Abstract
Objective
The glymphatic pathway, characterised as a cerebral drainage system, influences cognitive function in neurodegenerative diseases; however, evidence is limited in a normal ageing population. The aim of this study was to investigate the effect of glymphatic function on ageing-related cognitive decline.
Methods
We retrospectively reviewed the Cognitive Impairment, Retinopathy, and Cerebrovascular Lesions in the Elderly (CIRCLE) study, and participants with multi-model magnetic resonance imaging (MRI) scans and Mini-Mental State Examinations (MMSE) were enrolled. Glymphatic function was evaluated via the diffusion tensor imaging along the perivascular space (DTI-ALPS) index. Regression models were used to estimate the impact of the DTI-ALPS index on cognitive decline cross-sectionally and longitudinally. We further analysed the mediation effect of the DTI-ALPS on age and cognitive function.
Results
A total of 633 participants were included in this study (48.2% female; mean age, 62.8 ± 8.9 years). The DTI-ALPS index was positively associated with cognitive function cross-sectionally (β = 0.108, P = 0.003), and was an independent protective factor for cognitive decline longitudinally (odds ratio (OR) = 0.029, P = 0.007). The DTI-ALPS index declined progressively with ageing (r = −0.319, P <0.001), and the decrease was more pronounced after 65 years of age. Furthermore, the DTI-ALPS index mediated the relationship between age and MMSE score (β = −0.016, P <0.001). The mediation effect accounted for 21.3%, which was higher in subjects aged over 65 years (25.3%) compared with those aged under 65 years (5.3%).
Conclusion
Glymphatic function played a protective role in normal ageing-related cognitive decline, which may serve as a potential therapeutic target against cognitive decline in future.
Keywords: glymphatic function, diffusion tensor imaging along the perivascular space (DTI-ALPS) index, cognition, ageing, older people
Key Points
Glymphatic function was a protective factor for ageing-related cognitive decline.
Glymphatic function decreased progressively with age.
Introduction
Cognitive decline is becoming increasingly prevalent worldwide as a consequence of an ageing population [1]. People with cognitive decline are prone to dementia, while those who have not progressed to dementia, cognitive decline interferes with their ability to work and engagement in social activities [2–4]. Currently, more than 1 in 9 adults older than 45 years have reported experiencing cognitive decline, and more than 55 million older adults worldwide live with dementia [5, 6]. Unfortunately, few effective treatments can halt the progression of cognitive decline.
In recent years, the concept of the glymphatic system has provided novel insight into the mechanisms of cognitive impairment [7, 8]. As a highly organised waste clearance pathway of the central nervous system, the glymphatic system facilitates the flow of cerebrospinal fluid from arterial perivascular spaces to the interstitium and then to the venous perivascular spaces, ultimately clearing metabolites and waste products from the brain [9]. Accumulating findings from rodent models suggest that decreased glymphatic function leads to amyloid-β and tau accumulation [10, 11]. Consequently, the glymphatic system has been implicated in Alzheimer’s disease (AD) and other neurodegenerative diseases [12]. Encouragingly, some animal intervention research has shown that cognition could be improved by accelerating glymphatic clearance [13, 14], indicating that glymphatic function may be a potential target for the treatment of cognitive decline.
The majority of previous studies on the glymphatic system were conducted using rodent models, as human research is limited due to the requirement for the intrathecal-administered contrast agent to assess glymphatic function [15]. The diffusion tensor imaging along the perivascular space (DTI-ALPS) index offers an opportunity to non-invasively investigate the human glymphatic system [16]. Identified by projection and association fibres orthogonal to the perivascular space, the DTI-ALPS index has been proven to reflect glymphatic function in our previous study, as well as in other neurological disorders including AD, Parkinson’s disease (PD) and neuromyelitis optic spectrum disorder [17–20]. Recent evidence based on this method suggests an association between glymphatic function and amyloid deposition [21], and glymphatic function might act as a significant mediator in the relationship between amyloid deposition and cognitive dysfunction in AD [22].
Therefore, the aim of this study was to clarify whether glymphatic function played a role in ageing-related cognitive decline by investigating the effect of the DTI-ALPS index on cognition cross-sectionally and longitudinally using the CIRCLE study. We further explored the exact contribution of glymphatic function to cognition during normal ageing by measuring the mediation effect of DTI-ALPS on age and cognitive function.
