Abstract
Background
Longitudinal serology studies can assist in analyzing the kinetics of antibodies to SARS-CoV-2, helping to inform public health decision making. Our study aims to characterize circulating antibody trends over 18 months in vaccinated participants with and without evidence of COVID-19 infection.
Methods
A cohort of health care workers employed at Boston Medical Center was followed to collect serum samples and survey data over 6 time points from July 2020 through December 2021 (N = 527). History of SARS-CoV-2 infection, vaccination, and booster status were confirmed, where possible, through electronic medical records. Serum was assessed for the qualitative and semiquantitative detection of IgG antibody levels (anti-nucleoprotein [anti-N] and anti-spike [anti-S], respectively). Piecewise regression models were utilized to characterize antibody kinetics over time.
Results
Anti-S IgG titers remained above the positivity threshold following infection and/or vaccination throughout the 18-month follow-up. Among participants with no evidence of COVID-19 infection, titers declined significantly faster in the initial 90 days after full vaccination (β = −0.056) from December 2020 to March 2021 as compared with the decline observed following booster dose uptake (β = −0.023, P < 0.001). Additionally, COVID-19 infection prior to vaccination significantly attenuated the decline of anti-S IgG when compared with no infection following vaccine uptake (P < 0.001). Lastly, fewer participants contracted Omicron when boosted (12.7%) compared to fully vaccinated (17.6%). Regardless of vaccination status, participants who were Omicron positive had lower anti-S IgG titers than those who did not test positive, but this difference was not significant.
Conclusions
These findings provide novel 18-month kinetics of anti-S IgG antibodies and highlight the durability of hybrid immunity, underlining the strong humoral response stimulated by combined infection and vaccination.
Keywords: antibodies, COVID-19, immunity, SARS-CoV-2, vaccines
Our results highlight a rapid decline in antibodies following initial vaccination as compared with attenuated decline following booster dose uptake. Our results also indicate differences in circulating antibody decay among vaccinated health care workers with previous COVID-19 infection vs those without previous infection.
As of December 2021, the Centers for Disease Control and Prevention estimated that 94.7% of the US population had developed antibodies to SARS-CoV-2, be it through infection and/or vaccination [1]. Resulting from the fact that >80 000 000 Americans have had COVID-19 infection and approximately 219 000 000 have been vaccinated against the disease, evaluation of hybrid immunity to COVID-19 is pertinent [2]. This concept, developing vaccine- and infection-derived antibodies, may increase an individual's level of protection and is especially relevant given the increase in breakthrough COVID-19 infections and simultaneous monovalent and, more recently, bivalent booster dose uptake [3, 4]. As a result, our understanding of the immune response to SARS-CoV-2 continues to evolve, especially within the context of hybrid immunity [5].
Currently, we know that infection with SARS-CoV-2 evokes a humoral and cellular immune response. One key aspect of the immune reaction is the production of serum antibodies to 2 protein regions of the virus—specifically, anti-nucleoprotein (anti-N) (nucleoprotein) and anti-spike (anti-S) RBD (spike receptor-binding domain) regions [3, 6]. Most infected individuals will develop these anti-N and anti-S IgG antibodies within weeks of symptom onset, often peaking between 3 and 7 weeks [5, 7]. Available COVID-19 vaccines in the United States from Pfizer-BioNTech and Moderna rely on humoral and cellular response to provide protection [5, 7–9]. Humoral response to these vaccines can be measured by anti-S IgG assays. To date, there is still no known circulating antibody titer widely accepted to confer protection from infection; as such, continued population-level serum antibody testing can assist in characterizing hybrid immunity and potentially provide insight about future vaccine doses.
Health care workers (HCWs) are at an increased risk for exposure to SARS-CoV-2 and for acquiring COVID-19 infection [10]. Given the high rate of vaccination and booster dose uptake in this population, they provide an opportunity to better understand hybrid immunity and how it may influence serum antibody kinetics and the incidence of infection over time [11, 12]. In an effort to elucidate how public health strategies may better protect this vulnerable population through future waves of COVID-19 infection, long-term follow-up of SARS-CoV-2 serum antibodies is crucial. During the initial COVID-19 infection surge at Boston Medical Center (BMC), a safety net academic hospital in Boston, Massachusetts, our group conceived a study to assess seroprevalence within a cohort of HCWs [13]. A subset of this BMC HCW population was then followed longitudinally with serology data collected at approximately 3-month intervals from July 2020 through December 2021.
Earlier analyses of this cohort describe different trends in relation to infection and vaccination up to 15 months postinfection and 12 months postvaccination, encouraging investigation of antibody kinetics [14]. Furthermore, in conjunction with other studies, these findings suggest that with the onset of breakthrough infections from new variants and the uptake of booster doses, anti-S RBD IgG levels can remain well above assay positivity thresholds for more than a year from initial exposure despite a universal decline in concentration [8, 14]. The durability of antibodies to SARS-CoV-2 following COVID-19 infection has been observed for up to 16 months from symptom onset, calling into question the effect of vaccine uptake on circulating antibodies among those with previous COVID-19 infection and infection-naive individuals [15, 16].
We aim to add to the growing body of research characterizing SARS-CoV-2 antibody kinetics beyond 12 months from COVID-19 infection and/or full vaccination [15–19]. These present analyses of circulating antibodies up to 15 months from full vaccination among HCWs who were infection naive and previously infected can help to provide understanding of long-term antibodies to COVID-19 during this evolving pandemic.
