICI regimen, duration of ICI therapy, and number of SIT doses administered, coupled with the relatively small size of this study prevent definitive conclusions from this analysis. Definitively addressing the effect of specific SIT regimens on irEC recurrence will require prospective, randomized studies.
The management strategy in the control group after resolution of initial symptoms was either to rechallenge with the same ICI regimen (34.4%) or switch to a new ICI regimen (65.6%) with a cumulative irEC recurrence rate of 37.7%. Many of the patients in the control group switched from a CTLA-4 blocking regimen to a PD-(L)1 blocking regimen which may have contributed to a lower risk of recurrence. In comparison, patients who were concurrently treated with SIT had lower rates of ICI regimen change (31.2%). In all groups, ICI was held for a relatively similar amount of time.
The use of SIT in irEC was based on its success in patients with IBD.28 Vedolizumab is a human monoclonal IgG1 antibody that targets α4β7, a homing molecule specifically involved in T cell trafficking to the GI tract. α4β7 is not involved in T cell homing to other organs, which limits the extraintestinal side effects of vedolizumab.29 30 Recent analysis of the T cell infiltrate in irEC demonstrated expression of ITGA4 and ITGB7 which encode the subunits that form the α4β7 integrin supporting the use of vedolizumab in treating irEC.31 Cumulative safety data in patients with IBD do not show an increased risk of malignancy in patients treated with vedolizumab.32 The selective mechanism of action of vedolizumab reduces the risk of it interfering with antitumor effects of ICI when used for malignancies outside of the GI tract. This makes vedolizumab a good candidate for long-term therapy after ICI resumption, however prospective data are lacking.
The use of infliximab is similarly supported by its established role in IBD and also by murine models demonstrating that TNFα produced in the setting of PD-1 blockade leads to impaired CD8+ tumor infiltrating T lymphocyte responses and increased activation-induced T cell death.33–35 Concurrent treatment of mice with melanoma or colon cancer with ICI and anti-TNFα led to improved antitumor responses and survival, which was attributable to increased intratumor CD8+ T cell numbers and viability.33 34
We observed no differences in survival outcomes in patients receiving concurrent ICI and control patients. Despite the heterogeneity of our patient population, this demonstrates the safety of concurrent SIT as an approach to restarting ICI after irEC resolution. While inconclusive, some data do support an association between irAEs and improved antitumor responses.8 36–38 Severe adverse events often require treatment suspension or discontinuation in addition to immunosuppression, both of which may limit the magnitude of benefit in antitumor response associated with developing an irAE. We have recently reported a shorter time to resolution of irEC symptoms in patients treated with infliximab compared with vedolizumab as first-line SIT.39 Combined with the subgroup analysis showing that vedolizumab is associated with a lower risk of irEC recurrence, this could be a good justification to use infliximab as the initial SIT for symptom control then transition to vedolizumab for concurrent long-term management. We do not have complete data on the outcomes of patients who developed irEC recurrence and how the second episode of irEC was managed; however, stopping ICI therapy after recurrent irEC was not associated with refractory colitis in our experience, and recurrence could be managed with resumption of systemic glucocorticoids and transition to an alternative form of SIT. The decision to continue or stop ICI therapy after recurrent irEC in our cohort was at the discretion of the treating physician after considering multiple factors, with the difficulty in managing recurrent irEC likely one of those considerations. This is an important area of future study to determine whether switching to an alternative SIT is sufficient to control the symptomatic recurrence in patients with recurrent irEC after treatment with concurrent SIT.
The major limitations of this study are its retrospective nature, the heterogeneity of the primary malignancies, and the SIT protocol variations. The dose and timing of glucocorticoid relative to SIT initiation were not available in our cohort and may differ among treatment groups which could have had an impact on the risk of irEC recurrence and overall survival. Additionally, we did not have routine endoscopic confirmation of irEC resolution in the entire cohort and endoscopic remission likely decreases the risk of recurrence after ICI resumption. The changes made to the ICI regimens in response to irEC and the variations in those changes between groups could have a significant impact on patient outcomes and risk of recurrence of irAEs as well.
Conclusion
This is the first and largest multicenter study of treatment for irEC with concurrent SIT in combination with ICI therapy to facilitate ongoing cancer treatment and reduce irEC recurrence. We observed that concurrent SIT with ICI is safe and is associated with lower rates of severe irEC symptom recurrence and better endoscopic outcomes. This work emphasizes the importance of conducting prospective, placebo-controlled trials that compare current regimens used for treatment of irAEs as well as resumption of ICI after irAEs.
Footnotes
Twitter: @YRBadran
YRB and FZ contributed equally.
SMD and BED contributed equally.
DMF, YW and MD contributed equally.