Materials and methods
Study subjects
We retrospectively reviewed the individuals recruited in the CIRCLE study (ClinicalTrials.gov ID: NCT03542734) between January 2010 and December 2021. The CIRCLE study is a single-centre prospective observational study, aiming to enrol 1,000 individuals to explore the mechanisms of cognitive impairment. Adults aged ≥40 years and free of known dementia or stroke are included, and those with MRI contraindications, serious head injury, intracranial surgery or cancer are excluded. Participants undergo a neuropsychological test and multi-modal magnetic resonance imaging (MRI) scan. Criteria for selecting the subjects in this study were: (1) age ≥40 years; (2) free of known dementia or stroke; (3) completed multi-model MRI scan and Mini-Mental State Examinations (MMSE) evaluation; and (4) had written informed consent. Exclusion criteria were: failed calculation of the DTI-ALPS index due to severe head motion. We also retrieved participants’ demographic and clinical data including age, gender, years of education and co-morbid conditions. In our study, hypertension, diabetes, hyperlipidaemia and smoking were described as vascular risk factors. Participants who completed the follow-up neuropsychological assessment were included in the longitudinal study.
Ethics statement
The study was approved by the Human Ethics Committee of the Second Affiliated Hospital of Zhejiang University, School of Medicine. All clinical investigation was conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Neuropsychological assessment
To assess the cognitive function of each subject, the Chinese version of the MMSE translated from the original version was administered through face-to-face interviews [23]. Cognitive decline was defined as a decrease of three or more points of the MMSE in a follow-up visit [24, 25].
MRI protocol
All subjects underwent multi-model MRI using a 3.0 T MRI scanner (GE) with an 8-channel brain-phased array coil. The imaging parameters of 3D-T1 were: repetition time = 7.3 ms, echo time = 3.0 ms, flip angle = 8°, thickness = 1 mm, field of view = 25 × 25 cm2, matrix = 250 × 250; T2 fluid attenuated inversion recovery (T2 FLAIR): repetition time = 8,400 ms, echo time = 150 ms, field of view = 24 × 24 cm2, matrix size = 256 × 256, inversion time = 2,100 ms, slice thickness = 4.0 mm with no gap between slices. Diffusion tensor imaging (DTI): maximum b-value = 1,000 s/mm2, 30 non-collinear directions, repetition time = 4,600 ms, echo time = 69.3 ms, slice thickness = 2 mm, slice gap = 1 mm, matrix size = 160 × 160, field of view = 26 × 26 cm2. Enhanced T2 Star Weighted Angiography (ESWAN): echo time = 4.5 ms, inter-echo spacing = 4.5 ms, repetition time = 58 ms, field of view = 24 × 24 cm2, matrix size = 256 × 256, flip angle = 20°, slice thickness = 2 mm with no slice gap.
Evaluation of imaging
The DTI-ALPS index calculation was consistent with previous studies [16, 26]. First, the ESWAN raw data were processed to Phase images on the workstation (ADW4.4, GE). Second, diffusivity maps of DTI data in the direction of the x-axis (right–left), y-axis (anterior–posterior), z-axis (inferior–superior) and colour-coded fractional anisotropy (FA) maps were processed using DTI Studio (https://www.mristudio.org). Where the deep medullary veins were vertical to the ventricle body on Phase images, two 5 mm diameter regions of interest (ROIs) were placed on the projection fibres and the association fibres of the colour-coded FA map. The diffusivities in the directions of the x-axis (Dx), y-axis (Dy) and z-axis (Dz) of ROIs on projection fibres and association fibres were recorded as Dxproj, Dyproj, Dzproj, Dxassoc, Dyassoc, Dzassoc, respectively. The DTI-ALPS index was then calculated as [(Dxproj + Dxassoc)/(Dyproj + Dzassoc)]. A neurologist (Z.K., with 8 years of experience) placed ROIs and calculated the DTI-ALPS index independently for all subjects. For inter-observer reliability analysis, another neurologist (R.W., with 4 years of experience) placed ROIs and calculated the DTI-ALPS index independently for 50 subjects. For intra-observer reliability analysis, Z.K. re-placed ROIs and re-calculated the DTI-ALPS index for 50 subjects after 3 month intervals. The observers were blinded to the clinical data during measurements. The workflow of DTI-ALPS index processing is shown in Figure 1.
Figure 1.