METHODS
Study Design and Population
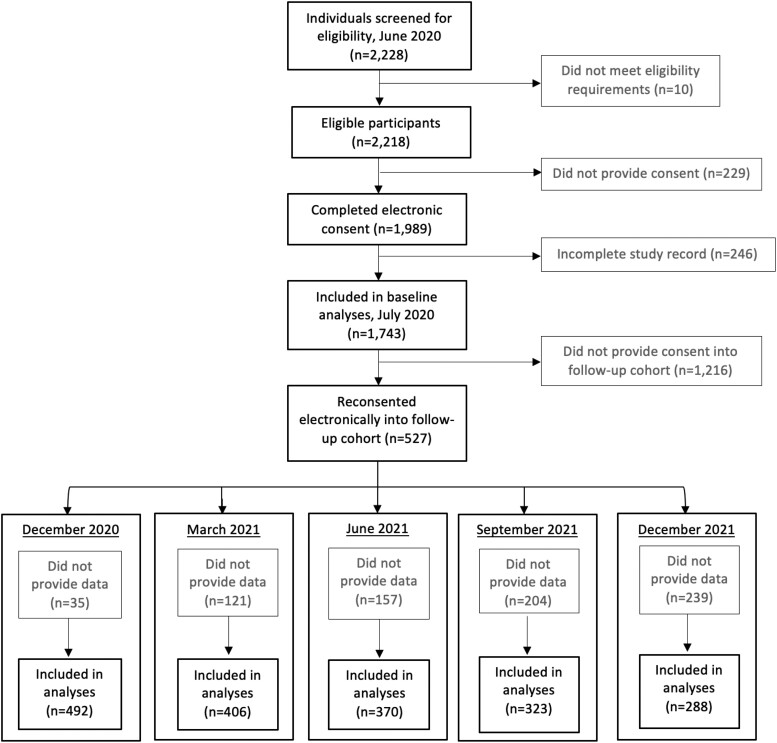
In June 2020, 2228 BMC HCWs were screened for participation into an initial cross-sectional COVID-19 seroprevalence study. Eligible participants were HCWs aged ≥18 years who were working on-site during the initial COVID-19 wave from March 13 to May 31, 2020 (Figure 1). In brief, eligible HCWs completed electronic informed consent, provided a serum sample, and filled out a survey covering sociodemographic and health-related information (eg, symptoms, comorbidities, COVID-19 history). Further details of recruitment have been described elsewhere [13]. Participants were offered SARS-CoV-2 antibody results upon assay emergency use authorization.
Figure 1.
Participant enrollment and follow-up from June 2020 through December 2021.
Following baseline data collection in July 2020, a smaller cohort of participants was given the opportunity to reconsent electronically into this present longitudinal cohort study. This cohort was selected by emailing all baseline participants who provided consent for recontact by study personnel. Participants were followed in approximately 3-month intervals following the July 2020 baseline data collection—in December 2020, March 2021, June 2021, September 2021, and December 2021—for a total observation time of 18 months (Figure 1). Follow-up cohort participants provided additional serum samples and updated survey data, including COVID-19 exposure, vaccination status, reverse transcription–polymerase chain reaction (RT-PCR) results, and infection prevention measures at each visit. This project was approved by the Institutional Review Board at BMC (H-40503).
At each time point, serum samples from participants were collected within 2 weeks of survey data collection and analyzed immediately for SARS-CoV-2 anti-N IgG and anti-S RBD IgG in the clinical pathology laboratory at BMC on the Abbott ARCHITECT i2000SR System per the manufacturer's instructions (Supplementary Methods).
COVID-19 Positivity and Vaccination Status
Criteria for COVID-19 positivity included any instance of the following: self-reported positive COVID-19 test result on a survey instrument, electronic medical record (EMR)–confirmed positive laboratory test result (RT-PCR), or new positivity on an anti-N IgG test between serology time points. EMR review also provided dates of primary vaccination series and booster, if applicable. COVID-19 positivity was considered a breakthrough infection if the initial infection occurred at least 2 weeks following completion of the primary vaccination series. Participants were classified with multiple infections if they met the aforementioned COVID-19 positivity definition, followed by a period with either a negative RT-PCR test result or negative anti-N IgG assay result, followed by another instance of COVID-19 positivity.
Following serum collection in early December 2021, BMC hospital testing protocol changes allowed for the use of self-administered COVID-19 antigen tests to diagnose infection, coinciding with the rise of the Omicron variant as the dominant subvariant in New England by the end of December 2021 [20]. As such, post hoc analyses included screening participants' EMRs for COVID-19 infection attributed to the Omicron variant as captured by RT-PCR or antigen testing from December 2021 through February 2022.
Seroprevalence Measurements
At each time point, frequency and percentage positivity were calculated for anti-N IgG and anti-S IgG in the cohort based on assay positivity. These calculations included all participants who provided serum samples for each time point.
Piecewise Regression and Statistical Analyses
Piecewise regression analyses were used to investigate log2-transformed anti-S IgG trends by vaccination and cumulative infection (any variant) status over time within the intervals of 0–89, 90–179, 180–269, and 270–349 days after full vaccination (2 weeks after completion of a primary vaccine series). In addition to intuitive interpretation, the application of this modeling allowed for the rate of change in post–primary series vaccination antibody concentrations to be investigated. Furthermore, separation into 90-day knots with distinction for COVID-19 infection and vaccination dose uptake provided additional context to these regression analyses. Most participants received vaccine doses within similar time windows, allowing for the creation of these knots corresponding to study visits. This model examines the association between antibody levels (dependent variable) and time from full vaccination (independent variable) within the 4 time segments. The following participants were excluded from analyses: those missing primary series vaccination dates (n = 83); those with evidence of multiple COVID-19 infections (n = 4); individuals who were immunocompromised and reported instances of cancer, HIV positivity, or receipt of an organ transplant (n = 34); and participants in the Pfizer-BioNTech vaccine trial (n = 12). T tests were used with a significance level of <0.05 to compare the regression slopes of serum anti-S IgG concentration regression between vaccination and infection groups by days from full vaccination. Separate piecewise regression with a continuous line and additive models (natural splines) were attempted but not selected, as they oversimplified observed trends within time intervals (data not shown).
Questionnaire data were recorded and managed in REDCap tools hosted at Boston University (Clinical and Translational Science Institute; 1UL1TR0001430). Categorical data are presented as frequencies, and missing responses <5% were excluded from analysis or are otherwise indicated.
Box plots were created to visualize December 2021 log2-transformed median anti-S IgG concentrations, with whiskers depicting 1.5 times the IQR by vaccination and subsequent Omicron infection status. Mood's median test was used to evaluate differences in median anti-S IgG concentration by Omicron infection status. P < 0.05 was considered statistically significant. Analyses were run via Statistical Analysis Software version 9.4 (SAS Institute) and R version 3.5.3 (“stats” package; R Foundation for Statistical Computing).
