Abstract
Axicabtagene ciloleucel (axi-cel) was found to have superior clinical outcomes compared to standard of care (SOC; salvage chemoimmunotherapy, followed by high-dose therapy with autologous stem cell rescue for responders) for second-line large B-cell lymphoma (2L LBCL) in the pivotal ZUMA-7 trial. The aim of this analysis was to evaluate the cost effectiveness of using axi-cel compared to the current standard 2L LBCL therapy. A 3-state partitioned-survival model estimated the cost effectiveness and budget impact from a payer perspective in the United States. Clinical outcomes were extrapolated based on the pivotal trial. The model calculated expected quality-adjusted life years (QALYs), total costs (in United States dollars [USD], and the incremental cost-effectiveness ratio (ICER), along with the budget impact. Sensitivity and scenario analyses were performed. The proportion alive at 10 years was estimated as 48% for axi-cel and 38% for SOC; median overall survival was estimated at 59 and 24 months for axi-cel and SOC, respectively. Over a lifetime horizon, the model estimated a total of 5.56 and 7.08 QALYs for SOC and axi-cel, respectively, of which 41% and 74% were in the event-free state, respectively. Incremental QALYs and costs were 1.51 and $100,366 USD, resulting in an ICER of $66,381 USD per QALY for axi-cel versus SOC. Despite crossover to subsequent CAR T in the SOC arm, second-line CAR T use was found to improve the quality and length of life compared to SOC. Cost offsets due to subsequent CAR T use led to a limited incremental cost difference. Treatment with axi-cel is a cost-effective option that addresses an important unmet clinical need for patients with LBCL who relapse or are refractory to front-line therapy.
Keywords: Axicabtagene ciloleucel, Chimeric antigen T-cell therapy, Large B-cell lymphoma, Cost-effectiveness analysis, Health economics
Large B-cell lymphoma (LBCL) is the most common subtype of non-Hodgkin lymphoma. In 2016, around 28,000 new cases were diagnosed in the United States (US) [1]. Overall, 10% to 15% have primary refractory LBCL, and an additional 20% to 25% of patients with LBCL will ultimately relapse from first-line therapy after an initial response [2]. Before chimeric antigen receptor (CAR) T-cell therapies, potentially curative treatments for patients with second-line LBCL who would receive salvage immunochemotherapy followed by high-dose chemotherapy included autologous stem cell transplantation (auto-SCT), and then allogeneic-SCT for those in whom auto-SCT fails. However, event-free survival (EFS) and overall survival (OS) for second-line LBCL patients treated with salvage chemotherapy are poor: less than 20% of SCT-intended patients are event free at 2 years, and around 30% are alive at 2 years, with a median survival of 11 months [3].
Multiple CAR T therapies have recently been approved for the treatment of third-line (3L) LBCL in the US, and in relapsed/refractory LBCL, after 2 or more prior lines, CAR T-cell therapies have shown high response rates and prolonged survival [4–6]. Recently presented long-term follow-up data from the pivotal phase 2 ZUMA-1 trial found that treatment with axicabtagene ciloleucel (axi-cel, Kite Pharma) resulted in 43% alive at 5 years, further supporting the potentially curative treatment option for many patients [7]. The comparative SCHOLAR-1 study also found that patients treated with axi-cel had a 73% reduction in the risk of death (hazard ratio [HR] = 0.27; 95% confidence interval [CI], 0.00-0.38) compared to salvage regimens [8]. Previous cost-effectiveness analyses of axi-cel in the 3L setting have found that treatment with axi-cel is associated with incremental gains in survival over chemotherapy and is a cost-effective use of healthcare resources [9,10].
The pivotal ZUMA-7 (NCT03391466) trial is the first and largest phase 3 randomized study in 2L LBCL evaluating the efficacy of a CAR T-cell therapy versus standard of care (SOC) (salvage chemotherapy followed by high-dose therapy with auto-SCT rescue for responders) [11]. At a median follow-up of 24.9 months, the primary analysis found that axi-cel demonstrated a 60.2% improvement in EFS compared with patients receiving SOC (HR = 0.398; 95% CI, 0.32 to 0.51; P< .0001) [11]. In addition, despite 56% of patients in the SOC arm receiving a subsequent CAR T-cell therapy, a trend toward improved OS was observed (HR = 0.73; 95% CI, 0.53 to 1.01; P= .03) [7]. These data supported axi-cel’s recent approval by the Food and Drug Administration in the US for the treatment of adult patients with LBCL who relapse or are refractory to first-line chemoim-munotherapy [12]. However, these benefits come at a higher upfront cost and must be examined formally in an economic evaluation. To inform healthcare decision-making, we conducted an economic evaluation of axi-cel compared to SOC, to investigate the long-term economic and humanistic implications of administering axi-cel in the 2L setting for LBCL.