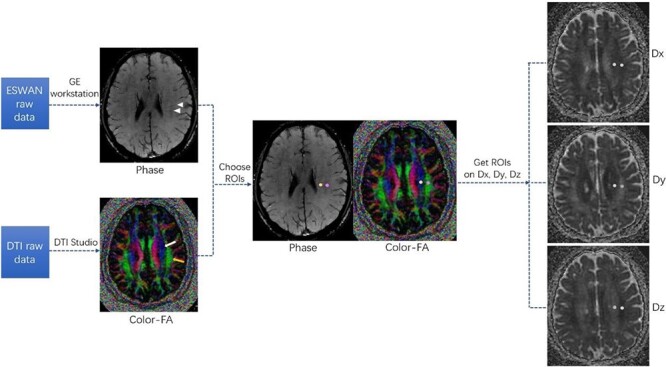
Workflow of diffusion tensor imaging along the perivascular space (DTI-ALPS) index processing. Phase image and colour-fractional anisotropy (FA) maps were obtained from ESWAN and DTI raw data, respectively. At the location of deep medullary veins (triangular arrows in the Phase image) vertical to the ventricle body, two regions of interest (ROIs) in the area of the projection fibres (white arrow on the colour-FA image) and the association fibres (yellow arrow on the colour-FA image) were placed on the colour-FA map. The diffusivities of ROIs in the directions of the x-axis (Dx), y-axis (Dy) and z-axis (Dz) were obtained accordingly. The DTI-ALPS index was then calculated as [(Dxproj + Dxassoc)/(Dyproj + Dzassoc)].
Cerebral volume, referred to as the volume of brain parenchyma, was derived from the 3D-T1 segmentation using the Statistical Parametric Mapping version 12 software package (SPM12; London, UK; https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). The white matter hyperintensities (WMH) volume was automatically processed on T2 FLAIR images using the lesion segmentation tool (LST) toolbox [27] in SPM12.
Statistics
All analyses were carried out using statistical product and service solutions (SPSS) software (version 22.0). The correlations between variables were assessed using Pearson’s or Spearman’s correlations. Clinical and neuroimaging characteristics of the two groups were compared via t-test, Wilcoxon rank sum and chi-squared tests as appropriate. WMH volumes were natural log transformed to normalise their distribution and preliminary analyses were conducted to ensure no violation of the multicollinearity assumptions. Associations between the DTI-ALPS index, MMSE score and cognitive decline were tested using a multivariable linear regression model and binary logistic regression model, respectively; age, years of education and other confounders were covariates. One-way analysis of variance (ANOVA) was carried out in different age groups, and the least significance difference (LSD) method was applied for the post-hoc test. Mediation analyses were performed using plugin called “Process” (version 3.2) for SPSS. Age was regarded as an exposure variable, the DTI-ALPS index was a potential mediator variable and the MMSE score was an outcome variable. Linear regression was used to test for risk factors of the DTI-ALPS index. All analyses were performed blinded to the participant identifying information, and a P value of <0.05 was considered statistically significant.
Results
Participant characteristics
A total of 633 older adults were included in the final analysis after excluding 18 participants whose DTI-ALPS index calculation failed due to severe head motion. The average age of the participants was 62.8 ± 8.9 years and 48.2% were female. The average DTI-ALPS index was 1.414 ± 0.193. The inter- and intra-observer correlation coefficients of the DTI-ALPS index were 0.901 and 0.916, respectively. The average MMSE score was 25.6 ± 4.1. Table 1 shows the demographic, clinical and imaging characteristics of the participants.
Table 1.
The baseline characteristics and multivariable linear regression for the MMSE score
| Total n = 633 | Model 1 | Model 2 | |||
|---|---|---|---|---|---|
| β | P value | β | P value | ||
| Age, years, mean ± SD | 62.8 ± 8.9 | −0.125 | 0.001 | −0.113 | 0.015 |
| Female, n (%) | 305 (48.2) | −0.116 | 0.006 | −0.074 | 0.104 |
| Years of education, median (IQR) | 8 (5–12) | 0.484 | <0.001 | 0.481 | <0.001 |
| Hypertension, n (%) | 361 (57.0) | −0.043 | 0.203 | −0.045 | 0.186 |
| Smoking, n (%) | 184 (29.1) | −0.030 | 0.456 | −0.028 | 0.489 |
| DTI-ALPS index, mean ± SD | 1.414 ± 0.193 | 0.101 | 0.004 | 0.108 | 0.003 |
| Cerebral volume, ml, mean ± SD | 1108.9 ± 117.5 | 0.095 | 0.026 | ||
| logWMH volume, ml, mean ± SD | 0.535 ± 0.641 | 0.059 | 0.189 | ||
| Diabetes, n (%) | 107 (16.9) | ||||
| Hyperlipidaemia, n (%) | 118 (18.6) | ||||
DTI-ALPS, diffusion tensor imaging along the perivascular space; MMSE, Mini-Mental State Examination; WMH, white matter hyperintensities; SD, Standard Deviation. Bold indicates p < 0.05.