Contributors: YRB contributed to the analysis of the results, writing and review of the manuscript. FZ contributed to the collection of the raw data. SD contributed to the collection of the raw data and writing of the manuscript. DL and BD contributed to the collection of the raw data. P-YL and NH performed the statistical analysis. WQ contributed to the statistical analysis. HA-S, AT, MA, JAT, and MP contributed to the interpretation of the data and review of the manuscript. DF, YW, and MD conceived of the study and contributed to the writing, analysis, and review of the manuscript. DF, YW, and MD are equally responsible for the overall content, accepting full responsibility for the work and conduct of the study, they each had access to the data, and jointly controlled the decision to publish.
Funding: Funding was provided by R01A169188, the Fariborz Maseeh Award for Innovative Medical Education, and The Peter and Ann Lambertus Family Foundation (MD), and by the Cancer Center Support Grant P30 CA08748 from the National Institutes of Health/National Cancer Institute (MP and DF).
Competing interests: Unrelated to this work, YRB has consulting fees from Aditum Bio and Goodpath. MD has research funding from Novartis and Eli Lilly, has received consulting fees from Tillotts Pharma, ORIC Pharmaceuticals, Partner Therapeutics, SQZ Biotech, AzurRx, Mallinckrodt Pharmaceuticals, and Moderna, and is a member of the Scientific Advisory Board for Neoleukin Therapeutics. DF has received consulting fees from Kaleido Biosciences, AzurRx, Mallinckrodt Pharmaceuticals, and Equillium. MP has received consulting fees from BMS, Merck, Array BioPharma, Novartis, Incyte, NewLink Genetics, Aduro, Eisai, Pfizer; honoraria from BMS and Merck; institutional support from RGenix, Infinity, BMS, Merck, Array BioPharma, Novartis, and AstraZeneca.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request as part of a collaborative agreement.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Baumeister SH, Freeman GJ, Dranoff G, et al. Coinhibitory pathways in Immunotherapy for cancer. Annu Rev Immunol 2016;34:539–73. 10.1146/annurev-immunol-032414-112049 [DOI] [PubMed] [Google Scholar]
- 2. Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint blockade in cancer therapy. JCO 2015;33:1974–82. 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Longo DL, Postow MA, Sidlow R, et al. Immune-related adverse events associated with immune Checkpoint blockade. N Engl J Med 2018;378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 4. Dougan M, Dranoff G, Dougan SK. Cancer Immunotherapy: beyond Checkpoint blockade. Annu Rev Cancer Biol 2019;3:55–75. 10.1146/annurev-cancerbio-030518-055552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dougan M, Luoma AM, Dougan SK, et al. Understanding and treating the inflammatory adverse events of cancer Immunotherapy. Cell 2021;184:1575–88. 10.1016/j.cell.2021.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dougan M. Checkpoint blockade toxicity and immune homeostasis in the gastrointestinal tract. Front Immunol 2017;8:1547. 10.3389/fimmu.2017.01547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pauken KE, Dougan M, Rose NR, et al. Adverse events following cancer Immunotherapy: obstacles and opportunities. Trends Immunol 2019;40:511–23. 10.1016/j.it.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Abu-Sbeih H, Mao E, et al. Immune-Checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunotherapy Cancer 2018;6:37. 10.1186/s40425-018-0346-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thompson JA, Schneider BJ, Brahmer J, et al. NCCN guidelines insights: management of Immunotherapy-related toxicities, version 1.2020. J Natl Compr Canc Netw 2020;18:230–41. 10.6004/jnccn.2020.0012 [DOI] [PubMed] [Google Scholar]
- 10. Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced Melanoma who discontinued treatment with Nivolumab and Ipilimumab because of adverse events: A pooled analysis of randomized phase II and III trials. JCO 2017;35:3807–14. 10.1200/JCO.2017.73.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune Checkpoint inhibitors: consensus recommendations from the society for Immunotherapy of cancer (SITC). J Immunother Cancer 2017;5:95. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune Checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. JCO 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dougan M, Wang Y, Rubio-Tapia A, et al. AGA clinical practice update on diagnosis and management of immune Checkpoint inhibitor colitis and hepatitis: expert review. Gastroenterology 2021;160:1384–93. 10.1053/j.gastro.2020.08.063 [DOI] [PubMed] [Google Scholar]
- 14. Bishu S, Melia J, Sharfman W, et al. Efficacy and outcome of tofacitinib in immune Checkpoint inhibitor colitis. Gastroenterology 2021;160:932–4. 10.1053/j.gastro.2020.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bai X, Hu J, Betof Warner A, et al. Early use of high-dose glucocorticoid for the management of irAE is associated with poorer survival in patients with advanced Melanoma treated with anti-PD-1 monotherapy. Clinical Cancer Research 2021;27:5993–6000. 10.1158/1078-0432.CCR-21-1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell Death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 2018;36:2872–8. 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 17. Poetker DM, Reh DD. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol Clin North Am 2010;43:753–68. 10.1016/j.otc.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 18. Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of Ipilimumab-induced Hypophysitis is associated with reduced survival in patients with Melanoma. Cancer 2018;124:3706–14. 10.1002/cncr.31629 [DOI] [PubMed] [Google Scholar]