MATERIAL AND METHODS
Cost-Effectiveness Model Overview
A partitioned survival model based on the patients enrolled in the ZUMA-7 trial (n = 11) was developed in Microsoft Excel in line with best practice guidelines [13]. The model follows a theoretical cohort of 2L LBCL patients over time, as they move between the mutually exclusive health states as event-free, post-event, or dead. The model estimates the proportion of the cohort in each health state at a given time based on the extrapolated survival curves and assigns costs and utilities based on the health state occupied. Reported Kaplan-Meier data from the trial informed EFS and OS inputs (OS in Supplementary Figure S1), and time to event outcomes beyond the study observation period were extrapolated using mixture cure models (MCM). Associated direct medical expenditures (costs) and quality of life with the health states were included over a lifetime time horizon. The analysis was conducted from a US third-party commercial payer perspective. Modeled outcomes include costs, life years (LYs) and quality-adjusted life years (QALYs) (all discounted at 3.0% per year) and the incremental cost effectiveness ratio (ICER). Model input values and data sources are summarized in Table 1, with additional detail provided in the below sections and in the Supplementary Materials.
Table 1.
Model Input Values, Distributions, Variations and Data Sources
| Model input | Point Estimate | Distribution | Variation | Source |
|---|---|---|---|---|
| Health state utility values | ||||
| Axi-cel, on-treatment | 0.740 | Beta | 0.031 | Roth 2018 [9] |
| SOC, on-treatment | 0.673 | Beta | 0.038 | Roth 2018 [9] |
| Pre-event, off-treatment | 0.823 | Beta | 0.041 | Sullivan 2006 [22] |
| Post-event | 0.710 | Beta | 0.036 | NICE (TA567) [23] |
| Direct costs* | ||||
| Axi-cel acquisition | $399,000 | N/A | $79,800 | List price |
| Lisocabtagene maraleucel acquisition | $410,300 | N/A | $82,060 | RED BOOK [18] |
| Tisagenlecleucel acquisition | $373,000 | N/A | $74,600 | RED BOOK [18] |
| CAR T-cell administration† | $72,977 | Gamma | $14,595 | Liu 2021 [24] |
| Leukapheresis, cost | $1,173 | Gamma | $235 | RED BOOK [18]; CMS 2021 [19] |
| Bridging/conditioning chemotherapy | $5,231 | N/A | N/A | RED BOOK [18]; CMS 2021 [19] |
| R-ICE | $7,873 | N/A | N/A | RED BOOK [18]; CMS 2021 [19] |
| R-ESHAP | $6,950 | N/A | N/A | RED BOOK [18]; CMS 2021 [19] |
| R-GDP | $7,035 | N/A | N/A | RED BOOK [18]; CMS 2021 [19] |
| R-DHAP | $6,973 | N/A | N/A | RED BOOK [18]; CMS 2021 [19] |
| HDT | $10,569 | N/A | N/A | RED BOOK [18]; CMS 2021 [19] |
| Chemotherapy inpatient administration | $3,642 | Gamma | $728 | Dasta 2005 [25] |
| Chemotherapy outpatient administration | $1,183 | Gamma | $237 | CMS 2021 [19] |
| Stem cell harvest | $78,358 | Gamma | $15,672 | Pelletier 2018 [26] |
| Auto-SCT | $44,506 | Gamma | $8901 | Pelletier 2018 [26] |
| Auto-SCT follow-up† | $13,949 | Gamma | $2790 | Pelletier 2018 [26] |
| Allo-SCT(+) | $355,387 | Gamma | $71,077 | Broder 2017 [27] |
| Allo-SCT follow-up§ | $313,495 | Gamma | $62,699 | Broder 2017 [27] |
| End of life care | $19,406 | Gamma | $3881 | Kutikova 2006 [28] |
| Pre-event resource utilization | $2,217 | Gamma | $443 | See Supplementary Table S4 |
| Post-event resource utilization‖ | $2,429 | Gamma | $486 | |
| BIM inputs | ||||
| Axi-cel market uptake¶ | 2%-6% | N/A | N/A | Data on file |
| Year 1 | 9%-13% | |||
| Year 2 | 12%-16% | |||
| Year 3 | 14%-18% | |||
| Year 4 | 16%-20% | |||
| Year 5 | ||||
| Incidence of LBCL | 54/1,000,000 | N/A | N/A | SEER [29] |
| Primary refractory disease | 15% | N/A | N/A | Sehn 2021 [2] |
| Relapsed disease | 25% | N/A | N/A | Sehn 2021 [2] |
| Proportion relapsed within 12 months | 70% | Maurer 2014 [30] | ||
| Proportion intended for auto-SCT | 50% | N/A | N/A | Friedberg 2011 [31] |
Allo-SCT indicates allogeneic stem cell transplantation; BIM, budget impact model; CMS, Center for Medicaid Services[CT, computerized tomography; HDT, high-dose therapy; R-DHAP, rituximab, dexamethasone, cytarabine, cisplatin; R-ESHAP, rituximab, etoposide, cytarabine, cisplatin; R-GDP, rituximab, gemcitabine, dexamethasone, cisplatin; R-ICE, rituximab, ifosfamide, carboplatin, etoposide.