RESULTS
Population Demographics and Vaccine Uptake
In total 527 BMC HCWs reconsented into the follow-up cohort from the baseline cohort (n = 1743). Subsequently, 492 participants returned in December 2020, 406 in March 2021, 370 in June 2021, 323 in September 2021, and 288 in December 2021 (Figure 1). Within the follow-up cohort (N = 527), the majority was female (77.0%). The median age was 40 years (SD, 12.6). The majority was between 30 and 39 years old throughout follow-up; however, the age distribution changed significantly (P = 0.006), with a higher proportion of younger participants included in earlier time points as compared with later time points. Occupation was most often reported as nurse (41.2%) or physician (31.5%). The remaining participants fell into the categories of patient-facing allied health (13.3%), administrative (10.3%), or non–patient-facing allied health (3.0%). Our cohort mostly identified as White (81.6%) or Asian (8.4%). Hispanic or Latinx ethnicity was indicated by 7.4% of participants. Overall demographic characteristics remained comparable across all time points with the exception of participant age (Table 1). Within our cohort, 34 participants (6.4%) were considered immunocompromised as a result of cancer, HIV positivity, and/or organ transplantation. Outside of these conditions, the cohort was largely healthy; the most prevalent comorbidities were high cholesterol (12.5%), asthma (10.6%), and hypertension (9.7%) (Supplementary Table 1).
Table 1.
Cohort Demographics Across Time Points
| Participants, No. (%)a | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Jul 2020 | Dec 2020 | Mar 2021 | Jun 2021 | Sep 2021 | Dec 2021 | P Value |
| Total | 527 | 492 | 406 | 370 | 323 | 288 | |
| Gender | … | … | … | … | … | … | .8110 |
| Female | 406 (77.04) | 378 (76.83) | 315 (77.59) | 297 (80.27) | 256 (79.26) | 228 (79.17) | |
| Male | 119 (22.58) | 112 (22.76) | 90 (22.17) | 72 (19.46) | 66 (20.43) | 59 (20.49) | |
| Age, y | … | … | … | … | … | … | .0006 |
| 20–29 | 95 (18.03) | 80 (16.26) | 49 (12.07) | 37 (10.0) | 30 (9.29) | 26 (9.03) | |
| 30–39 | 164 (31.12) | 158 (32.11) | 126 (31.03) | 112 (30.27) | 86 (26.63) | 76 (26.39) | |
| 40–49 | 96 (18.22) | 91 (18.50) | 82 (20.20) | 79 (21.35) | 68 (21.05) | 68 (23.61) | |
| 50–59 | 112 (21.25) | 98 (19.92) | 86 (21.18) | 77 (20.81) | 74 (22.91) | 63 (21.88) | |
| 60–69 | 60 (11.39) | 64 (13.01) | 62 (15.27) | 64 (17.30) | 64 (19.81) | 55 (19.10) | |
| Body mass index, kg/m2 | … | … | … | … | … | … | .9360 |
| Underweight | 9 (1.71) | 6 (1.22) | 3 (0.74) | 1 (1.89) | 4 (1.24) | 3 (1.04) | |
| Normal | 250 (47.44) | 228 (46.34) | 182 (44.83) | 174 (47.03) | 143 (44.27) | 132 (45.83) | |
| Overweight | 156 (29.60) | 152 (30.89) | 129 (31.77) | 103 (27.84) | 99 (30.65) | 85 (29.51) | |
| Obese | 112 (21.25) | 106 (21.54) | 92 (22.66) | 86 (23.24) | 77 (23.84) | 68 (23.61) | |
| Race | … | … | … | … | … | … | .9645 |
| Asian | 44 (8.35) | 41 (8.33) | 34 (8.37) | 31 (8.38) | 26 (7.03) | 26 (9.03) | |
| Black | 20 (3.08) | 17 (8.33) | 12 (2.96) | 13 (3.51) | 9 (2.79) | 8 (2.78) | |
| White | 430 (81.59) | 404 (82.11) | 312 (76.85) | 290 (78.38) | 260 (80.50) | 231 (80.21) | |
| Native American/PI | 2 (0.38) | 1 (0.19) | 1 (0.19) | 1 (0.19) | 1 (0.19) | 1 (0.19) | |
| Other | 28 (5.32) | 21 (4.92) | 16 (3.94) | 9 (2.43) | 8 (2.48) | 6 (2.08) | |
| Hispanic/Latinx | … | … | … | … | … | … | .2370 |
| Yes | 39 (7.40) | 38 (7.72) | 25 (6.16) | 21 (5.68) | 14 (4.33) | 13 (4.51) | |
| No | 487 (92.41) | 453 (92.07) | 380 (93.60) | 348 (94.05) | 309 (95.67) | 275 (95.49) | |
| Occupationb | … | … | … | … | … | … | .9999 |
| Administrative | 54 (10.25) | 52 (10.57) | 42 (10.34) | 43 (11.62) | 40 (12.38) | 31 (10.76) | |
| Allied health | |||||||
| Non–patient facing | 16 (3.04) | 16 (3.25) | 12 (2.96) | 11 (2.97) | 12 (3.72) | 12 (4.17) | |
| Patient facing | 70 (13.28) | 65 (13.21) | 54 (13.30) | 46 (12.43) | 35 (10.84) | 35 (12.15) | |
| Physician | 166 (31.50) | 152 (30.89) | 128 (31.53) | 119 (23.16) | 98 (30.34) | 91 (31.06) | |
| Nurse | 217 (41.18) | 203 (41.26) | 166 (40.89) | 147 (39.73) | 135 (41.80) | 117 (40.63) | |
| COVID-19 vaccination statusc | … | … | … | … | … | … | |
| Not vaccinated | 527 (100.00) | 441 (89.63) | 50 (12.32) | 20 (5.41) | 11 (3.41) | 2 (0.69) | |
| Fully vaccinated | … | 12 (2.44) | 356 (87.68) | 250 (94.59) | 311 (96.28) | 74 (25.69) | |
| 1 booster dose | … | … | … | … | 1 (0.31) | 212 (73.61) | |
Abbreviation: PI, Pacific Islander.