Model 1: adjusted for age, female, years of education, hypertension and smoking.
Model 2: adjusted for age, female, years of education, hypertension, smoking, cerebral volume and logWMH volume.
Cross-sectional and longitudinal correlation between the DTI-ALPS index and cognition
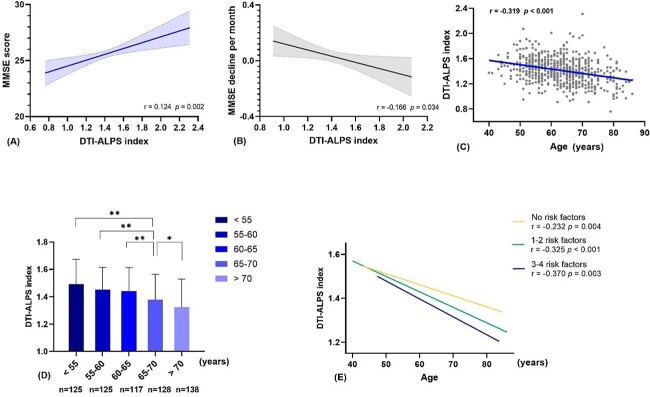
In the cross-sectional study, the DTI-ALPS index was positively associated with the MMSE score (r = 0.124, P = 0.002) (Figure 2A). Furthermore, age, years of education, history of hypertension, history of smoking, cerebral volume and logWMH volume were all associated with the MMSE score (all P <0.05) (Supplementary Table 1). As Table 1 shows, the DTI-ALPS index was independently related to the MMSE score after adjusting for age, gender, years of education, history of hypertension and history of smoking in multivariable linear regression model 1 (β = 0.101, P = 0.004), and the association was more pronounced after additionally adjusting for cerebral volume and WMH volume in multivariable linear model 2 (β = 0.108, P = 0.003).
Figure 2.
(A) Correlation between the DTI-ALPS index and MMSE score. (B) Correlation between the DTI-ALPS index and MMSE decline per month. (C) Scatter plot shows the correlation of the DTI-ALPS index and age. (D) The DTI-ALPS index in different age groups. * and ** indicates P <0.05 and P <0.01, respectively in post-hoc analysis between the two groups. (E) Correlations of age and the DTI-ALPS index with a different number of vascular risk factors. DTI-ALPS, diffusion tensor imaging along the perivascular space; MMSE, Mini-Mental State Examination.
In the longitudinal study, a total of 164 participants completed the follow-up neuropsychological assessment. The comparison of baseline characteristics in participants with and without follow-up visits are listed in Supplementary Table 2. The average follow-up interval was 16.9 ± 9.8 months, with 32 participants (19.5%) developing cognitive decline (decrease of MMSE ≥3).
As Table 2 shows, compared with participants without cognitive decline, those with cognitive decline were older and had fewer years of education, smaller cerebral volume and a lower DTI-ALPS index (all P <0.05). Binary logistic regression suggested that a lower DTI-ALPS index was an independent predictor of cognitive decline (OR = 0.029, 95% CI: 0.002–0.386, P = 0.007), when adjusting for age, years of education and cerebral volume. Figure 2B presents the negative correlation between the DTI-ALPS index and MMSE decline per month (r = −0.166, P = 0.034).
Table 2.
Univariate analysis and binary logistic regression for cognitive decline
| Cognitive decline n = 32 | Non-cognitive decline n = 132 | P value | Cognitive decline | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | ||||
| Age, years, mean ± SD | 66.1 ± 8.3 | 62.3 ± 8.9 | 0.030 | 0.996 | 0.943–1.052 | 0.893 |
| Female, n (%) | 11 (34.4) | 60 (45.5) | 0.256 | |||
| Years of education, median (IQR) | 5 (2–8) | 9 (5–12) | 0.001 | 0.871 | 0.788–0.963 | 0.007 |
| Hypertension, n (%) | 19 (59.4) | 83 (62.9) | 0.714 | |||
| Diabetes, n (%) | 6 (18.8) | 25 (18.9) | 0.980 | |||
| Hyperlipidaemia, n (%) | 3 (9.4) | 30 (22.7) | 0.149 | |||
| Smoking, n (%) | 8 (25.0) | 41 (31.1) | 0.502 | |||
| DTI-ALPS index, mean ± SD | 1.318 ± 0.157 | 1.432 ± 0.194 | 0.003 | 0.029 | 0.002–0.386 | 0.007 |
| Cerebral volume, ml, mean ± SD | 1063.2 ± 96.5 | 1114.3 ± 106.4 | 0.014 | 0.998 | 0.993–1.002 | 0.266 |
| logWMH volume, ml, mean ± SD | 0.876 ± 0.618 | 0.632 ± 0.706 | 0.075 | |||
| Baseline MMSE, mean ± SD | 25.6 ± 4.2 | 26.4 ± 3.4 | 0.320 | |||
| Follow-up interval, month, mean ± SD | 17.7 ± 9.1 | 16.7 ± 10.0 | 0.624 | |||
DTI-ALPS, diffusion tensor imaging along the perivascular space; SD, standard deviation; IQR, interquartile range; MMSE, Mini-Mental State Examination; WMH, white matter hyperintensities. Bold indicates p < 0.05.