All costs reported in 2021 USD.
Inpatient administration costs include the management of all AEs with the exception of B-cell aplasia.
Auto-SCT follow-up applied monthly up to a maximum of 24 months.
Only as a subsequent treatment; allo-SCT follow-up applied as a one-off cost.
Post-event resource utilization reverts to $0 at 5 years.
SOC is assumed to account for the remainder of the market share.
Model Structure
The partitioned survival model included three mutually exclusive health states: event-free, post-event and death (Figure 1). Sub-states were used to model time on or off treatment to account for treatment related costs and adverse events. In the event-free state, patients were on-treatment with either 2L axi-cel or SOC. Treatments in the post-event state were based on third or higher lines observed in the ZUMA-7 clinical trial [14]. Model cycle length was 1 month, and half-cycle corrections were applied.
Figure 1.
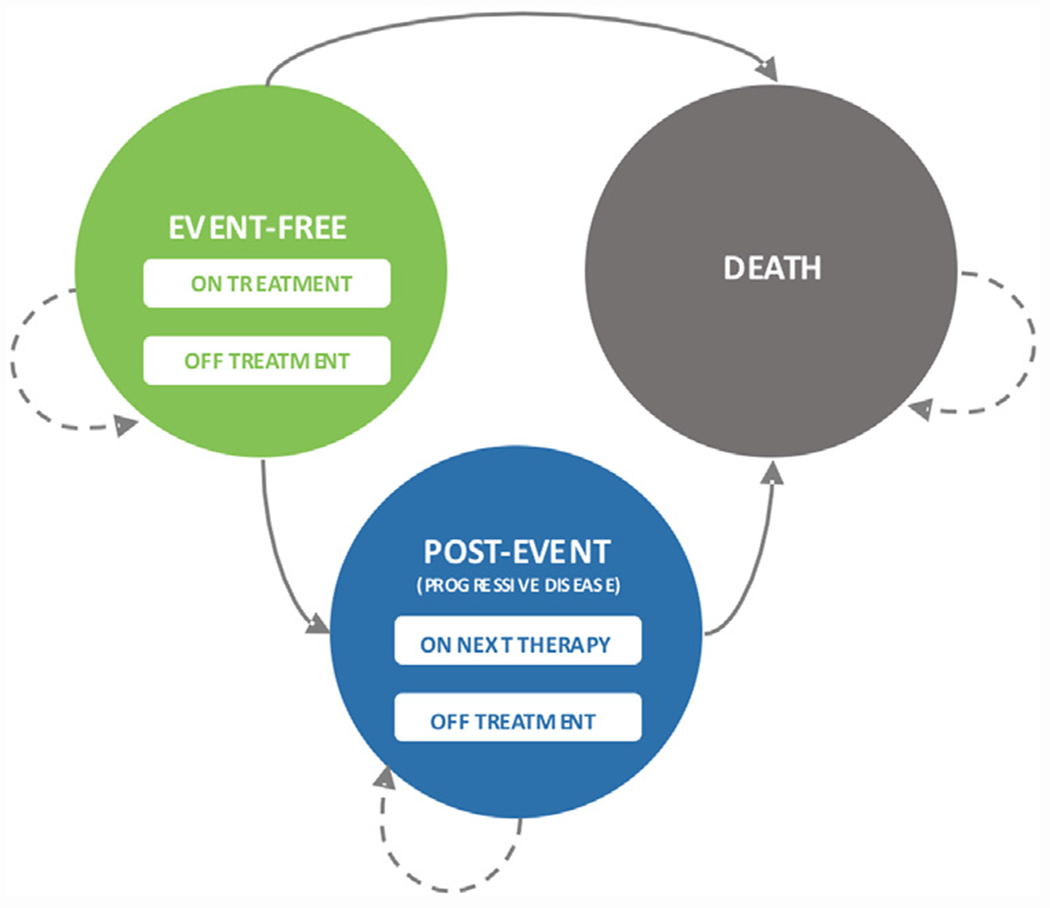
Model structure. Mutually exclusive health states are depicted by the circles. Arrows represent transitions between or within states; death is an absorbing state. Transition between states was driven by extrapolations of time to event data from the ZUMA-7 trial.