Column percentages may not add to 100% given missing data ranging from 0.19% to 11.23%. P values are based on χ2 test.
Administrative: director, supervisor, manager, chair, admissions personnel. Allied health, non–patient facing: laboratory personnel, laboratory technologist, radiology. Allied health, patient facing: technologist, medical assistant, speech pathologist, team leader, occupational therapist, dentist, phlebotomy, patient education, pharmacy, other. Physician: attending or resident medical doctor/doctor of osteopathy. Nurse: registered nurse, advanced practice registered nurse, nurse technician.
Full vaccination: completion of a primary vaccine series (2 doses of Moderna mRNA-1273 or Pfizer-BioNTech BNT162b2 or 1 dose of Johnson & Johnson/Janssen). Booster dose: 1 additional vaccine dose following a primary series.
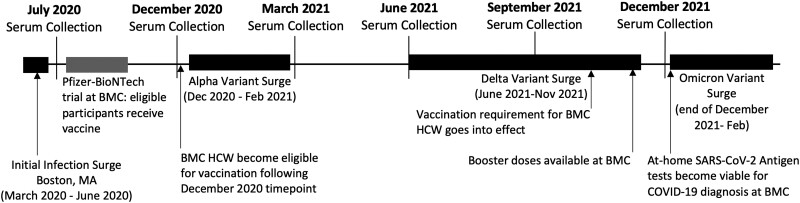
Vaccines were available to all BMC HCWs in mid-December 2020 (following serology analyses), and most participants (87.7%) reported full vaccination between January and February 2021, as captured at the March 2021 time point (Figure 2). In October 2021, BMC required all employees to be vaccinated against COVID-19, and by December 2021, 99.3% of our cohort was fully vaccinated. Of these, 54.2% received Pfizer-BioNTech (BNT162b2), 41.5% Moderna mRNA-1273, and 1.7% Johnson & Johnson (Janssen; data not shown). Two participants did not report full vaccination by December 2021 and were excluded from analyses: 1 had received 1 dose of Moderna mRNA-1273 in October 2021 and was recovering from COVID-19, for which hospital policy allowed for deferred full vaccination, and 1 did not have an EMR to cross-reference (Table 1).
Figure 2.
Timeline of major COVID-19 events and Boston Medical Center (BMC) hospital updates from July 2020 through December 2021. HCW, health care worker.
Booster doses were approved for all HCWs in the United States on 22 September 2021 and were captured in our cohort beginning in early December 2021, with 73.6% reporting booster uptake by this time point.
Incidence of COVID-19 Infection and Anti-N IgG Seroprevalence
Among all enrolled participants, 8.7% had self-reported a positive COVID-19 test result by July 2020. In December 2020, 4.7% reported a positive test result, and incidence continued to decline in March 2021 (3.2%), June 2021 (1.1%), and September (0.9%). However, in December 2021, incidence of COVID-19 infection rose to 2.4%. Beginning in June 2021, all COVID-19 positivity was classified as breakthrough infections, occurring at least 2 weeks after full vaccination. In total, there were 22 breakthrough cases of COVID-19 in this cohort. An additional 4 participants were identified as experiencing multiple COVID-19 infections over follow-up (Supplementary Table 2).
Following the last serology assessment, post hoc review of EMR data in February 2022 suggested that 13.9% of the December 2021 cohort tested positive for COVID-19 by a laboratory or self-reported at-home antigen test. In total, 138 HCWs in our cohort (26.2%) tested positive for COVID-19 from July 2020 through February 2022.
Over the 18 months, seroprevalence trends of anti-N IgG in our entire cohort generally paralleled COVID-19 infection rates (Supplementary Table 2). Anti-N IgG seroprevalence decreased from its peak of 9.5% in July 2020 to 6.9% in December 2020 but increased to 8.6% in March 2021 before declining to 5.7% in June 2021. Anti-N IgG seroprevalence then plateaued between the September (3.4%) and December (3.5%) 2021 time points.
Anti-S RBD IgG Seroprevalence
Seroprevalence of anti-S IgG expectedly mirrored vaccine uptake for the whole cohort. In July and December 2020, before widespread vaccination, 11.4% and 17.9% were seropositive for anti-S IgG, attributable to COVID-19 infection and vaccine trial participation. Following widespread vaccination, 95.6%, 98.4%, 99.1%, and 100.0% were seropositive for anti-S IgG in March 2021, June 2021, September 2021, and December 2021, respectively (Figure 2, Supplementary Table 2).
18-Month Trends of Anti-S IgG and Piecewise Regression Analyses
The kinetics of serum anti-S IgG within this cohort are described for up to 349 days from initial vaccine regimen completion in 4 time segments, defined as 0–89, 90–179, 180–269, and 270–349 days after full vaccination (Figures 3 and 4, Supplementary Figure 1, Supplementary Tables 3 and 4).
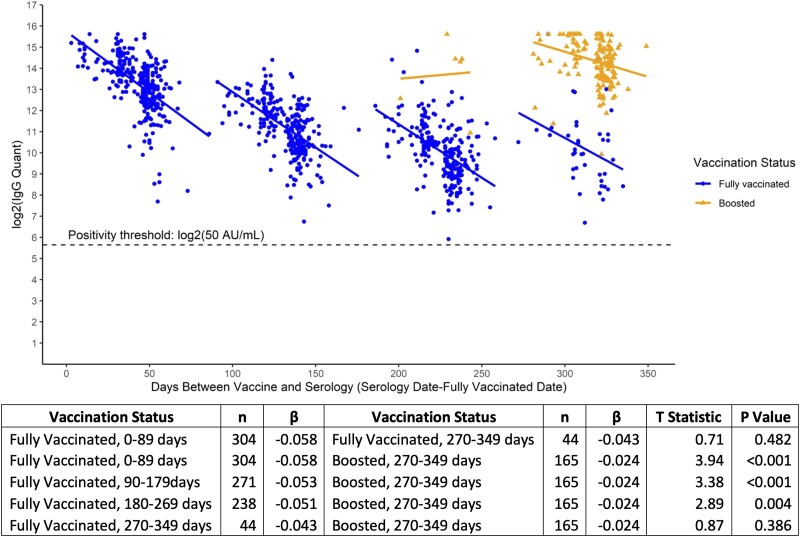
Figure 3.