Association between age and the DTI-ALPS index
As Figure 2C and D show, the DTI-ALPS index decreased with ageing (r = −0.319, P <0.001). One-way ANOVA indicated a significant difference of the DTI-ALPS index among different age groups (F = 17.3, P <0.001), and the post-hoc test showed the DTI-ALPS index of groups above 65 years old was significantly lower than other groups (all P <0.05). Moreover, participants were divided into three groups with vascular risk factors 0, 1–2 and 3–4, respectively. As Figure 2E shows, the DTI-ALPS index of participants with more risk factors decreased faster, as demonstrated by higher absolute values of correlation coefficients between the DTI-ALPS index and age.
The role of the DTI-ALPS index between age and cognition function
There was a significant mediation effect of the DTI-ALPS index in the relationship on age and MMSE score, after controlling for sex and years of education (β = −0.016, 95% CI: –0.028 to –0.006, P <0.001). The mediation effect accounted for 21.3% of the total effect (Figure 3A). Due to the rapid decline of the DTI-ALPS index after 65 years of age, mediation analyses were conducted separately on participants under and over 65 years old. As Figure 3B and C shows, the mediation effect was only 5.3% under 65 years of age, while this effect was up to 25.3% over 65 years of age.
Figure 3.
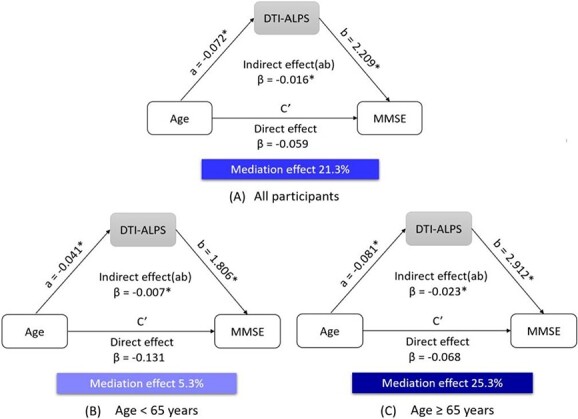
Mediation analysis of the DTI-ALPS index on age and MMSE score in (A) all participants, (B) under 65 years old and (C) over 65 years old. DTI-ALPS, diffusion tensor imaging along the perivascular space; MMSE, mini-mental state examination. * indicates p < 0.05.
Risk factors associated with the DTI-ALPS index
Males and participants with a history of hypertension or diabetes had a significantly lower DTI-ALPS index (all P <0.05, Supplementary Figure 1). No significant differences were observed in participants with or without a history of hyperlipidaemia or smoking. Furthermore, linear regression analysis found that ageing (β = −0.296, P <0.001), male (β = −0.120, P = 0.001), history of hypertension (β = −0.089, P = 0.019) and diabetes (β = −0.100, P = 0.008) were independent risk factors for a low DTI-ALPS index (Supplementary Table 3).
Discussion
Our study found that glymphatic function was a protective factor for cognitive decline in a relatively large population, and ageing was closely related to glymphatic dysfunction. Furthermore, glymphatic dysfunction contributed 21.3% to normal ageing-related cognitive decline.