Time to Event Outcomes
EFS, time to next treatment (TTNT), and OS were fit independently and extrapolated over the lifetime horizon from the Kaplan-Meier curves for respective trial arms (Figure 2) [15]. Based on previous research on axi-cel in LBCL, MCMs were deemed appropriate to predict the shape of the curves [16]. The method for selecting the base case curves was based on best statistical fit and clinical plausibility. Additional details on model selection and MCM methods are described in the Supplementary Materials.
Figure 2.
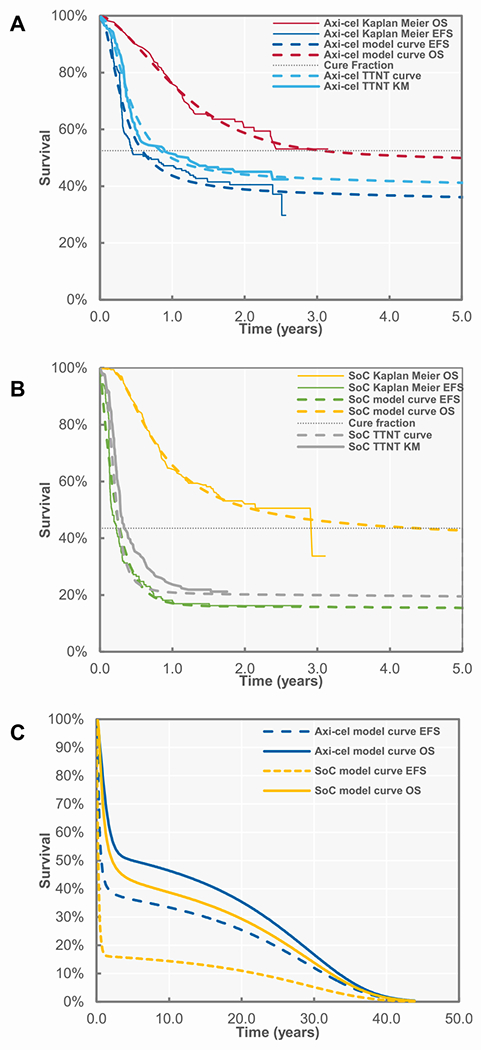
Survival plots (A) axi-cel, (B) standard of care, and (C) modeled extrapolated survival. TTNT indicates time to next treatment.
Costs
All costs are reported in 2021 US dollars (USD) and were inflated using the Consumer Price Index in the US [17]. Costs of drugs at list price were taken from RedBook [18], current as of December 2021; costs associated with the administration of treatments were obtained from the Center for Medicaid Services Physician Fee Schedule [19].
Cost of 2L Therapies
The cost of treatment with axi-cel included acquisition cost, infusion-related costs, leukapheresis, bridging corticosteroids, and conditioning chemotherapy (Table 1). Costs for 2L therapies in the SOC arm included salvage chemotherapy, high-dose chemotherapy, and auto-SCT-related costs. These were applied as a weighted average based on the frequencies of 2L treatment received in the ZUMA-7 trial. Of the 179 patients in the SOC arm, 168 received one of the four allowed salvage chemotherapies available in the trial (Supplementary Table S1). Of the of 80/179 patients who responded to salvage chemotherapy, 64/179 received high-dose therapy, and 62/179 underwent transplant in the second-line [14]. Additional detail is provided in the Supplementary Material.
Cost of Subsequent Therapies
Distribution and costs of subsequent therapies are presented in Supplementary Table S2. Costs were applied as a one-off cost at time of initiation of the post-event subsequent treatment, based on the TTNT curve. Duration of treatment was not modeled and an average number of treatment cycles were estimated. All patients receiving a CAR T-cell therapy as a subsequent treatment were costed with the same assumptions as in the 2L (Table 1). Patients who did not have a TTNT event by 5 years were assumed to achieve longterm remission, thus not requiring subsequent treatment.