Piecewise regression models of log2-transformed serum anti-S RBD (anti-Spike receptor binding domain) SARS-CoV-2 IgG antibody concentrations (AU/mL) by days between full vaccination and serology date, including vaccination status among vaccinated participants without evidence of COVID-19 infection.
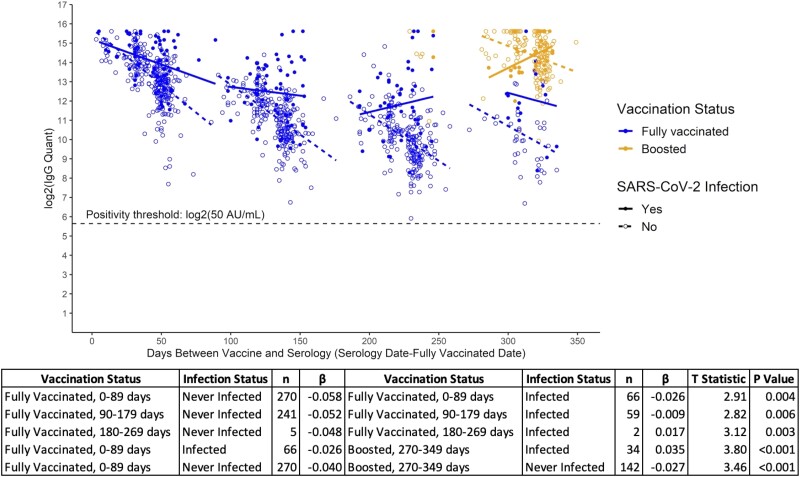
Figure 4.
Piecewise regression model of log2-transformed serum anti-S RBD (anti-Spike receptor binding domain) SARS-CoV-2 IgG antibody concentrations (AU/mL) by days between vaccination and serology, including vaccination and dichotomous infection status.
Based on log2-transformed serum anti-S IgG trends by vaccination status, the decay of serum antibodies among participants with no evidence of COVID-19 infection in the 90 days following full vaccination (β = −0.058) was greater than the decay observed from 270 to 349 days from vaccination (β = −0.043); however, this difference did not reach statistical significance (P = 0.482). The decline after full vaccination was greater at 0–89 days (β = −0.058, P < 0.001), 90–179 (β = −0.053, P < 0.001), and 180–269 (β = −0.051, P = 0.004) as compared with that observed following booster dose uptake (β = −0.024). Beyond 270 days, these observed differences between fully vaccinated and boosted individuals do not reach significance (Figure 3, Supplementary Tables 3A and 4A). Potential confounding by age, body mass index, gender, or race was assessed, and models remained unadjusted as no factor significantly affected the model (P > 0.05, data not shown).
As with the vaccinated-only participants, circulating anti-S IgG concentrations remained above the assay positivity threshold across all time points for individuals with evidence of COVID-19 infection in addition to their vaccination status (Figure 4, Supplementary Tables 3B and 4B). However, those with evidence of infection—as defined by self-reported positivity, EMR-confirmed laboratory test positivity, and/or anti-N IgG positivity—exhibited a significantly slower decline in all knots as compared with infection-naive participants. Specifically, while both groups experienced declining concentrations of anti-S IgG within the first 90 days (days 0–89), those without evidence of infection had a significantly sharper decline (infected vs never infected: β= −0.026 vs −0.058, P = 0.004). This trend persisted between 90 and 179 days (β = −0.009 vs −0.052, P = 0.006). Between 180 and 269 days, individuals with evidence of COVID-19 infection saw increasing titers of anti-S IgG, potentially attributable to infection after vaccination (Figure 4, Supplementary Tables 3B and 4B). Following booster dose uptake, increasing circulating antibodies among those with evidence of infection (β = 0.035) also suggested breakthrough COVID-19 disease after vaccination in this group, as participants who were boosted but uninfected conversely experienced a decline (β = −0.027) (Figure 4, Supplementary Tables 3B and 4B).
These results prompted stratification of infected and vaccinated participants by timing of infection (infected before vs after vaccination; ie, breakthrough infections). Participants with previous COVID-19 infection before vaccination exhibited a significantly slower decline in circulating anti-S IgG within the first 90 days following vaccination (infected prior vs never infected: β = −0.019 vs −0.058, P < 0.001) (Figure 4, Supplementary Figure 1, Supplementary Tables 3B and 4B–C).
The COVID-19 infections after vaccination (n = 22) observed in this cohort contributed to an increasing concentration of circulating anti-S IgG antibodies (Supplementary Figures 1 and 2). The incidence of infection after vaccination was variable through the follow-up period, and circulating titers of anti-S IgG fell within broad ranges for these participants. No significant differences in anti-S IgG regression were observed between the slopes of infection-naive participants and those with evidence of breakthrough infection after vaccination at any time point (P > 0.05) (Supplementary Table 4C).
Participants who were immunocompromised were omitted from the present piecewise regression analyses. Separate investigation of this group (n = 34) revealed differences in antibody decay by COVID-19 infection status that mirrored trends observed in the larger cohort (Supplementary Figure 3).
Participants in the Pfizer-BioNTech vaccine trial (n = 12) were fully vaccinated by September 2020, resulting in observed anti-S IgG antibody positivity in December 2020. These participants were omitted from the present piecewise regression analyses, but additional analyses revealed that all remained above the positivity threshold for the anti-S IgG II assay for 15 months following full vaccination and showed a strong increase in circulating anti-S IgG antibodies following booster dose uptake (Supplementary Figure 4).