- 19. Abu-Sbeih H, Ali FS, Wang X, et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune Checkpoint inhibitor-induced colitis. J Immunotherapy Cancer 2019;7:93. 10.1186/s40425-019-0577-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson DH, Zobniw CM, Trinh VA, et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related Enterocolitis. J Immunother Cancer 2018;6:103. 10.1186/s40425-018-0412-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verheijden RJ, May AM, Blank CU, et al. Association of anti-TNF with decreased survival in steroid refractory Ipilimumab and anti-Pd1-treated patients in the Dutch Melanoma treatment Registry. Clinical Cancer Research 2020;26:2268–74. 10.1158/1078-0432.CCR-19-3322 [DOI] [PubMed] [Google Scholar]
- 22. Lesage C, Longvert C, Prey S, et al. Incidence and clinical impact of anti-Tnfalpha treatment of severe immune Checkpoint inhibitor-induced colitis in advanced Melanoma: the Mecolit survey. J Immunother 2019;42:175–9. 10.1097/CJI.0000000000000268 [DOI] [PubMed] [Google Scholar]
- 23. Abu-Sbeih H, Ali FS, Alsaadi D, et al. Outcomes of Vedolizumab therapy in patients with immune Checkpoint inhibitor-induced colitis: a multi-center study. J Immunotherapy Cancer 2018;6:142. 10.1186/s40425-018-0461-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Badran YR, Cohen JV, Brastianos PK, et al. Concurrent therapy with immune Checkpoint inhibitors and Tnfalpha blockade in patients with gastrointestinal immune-related adverse events. J Immunother Cancer 2019;7:226. 10.1186/s40425-019-0711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying Prognostic Comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 26. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-Aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625–9. 10.1056/NEJM198712243172603 [DOI] [PubMed] [Google Scholar]
- 27. Abu-Sbeih H, Ali FS, Naqash AR, et al. Resumption of immune Checkpoint inhibitor therapy after immune-mediated colitis. JCO 2019;37:2738–45. 10.1200/JCO.19.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scribano ML. Vedolizumab for inflammatory bowel disease: from randomized controlled trials to real-life evidence. World J Gastroenterol 2018;24:2457–67. 10.3748/wjg.v24.i23.2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lamb CA, O’Byrne S, Keir ME, et al. Gut-selective integrin-targeted therapies for inflammatory bowel disease. J Crohns Colitis 2018;12(suppl_2):S653–68. 10.1093/ecco-jcc/jjy060 [DOI] [PubMed] [Google Scholar]
- 30. Wang C, Hanly EK, Wheeler LW, et al. Effect of Alpha4Beta7 blockade on intestinal lymphocyte Subsets and Lymphoid tissue development. Inflamm Bowel Dis 2010;16:1751–62. 10.1002/ibd.21266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luoma AM, Suo S, Williams HL, et al. Molecular pathways of colon inflammation induced by cancer Immunotherapy. Cell 2020;182:655–71. 10.1016/j.cell.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Card T, Ungaro R, Bhayat F, et al. Vedolizumab use is not associated with increased malignancy incidence: GEMINI LTS study results and post-marketing data. Aliment Pharmacol Ther 2020;51:149–57. 10.1111/apt.15538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bertrand F, Montfort A, Marcheteau E, et al. Tnfalpha blockade overcomes resistance to anti-PD-1 in experimental Melanoma. Nat Commun 2017;8:2256. 10.1038/s41467-017-02358-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perez-Ruiz E, Minute L, Otano I, et al. Prophylactic TNF blockade Uncouples efficacy and toxicity in dual CTLA-4 and PD-1 Immunotherapy. Nature 2019;569:428–32. 10.1038/s41586-019-1162-y [DOI] [PubMed] [Google Scholar]
- 35. Zheng L, Fisher G, Miller RE, et al. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature 1995;377:348–51. 10.1038/377348a0 [DOI] [PubMed] [Google Scholar]
- 36. Morimoto K, Yamada T, Takumi C, et al. Immune-related adverse events are associated with clinical benefit in patients with non-small-cell lung cancer treated with Immunotherapy plus chemotherapy: A retrospective study. Front Oncol 2021;11:630136. 10.3389/fonc.2021.630136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feng Y, Roy A, Masson E, et al. Exposure-response relationships of the efficacy and safety of Ipilimumab in patients with advanced Melanoma. Clin Cancer Res 2013;19:3977–86. 10.1158/1078-0432.CCR-12-3243 [DOI] [PubMed] [Google Scholar]
- 38. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic Melanoma treated by CTL-associated Antigen-4 blockade. Clin Cancer Res 2007;13(22 Pt 1):6681–8. 10.1158/1078-0432.CCR-07-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zou F, Faleck D, Thomas A, et al. Efficacy and safety of Vedolizumab and Infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: a two-center observational study. J Immunother Cancer 2021;9:e003277. 10.1136/jitc-2021-003277 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-007195supp001.pdf (364.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data are available upon reasonable request as part of a collaborative agreement.