Cost of Adverse Events
Grade 3 and greater adverse events (AEs) associated with axi-cel treatment, with the exception of B-cell aplasia, were assumed to occur during the initial treatment and hospitalization period and were thus not costed separately. The incidence of grade 3 and greater AEs for the SOC arm were informed by the ZUMA-7 trial (Supplementary Table S3). Because safety data were not available for all subsequent treatments, the cost of managing treatment-related AEs for subsequent treatments, including CAR T-cell therapies, was not considered. Costs for managing AEs are presented in Supplementary Table S3.
Health State Resource Utilization
Resource utilization included annual hospitalization, physician visits, lab testing and disease management costs (Supplementary Table S4) and were assumed to be equivalent for axi-cel and SOC (Supplementary Table S4) [14]. A one-time cost for end of life care was assigned to patients upon death. It was assumed that no LBCL-related resource use was incurred for those patients who remained event free after 5 years.
Health-Related Quality of Life
Utility values for on-treatment, pre-event off-treatment, and post-event are presented in Table 1 with additional detail in the Supplementary Materials. It was assumed that utility values revert back to that of the age-matched general population for patients who remain event-free for 5 years.
Sensitivity and Scenario Analyses
Input uncertainty was evaluated through both one-way and probabilistic sensitivity analyses. One-way sensitivity analyses, where costs and utilities were varied by ±20%, are presented as tornado diagrams, displaying the most influential parameters on model results. Probabilistic sensitivity analyses, where all parameters are simultaneously varied across predetermined distributions (gamma for costs, beta for bounded estimates), are presented as a cost-effectiveness plane. The probabilistic sensitivity analysis was run until model convergence. Alternate distributions of time-to-event data were explored in scenario analyses, including more optimistic and more pessimistic curves. A scenario analysis specific to subsequent treatments used from participating US clinical sites in the ZUMA-7 trial was performed. Subsequent treatment data were reanalyzed based on treatments used only by US patients to account for potential impacts on costs (Supplementary Table S5).
Budget Impact Model
A budget impact model was built using the cost-effectiveness model engine and included the cost of 2L treatments, adverse events in the 2L, as well as 3L treatment-related costs. The budget impact was calculated based on the difference of a future practice where CAR T-cell therapy is included as a treatment option for patients in the 2L versus the current practice where no CAR T-cell therapy is included in the 2L. Epidemiological and projected treatment pattern inputs are presented in Table 1, with additional detail in the Supplementary Material.
RESULTS
Cost-Effectiveness Analysis
In the base case analysis, the model estimated 5-year EFS to be 36.1% and 15.4% for axi-cel and SOC, respectively. The 10-year OS in the model was estimated as 46% for axi-cel and 38% for SOC (Figure 2) with a median OS of 59 and 24 months for axi-cel and SOC, respectively. Based on results from the MCM fitting to the OS curve, 53% and 42% of axi-cel and SOC patients, respectively, were considered long-term responders and of patients alive at 10 years, patients treated with axi-cel were 94% more likely to be event-free. Total discounted treatment-related costs (including both 2L and subsequent treatment) were $546,704 USD for axi-cel and $440,099 USD for SOC (Supplementary Table S6). Costs for subsequent therapy were higher among those receiving SOC in the 2L (Supplementary Figure S2). Among patients receiving 2L SOC, the highest proportion of subsequent treatment costs was for CAR-T therapies; among patients receiving 2L axi-cel, the highest proportion of subsequent treatment costs were for SCT. Further, use of CAR-T therapies were a primary constituent of costs in every line of therapy following SOC in the 2L (Supplementary Figure S3). Over the lifetime time horizon, incremental total costs were $100,366 USD and the model estimated 7.08 and 5.56 discounted QALYs for axi-cel and SOC, respectively, for a difference of 1.51. Treatment with axi-cel resulted in an ICER of $66,381 USD per QALY gained (Table 2).
Table 2.
Cost-Effectiveness Results (Discounted) in Base Case, 2021 USD
| AXI-CEL | SOC | Difference | |
|---|---|---|---|
| Life years | 9.14 | 7.59 | 1.55 |
| Event-free | 6.48 | 2.86 | 3.63 |
| Post-event | 2.66 | 4.73 | −2.07 |
| Quality-adjusted life years | 7.08 | 5.56 | 1.55 |
| Event-free | 5.23 | 2.28 | 2.95 |
| Post-event | 1.84 | 3.28 | −1.44 |
| Total costs | $635,794 | $535,428 | $100,366 |
| Second-line treatment-related | $449,786 | $95,319 | $354,467 |
| Subsequent treatment-related | $96,917 | $344,779 | −$247,862 |
| Disease management* | $85,658 | $77,282 | $8376 |
| ICER, axi-cel versus SOC | $66,381 |
Includes terminal care.