Omicron Variant Susceptibility
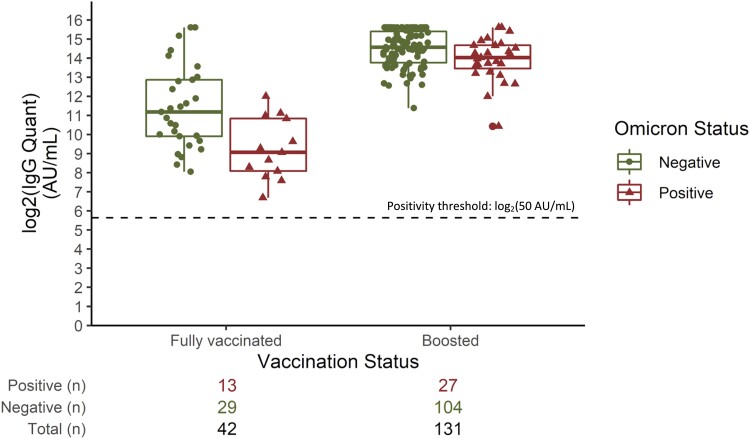
The Omicron variant surge began in Boston in December 2021 and by the end of December was responsible for more than half the COVID-19 cases in Massachusetts [20]. The last serologic time point for this study was completed in early December, and post hoc EMR analyses suggested that approximately 13.9% (n = 40) of our December cohort was COVID-19 positive from 20 December 2021 through 20 February 2022, likely attributable to the Omicron variant. During this surge, 13 of 74 (17.6%) fully vaccinated HCWs and 27 of 212 (12.7%) boosted HCWs tested positive for COVID-19. Specifically, fully vaccinated participants who tested negative for Omicron had suggestively higher median anti-S IgG concentrations in December 2021 (2326.4 AU/mL) as compared with fully vaccinated individuals who subsequently tested positive for COVID-19 (535.6 AU/mL); however, this difference was not statistically significant (P = 0.099) (Supplementary Table 5, Figure 5). Similarly, while results did not reach statistical significance, boosted individuals who tested negative for the Omicron variant had higher median anti-S IgG concentrations in December 2021 (24 260.3 AU/mL) than those who subsequently tested positive for the Omicron variant (16 711.8 AU/mL; P = 0.059). Furthermore, upon stratification by COVID-19 infection history and Omicron status, participants who were Omicron positive had suggestively lower anti-S IgG levels relative to those who tested negative for Omicron regardless of infection history, but these analyses were also not statistically significant (P > 0.05).
Figure 5.
Box plots of log2-transformed serum anti-S RBD (anti-Spike receptor binding domain) IgG antibody concentrations in December 2021 by vaccination status, including subsequent infection with the COVID-19 Omicron variant from December 2021 through February 2021. Data are presented as median (line), IQR (box), and IQR × 1.5 (whiskers).
DISCUSSION
As the COVID-19 pandemic evolves, understanding longitudinal trends of serum antibodies to SARS-CoV-2 is vital for informing public health decision making. Here, we are among the first to report SARS-CoV-2 antibody seroprevalence and kinetics from a cumulative 18 months of follow-up among HCWs at BMC in Boston. Throughout the follow-up time, antibody levels in this population consistently remained above the positivity threshold for the anti-S IgG assay and were heightened with the onset of COVID-19 infection and/or booster dose uptake. Our data highlight the importance of vaccination and help to characterize the effect of vaccination and COVID-19 infection on circulating anti-S IgG antibodies.
COVID-19 infections were primarily recorded in our cohort before December 2020 (13.4%) and from December 2021 to February 2022 (14.5%), coinciding with the initial infection surge from March through December 2020 and from the Omicron variant surge beginning in the end of December 2021, respectively, in Massachusetts. Anti-N IgG seroprevalence generally paralleled COVID-19 infection, yet higher seroprevalence-to-incidence ratios support the longevity of these circulating antibodies [6, 21, 22]. These data can reinforce the utility of anti-N antibody testing as a potential tool to capture previous asymptomatic infection while understanding that, despite data showing 7-month longevity of anti-N IgG after symptom onset, the waning of antibodies with time after infection ought to be considered [13, 22].
A majority of participants received a full COVID-19 vaccine regimen by February 2021, and 100% of our cohort was seropositive for anti-S IgG by December 2021, curbing the spread of infection and contributing to the <8% incidence of COVID-19 infection observed from December 2020 to December 2021. In spite of a documented decrease in vaccine effectiveness against the Delta variant, these fully vaccinated HCWs did not experience a similar incidence of infection, potentially highlighting an effective use of personal protective equipment and/or booster dose efficacy during the Delta variant surge when compared with Massachusetts population trends [13, 23–25]. However, even with widespread vaccination and booster dose uptake within this cohort, the known immune evasion of the Omicron variant is supported by the strong increase in infection observed after the December 2021 time point. This is potentially attributable to the increased number of mutations in the RBD region of the Omicron variant, which contribute to significant structural differences as compared with the Delta variant [26, 27] .
This is the first study to report anti-S RBD IgG antibody concentration trends for a cumulative 18 months, beginning at the start of the COVID-19 pandemic in July 2020. Our study characterizes participants’ circulating antibodies up to 15 months from vaccination in those with and without evidence of COVID-19 infection. Serum anti-S IgG concentrations are variable among individuals and across all time points in this cohort, a phenomenon explored by others [3, 17, 28]. Generally, anti-S IgG concentrations peaked following completion of primary series vaccination and declined until breakthrough COVID-19 infection and/or booster dose uptake. Our current findings support the decline in circulating anti-S IgG antibodies observed by our group and others in the months following vaccination in individuals who were COVID-19 naive [14–16, 29]. Within this cohort, we observed a 92% decline in anti-S IgG antibodies among infection-naive participants following vaccination, while Salvagno et al characterized 85% and 93% rates of decline for spike trimeric IgG and RBD IgG, respectively, in a population of HCWs [14, 29].
Among vaccinated individuals, the additional immunologic event of COVID-19 infection clearly plays a role in circulating antibody concentrations. It is evident that hybrid immunity imparted by prior infection significantly curbs the initial decline in circulating antibodies seen following vaccination as compared with no previous infection. Similar differences in antibody kinetics have also been noted between individuals who recovered from COVID-19 before vaccination and infection-naive individuals in the months following vaccination [18, 30].