Previous research has suggested that glymphatic dysfunction is related to cognitive decline in multiple neurodegenerative diseases, including AD, PD and idiopathic normal pressure hydrocephalus [19, 28]; with Nedergaard et al. highlighting that glymphatic failure might be the final common pathway to dementia for all neurodegenerative diseases [7]. This study has demonstrated that cognitive decline caused by natural ageing might also be related to glymphatic dysfunction. In addition, Zhang et al. speculated that glymphatic system impairment, as a result of ageing and diabetes, may predispose the development of cognitive dysfunction [29]. The deposition of metabolic products and consequent inflammation might be the core mechanisms. Accumulating evidence suggests that pathological proteins and iron deposition in the brain are cleared via the glymphatic pathway [11, 30, 31]. It has been shown that the deposition of pathological proteins could trigger neurotoxicity and inflammation [7, 32], and iron accumulation can compromise synaptic transmission and neuronal function [33]. Moreover, the impaired glymphatic system could downregulate the clearance of inflammatory cells and aggravate inflammation [34, 35]. Ultimately, the deposited metabolic products and inflammation could induce a cascade of pathological damage leading to cognitive impairment.
Our previous study reported the close relationship between ageing and glymphatic dysfunction in patients who underwent glymphatic MRI [36]. The current study once again proves the phenomenon in a much larger cohort with normal ageing participants, allowing clarification of the relationship between ageing and glymphatic function. The changes of cerebral vasculature with ageing might be the main mechanism [12], as cerebral arteries become stiffer with ageing leading to the retention of interstitial fluid in dilated perivascular spaces and impairment of the glymphatic system [37]. Furthermore, weakened arterial pulsations would slow down the glymphatic flow [38]. In addition to cerebral vasculature, previous studies have shown that the efficacy of glymphatic fluid transport was directly correlated with the quality of sleep [39, 40]. Sleep impairment is prevalent in older people, whose disrupted sleep architecture may sharply diminish the clearance of brain fluid and export of protein waste [41]. Moreover, the injury of meningeal lymphatics via ageing also exacerbates glymphatic dysfunction [36].
Furthermore, it is noteworthy that glymphatic function mediates the relationship between ageing and cognitive decline, since emerging evidence has shed light on the treatment of cognitive impairment by ameliorating glymphatic function. A rodent study showed voluntary exercise accelerated glymphatic clearance, attenuated the accumulation of amyloid plaques and ultimately protected mice against a decline in spatial cognition [42]. Another rodent study found a polyunsaturated fatty acid supplement could improve glymphatic transport and protect cognitive function [13]. Moreover, photobiomodulation therapy and digoxin, a traditional cardiovascular drug, has also shown protective effects on glymphatic function and cognition [43, 44]. The interventions mentioned above may be translated from animal to human one day, and it is worth considering the glymphatic pathway as a potential prevention or treatment target for cognitive decline in the future.
Additionally, our study revealed that participants with hypertension or diabetes exhibited decreased glymphatic function, which was accordant with previous animal studies. Specifically, spontaneous hypertensive rats showed impaired glymphatic transport compared with their wild phenotypes [45]. Type 2 diabetes mellitus rats also demonstrated suppressed clearance of interstitial fluid in the hippocampus and hypothalamus [46]. As arterial pulsations drive bulk glymphatic flow in the perivascular space [38], stiffness of the arterial wall caused by vascular risk factors might weaken arterial pulsations, reducing glymphatic flow. Our finding provides new evidence for cognitive decline in participants with hypertension and diabetes, and again highlights the importance of blood pressure and glucose control as they are not only vascular risk factors that may cause a series of complications but also considerable risk factors for the glymphatic system.
Due to practical constraints, some limitations of the current study need to be addressed. First, the DTI-ALPS index was calculated based on the diffusion of perivascular space and may not be directly equivalent to glymphatic function, although it has been demonstrated by glymphatic MRI and has been widely applied in multiple diseases. Second, glymphatic activity is mostly active during sleep, whereas the participants were scanned during the daytime, which may not represent intact glymphatic function. Third, it was only a preliminary imaging study that lacked pathology or other biomarkers, which needs to be confirmed by further studies. Fourth, cognition function was assessed using the MMSE in our study. Although the MMSE is widely used for screening dementia, it is susceptible to educational level, and is of low sensitivity to mild cognitive impairment and longitudinal cognitive decline. Future studies should consider the Montreal Cognitive Assessment (MoCA) or other additional tests. Lastly, the sample of our longitudinal analysis was smaller than the cross-sectional analysis, which might lead to potential bias. A large follow-up sample needs to be assessed in the future.
Conclusion
In general, glymphatic function could protect aged people against cognitive decline. While the glymphatic system is vulnerable to ageing and vascular risk factors, it might be a prevention or treatment target for cognitive decline with normal ageing.
Supplementary Material
Contributor Information
Junjun Wang, Department of Neurology, the Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou 310009, China; Department of Neurology, Zhejiang Hospital, Hangzhou 310012, China.