One-way deterministic sensitivity analyses found that the ICER was most sensitive to the axi-cel treatment cost (varied by ±20%), subsequent treatment patterns in the SOC arm, the number of inpatient days for axi-cel administration and post-event utilities (Supplementary Figure S4). The probabilistic sensitivity analysis found that, at a willingness to pay threshold of $150,000 USD per QALY, axi-cel was cost-effective versus SOC in 75% of the simulations (Supplementary Figures S5, S6).
When the gamma curve (a less favorable but still clinically plausible extrapolation) was selected for the extrapolation of axi-cel OS, the model estimated 10-year OS was 44.9%, and the ICER increased to $73,503 USD per QALY (Supplementary Table S7). The Gompertz curve (a more optimistic) and loglogistic curve (less optimistic for efficacy with a cure fraction of 44%) resulted in ICERs of $60,709 USD and $118,171 USD per QALY, respectively. When only considering US clinical practice treatment patterns, incremental costs of axi-cel versus SOC were reduced to $91,374 USD and axi-cel remained cost-effective with an ICER of $60,434 USD per QALY.
Budget Impact Analysis
It was estimated that 9 patients in a million member plan would be eligible for axi-cel treatment as 2L LBCL patients. The introduction of axi-cel to 2L treatment of patients with LBCL leads to a total 5-year cumulative budget impact ranging of $0.03 USD per member per month (PMPM) for the lower bound to $0.07 USD PMPM for the upper bound (Supplementary Table S8).
DISCUSSION
Patients with LBCL in whom a front-line therapy has failed have few curative intent treatment options, with associated limited long-term remission and prolonged survival. This analysis suggests increased overall survival from treatment with axi-cel as observed with 10-year estimated OS of 46.4% and 37.5% for axi-cel and for SoC, respectively, and a higher proportion of long-term responders for patients treated with axicel compared to SoC (53% versus 43%). This finding is consistent with the higher proportion of patients that received a potentially curative treatment option in the axi-cel arm of the trial and is reflected by the increased incremental QALYs of treatment with axi-cel. Furthermore, treatment with axi-cel in the second-line resulted in a budget impact of ~$0.01 PMPM due to the small population of patients eligible for treatment and cost-offsets of subsequent treatment.
This analysis estimated incremental costs were $103,808 USD higher with axi-cel; however, there were important offsets compared to SOC. Although 2L treatment costs were lower for the SOC arm due to the low cost of salvage chemotherapy, subsequent treatment costs represented nearly 80% of the total treatment-related costs for the SOC arm, including $266,778 USD from CAR T-cell therapy use as a subsequent treatment. Additionally, the higher proportion of long-term responders after treatment with axi-cel limited the disease management costs in the post-event health state, further reducing the difference in cost between arms. As with the cost-effectiveness analysis, as 2L CAR T uptake was increased in the budget impact analysis, the costs associated with CAR T therapies as a subsequent treatment decreased.
We found the ICER to be $66,381 USD per QALY gained, suggesting that 2L treatment with axi-cel was highly cost-effective versus SOC in the US. These results were maintained over a wide range of sensitivity and scenario analyses. Given uncertainty around long-term survival, when a more conservative yet still clinically plausible curve (i.e., demonstrating a benefit for axi-cel treatment vs. SoC) was used to extrapolate OS, the ICER increased to $73,503 USD per QALY, still below the accepted threshold for cost-effectiveness in the US [20]. Sensitivity analyses found that the biggest cost drivers of the cost-effectiveness and budget impact analysis were subsequent treatment patterns in the SOC arm, including the use of CART-cell therapies and the cost of auto-SCT.
The increase in length and quality of life from treatment with axi-cel resulted in incremental QALYs of 1.51, a large and meaningful improvement. Furthermore, 74% of the QALYs were in the EFS state for the axi-cel cohort compared to 41% for SoC, suggesting that patients treated with axi-cel spend a greater proportion of their lives with the higher quality-of-life benefit of EFS. These gains in efficacy estimated in this economic analysis align with what was observed in the ZUMA-7 trial, including prolonged EFS, contributing to incremental QALYs. Although the utilities in this economic model were taken from the literature, as observed in the ZUMA-7 trial, treatment with axi-cel results in a quicker return to baseline in quality of life compared to SOC [21]. In addition to the benefit observed in EFS health state, a trend of improved OS was observed. This trend is likely influenced by the receipt of CAR T therapies outside the protocol in the ZUMA-7 trial, which may have confounded the OS analysis [11]. Notwithstanding, the benefit of treatment with axi-cel translates into important incremental long-term benefits for patients.