To better understand trends by COVID-19 infection onset, we categorized COVID-19 positivity into “infected before full vaccination” and “infected after full vaccination (ie, breakthrough).” Infection after full vaccination appears to stimulate strong increases in circulating antibodies, although the small sample size limits major conclusions. Of interest, among 4 participants, antibody titers failed to increase following COVID-19 infection after full vaccination, potentially aligning with suggestions of immune fatigue related to SARS-CoV-2 similar to that observed for other viruses [31]. Our data suggest that an additional immunologic event supports higher serum IgG levels, yet the timing of infection in relation to vaccination may not affect serum antibody kinetics.
The subcohort analysis of HCWs enrolled in the Pfizer-BioNTech COVID-19 vaccine clinical trial provided internal support for long-term antibody kinetics observed in our cohort. Of interest, all remained above the positivity threshold for the anti-S IgG II assay for 15 months following full vaccination and showed a strong increase in circulating anti-S IgG antibodies following booster dose uptake, thus supporting the durability of these antibodies. Vaccine trial participants never contracted COVID-19 infection. While initial boosters utilized the original wild type strain of SARS-CoV-2, they seem to have helped reduce breakthrough and reinfections. It is, however, still unknown what titers of circulating antibodies correlate with protection from severe COVID-19 infection, especially as SARS-CoV-2 continues to mutate.
It is understood that given the substantial antigenic differences, the SARS-CoV-2 Omicron variant can evade the humoral immunity imparted by previous COVID-19 infection and vaccination [26, 27, 32]. Though inconclusive, serum anti-S IgG concentrations within our cohort, as measured by vaccination status prior to the Omicron variant surge, aligned with previous reports suggesting that individuals who would later be infected had lower median titers [33]. However, evidence of Omicron infection among boosted participants highlights the presence of additional mediators of immunity. The significant mutations observed in the Omicron variant spike protein are known to contribute to the humoral and cellular immunity-evading properties, yet studies show that T-cell responses to the variant may remain effective following vaccination [34]. Factors such as neutralizing capabilities of antibodies need to be evaluated to better understand the relationship between antibodies and their effectiveness against different variants. Collectively our data suggest a heterogenous antibody response among vaccinated participants with or without a history of COIVD-19 infection. Together, these findings warrant further investigation into the immunity-evading properties of the Omicron variant as well as analysis surrounding variant-specific booster doses, which will be vital in preparation for future waves of the pandemic.
Our results are strengthened by the extended follow-up time of 18 months, beginning in July 2020, as well as our thorough survey instrument and data collection techniques. We limited misclassification by confirming vaccination status and COVID-19 infection, where possible, using EMR data. In addition, our cohort design allowed for greater retention of participants over follow-up but could decrease the clarity of regression results for those with limited data points. We recognize that the humoral immunity evaluated presently is only one aspect of the greater immune profile against SARS-CoV-2. Furthermore, though serum antibodies can be readily measured, considerable variation exists among SARS-CoV-2 serologic assays, resulting from different characteristics and manufacturers, and thus are not interchangeable [35]. Virus neutralization or pseudoneutralization assays, the gold standard for evaluating protective antibody status, were unavailable to us at the time of these analyses. Results from these analyses are generalizable primarily to HCWs.
In conclusion, the described results at BMC are among the first to provide data from 18 months of follow-up among a cohort of HCWs, helping to identify important trends in the kinetics of serum antibodies to SARS-CoV-2. HCWs infected with COVID-19 prior to vaccination experienced slower anti-S IgG antibody decline as compared with noninfected individuals following vaccination. Furthermore, booster dose uptake significantly increased anti-S IgG titers and attenuated antibody waning following initial vaccination regardless of infection status. Our findings regarding serum anti-N IgG and anti-S IgG antibodies through the Alpha, Delta, and Omicron surges are important for characterizing humoral immunity.
Supplementary Material
Contributor Information
Maura C Dodge, Department of Pathology and Laboratory Medicine, Boston Medical Center, Boston, Massachusetts, USA.
Lei Ye, Department of Biostatistics, Abbott Core Diagnostics, Abbott Park, Illinois, USA.
Elizabeth R Duffy, Department of Pathology and Laboratory Medicine, Chobanian and Avedisian School of Medicine, Boston University, Boston, Massachusetts, USA.
Manisha Cole, Department of Pathology and Laboratory Medicine, Chobanian and Avedisian School of Medicine, Boston University, Boston, Massachusetts, USA.
Susan H Gawel, Department of Biostatistics, Abbott Core Diagnostics, Abbott Park, Illinois, USA.
Martha M Werler, Department of Epidemiology, School of Public Health, Boston University, Boston, Massachusetts, USA.
David Daghfal, Department of Biostatistics, Abbott Core Diagnostics, Abbott Park, Illinois, USA.
Chris Andry, Department of Pathology and Laboratory Medicine, Boston Medical Center, Boston, Massachusetts, USA; Department of Pathology and Laboratory Medicine, Chobanian and Avedisian School of Medicine, Boston University, Boston, Massachusetts, USA.
Yachana Kataria, Department of Pathology and Laboratory Medicine, Boston Medical Center, Boston, Massachusetts, USA; Department of Pathology and Laboratory Medicine, Chobanian and Avedisian School of Medicine, Boston University, Boston, Massachusetts, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Y. K. designed the project. M. C. D., E. R. D., M. C., C. A., and Y. K. provided administrative support. M. C. D., L. Y., S. H. G., M. M. W., D. D., and Y. K. were involved with data analysis and interpretation. All authors prepared and approved the manuscript.
Acknowledgments . We acknowledge the clinical chemistry, phlebotomy, and central receiving staff in the Department of Pathology and Laboratory Medicine at BMC for their work to help accomplish this study.
Financial support. This work was funded, in part, by BMC Development Philanthropy Funds for COVID-19 Research and Abbott Core Diagnostics.
Patient consent . All participants provided written consent prior to enrollment in the study. The study design was approved by the BMC Institutional Review Board (H-40503) and conforms to standards currently applied in the United States.