Ying Zhou, Department of Neurology, the Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou 310009, China.
Kemeng Zhang, Department of Neurology, the Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou 310009, China.
Wang Ran, Department of Neurology, the Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou 310009, China.
Xiao Zhu, Department of Neurology, the Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou 310009, China.
Wansi Zhong, Department of Neurology, the Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou 310009, China.
Yuping Chen, Department of Neurology, the Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou 310009, China.
Jiaping Li, Department of Neurology, the Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou 310009, China.
Jianzhong Sun, Department of Radiology, the Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou 310009, China.
Min Lou, Department of Neurology, the Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou 310009, China.
Declaration of Conflicts of Interest
None.
Data Availability
Due to privacy issues of data, it is not available to the community via open repository. The datasets generated during the present study are available from the corresponding author on reasonable request. Considerations will be made based on the review of reasons for requesting the data and the procedures for ensuring data privacy.
Declaration of Sources of Funding
This work was supported by the National Natural Science Foundation of China (81,971,101, 82,171,276 and 82,101,365).
References
- 1. Livingston G, Huntley J, Sommerlad A et al. Dementia prevention, intervention, and care: 2020 report of the Lancet commission. Lancet 396: 413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuehn B. Discussions about cognitive decline. JAMA 2018; 320: 750. [DOI] [PubMed] [Google Scholar]
- 3. Martino Adami P, Orellana A, García P et al. Matrix metalloproteinase 10 is linked to the risk of progression to dementia of the Alzheimer's type. Brain 2022; 145: 2507–17. [DOI] [PubMed] [Google Scholar]
- 4. Hamel R, Köhler S, Sistermans N et al. The trajectory of cognitive decline in the pre-dementia phase in memory clinic visitors: findings from the 4C-MCI study. Psychol Med 2015; 45: 1509–19. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization, Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia (15 March 2023, date last accessed).
- 6. Christopher A, Erin D, Lisa C. Subjective cognitive decline among adults aged ≥45 years – United States, 2015–2016. Centers for Disease Control and Prevention–Morbidity and Mortality Weekly Report 2018; 67: 754–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nedergaard M, Goldman S. Glymphatic failure as a final common pathway to dementia. Science 2020; 370: 50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Plog B, Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol 2018; 13: 379–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rasmussen M, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. The Lancet Neurology 2018; 17: 1016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Da Mesquita S, Louveau A, Vaccari A et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature 2018; 560: 185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishida K, Yamada K, Nishiyama R et al. Glymphatic system clears extracellular tau and protects from tau aggregation and neurodegeneration. J Exp Med 2022; 219: 1–9. 10.1084/jem.20211275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carlstrom L, Eltanahy A, Perry A et al. A clinical primer for the glymphatic system. Brain 2021; 145: 843–57. [DOI] [PubMed] [Google Scholar]
- 13. Liu X, Hao J, Yao E et al. Polyunsaturated fatty acid supplement alleviates depression-incident cognitive dysfunction by protecting the cerebrovascular and glymphatic systems. Brain Behavior Immunity 2020; 89: 357–70. [DOI] [PubMed] [Google Scholar]
- 14. He X, Li G, Li L et al. Overexpression of Slit2 decreases neuronal excitotoxicity, accelerates glymphatic clearance, and improves cognition in a multiple microinfarcts model. Mol Brain 2020; 13: 135. 10.1186/s13041-020-00659-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ringstad G, Vatnehol S, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 2017; 140: 2691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taoka T, Masutani Y, Kawai H et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol 2017; 35: 172–8. [DOI] [PubMed] [Google Scholar]
- 17. Zhang W, Zhou Y, Wang J et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage 2021; 238: 118257. 10.1016/j.neuroimage.2021.118257. [DOI] [PubMed] [Google Scholar]
- 18. Steward C, Venkatraman V, Lui E et al. Assessment of the DTI-ALPS parameter along the perivascular space in older adults at risk of dementia. Journal Neuroimaging 2021; 31: 569–78. [DOI] [PubMed] [Google Scholar]
- 19. Chen H, Chen P, Lu C et al. Associations among cognitive functions, plasma DNA, and diffusion tensor image along the perivascular space (DTI-ALPS) in patients with Parkinson's disease. Oxidative Medicine Cellular Longevity 2021; 2021: 4034509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cacciaguerra L, Carotenuto A, Pagani E et al. MRI evaluation of perivascular space abnormalities in neuromyelitis optica. Ann Neurol 2022; 92: 173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamagata K, Andica C, Takabayashi K et al. Association of MRI indices of glymphatic system with amyloid deposition and cognition in mild cognitive impairment and Alzheimer disease. Neurology 2022; 99: e2648–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hsu J, Wei Y, Toh C et al. MRI images implicate glymphatic alterations mediate cognitive dysfunction in AD. Ann Neurol 2023; 93: 164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Z, Zhang M. The application of mini-mental state examination in Chinese. Shanghai Arch Psychiatry 1989; 3: 108–11. [Google Scholar]