The ZUMA-7 trial data used to conduct this economic evaluation is the first randomized controlled trial to compare a CAR T-cell therapy versus SOC, with the largest sample size to date and longest duration of follow-up (median 24.9 months). Given that both CAR T and auto-SCT are potentially curative and require upfront costs, this cost-effectiveness analysis may support clinical decisions for determining treatment pathways and timing for treatments with curative intent. The ZUMA-7 trial suggests that 64% of transplant-intended patients did not receive auto-SCT; furthermore, at least 56% of patients in the SoC arm received a subsequent treatment with curative intent. This results in both increased overall costs and a higher risk of death before patients can receive 3L therapy.
This economic analysis has limitations. Although all available data from the trial were used to inform the economic models, the cost of managing adverse events and related disutilities for subsequent treatments were not included in this economic analysis. Thus the ICER presented could be conservative, as the impact on patients due to delayed or reduced need for subsequent treatments was not accounted for. The OS curves selected for extrapolation were based on statistical criteria and clinical plausibility, however, as with many economic analyses, survival benefits may be driven by the selection in curve. Scenario analyses where survival along with other parameters extrapolations were varied found that treatment with axi-cel remained cost-effective in the majority of simulations. It should also be noted that the use of CAR T therapies as a subsequent treatment in the model were based on observed use in the ZUMA-7 trial and may not be reflective of real-world treatment patterns, which would vary due to logistical, socio-economic, or provider considerations.
CONCLUSIONS
This economic analysis compared 2L axi-cel versus SOC, where we found that 2L axi-cel use improved survival and increased time without disease progression, resulting in a substantial gain in QALYs. Furthermore, 2L CAR T has limited financial impact due to the offset of subsequent CAR T use and reduced disease progression costs. Treatment with axi-cel for patients with LBCL who relapse or are refractory to front-line therapy is cost-effective compared to a less dependable strategy of SOC, where despite completing therapy, a minority of patients make it to definitive therapy in 2L and many patients proceed to 3L CAR-T.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Lianne Barnieh of the Maple Health Group for medical editorial assistance under the guidance of the authors, which was funded by Kite Pharma in accordance with Good Publications Practice guidelines.
Findings from this study have been presented at the 48th Annual Meeting of the European Society for Blood and Marrow Transplantation and the 2022 Tandem Meetings of the American Society for Transplantation and Cellular Therapy and Center for International Blood and Marrow Transplant Research.
Financial disclosure:
Kite Pharma funded this study. The funders of the study had a role in the study design, data interpretation and editing of the manuscript. The funders had no role in the data analysis. M-A,P, has also received support in part from the Memorial Sloan Kettering Cancer Center Core grant (P30 CA008748) from the National Institutes of Health/National Cancer Institute.
Conflict of interest statement:
M-A.P. has received institutional research support clinical trials from Incyte, Kite Pharma, Gilead Sciences, Miltenyi Biotec, Nektar Therapeutics and Novartis; honoraria from Abbvie, Allovir, Astellas, Bristol-Myers Squibb, Celgene, Equilium, Exevir, Incyte, Karyopharm, Kite Pharma, Gilead Sciences, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, OrcaBio, Takeda, VectivBio AG, and Vor Biopharma. M-A.P. serves on the Data Safety Monitoring Board for Cidara Therapeutics, Medigene, Sellas Life Sciences and Servier; and on the Scientific Advisory Board for NexImmune. M-A.P. has ownership interests in NexImmune and Omeros. J.K. has received research support from Roche, Astra Zeneca and Merck; consulting fees from Abbvie, Antengene, BMS, Gilead, Karyopharm, Medison Ventures, Merck, Roche and Seattle Genetics; and honoraria from Abbie, Amgen, Astra Zeneca, Bristol-Myers Squibb, Gilead, Incyte, Janssen, Karyopharm, Merck, Novartis, Pfizer, Roche, and Seattle Genetics. J.K. serves as the Scientific Advisory Board Chair for Lymphoma Canada and on the Data Safety Monitoring Board for Karyopharm. R.B., F.E.M., and N.J.S. are employees of Maple Health Group, who were contracted by Kite Pharma to conduct the work contained in this manuscript. J.T.S., S.V., and A.P. are employees of Kite Pharma. J.T.S. holds stock or other ownership in Gilead Sciences.
Footnotes
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jtct.2022.08.010.