Data availability . Data sets generated during and/or analyzed during the current study are not publicly available, as they reflect a sensitive patient population but may be made available upon reasonable request to the corresponding author.
References
- 1. Jones JM, Opsomer JD, Stone M, et al. Updated US infection- and vaccine-induced SARS-CoV-2 seroprevalence estimates based on blood donations, July 2020–December 2021. JAMA 2022; 328:298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . COVID-19 data tracker. Accessed Dec 2022. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html
- 3. Uprichard SL, O’Brien A, Evdokimova M, et al. Antibody response to SARS-CoV-2 infection and vaccination in COVID-19-naïve and experienced individuals. Viruses 2022; 14:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bates TA, McBride SK, Leier HC, et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol 2022; 7:eabn8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science 2022; 375:1122–7. [DOI] [PubMed] [Google Scholar]
- 6. Brochot E, Demey B, Touzé A, et al. Anti-spike, anti-nucleocapsid and neutralizing antibodies in SARS-CoV-2 inpatients and asymptomatic individuals. Front Microbiol. 2020; 11:584251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Post N, Eddy D, Huntley C, et al. Antibody response to SARS-CoV-2 infection in humans: a systematic review. PLoS One 2020; 15:e0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur 2021; 10:100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med 2020; 383:2439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen LH, Drew DA, Graham MS, et al. Coronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health 2020; 5:e475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Nurses Association. COVID vaccine facts for nurses survey. Accessed Dec 2022. Available at: https://www.nursingworld.org/news/news-releases/2021/ana-supports-mandated-covid-19-vaccinations-for-nurses-and-all-health-care-professionals/
- 12. American Medical Association . Accessed Dec 2022. Available at: https://www.ama-assn.org/system/files/2021-06/physician-vaccination-study-topline-report.pdf
- 13. Kataria Y, Cole M, Duffy E, et al. Seroprevalence of SARS-CoV-2 IgG antibodies and risk factors in health care workers at an academic medical center in Boston, Massachusetts. Sci Rep 2021; 11:9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dodge MC, Cole M, Duffy ER, Werler MM, Kataria Y. Fifteen-month follow-up of anti-spike receptor-binding domain SARS-CoV-2 antibodies among healthcare workers in Boston, MA. J Appl Lab Med 2022;7:1430–7. [DOI] [PubMed] [Google Scholar]
- 15. Zhang S, Xu K, Li C, et al. Long-term kinetics of SARS-CoV-2 antibodies and impact of inactivated vaccine on SARS-CoV-2 antibodies based on a COVID-19 patients cohort. Front Immunol 2022; 13:829665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang Y, Yang M, Peng Y, et al. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nat Microbiol 2022; 7:423–33. [DOI] [PubMed] [Google Scholar]
- 17. Congrave-Wilson Z, Cheng WA, Lee Y, et al. Twelve-month longitudinal serology in SARS-CoV-2 naïve and experienced vaccine recipients and unvaccinated COVID-19-infected individuals. Vaccines 2022; 10:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarjomaa M, Diep LM, Zhang C, et al. SARS-CoV-2 antibody persistence after five and twelve months: a cohort study from South-Eastern Norway. PLoS One 2022; 17:e0264667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Decru B, Van Elslande J, Steels S, et al. IgG anti-spike antibodies and surrogate neutralizing antibodies decline faster 3 to 10 months after BNT162b2 vaccination than after SARS-CoV-2 infection in healthcare workers. Front Immunol 2022; 13:909910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Massachusetts Department of Public Health- COVID-19 Response Reporting. Accessed Dec 2022. Available at: https://www.mass.gov/info-details/covid-19-response-reporting#covid-19-weekly-public-health-report-(archive)
- 21. Van Elslande J, Oyaert M, Ailliet S, et al. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J Clin Virol 2021; 136:104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ortega N, Ribes M, Vidal M, et al. Seven-month kinetics of SARS-CoV-2 antibodies and role of pre-existing antibodies to human coronaviruses. Nat Commun 2021; 12:4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy, and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Inf 2022; 28:202–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bian L, Gao Q, Gao F, et al. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev Vaccines 2021; 20:1201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niikura R, Fujishiro M, Nakai Y, et al. International observational survey of the effectiveness of personal protective equipment during endoscopic procedures performed in patients with COVID-19. Digestion 2021; 102:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature 2022; 604:553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022; 602:671–5. [DOI] [PubMed] [Google Scholar]
- 28. Sasso BL, Agnello L, Giglio RV, et al. Longitudinal analysis of anti-SARS-CoV-2 S-RBD IgG antibodies before and after the third dose of the BNT-162b2 vaccine. Sci Rep 2022; 12:8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salvagno G, Henry B, Pighi L, De Nitto S, Gianfilippi G, Lippi G. The pronounced decline of SARS-CoV-2 spike trimeric IgG and RBD IgG in baseline seronegative individuals six months after BNT162b2 vaccination is consistent with the need for vaccine boosters. Clin Chem Lab Med 2021; 60:e29–31. [DOI] [PubMed] [Google Scholar]
- 30. Dakovic Rode O, Bodulić K, Zember S, et al. Decline of anti-SARS-CoV-2 IgG antibody levels 6 months after complete BNT162b2 vaccination in healthcare workers to levels observed following the first vaccine dose. Vaccines 2022; 10:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaminski H, Lemoine M, Pradeu T. Immunological exhaustion: how to make a disparate concept operational? PLoS Pathog 2021; 17:e1009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andrews N, Stowe J, Kirsebom F, et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Eng J Med 2022; 386:1532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Möhlendick B, Čiučiulkaitė I, Elsner C, et al. Individuals with weaker antibody responses after booster immunization are prone to Omicron breakthrough infections. Front Immunol 2022; 13:907343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Z, Zhang Y, Wang M, et al. Humoral and cellular immune responses of COVID-19 vaccines against SARS-cov-2 Omicron variant: a systemic review. Int J Biol Sci 2022; 18:4629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Infantino M, Pieri M, Nuccetelli M, et al. The WHO international standard for COVID-19 serological tests: towards harmonization of anti-spike assays. Int Immunopharmacol 2021; 100:108095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.