- 24. Hensel A, Angermeyer M, Riedel-Heller S. Measuring cognitive change in older adults: reliable change indices for the mini-mental state examination. Journal of Neurology, Neurosurgery, Psychiatry 2007; 78: 1298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stringa N, van Schoor NM, Milaneschi Y et al. Physical activity as moderator of the association between APOE and cognitive decline in older adults: results from three longitudinal cohort studies. J Gerontol A Biol Sci Med Sci 2020; 75: 1880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yokota H, Vijayasarathi A, Cekic M et al. Diagnostic performance of glymphatic system evaluation using diffusion tensor imaging in idiopathic normal pressure hydrocephalus and mimickers. Current Gerontology Geriatrics Research 2019; 2019: 5675014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmidt P, Gaser C, Arsic M et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage 2012; 59: 3774–83. [DOI] [PubMed] [Google Scholar]
- 28. Reeves B, Karimy J, Kundishora A et al. Glymphatic system impairment in Alzheimer's disease and idiopathic normal pressure hydrocephalus. Trends Mol Med 2020; 26: 285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang L, Chopp M, Jiang Q, Zhang Z. Role of the glymphatic system in ageing and diabetes mellitus impaired cognitive function. Stroke Vasc Neurol 2019; 4: 90–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iliff J, Wang M, Liao Y et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012; 4: 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou W, Shen B, Shen WQ, Chen H, Zheng YF, Fei JJ. Dysfunction of the glymphatic system might be related to iron deposition in the normal aging brain. Front Aging Neurosci 2020; 12: 559603. 10.3389/fnagi.2020.559603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jucker M, Walker LJN. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 2013; 501: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferreira A, Neves P, Gozzelino R. Multilevel impacts of iron in the brain: the cross talk between neurophysiological mechanisms, cognition, and social behavior. Pharmaceuticals 2019; 12: 126–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Filiano AJ, Gadani SP, Kipnis J. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat Rev Neurosci 2017; 18: 375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mogensen F, Delle C, Nedergaard M. The glymphatic system (En) during inflammation. Int J Mol Sci 2021; 22: 7698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou Y, Cai J, Zhang W et al. Impairment of the glymphatic pathway and putative meningeal lymphatic vessels in the aging human. Ann Neurol 2020; 87: 357–69. [DOI] [PubMed] [Google Scholar]
- 37. Sun B, Wang L, Yang T et al. Lymphatic drainage system of the brain: a novel target for intervention of neurological diseases. Prog Neurobiol 2018; 163–4: 118–43. [DOI] [PubMed] [Google Scholar]
- 38. Iliff J, Wang M, Zeppenfeld D et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 2013; 33: 18190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hablitz L, Vinitsky H, Sun Q et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv 2019; 5: eaav5447. 10.1126/sciadv.aav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xie L, Kang H, Xu Q et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342: 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shokri-Kojori E, Wang G, Wiers C, et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. PNAS 2018; 115: 4483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. He XF, Liu DX, Zhang Q et al. Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front Mol Neurosci 2017; 10: 144. 10.3389/fnmol.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salehpour F, Khademi M, Bragin DE, DiDuro JO. Photobiomodulation therapy and the glymphatic system: promising applications for augmenting the brain lymphatic drainage system. Int J Mol Sci 2022; 23: 2975–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cao J, Yao D, Li R et al. Digoxin ameliorates glymphatic transport and cognitive impairment in a mouse model of chronic cerebral hypoperfusion. Neurosci Bull 2022; 38: 181–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mortensen K, Sanggaard S, Mestre H et al. Impaired glymphatic transport in spontaneously hypertensive rats. J Neurosci 2019; 39: 6365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jiang Q, Zhang L, Ding G et al. Impairment of the glymphatic system after diabetes. Journal of Cerebral Blood Flow Metabolism 2017; 37: 1326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to privacy issues of data, it is not available to the community via open repository. The datasets generated during the present study are available from the corresponding author on reasonable request. Considerations will be made based on the review of reasons for requesting the data and the procedures for ensuring data privacy.