REFERENCES
- 1.Teras LR DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66:443–459. [DOI] [PubMed] [Google Scholar]
- 2.Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021;384:842–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Imhoff GW, McMillan A, Matasar MJ, et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD Study. J Clin Oncol. 2017;35:544–551. [DOI] [PubMed] [Google Scholar]
- 4.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 6.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson C, Locke F, Ghobadi A, et al. Long-term (4- and 5-year) overall survival in ZUMA-1, the pivotal study of axicabtagene ciloleucel (axi-cel) in patients with refractory large B-cell lymphoma (LBCL). Blood. 138. 202120212021:1764. [Google Scholar]
- 8.Neelapu SS, Locke FL, Bartlett NL, et al. Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs salvage chemotherapy in refractory large B-cell lymphoma. Blood Adv. 2021;5:4149–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth JA, Sullivan SD, Lin VW, et al. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J Med Econ. 2018;21:1238–1245. [DOI] [PubMed] [Google Scholar]
- 10.Whittington MD, McQueen RB, Ollendorf DA, et al. Long-term survival and cost-effectiveness associated with axicabtagene ciloleucel vs chemotherapy for treatment of B-cell lymphoma. JAMA Netw Open. 2019;2(2):e190035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–654. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. Kite Pharma Inc. YESCARTA [package insert]. Available at https://www.fda.gov/media/108377/download. Accessed: February 8 2022.
- 13.Ramsey S, Willke R Briggs A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISpOr RCT-CEA Task Force report. Value Health. 2005;8:521–533. [DOI] [PubMed] [Google Scholar]
- 14.KITE. Data on File. 2021. [Google Scholar]
- 15.Latimer N NICE DSU Technical Support Document 14: Undertaking survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data. 2011. [PubMed] [Google Scholar]
- 16.Vadgama S, Mann J, Bashir Z, Spooner C, Collins GP, Bullement A. Predicting survival for chimeric antigen receptor T-cell therapy: a validation of survival models using follow-up data from ZUMA-1. Value Health. 25. 202220222022:1010–1017. [DOI] [PubMed] [Google Scholar]
- 17.United States Department of Labor. CPI for All Urban Consumers, Medical care services. Available at https://beta.bls.gov/dataViewer/view/timeseries/CUSR0000SAM2. Accessed: August 12, 2021.
- 18.RED BOOK. Available at https://www.ibm.com/products/micromedex-red-book. Accessed: August 12, 2021.
- 19.Centers for Medicare & Medicaid Services. Physician Fee Schedule. Available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched. Accessed: August 12, 2021.
- 20.ICER. 2020-2023 Value Assessment Framework. Institute for Clinical and Economic Review; 2020. January 31, 2020 (updated October 23, 2020). [Google Scholar]
- 21.Elsawy M, Chavez J, Avivi I, Larouche J-F, Wannesson L, Cwynarski K, et al. Patient-reported outcomes in a phase 3, randomized, open-label study evaluating the efficacy of axicabtagene ciloleucel (axi-cel) versus standard of care therapy in patients with relapsed/refractory large B-cell lymphona (ZuMa-7). Atlanta, Georgia: American Society of Hematology; 2021. [Google Scholar]
- 22.Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NICE. Tisagenlecleucel for treating relapsed or refractory diffuse large B-cell lymphoma after2 or more systemic therapies [TA567]. London, UK: National Institute for Health and Care Excellence; 2019. [Google Scholar]
- 24.Liu R Oluwole OO, Diakite I, Botteman MF, Snider JT, Locke FL. Cost effectiveness of axicabtagene ciloleucel versus tisagenlecleucel for adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy in the United States. J Med Econ. 2021;24:458–468. [DOI] [PubMed] [Google Scholar]
- 25.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33:1266–1271. [DOI] [PubMed] [Google Scholar]
- 26.Pelletier EM, Smith PJ, Dembek CJ. Payer costs of autologous stem cell transplant: results from a U.S. Claims Data Analysis. Blood. 2008;112:2373. [Google Scholar]
- 27.Broder MS, Quock TP, Chang E, et al. The cost of hematopoietic stem-cell transplantation in the United States. Am Health Drug Benefits. 2017;10:366–374. [PMC free article] [PubMed] [Google Scholar]
- 28.Kutikova L, Bowman L, Chang S, Long SR, Arning M, Crown WH. Medical costs associated with non-Hodgkin’s lymphoma in the United States during the first two years of treatment. Leuk Lymphoma. 2006;47:1535–1544. [DOI] [PubMed] [Google Scholar]
- 29.Surveillance EaERP. Cancer Stat Facts: NHL - Diffuse Large B-Cell Lymphoma (DLBCL). Available at https://seer.cancer.gov/statfacts/html/dlbcl.html. Accessed: February 8 2022.
- 30.Maurer MJ, Ghesquieres H, Jais JP, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32:1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011:498–505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


